Abstract
The host response against foreign materials designates the biocompatibility of intravenously administered microcapsules and thus, widely affects their potential for subsequent clinical use as artificial oxygen/drug carriers. Therefore, body distribution and systemic parameters, as well as markers of inflammation and indicators of organ damage were carefully evaluated after administration of short-chained poly (vinyl alcohol, (PVA)) solution or poly (ethylene glycol (PEG))-shielded perfluorodecalin-filled poly (d,l-lactide-co-glycolide, PFD-filled PLGA) microcapsules into Wistar rats. Whereas PVA infusion was well tolerated, all animals survived the selected dose of 1247 mg microcapsules/kg body weight but showed marked toxicity (increased enzyme activities, rising pro-inflammatory cytokines and complement factors) and developed a mild metabolic acidosis. The observed hypotension emerging immediately after start of capsule infusion was transient and mean arterial blood pressure restored to baseline within 70 min. Microcapsules accumulated in spleen and liver (but not in other organs) and partly occluded hepatic microcirculation reducing sinusoidal perfusion rate by about 20%.
Intravenous infusion of high amounts of PFD-filled PLGA microcapsules was tolerated temporarily but associated with severe side effects such as hypotension and organ damage. Short-chained PVA displays excellent biocompatibility and thus, can be utilized as emulsifier for the preparation of drug carriers designed for intravenous use.
Keywords: Artificial oxygen carriers, Biodegradable microcapsules, Perfluorocarbon, Poly (lactic/glycolic) acid (PLGA, PLA), Poly (vinyl alcohol), Biocompatibility
Abbreviations: ALAT, alanine aminotransferase; ANOVA, one-way analysis of variance; ASAT, aspartate aminotransferase; BE, base excess; CARPA, complement activation-related pseudoallergy; CK, creatine kinase; C3, complement factor 3; C4a, complement factor 4a; DAPI, 4',6-diamidin-2-phenylindol; FITC-dextran, fluorescein isothiocyanate-dextran 150,000; IFN-?, interferon-gamma; IL, interleukin; IVM, intravital microscopy; LDH, lactate dehydrogenase; MAP, mean arterial blood pressure; PEG, poly (ethylene glycol); PFD, perfluorodecalin; PLGA, poly (d,l-lactide-co-glycolide); pO2, pCO2, oxygen and carbon dioxide partial pressures; PVA, poly (vinyl alcohol); TNF-a, tumor necrosis factor alpha
Graphical abstract
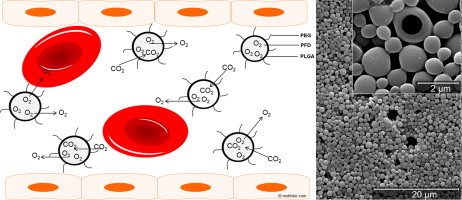
1. Introduction
Artificial blood substitutes are urgently needed to guarantee the rising blood supply of the population. Packed red cells are inadequately available, require cold storage conditions, display a short shelf-life and are associated with problems such as blood group compatibility and risk of transmission of various diseases [1]. Thus, alternatives such as perfluorocarbon-based oxygen carriers have moved into the focus of medical research. Perfluorocarbons (PFCs) are fluorinated hydrocarbons dissolving effectively the main respiratory gases oxygen and carbon dioxide in a manner that depends linearly on the partial pressure of the correspondent gas [2]. Their known chemical and biological inertness, due to the strength of the carbon-fluorine bonds, make them perfect candidates for medical applications but also evoke galenical problems [2]. So far, PFCs were always engineered as oil-in-water emulsions (in which PFC constitutes the oil phase) rendering them blood-compatible for intravenous administration [1]. However, typical problems such as biological incompatibility of the used emulsifiers, coalescence and flocculation of emulsion droplets leading to an increased particle size could not be satisfactorily eliminated [3]. We tried to overcome these problems by engineering biocompatible poly (ethylene glycol)-coated poly (d,l-lactide-co-glycolide) microcapsules (PLGA microcapsules) with a PFC core [4,5]. PLGA and poly (ethylene glycol) (PEG) are metabolizable, harmless compounds, that are approved by the Food and Drug Administration for internal use in humans, thus representing ideal raw materials for the design of intravenously applicable drug carriers [5]. To our knowledge, in vivo data are missing about intravenously administered microparticles made of PEG and PLGA, although these two materials are very common substances that have been excessively explored. Moreover, there generally exist only very few in vivo data about microparticles designed for intravenous application [6,7]. Certainly this is because the design of microcarrier systems for intravascular use represents a special challenge [7]. In difference to ultrasonic contrast agents or usual drug carriers, the dedication of microcapsules as artificial oxygen carriers requires the safe intravenous application of very high amounts of the pharmaceutical (1/10–1/3 of the blood volume for oxygen carriers, in contrast to 1/20,000 for ultrasonic contrast agents [8]).
The principal suitability of PFCs as artificial oxygen carrier is widely recognized in the literature [1,9] and the general feasibility of intravenous administration of our PFD-filled PLGA microcapsules has already been demonstrated [5]. Favorably, the pharmaceutical agent PFD must only be encapsulated and the intact capsule wall must allow an effective gas exchange. In the present work we focused on the detailed investigation of side effects caused by the intravenous administration of very high amounts of PFD-filled PLGA microcapsules to further the use as artificial oxygen carriers, because until now, none of the current formulations of hemoglobin-based or perfluorocarbon-based artificial oxygen carriers can be infused without toxic effects in sufficient amounts in order to preserve aerobic metabolism in all tissues [10].
2. Materials
2.1. Chemicals
Poly (d,l-lactide-co-glycolide) (PLGA) (50:50) produced by LACTEL (B6013-2P, inherent viscosity in chloroform 0.67 dl/g) was purchased from NRC Nordmann Rassmann (Hamburg, Germany). Poly (d,l-lactide-co-glycolide) (50:50) copolymers covalently attached with poly (ethylene glycol) (PEG) (RESOMER PEG Sample CR, RGPd50155, inherent viscosity in chloroform 0.50 dl/g) were obtained from Boehringer Ingelheim (Ingelheim, Germany). Nile red, fluorescein isothiocyanate-dextran 150,000 (FITC-dextran) and poly (vinyl alcohol) (PVA, Mw 9000–10,000, 80% hydrolyzed) came from Sigma (Deisenhofen, Germany). Perfluorodecalin (PFD) was from F2 Chemicals (Preston, United Kingdom). Isoflurane (Forene®) was obtained from Abbott (Wiesbaden, Germany), ketamine 10% from Ceva (Düsseldorf, Germany) and lidocaine (Xylocain® 1%) from AstraZeneca (Wedel, Germany). Portex® catheters (0.58 mm i.d./0.96 mm o.d.) were purchased from Smiths Medical International (Hythe, United Kingdom). Medical oxygen was obtained from Air Liquide (Düsseldorf, Germany), ringer solution from Fresenius (Bad Homburg, Germany) and sterile NaCl 0.9% Ecoflac from B. Braun, Melsungen, Germany. Paraffin (Paraplast Tissue Embedding Medium REF 501,006) was from McCormick Scientific (St. Louis, USA). Cryomatrix (Shandon Cryomatrix) was purchased from Thermo, Fisher Scientific (Waltham, USA) and Mounting Medium (Vectashield Hard Set Mounting Medium with DAPI, H-1500) from Vector Laboratories (Burlingame, USA).
3. Methods
3.1. Capsule preparation
PFD-filled PEG-PLGA microcapsules (diameter of 1.5 ± 0.8 µm) were prepared by an emulsion-evaporation procedure as described before [5]. Briefly, an organic solution of 100 mg PLGA (92% PLGA, 8% PEG-PLGA) and 100 µl PFD in 6 ml methylene chloride was emulsified into 20 ml of an aqueous solution of 1% PVA by using an Ultraturrax T25 (IKA Werke, Staufen, Germany) operating with an S25KV-25G-IL dispersing tool at a velocity of 10,200 rpm. For preparation of PEG-PLGA microspheres (unfilled polymer particles with a diameter of 1.5 ± 0.8 µm) Ultraturrax velocity was changed to 5400 rpm. The used methylene chloride was evaporated under magnetic stirring. Microcapsules (independent of the capsule type) were centrifuged (199.6 g, 20 min, Biofuge primo R, Heraeus, Hanau, Germany) and washed three times with sterile 0.9% NaCl. The final pellet was resuspended in 10 ml sterile 0.9% NaCl and subsequently filtered using a 2.7 µm pore size syringe filter (Whatman, Dassel, Germany). For the preparation of PFD-filled PEG-PLGA microcapsules with a lower size of 1 ± 0.5 µm, Ultraturrax velocity was changed to 14,000 rpm and a 1.6 µm pore size syringe filter (Whatman, Dassel, Germany) was used as described previously [11].
For evaluating frozen sections of various organs after contact with PFD-filled PEG-PLGA microcapsules (diameter of 1.5 µm), 100 µl of a stock solution of Nile red (0.057 mg/ml in methylene chloride) were added to the methylene chloride phase prior to emulsification [12].
All capsules were prepared freshly and were used the same day for animal experiments.
3.2. Animal experiments
3.2.1. Animals
A total number of 60 male Wistar rats (Rattus norvegicus, 448 ± 23 g) were obtained from the central animal unit of the Essen University Hospital. Animals were kept under standardized conditions of temperature (22 ± 1 °C), humidity (55 ± 5%), and 12/12-h light/dark cycles with free access to food (ssniff-Spezialdiäten, Soest, Germany) and water. All animals received humane care according to the standards of Annex III of the directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes [13]. The experimental protocol was approved by the local committee for animal protection based on the local animal protection act.
3.2.2. Anesthesia, analgesia, and surgical procedure
Rats were anesthetized with isoflurane (2.0% in 100% medical O2 at 4.0 l/min for induction, 1.5–2.0% isoflurane in 100% medical O2 at 1.0 l/min throughout the experiment) through face masks connected to a vaporizer (Isofluran Vet. Med. Vapor; Draeger, Luebeck, Germany) and received ketamine (50 mg/kg body weight, subcutaneously) into the right chest wall for analgesia. After local lidocaine administration (5 mg/kg body weight subcutaneously), a median skin-deep inguinal incision of about 2 cm was made along the right groin and a Portex catheter (0.58 mm ID, 0.96 mm OD) was placed within the right femoral artery and the right femoral vein. Each catheter was fixed with a surgical suture. At the end of experiment, animals were sacrificed by resection of the heart, liver, spleen, lung, kidney, brain, M. gastrocnemius and small intestine under deep isoflurane anesthesia.
3.2.3. Study groups
In the main setting we compared three experimental groups. One group (n = 14) received PFD-filled PLGA microcapsules (1.5 µm), one group (n = 9) was medicated with a sterile solution of 0.25% PVA (maximum possible concentration of PVA remainder from capsule synthesis) and one group (n = 9) was treated with 0.9% NaCl. All solutions were infused continuously for 30 min into the right femoral vein using a syringe pump (20 ml/kg body weight × h). The total volume of 20 ml/kg body weight was chosen, because this is a typical volume (5–30 ml/kg body weight) for studies with oxygen carriers [2,14]. The short infusion time of 30 min was selected as pre-hospital treatment of trauma or other severely injured patients (the potential target population for artificial oxygen carriers) should not exceed 30–40 min [15,16]. After the stop of infusion, 12 animals were monitored for 4 h. As high blood volumes were required for determination of cytokines and complement factors, for 20 animals the main setting was shortened from 270 to 150 min and 90 min, respectively (NaCl and PVA each n = 3 and PFD-filled PLGA microcapsules 1.5 µm n = 4 for both time points).
The frozen section procedure (see below) was performed in an additional setting only slightly differing from the main setting (see section frozen section procedure) with 2 groups, one receiving 1 µm PFD-filled PLGA microcapsules (n = 2) and one receiving 1.5 µm PFD-filled PLGA microcapsules (n = 2).
For the assessment of hepatic microcirculation, in vivo microscopy (intravital microscopy, IVM) was performed in an extra, independent setting (see section in vivo microscopy) with 4 groups: PFD-filled PLGA microcapsules (1 and 1.5 µm), PLGA microspheres (1.5 µm) and 0.9% NaCl each n = 6.
3.2.4. Biomonitoring
Systolic blood pressure, diastolic blood pressure and mean arterial blood pressure (MAP) were recorded continuously via the femoral artery catheter that was connected to a pressure transducer and displayed on a monitor. Ringer solution was delivered at 3 ml/h to keep the catheter functional. Heart rates were determined from systolic blood pressure spikes. The breathing rate was determined by counting the ventilation movements per minute every 10 min. The core body temperature of the rats was continuously monitored using a rectal sensor; cooling below 37 °C was prevented by both an underlying thermostat-controlled operating table and by covering the animals with aluminum foil.
3.2.5. Assessment of blood and plasma parameters
Blood samples (0.5 ml) for both blood gas analysis and the monitoring of released enzymes activities in plasma in the main setting (see study groups) were taken from the femoral artery catheter before the start of infusion (after catheterization of A. femoralis) and subsequently 90, 150, 210 and 270 min after start of the infusion using a 2 ml syringe containing 80 IU electrolyte-balanced heparin (Pico50, Radiometer Medical, Br?nsh?j, Denmark). Inflammation parameters were determined only at the end of experiment in each setting at 90 min, 150 min or 270 min. For each blood sampling animals were substituted with a 0.5 ml bolus of 0.9% NaCl via the femoral artery (with the additional effect to keep the catheter functional). In order to obtain plasma, blood was centrifuged at 4000g for 15 min at room temperature. The gained plasma was stored at 4 °C until use (maximal within 4 h).
3.2.5.1. Blood gas analysis
Arterial blood pH, oxygen and carbon dioxide partial pressures (pO2, pCO2), base excess (BE), bicarbonate and lactate were assessed with a blood gas analyzer (ABL 715, Radiometer, Copenhagen, Denmark).
3.2.5.2. Enzyme activities
The plasma activity of lactate dehydrogenase (LDH) as a general marker of cell injury, creatine kinase (CK) as a marker for muscle cell injury, aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) as markers for hepatocyte injury as well as the plasma creatinine concentration as a marker of renal function were determined using a fully automated clinical chemistry analyzer (Vitalab Selectra E, VWR International, Darmstadt, Germany) using commercially available reagent kits (DiaSys, Holzheim, Germany).
3.2.5.3. Inflammation parameters
The plasma concentration of the cytokines Interferon-gamma (IFN-?), Interleukin-1alpha (IL-1a), IL-1ß, IL-4, IL-5, IL-6, IL-10 and tumor necrosis factor alpha (TNF-a) were determined by using a rat-specific multiplex bead suspension array (Bio-Plex Cytokine Assay) in conjunction with a Bio-Plex Array Reader (Bio-Rad, Muenchen, Germany).
The plasma concentration of complement factors 3 (C3) and 4a (C4a) were assessed with rat-specific ELISA kits (Rat complement fragment 4a ELISA kit, Cusabio, Wuhan, China, and Rat Complement Factor 3, GenWay Biotech Inc, San Diego, CA, USA) according to the manufacturer's instructions.
3.2.6. Assessment of microcirculation and physiological functions
3.2.6.1. In vivo microscopy
In vivo microscopy analysis (IVM) was performed with a Leica DMLM epifluorescence microscope (Leica Microsystems, Wetzlar, Wetzlar, Germany, ×160 magnification). Anesthesia Analgesia, and surgical procedures were the same as in the setting without IVM. The left lateral liver lobe was exteriorized on a specially designed stage 40 min after catheterization of the femoral vessels. The abdominal cavity was kept moist. One milliliter/kilogram body weight of fluorescein isothiocyanate-dextran 150,000 (FITC-dextran, 5% solution in 0.9% NaCl) was injected intravenously for fluorescent staining of liver microcapillaries [17]. Fifteen min after application of FITC-dextran, PFD-filled PLGA microcapsules (either 1.5 or 1 µm), PLGA microspheres (1.5 µm) or 0.9% NaCl solution were infused continuously for 30 min into the right femoral vein using a syringe pump (20 ml/kg body weight × h). During the measurement, animals were biomonitored continuously as described.
The following parameters were determined in five randomly selected acinar areas and postsinusoidal venules:
-
(1)
Diameters of sinusoids and postsinusoidal venules by an image analysis software (Kappa ImageBase 2.8.2.11051, Kappa Optronics GmbH, Gleichen, Germany).
-
(2)
The number of perfused vessels (the ratio of perfused sinusoids to all sinusoids visible in a defined acinar area) (%) by analyzing full video sequences by an examiner blinded to the experimental groups.
3.2.6.2. Frozen section procedure
In order to evaluate the in vivo distribution of PFD-filled PLGA microcapsules, cryosections of liver, spleen, lung, kidney, brain, heart and M. Gastrocnemius were prepared. Anesthesia, analgesia, and surgical procedures were the same as in the setting without frozen section procedure. Fluorescent-stained microcapsules (1.5 or 1 µm) were infused continuously for 30 min into the right femoral vein using a syringe pump (20 ml/kg body weight × h) 40 min after catheterization of the femoral vessels. Microcapsules were allowed to circulate for 40 min. Afterwards the organs were cryopreserved and frozen. Cryosections of about 5 µm were prepared and nuclei were stained with 4',6-diamidin-2-phenylindol (DAPI). Evaluation of the in vivo distribution of the infused microcapsules was succeeded by fluorescence microscopy (×200 magnification), using an Axio Imager.A1 microscope equipped with an AxioCam MRc camera and an AxioVision Rel. 4.6 software (Zeiss, Jena, Germany).
3.2.7. Histological evaluation of the spleen, liver, lung and small intestine
For histological examinations liver, spleen, small intestine and lung were resected. Subsequent to its resection, the small intestine was immediately cut into 10 pieces of equal length (9.5–10.5 cm). The median liver lobe, the spleen and the forth segment of the small intestine (serially numbered from jejunum to ileum) were fixed in formalin (10% neutral buffered) for 24–48 h. Before the thorax was opened, the lung was filled with 5 cm3 air via a canula after tracheotomy, then harvested, filled with formalin (5 ml) to ensure complete unfolding and finally submersed in formalin, as the other organs. Paraffin-embedded sections were stained with hematoxylin-eosin and evaluated in a blinded manner. Histological changes in the small intestine were scored on a scale from 0 to 8 [adaptation of the Park/Chiu system [18,19]]. Using light microscopy, spleen sections (×100 and ×400 magnification) were assessed for integrity of red and white pulp, lung sections (×100 and ×400 magnification) were scanned for swelling of alveolar walls caused by bleeding or accumulation of water into the tissue and alveolar walls, liver sections (×100 and ×400 magnification) were investigated for disruption of parenchyma and vacuoles using the AxioVision Rel. 4.6 software (Zeiss, Jena, Germany).
3.3. Statistics
Biochemical assays were run in duplicate unless stated otherwise. Data are expressed as mean values ± SEM. Comparisons among multiple groups were performed with one-way analysis of variance (ANOVA) either for nonrecurring or for repeated measures followed by Dunnett (Figs. 1, 3, S1, S2, S4 and S6) or Sidak (Fig. 4) post-hoc analysis by using the graph pad prism 6.02 software (GraphPad Software, La Jolla, USA). Only data presented in the supporting information Fig. S5 was analyzed with two-way ANOVA followed by Dunnett post-hoc analysis. A p-value <0.05 was considered to indicate significance.
Fig. 1.
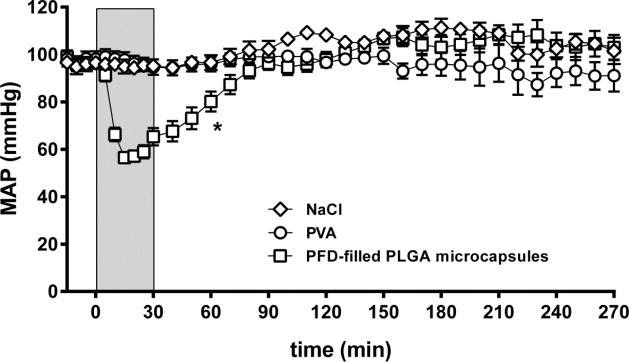
Effect of capsule infusion on mean arterial blood pressure. PFD-filled PLGA microcapsules (1.5 µm), 0.25% PVA or 0.9% NaCl were infused for 30 min (light gray, 20 ml/kg body weight × h). The values plotted are mean ± SEM of 14-6 (capsules), 9-3 (PVA and NaCl) individual experiments, *p < 0.05 compared to NaCl group for time-matched data (0–90 min).
Fig. 3.
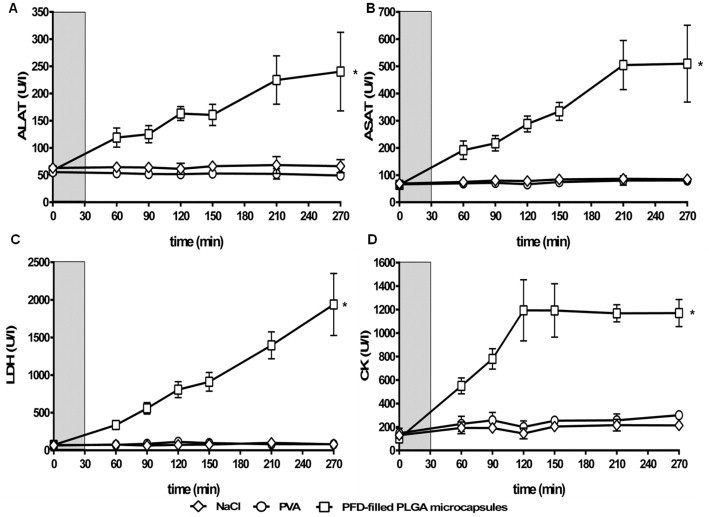
Effect of capsule infusion on organ/tissue damage: ALAT (A), ASAT (B), LDH (C) and CK (D). PFD-filled PLGA microcapsules (1.5 µm), 0.25% PVA or 0.9% NaCl were infused for 30 min (light gray, 20 ml/kg body weight × h). The values plotted are mean ± SEM of 14-6 (capsules), 9-3 (PVA and NaCl) individual experiments, *p < 0.05 compared to NaCl group.
Fig. 4.
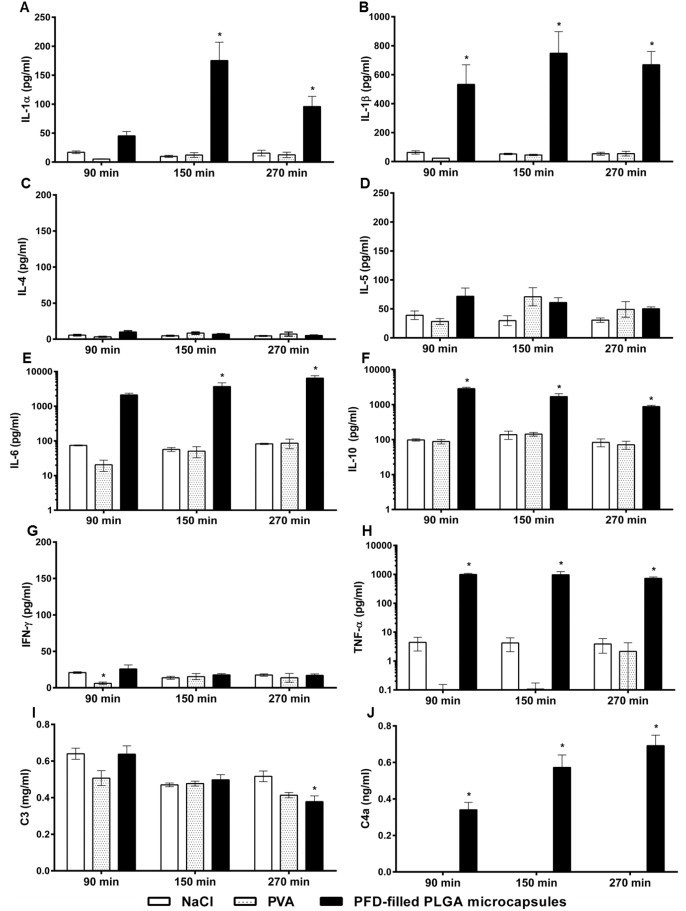
Effect of capsule infusion on release of cytokines and complement factors. PFD-filled PLGA microcapsules (1.5 µm), 0.25% PVA or 0.9% NaCl were infused for 30 min (20 ml/kg body weight × h). The values plotted are mean ± SEM of 6-4 (capsules) or 3 (PVA and NaCl) individual experiments, *p < 0.05 compared to NaCl group. The detection limit of the used ELISA was 0.08 ng/ml. Note: logarithmic scale was used for Fig. 4E, F and H. C4a level in NaCl and PVA groups at all time-points measured were below detection limit.
4. Results
4.1. Systemic reactions
4.1.1. Hemodynamics and other systemic parameters
MAP in the NaCl control group remained in the physiological range (90–120 mmHg) throughout the whole experiment (Fig. 1). MAP in the PVA group differed only slightly from control (91–100 mmHg). In sharp contrast, animals receiving PFD-filled PLGA microcapsules with a diameter of 1.5 µm experienced a significant, temporary descent of MAP to 57 mmHg shortly after start of infusion, regaining baseline conditions after about 70 min. In all three groups heart rates stayed constant at about 300 bpm (Fig. S1A). In the PVA and microcapsule groups breathing rates increased from baseline level (about 50 breaths per min) to about 60 breaths per min from 0 to 80 min normalizing afterwards to baseline level (Fig. S1B). The body temperature of all animals was inconspicuous and persisted around 37 °C (Fig. S1C).
4.1.2. Acid–base and metabolic status
The pH of all animals was slightly acidotic at about 7.3 (Fig. S2A). pCO2 was mildly elevated to about 55 mmHg (Fig. S2B) while pO2 was inconspicuous and varied around 500 mmHg in all three experimental groups (Fig. S1D). Base excess diminished somewhat in the NaCl and PVA treated animals, but did not move out of the physiological range (+3 to -3 mmol/l, Fig. S2C). However, the animals treated with microcapsules suffered from a significant decline from –0.9 mmol/l (0 min) to -5.9 mmol/l (120 min), compared to NaCl-infused animals. Blood lactate concentration in the NaCl and PVA groups increased only slightly to 1.2 and 1.0 mmol/l, respectively (Fig. S2D). In contrast, in the animals treated with microcapsules, lactate concentration significantly increased. Values doubled from 0.8 mmol/l (0 min) to 1.7 mmol/l (120 min) finally descending to control level at the end of the experiment (Fig. S2D).
4.2. Organ distribution and organ/tissue damage
Assessment of cryosections revealed an excessive accumulation of PFD-filled PLGA microcapsules (1.5 µm) in spleen and liver (Fig. 2A–B) but displayed very few capsules in other organs, i.e., lung (Fig. 2C), brain, kidney, heart and M. Gastrocnemius (Fig. S3). Identical results were obtained after infusion of 1 µm-sized PFD-filled PLGA microcapsules (data not shown).
Fig. 2.
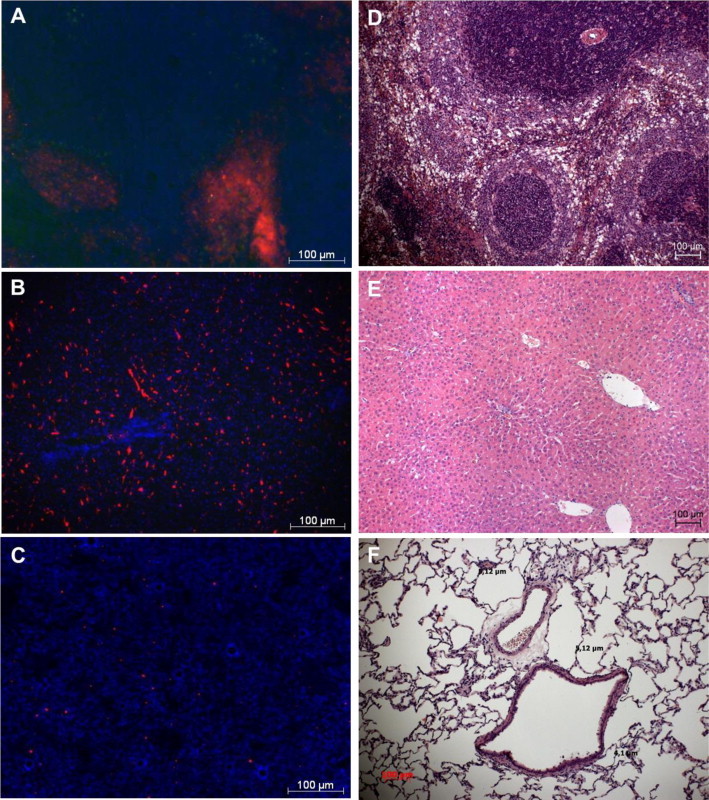
Organ distribution of PFD-filled PLGA microcapsules and effect of capsule infusion on tissue damage. PFD-filled PLGA microcapsules (1.5 µm) were stained with Nile red and were allowed to circulate for about 40 min within the blood circulation, before organs were cryopreserved and frozen. Cryosections of spleen (A), liver (B) and lung (C) were prepared and nuclei were stained with DAPI (blue) for fluorescence microscopic analysis (×200 magnification). For assessment of the effect of capsule infusion on tissue damage, unstained PFD-filled PLGA microcapsules (1.5 µm) were infused for 30 min (20 ml/kg body weight × h). Microscopic assessment (×100 magnification) of spleen (D), liver (E) and lung tissue (F).
Infusion of NaCl or PVA did not affect plasma activities of the enzymes ALAT, ASAT, CK and LDH (Fig. 3). In contrast, after infusion of PFD-filled PLGA microcapsules plasma activities of ALAT (3.6-fold), ASAT (6-fold), CK (5-fold) and LDH (24-fold) were significantly elevated if compared to the NaCl group at the end of experiment (Fig. 3). Creatinine concentrations oscillated around 0.7 mg/dl in all three experimental groups (Fig. S4).
Hematoxylin-eosin-staining revealed changes of spleen and liver architecture. Compared with NaCl- or PVA-infused animals (free of pathological indications), spleens of animals treated with PFD-filled PLGA microcapsules showed beginnings of dissolving tissue structures of white pulp and red pulp (Fig. 2D). Liver structures were still distinguishable but offered some vacuoles (Fig. 2E), while lung tissue was intact and did not display any sign of thickened alveoli walls (Fig. 2F).
Microscopic investigations of the mucosa of the fourth segment of the small intestine did not reveal distinct pathological changes in any group (data not shown). In contrast, in the group of animals treated with PFD-filled PLGA microcapsules macroscopic assessment of the serosa revealed some petechiae scattered over the entire jejunum.
4.3. Effects on inflammation parameters
Plasma concentrations of IL-1a, IL-1ß, IL-6, IL-10 and TNF-a displayed similar characteristics. After the infusion of PFD-filled PLGA microcapsules, pro-inflammatory cytokines (such as IL-1a, IL-1ß, IL-6, TNF-a) were strongly elevated compared to NaCl controls, whereas infusion of PVA did not provoke any immune response (Fig. 4A–H). Notably, 90 min after begin of microcapsule infusion a clear increase of IL-10 took place and persisted until the end of the experiment (Fig. 4F). Irrespective of the treatment group, plasma levels of both IL-4 (<14.1 pg/ml) and IFN-? (<35.0 pg/ml) were unaffected and only very small amounts of IL-5 (<113.0 pg/ml, not significantly different between the treatment groups) were released (Fig. 4C/D/G).
The application of NaCl did not alter the concentration of complement factor 3 (C3) in plasma. Only at 270 min, C3 level in the group treated with PFD-filled PLGA microcapsules was reduced significantly when compared to the NaCl group at 270 min (Fig. 4I). Infusion of NaCl or PVA did not affect the plasma amount of complement factor 4a (C4a) either. In both groups all values stayed below the assay’s detection limit (Fig. 4J). In contrast, after infusion of PFD-filled PLGA microcapsules, the plasma concentration of C4a increased and differed significantly from the respective NaCl control at all time-points monitored (Fig. 4J).
4.4. Effects on microcirculation
The diameters of sinusoids and postsinusoidal venules increased about 23% and 20% already 10 min after start of infusion of PFD-filled PLGA microcapsules (1.5 µm and 1 µm, respectively) whereas infusion of PLGA microspheres caused a decrease of vessel diameter of 15% compared to NaCl control group (Fig. S5). During the following 20 min of infusion, diameters of sinusoids and postsinusoidal venules of all microcapsule treated animals normalized, thereby reaching the baseline level at the end of the infusion (Fig. S5).
After infusing 0.9% NaCl, the number of perfused vessels and MAP remained at baseline levels (100%, 100 mmHg, respectively, Fig. 5A). In contrast, at the very beginning of the infusion of PFD-filled PLGA microcapsules (1.5 µm) MAP (mmHg) and the number of perfused vessels (%) decreased to about 60 mmHg/50% from baseline. Only MAP regenerated during the following observation time, whereas the number of perfused vessels only reached 80% of baseline level (Fig. 5B). Infusion of PFD-filled PLGA microcapsules (diameter 1 µm) revealed identical results as obtained with 1.5 µm microcapsules (Fig. S6). After treatment with PLGA microspheres MAP did not decrease but stayed in the physiological range (85–100 mmHg) throughout the whole experiment (similar to the NaCl group) and the number of perfused vessels slowly decreased leveling off at 80% of baseline level after 150 min (Fig. 5C).
Fig. 5.
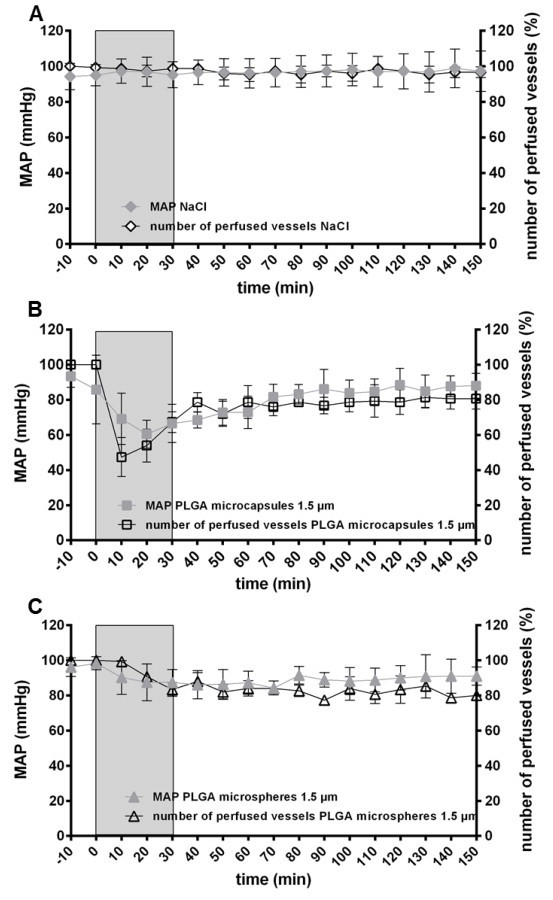
Effect of capsule infusion on hepatic microcirculation. IVM was performed to determine the number of perfused vessels of the hepatic microvascular system after intravenous administration (30 min, light gray, 20 ml/kg body weight × h) of either 0.9% NaCl (A), PFD-filled PLGA microcapsules (1.5 µm, B) or PLGA microspheres (1.5 µm, C). The values plotted are mean ± SEM of 6 individual experiments.
5. Discussion
5.1. Study rationale
Blood losses up to a hemoglobin level of 10 g/dl are generally well tolerated by patients. Intervention with red blood cell concentrates or artificial oxygen carriers becomes necessary when hemoglobin level would further decrease. If and how many red blood cell concentrates are transfused (in default of approved artificial oxygen carriers) not only depends on the individual patient’s condition but also on the hospital’s practice, whereas at a hemoglobin decrease below 6 g/dl transfusion of red blood cell concentrates is generally indicated [20]. If transfusion is inalienable, until now, usually 2 or more red blood cell concentrates are required, as the average increase in hemoglobin level per unit red blood cell concentrate is only 1.0 g/dl [21]. Therefore, the tolerance of high quantities of foreign particles in the intravascular system is a prerequisite for the successful use of microcapsules as artificial oxygen carriers and implies both, the occurrence and the monitoring of new toxicity profiles. So far the infusion of 1247 mg PFD-filled PLGA microcapsules/ kg body weight is clearly higher than common dosing of intravenously administered polymeric pharmaceuticals (ca. 23 mg/kg body weight [7], 440 mg/kg body weight [22]). Not only quantitatively but also in relation to the blood volume the amount of infused microcapsules is high (1/6 of the blood volume, assuming the calculation of the rat’s blood volume proposed by Lee et al. [23]), which is in the same order of magnitude as demonstrated for PLA50 nanoparticles (also 1/6) [22] but much higher than similar-sized microparticles (1/28) [7] or ultrasound contrast agents (1/20,000) [8]. Assuming a mean blood volume of 4–6 l for humans, 1/6 (0.67–1.0 l) corresponds to 2.4–3.6 red blood cell concentrates (0.28 l).
5.2. Effects on MAP
Transient systemic hypotension after infusion of PFC-based artificial oxygen carriers has been described long ago for PFC-based emulsion systems [24,25] and latterly also for capsule-based PFC-containing systems [26]. This undesirable side effect has been attributed to the action of the emulsifier without [27,28] or in combination with the PFC [29]. In order to safely exclude any emulsifier-caused side effects, a control group receiving PVA only (without microcapsules) was implemented, although the short-chained PVA used in this study is known as biocompatible and eliminable via the kidneys [30,31]. As MAP remained stable in the PVA group (Fig. 1), our results are comparable to the data of Ingram et al., suggesting, that only the combined action of PFC and emulsifier is responsible for transient hypotension[29]. Furthermore, hypotension only occurred after application of PFD-filled PLGA microcapsules (Fig. 1,Fig. 5B) but not after application of PFD-free PLGA microspheres (Fig. 5C). Due to the fact that transient hypotension was also described after intravenous application of PFD-filled poly(n-butyl-cyanoacrylate) nanocapsules [26], transient hypotension may be a general complication of perfluorocarbon-based products irrespective of the type of galenical packaging (emulsion or capsule) of the oxygen carrier.
The proposed mechanism for this transient hypotension would be the prompt activation of the complement system leading to the release of vasoactive substances [28,29,32,33]. As anaphylatoxins can regulate vasodilation and increase permeability of small blood vessels [34,35], a decline of C3 concentration in plasma and increase of the anaphylatoxin C4a after infusion of microcapsules (but no changes after treatment with PVA) are in line with this hypothesis (Fig. 4I,J). Activation of both, the classical and the alternative pathway of the complement system are possible by contact of blood with artificial particles [36,37]. The clear increase of C4a (not part of the alternative pathway [38,39]), should support an involvement of the classical pathway. Activation of the classical pathway (initiated by the adsorption of plasma proteins such as IgG and albumin) can also amplify the alternative pathway mediating primarily the reaction against foreign biomaterials [40]. Since PEG-shielding can only partly reduce protein adsorption on surfaces of PLGA particles [5,37], adsorbed IgG may mediate activation and binding of C3b to the capsules’ surface as proposed by Nilsson et al. [38]. A complement activation-related pseudoallergy (CARPA) that is already confirmed for different nanoparticles, polymers and emulsifiers such as Cremophor EL or Tween [41,42] was not responsible for transient hypotension (Figs. S1 and S2). Even though in contradiction to CARPA symptoms in pigs and dogs [41,43] this is not surprising, as rats are especially insensitive to CARPA [44].
Another explanation for transient hypotension would be an involvement of nitric oxide-mediated (NO) pathways. Short- and long-term regulation of NO production in response to shear stress on the endothelial membrane of vasculature (as potentially caused by the heavy-weight PFD-filled microcapsules) is well-known [45,46]. Additionally, the formation of relatively stable S-nitrosothiols (believed to act as biological metabolites and carriers of NO) in the blood in presence of perfluorocarbons (as PFD) can induce NO releasevia synthesis of intermediates, that are highly effective in nitrosating other compounds [47–49]. This process can also be triggered by shear force on endothelial cells [50].
However, an effect on MAP evoked primarily by the release of cytokines seems unlikely, although release of cytokines from monocytes, macrophages and lymphocytes after contact with PLGA is described in vitro and in vivo [51–54]. The cytokine profile after infusion of PFD-filled PLGA microcapsules (Fig. 4A–H) is in line with in vitro data from mononuclear cells cultured in the presence of poly-l-lactide [52] and macrophages cultured in the presence of PLGA microparticles (diameter of 6.5 µm) [53]. The strong release of IL-10 may counteract the effects induced by the pro-inflammatory cytokines, avoiding an excessive immune response by, among others, influencing production of TNF-a [51,52]. Nevertheless a direct relation between release of cytokines and transient hypotension cannot be propagated, as cytokine levels in plasma remained elevated until the end of the experiment (Fig. 4A–H), whereas MAP normalized after 70 min (Fig. 1,Fig. 5B). Likewise, the reported appearance of allergic and anaphylactic reactions to some PFC-containing emulsions [9] leading to hypotension cannot be used as an argument in the present study as the IL-5 level obtained after application of PLGA microcapsules did not differ significantly from corresponding NaCl control values (Fig. 4D).
5.3. Effect on microcirculation and distribution pattern
Hepatic sinusoidal blood flow depends among other factors on MAP and sinusoidal diameter, whereupon reduced sinusoid diameter causes impairment of sinusoidal blood flow [55]. Decreased number of perfused vessels is certainly mostly entailed by the prompt and radical drop of MAP after application of PFD-filled PLGA microcapsules (Fig. 5B), because undesirable artifacts due to liver exteriorization and IVM handling on hepatic microcirculation could be excluded (Fig. 5A). Additional aggravation by a reduced sinusoidal diameter cannot be identified as driving force for this decrease, since the diameter at this time was even increased from baseline level; possibly be a sort of counteraction. Because both, hypotension and increase of sinusoidal diameter were only transient and quickly restored to baseline level, the just partial recovering of the number of perfused vessels probably indicates a PFD-filled PLGA microcapsules-mediated obstruction of microcapillaries (Fig. 5B). The fact that the number of perfused vessels dropped likewise but temporarily-delayed (synchronized with a reduction of the sinusoidal diameter) while MAP remained in the physiological range after treatment with microspheres, should further substantiate the fractional occlusion of hepatic microcirculation by PLGA microcapsules (Fig. 5B). This can be verified by the accumulation of PFD-filled PLGA microcapsules only in spleen and liver (Fig. 2A and B). The same distribution pattern also applies for intravenously administered PLGA nanoparticles [56] and 2 µm-sized silicon dioxide particles [57]. While other investigators additionally observed an accumulation of microparticles in lung tissue [57,58], our results showed neither enrichment in that organ nor swollen alveolar walls (Fig. 2C and F).
5.4. Effects on acid–base metabolism and tissue oxygenation
Persisting microcirculatory impairment can lead to tissue hypoxia and the development of subsequent organ dysfunction [55]. Thus, the function of oxygen carriers is to improve the microcirculation as already established for a PFC-based emulsion and a hemoglobin-based suspension [55,59]. However, animals treated with PFD-filled PLGA microcapsules experienced a mild metabolic acidosis (Fig. S2) corresponding to the data of Sedova et al. describing the same phenomenon after infusion of a PFC-containing emulsion [60].
Probably lactate formation in muscle tissue increases due to transient hypotension and reduced number of perfused vessels of sinusoids. This can entail an undersupply of affected tissue areas with oxygen triggering anaerobic glycolysis [61]. The increase in enzyme activities in plasma, such as LDH as a general marker of cell injury and CK as a marker for muscle cell injury, should further support the assumption of cellular damage (Fig. 3C and D). An increased LDH level was also described after contact of silica nanoparticles with human endothelial cells and after pulmonary exposition of rats to polystyrene particles [62]. In contrast, intravenous injection of nanoporous silicon microparticles provoked only a mild increase in LDH plasma activity [63]. As these data were obtained 24 h post injection of microparticles, they differ from the acute response (only 4 h post infusion of PLGA microcapsules) monitored in this study as expected. Reduced perfusion and hypoxia also damaged liver and spleen tissue. Pathological tissue alteration and increase in plasma activities of liver tissue-associated enzymes such as ALAT and ASAT after infusion of PFD-filled PLGA microparticles further emphasize liver damage (Figs. 2 and 3). This matches data on PFC-containing emulsions describing a transient increase of plasma transaminase activities because of accumulation of emulsion droplets in Kupffer cells [64].
The conventional in vivo degradation of PLGA to lactic and glycolic acid in the presence of oxygen [65], with all metabolites entering the tricarboxylic acid cycle (further degradation into H2O and CO2) [66] or, the alternative elimination of glycolic acid via the kidney [67] certainly were not the reason for the observed acidosis, as PLGA is attributed with an in vivo degradation time of 50–60 days [68]. Although this degradation process of PLGA is primarily a hydrolytic process (drivable by enzymes, such as trypsin, but not dependent on their presence [69]) contact with blood (not containing the relevant enzymes) does not enhance the degradation of PLGA microcapsules. In line with this, degradation studies with PEG-PLGA capsules show a decay rate of only about 15% 1–3 h after intravenous administration [70] and studies with PLGA films describe a drop in pH only after an in vitro degradation period of 30 days [71]. Hence, the impact of these degradation mechanisms becomes more important in a later phase and thus, cannot explain the observed mild, metabolic acidosis.
6. Conclusions
Generally, the infusion of high quantities of PFD-filled PLGA microcapsules was tolerated as all animals survived the selected observation period. Nevertheless, the findings of this study imply a new toxicity profile compromised with severe side effects that are only partially described for other intravenously infused foreign particles and for the used materials such as PFD so far. As expected from previous studies about the pharmacokinetics and biodisposition of different PVAs [30,31], in the present investigation, short-chained PVA was completely harmless in vivo during an observation time of 4 h.
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance by Angela Wensing. Furthermore, the authors would like to thank Tanja Hinkeldein from the Department of Nephrology of the University Hospital Essen for help with the frozen section procedure and the team of the department of Pathology of the University Hospital Essen for hematoxylin–eosin staining of histological sections.
Appendix A. Supplementary Material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.rinphs.2014.04.001.
Appendix A. Supplementary Materials
Supplementary Materials for Safety of poly (ethylene glycol)-coated perfluorodecalin-filled poly(lactide-co-glycolide) microcapsules following intravenous administration of high amounts in rats.
References
- 1.Castro C.I., Briceno J.C. Perfluorocarbon-based oxygen carriers: review of products and trials. Artificial Organs. 2010;34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. 20698841 [DOI] [PubMed] [Google Scholar]
- 2.Clark M.C., Weiman D.S., Pate J.W., Gir S. Perfluorocarbons: future clinical possibilities. Journal of Investigative Surgery: the Official Journal of the Academy of Surgical Research. 1997;10:357–365. doi: 10.3109/08941939709099599. 9654392 [DOI] [PubMed] [Google Scholar]
- 3.Kuznetsova I.N. Perfluorocarbon emulsions: stability in vitro and in vivo (a review) Pharmaceutical Chemistry Journal. 2003;37:20–25. [Google Scholar]
- 4.Bauer J., Zahres M., Zellermann A., Kirsch M., Petrat F., de Groot H., Mayer C. Perfluorocarbon-filled poly(lactide-co-gylcolide) nano- and microcapsules as artificial oxygen carriers for blood substitutes:a physico-chemical assessment. Journal of Microencapsulation. 2010;27:122–132. doi: 10.3109/02652040903052002. 20121485 [DOI] [PubMed] [Google Scholar]
- 5.Ferenz K.B., Waack I.N., Mayer C., de Groot H., Kirsch M. Long-circulating poly(ethylene glycol)-coated poly(lactid-co-glycolid) microcapsules as potential carriers for intravenously administered drugs. Journal of Microencapsulation. 2013;30 doi: 10.3109/02652048.2013.770098. 23489015 [DOI] [PubMed] [Google Scholar]
- 6.Li C., Yang D.J., Kuang L.-R., Wallace S. Polyamino acid microspheres: preparation, characterization and distribution after intravenous injection in rats. International Journal of Pharmaceutics. 1993;94:143–152. [Google Scholar]
- 7.Shishatskaya E., Goreva A., Kalacheva G., Volova T. Biocompatibility and resorption of intravenously administered polymer microparticles in tissues of internal organs of laboratory animals. Journal of Biomaterials Science. Polymer Edition. 2011;22:2185–2203. doi: 10.1163/092050610X537138. 21067658 [DOI] [PubMed] [Google Scholar]
- 8.Schutt E.G., Klein D.H., Mattrey R.M., Riess J.G. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: the key role of perfluorochemicals. Angewandte Chemie (International Ed. in English) 2003;42:3218–3235. doi: 10.1002/anie.200200550. 12876730 [DOI] [PubMed] [Google Scholar]
- 9.Vorob’ev S.I. First- and second-generation perfluorocarbon emulsions. Pharmaceutical Chemistry Journal. 2009;43:209–218. [Google Scholar]
- 10.Cabrales P., Intaglietta M. Blood substitutes: evolution from noncarrying to oxygen- and gas-carrying fluids. ASAIO Journal (American Society for Artificial Internal Organs: 1992) 2013;59:337–354. doi: 10.1097/MAT.0b013e318291fbaa. 23820271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsch M., Bramey T., Waack I.N., Petrat F., Mayer C., De Groot H. The necessity for the coating of perfluorodecalin-filled poly(lactide-co-glycolide) microcapsules in the presence of physiological cholate concentrations: Tetronic-908 as an exemplary polymeric surfactant. Journal of Microencapsulation. 2012;29:30–38. doi: 10.3109/02652048.2011.629743. 22047544 [DOI] [PubMed] [Google Scholar]
- 12.Pisani E., Tsapis N., Paris J., Nicolas V., Cattel L., Fattal E. Polymeric nano/microcapsules of liquid perfluorocarbons for ultrasonic imaging: physical characterization. Langmuir: The ACS Journal of Surfaces and Colloids. 2006;22:4397–4402. doi: 10.1021/la0601455. 16618193 [DOI] [PubMed] [Google Scholar]
- 13.Directive 2010/63/EU on the protection of animals used for scientific purposes. Institute for Health and Consumer Protection; Ispra, Italy: 2010. [Google Scholar]
- 14.Shimbo D., Abumiya T., Shichinohe H., Nakayama N., Kazumata K., Houkin K. Post-ischemic intra-arterial infusion of liposome-encapsulated hemoglobin can reduce ischemia reperfusion injury. Brain Research. 2014;1554:59–66. doi: 10.1016/j.brainres.2014.01.038. 24486612 [DOI] [PubMed] [Google Scholar]
- 15.McCoy C.E., Menchine M., Sampson S., Anderson C., Kahn C. Emergency medical services out-of-hospital scene and transport times and their association with mortality in trauma patients presenting to an urban Level I Trauma Centre. Annals of Emergency Medicine. 2013;61:167–174. doi: 10.1016/j.annemergmed.2012.08.026. 23142007 [DOI] [PubMed] [Google Scholar]
- 16.Weerheijm D.V., Wieringa M.H., Biert J., Hoogerwerrf N. Optimizing transport time from Accidentto Hospital: When to drive and when to Fly? International Scholarly Research Network Emergency Medicine. 2012;(508579) [Google Scholar]
- 17.Richter S., Vollmar B., Mucke I., Post S., Menger M.D. Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers. Journal of Physiology. 2001;531:193–201. doi: 10.1111/j.1469-7793.2001.0193j.x. 11179403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu C.J., McArdle A.H., Brown R., Scott H.J., Gurd F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Archives of Surgery (Chicago, Ill.: 1960) 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. 5457245 [DOI] [PubMed] [Google Scholar]
- 19.Park P.O., Haglund U., Bulkley G.B., Falt K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574–580. 2159192 [PubMed] [Google Scholar]
- 20.Carson J.L., Carless P.A., Hebert P.C. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews. 2012;4 doi: 10.1002/14651858.CD002042.pub3. CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards J., Morrison C., Mohiuddin M., Tchatalbachev V., Patel C., Schwickerath V.L., Menitove J.E., Singh G. Patient blood transfusion management: discharge hemoglobin level as a surrogate marker for red blood cell utilization appropriateness. Transfusion. 2012;52:2445–2451. doi: 10.1111/j.1537-2995.2012.03591.x. 22413968 [DOI] [PubMed] [Google Scholar]
- 22.Plard J.-P., Bazile D. Comparison of the safety profiles of PLA50 and me.PEG-PLA50 nanoparticles after single dose intravenous administration to rat. Colloids and Surfaces B: Biointerfaces. 1999;16:173–183. [Google Scholar]
- 23.Lee H.B., Blaufox M.D. Blood volume in the rat. Journal of Nuclear Medicine: Official Publication, Society Of Nuclear Medicine. 1985;26:72–76. [PubMed] [Google Scholar]
- 24.Tremper K.K., Friedman A.E., Levine E.M., Lapin R., Camarillo D. The preoperative treatment of severely anemic patients with a perfluorochemical oxygen-transport fluid, fluosol-DA. New England Journal of Medicine. 1982;307:277–283. doi: 10.1056/NEJM198207293070503. 7045667 [DOI] [PubMed] [Google Scholar]
- 25.Waxman K., Cheung C.K., Mason G.R. Hypotensive reaction after infusion of a perfluorochemical emulsion. Critical Care Medicine. 1984;12:609–610. doi: 10.1097/00003246-198407000-00016. 6610534 [DOI] [PubMed] [Google Scholar]
- 26.Stephan C., Schlawne C., Grass S., Waack I.N., Ferenz K.B., Bachmann M., Barnert S., Schubert R., Bastmeyer M., De Groot H., Mayer C. Artificial oxygen carriers based on perfluorodecalin-filled poly(n-butyl-cyanoacrylate) nanocapsules. Journal of Microencapsulation. 2014 doi: 10.3109/02652048.2013.843600. 24124886 epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Mattrey R.F., Hilpert P.L., Long C.D., Long D.M., Mitten R.M., Peterson T. Hemodynamic effects of intravenous lecithin-based perfluorocarbon emulsions in dogs. Critical Care Medicine. 1989;17:652–656. doi: 10.1097/00003246-198907000-00011. 2736927 [DOI] [PubMed] [Google Scholar]
- 28.Vercellotti G.M., Hammerschmidt D.E., Craddock P.R., Jacob H.S. Activation of plasma complement by perfluorocarbon artificial blood: Probable mechanism of adverse pulmonary reactions in treated patients and rationale for corticosteroids prophylaxis. Blood. 1982;59:1299–1304. 7082830 [PubMed] [Google Scholar]
- 29.Ingram D.A., Forman M.B., Murray J.J. Activation of complement by fluosol attributable to the pluronic detergent micelle structure. Journal of Cardiovascular Pharmacology. 1993;22:456–461. doi: 10.1097/00005344-199309000-00017. 7504138 [DOI] [PubMed] [Google Scholar]
- 30.Kaneo Y., Hashihama S., Kakinoki A., Tanaka T., Nakano T., Ikeda Y. Pharmacokinetics and biodisposition of poly(vinyl alcohol) in rats and mice. Drug Metabolism and Pharmacokinetics. 2005;20:435–442. doi: 10.2133/dmpk.20.435. 16415529 [DOI] [PubMed] [Google Scholar]
- 31.Tabata Y., Murakami Y., Ikada Y. Tumor accumulation of poly(vinyl alcohol) of different sizes after intravenous injection. Journal of Controlled Release: Official Journal of the Controlled Release Society. 1998;50:123–133. doi: 10.1016/s0168-3659(97)00129-6. 9685879 [DOI] [PubMed] [Google Scholar]
- 32.Moghimi S.M., Andersen A.J., Ahmadvand D., Wibroe P.P., Andresen T.L., Hunter A.C. Material properties in complement activation. Advanced Drug Delivery Reviews. 2011;63:1000–1007. doi: 10.1016/j.addr.2011.06.002. 21689701 [DOI] [PubMed] [Google Scholar]
- 33.Szebeni J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. 16140450 [DOI] [PubMed] [Google Scholar]
- 34.Davies D.H. Immune System, Encyclopedia of Life Sciences. John Wiley & Sons; Chichester: 2008. [Google Scholar]
- 35.Klos A., Tenner A.J., Johswich K.O., Ager R.R., Reis E.S., Kohl J. The role of the anaphylatoxins in health and disease. Molecular Immunology. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. 19477527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson J., Ekdahl K.N., Larsson R., Nilsson U.R., Nilsson B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. Journal of Immunology (Baltimore, Md.: 1950) 2002;168:5786–5791. doi: 10.4049/jimmunol.168.11.5786. 12023380 [DOI] [PubMed] [Google Scholar]
- 37.Yang A., Liu W., Li Z., Jiang L., Xu H., Yang X. Influence of polyethyleneglycol modification on phagocytic uptake of polymeric nanoparticles mediated by immunoglobulin G and complement activation. Journal of Nanoscience and Nanotechnology. 2010;10:622–628. doi: 10.1166/jnn.2010.1738. 20352902 [DOI] [PubMed] [Google Scholar]
- 38.Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. The role of complement in biomaterial-induced inflammation. Molecular Immunology. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. 16905192 [DOI] [PubMed] [Google Scholar]
- 39.Vonarbourg A., Passirani C., Saulnier P., Benoit J.P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. 16650890 [DOI] [PubMed] [Google Scholar]
- 40.Andersson J., Ekdahl K.N., Lambris J.D., Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. 15522749 [DOI] [PubMed] [Google Scholar]
- 41.Szebeni J., Bedocs P., Csukas D., Rosivall L., Bunger R., Urbanics R. A porcine model of complement-mediated infusion reactions to drug carrier nanosystems and other medicines. Advanced Drug Delivery Reviews. 2012;64:1706–1716. doi: 10.1016/j.addr.2012.07.005. 22820530 [DOI] [PubMed] [Google Scholar]
- 42.Weiszhar Z., Czucz J., Revesz C., Rosivall L., Szebeni J., Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences. 2012;45:492–498. doi: 10.1016/j.ejps.2011.09.016. 21963457 [DOI] [PubMed] [Google Scholar]
- 43.Qiu S., Liu Z., Hou L., Li Y., Wang J., Wang H., Du W., Wang W., Qin Y. Complement activation associated with polysorbate 80 in beagle dogs. International Immunopharmacology. 2013;15:144–149. doi: 10.1016/j.intimp.2012.10.021. 23159336 [DOI] [PubMed] [Google Scholar]
- 44.Szebeni J., Alving C.R., Rosivall L., Bunger R., Baranyi L., Bedocs P., Toth M., Barenholz Y. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. Journal of Liposome Research. 2007;17:107–117. doi: 10.1080/08982100701375118. 17613700 [DOI] [PubMed] [Google Scholar]
- 45.Balligand J.L., Feron O., Dessy C. eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiological Reviews. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. 19342613 [DOI] [PubMed] [Google Scholar]
- 46.Kerwin J.F., Jr., Heller M. The arginine-nitric oxide pathway: A target for new drugs. Medicinal Research Reviews. 1994;14:23–74. doi: 10.1002/med.2610140103. 7508539 [DOI] [PubMed] [Google Scholar]
- 47.Cabrales P., Vasquez B.Y.S., Negrete A.C., Intaglietta M. Perfluorocarbons as gas transporters for O2, NO, CO and volatile anesthetics. Transfusion Alternatives in Transfusion Medicine. 2007;9:294–303. [Google Scholar]
- 48.Ortiz D., Cabrales P., Briceno J.C. Transport of nitric oxide by perfluorocarbon emulsion. Biotechnology Progress. 2013;29:1565–1572. doi: 10.1002/btpr.1797. 23966236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafikova O., Sokolova E., Rafikov R., Nudler E. Control of plasma nitric oxide bioactivity by perfluorocarbons: Physiological mechanisms and clinical implications. Circulation. 2004;110:3573–3580. doi: 10.1161/01.CIR.0000148782.37563.F8. 15557364 [DOI] [PubMed] [Google Scholar]
- 50.Huang B., Chen S.C., Wang D.L. Shear flow increases S-nitrosylation of proteins in endothelial cells. Cardiovascular Research. 2009;83:536–546. doi: 10.1093/cvr/cvp154. 19447776 [DOI] [PubMed] [Google Scholar]
- 51.Jones J.A., Chang D.T., Meyerson H., Colton E., Kwon I.K., Matsuda T., Anderson J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. Journal of Biomedical Materials Research. Part A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. 17503526 [DOI] [PubMed] [Google Scholar]
- 52.Marques A.P., Reis R.L., Hunt J.A. Cytokine secretion from mononuclear cells cultured in vitro with starch-based polymers and poly-L-lactide. Journal of Biomedical Materials Research. Part A. 2004;71:419–429. doi: 10.1002/jbm.a.30155. 15472922 [DOI] [PubMed] [Google Scholar]
- 53.Nicolete R., dos Santos D.F., Faccioli L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. International Immunopharmacology. 2011;11:1557–1563. doi: 10.1016/j.intimp.2011.05.014. 21621649 [DOI] [PubMed] [Google Scholar]
- 54.Thasneem Y.M., Sajeesh S., Sharma C.P. Effect of thiol functionalization on the hemo-compatibility of PLGA nanoparticles. Journal of Biomedical Materials Research. Part A. 2011;99:607–617. doi: 10.1002/jbm.a.33220. 21953904 [DOI] [PubMed] [Google Scholar]
- 55.Paxian M., Keller S.A., Huynh T.T., Clemens M.G. Perflubron emulsion improves hepatic microvascular integrity and mitochondrial redox state after hemorrhagic shock. Shock (Augusta, Ga.) 2003;20:449–457. doi: 10.1097/01.shk.0000090601.26659.87. 14560110 [DOI] [PubMed] [Google Scholar]
- 56.Le Ray A.M., Gautier J.C., Benoît J.P. Fate of [14C]poly(DL-lactide-co-glycolide) nanoparticles after intravenous and oral administration to mice. International Journal of Pharmaceutics. 1994;106:201–211. [Google Scholar]
- 57.Martin F.J., Melnik K., West T., Shapiro J., Cohen M., Boiarski A.A., Ferrari M. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: preliminary findings. Drugs in Research and Development. 2005;6:71–81. doi: 10.2165/00126839-200506020-00002. 15818779 [DOI] [PubMed] [Google Scholar]
- 58.Dandagi P.M., Mastiholimath V.S., Patil M.B., Gupta M.K. Biodegradable microparticulate system of captopril. International Journal of Pharmaceutics. 2006;307:83–88. doi: 10.1016/j.ijpharm.2005.10.025. 16310990 [DOI] [PubMed] [Google Scholar]
- 59.Zapletal C., Bode A., Lorenz M.W., Gebhard M.M., Golling M. Effects of hemodilution with a hemoglobin-based oxygen carrier (HBOC-201) on ischemia/reperfusion injury in a model of partial warm liver ischemia of the rat. Microvascular Research. 2009;78:386–392. doi: 10.1016/j.mvr.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Sedova L.A., Kochetygov N.I., Berkos M.V., Pjatowskaja N.N. Side reaction caused by the perfluorocarbon emulsions in intravenous infusion to experimental animals. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology. 1998;26:149–157. doi: 10.3109/10731199809119773. 9564433 [DOI] [PubMed] [Google Scholar]
- 61.Robergs R.A., Ghiasvand F., Parker D. Biochemistry of exercise-induced metabolic acidosis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. 15308499 [DOI] [PubMed] [Google Scholar]
- 62.Brown D.M., Wilson M.R., MacNee W., Stone V., Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles:a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicology and Applied Pharmacology. 2001;175:191–199. doi: 10.1006/taap.2001.9240. 11559017 [DOI] [PubMed] [Google Scholar]
- 63.Tanaka T., Godin B., Bhavane R., Nieves-Alicea R., Gu J., Liu X., Chiappini C., Fakhoury J.R., Amra S., Ewing A., Li Q., Fidler I.J., Ferrari M. In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice. International Journal of Pharmaceutics. 2010;402:190–197. doi: 10.1016/j.ijpharm.2010.09.015. 20883755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flaim S.F. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology. 1994;22:1043–1054. doi: 10.3109/10731199409138801. 7849908 [DOI] [PubMed] [Google Scholar]
- 65.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery Carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. 22577513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. 12628320 [DOI] [PubMed] [Google Scholar]
- 67.Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. 11055295 [DOI] [PubMed] [Google Scholar]
- 68.Pisal D.S., Kosloski M.P., Balu-Iyer S.V. Delivery of therapeutic proteins. Journal of Pharmaceutical Sciences. 2010;99:2557–2575. doi: 10.1002/jps.22054. 20049941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai Q., Shi G., Bei J., Wang S. Enzymatic degradation behavior and mechanism of poly(lactide-co-glycolide) foams by trypsin. Biomaterials. 2003;24:629–638. doi: 10.1016/s0142-9612(02)00377-0. 12437957 [DOI] [PubMed] [Google Scholar]
- 70.Esmaeili F., Ghahremani M.H., Esmaeili B., Khoshayand M.R., Atyabi F., Dinarvand R. PLGA nanoparticles of different surface properties: preparation and evaluation of their body distribution. International Journal of Pharmaceutics. 2008;349:249–255. doi: 10.1016/j.ijpharm.2007.07.038. 17875373 [DOI] [PubMed] [Google Scholar]
- 71.Lu L., Garcia C.A., Mikos A.G. In vitro degradation of thin poly(D,L-lactic-co-glycolic acid) films. Journal of Biomedical Materials Research. 1999;46:236–244. doi: 10.1002/(sici)1097-4636(199908)46:2<236::aid-jbm13>3.0.co;2-f. 10380002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials for Safety of poly (ethylene glycol)-coated perfluorodecalin-filled poly(lactide-co-glycolide) microcapsules following intravenous administration of high amounts in rats.


