Abstract
Following exposure to microgravity, there is a reduced ability of astronauts to augment peripheral vascular resistance, often resulting in orthostatic hypotension. The purpose of this study was to test the hypothesis that mesenteric arteries and veins will exhibit diminished vasoconstrictor responses after spaceflight. Mesenteric arteries and veins from female mice flown on the Space Transportation System (STS)-131 (n=11), STS-133 (n=6), and STS-135 (n=3) shuttle missions and respective ground-based control mice (n=30) were isolated for in vitro experimentation. Vasoconstrictor responses were evoked in arteries via norepinephrine (NE), potassium chloride (KCl), and caffeine, and in veins through NE across a range of intraluminal pressures (2–12 cmH2O). Vasoconstriction to NE was also determined in mesenteric arteries at 1, 5, and 7 d postlanding. In arteries, maximal constriction to NE, KCl, and caffeine were reduced immediately following spaceflight and 1 d postflight. Spaceflight also reduced arterial ryanodine receptor-3 mRNA levels. In mesenteric veins, there was diminished constriction to NE after flight. The results indicate that the impaired vasoconstriction following spaceflight occurs through the ryanodine receptor-mediated intracellular Ca2+ release mechanism. Such vascular changes in astronauts could compromise the maintenance of arterial pressure during orthostatic stress.—Behnke, B. J., Stabley, J. N., McCullough, D. J., Davis, R. T., III, Dominguez, J. M., II, Muller-Delp, J. M., Delp, M. D. Effects of spaceflight and ground recovery on mesenteric artery and vein constrictor properties in mice.
Keywords: orthostatic hypotension, microgravity, microcirculation
Orthostatic hypotension was found to manifest among the astronauts of Project Mercury after only brief excursions into space, making this condition one of the first medical complications reported to occur in astronauts (1, 2). Following short-term flight (2–3 wk) on the space shuttle, 20–50% of astronauts exhibit orthostatic hypotension when reexposed to normal gravitational stress (3–5), with the prevalence of this condition rising markedly after longer term (4–6 mo) habitation in microgravity (6). This orthostatic hypotension is due, in part, to a diminished ability to elevate peripheral vascular resistance (PVR; refs. 3, 7–9). One possible mechanism for the impaired ability to elevate PVR following spaceflight is a reduced magnitude or rate of vasoconstriction of the arterial resistance vasculature. Ground-based human and animal models used to simulate the effects of microgravity suggest decrements in vasoconstrictor responsiveness of arterial segments in various regions of the body, including the splanchnic circulation (10–13), as a potential mechanism contributing to orthostatic hypotension in astronauts.
In the face of a spaceflight-associated reduction in PVR, cardiac output would have to be augmented in order to maintain mean arterial pressure (MAP) during an orthostatic challenge. However, the ability to augment cardiac output following simulated microgravity is diminished, owing to decrements in stroke volume (14, 15). Venous filling pressure can affect stroke volume via the Starling mechanism and is largely regulated by systemic venous capacitance, of which the splanchnic region has the greatest capacitance (16). Because veins from the mesentery are responsive to sympathetic activity (17), active baroreceptor-mediated venoconstriction would mobilize mesenteric venous blood volume centrally to maintain venous filling pressure and stroke volume, while a diminished adrenergic and/or myogenic constriction of mesenteric veins after spaceflight would impair this process. Previous investigations simulating microgravity in rats have demonstrated diminished adrenergic and pressure-induced constriction of mesenteric veins (13, 18). However, little is known about the effects of spaceflight on the intrinsic vaso- or venoconstrictor properties of the mesenteric vasculature or whether putative alterations in vasomotor responses are reversible. Therefore, the purpose of this study was to determine the effects of microgravity on intrinsic vasomotor properties of mesenteric arteries and veins from mice flown on the Space Transportation System (STS)-131, STS-133, and STS-135 shuttle missions. Based on previous results from studies using ground-based simulations of microgravity (12, 13, 18), we hypothesized that spaceflight will diminish the magnitude of adrenergic KCl- and caffeine-evoked vasoconstriction in mesenteric arteries, and that this decrement will be related to altered sarcoplasmic reticulum Ca2+ handling; and that spaceflight will blunt adrenergic constriction of mesenteric veins across a range of intraluminal pressures. In addition, we hypothesized that the putative decrements in adrenergic vasoconstriction are reversible within 1 wk of return to Earth's gravitational influences.
MATERIALS AND METHODS
Experimental procedures were approved by the Institutional Animal Care and Use Committee at the National Aeronautics and Space Administration (NASA) and conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996).
Animals
Experiments described herein were performed on distinct animal groups from three separate NASA space shuttle missions. Female C57BL/6 mice (16–23 wk old; n=11; Charles River, Raleigh, NC, USA) were flown on the STS-131 mission for 15 d. Microvessel dissection procedures commenced ∼2.5 h after the landing of the space shuttle, following isoflurane anesthesia and euthanasia via exsanguination. Female BALB/cJ mice (12–13 wk old) were flown on the STS-133 mission for 13 d. Animals were euthanized with a high dose injection of xylazine and ketamine (i.p., 30 mg/kg body weight xylazine plus 300 mg/kg body weight ketamine), and microvessel dissection procedures commenced precisely 1 d (R+1; n=2), 5 d (R+5; n=2), and 7 d (R+7; n=2) after the return landing. Female C57BL/6 mice (9 wk old; n=3) were flown on the STS-135 mission for 13 d. Microvessel dissection procedures commenced ∼3–4 h after landing, following isoflurane anesthesia and euthanasia via cardiac puncture and exsanguination.
Spaceflight group (SF) mice were housed in NASA's animal enclosure modules (AEMs) located on the orbiter's middeck, maintained on a 12-h light-dark cycle, and provided food (19) and water ad libitum. Age-matched ground-based control group (GC) animals were housed in identical AEMs within an orbital environment simulator at Kennedy Space Center (KSC) to mimic the temperature, humidity, and CO2 of the space shuttle middeck. GC mice during the STS-131 (n=11) and STS-135 (n=9) missions were housed for the duration of each mission, beginning 48 h after launch and ending 48 h after landing. Aged-matched GC animals during the STS-133 mission were housed beginning and ending simultaneously to SF animals; experiments with GC mice (total n=10) were performed simultaneously at the R+1 (n=3), R+5 (n=4), and R+7 (n=3) time points. All experimental procedures conducted on SF animals were duplicated on GC animals. The KSC veterinarian deemed all animals healthy prior to SF and GC experiments. All in vitro laboratory experiments involving animals from each space shuttle flight were identically performed as described below.
Microvessel preparation
Mesenteric tissue was dissected from the carcass, placed in physiological saline solution (PSS) buffer on ice, and transported in a staggered fashion from KSC to the University of Florida campus in Gainesville, FL (∼3-h drive). Distal arcading resistance arteries (STS-131, STS-133) and veins (STS-135) from the mesentery were then dissected free using a stereomicroscope (Olympus SZX12; Olympus, Tokyo, Japan). Vessels were transferred to a Lucite chamber containing PSS, cannulated at each end with glass micropipettes, secured via 11-0 ophthalmic sutures (Alcon Laboratories, Inc., Fort Worth, TX, USA), and pressurized with PSS. After cannulation, the isolated vessel tissue chamber was transferred to the stage of an inverted microscope (Olympus IX70) interfaced in series with a video camera (Panasonic BG310; Panasonic, Tokyo, Japan), a horizontal video caliper (307A; Colorado Video, Boulder, CO, USA), a data-acquisition system (Powerlab; ADInstruments, Colorado Springs, CO, USA), a video cassette recorder (VCR; Panasonic AG-7300), and a video monitor (Panasonic WV-BM1410). Vessels were equilibrated for 15 min at 37°C with an intraluminal pressure of 108 cmH2O for arteries (20) and 4 cmH2O for veins prior to investigation of vasoconstrictor and myogenic properties. The distance between cannulating micropipettes was adjusted following pressurization so that the vessel axial length was straight but not stretched. Bathing PSS was replaced every 15 min during equilibration and throughout the experimental procedures. Intraluminal diameters were continuously measured via videomicroscopic techniques (12, 13).
Mesenteric arteries
Intraluminal pressure was maintained and manipulated by way of two independent reservoirs connected to the glass micropipettes cannulating mesenteric arteries. The basal pressure of 108 cmH2O was achieved by setting both reservoirs to the same hydrostatic level. Active myogenic behavior was determined via stepwise increases in intraluminal pressure (i.e., from 100 to 180 cmH2O) by raising the height of both reservoirs in 20-cmH2O increments. Intraluminal pressure was then decreased in 20-cmH2O increments from 180 to 100 cmH2O before return to basal pressure. Vasoconstrictor responses to increasing concentrations of KCl (10–100 mM) were measured to investigate the contribution of voltage-gated Ca2+ channels. Target isotonic K+ bath concentrations were achieved by balancing concentrations of NaCl and KCl in PSS such that bath osmolarity was maintained. Vasoconstrictor responses to the cumulative addition of norepinephrine (NE, 10−9 to 10−4 M) were measured to investigate α-adrenoreceptor-mediated vasoconstriction. Vasoconstriction through intracellular Ca2+ release from the sarcoplasmic reticulum was determined by measuring arterial responses to caffeine (15 mM) as previously described (12, 21).
Maximal intraluminal diameter and medial wall thickness were determined after two 15-min incubations in Ca2+-free PSS at 108 cmH2O. A bolus dose of sodium nitroprusside (SNP; 10−4 M) (22, 23) was added during the second 15-min incubation in Ca2+-free PSS in order to ensure complete smooth muscle relaxation. Medial wall thickness was taken as the mean of three separate wall thickness measurements from positions randomly selected along the microvessel.
In a second set of mesenteric arteries, passive vessel behavior was determined using identical procedures as that for the active myogenic response, except that the arteries were incubated twice for 15 min in Ca2+-free PSS with SNP (10−4 M) present during the second incubation prior to and during determination of the pressure-diameter relation.
During the NE dose-response test described above, a VCR was used to record the mesenteric artery constriction at 30 frames/s. To characterize mesenteric artery vasoconstrictor dynamics (12), the temporal response to NE was evaluated during the 10−4 M NE dose by using a video caliper (307A; Colorado Video) to conduct frame-by-frame measurements of intraluminal diameter, beginning at the onset of vasoconstriction and proceeding until a steady state was observed. Time and diameter values were curve-fit to a monoexponential-plus decay model (24, 25) using an iterative least-squares technique by means of a commercial graphing/analysis package (KaleidaGraph 3.5; Synergy Software, Inc., Reading, PA, USA). A user-defined function to the data was fit using the following equation:
where ΔDt is the change in diameter at time t, Db is baseline diameter, ΔDss is the change in diameter from baseline to the steady-state value, TD is the time delay, and τ is the time constant of the response, which estimates the time taken to reach 63% of the final exponential response. From the mathematical modeling results, the mean response time (MRT; TD + τ) and the rate of vasoconstriction (ΔD/τ) were calculated.
To determine whether alterations in adrenergic vasoconstriction of arteries returns to normal within 1 wk of return to terrestrial gravity, experiments were performed on days R+1, R+5, and R+7 after landing. Mesenteric arteries were dissected and prepared for in vitro experimentation. NE dose responses, maximal intraluminal diameter, and medial wall thickness measurements were performed as described above.
Mesenteric veins
Basal intraluminal pressure of mesenteric veins was set at 4 cmH2O. NE dose response curves were determined as described above for mesenteric arteries. Immediately after incubation in the final concentration of 10−4 M NE, intraluminal pressure was decreased to 2 cmH2O for 3 min before increasing intraluminal pressure in a stepwise fashion up to 12 cmH2O in 2-cmH2O increments to determine the pressure-diameter relation. Maximal intraluminal diameter and medial wall thickness at 4 cmH2O were determined as described above for mesenteric arteries.
mRNA expression
Additional mesenteric arteries isolated from SF and GC mice from the STS-131 mission were snap-frozen and stored at −80°C for later analysis of mRNA levels as described previously (12, 26, 27). The RNAqueous-Micro Kit (Applied Biosystems/Ambion, Austin, TX, USA), specified for use in microdissected tissue, was used to isolate total RNA from mesenteric arteries. Vessels immersed in 100 μl of lysis buffer were homogenized using a pellet mixer (Argos Technologies, Elgin, IL, USA). The lysate solution was then passed through the RNAqueous silica filter for total RNA isolation. Total RNA was reverse transcribed into complimentary DNA (cDNA) via the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). cDNA was then used in the real-time PCR (StepOnePlus; Applied Biosystems) to determine mRNA levels by way of TaqMan gene expression assays and endogenous controls (Applied Biosystems) specific for ryanodine receptor 2 (RyR-2; Mm00465877_m1), ryanodine receptor 3 (RyR-3; Mm01328421_m1), and 18S ribosomal RNA (4319413E). On the basis of data from a previous study where no ryanodine receptor 1 mRNA expression was detected in mesenteric arteries from hindlimb-unloaded rats (12), we elected only to probe for changes in RyR-2 and RyR-3 mRNA.
Solutions and drugs
PSS contained (in mM) 145 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.17 MgSO4, 2.0 CaCl2, 5.0 glucose, 2.0 pyruvate, 0.025 EDTA, and 3.0 MOPS, with pH 7.4. PSS was supplemented with bovine serum albumin (BSA; 1 g/100 ml; USB Corp., Cleveland, OH, USA) and passed through a 0.22-μm cellulose acetate filter (430015; Corning, Corning, NY, USA). Ca2+-free PSS preparation was identical except for the addition of 2 mM EDTA, the replacement of CaCl2 with 2.0 mM NaCl, and the exclusion of BSA. KCl and NE stock solutions were prepared in PSS. Caffeine (15 mM) was prepared in Ca2+-free PSS.
Statistical and data analyses
Intraluminal diameters were measured in micrometers and expressed as a percentage of vasoconstriction as follows:
where Db is the initial baseline intraluminal diameter measured before experimental intervention, and Dss is the steady-state intraluminal diameter measured after agonist addition. Spontaneous tone was expressed as a percentage of the maximal diameter (Dm) as follows:
The significance of differences in body mass, vessel characteristics, and mRNA levels were determined via Student's unpaired t tests. Pressure-response and concentration-response curves were evaluated by using repeated-measures analysis of variance with one within (intraluminal pressure or agonist concentration) and one between (experimental groups) factor. Planned contrasts were conducted at each intraluminal pressure or concentration level to determine whether differences existed between experimental groups (GC vs. SF). All values are presented as means ± se. An α level of 0.05 delineated significance.
RESULTS
Body and soleus muscle mass
For STS-131 mice, total body mass was not different between GC and SF mice (22.0±0.4 vs. 20.9±0.7 g, respectively). Total body mass of STS-133 GC mice (21.7±0.2 g) was greater than that of SF R+1 (18.7±0.3 g) and SF R+5 (20.3±0.1 g) mice, but not different from that of SF R+7 (20.6±0.7 g) mice. And total body mass of STS-135 mice was not different between groups (GC: 19.7±0.5 g; SF: 19.4±1.4 g). Right soleus muscle mass was greater in GC than SF mice in both the STS-131 (GC: 13.5±0.6 mg; SF: 10.4±0.4 mg) and STS-135 (GC: 6.0±0.3 mg; SF: 4.6±0.1 mg) missions; soleus muscle mass was not obtained from STS-133 animals.
Vessel characteristics
For mesenteric arteries pressurized at 108 cmH2O, maximal diameter, medial wall thickness, and spontaneous tone were not different between GC and SF groups on either the STS-131 or STS-133 missions (Table 1). For mesenteric veins pressurized at 4 cmH2O, medial wall thickness and spontaneous tone were not different between GC and SF mice from the STS-135 mission (Table 1). However, maximal diameter of mesenteric veins tended to be larger (P=0.078) in the SF mice (Table 1).
Table 1.
Vessel characteristics
| Characteristic | GC | SF |
|---|---|---|
| STS-131: mesenteric arteries | ||
| Maximal diameter (μm) | 170 ± 8 | 168 ± 12 |
| Medial wall thickness (μm) | 13.5 ± 0.7 | 13.8 ± 1.3 |
| Spontaneous tone (%) | 5.4 ± 1.7 | 5.2 ± 1.0 |
| STS-133: mesenteric arteries | ||
| R+1 | ||
| Maximal diameter (μm) | 159 ± 11 | 152 ± 25 |
| Medial wall thickness (μm) | 13.4 ± 0.7 | 13.0 ± 2.3 |
| Spontaneous tone (%) | 5.3 ± 0.9 | 4.8 ± 1.0 |
| R+5 | ||
| Maximal diameter (μm) | 171 ± 3 | |
| Medial wall thickness (μm) | 11.5 ± 0.8 | |
| Spontaneous tone (%) | 4.7 ± 1.8 | |
| R+7 | ||
| Maximal diameter (μm) | 154 ± 7 | |
| Medial wall thickness (μm) | 11.4 ± 1.9 | |
| Spontaneous tone (%) | 5.3 ± 1.5 | |
| STS-135: mesenteric veins | ||
| Maximal diameter (μm) | 236 ± 9 | 282 ± 37# |
| Medial wall thickness (μm) | 8.7 ± 0.9 | 9.6 ± 1.0 |
| Spontaneous tone (%) | 7.5 ± 1.7 | 6.3 ± 1.8 |
Values are means ± se for vessels from mice associated with the STS-131 (GC: n=11; SF: n=11), STS-133 (GC: n=10; SF: R+1, n=2; R+5, n=2; R+7, n=2) and STS-135 (GC: n=9; SF: n=3) space shuttle missions; n = number of animals studied.
P = 0.078 vs. GC.
Mesenteric artery vasoconstrictor responses
NE produced a dose-dependent increase in vasoconstriction of mesenteric arteries from both GC and SF animals (Fig. 1A). The vasoconstriction induced by NE was lower in the arteries from SF vs. GC (Fig. 1A). When quantifying temporal vasoconstrictor responses of mesenteric arteries to 10−4 M NE, both groups demonstrated a delay followed by a monoexponential decrease in lumen diameter. The monoexponential model provided a superb fit, as demonstrated by a high correlation coefficient (GC: r=0.983±0.006; SF: r=0.946±0.042). The speed of vasoconstriction was markedly slower in the SF group, resulting in a doubling in the time taken to reach a steady-state diameter after exposure to NE (GC: 10.0±2.5 s; SF: 20.6±5.6 s; P < 0.05). Arteries from the SF mice demonstrated a prolonged MRT (i.e., time delay + time constant; Fig. 1B) as well as an ∼50% reduction in the rate of vasoconstriction (Δ vasoconstriction ÷ time constant; Fig. 1B) vs. the GC response.
Figure 1.
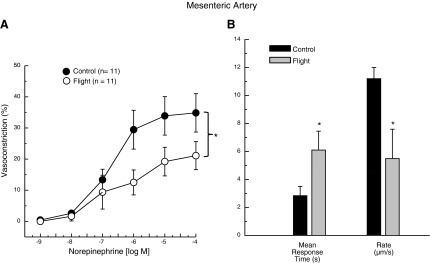
A) NE dose-response relations of mesenteric arteries from GC and 15-d STS-131 SF mice. B) Mean response time and rate of constriction after exposure to 10−4 M NE in mesenteric arteries from GC and SF mice. Values are means ± se; n = number of animals studied. *P < 0.05 between groups.
Increases in KCl concentration produced dose-dependent decrease in luminal diameter of mesenteric arteries from both groups; however, constriction evoked by KCl was lower in arteries from SF mice (Fig. 2A). Spaceflight did not alter active myogenic responses to pressure changes (Fig. 2B) or affect the passive-pressure response characteristics of mesenteric arteries (Fig. 2B). Vasoconstrictor responses to caffeine were lower in mesenteric arteries from SF vs. GC mice (Fig. 3A).
Figure 2.
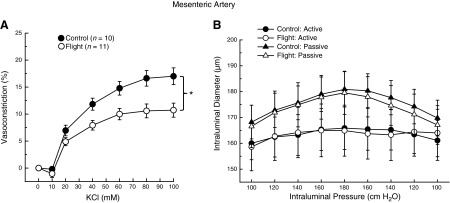
A) Potassium chloride dose-response relations of mesenteric arteries from GC and 15-d STS-131 SF mice. B) Active and passive diameter responses to changes in transmural pressure in mesenteric arteries from GC and 15-d STS-131 SF mice. Values are means ± se; n = number of animals studied. Active responses were different from passive responses within each group, but neither active nor passive diameter responses were different between vessels from GC and SF mice. *P < 0.05 between groups.
Figure 3.
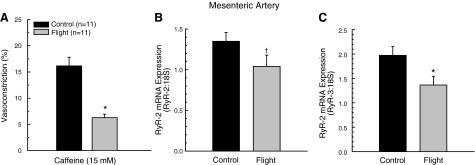
A) Maximal vasoconstrictor response to 15 mM caffeine in mesenteric arteries from GC and 15-d STS-131 SF mice. B, C) Effects of spaceflight on RyR-2 (B) and RyR-3 (C) mRNA expression in mesenteric arteries from GC and 15-d STS-131 SF mice. Values are means ± se; n = number of animals studied. *P < 0.05, †P < 0.10 vs. GC.
There was a tendency (P=0.064) for RyR-2 mRNA levels to be lower in mesenteric arteries after spaceflight (Fig. 3B), whereas the lower expression of RyR-3 mRNA was significant in the SF vs. GC arteries (Fig. 3C).
Time course of adrenergic vasoconstriction during ground recovery from spaceflight
Mesenteric artery vasoconstrictor responses to NE in GC mice were similar at the R+1, R+5, and R+7, time points, so the data were pooled to provide greater statistical power for determining potential differences between GC and SF vascular responses. NE-evoked constriction was significantly lower in the SF R+1 group relative to that from GC mice (Fig. 4A), and qualitatively similar to results obtained immediately after spaceflight (Fig. 1A). However, after 5 d (Fig. 4B) and 7 d (Fig. 4C) of ground recovery, mesenteric artery vasoconstriction was not different between groups.
Figure 4.
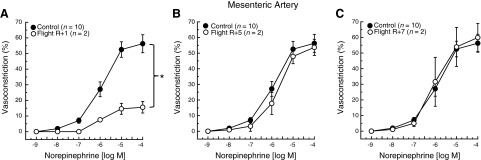
NE dose-response relations in mesenteric arteries from GC and 13-d STS-133 SF mice at R+1 d (A), R+5 d (B), and R+7 d (C) after return from flight. Values are means ± se; n = number of animals studied. *P < 0.05 between groups.
Mesenteric vein constrictor responses
There was a dose-dependent constriction of mesenteric veins in both SF and GC groups. Venoconstriction to NE was lower in veins from SF mice (Fig. 5A). Mesenteric vein luminal diameter increased as a function of increasing intraluminal pressure in the presence of 10−4 M NE in both groups. However, intraluminal diameter was greater at each pressure in veins from the SF group (Fig. 5B).
Figure 5.
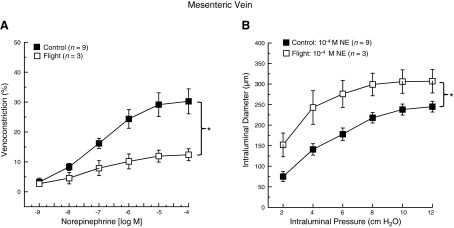
A) NE dose-response relations in mesenteric veins from GC and 13-d STS-135 SF mice. B) Intraluminal pressure-diameter relations in mesenteric veins from GC and 13-d STS-135 SF mice following preincubation in 10−4 M NE. Values are means ± se; n = number of animals studied. *P < 0.05 between groups.
DISCUSSION
The purpose of this study was to determine whether spaceflight alters the intrinsic vasoconstrictor responsiveness of mesenteric arteries and veins, and to examine whether putative reductions in vasoconstrictor responsiveness persist up to 1 wk after landing. Based on hindlimb unloading (HU) rodent models, which are ground-based studies to simulate a weightless environment (12, 13), we hypothesized that mesenteric vessel responses to NE, KCl, and caffeine would be impaired in SF mice. The results demonstrate that spaceflight diminishes the magnitude (Fig. 1A) and rapidity (Fig. 1B) of mesenteric artery vasoconstriction to the adrenergic agonist NE; microgravity diminishes vasoconstriction to KCl (Fig. 2A) and caffeine (Fig. 3A); and spaceflight does not affect myogenic vasoconstriction (Fig. 2B). The blunted constriction to caffeine, which is mediated through a sarcoplasmic reticulum Ca2+-release mechanism, was associated with lower arterial RyR-2 (Fig. 2B) and RyR-3 (Fig. 2C) mRNA expression. These microgravity-induced changes in arterial vasoconstriction occurred in the absence of any gross structural changes in mesenteric artery wall thickness or maximal interluminal diameter (Table 1), or by any alterations in the passive mechanical properties of the vessels, as indicated by an unaltered passive pressure-diameter response (Fig. 2B). The diminished adrenergic mesenteric artery vasoconstriction persisted 1 d postspaceflight (Fig. 4A), but was similar to that in GC mice at 5 and 7 d postflight (Fig. 4B, C). Finally, spaceflight blunted NE-evoked constriction of mesenteric veins (Fig. 5). These results suggest that on return to Earth, decrements in mesenteric arterial and venous constriction in astronauts could compromise the cardiovascular response to maintain arterial pressure during orthostatic stress.
Mechanistic basis for diminished arterial vasoconstriction
Following spaceflight, astronauts demonstrate a diminished ability to elevate PVR during orthostasis (3, 6, 8). This reduced PVR occurs despite elevations in sympathetic neural outflow (28) and higher levels of circulating plasma NE (29) during microgravity. Such observations support the contention that microgravity induces a hyporesponsiveness of resistance arteries to adrenergic stimuli. Results from the present study confirm that microgravity impairs both the magnitude and rate of mesenteric artery vasoconstriction to NE (Fig. 1A, B, respectively). In one of only two studies to previously examine the effects of spaceflight on intrinsic vasomotor responsiveness, Hatton et al. (30) reported that microgravity blunted NE-mediated vasoconstriction of mesenteric arteries from SF rats. However, because these animals were spontaneously hypertensive, the effects of spaceflight on vascular responses in normotensive animals was uncertain. In the second study, NE-mediated constriction of gastrocnemius feed arteries was found to be lower in SF mice (31). Collectively, experimental results with rats and mice confirm the contention that spaceflight compromises the vasoconstrictor responsiveness of the peripheral resistance vasculature to adrenergic stimulation.
To elucidate potential mechanisms of reduced vasoconstriction after spaceflight, vasoconstrictor responses that occur through discrete mechanisms were determined. NE and KCl cause vasoconstriction through both the influx of extracellular Ca2+ and intracellular Ca2+ release from the sarcoplasmic reticulum (32–34). In contrast, myogenic vasoconstriction appears to be more dependent on extracellular Ca2+ for smooth muscle contraction (35–38). Several studies have shown that while mobilization of intracellular Ca2+ stores may occur during myogenic vasoconstriction (39), release of intracellular Ca2+ is not an essential component for this vasoconstriction to occur (37, 38). Therefore, the differential effects of spaceflight to impair vasoconstriction elicited through both NE (Fig. 1A) and KCl (Fig. 2A), but not via a myogenic response (Fig. 2B), could reflect a deficit in intracellular Ca2+ release. To test this possibility, caffeine-induced vasoconstriction, which occurs through an intracellular Ca2+-release mechanism (32–34), was measured. The finding of a lower caffeine-mediated vasoconstriction of arteries from SF mice (Fig. 3A) suggests that microgravity adversely affects elevations in smooth muscle cell cytosolic Ca2+ levels through an impaired sarcoplasmic reticulum Ca2+-release mechanism. This conclusion is supported by the findings of reduced RyR-2 (Fig. 3B) and RyR-3 (Fig. 3C) mRNA levels in the mesenteric arteries from SF mice. These ryanodine receptor subtypes are important for intracellular release of Ca2+ in smooth muscle cells (40, 41).
Numerous ground-based studies using HU rats to simulate the effects of microgravity have found deficits in arterial vasoconstriction, including those of the abdominal and thoracic aorta (42–46), pulmonary arteries (47), femoral arteries (46, 48, 49), skeletal muscle arterioles (22, 50) and mesenteric arteries (12, 13, 51, 52). In mesenteric arteries, Colleran et al. (12) demonstrated lower NE-, KCl-, and caffeine-induced vasoconstriction, as well as decreased RyR-2 mRNA and protein after 14 d of HU. In addition, ryanodine-sensitive Ca2+ release from the sarcoplasmic reticulum is decreased in vascular smooth muscle cells isolated from mesenteric arteries of HU rats (53). Thus, the mechanism of the microgravity-induced impairment of mesenteric artery vasoconstrictor responsiveness in the present study appears to be consistent with that of ground-based studies with HU rats.
Alternative mechanisms have been proposed to underlie reductions in responsiveness of central arteries to NE, including enhanced endothelial-dependent vasodilation (offsetting vasoconstriction), and atrophic vascular remodeling resulting in a reduction in medial wall thickness and medial cross-sectional area. With regard to the first alternatively proposed mechanism, it is possible that arterial hyporesponsiveness to NE with simulated microgravity involves up-regulation of the endothelial NO/cGMP pathway, as differences in vasoconstriction were abolished with the ablation of the endothelial cell lining or inhibition of nitric oxide synthase activity (42, 47). However, Delp et al. (44) and Sangha et al. (48) have demonstrated in the abdominal aorta that the impairment of vasoconstriction persists regardless of whether the endothelium is removed or intact. And in perhaps the most relevant study, Hatton et al. (30) demonstrated an attenuated relaxation to the endothelium-dependent vasodilator acetylcholine in mesenteric arteries of rats after spaceflight, suggesting that endothelium-derived NO bioavailability is depressed rather than enhanced in arteries showing reduced vasoconstrictor responsiveness to NE.
The second alternative mechanism for the reduced vasoconstrictor responsiveness of mesenteric arteries is an atrophic structural remodeling (54, 55). Using both histomorphometric techniques and videographic methods in pressurized mesenteric artery segments to determine vessel medial wall thickness, medial cross-sectional area and intraluminal diameter, Lin et al. (54) have reported a thinner medial wall and diminished medial cross-sectional area with no change in maximal intraluminal diameter in mesenteric arteries from HU rats; this atrophic remodeling was associated with attenuated myogenic KCl- and phenylephrine-evoked vasoconstriction. Others, however, using similar histomorphometric and videographic methodologies, have found no alterations in gross vascular structure of mesenteric arteries in HU rats (12, 13, 56) or spaceflown rats (30) and mice (present study). Thus, the preponderance of evidence suggests that atrophic structural remodeling cannot account for decrements in vasoconstrictor responsiveness of mesenteric arteries in spaceflown and HU rodents.
Mechanistic basis for diminished venous constriction
Venular pressure-diameter responses are regulated by sympathetic activity in vivo (17) through α1 adrenoreceptors (57, 58). To our knowledge, the effects of microgravity on intrinsic venoconstrictor function have not been previously studied. Results from the present study demonstrate blunted NE-induced constriction of mesenteric veins (Fig. 5A) across a range of intraluminal pressures (Fig. 5B). These results are consistent with a previous observation of diminished α-receptor density in the vena cava of rats following spaceflight (59) as a potential mechanism for the diminished venoconstriction. Several studies using HU rats have likewise reported a decreased venoconstriction to NE in the vena cava and mesenteric veins (13, 18, 59, 60), but not in jugular and femoral veins (46). Similar to mesenteric arteries, another potential mechanism for spaceflight-associated impairment of venoconstriction is a blunting of intracellular Ca2+ release through the down-regulation of ryanodine receptors (61). Indeed, Morel et al. (60) demonstrated lower intracellular Ca2+ release in response to NE, angiotensin II, and caffeine in rat portal vein myocytes after 14 d of HU. Further, [3H]ryanodine binding in the same myocytes revealed a reduction in sarcoplasmic reticulum-based ryanodine receptors with no change in ryanodine receptor Ca2+ sensitivity or voltage-gated Ca2+ channel activity.
The primary unanswered question regarding vascular alterations associated with spaceflight is the stimulus for the change. Results from the present and previous studies (30, 31) show impaired contraction of smooth muscle cells of arteries and veins from SF rats and mice. In addition, pregnant rats flown on the Cosmos 1514 mission had prolonged labor (62), and rats flown on Cosmos 1667 had distended stomachs filled with food (63), indicating the possible impairment of uterine and stomach smooth muscle contraction with spaceflight. Also, lymphatic vascular smooth muscle cells in HU rats demonstrate an impaired ability to constrict (64). The apparent systemic nature of the smooth muscle dysfunction would seem to rule out local alterations in hydrostatic pressures or intravascular shear-stress as direct effector mechanisms. Rather, alterations in hydrostatic fluid pressures or other spaceflight-associated phenomena could trigger systemic release of some as of yet unknown factors that could be responsible for the smooth muscle dysfunction induced by microgravity. For example, cephalic fluid shifts elicit elevations in circulating levels of numerous substances (65), including endogenous hormones such as atrial natriuretic peptides (ANPs) and brain natriuretic peptides (BNPs) that serve to regulate fluid volume (66). We have previously demonstrated that both ANPs and BNPs reduce smooth muscle contractile responses to adrenergic stimulation in mesenteric arteries and veins, and that these peptides are elevated during simulated microgravity (13). Keil et al. (67) have also reported that ANP levels were lower in atrial tissue from rats flown on the Cosmos 2044/Bion 9 biosatellite, and suggested that the lower tissue concentrations reflect elevated ANP secretion during flight. However, others have reported unchanged (68) or decreased (69) plasma ANP concentrations with simulated microgravity. Therefore, whether these peptides or other circulating factors are responsible for microgravity-induced smooth muscle dysfunction remains to be determined.
Time course of arterial recovery from spaceflight
Impairment of vasoconstrictor responsiveness of mesenteric resistance arteries may underlie, at least in part, the orthostatic hypotension evident in humans following spaceflight (3, 6–9, 70), given that ∼20% of the increase in PVR comes from the splanchnic region on standing (71). The condition of orthostatic intolerance is usually corrected within 2 d of return to terrestrial gravity, while complete orthostatic recovery is achieved in a length of time equivalent to that of flight operations (5). Using ground-based models of simulated microgravity, structural and functional adaptations of arteries in various vascular beds require several days before either partial or full return to that observed in control animals (55). Results from the present study demonstrate that reductions in NE-induced vasoconstriction persist 1 d after spaceflight (Fig. 4A), but return to control levels within 5 d of ground recovery (Fig. 4B). The precise mechanisms for the recovered vasoconstriction are presently unknown, but the time frame of recovery (i.e., 2–5 d) is similar to that required to fully restore ryanodine receptor function and caffeine-induced Ca2+ release in mesenteric artery vascular smooth muscle cells after simulated microgravity (53).
Functional ramifications of vasomotor alterations
Stroke volume has been shown to be diminished during orthostasis following spaceflight (5, 15). Because stroke volume is affected by cardiac contractility and venous filling pressure, a blunting of the magnitude and rate of arterial vasoconstriction would not only affect PVR, but also compromise central venous filling pressure by increasing the volume of blood located within peripheral arterioles, venules, and small veins (71). Given the prodigious vascular capacitance of the splanchnic region (16), a diminished mesenteric vasoconstriction after spaceflight in humans could impair central mobilization of blood volume to support stroke volume and cardiac output. Indeed, a compromised ability to lower perfusion of splanchnic areas during lower-body negative pressure has been shown to occur following prolonged bedrest (10, 11). Intact vasoconstrictor mechanisms are also required for the redistribution of cardiac output away from visceral tissue to active skeletal muscles during exercise (71, 72). Thus, an impaired ability to reduce visceral blood flow in HU rats (14, 51) would adversely affect aerobic capacity and exercise tolerance. Therefore, dysfunction of vaso- and venoconstrictor mechanisms within the splanchnic vasculature in astronauts likely plays a fundamental role in the altered hemodynamics during exercise and orthostasis upon return to Earth.
CONCLUSIONS
The results of the present study demonstrate that spaceflight diminishes the magnitude (Fig. 1A) and rapidity (Fig. 1B) of mesenteric artery vasoconstriction to the adrenergic receptor agonist NE. Vasoconstriction evoked through voltage-gated Ca2+ channels (KCl; Fig. 2A) and sarcoplasmic reticulum Ca2+ release (caffeine; Fig. 3A) was also diminished in arteries from SF mice. The blunted constriction to caffeine was associated with lower arterial RyR-2 (Fig. 2B) and RyR-3 (Fig. 2C) mRNA, indicating that the vasoconstrictor dysfunction of mesenteric arteries from SF mice occurs through an impairment of the ryanodine receptor-mediated intracellular Ca2+ release mechanism. These changes in arterial vasoconstriction elicited by microgravity occurred in the absence of any gross structural changes in mesenteric artery medial wall thickness or maximal luminal diameter (Table 1), or through any alterations in the passive mechanical properties of the vessels (Fig. 2B). The adrenergic vasoconstrictor dysfunction persisted 1 d postspaceflight (Fig. 4A), but was normalized 5 d after landing (Fig. 4B). Finally, spaceflight blunted NE-evoked constriction of mesenteric veins (Fig. 5A). When applied to the human condition, the results suggest that the decrements in mesenteric arterial vasoconstriction could underlie the relative inability of astronauts to augment PVR and maintain MAP during orthostasis on return to Earth. Furthermore, the impairment of arterial and venous constriction could diminish the ability of astronauts to centrally mobilize blood volume to maintain venous filling pressure, stroke volume, and cardiac output during orthostatic and exercise stress.
Acknowledgments
These studies were originally flown on space shuttle mission STS-107, on which the orbiter Columbia tragically disintegrated during its return flight to Earth. This manuscript is dedicated to the STS-107 crew, who were willing to risk their lives to advance our understanding of the effects of microgravity on human health. The authors thank Blaze Emerson for technical assistance and Richard Boyle, Paula Dumars, Marianne Steele, Marilyn Vasquez, and Vera Vizir [U.S. National Aeronautics and Space Administration (NASA) Ames Research Center, Mountain View, CA, USA] for their logistical support.
This study was supported by grants from NASA (NNX08AQ62G and NNX09AP06G), the U.S. National Institutes of Health (AG-31317), the Florida Biomedical Research Program (1BN-02), and the Jane Adams Edmonds endowed doctoral fellowship (Department of Applied Physiology and Kinesiology, University of Florida).
Footnotes
- ΔDss
- change in diameter from baseline to steady-state value
- ΔDt
- change in diameter at time t
- AEM
- animal enclosure module
- ANP
- atrial natriuretic peptide
- BNP
- brain natriuretic peptide
- Db
- baseline diameter
- Dss
- steady-state intraluminal diameter
- Dm
- maximal diameter
- GC
- ground-based control group
- HU
- hindlimb unloading
- KSC
- Kennedy Space Center
- MAP
- mean arterial pressure
- MRT
- mean response time
- NE
- norepinephrine
- PVR
- peripheral vascular resistance
- PSS
- physiological saline solution
- R+1
- return landing plus 1 d
- R+5
- return landing plus 5 d
- R+7
- return landing plus 7 d
- RyR-2
- ryanodine receptor 2
- RyR-3
- ryanodine receptor 3
- SF
- spaceflight group
- SNP
- sodium nitroprusside
- STS
- Space Transortation System
- TD
- time delay
REFERENCES
- 1. Catterson A. D., McCutcheon H. A., Pollard R. A. (1963) Mercury project summary including results of the fourth manned orbital flight May 15 and 16, 1963. In Aeromedical Observations (NASA, ed.) pp. 299–326, National Aeronautics and Space Administration, Washington, DC [Google Scholar]
- 2. Bullard R. W. (1972) Physiological problems of space travel. Annu. Rev. Physiol. 34, 205–234 [DOI] [PubMed] [Google Scholar]
- 3. Buckey J. C., Jr., Lane L. D., Levine B. D., Watenpaugh D. E., Wright S. J., Moore W. E., Gaffney F. A., Blomqvist C. G. (1996) Orthostatic intolerance after spaceflight. J. Appl. Physiol. 81, 7–18 [DOI] [PubMed] [Google Scholar]
- 4. Blomqvist C. G., Stone H. L. (1983) Cardiovascular adjustments to gravitational stress. In Handbook of Physiology. The Cardiovascular System: Peripheral Circulation and Organ Blood Flow pp. 1027–1063, American Physiological Society, Bethesda, MD, USA [Google Scholar]
- 5. Watenpaugh D. E., Hargens A. R. (1996) The cardiovascular system in microgravity. In Handbook of Physiology. Environmental Physiology pp. 631–674, American Physiological Society, Bethesda, MD, USA [Google Scholar]
- 6. Meck J. V., Reyes C. J., Perez S. A., Goldberger A. L., Ziegler M. G. (2001) Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosom. Med. 63, 865–873 [DOI] [PubMed] [Google Scholar]
- 7. Arbeille P. F., G., Archaibou J., Potter J. M., Kotovskaya A. (1996) Cardiac and vascular adaptation to 0g with and withouth thigh cuffs (Antares 14 and Altair 21 day Mir spaceflights). Acta Astronaut. 36, 6–10 [DOI] [PubMed] [Google Scholar]
- 8. Mulvagh S. L., Charles J. B., Riddle J. M., Rehbein T. L., Bungo M. W. (1991) Echocardiographic evaluation of the cardiovascular effects of short-duration spaceflight. J. Clin. Pharmacol. 31, 1024–1026 [DOI] [PubMed] [Google Scholar]
- 9. Waters W. W., Ziegler M. G., Meck J. V. (2002) Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. J. Appl. Physiol. 92, 586–594 [DOI] [PubMed] [Google Scholar]
- 10. Arbeille P., Kerbeci P., Mattar L., Shoemaker J. K., Hughson R. (2008) Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest. Am. J. Physiol. Heart Circ. Physiol. 295, H1846–H1854 [DOI] [PubMed] [Google Scholar]
- 11. Arbeille P. P., Besnard S. S., Kerbeci P. P., Mohty D. M. (2005) Portal vein cross-sectional area and flow and orthostatic tolerance: a 90-day bed rest study. J. Appl. Physiol. 99, 1853–1857 [DOI] [PubMed] [Google Scholar]
- 12. Colleran P. N., Behnke B. J., Wilkerson M. K., Donato A. J., Delp M. D. (2008) Simulated microgravity alters rat mesenteric artery vasoconstrictor dynamics through an intracellular Ca(2+) release mechanism. Am. J. Physiol. 294, R1577–1585 [DOI] [PubMed] [Google Scholar]
- 13. Behnke B. J., Zawieja D. C., Gashev A. A., Ray C. A., Delp M. D. (2008) Diminished mesenteric vaso- and venoconstriction and elevated plasma ANP and BNP with simulated microgravity. J. Appl. Physiol. 104, 1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodman C. R., Sebastian L. A., Tipton C. M. (1995) Influence of simulated microgravity on cardiac output and blood flow distribution during exercise. J. Appl. Physiol. 79, 1762–1768 [DOI] [PubMed] [Google Scholar]
- 15. Levine B. D., Zuckerman J. H., Pawelczyk J. A. (1997) Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96, 517–525 [DOI] [PubMed] [Google Scholar]
- 16. Rowell L. B., Detry J. M., Blackmon J. R., Wyss C. (1972) Importance of the splanchnic vascular bed in human blood pressure regulation. J. Appl. Physiol. 32, 213–220 [DOI] [PubMed] [Google Scholar]
- 17. Shoukas A. A., Bohlen H. G. (1990) Rat venular pressure-diameter relationships are regulated by sympathetic activity. Am. J. Physiol. 259, H674–H680 [DOI] [PubMed] [Google Scholar]
- 18. Dunbar S. L., Berkowitz D. E., Brooks-Asplund E. M., Shoukas A. A. (2000) The effects of hindlimb unweighting on the capacitance of rat small mesenteric veins. J. Appl. Physiol. 89, 2073–2077 [DOI] [PubMed] [Google Scholar]
- 19. Sun G. S., Tou J. C., Liittschwager K., Herrera A. M., Hill E. L., Girten B., Reiss-Bubenheim D., Vasques M. (2010) Evaluation of the nutrient-upgraded rodent food bar for rodent spaceflight experiments. Nutrition 26, 1163–1169 [DOI] [PubMed] [Google Scholar]
- 20. Fenger-Gron J., Mulvany M. J., Christensen K. L. (1995) Mesenteric blood pressure profile of conscious, freely moving rats. J. Physiol. 488(Pt. 3), 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Callera G. E., Bendhack L. M. (1999) Contribution of sarcoplasmic reticulum calcium uptake and L-type calcium channels to altered vascular responsiveness in the aorta of renal hypertensive rats. Gen. Pharmacol. 33, 457–466 [DOI] [PubMed] [Google Scholar]
- 22. Delp M. D., Colleran P. N., Wilkerson M. K., McCurdy M. R., Muller-Delp J. (2000) Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am. J. Physiol. Heart Circ. Physiol. 278, H1866–H1873 [DOI] [PubMed] [Google Scholar]
- 23. McCurdy M. R., Colleran P. N., Muller-Delp J., Delp M. D. (2000) Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J. Appl. Physiol. 89, 398–405 [DOI] [PubMed] [Google Scholar]
- 24. Behnke B. J., Kindig C. A., Musch T. I., Koga S., Poole D. C. (2001) Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir. Physiol. 126, 53–63 [DOI] [PubMed] [Google Scholar]
- 25. Behnke B. J., Delp M. D. (2010) Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J. Appl. Physiol. 108, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donato A. J., Lesniewski L. A., Delp M. D. (2005) The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc. Res. 66, 393–401 [DOI] [PubMed] [Google Scholar]
- 27. Spier S. A., Delp M. D., Meininger C. J., Donato A. J., Ramsey M. W., Muller-Delp J. M. (2004) Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J. Physiol. 556, 947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ertl A. C., Diedrich A., Biaggioni I., Levine B. D., Robertson R. M., Cox J. F., Zuckerman J. H., Pawelczyk J. A., Ray C. A., Buckey J. C., Jr., Lane L. D., Shiavi R., Gaffney F. A., Costa F., Holt C., Blomqvist C. G., Eckberg D. L., Baisch F. J., Robertson D. (2002) Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J. Physiol. 538, 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norsk P., Damgaard M., Petersen L., Gybel M., Pump B., Gabrielsen A., Christensen N. J. (2006) Vasorelaxation in space. Hypertension 47, 69–73 [DOI] [PubMed] [Google Scholar]
- 30. Hatton D. C., Yue Q., Chapman J., Xue H., Dierickx J., Roullet C., Coste S., Roullet J. B., McCarron D. A. (2002) Blood pressure and mesenteric resistance arterial function after spaceflight. J. Appl. Physiol. 92, 13–17 [DOI] [PubMed] [Google Scholar]
- 31. Stabley J. N., Dominguez J. M., Dominguez C. E., Mora F., Ahlgren J., Behnke B. J., Muller-Delp J., Delp M. D. (2012) Spaceflight reduces vasoconstrictor responsiveness of skeletal muscle resistance arteries in mice. J. Appl. Physiol. 113, 1439–1445 [DOI] [PubMed] [Google Scholar]
- 32. Boittin F. X., Macrez N., Halet G., Mironneau J. (1999) Norepinephrine-induced Ca(2+) waves depend on InsP(3) and ryanodine receptor activation in vascular myocytes. Am. J. Physiol. 277, C139–C151 [DOI] [PubMed] [Google Scholar]
- 33. Coussin F., Macrez N., Morel J. L., Mironneau J. (2000) Requirement of ryanodine receptor subtypes 1 and 2 for Ca(2+)-induced Ca(2+) release in vascular myocytes. J. Biol. Chem. 275, 9596–9603 [DOI] [PubMed] [Google Scholar]
- 34. Steenbergen J. M., Fay F. S. (1996) The quantal nature of calcium release to caffeine in single smooth muscle cells results from activation of the sarcoplasmic reticulum Ca(2+)-ATPase. J. Biol. Chem. 271, 1821–1824 [DOI] [PubMed] [Google Scholar]
- 35. Davis M. J., Hill M. A. (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 79, 387–423 [DOI] [PubMed] [Google Scholar]
- 36. Miriel V. A., Mauban J. R., Blaustein M. P., Wier W. G. (1999) Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J. Physiol. 518(Pt. 3), 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe J., Horiguchi S., Karibe A., Keitoku M., Takeuchi M., Satoh S., Takishima T., Shirato K. (1994) Effects of ryanodine on development of myogenic response in rat small skeletal muscle arteries. Cardiovasc. Res. 28, 480–484 [DOI] [PubMed] [Google Scholar]
- 38. Watanabe J., Karibe A., Horiguchi S., Keitoku M., Satoh S., Takishima T., Shirato K. (1993) Modification of myogenic intrinsic tone and [Ca2+]i of rat isolated arterioles by ryanodine and cyclopiazonic acid. Circ. Res. 73, 465–472 [DOI] [PubMed] [Google Scholar]
- 39. Davis M. J., Donovitz J. A., Hood J. D. (1992) Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am. J. Physiol. 262, C1083–C1088 [DOI] [PubMed] [Google Scholar]
- 40. Zheng Y. M., Wang Q. S., Liu Q. H., Rathore R., Yadav V., Wang Y. X. (2008) Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J. Vasc. Res. 45, 469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Essin K., Gollasch M. (2009) Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J. Biomed. Biotechnol. 2009, 135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White A. R., Ryoo S., Bugaj L., Attarzadeh D. O., Thiyagarajan S., Chen K., Attwater S., Abbot B., Li D., Champion H. C., Shoukas A. A., Nyhan D., Hare J. M., Berkowitz D. E., Tuday E. C. (2010) Early changes in vasoreactivity after simulated microgravity are due to an upregulation of the endothelium-dependent nitric oxide/cGMP pathway. Eur. J. Appl. Physiol. 110, 395–404 [DOI] [PubMed] [Google Scholar]
- 43. Delp M. D., Brown M., Laughlin M. H., Hasser E. M. (1995) Rat aortic vasoreactivity is altered by old age and hindlimb unloading. J. Appl. Physiol. 78, 2079–2086 [DOI] [PubMed] [Google Scholar]
- 44. Delp M. D., Holder-Binkley T., Laughlin M. H., Hasser E. M. (1993) Vasoconstrictor properties of rat aorta are diminished by hindlimb unweighting. J. Appl. Physiol. 75, 2620–2628 [DOI] [PubMed] [Google Scholar]
- 45. Papadopoulos A., Delp M. D. (2003) Effects of hindlimb unweighting on the mechanical and structure properties of the rat abdominal aorta. J. Appl. Physiol. 94, 439–445 [DOI] [PubMed] [Google Scholar]
- 46. Purdy R. E., Duckles S. P., Krause D. N., Rubera K. M., Sara D. (1998) Effect of simulated microgravity on vascular contractility. J. Appl. Physiol. 85, 1307–1315 [DOI] [PubMed] [Google Scholar]
- 47. Nyhan D., Kim S., Dunbar S., Li D., Shoukas A., Berkowitz D. E. (2002) Impaired pulmonary artery contractile responses in a rat model of microgravity: role of nitric oxide. J. Appl. Physiol. 92, 33–40 [DOI] [PubMed] [Google Scholar]
- 48. Sangha D. S., Vaziri N. D., Ding Y., Purdy R. E. (2000) Vascular hyporesponsiveness in simulated microgravity: role of nitric oxide-dependent mechanisms. J. Appl. Physiol. 88, 507–517 [DOI] [PubMed] [Google Scholar]
- 49. Ma J., Zhang L. F., Yu Z. B. (1996) Effects of 14-day tail suspension on vasoreactivity of arteries from different parts of the body in rats. J. Gravit. Physiol. 3, 9–10 [PubMed] [Google Scholar]
- 50. Delp M. D. (1999) Myogenic and vasoconstrictor responsiveness of skeletal muscle arterioles is diminished by hindlimb unloading. J. Appl. Physiol. 86, 1178–1184 [DOI] [PubMed] [Google Scholar]
- 51. McDonald K. S., Delp M. D., Fitts R. H. (1992) Effect of hindlimb unweighting on tissue blood flow in the rat. J. Appl. Physiol. 72, 2210–2218 [DOI] [PubMed] [Google Scholar]
- 52. Overton J. M., Tipton C. M. (1990) Effect of hindlimb suspension on cardiovascular responses to sympathomimetics and lower body negative pressure. J. Appl. Physiol. 68, 355–362 [DOI] [PubMed] [Google Scholar]
- 53. Xue J. H., Chen L. H., Zhao H. Z., Pu Y. D., Feng H. Z., Ma Y. G., Ma J., Chang Y. M., Zhang Z. M., Xie M. J. (2011) Differential regulation and recovery of intracellular Ca2+ in cerebral and small mesenteric arterial smooth muscle cells of simulated microgravity rat. PLoS. One 6, e19775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin L. J., Gao F., Bai Y. G., Bao J. X., Huang X. F., Ma J., Zhang L. F. (2009) Contrasting effects of simulated microgravity with and without daily -Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. J. Appl. Physiol. 107, 1710–1721 [DOI] [PubMed] [Google Scholar]
- 55. Zhang L. F. (2001) Vascular adaptation to microgravity: what have we learned? J. Appl. Physiol. 91, 2415–2430 [DOI] [PubMed] [Google Scholar]
- 56. Wilkerson M. K., Muller-Delp J., Colleran P. N., Delp M. D. (1999) Effects of hindlimb unloading on rat cerebral, splenic, and mesenteric resistance artery morphology. J. Appl. Physiol. 87, 2115–2121 [DOI] [PubMed] [Google Scholar]
- 57. Kong J. Q., Taylor D. A., Fleming W. W. (1994) Functional distribution and role of alpha-1 adrenoceptor subtypes in the mesenteric vasculature of the rat. J. Pharmacol. Exp. Ther. 268, 1153–1159 [PubMed] [Google Scholar]
- 58. Leech C. J., Faber J. E. (1996) Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am. J. Physiol. 270, H710–H722 [DOI] [PubMed] [Google Scholar]
- 59. Sayet I., Neuilly G., Mironneau J., Mironneau C. (1995) Influence of spaceflight, hindlimb suspension, and venous occlusion on alpha 1-adrenoceptors in rat vena cava. J. Appl. Physiol. 78, 1882–1888 [DOI] [PubMed] [Google Scholar]
- 60. Morel J. L., Boittin F. X., Halet G., Arnaudeau S., Mironneau C., Mironneau J. (1997) Effect of a 14-day hindlimb suspension on cytosolic Ca2+ concentration in rat portal vein myocytes. Am. J. Physiol. 273, H2867–H2875 [DOI] [PubMed] [Google Scholar]
- 61. Dabertrand F., Porte Y., Macrez N., Morel J. L. (2011) Spaceflight regulates ryanodine receptor subtype 1 in portal vein myocytes in the opposite way of hypertension. J. Appl. Physiol. 112, 471–480 [DOI] [PubMed] [Google Scholar]
- 62. Serova L. V., Denisova L. A., Makeeva V. F., Chelnaya N. A., Pustynnikova A. M. (1984) The effect of microgravity on the prenatal development of mammals. Physiologist 27, S107–S110 [Google Scholar]
- 63. Gazenko O. G., Ilyin Y. A., Savina Y. A., Kaplanskiy A. S., Oganov V. S., Popova I. A., Smimov K. V., Konstantinova I. V. (1987) Experiments with rats flown aboard Cosmos-1667 biosatellite (main objectives, conditions and results). Kos. Biol. Aviak. Med. 21, 8–16 [Google Scholar]
- 64. Gashev A. A., Delp M. D., Zawieja D. C. (2006) Inhibition of active lymph pump by simulated microgravity in rats. Am. J. Physiol. Heart Circ. Physiol. 290, H2295–H2308 [DOI] [PubMed] [Google Scholar]
- 65. Convertino V. A. (1997) Conditions of reduced gravity. In Clinical Autonomic Disorders (Low P. A., ed) Vol. 33, pp. 429–440, Lippincott-Raven, Philadelphia [Google Scholar]
- 66. Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. (1990) Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 70, 665–699 [DOI] [PubMed] [Google Scholar]
- 67. Keil L. C., Evans J., Grindeland R., Popova I. A. (1994) Natriuretic peptide content of atria from rats exposed to 14 days of spaceflight. In Final Reports of the U.S. Experiments Flown on the Soviet Biosatellite Cosmos 2044, 1 (Connolly J. P., Grindeland R. E., Ballard R. W., eds) NASA TM-108802, pp. 374–379, National Aeronautics and Space Administration, Washington, DC [Google Scholar]
- 68. Neri G., Bova S., Malendowicz L. K., Mazzocchi G., Nussdorfer G. G. (2002) Simulated microgravity impairs aldosterone secretion in rats: possible involvement of adrenomedullin. Am. J. Physiol. 283, R832–R836 [DOI] [PubMed] [Google Scholar]
- 69. Fareh J., Bayard B., Gabrion J., Thibault G., Oliver J., Bouille C., Gauquelin G., Gharib C. (1994) Cardiac and plasma atrial natriuretic peptide after 9-day hindlimb suspension in rats. J. Appl. Physiol. 76, 641–649 [DOI] [PubMed] [Google Scholar]
- 70. Baevsky R. M., Baranov V. M., Funtova I. I., Diedrich A., Pashenko A. V., Chernikova A. G., Drescher J., Jordan J., Tank J. (2007) Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J. Appl. Physiol. 103, 156–161 [DOI] [PubMed] [Google Scholar]
- 71. Rowell L. B. (1993) Human Cardiovascular Control, Oxford University Press, New York [Google Scholar]
- 72. Armstrong R. B., Delp M. D., Goljan E. F., Laughlin M. H. (1987) Distribution of blood flow in muscles of miniature swine during exercise. J. Appl. Physiol. 62, 1285–1298 [DOI] [PubMed] [Google Scholar]


