Abstract
Introduction
Measles virus is a major human pathogen responsible for approximately 150,000 measles deaths annually. The disease is vaccine preventable and eradication of the virus is considered feasible in principle. However, a herd immunity exceeding 95% is required to prevent sporadic viral outbreaks in a population. Declining disease prevalence combined with public anxieties about vaccination safety has increased vaccine refusal especially in the European region, which has resulted in measles resurgence in some areas.
Areas covered
Here, we discuss whether synergizing effective measles therapeutics with vaccination could contribute to solving an endgame conundrum of measles elimination by accelerating the eradication effort. Based on an anticipated use for protection of high-risk contacts of confirmed measles cases through post-exposure prophylaxis, we identify key elements of the desirable drug profile, review current disease management strategies and the state of experimental inhibitor candidates, evaluate the risk associated with viral escape from inhibition, and consider the potential of measles therapeutics for the management of persistent viral infection of the CNS. Assuming a post-measles world with waning measles immunity, we contemplate the possible impact of therapeutics on controlling the threat imposed by closely related zoonotic pathogens of the same genus as measles virus.
Expert opinion
Efficacious therapeutics given for post-exposure prophylaxis of high-risk social contacts of confirmed index cases may aid measles eradication by closing herd immunity gaps due to vaccine refusal or failure in populations with overall good vaccination coverage. The envisioned primarily prophylactic application of measles therapeutics to a predominantly pediatric and/or adolescent patient population dictates the drug profile; the article must be safe and efficacious, orally available, shelf-stable at ambient temperature, and amenable to cost-effective manufacture.
2.1 Measles and measles pathophysiology
Measles is a highly communicable disease that is caused by measles virus (MeV), an enveloped virus that contains a single-stranded RNA genome of negative polarity (figure 1). The virus belongs to the genus morbillivirus in the paramyxovirus family and spreads through the respiratory route. While naturally occurring measles is limited to humans, other morbilliviruses such as canine distemper virus (CDV), phocine distemper virus, and peste des petits ruminants virus (PPRV) cause major morbidity and mortality in livestock and wild animals. Within the morbillivirus genus, MeV is most closely related to rinderpest virus, which was recently declared eradicated (1-3). In fact, an ancestral predecessor is considered to have first entered the human population when humans and cattle started to live in proximity (4). This zoonotic transgression presumably happened 5,000-10,000 years ago when human communities reached sizes sufficient to sustain continued MeV presence in the population.
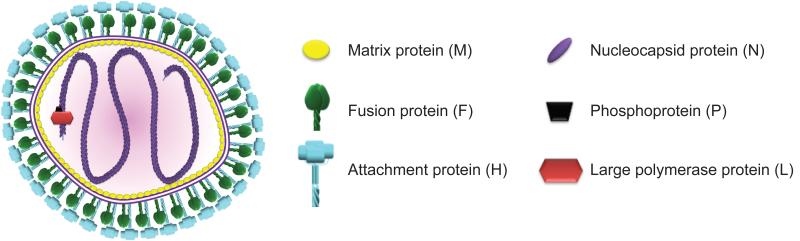
Figure 1.
Schematic representation of an MeV particle. The viral envelope (purple double line) is densely coated by viral attachment and fusion glycoprotein oligomers, which in a concerted action mediate fusion of the envelope with cellular membranes for viral entry upon receptor binding. Short cytosolic domains of the envelope glycoproteins are thought to interact with the matrix protein layer, which also stands in contact with the viral genome and organizes particle assembly. The MeV genome consists of a single non-segmented RNA strand of negative polarity that is tightly encapsidated by the N protein in a ribonucleoprotein complex. The large (L) subunit of the viral RNA-dependent RNA-polymerase complex is together with the P protein polymerase cofactor responsible for genome replication and the synthesis of viral mRNAs.
Morbilliviruses are predominantly associated with acute disease, although we will also discuss in this review the potential of therapeutics for improved management of rare measles complications due to persistent infection. Being recognized as one of the most infectious human pathogens known, basic reproductive numbers (R0 values) are estimated to range from 12 to 18 (5-7), but anecdotal evidence suggests that far higher (>200) R0 rates are possible when groups of immunologically naïve people are confronted with an index case under conditions of close spatial confinement (8). This high infectivity is reflected in the high disease prevalence in the pediatric group: over 90% of children contracted measles by the age of 15 before the live-attenuated vaccine became widely available. In 1980, the virus was responsible for an estimated 2.6 million deaths per year globally (9).
Whereas infection by several other human pathogens of the paramyxovirus family such as respiratory syncytial virus and the human parainfluenzaviruses remains limited to the respiratory tract, rapid progression to systemic infection and viremia is a hallmark of morbillivirus infection (10). Transmitted mostly through respiratory droplets, MeV is inherently lymphotropic and considered to first infect alveolar macrophages and dendritic cells in the respiratory tract (11, 12). Following initial local amplification in lung-associated mononuclear cells, the virus spreads to lymphocytes in draining lymph nodes, where massive replication sets the stage for systemic host invasion and viral spread from lymphatic tissues to epithelial cells (10). Extensive studies in non-human primate models of MeV infection have revealed that humoral immunity is essential for lasting protection, but efficient viral clearance depends on the rapid expansion of MeV-specific CD8+ T cells (4, 13). The innate and adaptive host immune responses are major contributors to the symptoms of measles, i.e. fever, cough, and the characteristic measles rash, which is due to massive CD4+ and CD8+ T cell infiltration to the sites of MeV replication.
The mostly immunopathological nature of measles must be taken into account when conceiving possible therapeutic intervention strategies. The disease commences with a long incubation period of approximately 10 days, followed by three to five days of prodromal illness characterized by fever, cough, conjunctivitis, and the emergence of Koplik’s spots, white lesions on the mucosa lining the cheeks. Shortly after the onset of rash, CD8+ T cell-mediated viral clearance begins, marking the beginning of the recovery phase. Patients become infectious towards the end of the incubation period and continue to transmit the virus through the prodromal phase and the beginning of the rash period (4, 10). Despite a strong MeV-specific immune response and the induction of robust immune memory, the virus paradoxically induces a months-long immunosuppression that includes general lymphopenia (10, 14), lymphocyte proliferation unresponsiveness (15, 16), altered cytokine profiles and elevated IL-10 concentrations (17), and a shift from Th1 towards Th2 responses that set the stage for a high risk of secondary infections (18, 19).
2.2 Potential for measles eradication
A live-attenuated MeV vaccine is the only strategy available to date to prevent measles. Based on the MeV Edmonston strain that was isolated in the 1950s (20), the vaccine strain genotype ceased from endemic transmission decades ago. However, only a single MeV serotype exists thus far and the vaccine strain induces robust cross-neutralization of all currently endemic MeV genotypes (21). The current vaccine is safe and efficacious, but requires an uninterrupted cold chain and trained healthcare professionals for dissemination. Due to the high MeV attack rates, a herd immunity of approximately 95% is required to prevent sporadic MeV outbreaks (5), which cannot be achieved with a single dose of the vaccine. Current practice therefore recommends two doses, the first of which should be delivered at 12 to 15 months of age according to the guidelines of the Advisory Committee on Immunization Practices (ACIP) (22) to balance neutralization of the vaccine virus by maternal antibodies with the risk of acquiring measles (23).
Spearheaded by the Measles Initiative launched in 2001, major progress has been made to reduce disease burden globally by increasing vaccination coverage. Global measles mortality was reduced by approximately 71% between 2000 and 2007, from an estimated 548,000 to 197,000 deaths annually (24). In 2008, the WHO launched an investigation of the general feasibility of global measles eradication (25) and concluded in 2010 that MeV meets critical eradication requirements (26): the disease is restricted to humans and the virus lacks an animal reservoir; a cost-effective vaccine for intervention exists that induces durable protection; and diagnostic tests and an effective surveillance system are available.
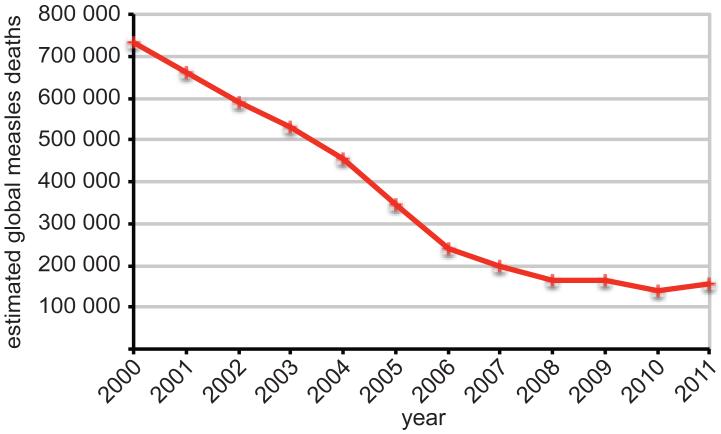
However, several potential obstacles are discussed that distinguish the anti-measles program from the successful smallpox eradication campaign (27): i) in contrast to measles, smallpox is visibly disfiguring, resulting in acute disease awareness in the public; ii) swiftness of the smallpox program bypassed a protracted endgame conundrum – the last case of smallpox was detected in 1978 (28), little over a decade after the World Health Assembly officially endorsed the eradication program; and iii) largely unchallenged public faith in vaccination prevented the formation of broad anti-vaccination campaign. The major achievements towards MeV eradication fall predominantly into the 2000-2007 period, while annual measles deaths have plateaued at around 150,000 since then (29, 30) (figure 2). Funding shortfalls and reduced political commitment have contributed to widespread MeV activity since 2008, and in 2012 countries in Europe, Africa, South Asia and Southeast Asia reported large outbreaks. A global Measles and Rubella strategic plan 2012-2020 was therefore launched in 2012 with the goal to achieve at least 90% coverage nationally, and 80% in every district (31).
Figure 2.
Estimated number of global measles deaths in the 2000 to 2011 period. Source: WHO.
Where insufficient measles immunity is due to limited access to vaccination, heightened efforts through routine immunizations and targeted campaigns – India for instance aimed to reach more than 130 million children in a large 2011-2013 measles catch-up vaccination drive – are expected to reduce disease burden in the next years. However, in particular the European region experiences deliberate vaccine refusal, rather than poor vaccine availability, as the major cause for continued endemic transmission of the virus and large outbreaks, creating a significant obstacle to disease eradication (27). In fact, Europe reported an approximately four-fold increase to over 30,000 measles cases in 2011 compared to the 2009 case numbers (32). Due to high measles activity in 2013 in Germany for instance, the country will miss 2015 elimination targets (33).
Public reservations against the measles vaccine can be traced to two major sources: concerns about vaccination safety, and religious and moral concerns. The former is largely based on a fraudulent report that linked the trivalent MMR vaccine to the development of autism (34). Although the study was formally retracted (35) and subsequent analyses revealed no credible connection between autism and MMR vaccination (35-37), the concerns persist despite major educational efforts (38). While safety concerns are not specific to certain communities, religious and moral objections are often linked to specific congregations or religious subgroups (27).
A major contributing factor to MMR vaccination refusal in the developed world is that measles control suffers, paradoxically, from its own success, since disease awareness increasingly disappears from public memory as prevalence declines (39, 40). As a consequence, risk perception changes and individuals feel less threatened by the actual disease than the conceived possible side effects of vaccination (27, 41). In this scenario, the challenges to achieving disease eradication grow increasingly larger the more drawn-out the endgame of elimination becomes (27). Decreasing acceptance of continued vaccination may also lead to a decline in immunity in regions with currently high vaccination coverage such as North America (42).
Further emphasizing the need for uninterrupted public compliance with vaccination is the analysis of the 2011 measles epidemic in Quebec, the largest outbreak in over a decade in North America. Adolescents of 12-17 years of age were the major driving force of this epidemic; 4.7% of students of the high school at the center of the outbreaks were unvaccinated, while 48% of cases at this school had received the recommended two doses of the vaccine (23). Secondary vaccine failure was identified as the most likely reason for continued susceptibility of the vaccinated students, since US-based data suggest that measles antibodies persist over a 10-year period after the second vaccine dose even in the absence of natural boosting through wild type virus (43). If that conclusion is correct, the Canadian outbreak reveals that even if a 100% two-dose coverage could be established, 5-6% of the vaccinees would remain susceptible to the virus, and the population overall would thus barely meet the required elimination target (23).
2.3 Synergizing vaccine and therapeutics for an anti-measles platform
Without doubt, the measles vaccine has had a major impact on reducing global child mortality, and any eradication attempt must rest on the herd immunity foundation established through vaccination. Substantial efforts are made to increase vaccine uptake through social mobilization and improved dialogue with community leaders, healthcare professionals and policy makers to improve the understanding of risks, increase the transparency of the decision process (23, 44), and advance the current vaccine. The ideal future vaccine would be thermo-stable, efficacious in neonates after a single dose, and support needle-free delivery (38, 45). Equipped with the current vaccine and confronted with the scenario that public resolve and political support will be thoroughly tested during a prolonged endgame of elimination, however, the time may have come for a non-partisan evaluation of whether synergizing prophylactic efforts with effective therapeutics may shorten the timeline to viral control and, ultimately, contribute to sealing the deal on viral eradication.
Since the disease is mostly a result of host immunopathology (4, 10) and viral titers rapidly decline shortly after the onset of rash due to the cell-mediated immune response, the possible benefit of antiviral therapy for improved management of established disease seems minimal. However, the long incubation period of measles combined with the high attack rates of the virus open a window of opportunity for post-exposure treatment of family and social contacts (i.e. pre-school and school contacts, community subgroups) of a confirmed index case. In developed countries, vaccination records are expected to ensure that post-exposure therapy efforts are directed primarily to unvaccinated contacts or single-dose vaccinees. The effectiveness of post-exposure vaccination was found limited, and may depend on timing of vaccination (some protection may be gained if administered within 72 hours of the original infection) and the nature of the exposure (22).
The experience of the 2011 Quebec measles outbreak suggests that in geographical regions that enjoyed extended periods of vaccine-interrupted endemic transmission and thus a low likelihood of natural boosting through wild type virus, groups of 2-dose vaccinees with waning immunity or secondary vaccination failure accumulated undetected. Canada launched mass immunization campaigns in 1996-1997 to provide first and/or second vaccine doses to children 1 to 17 years of age (46, 47), and 2-dose vaccination coverage was subsequently considered to exceed 90% by the age of school entry (23, 48-50). Yet in 2011, the adolescent age group (12-17 years of age) showed the highest incidence rate (75.6 per 100,000) and largest case number (56%) of all age groups (23). To prevent epidemic outbreaks, fully vaccinated (two recorded doses) members of contact age groups with low likelihood of previous boosting by wild type virus (i.e. born after endemic transmission was interrupted in the geographical region) should therefore be considered for post-exposure therapy in addition to the unvaccinated or single-dose vaccinees.
Natural infection by non-attenuated wild type MeV leads to more robust protection than vaccine-mediated immunity (51-53). While currently not experimentally tested for MeV, this observation supports the hypothesis that post-exposure pharmacological attenuation of wild type MeV, taking place after the initial immune priming of the host by the virus, may induce an immune response at least equal to that achievable by vaccination. If correct, post-exposure treatment has the potential to not only prevent individual disease, alleviate MeV-induced immunosuppression, halt transmission and block a mounting local epidemic, but also to contribute to closing the herd immunity gap due to vaccine refusal, failure, or waning immunity.
Independent of whether post-exposure therapy may or may not coincide with the induction of protective immunity, uninterrupted vaccination must remain the cornerstone of the MeV eradication campaign. It is therefore imperative to ensure that efficacious therapeutics, once available, will be perceived by the broad public as an additional component of an integrated anti-measles platform, not a stand-alone alternative to vaccination. Policy makers, health organizations and health care professionals will be equally required to transparently communicate that the anticipated therapeutic benefit to viral eradication – preventing epidemics due to herd immunity gaps that originate from vaccine refusal by population subgroups or vaccine failure – can only be realized through synergizing with vaccination.
2.4 Desirable drug profile
The envisioned primarily prophylactic application of measles therapeutics to a predominantly pediatric and/or adolescent patient population determines the required drug profile: the article must be safe and efficacious, orally available, shelf-stable at ambient temperature, and amenable to cost-effective manufacture (table 1). These diverse requirements are best addressed by small-molecule therapeutics (54).
Table 1.
Key elements of the desirable drug profile of a measles therapeutic developed for use in post-exposure prophylaxis.
| Development stage | Go/no go decision criterion | Rationale |
|---|---|---|
| Allows cost-effective manufacture with existing expertise and facilities |
Target budget range ≤ $100.00 per treatment course |
|
| Fundamental requests |
Suitable for oral delivery | Patient compliance; Systemic distribution required |
| Shelf-stable at ambient temperature for extended periods |
Cost-effective stock piling; Allows use in developing countries without uninterrupted cold chain |
|
|
| ||
|
In vitro efficacy: Potent reduction of MeV load in cell culture and primary cells |
Prophylactic use imposes high constraints on tolerability |
|
| Hit/lead profile |
In vitro toxicity: SI >>100 in cell culture and primary cells |
|
| MeV-specific with defined mechanism of antiviral activity |
Reduced risk of adverse effects; Proactive view on resistance pathways |
|
| Clear ADME profile | Reduced risk of adverse effects | |
|
| ||
| Orally bioavailability >30% | Systemic spread of MeV requires that blood drug levels reach effective concentrations after oral delivery |
|
| Pharmacokinetics: Trough blood levels exceed in vitro active concentrations |
||
| Therapeutic candidate profile |
Efficacious in post-exposure prophylaxis | Reduction of virus load; Suppression/reduction of disease |
| Tolerability: no observable toxicity at efficacious dose after repeat exposure |
Prophylactic courses of up to 20-day duration envisioned |
|
| Robust resistance package | Viral escape and/or co-infection with wild type MeV does not result in enhanced disease |
|
Based on a maximal incubation/prodromal period of up to 15 days from primary infection to the onset of rash (4), it can be reasonably assumed that extending treatment cycles beyond 17-20 days of duration after the last contact with a confirmed measles case would provide little additional benefit to the patient. Even with this limitation to treatment lengths, pharmacokinetic profiles of a clinical candidate must support dosing regimens not exceeding twice-daily to ensure reasonable patient compliance over a two-to-three week period.
Targeting of a pediatric patient population, in particular when commencing prior to diagnostic proof of infection, mandates an outstanding safety profile of any seriously considered drug candidate. At least in the case of the identifiable non-vaccinated, the problem of dosing prior to confirmation of actual infection may be partially offset by the high attack rate of MeV, which translates into a high likelihood of successful viral spread to the majority of immunologically naïve social contacts. While one approach may be to commence the preemptive treatment of high-risk vaccinated contacts of a confirmed case (i.e. the 12-17 year-old vaccinees in the 2011 Quebec outbreak) only when first symptoms indicative of MeV infection emerge at the prodromal stage, this strategy may compromise efficacy and, at least in the case of widespread epidemics with a high number of potential high-risk contacts, lack feasibility.
Anticipated treatment costs and compliance issues exclude all routes of delivery requiring injection and/or administration by healthcare professionals for MeV therapeutics, and all drug articles that by nature depend on injectable formulations. Respiratory delivery, for instance through aerosol or dry-powder inhalation, would not be subject to these limitations and could therefore serve as an acceptable alternative to oral administration. However, highest drug levels can then be expected to be found in the respiratory tract, while rapid progression to systemic invasion and viremia is a hallmark of all natural morbillivirus infections (10, 55, 56). Since the stage of actual disease progression would be unknown in a post-exposure prophylactic treatment setting, respiratory delivery may likely show little efficacy if treatment commences in the incubation period but after viral spread beyond the respiratory tract.
Poor thermo-stability must not necessarily constitute an exclusion criterion for an otherwise promising drug candidate targeted at patient groups in the developed world, but two considerations make shelf-stability at ambient temperature highly desirable: i) an efficacious, orally available antiviral not requiring trained healthcare personnel for distribution may also make a valuable contribution to controlling epidemic outbreaks in developing countries – especially if exposed and preemptively treated individuals do not become infectious but develop protective immunity; and ii) cost-effective stockpiling of a measles therapeutic may be desirable at the current stage of global measles control to create a rapid response measure for controlling local outbreaks in areas with generally high vaccination coverage. Stocked therapeutics may become an important first-line defense in the last phases of the eradication endgame, when measles will increasingly be perceived by the public as an historical disease, further eroding public resolve and political will to continue vaccination.
The estimated cost of the measles vaccine is approximately $1.00 per dose (57). If this number is used as a benchmark for a measles treatment course, the available budget would amount to 2.5 cents per dose, assuming a 20-day treatment cycle and twice daily dosing. However, an acceptable cost of $100.00 per treatment cycle seems more realistic for a small-molecule agent based on the precedence set by oseltamivir (Tamiflu) treatment of influenza virus infections (58). Importantly, cost-effectiveness simulations of oseltamivir for post-exposure prophylaxis in children in the United States returned effectiveness ratios similar to those of influenza vaccination (58). Even with a $100.00/treatment cycle budget cap, the feasibility of cost-effective production must be evaluated critically at an early stage of a measles therapeutic development campaign, since candidate classes inherently associated with high production and/or delivery costs such as larger peptidic antivirals or antisense inhibitors cannot seriously provide a viable treatment option.
2.5 Measles management strategies and developmental inhibitor candidates
Currently, no specific antiviral therapeutics are available for the treatment of measles. In the developing world, vitamin A deficiency was recognized as a risk factor for severe measles (59). To improve disease management, the WHO therefore recommends two consecutive doses of vitamin A for all infected children 1 year of age or older, and lower doses for younger patients (60). However, the benefits of vitamin A supplementation may be most substantial in developing countries with vitamin A deficiency, and are suggested to be most pronounced in children under the age of two (61, 62). High-dose ribavirin treatment, approved against some paramyxoviruses, was explored alone or together with α-interferon, mostly for the treatment of persistent MeV infection of the CNS. Whereas some of these studies showed efficacy against MeV (63-68), others reported intermittent effects (69, 70) or lack of efficacy (71). Unclear therapeutic benefit, severe side effects associated with ribavirin combined with α-interferon (72, 73) and high costs rule out the use of these drugs for post-exposure prophylaxis campaigns. MeV-specific immunoglobulin (IG) therapy may prevent disease (74) and was recommended for the protection of exposed immunocompromised and infants, but is expensive, requires an uninterrupted cold chain and sterile materials, does not support the development of protective immunity, and is not feasible for the control of larger measles epidemics.
A diverse array of experimental antivirals for measles therapy has been suggested in the literature that differ widely by chemical nature, mechanism of antiviral activity, and developmental status. Candidates considered include antisense molecules (75, 76), peptide-conjugated morpholino oligomers (77), therapeutic peptides (78-81), natural extracts or semisynthetic compounds (82-91), and small-molecule drug candidates (92-102). The state of individual compounds was previously reviewed in detail (103, 104). In many cases, suggested substances were only moderately active, highly cytotoxic, inactive when added to cells post-infection in tissue culture, or the active ingredient remained uncharacterized, eliminating them from consideration for serious development. The three best characterized candidates are morpholino oligomers (77), peptide entry inhibitors (80, 81), and small-molecule allosteric inhibitors of the viral RNA-dependent RNA-polymerase (RdRp) complex (102, 105, 106). Of these, morpholino oligomers showed only intermediate inhibitory activity (5-15 μM active concentration range) in cell culture and effective in vivo delivery is challenging. Rather than reiterating previous discussions, we will therefore focus here on peptidic entry inhibitors and small-molecule RdRp inhibitors.
Large (>30 residues) inhibitory peptides derived from the heptad repeat (HR) region of the measles virus fusion protein, the protein that mediates merger of the viral envelope with cellular membranes for delivery of the viral genome into the target cell (107), were first discovered approximately 20 years ago (80). More recent modifications include peptide dimerization and the addition of cholesterol moieties (81). Free peptide and cholesterol-conjugated peptide dimers potently blocked viral entry with nanomolar active concentrations by a mechanism considered analogous to that of the peptidic HIV-1 entry inhibitor enfuvirtide (Fuzeon) (108, 109); at a late stage of the viral entry process, different HR domains of the viral fusion proteins fold onto each other, forming a thermodynamically highly stable fusion core, which is intimately linked to successful fusion pore formation (107). During treatment, the inhibitory peptides are thought to compete with the endogenous HR domains for integration into the fusion core structure, preventing productive membrane fusion (see (110, 111) for illustration). Although clinically efficacious, enfuvirtide has highlighted some of the challenges associated with heptad repeat domain-derived antivirals: delivery by subcutaneous injection is required, which necessitates sterile materials and trained healthcare professionals; treated patients develop severe injection-site reactions; manufacture is costly (i.e. enfuvirtide therapy costs are estimated at $25,000/anno, corresponding to over $1,400 for a 20-day measles prophylaxis assuming equivalent production costs); and shelf-storage stability is limited (112). Considering that the primary target for a measles therapeutic consists of high-risk pediatric/adolescent patients without confirmation of actual infection, a treatment strategy necessitating visits to a healthcare provider for prophylactical injections for up to 20 consecutive days must realistically be considered to be entirely unacceptable to patients and their parents. This may change when patients become symptomatic, but very likely the window of opportunity for efficacious intervention will have permanently closed by that point. Combined, the staggering production and delivery costs and the anticipated lack of patient compliance therefore make peptidic entry inhibitors ill-suited for post-exposure measles prophylaxis.
In our opinion, orally available small-molecule measles drugs that can be produced cost-effectively with existing manufacturing expertise and facilities represent the only realistic path forward. Of the various small-molecule candidate classes discussed in the literature, only four show MeV selectivity and a defined molecular target and/or mechanism of inhibitory activity (table 2). However, lead compounds of three of these classes are compromised by intermediate antiviral potency (active concentrations >0.5 μM) in cell culture, questioning their developmental potential. In addition to small-molecule MeV entry inhibitors, a class of allosteric small-molecule blockers of the viral polymerase was described with nanomolar potency against all currently endemic MeV genotypes worldwide (102). Members of this class target the large protein subunit of the viral RdRp complex, which contains all enzymatic centers required for genome replication and the synthesis of viral mRNAs (113). Synthetic hit-to-lead optimization has yielded highly effective analogs with selectivity indices (SI (CC50/EC50) > 300), clear safety profiles, and good metabolic stability when incubated with human S9 hepatocyte subcellular fractions (106). A highly water-soluble therapeutic candidate of this class shows approximately 30% oral bioavailability and promising pharmacokinetic parameters, resulting in micromolar drug blood concentrations after a single oral dose. While an in-depth assessment of the therapeutic efficacy of this compound class is still ongoing, this lead candidate thus far meets key features of the described drug profile.
Table 2.
MeV-specific small-molecule inhibitors with proposed or known molecular target.
| Compound | Active Concentration |
Target | Ref. |
|---|---|---|---|
| 5′-Fluoro-5′- deoxyaristeromycin |
13 μM | S-adenosylhomocysteine hydrolase |
(93) |
| Coumarin analogs | 0.6 μM | possibly RdRp | (95) |
| AS-48 | 0.6-2 μM (wild type isolates) |
MeV fusion protein | (97, 98, 147) |
| ERDRP-0519 | ~60 nM (wild type isolates) |
L protein subunit of the MeV RdRp complex |
(102, 105, 106) |
2.6 Measles therapeutics and viral resistance
The inherently high mutation rates of RNA virus polymerases support the rapid appearance of a swarm of viral quasispecies (114). Although experimental assessment recently revealed that MeV mutations rates in vivo are in the order of 1.8×10−6 per base and replication event (115) and thus several orders of magnitude lower than originally assumed (116), escape mutations mediating viral resistance to an inhibitor will certainly emerge in the field. The precedence set by antiretroviral therapy suggests, for instance, that allosteric reverse transcriptase inhibitors are predominantly associated with primary site resistance (117, 118), while escape from inhibition by the peptidic entry inhibitor enfuvirtide can be based on primary and secondary resistance mechanisms (119-121).
In fact, high frequencies of viral escape have severely diminished the value of allosteric RT blockers for antiretroviral therapy (117). However, it must be explored whether this limitation equally affects viral pathogens that are, unlike HIV, predominantly associated with acute disease. It is well accepted that HIV quasispecies develop during persistent infection, but go through a genetic bottleneck at transmission and typically do not spread (122). In the case of MeV, patients become infectious during a narrow 7-day window, approximately ranging from day 8 to 15 post-infection (10), and progression to persistent infection is rare. This disease profile demands continued spread to sustain the virus in a population, making MeV vulnerable to changes in viral fitness, since even slight reductions in transmission success should render the virus clinically insignificant. For instance, hot spots of viral escape from the lead class of allosteric RdRp inhibitors were identified experimentally and found to locate to regions of the viral L protein that are completely conserved among different MeV genotypes (105). Sequence conservation suggests that these L microdomains are under selective control in vivo, preventing the development of sequence heterogeneity. Indeed, genetically controlled MeV recombinants harboring individual resistance mutations mostly showed reduced growth rates even under cell culture conditions (105).
As a major developmental go/no go decision-making point, a complete resistance package must be generated for every new MeV clinical candidate prior to introduction in the field. This assessment should include the identification of resistance hot spots through in vitro and in vivo viral adaptation to the article, the generation of defined resistant recombinants in a pathogenic genetic background, and pathogenicity assessment in a relevant animal model after infection with a resistant variant alone or in combination with wild type virus. Although recombination events are very rare in members of the paramyxovirus family, it is imperative to convincingly address the potential of resistant variants to induce enhanced disease, especially under conditions supporting co-circulation with wild type virus. Therapeutic candidates associated with the risk of increasing disease severity should reasonably be excluded early from further consideration.
2.7 Use of therapeutics for the management of measles complications and persistent infection?
The majority of severe complications associated with measles are due to the months-long immunosuppressive effect of the virus. Bacterial secondary infections, for instance by Streptococcus pneumonia and Haemophilus influenzae, are a frequent cause of bacterial pneumonia after measles (4) and best managed by the rapid initiation of an effective antibiosis (123).
MeV has been associated in the literature with a wide array of human diseases ranging from multiple sclerosis and Paget’s disease to Crohn’s disease, epilepsy and systemic lupus erythematosis (comprehensively reviewed in (124)). However, these associations were mostly tenuous, based on flawed evidence or individual, anecdotal reports (124). Validated measles complications originate from viral spread to the CNS and include acute demyelinating encephalomyelitis (ADEM) (125), measles inclusion body encephalitis (MIBE) (124), and subacute sclerosing panencephalitis (SSPE) infection (126, 127). All of these neurologic sequelae are comparably rare, SSPE for instance occurs with a frequency of 1:10,000-100,000 cases (128), but only MIBE and SSPE are linked to active virus replication in the brain. In contrast, ADEM is believe to be based on an auto-immune reaction (124) and must therefore be excluded as a possible candidate for disease management through antiviral therapeutics.
Both MIBE and SSPE are fatal complications due to progressive neurological deterioration. MIBE affects the immunocompromised and occurs within two to six months after measles (129, 130), whereas SSPE presents as a delayed sequelae that develops approximately 8 years after the acute infection (4, 126). SSPE variants of MeV were extensively characterized and found to have accumulated major changes in the matrix and envelope glycoproteins (131, 132) that substantially compromise the membrane fusion machinery of the virus and its ability to form free particles (124). In contrast, all components of the viral polymerase are fully functional, and it was suggested that SSPE virus genomes may spread between densely packed neurons in the CNS by direct transfer from cytoplasm to cytoplasm, possibly involving trans-synaptic transmission (124, 133, 134). If SSPE disease progression indeed becomes increasingly independent of bona fide fusion pore formation, blockers of the viral polymerase function should be considered preferentially for management of SSPE, while MeV fusion and entry inhibitors may be less suitable for this task.
Independent of the best-suited mechanism of antiviral activity, the feasibility of both MIBE and SSPE therapy is hampered by the obstacle that most cases occur in the developing world. Especially for late-onset SSPE, the molecular basis for progressive deterioration of neurological function remains frequently undiagnosed in developing countries, whereas cases in the developed world are extremely rare. While the possible benefit of measles therapeutics for management of MIBE and SSPE must be examined when potent inhibitors become available, the anticipated very low case numbers render the overall impact on human health minimal in comparison with that envisioned for prevention of acute disease through post-exposure prophylaxis.
2.8 Broader impact of therapeutics on morbillivirus disease and control
Should the efficacy of an inhibitor class against related animal pathogens of the morbillivirus genus be valued as an important selection criterion when identifying a therapeutic candidate? Beyond the potential impact of therapeutics on measles eradication, two additional indication areas are conceivable for pan-morbillivirus inhibitors: veterinary use to block epidemic transmission in livestock and use as a rapid control measure in the case of re-introduction of morbilliviruses into the human population through cross-species infection by animal paramyxoviruses in a post-measles world.
The WHO has declared rinderpest virus, the animal morbillivirus most closely related to MeV, eradicated in 2011 (1-3). In contrast, PPRV, which predominantly infects sheep and goats,is widely distributed across West and Central Africa, Arabia, the Middle East and Southern Asia (135). PPRV is associated with 50-80% mortality in a naïve animal population (136), spreads extensively, and causes a major economic burden for local farming communities (135). Rapid protection of non-symptomatic animals in an infected herd through pan-morbillivirus inhibitors could save the majority of the herd and thereby lift a substantial obstacle to subsistence farming in many developing countries.
At present, it is unclear whether measles vaccination should (and can) be continued indefinitely, or whether immunization can be stopped after successful eradication of the virus (137). Public resolve and financial commitment to vaccination will likely falter rapidly in a post-measles world. However, one must consider the possibility that animal morbilliviruses may cross the species barrier and occupy the niche vacated by MeV. Successful smallpox eradication provides some basis for this concern, since related monkeypox and cowpox viruses have infected humans more frequently since smallpox immunization was discontinued (138, 139).
MeV has emerged from an ancestral animal morbillivirus that has crossed the species barrier into the human population. Currently, transition of, for instance, CDV into humans is blocked by cross-protection of MeV neutralizing antibodies against CDV (140, 141). Although viewed unlikely (142), adaptation of CDV to replication in the human host cannot be excluded in the absence of measles immunity (141, 143-145) and was considered to have the potential to amount to a major threat to human health (146). Stockpiling a pan-morbillivirus inhibitor provides reassurance that we will have the means to decisively silence local outbreaks of human infection by zoonotic morbilliviruses with – initially – limited human-to-human transmission, once large-scale measles vaccine production has ceased.
2.9 Conclusion
Major progress has been made in the past decade by the Measles Initiative to reduce the number of annual measles cases globally, and worldwide eradication of the virus is considered feasible in principle. However, after a dramatic >70% drop in annual case fatalities between 2000 and 2007, global measles mortality rates have been stagnant at approximately 150,000 deaths per year in the last years. In some countries in the European region, a resurgence of the virus was experienced after epidemic transmission was declared interrupted. This development is largely due to vaccine refusal based on philosophical or religious motives, and, predominantly, parental concerns about vaccination safety, and had lead to insufficient vaccine coverage to prevent endemic transmission of the virus. Novel small-molecule MeV antivirals may aid measles eradication efforts by synergizing through post-exposure prophylaxis of social contacts of confirmed index cases and strategic outbreak response.
3. Expert opinion
Measles vaccine refusal could amount to a major obstacle to viral eradication in late stages of the elimination campaign in particular, since public risk perception shifts with decreasing viral prevalence in the population. As the disease itself fades from public memory, adverse effects associated with vaccination increasingly drive public anxiety, further threatening compliance.
Since the problem magnifies with the length of periods with low disease incidence, accelerating the effort holds high promise to solve the endgame conundrum. In addition to social mobilization and public education to increase confidence in the campaign in both the developed and developing world, synergizing the measles vaccine with effective therapeutics may reduce disease burden and shorten the timeline to eradication. These goals could be achieved through post-exposure prophylactic therapy of high-risk social contacts of confirmed measles cases to rapidly quench epidemic outbreaks that are fueled by vaccine refusal or failure, or waning immunity in areas that have enjoyed extended periods of interrupted endemic transmission.
In contrast to post-exposure prophylaxis, we consider the anticipated benefit of antivirals for management of symptomatic disease to be minimal, because viral titers drop shortly after rash onset and the disease is predominantly immunopathological by nature. While effective therapeutics could provide new perspectives for the treatment of persistent MeV infections of the CNS, the overall health impact would be small since the complications are rare and most late-onset cases remain likely undiagnosed.
The envisioned predominant use of a measles therapeutic for post-exposure prophylaxis in the pediatric/adolescents group dictates key features of the required drug profile. The article must be orally efficacious, possess an outstanding safety profile, return high systemic drug levels and show pharmacokinetics parameters suitable for once or twice daily dosing regimens, be amenable to cost-effective manufacture, and ideally be shelf-stable for extended periods at ambient temperature. Compromising on any of these criteria early in the discovery process will, in our opinion, undermine any prospect of long-term success and derail the development program at a later stage.
Of the many experimental MeV-specific inhibitor candidates reported in the literature, heptad repeat-derived peptidic viral entry inhibitors and allosteric small-molecule blockers of the measles polymerase are among the most advanced and best characterized. Although highly potent and clinically efficacious for antiretroviral therapy, peptidic MeV entry inhibitors are incompatible with central requirements of the drug profile since they are expensive to manufacture and unsuitable for oral formulations. Staggering production costs and reasonably anticipated patient refusal of prophylactical injections on multiple consecutive days render them particularly ill-suited for measles prophylaxis.
Small-molecule drugs are by nature better matched to meet the diverse demands placed on a measles therapeutic. The currently most advanced MeV polymerase blocker class shows reasonable promise based on high oral bioavailability, pharmacokinetic profile, shelf-stability, and ease of cost-effective production scale-up. Cell culture-based safety profiles are encouraging, but biotoxicity issues and the potential for drug-drug interactions have stalled numerous small-molecule drug development campaigns and must be addressed rigorously, especially when considering the envisioned prophylactic use in a pediatric patient population.
Mounting viral resistance to the inhibitor could present another obstacle compromising the therapeutic value of a measles therapeutic. Indeed, resistance to all classes of current inhibitor candidates can be induced through viral adaptation in cell culture. However, MeV requires continuous transmission to sustain its presence in a population and should therefore be sensitive to changes in overall fitness. Recombination events are rare in the paramyxovirus family, but a complete resistance package must be generated in a relevant animal model to ensure that viral escape and/or co-circulation of resistant and wild type virus does not result in the development of enhanced disease.
To reduce the potential for adverse effects, a prophylactically used measles drug should be MeV-specific rather than broadly active and/or target host cell factors required for virus replication. However, major animal pathogens of the morbillivirus genus share high sequence homology and identify with MeV. Should the drug indication spectrum extend to related morbilliviruses, veterinary use should be considered. A cheap, effective, and shelf-stable PPRV inhibitor, for instance, could be game changing for subsistence farming in many developing countries by saving the majority of susceptible animals in a herd with individual PPRV cases. In addition, a pan-morbillivirus inhibitor could serve as a first line defense against reintroduction of morbilliviruses into the human population through cross-species infection of humans by zoonotic pathogens of the genus such as CDV.
A very exciting perspective for capitalizing on synergistic effects between vaccine and therapeutics emerges from the hypothesis that therapeutics given for post-exposure prophylaxis may lead to a pharmaceutical attenuation of the virus in the incubation phase. In a best case scenario, treated patients would remain symptom free, do not become infectious and are spared from post-measles immunosuppression, but would profit from initial immune priming by wild type MeV and subsequent immune-clearance of pharmaceutically attenuated virus. Should patients indeed emerge from post-exposure treatment disease free and with protective anti-MeV immunity, therapeutics could have major value in solving the measles eradication endgame conundrum by closing herd immunity gaps due to vaccine refusal or failure.
References
- 1.Cima G. Rinderpest eradicated. International organizations declare “cattle plague” dead. Journal of the American Veterinary Medical Association. 2011;239:11–15. doi: 10.2460/javma.239.1.11. [DOI] [PubMed] [Google Scholar]
- 2.OIE declares rinderpest eradicated. The Veterinary record. 2011;168:573. doi: 10.1136/vr.d3408. [DOI] [PubMed] [Google Scholar]
- 3.Rinderpest officially eradicated. The Veterinary record. 2011;169:3. doi: 10.1136/vr.d4112. [DOI] [PubMed] [Google Scholar]
- 4.Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–164. doi: 10.1016/S0140-6736(10)62352-5. * Comprehensive overview of the disease and infectious agent.
- 5.Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin DE. Measles Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5 ed Vol. 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1551–1585. [Google Scholar]
- 7.Hethcote HW. The mathematics of infectious disease. SIAM Review. 2000;42:599–653. [Google Scholar]
- 8.Christensen PE, Schmidt H, Bang HO, et al. An epidemic of measles in southern Greenland, 1951; measles in virgin soil. II. The epidemic proper. Acta medica Scandinavica. 1953;144:430–449. doi: 10.1111/j.0954-6820.1953.tb15717.x. [DOI] [PubMed] [Google Scholar]
- 9.Strebel PM, Cochi SL, Hoekstra E, et al. A world without measles. J Infect Dis. 2011;204(Suppl 1):S1–3. doi: 10.1093/infdis/jir111. [DOI] [PubMed] [Google Scholar]
- 10.de Vries RD, Mesman AW, Geijtenbeek TB, et al. The pathogenesis of measles. Curr Opin Virol. 2012;2:248–255. doi: 10.1016/j.coviro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Lemon K, de Vries RD, Mesman AW, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011;7:e1001263. doi: 10.1371/journal.ppat.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries RD, Lemon K, Ludlow M, et al. In vivo tropism of attenuated and pathogenic measles virus expressing green fluorescent protein in macaques. Journal of virology. 2010;84:4714–4724. doi: 10.1128/JVI.02633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Permar SR, Klumpp SA, Mansfield KG, et al. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J Infect Dis. 2004;190:998–1005. doi: 10.1086/422846. [DOI] [PubMed] [Google Scholar]
- 14.Griffin DE. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176–189. doi: 10.1111/j.1600-065X.2010.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch RL, Griffin DE, Johnson RT, et al. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin Immunol Immunopathol. 1984;31:1–12. doi: 10.1016/0090-1229(84)90184-3. [DOI] [PubMed] [Google Scholar]
- 16.Avota E, Avots A, Niewiesk S, et al. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nature medicine. 2001;7:725–731. doi: 10.1038/89106. [DOI] [PubMed] [Google Scholar]
- 17.Moss WJ, Ryon JJ, Monze M, et al. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J Infect Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- 18.Griffin DE, Ward BJ. Differential CD4 T cell activation in measles. J Infect Dis. 1993;168:275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- 19.Griffin DE, Cooper SJ, Hirsch RL, et al. Changes in plasma IgE levels during complicated and uncomplicated measles virus infections. J Allergy Clin Immunol. 1985;76:206–213. doi: 10.1016/0091-6749(85)90703-1. [DOI] [PubMed] [Google Scholar]
- 20.Griffin DE, Oldstone MB. Measles. History and basic biology. Introduction. Curr Top Microbiol Immunol. 2009;329:1. [PubMed] [Google Scholar]
- 21.Bellini WJ, Rota PA. Biological feasibility of measles eradication. Virus research. 2011;162:72–79. doi: 10.1016/j.virusres.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 22.McLean HQ, Fiebelkorn AP, Temte JL, et al. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2013;62:1–34. [PubMed] [Google Scholar]
- 23.De Serres G, Markowski F, Toth E, et al. Largest measles epidemic in North America in a decade--Quebec, Canada, 2011: contribution of susceptibility, serendipity, and superspreading events. The Journal of infectious diseases. 2013;207:990–998. doi: 10.1093/infdis/jis923. *Evaluation of a large measeles outbreak in a population with high vacciantion coverage.
- 24.Progress in global control and regional elimination of measles: 2001-2011. Weekly Epidemiological Record. 2013 [Google Scholar]
- 25.WHO . Sixty-first World Health Assembly. Geneva, Switzerland: 2008. [Google Scholar]
- 26.World Health O Proceedings of the Global Technical Consultation to assess the feasibility of measles eradication, 28-30 July 2010. The Journal of infectious diseases. 2011;204(Suppl 1):S4–13. doi: 10.1093/infdis/jir100. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Victor DS, Omer SB. Vaccine refusal and the endgame: walking the last mile first. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120148. doi: 10.1098/rstb.2012.0148. *Detailed examination of the reasons for public vaccine refusal and possible countermeasures.
- 28.Hopkins DR. Smallpox: ten years gone. American journal of public health. 1988;78:1589–1595. doi: 10.2105/ajph.78.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Measles. 2013 http://www.who.int/mediacentre/factsheets/fs286/en/index.html. [Online.]
- 30.Simons E, Ferrari M, Fricks J, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379:2173–2178. doi: 10.1016/S0140-6736(12)60522-4. *Comprehensive summary of the progress in global measles deaths reduction.
- 31.Lazzarin A. Enfuvirtide: the first HIV fusion inhibitor. Expert Opin Pharmacother. 2005;6:453–464. doi: 10.1517/14656566.6.3.453. [DOI] [PubMed] [Google Scholar]
- 32.Number of measles cases. 2011. 2011. posting date. European Center for Disease Control and Prevention. [Online.] [Google Scholar]
- 33.Santibanez S, Mankertz A. [Molecular surveillance shows progress in measles elimination process] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:1238–1242. doi: 10.1007/s00103-013-1795-1. [DOI] [PubMed] [Google Scholar]
- 34.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 35.Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation. Lancet. 2004;363:750. doi: 10.1016/S0140-6736(04)15715-2. [DOI] [PubMed] [Google Scholar]
- 36.Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. The New England journal of medicine. 2002;347:1477–1482. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- 37.DeStefano F, Thompson WW. MMR vaccine and autism: an update of the scientific evidence. Expert review of vaccines. 2004;3:19–22. doi: 10.1586/14760584.3.1.19. [DOI] [PubMed] [Google Scholar]
- 38.Goodson JL, Chu SY, Rota PA, et al. Research priorities for global measles and rubella control and eradication. Vaccine. 2012;30:4709–4716. doi: 10.1016/j.vaccine.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown KF, Long SJ, Ramsay M, et al. U.K. parents’ decision-making about measles-mumps-rubella (MMR) vaccine 10 years after the MMR-autism controversy: a qualitative analysis. Vaccine. 2012;30:1855–1864. doi: 10.1016/j.vaccine.2011.12.127. [DOI] [PubMed] [Google Scholar]
- 40.Smith PJ, Humiston SG, Marcuse EK, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011;126(Suppl 2):135–146. doi: 10.1177/00333549111260S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black S, Rappuoli R. A crisis of public confidence in vaccines. Sci Transl Med. 2010;2:61mr61. doi: 10.1126/scitranslmed.3001738. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease C, Prevention Measles - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:253–257. [PubMed] [Google Scholar]
- 43.LeBaron CW, Beeler J, Sullivan BJ, et al. Persistence of measles antibodies after 2 doses of measles vaccine in a postelimination environment. Archives of pediatrics & adolescent medicine. 2007;161:294–301. doi: 10.1001/archpedi.161.3.294. [DOI] [PubMed] [Google Scholar]
- 44.Larson HJ, Cooper LZ, Eskola J, et al. Addressing the vaccine confidence gap. Lancet. 2011;378:526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 45.Griffin DE, Pan CH, Moss WJ. Measles vaccines. Front Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- 46.Canadian Immunization Guide. 5th e. Health Canada; Ottawa: 1998. National Advisory Committee on Immunization; pp. 118–124. [Google Scholar]
- 47.King A, Varughese P, De Serres G, et al. Measles elimination in Canada. The Journal of infectious diseases. 2004;189(Suppl 1):S236–242. doi: 10.1086/378499. [DOI] [PubMed] [Google Scholar]
- 48.Boulianne N, Audet D, Ouakki M. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2006. Institut National de Santé Publique du Québec; Quebec City, Canada: 2007. 2007. posting date. http://www.inspq.qc.ca/pdf/publications/678-VaccinationEnfantsen2006.pdf. [Online.] [Google Scholar]
- 49.Boulianne N, Bradet R, Audet D, et al. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2008. Institut National de Santé Publique du Québec; Quebec City, Canada: 2009. 2009. posting date. http://www.inspq.qc.ca/pdf/publications/1045_CouverturVaccinEnfants1et2Ans2008.pdf. [Online.] [Google Scholar]
- 50.Boulianne N, Bradet R, Audet D, et al. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2010. Institut National de Santé Publique du Québec; Quebec City, Canada: 2011. 2011. posting date. http://www.inspq.qc.ca/pdf/publications/1318_EnqueteCouvVaccinEnfants1Et2AnsQc2010.pdf. [Online.] [Google Scholar]
- 51.Putz MM, Bouche FB, de Swart RL, et al. Experimental vaccines against measles in a world of changing epidemiology. Int J Parasitol. 2003;33:525–545. doi: 10.1016/s0020-7519(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 52.Mossong J, Nokes DJ, Edmunds WJ, et al. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am J Epidemiol. 1999;150:1238–1249. doi: 10.1093/oxfordjournals.aje.a009951. [DOI] [PubMed] [Google Scholar]
- 53.Mossong J, O’Callaghan CJ, Ratnam S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine. 2000;19:523–529. doi: 10.1016/s0264-410x(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 54.Ganellin CR, Jefferis R, Roberts SM. Introduction to biological and small molecule drug research and development: theory and case studies. Academic Press; Oxford: 2013. p. 1. volume. [Google Scholar]
- 55.de Vries RD, McQuaid S, van Amerongen G, et al. Measles immune suppression: lessons from the macaque model. PLoS Pathog. 2012;8:e1002885. doi: 10.1371/journal.ppat.1002885. *Excellent analysis of the molecular basis for MeV immunosuppression.
- 56.von Messling V, Milosevic D, Cattaneo R. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci U S A. 2004;101:14216–14221. doi: 10.1073/pnas.0403597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention Global Health - Measles, Rubella, and CRS. http://www.cdc.gov/globalhealth/measles/why/infographic.htm. [Online.]
- 58.Talbird SE, Brogan AJ, Winiarski AP. Oseltamivir for influenza postexposure prophylaxis: economic evaluation for children aged 1-12 years in the U.S. American journal of preventive medicine. 2009;37:381–388. doi: 10.1016/j.amepre.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Barclay AJ, Foster A, Sommer A. Vitamin A supplements and mortality related to measles: a randomised clinical trial. Br Med J (Clin Res Ed) 1987;294:294–296. doi: 10.1136/bmj.294.6567.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strategies for reducing global measles mortality. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record. Health Section of the Secretariat of the League of Nations. 2000;75:411–416. [PubMed] [Google Scholar]
- 61.D’Souza RM, D’Souza R. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2002:CD001479. doi: 10.1002/14651858.CD001479. [DOI] [PubMed] [Google Scholar]
- 62.Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis. 2000;182(Suppl 1):S122–133. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 63.Stogner SW, King JW, Black-Payne C, et al. Ribavirin and intravenous immune globulin therapy for measles pneumonia in HIV infection. South Med J. 1993;86:1415–1418. doi: 10.1097/00007611-199312000-00023. [DOI] [PubMed] [Google Scholar]
- 64.Hosoya M. [Therapy and prognosis in subacute sclerosing panencephalitis] Nippon Rinsho. 2007;65:1483–1486. [PubMed] [Google Scholar]
- 65.Tomoda A, Nomura K, Shiraishi S, et al. [Trial of intraventricular ribavirin and interferon-alpha combination therapy for subacute sclerosing panencephalitis (SSPE) in Japan] No To Hattatsu. 2003;35:321–326. [PubMed] [Google Scholar]
- 66.Gururangan S, Stevens RF, Morris DJ. Ribavirin response in measles pneumonia. J Infect. 1990;20:219–221. doi: 10.1016/0163-4453(90)91094-t. [DOI] [PubMed] [Google Scholar]
- 67.Forni AL, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis. 1994;19:454–462. doi: 10.1093/clinids/19.3.454. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez H, Banks G, Smith R. Ribavirin: a clinical overview. Eur J Epidemiol. 1986;2:1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 69.Campbell C, Levin S, Humphreys P, et al. Subacute sclerosing panencephalitis: results of the Canadian Paediatric Surveillance Program and review of the literature. BMC Pediatr. 2005;5:47. doi: 10.1186/1471-2431-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara S, Kimura H, Hoshino Y, et al. Combination therapy with intraventricular interferon-alpha and ribavirin for subacute sclerosing panencephalitis and monitoring measles virus RNA by quantitative PCR assay. Brain Dev. 2003;25:367–369. doi: 10.1016/s0387-7604(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 71.del Toro-Riera M, Macaya-Ruiz A, Raspall-Chaure M, et al. [Subacute sclerosing panencephalitis: combined treatment with interferon alpha and intraventricular ribavirin] Rev Neurol. 2006;42:277–281. [PubMed] [Google Scholar]
- 72.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 74.Atkinson W, Hamborsky J, McIntyre L, et al. Epidemiology and Prevention of Vaccine-Preventable Diseases. 10 ed. Public Health Foundation; Washington DC.: 2008. Centers for Disease Control and Prevention. [Google Scholar]
- 75.Koschel K, Brinckmann U, Hoyningen-Huene VV. Measles virus antisense sequences specifically cure cells persistently infected with measles virus. Virology. 1995;207:168–178. doi: 10.1006/viro.1995.1063. [DOI] [PubMed] [Google Scholar]
- 76.Bell AF, Whitton JL, Fujinami RS. Antisense-mediated resistance to measles virus infection in HeLa cells. J Infect Dis. 1997;176:258–261. doi: 10.1086/517261. [DOI] [PubMed] [Google Scholar]
- 77.Sleeman K, Stein DA, Tamin A, et al. Inhibition of measles virus infections in cell cultures by peptide-conjugated morpholino oligomers. Virus Res. 2009;140:49–56. doi: 10.1016/j.virusres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 78.Miller FA, Dixon GJ, Arnett G, et al. Antiviral activity of carbobenzosy di- and tripeptides on measles virus. Appl Microbiol. 1968;16:1489–1496. doi: 10.1128/am.16.10.1489-1496.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicolaides E, DeWald H, Lipnik M, et al. Potential antiviral agents. Carbobenzoxy di- and tripeptides active against measles and herpes viruses. J Med Chem. 1968;11:74–79. doi: 10.1021/jm00307a016. [DOI] [PubMed] [Google Scholar]
- 80.Lambert DM, Barney S, Lambert AL, et al. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci U S A. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welsch JC, Talekar A, Mathieu C, et al. Fatal Measles Infection Prevented by Brain-Penetrant Fusion Inhibitors. Journal of virology. 2013 doi: 10.1128/JVI.02436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parker ME, Chabot S, Ward BJ, et al. Traditional dietary additives of the Maasai are antiviral against the measles virus. J Ethnopharmacol. 2007;114:146–152. doi: 10.1016/j.jep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 83.Petricevich VL, Mendonca RZ. Inhibitory potential of Crotalus durissus terrificus venom on measles virus growth. Toxicon. 2003;42:143–153. doi: 10.1016/s0041-0101(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 84.Wachsman MB, Ramirez JA, Galagovsky LR, et al. Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivir Chem Chemother. 2002;13:61–66. doi: 10.1177/095632020201300105. [DOI] [PubMed] [Google Scholar]
- 85.Olila D, Olwa O, Opuda-Asibo J. Screening extracts of Zanthoxylum chalybeum and Warburgia ugandensis for activity against measles virus (Swartz and Edmonston strains) in vitro. Afr Health Sci. 2002;2:2–10. [PMC free article] [PubMed] [Google Scholar]
- 86.Cos P, Hermans N, De Bruyne T, et al. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. J Ethnopharmacol. 2002;79:155–163. doi: 10.1016/s0378-8741(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 87.Huang SP, Shieh GJ, Lee L, et al. Inhibition effect of shengma-gegen-tang on measles virus in Vero cells and human peripheral blood mononuclear cells. Am J Chin Med. 1997;25:89–96. doi: 10.1142/S0192415X97000123. [DOI] [PubMed] [Google Scholar]
- 88.McWhorter JH. Spicebush. A Cherokee remedy for the measles. N C Med J. 1996;57:306. [PubMed] [Google Scholar]
- 89.Lin YM, Flavin MT, Schure R, et al. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 90.Kurokawa M, Ochiai H, Nagasaka K, et al. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 1993;22:175–188. doi: 10.1016/0166-3542(93)90094-y. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi T, Hayashi K, Maeda M, et al. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J Nat Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 92.Barnard DL, Stowell VD, Seley KL, et al. Inhibition of measles virus replication by 5′-nor carbocyclic adenosine analogues. Antivir Chem Chemother. 2001;12:241–250. doi: 10.1177/095632020101200405. [DOI] [PubMed] [Google Scholar]
- 93.Li W, Yin X, Schneller SW. 5′-Fluoro-5′-deoxyaristeromycin. Bioorganic & medicinal chemistry letters. 2008;18:220–222. doi: 10.1016/j.bmcl.2007.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang N, Chen HM, Sood R, et al. in vitro inhibition of the measles virus by novel ring-expanded (‘fat’) nucleoside analogues containing the imidazo[4,5-e]diazepine ring system. Bioorganic & medicinal chemistry letters. 2002;12:3391–3394. doi: 10.1016/s0960-894x(02)00762-x. [DOI] [PubMed] [Google Scholar]
- 95.Barnard DL, Xu ZQ, Stowell VD, et al. Coumarins and pyranocoumarins, potential novel pharmacophores for inhibition of measles virus replication. Antivir Chem Chemother. 2002;13:39–59. doi: 10.1177/095632020201300104. [DOI] [PubMed] [Google Scholar]
- 96.Santagati NA, Bousquet E, Garozzo A, et al. Synthesis and anti-measles virus activity of new isoquinolin-4-one derivatives. Farmaco. 2003;58:1217–1225. doi: 10.1016/j.farmac.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Plemper RK, Doyle J, Sun A, et al. Design of a small-molecule entry inhibitor with activity against primary measles virus strains. Antimicrob Agents Chemother. 2005;49:3755–3761. doi: 10.1128/AAC.49.9.3755-3761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plemper RK, Erlandson KJ, Lakdawala AS, et al. A target site for template-based design of measles virus entry inhibitors. Proc Natl Acad Sci U S A. 2004;101:5628–5633. doi: 10.1073/pnas.0308520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun A, Prussia A, Zhan W, et al. Nonpeptide inhibitors of measles virus entry. J Med Chem. 2006;49:5080–5092. doi: 10.1021/jm0602559. [DOI] [PubMed] [Google Scholar]
- 100.Sun A, Chandrakumar N, Yoon JJ, et al. Non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase complex activity: Synthesis and in vitro evaluation. Bioorganic & medicinal chemistry letters. 2007;17:5199–5203. doi: 10.1016/j.bmcl.2007.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun A, Yoon JJ, Yin Y, et al. Potent Non-Nucleoside Inhibitors of the Measles Virus RNA-Dependent RNA Polymerase Complex. J Med Chem. 2008;51:3731–3741. doi: 10.1021/jm701239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White LK, Yoon JJ, Lee JK, et al. Nonnucleoside inhibitor of measles virus RNA-dependent RNA polymerase complex activity. Antimicrob Agents Chemother. 2007;51:2293–2303. doi: 10.1128/AAC.00289-07. *First characterization of a promising small-molecule measles polymerase inhibitor class.
- 103.Plemper RK, Snyder JP. Measles control--can measles virus inhibitors make a difference? Current opinion in investigational drugs. 2009;10:811–820. [PMC free article] [PubMed] [Google Scholar]
- 104.Barnard DL. Inhibitors of measles virus. Antivir Chem Chemother. 2004;15:111–119. doi: 10.1177/095632020401500301. [DOI] [PubMed] [Google Scholar]
- 105.Yoon JJ, Krumm SA, Ndungu JM, et al. Target analysis of the experimental measles therapeutic AS-136A. Antimicrob Agents Chemother. 2009;53:3860–3870. doi: 10.1128/AAC.00503-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ndungu JM, Krumm SA, Yan D, et al. Non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase: synthesis, structure-activity relationships, and pharmacokinetics. J Med Chem. 2012;55:4220–4230. doi: 10.1021/jm201699w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plemper RK. Cell entry of enveloped viruses. Curr Opin Virol. 2011;1:92–100. doi: 10.1016/j.coviro.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kilby JM, Hopkins S, Venetta TM, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 109.Kilby JM, Lalezari JP, Eron JJ, et al. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults. AIDS Res Hum Retroviruses. 2002;18:685–693. doi: 10.1089/088922202760072294. [DOI] [PubMed] [Google Scholar]
- 110.De Clercq E. Antiviral drugs in current clinical use. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 111.Doms RW, Moore JP. HIV-1 membrane fusion: targets of opportunity. The Journal of cell biology. 2000;151:F9–14. doi: 10.1083/jcb.151.2.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jamjian MC, McNicholl IR. Enfuvirtide: first fusion inhibitor for treatment of HIV infection. Am J Health Syst Pharm. 2004;61:1242–1247. doi: 10.1093/ajhp/61.12.1242. [DOI] [PubMed] [Google Scholar]
- 113.Lamb RA, Parks GD. Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5 ed Vol. 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1449–1496. [Google Scholar]
- 114.Domingo E, Holland JJ. Mutation rates and rapid evolution of RNA viruses. In: Morse SS, editor. The evolutionary biology of viruses. Raven Press Ltd; New York, NY: 1994. pp. 161–184. [Google Scholar]
- 115.Zhang X, Rennick LJ, Duprex WP, et al. Determination of spontaneous mutation frequencies in measles virus under nonselective conditions. Journal of virology. 2013;87:2686–2692. doi: 10.1128/JVI.02146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schrag SJ, Rota PA, Bellini WJ. Spontaneous mutation rate of measles virus: direct estimation based on mutations conferring monoclonal antibody resistance. Journal of virology. 1999;73:51–54. doi: 10.1128/jvi.73.1.51-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem Biodivers. 2004;1:44–64. doi: 10.1002/cbdv.200490012. [DOI] [PubMed] [Google Scholar]
- 118.De Clercq E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antiviral research. 1998;38:153–179. doi: 10.1016/s0166-3542(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 119.Peuchant O, Capdepont S, Ragnaud JM, et al. Primary resistance to enfuvirtide (T20) in recently HIV-1 infected, antiretroviral-naive patients from the ANRS Aquitaine Cohort. Antiviral therapy. 2007;12:559–562. doi: 10.1177/135965350701200413. [DOI] [PubMed] [Google Scholar]
- 120.Lu J, Deeks SG, Hoh R, et al. Rapid emergence of enfuvirtide resistance in HIV-1-infected patients: results of a clonal analysis. Journal of acquired immune deficiency syndromes. 2006;43:60–64. doi: 10.1097/01.qai.0000234083.34161.55. [DOI] [PubMed] [Google Scholar]
- 121.Greenberg ML, Cammack N. Resistance to enfuvirtide, the first HIV fusion inhibitor. J Antimicrob Chemother. 2004;54:333–340. doi: 10.1093/jac/dkh330. [DOI] [PubMed] [Google Scholar]
- 122.Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 123.Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. The Cochrane database of systematic reviews. 2013;8:CD001477. doi: 10.1002/14651858.CD001477.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rima BK, Duprex WP. Morbilliviruses and human disease. The Journal of pathology. 2006;208:199–214. doi: 10.1002/path.1873. [DOI] [PubMed] [Google Scholar]
- 125.Nasr JT, Andriola MR, Coyle PK. ADEM: literature review and case report of acute psychosis presentation. Pediatr Neurol. 2000;22:8–18. doi: 10.1016/s0887-8994(99)00116-2. [DOI] [PubMed] [Google Scholar]
- 126.ter Meulen V, Stephenson JR, Kreth HW. Subacute sclerosing panencephalitis. In: Fraenkel-Conrat H, Wagner RR, editors. Comprehensive Virology. Plenum Press; New York, NY: 1983. pp. 105–159. [Google Scholar]
- 127.Modlin JF, Jabbour JT, Witte JJ, et al. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics. 1977;59:505–512. [PubMed] [Google Scholar]
- 128.Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. The Journal of infectious diseases. 2005;192:1686–1693. doi: 10.1086/497169. [DOI] [PubMed] [Google Scholar]
- 129.Turner A, Jeyaratnam D, Haworth F, et al. Measles-associated encephalopathy in children with renal transplants. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:1459–1465. doi: 10.1111/j.1600-6143.2006.01330.x. [DOI] [PubMed] [Google Scholar]
- 130.Freeman AF, Jacobsohn DA, Shulman ST, et al. A new complication of stem cell transplantation: measles inclusion body encephalitis. Pediatrics. 2004;114:e657–660. doi: 10.1542/peds.2004-0949. [DOI] [PubMed] [Google Scholar]
- 131.Billeter MA, Cattaneo R, Spielhofer P, et al. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. Ann N Y Acad Sci. 1994;724:367–377. doi: 10.1111/j.1749-6632.1994.tb38934.x. [DOI] [PubMed] [Google Scholar]
- 132.Rima BK, Duprex WP. Molecular mechanisms of measles virus persistence. Virus Res. 2005;111:132–147. doi: 10.1016/j.virusres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 133.Urbanska EM, Chambers BJ, Ljunggren HG, et al. Spread of measles virus through axonal pathways into limbic structures in the brain of TAP1 −/− mice. Journal of medical virology. 1997;52:362–369. doi: 10.1002/(sici)1096-9071(199708)52:4<362::aid-jmv3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 134.Lawrence DM, Patterson CE, Gales TL, et al. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. Journal of virology. 2000;74:1908–1918. doi: 10.1128/jvi.74.4.1908-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Banyard AC, Parida S, Batten C, et al. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. The Journal of general virology. 2010;91:2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 136.Kitching RP. The economic significance and control of small ruminant viruses in North Africa and West Asia. In: Thompson FS, editor. Increasing small ruminant productivity in semi-arid areas. Kluwer Academic Publishers-Dordrecht; The Netherlands: 1988. pp. 225–236. [Google Scholar]
- 137.Meissner HC, Strebel PM, Orenstein WA. Measles vaccines and the potential for worldwide eradication of measles. Pediatrics. 2004;114:1065–1069. doi: 10.1542/peds.2004-0440. [DOI] [PubMed] [Google Scholar]
- 138.Reynolds MG, Damon IK. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends in microbiology. 2012;20:80–87. doi: 10.1016/j.tim.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 139.Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Veterinary microbiology. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bieringer M, Han JW, Kendl S, et al. Experimental adaptation of wild-type canine distemper virus (CDV) to the human entry receptor CD150. PloS one. 2013;8:e57488. doi: 10.1371/journal.pone.0057488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Appel MJ, Shek WR, Shesberadaran H, et al. Measles virus and inactivated canine distemper virus induce incomplete immunity to canine distemper. Archives of virology. 1984;82:73–82. doi: 10.1007/BF01309369. [DOI] [PubMed] [Google Scholar]
- 142.Sanders R, Dabbagh A, Featherstone D. Risk analysis for measles reintroduction after global certification of eradication. The Journal of infectious diseases. 2011;204(Suppl 1):S71–77. doi: 10.1093/infdis/jir133. [DOI] [PubMed] [Google Scholar]
- 143.de Swart RL, Duprex WP, Osterhaus AD. Rinderpest eradication: lessons for measles eradication? Current opinion in virology. 2012;2:330–334. doi: 10.1016/j.coviro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 144.Stittelaar KJ, de Swart RL, Osterhaus AD. Vaccination against measles: a neverending story. Expert review of vaccines. 2002;1:151–159. doi: 10.1586/14760584.1.2.151. [DOI] [PubMed] [Google Scholar]
- 145.Qiu W, Zheng Y, Zhang S, et al. Canine distemper outbreak in rhesus monkeys, China. Emerging infectious diseases. 2011;17:1541–1543. doi: 10.3201/eid1708.101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Osterhaus A. Catastrophes after crossing species barriers. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2001;356:791–793. doi: 10.1098/rstb.2001.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun A, Prussia A, Zhan W, et al. Nonpeptide Inhibitors of Measles Virus Entry. J Med Chem. 2006;49:5080–5092. doi: 10.1021/jm0602559. [DOI] [PubMed] [Google Scholar]