Significance
Biological nitrogen fixation (BNF) is the largest natural source of new nitrogen (N) to terrestrial ecosystems. Tropical forest ecosystems are a putative global hotspot of BNF, but direct, spatially explicit measurements in the biome are virtually nonexistent. Nonetheless, robust estimates of tropical forest BNF are critical for understanding how these important ecosystems may respond to global change and assessing human perturbations to the N cycle. Here, we introduce a spatial sampling method to assess BNF and present evidence that tropical forest BNF is much lower than previously assumed. Our results imply that humans have roughly doubled N inputs to the tropical forest biome relative to N inputs through BNF.
Keywords: adaptive cluster sampling, free-living nitrogen fixation, nitrogen deposition, symbiotic nitrogen fixation
Abstract
Biological nitrogen fixation (BNF) is the largest natural source of exogenous nitrogen (N) to unmanaged ecosystems and also the primary baseline against which anthropogenic changes to the N cycle are measured. Rates of BNF in tropical rainforest are thought to be among the highest on Earth, but they are notoriously difficult to quantify and are based on little empirical data. We adapted a sampling strategy from community ecology to generate spatial estimates of symbiotic and free-living BNF in secondary and primary forest sites that span a typical range of tropical forest legume abundance. Although total BNF was higher in secondary than primary forest, overall rates were roughly five times lower than previous estimates for the tropical forest biome. We found strong correlations between symbiotic BNF and legume abundance, but we also show that spatially free-living BNF often exceeds symbiotic inputs. Our results suggest that BNF in tropical forest has been overestimated, and our data are consistent with a recent top-down estimate of global BNF that implied but did not measure low tropical BNF rates. Finally, comparing tropical BNF within the historical area of tropical rainforest with current anthropogenic N inputs indicates that humans have already at least doubled reactive N inputs to the tropical forest biome, a far greater change than previously thought. Because N inputs are increasing faster in the tropics than anywhere on Earth, both the proportion and the effects of human N enrichment are likely to grow in the future.
Over the last few decades, humans have dramatically altered the global nitrogen (N) cycle (1–3). Three main processes—Haber–Bosch fixation of atmospheric N2, widespread cultivation of leguminous N-fixing crops, and incidental N fixation during fossil fuel combustion—collectively add more reactive N to the biosphere each year than all natural processes combined (2). Although human perturbation of the N cycle has brought substantial benefits to society (most notably, an increase in crop production) (4), it has also had a number of negative effects on both ecosystems (5, 6) and people (7).
Although humanity’s large imprint on the global N cycle is clear, quantifying the extent of anthropogenic changes depends, in large part, on establishing baseline estimates of nonanthropogenic N inputs (1, 8, 9). Before recent human activities, biological N fixation (BNF) was the largest source of new N to the biosphere (9). Terrestrial BNF has been particularly challenging to quantify, because it displays high spatial and temporal heterogeneity at local scales, it arises from both symbiotic associations between bacteria and plants as well as free-living microorganisms (e.g., in leaf litter and soil) (10), and high atmospheric concentrations of N2 make direct flux measurements unfeasible. Consequently, spatial estimates of BNF have always been highly uncertain (11), and global rate estimates have fallen precipitously in the last 15 y (from 100–290 to ∼44 Tg N y−1) (9). This decline in BNF implies an increase in the relative magnitude of anthropogenic N inputs from 100–150% to 190–470% of BNF (9).
Historically, the largest anthropogenic changes to the N cycle have occurred in the northern temperate zone: first throughout the United States and western Europe and more recently, in China (12, 13). Large-scale estimates of BNF in natural ecosystems in these regions are consistently low (11), leading some to conclude that anthropogenic N inputs in the northern temperate zone exceed naturally occurring BNF and preindustrial atmospheric N deposition by an order of magnitude or more (1, 14). By contrast, the highest rates of naturally occurring BNF have been thought to occur in the evergreen lowland tropical rainforest biome (11), implying that, on a regional basis, human alteration of the tropical N cycle has been comparatively modest. However, in recent years, the tropics have seen some of the most dramatic increases in anthropogenic N inputs of any region on Earth—a trend that is likely to continue (2, 6, 13). Anthropogenic N inputs are increasing in tropical regions, primarily because of increasing fossil fuel combustion (13) and expanding high-N-input agriculture for both food and biofuels (6). These anthropogenic N inputs are having a measurable effect on tropical ecosystems (15). However, understanding and forecasting the effects of anthropogenic N depend, in part, on accurate estimates of BNF in lowland tropical rainforest.
Unfortunately, the paradigm that the tropics have high rates of BNF is based on a paucity of evidence and several tenuous assumptions. For example, an early global synthesis of terrestrial BNF (11)—which included contributions from both symbiotic and free-living sources—included only one measured estimate of symbiotic BNF from tropical forest (16 kg N ha−1 y−1) (16). That single estimate, scaled over thousands of square kilometers, represented the only direct evidence of high tropical BNF rates available at that time (Fig. 1). Subsequent modeled estimates (17) that indirectly estimated BNF have reinforced the notion that tropical BNF rates are high and dominated by the symbiotic form of fixation (Fig. 1). Such high estimates of symbiotic BNF are consistent with the large number of leguminous trees in tropical forest (18–20). However, many legume species do not form N-fixing nodules (21), and of those species that do, nodulation in individuals varies with soil nutrient status, N demand, and tree age (22). Several recent analyses (10, 22–24) indicate lower tropical forest BNF and suggest that symbiotic BNF may not be as important to total BNF as previously thought (Fig. 1), although few studies have simultaneously measured symbiotic and free-living BNF.
Fig. 1.
Previous estimates of BNF in tropical rainforest and BNF measured in this study. Percentages indicate the proportion of total BNF from symbiotic BNF. Cleveland et al. 1999 A (11) is a literature database-derived estimate of tropical forest BNF; Cleveland et al. 1999 B (11) is a modeled estimate of BNF based on the correlation between net primary productivity (NPP) and BNF derived with remotely sensed NPP and evergreen broadleaved forest (EBF) land cover classification. Central estimates and variance for Cleveland et al., 1999 A (11) and Reed et al. 2011 (10) represent the low, central, and high data-based estimates of BNF assuming 5%, 15%, and 15% legume cover, respectively. Central estimates and variance for Wang and Houlton 2009 (17) represent the modeled mean and SD of BNF predicted for the EBF biome. Central estimates and variance for Cleveland et al. 2010 (23) represent the low, central, and high estimates of symbiotic BNF plus free-living BNF or modeled BNF plus free-living BNF. Central estimates and variance for BNF in the four forest ages measured here (primary, 5–15 y, 15–30 y, and 30–50 y) represent means ± 1 SD (n = 3). Our estimate of BNF in a dynamic primary forest (gap dynamics) lacks SD, because it consisted of only two measurements: low and high estimates of forest turnover times equal to 150 and 75 y, respectively.
There is also a sound theoretical basis for questioning high estimates of BNF in tropical forest. Namely, high concentrations of soil N in the legume-rich tropics create something of a paradox. Although BNF could create N-rich conditions, the substantial energetic cost of BNF means—and some data show—that BNF should be suppressed under high N availability in primary forests (25). Because of high rates of net primary productivity and high N demand in secondary forests (26, 27), regenerating canopy gaps or abandoned agricultural land may have higher rates of BNF than late-successional forest ecosystems (26).
Resolving the uncertainty in the tropical (and global) N cycle requires that we overcome the enduring challenge of quantifying BNF in any ecosystem. How do we estimate large-scale rates of a process that displays extreme spatial heterogeneity at local scales? Whether using acetylene reduction assays, 15N tracer incubations, or the 15N natural abundance method, most past approaches to empirically estimate symbiotic BNF have relied on spatial extrapolations of BNF rates measured at the level of individual trees. Typically, such extrapolations are based on legume abundance (e.g., percent cover) and make species- or genera-level assumptions about nodulation status of putative N fixers. Here, we applied a method commonly used by community ecologists to measure rare species abundances—stratified adaptive cluster sampling (SACS) (28)—to measure symbiotic BNF. This approach could be used in any ecosystem, and in contrast to other methods, SACS generates unbiased estimates of mean symbiotic BNF (independent of legume abundance) and can more robustly capture the irregular distribution of nodules on the landscape. We simultaneously measured symbiotic and free-living BNF multiple times over the course of 1 y to generate spatially explicit rates of BNF inputs in primary and secondary (5–50 y old) lowland tropical forest in Costa Rica and then used the understanding gained from those estimates to revisit estimates of BNF and anthropogenic N inputs in the tropical forest biome.
Results and Discussion
Taken together, our data suggest far lower rates of total BNF in a region of mixed primary and secondary tropical forest than have been previously reported (Fig. 1). The mean rate of total BNF that we measured in primary forest was only 1.2 kg N ha−1 y−1, 10–20 times lower than previously published empirical (11.7 kg N ha−1 y−1) (10) or modeled rates (25.4–31.9 kg N ha−1 y−1) (Fig. 1) (11, 17). Secondary forest had higher total BNF than primary forest (6.2–14.4 kg N ha−1 y−1), and rates increased with age in the three successional forest age classes (although with substantial intraage variability) (Fig. 1). However, the primary forest BNF estimate does not explicitly account for the important role of frequent small-scale disturbances that create canopy gaps in primary forest. Gaps promote species turnover and thereby contribute to small-scale BNF variability in primary forest. Although the primary forest sites that we studied showed no signs of recent disturbance and lacked large canopy gaps, data from other Costa Rican rainforests suggest tree turnover times that average between 75 and 150 y (29). Assuming that secondary forest BNF approximately represents BNF in disturbed forest patches (a reasonable assumption based on a recent analysis in nearby Panama) (22), we suggest a time-integrated mean estimate for BNF of 5.7 kg N ha−1 y−1 for primary forest in this region (Fig. 1, gap dynamics). Although substantially higher than our estimate of BNF in undisturbed primary forest alone, this estimate is still low relative to previous estimates (Fig. 1).
Symbiotic BNF, which is typically assumed to be the dominant source of BNF in tropical forests, accounted for only 20–50% of total BNF in our study depending on forest age (Fig. 1). Therefore, free-living BNF may represent an equal, if not greater, source of new N to both primary and secondary tropical forests than symbiotic BNF. On a per-mass basis, symbiotic nodules have the highest rates of BNF measured in nature (30), whereas mass-based rates of free-living BNF tend to be much lower (10). However, free-living BNF is much more consistent across the landscape than symbiotic BNF. Thus, N inputs through widespread (but low) rates of free-living BNF may exceed N inputs through isolated (but high) rates of symbiotic BNF. Recent syntheses have made this argument (Fig. 1) (10, 23), but until now, direct evidence has been lacking. We note that, although soil and litter are likely the dominant sources of free-living BNF in tropical forest (10), if we included N inputs from unmeasured sources such as decaying wood (31), canopy epiphytes (32), or termites (33), the relative contribution of free-living BNF would almost certainly increase. Surprisingly, the proportion of the three sources of BNF that we measured (soil, litter, and symbiotic) was similar between primary and secondary forests (Fig. S1), raising questions about the biophysical and/or biogeochemical factors that regulate symbiotic and free-living BNF across forest age classes (10). However, different BNF rates between primary and secondary forest occurred, despite similar soil N and P availability among the sites (Table S1).
We measured low rates of symbiotic BNF in primary tropical forest, despite the presence of legumes in all our sites and an abundance of legumes in secondary forest sites. In fact, both legume abundance and legume basal area in our sites were similar to those in multiple well-studied tropical forests around the world (Table 1 and Fig. S2) (34). Therefore, we suggest that differences between our direct spatial measurement of symbiotic BNF and previous estimates reflect the ability of the SACS method to provide a spatially unbiased estimate of nodule biomass within a site and to generate more robust spatial estimates of symbiotic BNF.
Table 1.
Legume (Fabaceae) species abundance in five primary forest sites [Korup, Luqillo, Yasuni, Pasoh, and Barro Colorado Island (BCI)] that contribute to the Center for Tropical Forest Science plot network (34) as well as the primary forest (1°) and secondary forest (2°) measured in this study near the Piro Biological Station in southwest Costa Rica
| Fabaceae abundance and basal area | Site | ||||||
| Korup, Cameroon | Luqillo, Puerto Rico | Yasuni, Ecuador | Pasoh, Malaysia | BCI, Panama | Piro 1°, Costa Rica* | Piro 2°, Costa Rica* | |
| Family rank | 3 | 6 | 1 | 2 | 2 | 8 ± 2 | 5 ± 2 |
| Basal area (m2 ha−1) | 2.9 | 0.8 | 2.2 | 2.6 | 3.2 | 1.0 ± 0.5 | 9.1 ± 3.3 |
| Basal area (%) | 9.0 | 6.5 | 14.9 | 8.5 | 9.9 | 0.7 ± 0.39 | 10.3 ± 3.0 |
| Trees (ha−1) | 387.6 | 36.0 | 377.2 | — | 343.7 | 67.3 ± 20.7 | 371.1 ± 95.6 |
| Trees (%) | 5.9 | 2.7 | 13.0 | 3.3 | 7.5 | 8.2 ± 3.9 | 39.9 ± 9.9 |
| Total tree species (ha−1; all families) | 38 | 6 | 108 | — | 37 | 90 ± 6 | 58 ± 6 |
Means ± 1 SE.
Accurately scaling symbiotic BNF from nodules to ecosystems has been a major impediment to generating spatial estimates of symbiotic BNF in tropical forest. Scaling approaches that rely on legume abundance assume that legumes are actively fixing and/or that symbiotic BNF is correlated with tree size. However, studies using N isotopes have shown that only 36–53% of legumes are actively fixing in the Amazon (35, 36), and legume tree size and nodule biomass were only weakly correlated in a Panamanian rainforest (22). By comparing our estimates of BNF using the SACS method with two other methods that have scaled BNF based on legume abundance, we show that the SACS method was the proximate cause of the difference between the existing BNF estimates and our lower measurement of BNF. We used legume relative abundance in our plots (Table 1) to estimate BNF with the same regression equation that was used by Cleveland et al. (11). Compared with our measured BNF, the legume abundance-based regression predicted much higher rates of BNF in our primary forest sites (14–22 kg N ha−1 y−1) (Fig. 2)—BNF rates were similar to the rates that generated the biome-level estimate in the work by Cleveland et al. (11). Because legumes were more abundant in secondary forest sites, the regression approach predicted secondary forest BNF rates as high as 100 kg N ha−1 y−1, a value strikingly higher than the measured values (Fig. 2). Furthermore, our estimate of nodule biomass was 40% lower in primary forest and 45% lower in secondary forest than a legume-based maximum likelihood scaling approach used in Panama (22) that predicted much lower rates of symbiotic BNF than had been reported in the literature (16) (Fig. 2). Because the SACS method incorporated both isolated areas, where nodules are abundant, and extensive areas, where nodules are absent, we argue that it represents a significant advance in the measurement of symbiotic BNF in tropical forest.
Fig. 2.

Comparisons between the SACS approach used in this study and existing methods of spatially extrapolating BNF and nodule biomass. (A) Based on legume abundance in the plots that we used, the regression equation used by Cleveland et al. (11) predicts much higher BNF in both primary and secondary forests than we actually measured. In primary forests, the regression approach predicts a symbiotic BNF flux of 14–23 kg N ha−1 y−1; we measured a flux of 0.1–0.5 kg N ha−1 y−1. (B) Using maximum likelihood estimates of nodule biomass and legume abundance, Barron et al. (22) estimated nodule biomass, on a spatial basis, in both primary and secondary forests in Panama. Using the SACS design, we measured 45% less nodule biomass in primary forests near the Piro Biological Station and 40% less nodule biomass in secondary forests near the Piro Biological Station than estimated by Barron et al. (22) in Panama.
The SACS approach estimated nodule biomass independently of legume abundance, thereby providing the opportunity to correlate nodule biomass and legume abundance at the ecosystem scale. There has been a surprising lack of empirical evidence to validate the intuitive positive relationships among legume abundance, nodule biomass, and symbiotic BNF rates. For instance, ter Steege et al. (18) suggested that, across the Amazon basin, legume abundance and nodule biomass were negatively correlated, implying that legume abundance may not predict symbiotic BNF rates. Here, we observed strong positive correlations among the basal area of putatively N-fixing legumes (21), nodule biomass, and symbiotic BNF rates (Fig. S3). We argue that these relationships emerged because we measured BNF across a secondary successional chronosequence where the number and size of legumes varied. Both the shift in legume abundance through succession and the strength of these correlations provide empirical evidence for emerging theory and models that suggest that symbiotic BNF is up- and down-regulated by facultative mechanisms (25) or species replacement (37) to meet N demand. The correlations also provide compelling evidence that symbiotic BNF may be a function of legume basal area during succession and suggest a promising avenue for future work attempting to scale symbiotic BNF rates from legume basal area estimates.
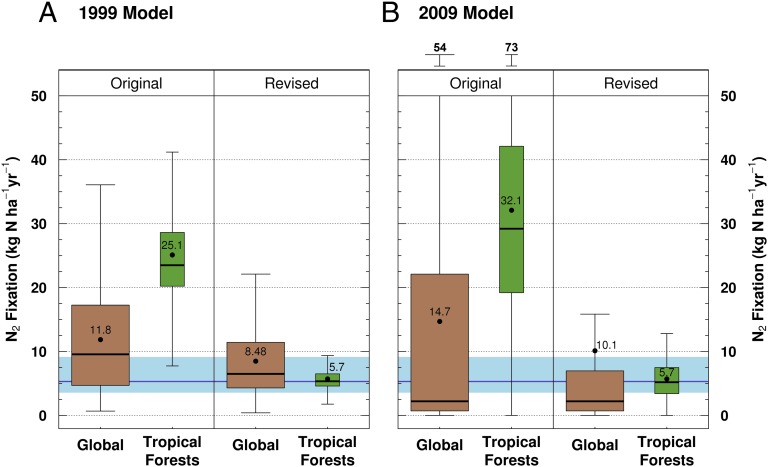
Although our estimates of BNF were collected in one set of sites, two lines of evidence suggest that the low rates of symbiotic BNF that we observed might be common in lowland tropical forest. First, as mentioned above, legume abundance and basal area were similar between our sites and other tropical forest sites around the world (Table 1 and Fig. S2) (34), implying that the low rates of BNF that we observed were not because of a lack of legumes. Second, a reanalysis of a recent lower global BNF estimate that used a top-down 15N isotope model approach (9) also implied lower rates of tropical forest BNF. Vitousek et al. (9) estimated that preindustrial global BNF was 58 Tg N y−1 (equal to 5.3 kg N ha−1 y−1 on vegetated land), much less than previous estimates of 128–195 Tg N y−1 (8, 11, 17). Because tropical forest is believed to contribute a disproportionate amount to global BNF (11), we argue that fixation in this biome cannot remain high if global BNF is one-half or one-third the value suggested by previous estimates. We show this point by constraining two existing global models of BNF (11, 17) with our empirically derived estimate of 5.7 kg N ha−1 y−1 in tropical forest, while holding BNF constant in all other biomes. With tropical forest BNF constrained, global BNF estimated by these models was reduced to the range predicted by the top-down isotope-based estimate (Fig. 3). Although we do not suggest that our empirically derived estimate of BNF represents the value for all tropical forests, it is noteworthy that our measured rate is much easier to reconcile with 58 Tg N y−1 global BNF than previous estimates (e.g., 25–30 kg N ha−1 y−1) (Fig. 1). We also note that it is the first, to our knowledge, field-based estimate of BNF that supports, by direct measurement, the top-down 15N isotope-based model estimate of BNF (9). Although some areas with exceptionally high rates of BNF undoubtedly exist in tropical forest, these high rates of BNF may either be transient (Fig. 1) (26) or lack sufficient spatial extent to generate high rates of BNF throughout the tropical rainforest biome.
Fig. 3.
Revising global BNF models with our gap-based estimate of BNF in tropical forests (5.7 kg N ha−1 y−1) places existing global estimates in the range of BNF measured by a top-down isotope model of global BNF (9) (dark blue horizontal line, mean; shaded blue horizontal area, high and low estimates). (A) By revising tropical BNF in the original 1999 model (11), we reduced global BNF from 11.8 to 8.5 kg N ha−1 y−1. (B) By revising tropical BNF in the original 2009 model (17), we reduced global BNF from 14.7 to 10.1 kg N ha−1 y−1. Dots represent means, with values noted. Black horizontal bars within box plots represent the median values, box plots represent 1 SEM, and whiskers represent the total range of estimates. Global mean BNF estimates using these two models remain substantially higher than the median value because of high legume-derived estimates of BNF in tropical savannas.
If, as multiple lines of evidence suggest, low rates of BNF are common in tropical forest, this finding would fundamentally alter our understanding of both the tropical N cycle and the impact that humans have had on it. In undisturbed tropical forest, where relatively high soil N availability is common (38–42), lower rates of BNF are consistent with theoretical tradeoffs in the energetics of nutrient acquisition (37, 43–45). Although high BNF has been invoked as a driver of those N-rich conditions, the maintenance of such high rates in the face of N abundance has always been paradoxical (25). The data that we present here support recent evidence suggesting that the majority of BNF-derived N that enters tropical forest may do so episodically (i.e., during periods of forest regeneration), when N demand and biomass accrual are high (26). Subsequent development of N-rich conditions may, therefore, result more from shifting patterns in nutrient limitation and forest growth strategies than from chronically high rates of BNF.
Low tropical forest BNF rates also imply that recent human activity has enriched the tropical N cycle far more than previously thought. Anthropogenically derived N deposition inputs in tropical regions are already similar to (or exceed) the rates of BNF that we report here (15, 46). In areas deforested for agriculture, massive per-area increases in N inputs are common because of fertilizer application and/or the growth of N-fixing crops—most notably, soy (47). Deforestation is often the result of economically expedient (and politically supported) agricultural extensification (sensu lato, ref. 48), after which exhausted agricultural areas are abandoned to secondary succession.
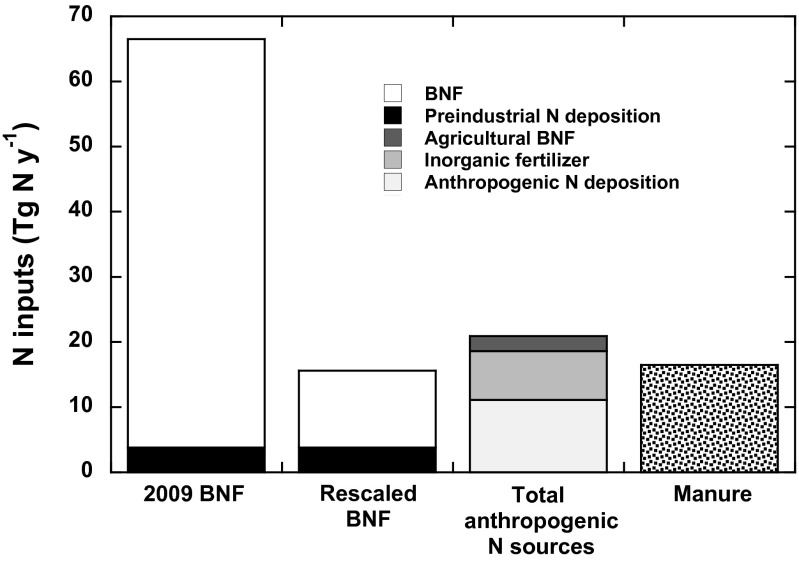
To provide context for the importance of BNF in evaluating the overall anthropogenic change in human vs. natural N inputs to the tropical forest biome, we compiled biome-wide atmospheric N deposition estimates (49), inorganic and organic fertilizer use data (50), and an estimate of agricultural N fixation based on crop area and yield statistics (51) within the historical tropical rainforest biome (i.e., the tropical rainforest biome in the absence of human activity) (52). Most strikingly, this analysis shows the importance of accurate BNF estimates in considering human changes to the tropical N cycle. In particular, anthropogenic N sources only represented 31% of total nonanthropogenic N inputs using prior assumptions of high forest BNF (Fig. 4). Using the lower BNF value indicated by our field study, we suggest that humans have increased the amount of reactive N entering the tropical rainforest biome by 134% (Fig. 4). We note that our estimate of anthropogenic N flux does not include organic fertilizer, like manure, which recycles both endogenous and exogenous N within ecosystems (assuming that most manure is not a net import from other biomes). However, in Fig. 4, we depict biome-level organic fertilizer inputs to illustrate that manure application in the tropics rivals the biome-wide forest BNF value derived from our estimate and that manure is an important alternative to inorganic fertilizer manufactured by the Haber–Bosch process (Fig. 4). The values for both agricultural BNF and fertilizer use are undoubtedly conservative: although they represent compilations of the currently available data (circa 2000), they do not integrate the fact that, over the past 15 y, both overall N fertilizer use and the extent of soy cultivation (an important source of agricultural BNF) have expanded dramatically in tropical regions (6, 53).
Fig. 4.
The rate of BNF in tropical rainforest generated using the SACS approach is much lower than previous estimates, and it implies that the human perturbation to the tropical N cycle is approximately four times greater than previous N fixation estimates would suggest. We estimated BNF using an existing (2009) model (17) that generated high estimates of BNF, and then, we estimated lower rates of BNF by downscaling the 2009 model (17) to our mean BNF estimate of 5.7 kg N ha−1 y−1. Anthropogenic N deposition was calculated as the difference between total tropical N deposition (49) and preindustrial N deposition (58). Manure was not treated as an anthropogenic N input, because we assumed that it is regionally produced and that it represents a recycling of previous anthropogenic or naturally fixed N. However, manure is a major source of N to tropical ecosystems nearly equivalent to all other anthropogenic N sources combined.
Regardless of the extent to which human activities have actually perturbed the tropical N cycle, there is no doubt that, as the proportion of anthropogenic N inputs increases relative to natural inputs, the region will continue to see significant ecological and socioeconomic impacts. In temperate regions, anthropogenic N inputs have contributed to shifts in species composition, even when below so-called critical loads—thresholds below which inputs of N are supposedly safe for ecosystems (54). Given that tropical regions contain much of the biological diversity on Earth (55), many tropical species may be vulnerable to the unintended, indirect effects of increasing anthropogenic N. Similarly, as seen in much of the temperate zone, increased anthropogenic N inputs could have positive effects on human health, but without effective management, they could also have profound negative effects (7). Low rates of BNF in tropical regions add urgency to growing calls to manage increasing N inputs in tropical biomes and require local, regional, and international policy instruments that, for the most part, are not yet in place (6).
Methods
We measured BNF in 12 0.5-ha plots (50 × 100 m) on the Osa Peninsula, Costa Rica near the Piro Biological Station (8°24′ N, 83°20′ W; 87 m above sea level) (56). SI Methods, sections 1 and 2 provide additional site description. We measured symbiotic, soil, and litter N fixation four times in 2012 and 2013, capturing all seasonal precipitation variation that the sites experience—January, May, July, and October. To measure symbiotic BNF, we measured nodule biomass during the early wet season at each site using SACS (28). SI Methods, section 2 and Fig. S4 give additional details. In total, we searched for nodules in ∼1,500 5.5-cm-wide × 10-cm-deep cores. We measured BNF rates on excised nodules, soil, and litter using the acetylene reduction assay. SI Methods, sections 3 and 4 give additional details. We aggregated the 5- to 15-, 15- to 30-, and 30- to 50-y forest age classes into the category of secondary forest. We measured primary forest turnover (gap dynamics) using two scenarios—low turnover (150 y) and high turnover (75 y) based on estimates in ref. 29. SI Methods, section 5 details measurement of BNF in each scenario. We measured the relationship between nodule biomass, symbiotic BNF, and basal area of putative N-fixing legumes using linear regression in the statistical package R (57). We constrained two existing spatially explicit global N2 fixation models (11, 17) by our empirically derived estimate of tropical N2 fixation and compared both previous and constrained models with the top-down global BNF measured in ref. 9. SI Methods, section 6 gives additional details on constraining the models. We quantified the extent of anthropogenic impacts on the tropical N cycle by comparing preindustrial N inputs (BNF and N deposition) with recent estimates of anthropogenic N inputs (postindustrial N deposition, agricultural BNF, and agricultural fertilization) across total historical tropical forest area. SI Methods, section 7 gives additional details.
Supplementary Material
Acknowledgments
We thank B. Vilchez and E. Ortiz for establishing the chronosequence plots; M. Lopez-Morales, R. Cole, S. Weintraub, and S. Alvarez-Clare for assistance in the field; S. Castle, E. Prag, N. Boote, and A. Ginter for assistance in the laboratory; R. Cole for sharing soil chemical data; J. Sprent and three anonymous reviewers for comments on our manuscript; and C. Nelson and D. Affleck for assistance with the stratified adaptive cluster sampling design. Grants from the Andrew W. Mellon Foundation, the National Science Foundation (Grant DEB-0919080), the Ecosystems Mission Area of the US Geological Survey, and the Blue Moon Fund supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320646111/-/DCSupplemental.
References
- 1.Vitousek PM, et al. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl. 1997;7(3):737–750. [Google Scholar]
- 2.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 3.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 4.Matson PA, Parton WJ, Power AG, Swift MJ. Agricultural intensification and ecosystem properties. Science. 1997;277(5325):504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- 5.Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451(7179):712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- 6.Austin AT, et al. Environment. Latin America’s nitrogen challenge. Science. 2013;340(6129):149. doi: 10.1126/science.1231679. [DOI] [PubMed] [Google Scholar]
- 7.Townsend AR, et al. Human health effects of a changing global nitrogen cycle. Front Ecol Environ. 2003;1(5):240–246. [Google Scholar]
- 8.Galloway JN, et al. Nitrogen cycles: Past, present, and future. Biogeochemistry. 2004;70(2):153–226. [Google Scholar]
- 9.Vitousek PM, Menge DNL, Reed SC, Cleveland CC. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos Trans R Soc Lond B Biol Sci. 2013;368(1621):20130119. doi: 10.1098/rstb.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed SC, Cleveland CC, Townsend AR. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu Rev Ecol Evol Syst. 2011;42:489–512. [Google Scholar]
- 11.Cleveland CC, et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem Cycles. 1999;13(2):623–645. [Google Scholar]
- 12.Dentener F, et al. Nitrogen and sulfur deposition on regional and global scales: A multimodal evaluation. Global Biogeochem Cycles. 2006;20:GB4003. [Google Scholar]
- 13.Liu X, et al. Enhanced nitrogen deposition over China. Nature. 2013;494(7438):459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- 14.Howarth RW, et al. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry. 1996;35(1):75–139. [Google Scholar]
- 15.Hietz P, et al. Long-term change in the nitrogen cycle of tropical forests. Science. 2011;334(6056):664–666. doi: 10.1126/science.1211979. [DOI] [PubMed] [Google Scholar]
- 16.Jordan C, et al. The nitrogen cycle in a ‘Terra Firme’ rainforest on oxisol in the Amazon territory of Venezuela. Plant Soil. 1982;67:325–332. [Google Scholar]
- 17.Wang Y-P, Houlton BZ. Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon-climate feedback. Geophys Res Lett. 2009;36:L24403. [Google Scholar]
- 18.ter Steege H, et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature. 2006;443(7110):444–447. doi: 10.1038/nature05134. [DOI] [PubMed] [Google Scholar]
- 19.Crews TE. The presence of nitrogen fixing legumes in terrestrial communities: Evolutionary vs ecological considerations. Biogeochemistry. 1999;46(1-3):233–246. [Google Scholar]
- 20.Sylvester-Bradley R, De Oliveira LA, De Podestá Filho JA, St. John TV. Nodulation of legumes, nitrogenase activity of roots and occurrence of nitrogen-fixing Azospirillum spp. in representative soils of central Amazonia. Agro-ecosyst. 1980;6(3):249–266. [Google Scholar]
- 21.Sprent JI. In: Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment. Werner D, Newton WE, editors. Berlin: Springer; 2005. pp. 113–141. [Google Scholar]
- 22.Barron AR, Purves DW, Hedin LO. Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia. 2011;165(2):511–520. doi: 10.1007/s00442-010-1838-3. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland CC, et al. Using indirect methods to constrain symbiotic nitrogen fixation rates: A case study from an Amazonian rain forest. Biogeochemistry. 2010;99(1-3):1–13. [Google Scholar]
- 24.Gehring C, Vlek PLG, de Souza LAG, Denich M. Biological nitrogen fixation in secondary regrowth and mature rainforest of central Amazonia. Agric Ecosyst Environ. 2005;111:237–252. [Google Scholar]
- 25.Hedin LO, Brookshire ENJ, Menge DNL, Barron AR. The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst. 2009;40:613–635. [Google Scholar]
- 26.Batterman SA, et al. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature. 2013;502(7470):224–227. doi: 10.1038/nature12525. [DOI] [PubMed] [Google Scholar]
- 27.Davidson EA, et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature. 2007;447(7147):995–998. doi: 10.1038/nature05900. [DOI] [PubMed] [Google Scholar]
- 28.Thompson SK. Sampling. New York: Wiley; 2002. [Google Scholar]
- 29.Hartshorn GS. Neotropical forest dynamics. Biotropica. 1980;12(2):23–30. [Google Scholar]
- 30.Boring LR, Swank WT, Waide JB, Henderson GS. Sources, fates, and impacts of nitrogen inputs to terrestrial ecosystems: Review and synthesis. Biogeochemistry. 1988;6(2):119–159. [Google Scholar]
- 31.Silvester WB, Sollins P, Verhoeven T, Cline SP. Nitrogen fixation and acetylene reduction in decaying conifer boles: Effects of incubation time, aeration, and moisture content. Can J For Res. 1982;12(3):646–652. [Google Scholar]
- 32.Fürnkranz M, et al. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2008;2(5):561–570. doi: 10.1038/ismej.2008.14. [DOI] [PubMed] [Google Scholar]
- 33.Yamada A, et al. Nitrogen fixation by termites in tropical forests, Thailand. Ecosystems. 2006;9(1):75–83. [Google Scholar]
- 34.Losos EC, Leigh EG. Tropical Forest Diversity and Dynamism. Chicago: University of Chicago Press; 2004. [Google Scholar]
- 35.Nardoto GB, et al. Basin-wide variations in Amazon forest nitrogen-cycling characteristics as inferred from plant and soil 15N:14N measurements. Plant Ecol Divers. 2014;7(1-2):173–187. [Google Scholar]
- 36.Pons TL, Perreijn K, van Kessel C, Werger MJA. Symbiotic nitrogen fixation in a tropical rainforest: 15N natural abundance measurements supported by experimental isotopic enrichment. New Phytol. 2007;173(1):154–167. doi: 10.1111/j.1469-8137.2006.01895.x. [DOI] [PubMed] [Google Scholar]
- 37.Menge DNL, Hedin LO. Nitrogen fixation in different biogeochemical niches along a 120 000-year chronosequence in New Zealand. Ecology. 2009;90(8):2190–2201. doi: 10.1890/08-0877.1. [DOI] [PubMed] [Google Scholar]
- 38.Jenny H. Causes of the high nitrogen and organic matter content of certain tropical forest soils. Soil Sci. 1950;69(1):63–70. [Google Scholar]
- 39.Vitousek OM, Sanford RL., Jr Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17(1):137–167. [Google Scholar]
- 40.Hedin LO, Vitousek PM, Matson PA. Nutrient losses over four million years of tropical forest development. Ecology. 2003;84(9):2231–2255. [Google Scholar]
- 41.Houlton BZ, Sigman DM, Hedin LO. Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA. 2006;103(23):8745–8750. doi: 10.1073/pnas.0510185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brookshire ENJ, Gerber S, Menge DNL, Hedin LO. Large losses of inorganic nitrogen from tropical rainforests suggest a lack of nitrogen limitation. Ecol Lett. 2012;15(1):9–16. doi: 10.1111/j.1461-0248.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- 43.Vitousek PM, Field CB. Ecosystem constraints to symbiotic nitrogen fixers: A simple model and its implications. Biogeochemistry. 1999;46(1-3):179–202. [Google Scholar]
- 44.Vitousek PM, et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry. 2002;57-58(1):1–45. [Google Scholar]
- 45.Menge DNL, Levin SA, Hedin LO. Evolutionary tradeoffs can select against nitrogen fixation and thereby maintain nitrogen limitation. Proc Natl Acad Sci USA. 2008;105(5):1573–1578. doi: 10.1073/pnas.0711411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boy J, Rollenbeck R, Valarezo C, Wilcke W. Amazonian biomass burning-derived acid and nutrient deposition in the north Andean montane forest of Ecuador. Global Biogeochem Cycles. 2008;22:GB4011. [Google Scholar]
- 47.Macedo MN, et al. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proc Natl Acad Sci USA. 2012;109(4):1341–1346. doi: 10.1073/pnas.1111374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinelli LA. Block changes to Brazil’s forest code. Nature. 2011;474(7353):579. doi: 10.1038/474579a. [DOI] [PubMed] [Google Scholar]
- 49. European Commission-Joint Research Center (2014) EDGAR 4.2: Emissions Database for Global Atmospheric Research. Available at http://edgar.jrc.ec.europa.eu/index.php. Accessed October 28, 2013.
- 50.Potter P, Ramankutty N, Bennet EM, Donner SD. Characterizing the spatial patterns of global fertilizer application and manure production. Earth Interact. 2010;14(2):1–22. [Google Scholar]
- 51.Monfreda C, Ramankutty N, Foley JA. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Global Biogeochem Cycles. 2008;22:GB1022. [Google Scholar]
- 52.Ramankutty N, Foley JA. Estimating historical changes in global land cover: Croplands from 1700 to 1992. Global Biogeochem Cycles. 1999;13:997–1027. [Google Scholar]
- 53.Townsend AR, Howarth RW. Fixing the global nitrogen problem. Sci Am. 2010;302(2):64–71. doi: 10.1038/scientificamerican0210-64. [DOI] [PubMed] [Google Scholar]
- 54.Payne RJ, Dise NB, Stevens CJ, Gowing DJ. BEGIN Partners Impact of nitrogen deposition at the species level. Proc Natl Acad Sci USA. 2013;110(3):984–987. doi: 10.1073/pnas.1214299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dirzo R, Raven PH. Global state of biodiversity and loss. Annu Rev Environ Resour. 2003;28(1):137–167. [Google Scholar]
- 56.Morales-Salazar M, et al. Diversidad y estructura horizontal en los bosques tropicales del Corredor Biológico de Osa, Costa Rica. Revista Forestal Mesoamericana Kurú. 2012;9(23):19–28. [Google Scholar]
- 57.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 58.Holland EA, Dentener FJ, Braswell BH, Sulzman JM. Contemporary and pre-industrial global reactive nitrogen budgets. Biogeochemistry. 1999;46(1-3):7–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.