Abstract
The dentate gyrus of the hippocampus is one of the few areas of the adult brain that undergoes neurogenesis. In the present study, cells capable of proliferation and neurogenesis were isolated and cultured from the adult rat hippocampus. In defined medium containing basic fibroblast growth factor (FGF-2), cells can survive, proliferate, and express neuronal and glial markers. Cells have been maintained in culture for 1 year through multiple passages. These cultured adult cells were labeled in vitro with bromodeoxyuridine and adenovirus expressing beta-galactosidase and were transplanted to the adult rat hippocampus. Surviving cells were evident through 3 months postimplantation with no evidence of tumor formation. Within 2 months postgrafting, labeled cells were found in the dentate gyrus, where they differentiated into neurons only in the intact region of the granule cell layer. Our results indicate that FGF-2 responsive progenitors can be isolated from the adult hippocampus and that these cells retain the capacity to generate mature neurons when grafted into the adult rat brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Das G. D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965 Jun;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46(3):315–323. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- Bayer S. A., Yackel J. W., Puri P. S. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982 May 21;216(4548):890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Boss B. D., Peterson G. M., Cowan W. M. On the number of neurons in the dentate gyrus of the rat. Brain Res. 1985 Jul 8;338(1):144–150. doi: 10.1016/0006-8993(85)90257-4. [DOI] [PubMed] [Google Scholar]
- Cameron H. A., Woolley C. S., McEwen B. S., Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993 Sep;56(2):337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Deloulme J. C., Baudier J., Sensenbrenner M. Establishment of pure neuronal cultures from fetal rat spinal cord and proliferation of the neuronal precursor cells in the presence of fibroblast growth factor. J Neurosci Res. 1991 Aug;29(4):499–509. doi: 10.1002/jnr.490290410. [DOI] [PubMed] [Google Scholar]
- Gensburger C., Labourdette G., Sensenbrenner M. Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro. FEBS Lett. 1987 Jun 8;217(1):1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Bell D. H. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984 Jun;4(6):1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. S., Bell D. H. Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp Brain Res. 1983;52(1):1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977 Sep 9;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Levison S. W., Goldman J. E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993 Feb;10(2):201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Lois C., Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994 May 20;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993 Jul;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Morshead C. M., van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992 Jan;12(1):249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R., Smith A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992 Sep;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Murphy M., Drago J., Bartlett P. F. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. J Neurosci Res. 1990 Apr;25(4):463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- Onifer S. M., Whittemore S. R., Holets V. R. Variable morphological differentiation of a raphé-derived neuronal cell line following transplantation into the adult rat CNS. Exp Neurol. 1993 Jul;122(1):130–142. doi: 10.1006/exnr.1993.1114. [DOI] [PubMed] [Google Scholar]
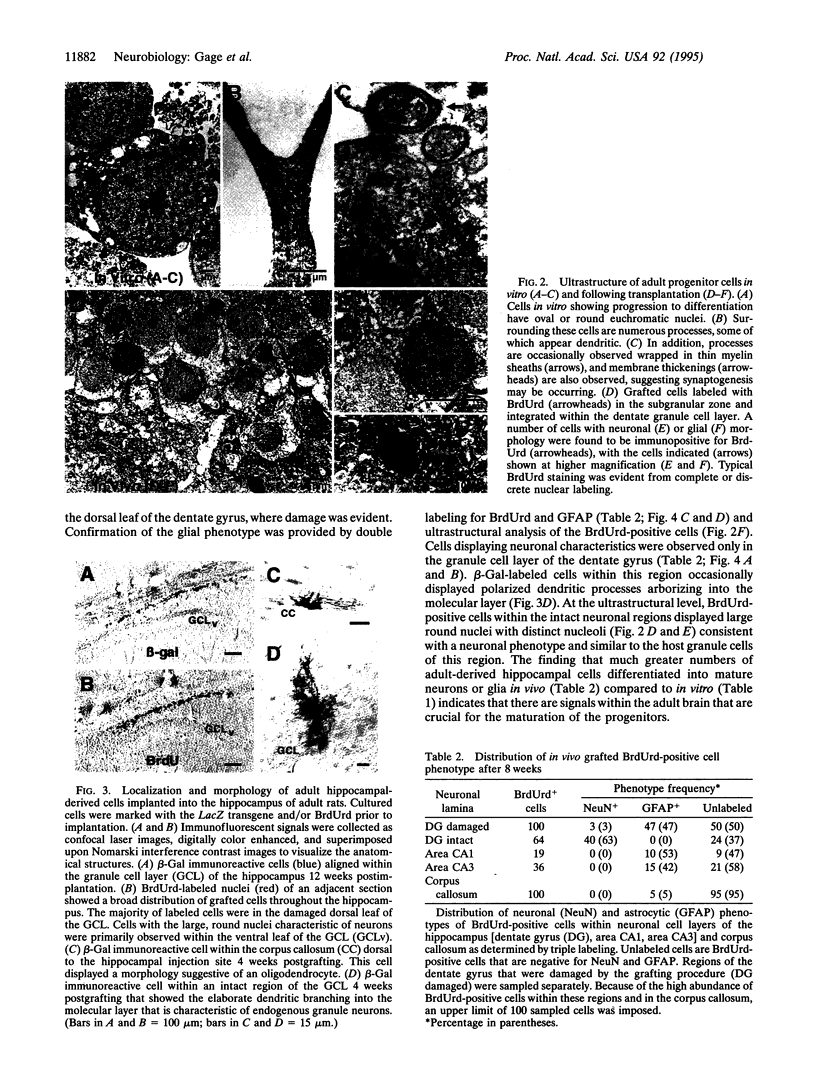
- Peterson D. A., Lucidi-Phillipi C. A., Eagle K. L., Gage F. H. Perforant path damage results in progressive neuronal death and somal atrophy in layer II of entorhinal cortex and functional impairment with increasing postdamage age. J Neurosci. 1994 Nov;14(11 Pt 2):6872–6885. doi: 10.1523/JNEUROSCI.14-11-06872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Gage F. H. Spinal cord neuroblasts proliferate in response to basic fibroblast growth factor. J Neurosci. 1994 Jun;14(6):3548–3564. doi: 10.1523/JNEUROSCI.14-06-03548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Peterson D. A., Schinstine M., Gage F. H. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz P. J., Cunningham M. G., McKay R. D. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 1991 Aug 23;66(4):713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards L. J., Kilpatrick T. J., Bartlett P. F. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M., Chikaraishi D. M., Kauer J. S. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991 Nov;11(11):3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. Y., Deitcher D. L., Walsh C., Arnold-Aldea S., Hartwieg E. A., Cepko C. L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992 Jan 10;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Stanfield B. B., Trice J. E. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72(2):399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Vietje B. P., Wells J. Selective lesions of granule cells by fluid injections into the dentate gyrus. Exp Neurol. 1989 Dec;106(3):275–282. doi: 10.1016/0014-4886(89)90160-x. [DOI] [PubMed] [Google Scholar]