Significance
Pharmacological and global KO studies have worked to elucidate the function of dopamine 1-class receptors (D1Rs and D5Rs) in hippocampal-dependent learning and memory. Yet, these manipulations are unable to restrict D1R from D5R activity within hippocampal subregions. We generated mice that lack D1Rs or D5Rs in dentate gyrus (DG) granule cells of the hippocampus. This allowed us to characterize the precise role of D1Rs and D5Rs in modulating c-Fos activity in the hippocampus and in Pavlovian fear conditioning. We demonstrate that DG D1R deletion, but not D5R deletion, increases DG granule cell baseline c-Fos activity, decreases DG and CA3 c-Fos activity in response to contextual exposure and to contextual fear conditioning, impairs contextual memory formation, and enhances generalization of the conditioned fear response.
Keywords: dopamine 1 receptor, dopamine 5 receptor, Pavlovian fear conditioning, memory generalization
Abstract
Activation of the hippocampal dopamine 1-class receptors (D1R and D5R) are implicated in contextual fear conditioning (CFC). However, the specific role of the D1R versus D5R in hippocampal dependent CFC has not been investigated. Generation of D1R- and D5R-specific in situ hybridization probes showed that D1R and D5R mRNA expression was greatest in the dentate gyrus (DG) of the hippocampus. To identify the role of each receptor in CFC we generated spatially restricted KO mice that lack either the D1R or D5R in DG granule cells. DG D1R KOs displayed significant fear memory deficits, whereas DG D5R KOs did not. Furthermore, D1R KOs but not D5R KOs, exhibited generalized fear between two similar but different contexts. In the familiar home cage context, c-Fos expression was relatively low in the DG of control mice, and it increased upon exposure to a novel context. This level of c-Fos expression in the DG did not further increase when a footshock was delivered in the novel context. In DG D1R KOs, DG c-Fos levels in the home cage was higher than that of the control mice, but it did not further increase upon exposure to a novel context and remained at the same level upon a shock delivery. In contrast, the levels of DG c-Fos expression was unaffected by the deletion of DG D5R neither in the home cage nor upon a shock delivery. These results suggest that DG D1Rs, but not D5Rs, contribute to the formation of distinct contextual representations of novel environments.
The hippocampus is crucial for aversive Pavlovian conditioning, such as contextual fear conditioning (CFC) (1, 2). In CFC, the conditioned stimulus (context) is paired with the unconditioned stimulus (footshock), and after pairing, the context serves as a cue to predict a potential footshock (3, 4). Although the role of dopamine has been studied in the context of reward learning (5), evidence suggests that midbrain dopaminergic neurons are also important for aversive Pavlovian conditioning (6–9). In line with this evidence, hippocampal encoding of novel and contextual information is linked to dopamine release via excitation of dopamine neurons of the midbrain (5, 10, 11). Additionally, delivery of aversive stimuli, such as a footshock, results in increased dopaminergic neuron activity (12). Moreover, inactivation of hippocampal D1Rs and D5Rs attenuates contextual fear memory (13). Thus, it follows that delivery of an aversive stimulus activates midbrain dopamine neurons that project to the hippocampus, which is crucial for encoding novel contextual cues (12, 14, 15). Activation of hippocampal D1Rs and D5Rs may then strengthen the encoding of novel contextual information during CFC.
The precise role of subregion-specific D1R or D5R activation in hippocampal-dependent learning and memory is unknown. This is in part due to the inability to discriminate between and spatially restrict D1R from D5R function (16–18), which is an important caveat because each receptor is involved in modulating distinct neuronal processes (19–22). Indeed, there is a lack of consensus of D1R and D5R expression patterns in the rodent hippocampus (23–27). Moreover, pharmacological findings are at odds with D1R and D5R global KO studies, which show that neither D1Rs nor D5Rs are required for fear conditioning (16, 17). Therefore, to reconcile these disparate findings and to test the necessity of D1R and D5R activation for CFC, it is necessary to functionally isolate and spatially restrict hippocampal D1R and D5R activity.
In this study, we found that D1Rs and D5Rs exhibit overlapping expression in dentate gyrus (DG) granule cells. DG D1R activation is necessary to increase c-Fos expression in the DG and CA3 to enhance novel contextual encoding. Moreover, DG D1R activation decreases generalization of the conditioned fear response to novel contexts. However, we found no role for DG D5Rs in modulating DG c-Fos expression or contextual fear learning and memory. In using our subregion-specific KO mice, we show that the hippocampal dopamine signal plays a definitive role in CFC.
Results
D1R and D5R Expression Exhibits Greatest Overlap in Dentate Gyrus Granule Cells.
The expression patterns of D1Rs and D5Rs have remained unclear, making it difficult to identify the function of these receptors in the modulation of hippocampal dependent learning and memory. Because D1Rs and D5Rs carry a high level of similarity in their amino acid sequence (18, 24), the use of antibodies to identify region specific D1R and D5R expression has not been effective (28). To overcome this issue, we have created D1R- and D5R-specific mRNA probes for in situ hybridization (ISH) experiments. We validated the specificity of each riboprobe by generating D1R−/− and D5R−/− mouse lines and tested each probe on brains slices of these constitutive KO mice (Fig. S1 B–E). Using the D1R-specific probe, we have shown that D1R mRNA is present throughout the cortical layers and expressed strongly in the striatum, olfactory tubercle (OT), olfactory bulb, and the DG (Fig. 1 A and B). The D5R mRNA is expressed strongly in the DG, CA3, and CA1 (Fig. 1D) and weakly in the paraventricular nucleus of the thalamus (Fig. S1D), cortex, the cerebellum, the striatum, and OT (Fig. 1C). These data are consistent with previous ISH studies (23, 24, 29). We have revealed, to our knowledge for the first time, that in the hippocampus, the DG is the area where both D1Rs and D5Rs are robustly expressed.
Fig. 1.
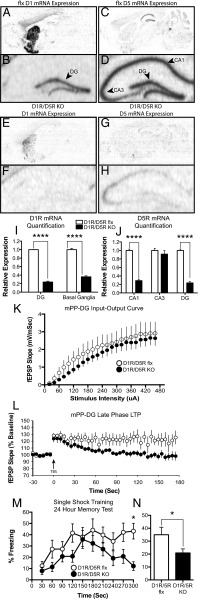
Characterization of forebrain D1R/5R KO mice. (A and B) D1R mRNA expression in flx mice. (C and D) D5R mRNA expression in flx mice. (E and F) D1R mRNA expression in D1R/5R KO mice. (G and H) D5R mRNA expression in D1R/5R KO mice. (I and J) Quantification of the D1R (DG flx and KO, n = 8; basal ganglia flx and KO, n = 5; I) and D5R mRNA (flx and KO, n = 8 for all subregions; J) signal in D1R/5R KO mice. (K) mPP-DG input–output curve (flx, n = 6; KO n = 9). (L) l-LTP at the mPP-DG synapse (flx, n = 6; KO, n = 8). (M) The 24-h contextual fear memory test (flx, n = 10; KO, n = 11). (N) Average of L. CP, caudate/putamen; CTX, cortex; NAcc, nucleus accumbens; OLB, olfactory bulb; OT, olfactory tubercle.
Comparison of Forebrain Restricted D1R and D5R KO Mice to Pharmacological Antagonist Studies.
Prior studies investigating the role of D1Rs and D5Rs in learning and memory have relied on pharmacological antagonists (13, 30). However, these antagonists block both D1Rs and D5Rs due to their high structural similarity (28). To connect prior literature using D1R/D5R antagonists, we generated mice lacking both D1Rs and D5Rs by crossing CaMKII-Cre mice (31) with floxed (flx) D1R/D5R mice, which resulted in forebrain-wide D1R and D5R deletion (D1R/D5R KO) (Fig. 1 E and G). Receptor deletion in the D1R/D5R KOs was quantified by measuring the grayscale gradient of the ISH image within each region of interest (32). Significant reduction of the D1R mRNA occurred in the DG (unpaired t test, P < 0.0001) and striatum (unpaired t test, P < 0.0001) (Fig. 1 E, F, and I) in the mutant mice. The DG D1R mRNA signal was nearly absent by 16 wk of age. The DG (unpaired t test, P < 0.0001) and CA1 (unpaired t test, P < 0.0001) D5R mRNA signal was significantly reduced in D1R/D5R KO mice, with near complete deletion by 28 wk of age (Fig. 1 G, H, and J). The levels of D5R mRNA expression were unaltered in the CA3 area of the KO animals compared with control mice (unpaired t test, P = 0.362) (Fig. 1J).
D1R/D5R deletion in the DG was also confirmed by observed deficits in synaptic plasticity at the medial perforant path (mPP)-DG synapse in KO animals. Previous studies have shown that pharmacological blockade of D1R/D5Rs inhibits mPP-DG late phase long term potentiation (l-LTP) (33); therefore, we assessed synaptic transmission and l-LTP at the mPP-DG synapse using an anesthetized in vivo preparation. An input–output curve was generated by stimulation of the mPP, with the recording electrode placed in the hilus of the DG. There was no significant difference between genotypes [two-way ANOVA, F(1, 13) = 0.4413, P = 0.5181] (Fig. 1K). A theta burst protocol was used to induce l-LTP at the mPP-DG synapse in flx and mutant mice (Fig. 1L). D1R/D5R KO mice showed initial potentiation (paired t test, 25–30 min, 113.1% ± 3.3%, P < 0.05). However, the slope of the fEPSP returned to baseline (paired t test, 175–180 min, 95.9% ± 4.7%, P = 0.492), whereas flx D1R/D5R mice exhibited robust l-LTP (paired t test, 175–180 min, 125.4% ± 10.0%, P < 0.05).
D1R/D5R KO mice show deficits in CFC. When given a 24-h fear memory test, D1R/D5R KO mice exhibited fear memory deficits as measured by reduced freezing levels in KO animals (unpaired t test, P < 0.05) (Fig. 1 M and N). The observed phenotype in mutant mice was not due to differences in pain sensitivity, gross motor dysfunction, or altered anxiety levels (Fig. S2 A–D). Because the amygdala is pivotal in processing fear memory, we trained mice on an amygdala-dependent cued-fear conditioning protocol where a discrete auditory cue predicts and coterminates with an aversive foot shock. D1R/D5R KO mice showed normal conditioning to tone (unpaired t test, P = 0.5533), suggesting that contextual fear memory deficits in the KO mice are not due to altered amygdalar function (Fig. S3A).
Identification of DG-Specific D1R and D5R Function in Contextual Fear Conditioning and Subregion-Specific c-Fos Activation.
We generated DG specific D1R KO and D5R KOs by crossing POMC-Cre mice (34) with flx D1R mice or flx D5R mice, respectively. D1R gene recombination was restricted to DG granule cells of the hippocampus. The D1R mRNA signal was nearly absent by 16 wk of age in D1R mutants (Fig. 2 A and B). Quantification of the D1R ISH signal in flx and KO mice showed significant reduction of D1R mRNA expression in the DG (unpaired t test, P = 6.27 × 10−8) with normal DG D5 mRNA expression (unpaired t test, P = 0.309) (Fig. 2 C–E). DG D5R KO animals exhibited maximum deletion of DG D5Rs (unpaired t test, P = 3.86 × 10−6) by 13 wk of age and showed normal D1R mRNA expression in the DG (unpaired t test, P = 0.250) (Fig. 2 F–J).
Fig. 2.
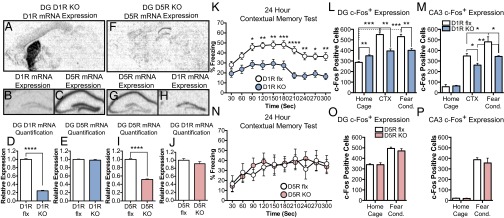
Characterization of DG-specific D1R and D5R KO lines. (A and B) D1R mRNA expression in D1 KO line. (C) D5R mRNA expression in D1 KO line. (D and E) Quantification of D1R (flx, n = 4; KO, n = 4; D) and D5R mRNA (flx, n = 4; KO, n = 3; E) expression in the DG. (F and G) D5R mRNA expression in D5 KO line. (H) D1R mRNA expression in D5 KO line. (I and J) Quantification of D5R (flx and KO, n = 4 for all subregions; I) and D1R mRNA (flx, n = 4; KO, n = 4; J) expression in the DG. (K) D1R KO line, contextual fear memory test (flx, n = 51; KO, n = 40). (L and M) D1R KO line, quantification of c-Foc+ neurons in the DG (L) and CA3 (M) neurons (DG and CA3; HC flx and KO, n = 3; CTX flx and KO, n = 5; FC flx and KO, n = 4). (N) DG D5R KO line, contextual fear memory test (flx, n = 9; KO, n = 11). (O and P) D5R KO line, quantification of c-Foc+ neurons in the DG (O) and CA3 (P) neurons (DG and CA3; HC flx, n = 3; KO, n = 2; FC flx and KO, n = 3). CTX, context exposure; FC, fear conditioning; HC, home cage.
DG D1R deletion impairs CFC, whereas DG D5R deletion does not. When mice were trained on a CFC paradigm, D1R KOs exhibited impaired fear memory compared with control mice [two-way ANOVA (time × genotype), F(9, 801)time = 9.963, P < 0.0001; F(1, 89)genotype = 20.36, P < 0.0001]. Bonferroni multiple comparisons test also shows deficits at multiple time points (Fig. 2K). DG D5R KO mice exhibited normal contextual fear memory when average freezing levels were compared between the mutant and control mice (unpaired t test, P = 0.908) (Fig. 2N). We also tested the necessity of DG D1Rs for short-term memory (1 and 3 h after training) and observed no fear memory deficits (unpaired t test, 1 h, P = 0.5584; 3 h, P = 0.3337) (Fig. S3B). DG D1R KOs showed normal cued fear conditioning during tone presentation (unpaired t test, P = 0.4877) (Fig. S3C). Moreover, neither the DG D1R KO nor the DG D5R KO mice exhibited gross differences in pain sensitivity, motor function, or anxiety (Fig. S2 E–L).
DG D1R, but not D5R, deletion impairs DG and CA3 c-Fos expression to novel contextual exposure and CFC. We used c-Fos expression levels to measure hippocampal subregion encoding of novel contextual information (35–38). We measured c-Fos+ neurons of the DG and CA3 in response to the animals’ home cage, exposure to a novel context or novel contextual exposure plus footshock (i.e., mice receiving CFC). Flx D1R controls showed a robust increase in the number of c-Fos+ neurons when exposed to a novel context (unpaired t test, P < 0.0005) or given CFC (unpaired t test, P < 0.0005), in comparison with the home cage group. Moreover, there was no significant difference in DG c-Fos+ neurons in flx D1R mice, when the context and CFC groups were compared (flx: unpaired t test, P < 0.599) (Fig. 2L). In contrast, compared with the control mice, D1R KOs showed significantly reduced c-Fos+ expression in DG granule cells to both the novel context exposure (unpaired t test, P < 0.01) and CFC (unpaired t test, P < 0.01) (Fig. 2L). Interestingly, in the home cage group, D1R KOs showed significantly enhanced c-Fos+ DG granule cells, compared with flx control (unpaired t test, P < 0.01) (Fig. 2L). No significant difference was found in the number of c-Fos+ neurons between the home cage group and novel context exposure (unpaired t test, P = 0.148) or between the home cage group and fear-conditioned groups in the D1R KOs (unpaired t test, P = 0.058) (Fig. 2L). However, a noticeable trend toward significance was observed between the home cage and fear-conditioned groups.
In both flx control and DG D1R KOs, the presentation of a footshock significantly increased the number of c-Fos+ neurons in CA3, compared with novel context exposure (flx: unpaired t test, P < 0.05; D1 KO: unpaired t test, P < 0.01) (Fig. 2M). Nevertheless, DG D1R deletion results in a significant decrease of c-Fos+ CA3 neurons in both the novel context exposure (unpaired t test, P < 0.05) and CFC groups (unpaired t test, P < 0.05), compared with control mice (Fig. 2M). Fear-conditioned DG D5R KOs did not exhibit any significant differences in c-Fos expression in the DG (unpaired t test, P = 0.406) or CA3 (unpaired t test, P = 0.568) compared with control mice (Fig. 2 O and P).
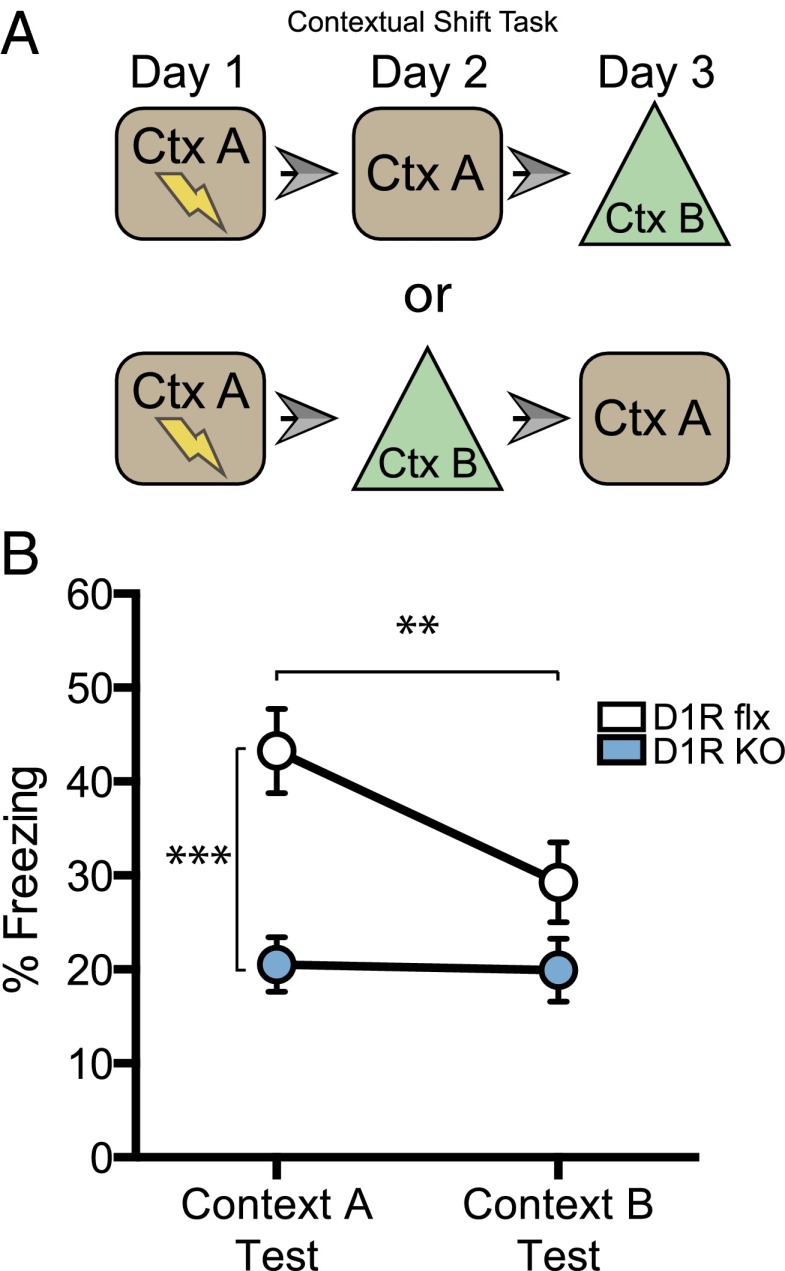
DG D1R deletion results in contextual generalization. Given the deficits in DG c-Fos expression in DG D1R KOs (Fig. 2L) and the observed CFC deficits in these mice (Fig. 2K), we hypothesized that the KOs have impaired encoding of contextual information. Thus, DG D1R KOs should confound contextual cues that accurately predict the delivery of a potential footshock. To test the role of DG D1R activation in using specific contextual cues as a predictor of shock, we trained mice in a contextual shift design task (4). Mice were trained on a CFC paradigm; 24 h after training, one group was placed back into the training context (context A) and a second group was placed into a unique but similar context (context B) (Fig. 3A). On the following day, mice that were first tested in context A were tested in context B, and mice first tested in context B were tested in context A. DG D1R KOs again showed an impairment in fear memory to context A compared with control mice (unpaired t test, P < 0.0005). In addition, control mice froze significantly more to context A than to context B, while DG D1R KOs froze equally to both contexts [two-way ANOVA (context × genotype), F(1, 35)interaction = 9.972, P < 0.0033] (Fig. 3B).
Fig. 3.
Contextual shift design task. (A) Schematic of training and testing schedule. (B) Contextual fear memory test in training context and nontraining context (flx = 20, KO = 17).
Discussion
DG D1Rs Contribute to the Formation of Novel Contextual Representations.
In this study we found that the overlapping expression of D1Rs and D5Rs is greatest in the DG of the hippocampus among all brain regions. The strong expression of DG D1Rs and D5Rs highlights the significance of the dopamine signal in hippocampal dependent memory processing. Additionally, we have shown that the loss of either both receptors or one of the two receptors in the DG results in an impairment of l-LTP at the mPP-DG synapse. Furthermore, we found that DG D1R deletion, but not D5R deletion, increases DG c-Fos expression in a familiar environment (i.e., home cage). However, D1R deletion decreases DG and CA3 c-Fos expression when the animal was exposed to a novel context or when they are fear conditioned in a novel context. Moreover, DG D1R KO deletion impairs contextual fear conditioning. The lack of enhanced c-Fos levels in DG D1R KOs to novel but not familiar contextual exposure supports the specific role of DG D1Rs in encoding novel contextual information. In addition, the observed enhancement of context generalization in the KO animals further supports the role of DG D1Rs in accurately encoding novel contextual information. It is likely that the freezing deficit observed in DG D1R KOs is due to this impairment in forming contextual representation.
Recent work, however, showed that DG-to-CA3 transmission is absent: There are no observed deficits in contextual fear conditioning (39). Our present results (Fig. 2K) seemingly go against these earlier findings. However, given that the DG D1R KOs exhibit a significant enhancement of baseline c-Fos activity, which does not significantly increase to novel context exposure or to fear conditioning (Fig. 2L), we propose that KO mice may transmit corrupt contextual information from the DG to CA3. Thus, CA3 is likely processing abnormal information from the DG resulting in disrupted encoding of novel contextual information leading to contextual fear memory deficits in DG D1R KO mice. Our reasoning is in line with hippocampal c-Fos activation and hippocampal lesion studies. First, hyperactivation of c-Fos expression in the DG results in impaired contextual fear memory (40). Second, hippocampal lesions result in the disruption of encoding contextual information and not the context–footshock association (1, 2, 41, 42). However, unlike the lesions studies, our DG D1R deletion does not simply lead to loss of function, but rather to a gain of impairment. That is, DG D1R deletion impairs the ability to build a distinct contextual representation of similar contexts because D1R deletion increases the transfer of inaccurate contextual information to CA3, which in turns results in CFC deficits.
Spatially Restricted KO Animals Clarify the Discrepancies Between Pharmacological and Global KO Studies.
Until now, experimental manipulations have been unable to adequately address the individual functions of D1Rs and D5Rs in hippocampal dependent memory. Pharmacological studies have provided evidence that both D1Rs and D5Rs or one of the two, are necessary for CFC, which has been at odds with D1R−/− and D5R−/− mutant studies that showed that neither D1Rs nor D5Rs were required for fear conditioning (13, 16, 17). There are several potential reasons for these discrepancies. First, the D1R/D5R antagonists used can alter other neuromodulatory receptor activity known to affect synaptic plasticity (43–47). Because there are functional differences between D1Rs and D5Rs, the pharmacological data may not accurately depict the necessity of D1R/D5Rs in hippocampal memory formation. Second, the lack of an observed fear conditioning phenotype in the D1R−/− and D5R−/− mice, may be due compensatory mechanisms (e.g., receptor up-regulation) of D1Rs and D5Rs in D5R−/− and D1R−/− mice, respectively. We have observed that D1R−/− mice generated in our laboratory lack motivation to seek food and water. These mice died soon after weaning unless food was directly and easily accessible, which is in agreement with previous studies reporting similar behavioral abnormalities (16, 48). Therefore, the global KO studies may not have provided data that is a true function of D1Rs or D5Rs in hippocampal dependent memory formation. Given these issues, it has been difficult to reconcile pharmacological and global KO studies, hindering the ability to accurately determine D1R from D5R function. Nonetheless, our current findings are in agreement with some of the pharmacological data, because we find that forebrain D1Rs or D5Rs, or both are required for fear memory and l-LTP at the mPP-DG synapse (Fig. 1 L and M). The genetic tools used in our current study have provided a unique advantage over the pharmacological studies by allowing us to investigate the precise role of each receptor subtype in the DG.
Experimental Procedures
Animal Sex.
All experimental animals in this manuscript are male.
Generation of the D1/D5 Receptor KO Mice.
To generate inbred C57BL/6 mouse lines with the dopamine D1R and D5R flanked by loxP sites, we made targeting constructs from BAC clones obtained from a mouse C57BL/6 genomic library (Fig. S1A). For both loci, the entire coding regions were flanked by two loxP sites, and a third loxP site was inserted with antibiotic resistance selection markers. The targeting constructs were introduced into C57BL/6 ES cells, and desired clones were selected by Southern blot analysis using external 3′ and 5′ probes. Two independent homologous recombination events for each construct were chosen for transfection with a plasmid carrying the Cre recombinase gene, and BALB/c blastocysts were injected with and clones in which the whole coding region was deleted, or clones in which the intact coding sequence was flanked by two loxP sites (i.e., floxed). Germ-line transmission was obtained when crossing chimaeras to B6 breeders. Crossing floxed mice to the CaMKII-Cre line (31), which expresses a transgene for cre recombinase in the adult forebrain, resulted in region and cell type specific deletion of the targeted genes. Mice were genotyped by PCR (Fig. S1B). Mice with a global deletion of both D1Rs and D5Rs did not survive past 2–4 wk of age. All experiments described in this paper were performed with flx D1R/D5R mice expressing the cre recombinase transgene, and their flx littermates as controls. The controls as well as their mutant littermates were viable, and showed no abnormalities in weight, general aspect, grooming, activity, breeding, and social behavior. All experiments with Forebrain D1R/D5R KOs and the control littermate’s occurred between the ages of 28 and 40 wk of age. All experiments with DG specific D1R or D5R KOs occurred between 16 and 24 wk of age.
ISH.
See ref. 34 for general ISH protocol.
D1R mRNA probe:
5′-ACAAAAGCACAATGGTGTTCCATCAGGAGCATCTCCATAGCAATCCAAG
CCATACCAGGAAGAGAGCCGCTTGCTTTCCACCTGTCTTCTGGGTTCAGTGCTCCAGGTCGCTGTTCCCTGGCATCCGCTGTCCCTAGATTCCCCAAGGAATCATAGGCTTTTAAGCATACTCTAAGAGTCTGGGGCCTCTTCCTGGTCAATCTCAGTCACTTTTGGGGATGCTGCCTCTTCTTCTGAGACACAGCCTAAAATACATGCATTTCTCCTTCAAGCCCCTGGTGCCACATCTCTCCAA ATGCC-3′
D5RmRNA probe:
5′-CCAAAATCCTGCTGTCTTCCAAGAGCACTGGCACTTGTGGTTTCTCTAGG
AGAAACACTGAGCACCAACTGGCAAAGCAAAGGTGACTGCCCCTCCTCCCAGCCACAAATGAATGTACTGTGCGCTTATGGAAACCACAACAAATCAGGGAGAAATCCCGGCCACAGGAAAGACCCTTCAACCTGCACTAAAGCAGCAGCCCGAGAACAGGGGGCTATGGTCCCAAAGTCTAGAAAGTCACAGACCATACCAGCAATTGCCACTCAGACCTGTCATTTAAAAAGCAACCCAGGTGCAAGTCACAGAACAAGCCTCTGTTAGAAAGGGTAAATTGAGGTGTACTTCTTAAAGGACCAGGT TCCACTTTCTCGTCTCTAAAGGGAACTCT-3′
Quantification of Receptor Deletion.
Quantification of hippocampal receptor deletion was measured from ISH images. The change of the 8-bit grayscale value gradient of these images was measured. Each region was quantified by the grayscale values described above using the software application ImageJ. The area of interest (e.g., the DG) was measured, and the mean grayscale value was calculated. The mean grayscale value depicts the concentration of the receptor. These values were normalized to the image background, within each image (32).
In Vivo Physiology.
Mice underwent mPP-DG l-LTP experiments and received isoflurane during the duration of recordings. A rectal thermometer was used to maintain the animal at 37 °C using a heating blanket. Two holes were made using a dental drill with the recording electrode placed into the hilus of the dentate gyrus (2 mm posterior from bregma and 1.5 mm lateral to the midline) and the stimulating electrode placed into the mPP (3 mm lateral from lambda) ipsilaterally to the recording electrode. Each electrode lowered to ∼1.5 mm from the brain surface. Recordings primarily occurred in the right hemisphere. However, when responses in the right hemisphere were not strong enough the recording and stimulating electrodes were placed in the left hemisphere. In each experiment, an input-output curve was assessed from 0 μA through 460 μA with 20-μA steps. Three recordings were taken at each step and averaged. A population spike of at least 5 mV was necessary for experimentation. l-LTP was induced by a theta burst protocol where six trains were given at 5 Hz, each train consisted of six pulses at 400 Hz, this was repeated six times with a 30-s interval.
c-Fos Experiments.
Mice were anesthetized with an overdose of avertin 60 min after contextual fear conditioning. The fully anesthetized animals were perfused transcardially with PBS, followed by 4% paraformaldehyde (PFA) in PBS. Brains were stored in fixative [4% (wt/vol) PFA in PBS] overnight at 4 °C, and then incubated overnight in 30% (wt/vol) sucrose. A cryostat was used to collect sagittal sections of 60-μm thickness. Sagittal sections were blocked with PBST (PBS with 0.3% Triton X-100) with 5% (vol/vol) normal goat serum for 1 h and then incubated with primary antibody at 4 °C for 24 h (rabbit anti-c-Fos 1:500, Santa Cruz; mouse anti-NeuN 1:1,000, Millipore). Slices then underwent three wash steps for 15 min each in PBST, followed by 3-h incubation with secondary antibody (1:200 AlexaFluor 488 anti-mouse, Invitrogen; 1:200 AlexFluor546 anti-rabbit, Invitrogen). Slices were then incubated for 30 min with NeuroTrace 640∕660 for Deep-Red Fluorescent Nissl Stain (Invitrogen), and underwent three more wash steps of 15 min each in PBS, followed by mounting and coverslipping on microscope slides. For quantification analysis of number of c-Fos–positive cells in DG and CA3 region in mice, sampling of c-Fos–positive cells was conducted throughout the dorsal DG. Ten sagittal hippocampal sections were used to count c-Fos–positive cells in DG and hippocampal CA3 region in a genotype- and treatment-blinded manner.
Behavioral Batteries.
Pain sensitivity.
A heating block with high walls was set to 50 °C. Mice were placed onto the heating block one at a time. The time from being set onto the heating block to the time the mouse rubs its paws was used as the index for pain sensitivity.
Open field activity.
Mice were handled for three consecutive days for 2 min per cage before the first day of open field test. Activity was measured by IR beam interruption and recorded in 1-min intervals over a 10-min period in a novel chamber (Digiscan apparatus, Accuscan Instruments). This was conducted for 3 consecutive days.
Rotarod.
Mice were placed on a rotating platform that increases in the rate of rotation over a 300-s window. The time from when the mice were placed onto the apparatus to the time they fell off was recorded.
Fear Conditioning.
All experiments were conducted using FreezeView software. Animals received either a single 0.5-mA or 0.75-mA unsignaled footshock at 118 s with a 2-s duration in a novel chamber. Mice remained in the chamber for 60 s after footshock. One, 3, or 24 h after CFC, mice were returned to same conditioning chambers where freezing was assessed for 5 min. In the contextual shift design task, mice were placed in either the training context 24 h after training or in a novel context (context B). Twenty-four hours after the first test, mice placed in the training context were then placed in context B and vise versa. In cue feared conditioning, mice received a single paired conditioned stimulus tone and unconditioned stimulus (30 s, 5 kHz, 75 dB, 0.75 mA, 2 s duration). Twenty-four hours after training, mice were placed into a similar context as compare with the training context and explored the context for 2 min, after which a tone presentation was given for 3 min.
Supplementary Material
Acknowledgments
We thank Y. Wang, W. Yu, C. Carr, N. Yechoor, J. Moyer, J. Zhou, J. Martin, and S. Cooke for experimental help; J. Z. Young, T. Ryan, R. Redondo, D. L. Buhl, J. Suh, A. Rivest, T. McHugh, and K. Mulroy for comments; and the members of S.T. laboratory for support. This work was supported by the RIKEN Brain Science Institute and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407395111/-/DCSupplemental.
References
- 1.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 3.Rescorla R, Wagner A. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. New York: Appletone-Century-Crofts; 1972. [Google Scholar]
- 4.Fanselow MS. Conditional and Unconditional Component of Post-Shock Freezing. Pak J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 5.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweifel LS, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14(5):620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Izumi T, Maki Y, Muraki I, Koyama T. Effect of the dopamine D(1/5) antagonist SCH 23390 on the acquisition of conditioned fear. Pharmacol Biochem Behav. 2000;66(3):573–578. doi: 10.1016/s0091-3057(00)00254-9. [DOI] [PubMed] [Google Scholar]
- 14.Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33(4):445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 15.Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32(18):6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Ghundi M, et al. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383(2):95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 17.Holmes A, et al. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115(5):1129–1144. [PubMed] [Google Scholar]
- 18.Sunahara RK, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, et al. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403(6767):274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- 20.Lee FJS, et al. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111(2):219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 21.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75(3):447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int J Dev Neurosci. 2000;18(7):669–677. doi: 10.1016/s0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 23.Fremeau RT, Jr, et al. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci USA. 1991;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiberi M, et al. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: Differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA. 1991;88(17):7491–7495. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laplante F, Sibley DR, Quirion R. Reduction in acetylcholine release in the hippocampus of dopamine D5 receptor-deficient mice. Neuropsychopharmacology. 2004;29(9):1620–1627. doi: 10.1038/sj.npp.1300467. [DOI] [PubMed] [Google Scholar]
- 26.Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31(11):4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangarossa G, et al. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012;22(12):2199–2207. doi: 10.1002/hipo.22044. [DOI] [PubMed] [Google Scholar]
- 28.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Lemberger T, et al. Expression of Cre recombinase in dopaminoceptive neurons. BMC Neurosci. 2007;8:4. doi: 10.1186/1471-2202-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y-Y, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87(7):1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 32.Lazic SE. Statistical evaluation of methods for quantifying gene expression by autoradiography in histological sections. BMC Neurosci. 2009;10:5. doi: 10.1186/1471-2202-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63(5):673–682. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, et al. c-fos regulates neuronal excitability and survival. Nat Genet. 2002;30(4):416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]
- 36.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 37.Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14(11):758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- 38.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 39.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 42.Lovett-Barron M, et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343(6173):857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarrindast MR, Honardar Z, Sanea F, Owji AA. SKF 38393 and SCH 23390 inhibit reuptake of serotonin by rat hypothalamic synaptosomes. Pharmacology. 2011;87(1-2):85–89. doi: 10.1159/000323232. [DOI] [PubMed] [Google Scholar]
- 44.Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: Rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22(9):3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hicks PE, Schoemaker H, Langer SZ. 5HT-receptor antagonist properties of SCH 23390 in vascular smooth muscle and brain. Eur J Pharmacol. 1984;105(3-4):339–342. doi: 10.1016/0014-2999(84)90628-9. [DOI] [PubMed] [Google Scholar]
- 46.Kojima T, et al. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anesthetized rats: An effect mediated via 5-HT1A receptors. Brain Res. 2003;959(1):165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- 47.Bischoff S, Heinrich M, Sonntag JM, Krauss J. The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol. 1986;129(3):367–370. doi: 10.1016/0014-2999(86)90449-8. [DOI] [PubMed] [Google Scholar]
- 48.Drago J, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91(26):12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.