Significance
Prostate cancer is the most common nonskin cancer in America and the fifth most common cancer worldwide. Inflammation is implicated in the initiation and progression of prostate cancer; however, sources of inflammation remain unidentified. Trichomonas vaginalis is a prevalent parasite that infects prostate epithelium and is associated with an increase in aggressive prostate cancer. Here, we demonstrate that a secreted T. vaginalis protein homologous to human macrophage migration inhibitory factor elicits antibodies in infected individuals, increases prostate cell proliferation and invasiveness, and induces cellular pathways linked to inflammation. This study demonstrates that a specific parasite-derived protein can mimic its human homolog to increase inflammation and cell proliferation, which, in turn, may result in the promotion and progression of prostate cancer.
Abstract
The human-infective parasite Trichomonas vaginalis causes the most prevalent nonviral sexually transmitted infection worldwide. Infections in men may result in colonization of the prostate and are correlated with increased risk of aggressive prostate cancer. We have found that T. vaginalis secretes a protein, T. vaginalis macrophage migration inhibitory factor (TvMIF), that is 47% similar to human macrophage migration inhibitory factor (HuMIF), a proinflammatory cytokine. Because HuMIF is reported to be elevated in prostate cancer and inflammation plays an important role in the initiation and progression of cancers, we have explored a role for TvMIF in prostate cancer. Here, we show that TvMIF has tautomerase activity, inhibits macrophage migration, and is proinflammatory. We also demonstrate that TvMIF binds the human CD74 MIF receptor with high affinity, comparable to that of HuMIF, which triggers activation of ERK, Akt, and Bcl-2–associated death promoter phosphorylation at a physiologically relevant concentration (1 ng/mL, 80 pM). TvMIF increases the in vitro growth and invasion through Matrigel of benign and prostate cancer cells. Sera from patients infected with T. vaginalis are reactive to TvMIF, especially in males. The presence of anti-TvMIF antibodies indicates that TvMIF is released by the parasite and elicits host immune responses during infection. Together, these data indicate that chronic T. vaginalis infections may result in TvMIF-driven inflammation and cell proliferation, thus triggering pathways that contribute to the promotion and progression of prostate cancer.
Prostate cancer is the most common noncutaneous cancer of men in the United States, affecting one in six men (1). Although the causes of prostate cancer are poorly understood, inflammation has been implicated in both initiation and progression of the disease (2, 3). The origin of inflammation in prostate cancer is unclear, although chronic infections are believed to promote and establish a tumor-enhancing proinflammatory environment.
Trichomonas vaginalis is the causative agent of the most common nonviral sexually transmitted infection, infecting ∼275 million people worldwide (4). T. vaginalis is a flagellated, protozoan parasite that infects the prostate epithelium (5, 6). Over 75% of men harboring T. vaginalis are asymptomatic and may not seek treatment, resulting in chronic inflammation (5). Several studies have positively associated T. vaginalis infection with increased incidence and severity of prostate cancer, as well as benign prostate hyperplasia (2, 6–9). The magnitude of the association between T. vaginalis seropositivity and overall prostate cancer risk is between 1.23 and 1.43 based on two large, nested case–control studies (7, 8). Additionally there is a statistically significant increase in risk of extraprostatic cancer [odds ratio (OR) = 2.17] or cancer-specific death (OR = 2.69) with T. vaginalis seropositive status (7).
Our research focuses on the potential contribution of a proinflammatory protein, T. vaginalis macrophage migration inhibitory factor (TvMIF), to prostate cancer, because the human homolog has a role in the growth and invasion of prostate cancer (10, 11). Human macrophage migration inhibitory factor (HuMIF) has been implicated in a broad array of conditions associated with inflammation, including autoimmunity, cell proliferation, angiogenesis, and tumorigenesis (12–15). Increased expression of HuMIF has been reported in several cancers, including prostate cancer (16–18). Studies show high expressers of HuMIF have a heightened risk of prostate cancer, as well as a significant increase in prostate cancer progression and drug resistance (17–23). Several clinical studies have shown HuMIF production correlates with both tumor aggressiveness and metastatic potential (23, 24). Additionally, the expression of the HuMIF receptor CD74 is increased in prostate cancer (10, 25).
HuMIF induces ERK1/2, MAPK, and Akt activation via binding with the extracellular domain of the MIF receptor CD74 (26). HuMIF has multiple functions with regard to regulating the immune system, including protecting monocytes and macrophages from activation-induced apoptosis, which results in sustained inflammation (27, 28). Consequently, HuMIF is implicated in the pathogenesis of several inflammatory and autoimmune diseases in addition to cancer (29, 30).
Recent studies have shown that several parasitic eukaryotes encode MIF-like proteins with considerable structural and biological similarity to their mammalian hosts (31–34). These parasite MIF proteins have been shown to modulate host immune responses and regulate pathways to promote parasite survival. Here, we characterize TvMIF and show that it can act as a molecular mimic of HuMIF. We find that TvMIF binding to the human CD74 receptor activates extracellular signal-regulated kinases (ERK)1/2 and Akt protein kinase/proapoptotic Bcl-2–associated death promoter (BAD) pathways as well as secretion of proinflammatory IL-8 from monocytes, reduces monocyte migration, and increases growth and invasiveness of benign prostate hyperplasia (BPH-1) and prostate cancer (PC3) cells. This research is, to our knowledge, the first to identify a human inflammatory cytokine mimic in T. vaginalis and to begin to explore the link between this sexually transmitted infection and prostate cancer.
Results
Characterization of TvMIF.
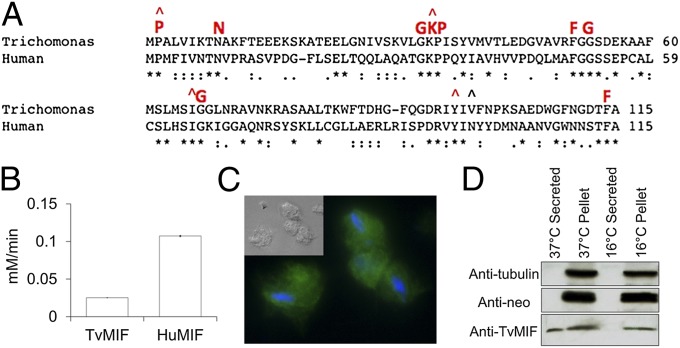
MIF is an evolutionarily conserved protein with a unique blend of hormone-like, cytokine-like, and enzymatic activities. Due to MIF’s involvement in inflammation and cancer, we hypothesized that if T. vaginalis secreted a protein of similar function during a chronic prostate infection, the actions of this protein could lead to increased risk of prostate cancer. The T. vaginalis genome (www.trichdb.org) encodes a single MIF homolog (Tvag_219770). This protein, TvMIF, has 31% identity to HuMIF and contains all nine invariant residues (33) found in other eukaryotic MIFs (marked in red in Fig. 1A). TvMIF was expressed as a histidine-tagged fusion protein and purified for in vitro studies. One characteristic of MIF is tautomerase activity (35, 36), and TvMIF possesses four of the five conserved tautomerase catalytic residues (Fig. 1A), so we tested whether TvMIF can tautomerize the substrate l-dopachrome methyl ester. As shown in Fig. 1B, TvMIF is able to do so, albeit at a rate fivefold less than HuMIF. Catalytically inactive HuMIF is greatly impaired in its ability to stimulate generation of superoxide in activated neutrophils (37); thus, the reduced tautomerase activity of TvMIF could decrease the molecule’s priming of host neutrophils. Enzymatic tautomerase activity is not necessary for biological functions, such as macrophage migration, countering the effects of glucocorticoids, or growth regulatory and invasion-promoting properties (38–40). Thus, our further analyses have focused on characterizing possible biological functions of TvMIF that could contribute to prostate cancer.
Fig. 1.
(A) Multiple Sequence Comparison by Log-Expectation (MUSCLE) alignment of TvMIF and HuMIF sequences with nine invariant residues (39) shown in red. Carats (^) indicate five residues comprising the tautomerase active site. (B) TvMIF tautomerase activity was measured using 10 μg/mL recombinant TvMIF or HuMIF; assays were carried out in triplicate, and data shown are mean ± SE. (C) Immunofluorescence image and phase (Inset) using anti-HA antibody (green) and DAPI stain (blue) on parasites expressing TvMIF-HA. (Magnification: 100×.) (D) Immunoblot analyses of supernatant and pellet fractions of T. vaginalis transfectants expressing TvMIF-HA incubated at 37 °C or 16 °C (secretion negative control). Anti-HA antibodies detect MIF. Anti–β-tubulin (Anti-tubulin) and anti-neomycin (Anti-neo) phosphotransferase antibodies serve as negative controls for cell lysis.
To examine TvMIF localization in vivo, we transfected T. vaginalis with a plasmid expressing TvMIF fused to an HA tag. Immunofluorescence assays revealed a cytoplasmic localization (Fig. 1C). Because HuMIF is known to be secreted, we tested whether TvMIF is secreted and found it present in secreted fractions (Fig. 1D). Together, these data indicate that T. vaginalis produces and secretes TvMIF into its extracellular environment in vitro.
Evidence of TvMIF Secretion in Vivo.
To examine whether TvMIF is released during T. vaginalis infection in humans, we evaluated 190 sera samples (111 anti–T. vaginalis-positive and 79 T. vaginalis-seronegative) for anti-TvMIF (Table 1). Sera were more frequently reactive to TvMIF in T. vaginalis-positive (56.8%) than in T. vaginalis-negative (11.4%) individuals (χ2 = 40.35, P < 0.0001, OR = 8.62). These data strongly suggest that TvMIF is expressed and secreted in vivo during T. vaginalis infection. False-negative, cross-reactive, or heterophilic antibodies or other unspecific antigen binding proteins may be responsible for reactivity in T. vaginalis-negative sera samples. Also, some T. vaginalis-seronegative patients may have had a previous T. vaginalis infection that was cleared, with detectable levels of circulating anti-TvMIF antibodies persisting in their sera. Remarkably, a higher proportion of male patients (78.7%) compared with female patients (30.0%) tested positive for anti-TvMIF antibodies (χ2 = 21.50, P < 0.0001, OR = 9.03). These data suggest either more TvMIF is released by parasites in a male host or the immune system of infected males responds more strongly to the TvMIF protein.
Table 1.
TvMIF is detected by ELISA in patients infected with T. vaginalis
| Sera sample | T. vaginalis-positive | T. vaginalis-negative |
| Male, α-TvMIF–positive | 48 (78.69%) | 6 (17.65%) |
| Male, α-TvMIF–negative | 13 (21.31%) | 28 (82.35%) |
| Female, α-TvMIF–positive | 15 (30.00%) | 3 (6.66%) |
| Female, α-TvMIF–negative | 35 (70.00%) | 42 (93.33%) |
Number and percentage of sera samples that tested positive or negative for T. vaginalis infection and anti-TvMIF antibodies from 190 patients sorted by gender.
TvMIF Inhibits Monocyte Migration and Induces IL-8 Secretion.
A standard biological activity of HuMIF is the ability to inhibit random migration of macrophages or monocytes (38, 41). To determine whether TvMIF exerts this activity, we examined its effect on monocyte migration using a Boyden chamber assay. We found that TvMIF inhibits ∼50% of monocyte migration, comparable to that observed using HuMIF (Table 2).
Table 2.
TvMIF inhibits migration of human monocytes
| Concentration | TvMIF | HuMIF |
| 10 ng/mL | 54 ± 17.5* | 42.9 ± 16.9** |
Percentage of inhibition of monocyte migration using 10 ng/mL TvMIF or HuMIF. Values are means of three independent experiments ± SEM. *P < 0.01; **P < 0.02. No inhibition of migration was measured using PBS (vehicle control).
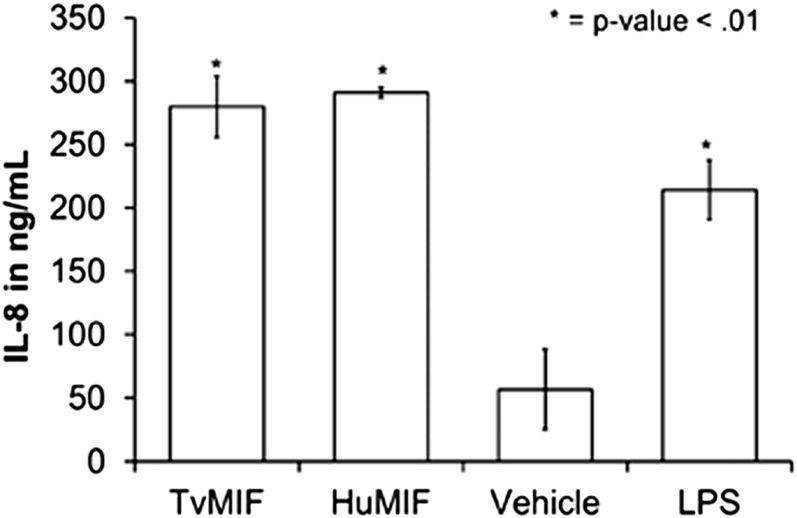
Mammalian MIF is proinflammatory and plays a role in various autoimmune and inflammatory diseases and cancers (15), so we examined whether TvMIF could induce secretion of the proinflammatory cytokine IL-8. Using human monocytes from three different donors, we show TvMIF is able to elicit approximately the same amount of IL-8 secretion from monocytes as HuMIF (Fig. 2). This result indicates TvMIF is capable of inducing secretion of proinflammatory cytokines from human monocytes, which may contribute to the inflammatory environment resulting from T. vaginalis infection. These data indicate that TvMIF can functionally mimic HuMIF in its interaction with monocytes.
Fig. 2.
TvMIF (1 ng/mL) or HuMIF (1 ng/mL) induces IL-8 production from human monocytes. Data shown are representative; the experiment was repeated using three different donors, and the same trend in IL-8 production was consistently observed. Data are the mean of quadruplicates per one assay ± SEM.
TvMIF Binds Human CD74 with an Affinity Similar to HuMIF.
Because HuMIF is a cytokine and mediator of inflammation and carcinogenesis, particularly in its extracellular form (42), we examined whether TvMIF could bind the HuMIF receptor CD74. Because CD74 is a membrane protein, a soluble, extracellular portion called sCD74114–232 (27) was used to determine the interaction between CD74 and TvMIF. An overlay of heteronuclear single quantum coherence spectra of free 15N-labeled sCD74114–232 (black) and bound 15N-labeled sCD74114–232 in the presence of TvMIF (red) is shown in Fig. 3A. Missing peaks that do not overlap between the black and red spectra are a result of peaks broadened beyond detection and indicate that sCD74114–232 interacts with TvMIF.
Fig. 3.
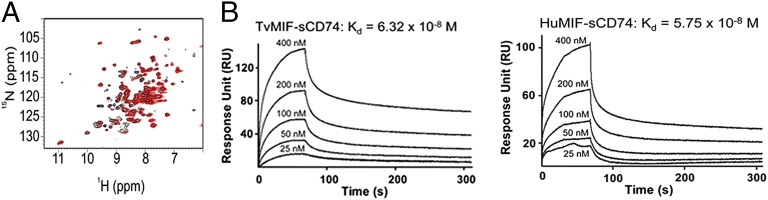
TvMIF interacts with the HuMIF receptor CD74. (A) NMR spectra of TvMIF and sCD74114–232 show incomplete overlap of red and black peaks, indicating that TvMIF and CD74 interact. (B) Surface plasmon resonance plots of various concentrations of TvMIF or HuMIF flowed over immobilized sCD74114–232 reveal that TvMIF and HuMIF have similar Kd values for the human receptor CD74.
To quantify the interaction and compare TvMIF and HuMIF binding, we examined real-time binding of sCD74114–232 to TvMIF and HuMIF using surface plasmon resonance. The analysis of sCD74114–232–TvMIF and sCD74114–232–HuMIF interaction revealed equilibrium dissociation constants (Kds) of 6.32 × 10−8 M and 5.75 × 10−8 M, respectively, indicating similar affinities for HuMIF and TvMIF binding of CD74 (Fig. 3B). These data indicate that TvMIF has the capacity to mimic HuMIF binding to the CD74 receptor.
TvMIF Induces Phosphorylation of ERK1/2, Akt, and BAD in BPH-1 Cells.
The binding of HuMIF to CD74 is known to trigger downstream pathways via phosphorylation of ERK1/2 and Akt. Given the ability of TvMIF to bind with high affinity to CD74 (Fig. 3B), we investigated the effect of TvMIF on these pathways. Extracellular MIF is thought to play a role in tumor growth by activating signaling cascades, such as p44/42 MAPK (ERK1/2) (26). These cascades, in turn, promote cell proliferation and activation of downstream effector proteins involved in the inflammatory response, such as secretion of IL-8 as previously shown (Fig. 2). HuMIF binding of CD74 is also known to activate Akt (43), which can then result in BAD phosphorylation leading to activation of antiapoptotic pathways (44).
To assess the ability of TvMIF to trigger signaling cascades, we used the BPH-1 cell line, which expresses the HuMIF receptor CD74 (Fig. S1). Immunoblot analyses of BPH-1 cells treated with 1 ng/mL (80 pM) TvMIF or HuMIF show that the amount of phosphorylated Akt and ERK1/2 is significantly increased by TvMIF and HuMIF over the vehicle control within 15 min (Fig. 4A and Fig. S2). Because MIF is implicated in cancer progression, we also examined whether TvMIF activates ERK phosphorylation in the PC3 cell line. Immunoblot analyses of PC3 cells treated with TvMIF or HuMIF indicate that there is a significant increase in ERK1/2 phosphorylation over vehicle control within 1 h (Fig. 4B and Fig. S2). We were unable to test for an increase in Akt phosphorylation in PC3 cells because the absence of phosphatase and tensin homolog (PTEN) results in very high basal levels of Akt and phosphorylated Akt. These data demonstrate that in addition to interacting with host MIF receptors (Fig. 3), TvMIF can activate host signaling pathways in vitro.
Fig. 4.
TvMIF activates ERK1/2 and Akt/BAD pathways in BPH-1 and PC3 cells, resulting in increased cell proliferation and invasion. (A) Serum-starved BPH-1 cells were exposed to 80 pM endotoxin-free rTvMIF or rHuMIF and tested by immunoblot analysis for phosphorylated-ERK Thr202/Tyr204 (p-ERK1/2), total ERK1/2 (ERK1/2), phosphorylated Akt (p-Akt), total Akt (Akt), BAD phosphorylated at ser136 (p-BAD), total BAD (BAD), and a β-tubulin (Tubulin) loading control, using the corresponding antibodies. (B) Serum-starved PC3 cells were exposed to 80 pM endotoxin-free TvMIF (Tv-4h) or HuMIF (Hu-4h) for 4 h and probed for p-ERK1/2, ERK1/2, and a β-tubulin loading control. The time course and quantification of BPH-1 and PC3 data are provided in Fig. S2. Negative (Neg) = 1× PBS vehicle control, and positive (Pos) = 5% FBS. (C) BPH-1 or PC3 cells were plated and dosed with 80 pM LPS-free rTvMIF or rHuMIF every 24 h for 72 h. Cell numbers were then assessed colorimetrically. The addition of either 1 ng/mL TvMIF or HuMIF increases proliferation. Mean fold increase in proliferation (∼1.2-fold for BPH-1 cells and ∼1.4-fold for PC3 cells) over untreated cells from three independent experiments done in quadruplicate, with ±SEM, is shown. (D) BPH-1 or PC3 cells preincubated with 80 pM LPS-free rTvMIF or rHuMIF for 24 h were added to BD Fluorblok Tumor Invasion Plates in the presence of 80 pM TvMIF or HuMIF for 48 h. Fold increase in invasion over vehicle control-treated cells ± SEM of three independent experiments in triplicate is shown. *P < 0.05.
The activation of Akt is known to provide a vital antiapoptotic signal via phosphorylation of BAD (45). Thus, we probed for BAD phosphorylation in BPH-1 cells in the presence of 80 pM TvMIF or HuMIF and its increase over time (Fig. 4A and Fig. S2). As encountered when probing for phosphorylation of Akt in PC3 cells, we were unable to detect significant phosphorylation of BAD over background levels due to very high basal levels in PTEN-deficient cells (46). Together, our data show that TvMIF binding to the human receptor CD74 functionally mimics HuMIF binding by triggering several downstream pathways involved in increasing cell proliferation and inflammation.
TvMIF Increases Growth of BPH-1 and PC3 Cells.
The binding of TvMIF to CD74, and subsequent phosphorylation of ERK1/2, Akt, and BAD, triggers downstream pathways involved in cell proliferation and inflammation. Thus, we examined whether TvMIF alters cell proliferation. Exposing the BPH-1 or PC3 cell line to 1 ng/mL (80 pM) TvMIF or HuMIF, we saw a 20% or 40% increase in growth, respectively, after 72 h of exposure (Fig. 4C). The increase in cell proliferation is similar whether using TvMIF or HuMIF, again indicating that TvMIF may be as potent as HuMIF in its effects on human cells. These data indicate that TvMIF released into the extracellular milieu during infection can increase growth of both benign and cancerous prostate cells, and hence contribute to progression of prostate cancer.
TvMIF Increases Invasiveness of BPH-1 and PC3 Cells.
Another characteristic of cancerous cells is increased invasive ability. Studies have shown that inhibition of HuMIF or its receptor can attenuate invasion of cancer cells (11). Thus, we investigated whether adding exogenous TvMIF would affect the invasiveness of BPH-1 or PC3 cells. Using a tumor invasion assay, we examined cell migration through a Matrigel-coated membrane. We found that 1 ng/mL TvMIF or HuMIF increases the invasiveness of both BPH-1 and PC3 cells ∼30% (Fig. 4D) compared with vehicle control. These data provide evidence that TvMIF may contribute to tumor progression and metastatic potential of prostate cells.
Discussion
MIF was discovered in 1966 as a soluble mediator that inhibited the random migration of monocytes (41, 47). Since then, HuMIF has been shown to be a versatile proinflammatory cytokine involved in an array of processes, including immunity, cell proliferation, and tumorigenesis (12, 15, 48). Here, we have characterized the MIF homolog found in the highly divergent, human-infective parasite T. vaginalis.
We have shown that TvMIF exhibits tautomerase activity, the ability to inhibit macrophage migration, and is proinflammatory. We have also demonstrated that TvMIF binds the human CD74 receptor with high affinity and is as potent as HuMIF in activating ERK in both BPH-1 and PC3 cell lines, as well as Akt/BAD phosphorylation in BPH-1 cells. Moreover, TvMIF was found to increase the growth and invasiveness of both BPH-1 and PC3 cells. Finally, we show that TvMIF is expressed in vivo, that it elicits a robust immune response in infected individuals, and that sera from infected men are significantly more reactive to TvMIF than sera from infected women.
The conservation in biological activity between TvMIF and HuMIF is notable considering the phylogenetic distance between T. vaginalis and its human host (49). This conservation could result from parasite/host coevolution and represent a form of molecular mimicry (50), or it could indicate that MIF has an unknown but crucial basic function that has been conserved in eukaryotes. Our data indicate that the parasite secretes TvMIF, which mimics HuMIF to bind to the human CD74 receptor, which, in turn, leads to increased cellular proliferation and invasiveness.
In fact, TvMIF interacts with the human CD74 with approximately the same affinity as HuMIF, which would allow it to compete or perhaps potentially cooperate with HuMIF. As prostate cancer progresses, levels of HuMIF and CD74 increase (10), resulting in a positive feedback loop that leads to inflammation and activation of cell proliferation and survival pathways (51–53). TvMIF could add fuel to this fire by stimulating CD74, because we show that TvMIF binding to CD74 can activate downstream ERK and Akt/BAD pathways. The effects exerted by TvMIF may likewise have an impact on host defenses and result in overall local immune dysregulation because effective host immunity depends, in part, on the appropriate balance of cytokines.
Indeed, we show that at 1 ng/mL, TvMIF can induce IL-8 secretion from monocytes. We also found that higher concentrations of either TvMIF or HuMIF could stimulate IL-6 secretion (Fig. S3). This result is consistent with reports that HuMIF potentiates secretion of IL-6 but does not induce secretion independently (54). It is possible that in cases of coinfection, other diseases, or chronic inflammation, TvMIF could synergize with other signals to release a broader array of inflammatory cytokines.
The presence of antibodies against TvMIF in human sera demonstrates that TvMIF is expressed during infection. Secretion of TvMIF during infection would allow it access to host receptors and other host cells in the vicinity of infection. Interestingly, sera from males infected with T. vaginalis were more reactive to anti-TvMIF antibodies than female-infected patient sera. This finding is particularly notable because women often have a more active immune system (55) and typically have greater risk for contraction and a heavier sexually transmitted infection burden due to their greater mucosal surface area (56). One hypothesis for this difference is that TvMIF is either produced or secreted by parasites to a greater extent in male patients due to potential involvement of TvMIF in parasite survival in the harsh environment of the penile urethra or prostate. Alternatively, TvMIF may be expressed in the context of inflammation more frequently in male patients, leading to the formation of an adaptive immune response to TvMIF that would otherwise be an inert antigen due to its resemblance to HuMIF.
The ability of TvMIF to increase proliferation and invasiveness of BPH-1 and PC3 cells in vitro further supports a role for TvMIF in human prostate cancer. Moreover, TvMIF-induced IL-8 could contribute to angiogenesis (57, 58), thus exacerbating the cycle of inflammation, carcinogenesis, and metastasis. A link between T. vaginalis infection, inflammation, and cancer is reinforced by clinical evidence correlating T. vaginalis infection and increased risk of both benign prostate hyperplasia and prostate cancer (6, 7, 9). The development of a chronic infection animal model for T. vaginalis or future studies of prostate tissues from T. vaginalis-infected patients will be important to validate our in vitro data and to assess further whether T. vaginalis is responsible for causing a subset of human prostate cancers. It is notable that other chronically infective pathogens, such as Helicobacter pylori and Schistosoma hematobium, and viruses, such as hepatitis B virus or EBV, are associated with increased cancer risk and even considered causative (59–61). Approximately 17% of the global burden of cancer is related to chronic infections (61) as chronic inflammation may increase cancer susceptibility. In fact, the Hanahan cycle of cancer (62) defines inflammation and uncontrolled cell growth, both activities promoted by TvMIF, as hallmarks of cancer.
Taken together, these analyses indicate that through a combination of chronic inflammation and secreted bioactive molecules, such as TvMIF, T. vaginalis infection can have an impact on host immune system regulation and play a role in increased risk of human prostate cancer. These studies underscore the importance of understanding the impact of parasite-secreted molecules on human infection and the need to investigate subclinical or “asymptomatic” aspects of parasite biology. More studies on T. vaginalis and cancer, along with parasite mimics of human cytokines, will aid in understanding the extent to which this chronically infective parasite affects its hosts.
Materials and Methods
T. vaginalis Cell Culture.
T. vaginalis strain G3 was cultured as previously described (63). Tvag_219770 (TvMIF) was cloned into the Master-Neo-(HA)2 plasmid (64) and transfected as previously described (65), and it was maintained with 100 μg/mL G418 (Invitrogen). Parasites were grown at 37 °C and passaged daily for ≤2 wk.
Secretion Assay.
A secretion assay was performed as described (66). Western blot analysis was performed using anti-HA (1:5,000; Covance) to detect TvMIF, anti–β-tubulin (1:1,000; Sigma), and anti-neomycin phosphotransferase II (1:2,500; Jackson Labs). Secondary antibodies were anti-mouse–HRP (1:25,000) and anti-rabbit–HRP (1:25,000).
Human Cell Culture.
BPH-1 and PC3 cells were grown as described (67, 68). Monocytes were obtained from the University of California, Los Angeles (UCLA) Virology Core in Iscove’s modified Dulbecco’s medium (Invitrogen) + 5% (vol/vol) human antibody serum + 15% (vol/vol) FBS and used the same day for experiments.
Production of Recombinant Macrophage Migration Inhibitory Factor.
TvMIF or HuMIF was cloned into the pET200 expression vector with a C-terminal His-tag and transformed into Escherichia coli BL21 cells (Invitrogen). Protein was purified using His FastFlow Columns (GE Healthcare). For cell culture assays, protein was dialyzed into PBS and cleaned using Detoxi-Gel Endotoxin Removing Columns (Thermo Scientific). Protein was checked for LPS using a ToxinSensor Chromogenic LAL Endotoxin Assay Kit (Genscript) and found to have <0.005 EU/mL LPS.
The pET28a plasmid containing sCD74114–232 gene was kindly provided by the laboratory of R. Bucala (Yale University, New Haven, CT). The sCD74 protein was expressed in BL21 E. coli. The 15N-labeled sCD74114–232 was produced by growing bacteria in minimal medium consisting of 1 mM magnesium sulfate, 0.1 mM calcium chloride, 0.5 μg/mL thiamine, 100 μg/mL ampicillin, 1 g/L 15NH4Cl as the main nitrogen source (CIL), and 5 g/L glucose as the main carbon source. The protein was purified using nitrilotriacetic acid affinity chromatography to greater than 90% purity as previously described (27).
Tautomerase Assay.
TvMIF or HuMIF dopachrome tautomerase activity was measured as previously described (33).
Macrophage Migration Assay.
CD14+ monocytes were isolated by magnetic cell sorting using MACS anti-CD14–labeled magnetic microbeads (Miltenyi Biotec Italia) following peripheral blood mononuclear cell purification from buffy coats of healthy donors by Ficoll gradient centrifugation in Histopaque 1077 (Sigma–Aldrich).
Monocyte migration assays were performed using a Chemicon QCM chemotaxis 5-μm, 24-well cell migration assay kit (Millipore), according to the manufacturer’s directions. The migration rate was quantified by reading fluorescence emission at 480/520 nm using a GENios microplate reader (Tecan).
NMR Data Collection and Processing.
NMR data were collected at 40 °C on a Varian 600-Mhz NMR spectrometer. All titration experiments were performed using 200 μM H-15N–labeled sCD74114–232 and a series of concentrations (50 μM, 100 μM, 200 μM, and 400 μM) of protonated TvMIF in 20 mM NaPO4 and 1 mM EDTA (pH 7.4). At each titration point, a heteronuclear single quantum coherence spectrum was collected at 40 °C.
Real-Time Binding Studies.
Surface plasmon resonance (GE Healthcare BIA T100) was used to measure the real-time binding of sCD74114–232 to TvMIF and HuMIF. sCD74114–232 was immobilized onto CM5 sensor chips (GE Healthcare) by amine coupling following the manufacturer’s instructions. sCD74114–232 binding was measured for 60 s at 25 °C, followed by 260 s of dissociation. BIAevaluation was used to determine the equilibrium affinity constant. Three independent experiments were performed.
Cytokine Detection.
Primary human monocytes from UCLA’s Virology Core were incubated in serum-free AimV (Invitrogen) for 2 h before exposure to 1 ng/mL endotoxin-free TvMIF or HuMIF, LPS (Sigma), or Dulbecco’s PBS (vehicle control; Invitrogen). Supernatants were collected and assayed for IL-8 using an ELISA kit (AssayPro) according to the manufacturer’s instructions. Additional cytokines were examined using a BD Biosciences Cytokine Bead Array according to the manufacturer’s instructions.
Growth Assays.
Five thousand BPH-1 or PC3 cells per well were plated in quadruplicate in a 96-well plate and treated with 1 ng/mL TvMIF, HuMIF, or Dulbecco’s PBS as a vehicle control. The media were changed daily, and 24-h time points were quantified using a Promega CytotoxOne Membrane Integrity Assay kit. Results were quantified as the fold increase over vehicle control.
Invasion Assays.
Invasion assays were performed according to the manufacturer’s instructions (BD Biosciences). BPH-1 or PC3 cells were preincubated with 1 ng/mL TvMIF or HuMIF for 24 h. Cells were trypsinized, and 1 × 105 cells were added to the apical chamber of each well of a BD Fluoroblok Tumor Invasion System with 1 ng/mL TvMIF or HuMIF. Cells that migrated to the lower chamber were labeled after 48 h with 5 μM Calcein AM (BD Biosciences). Invasiveness was quantified by measuring the fluorescence of migrated TvMIF- or HuMIF-treated cells relative to samples treated with vehicle (PBS) control.
MIF Activation of ERK1/2, Akt, and BAD.
BPH-1 and PC3 cells were serum-starved for at least 24 h before experiments. TvMIF (1 ng/mL) or HuMIF (1 ng/mL) was added, and at various time points, cells were washed before the addition of ice-cold radioimmunoprecipitation assay buffer + 1X HALT phosphatase inhibitor (Thermo Scientific) + 1X HALT protease inhibitor (Thermo Scientific). Cell lysates were collected by scraping and spun to remove debris. Protein content was quantified using the BCA Protein Assay Kit (Pierce). Western blots were blocked with 3% (wt/vol) BSA⋅Tris-buffered saline and Tween 20 and incubated with rabbit anti-ERK1/2, anti–phospho-ERK1/2, anti-Akt, or anti–phospho-Akt antibodies (all at 1:1,000; all from Cell Signaling) and anti–β-tubulin (1:5,000; AbCam) as primary antibodies. Anti-rabbit–HRP secondary antibody (1:25,000; Jackson Laboratories) was used before addition of ECL for measurement of antibody binding by chemiluminescence. When needed, membranes were stripped with 200 mM NaOH and 0.1% TX-100, and probed for total ERK1/2, Akt, or β-tubulin. Quantification performed with ImageJ (National Institutes of Health; data shown in Fig. S2) is the mean fold increase relative to negative control (loading normalized with tubulin) from three independent experiments with two exposures quantified per experiment.
Assaying Immunogenicity of TvMIF.
Sera from 190 patients (95 male and 76 female) previously tested for the presence of anti-T. vaginalis antibodies (111 positive results and 79 negative results) (69) by indirect ELISA were used to evaluate TvMIF immunogenicity during human infection. The collection of human sera samples was approved by the Bioethics Committee of University of Sassari (reference no. 803/CE). Informed consent was obtained from all donors and the samples were entered into a database and processed anonymously.
Briefly, TvMIF was used to coat 96-well microtiter plates (0.5 μg per well; Nunc). Sera were diluted 1:200 and tested for TvMIF reactivity by incubating plates with polyclonal anti-human antibodies. Three newborn serum samples were used as negative controls. Cutoff values were established using the mean value of control sera + 3 SDs. A serum sample with an ELISA reading above the cutoff value was classified as positive.
Supplementary Material
Acknowledgments
We thank our colleagues for helpful discussions, Dr. Gil Lustig and Simona Santona for technical assistance, and Dr. Brian Janssen and Angelica Riestra for comments on the manuscript. This work was supported by National Institutes of Health Grants AI03182 and AI105779 (to P.J.J.). O.T. was supported by Microbial Pathogenesis Training Grant T32-AI07323, a Warsaw Fellowship, a Graduate Division Dissertation Year Fellowship, and Medical Scientist Training Program Grant T32GM008042. D.D. was supported by Fondazione Banco di Sardegna Grant 2012-1089. P.L.F. and A.R.C. were supported by Legge Regionale 7/2007 Regione Autonoma della Sardegna Grant CRP 25578.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321884111/-/DCSupplemental.
References
- 1.Rigamonti N, Bellone M. Prostate cancer, tumor immunity and a renewed sense of optimism in immunotherapy. Cancer Immunol Immunother. 2012;61(4):453–468. doi: 10.1007/s00262-012-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutcliffe S, Platz EA. Inflammation and prostate cancer: A focus on infections. Curr Urol Rep. 2008;9(3):243–249. doi: 10.1007/s11934-008-0042-z. [DOI] [PubMed] [Google Scholar]
- 3.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (2012) Global incidence and prevalence of selected curable sexually transmitted infections – 2008 (WHO, Geneva)
- 5.Gardner WA, Jr, Culberson DE, Bennett BD. Trichomonas vaginalis in the prostate gland. Arch Pathol Lab Med. 1986;110(5):430–432. [PubMed] [Google Scholar]
- 6.Mitteregger D, et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Med Microbiol Immunol (Berl) 2012;201(1):113–116. doi: 10.1007/s00430-011-0205-2. [DOI] [PubMed] [Google Scholar]
- 7.Stark JR, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst. 2009;101(20):1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutcliffe S, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(5):939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- 9.Taylor ML, Mainous AG, 3rd, Wells BJ. Prostate cancer and sexually transmitted diseases: A meta-analysis. Fam Med. 2005;37(7):506–512. [PubMed] [Google Scholar]
- 10.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177(12):8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 11.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)—The potential missing link. QJM. 2010;103(11):831–836. doi: 10.1093/qjmed/hcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucala R, Donnelly SC. Macrophage migration inhibitory factor: A probable link between inflammation and cancer. Immunity. 2007;26(3):281–285. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Bernhagen J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 15.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): Mechanisms of action and role in disease. Microbes Infect. 2002;4(4):449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 16.del Vecchio MT, et al. Macrophage migration inhibitory factor in prostatic adenocarcinoma: Correlation with tumor grading and combination endocrine treatment-related changes. Prostate. 2000;45(1):51–57. doi: 10.1002/1097-0045(20000915)45:1<51::aid-pros6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Hussain F, et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol Cancer Ther. 2013;12(7):1223–1234. doi: 10.1158/1535-7163.MCT-12-0988. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Siegler KL, Iczkowski KA, Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer. 2005;5:73. doi: 10.1186/1471-2407-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawadros T, et al. Release of macrophage migration inhibitory factor by neuroendocrine-differentiated LNCaP cells sustains the proliferation and survival of prostate cancer cells. Endocr Relat Cancer. 2013;20(1):137–149. doi: 10.1530/ERC-12-0286. [DOI] [PubMed] [Google Scholar]
- 20.Yu DS, Lin JC, Hsieh DS, Chang SY, Lee CF. Modulation of MDR-1 gene by MIF and GSTpi with drug resistance generation in hormone independent prostate cancer. Arch Androl. 2006;52(4):283–291. doi: 10.1080/01485010600630116. [DOI] [PubMed] [Google Scholar]
- 21.Meyer-Siegler KL, Bellino MA, Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. 2002;94(5):1449–1456. doi: 10.1002/cncr.10354. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology. 1996;48(3):448–452. doi: 10.1016/S0090-4295(96)00207-5. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Siegler KL, et al. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 2007;8(8):646–652. doi: 10.1038/sj.gene.6364427. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, et al. Macrophage migration inhibitory factor expression in ovarian cancer. Am J Obstet Gynecol. 2007;196(4):348.e1-5. doi: 10.1016/j.ajog.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Fromont G, et al. Biological significance of perineural invasion (PNI) in prostate cancer. Prostate. 2012;72(5):542–548. doi: 10.1002/pros.21456. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem. 1999;274(25):18100–18106. doi: 10.1074/jbc.274.25.18100. [DOI] [PubMed] [Google Scholar]
- 27.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197(11):1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell RA, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: Regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99(1):345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calandra T, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6(2):164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 30.Morand EF. New therapeutic target in inflammatory disease: Macrophage migration inhibitory factor. Intern Med J. 2005;35(7):419–426. doi: 10.1111/j.1445-5994.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 31.Augustijn KD, et al. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect Immun. 2007;75(3):1116–1128. doi: 10.1128/IAI.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcone FH, et al. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol. 2001;167(9):5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- 33.Kamir D, et al. A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J Immunol. 2008;180(12):8250–8261. doi: 10.4049/jimmunol.180.12.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommerville C, et al. Biochemical and immunological characterization of Toxoplasma gondii macrophage migration inhibitory factor. J Biol Chem. 2013;288(18):12733–12741. doi: 10.1074/jbc.M112.419911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosengren E, et al. The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase. FEBS Lett. 1997;417(1):85–88. doi: 10.1016/s0014-5793(97)01261-1. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren E, et al. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 37.Swope M, Sun HW, Blake PR, Lolis E. Direct link between cytokine activity and a catalytic site for macrophage migration inhibitory factor. EMBO J. 1998;17(13):3534–3541. doi: 10.1093/emboj/17.13.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermanowski-Vosatka A, et al. Enzymatically inactive macrophage migration inhibitory factor inhibits monocyte chemotaxis and random migration. Biochemistry. 1999;38(39):12841–12849. doi: 10.1021/bi991352p. [DOI] [PubMed] [Google Scholar]
- 39.Bendrat K, et al. Biochemical and mutational investigations of the enzymatic activity of macrophage migration inhibitory factor. Biochemistry. 1997;36(49):15356–15362. doi: 10.1021/bi971153a. [DOI] [PubMed] [Google Scholar]
- 40.Fingerle-Rowson G, et al. A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol Cell Biol. 2009;29(7):1922–1932. doi: 10.1128/MCB.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 42.Verjans E, et al. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer. 2009;9:230. doi: 10.1186/1471-2407-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lue H, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26(35):5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 44.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 45.Bifulco C, McDaniel K, Leng L, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor. Curr Pharm Des. 2008;14(36):3790–3801. doi: 10.2174/138161208786898608. [DOI] [PubMed] [Google Scholar]
- 46.Scherbakova EA, Stromskaya TP, Rybalkina EY, Kalita OV, Stavrovskaya AA. Role of the PTEN protein in the multidrug resistance of prostate cancer cells. Mol Biol. 2008;42(3):430–435. [PubMed] [Google Scholar]
- 47.David JR. Delayed hypersensitivity in vitro: Its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke G, Armstrong ME, Donnelly SC. Macrophage migration inhibitory factor (MIF), enzymatic activity and the inflammatory response. Biofactors. 2009;35(2):165–168. doi: 10.1002/biof.27. [DOI] [PubMed] [Google Scholar]
- 49.Vanácová S, Yan W, Carlton JM, Johnson PJ. Spliceosomal introns in the deep-branching eukaryote Trichomonas vaginalis. Proc Natl Acad Sci USA. 2005;102(12):4430–4435. doi: 10.1073/pnas.0407500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Shakra M, Buskila D, Shoenfeld Y. Molecular mimicry between host and pathogen: Examples from parasites and implication. Immunol Lett. 1999;67(2):147–152. doi: 10.1016/s0165-2478(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 51.Beswick EJ, Reyes VE. Macrophage migration inhibitory factor and interleukin-8 produced by gastric epithelial cells during Helicobacter pylori exposure induce expression and activation of the epidermal growth factor receptor. Infect Immun. 2008;76(7):3233–3240. doi: 10.1128/IAI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gore Y, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283(5):2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 53.Starlets D, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107(12):4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 54.Calandra T, Bucala R. Macrophage migration inhibitory factor: A counter-regulator of glucocorticoid action and critical mediator of septic shock. J Inflamm. 1995-1996;47(1-2):39–51. [PubMed] [Google Scholar]
- 55.Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev. 2012;11(6-7):A486–A492. doi: 10.1016/j.autrev.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 56.Madkan VK, Giancola AA, Sra KK, Tyring SK. Sex differences in the transmission, prevention, and disease manifestations of sexually transmitted diseases. Arch Dermatol. 2006;142(3):365–370. doi: 10.1001/archderm.142.3.365. [DOI] [PubMed] [Google Scholar]
- 57.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 58.Koch AE, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 59.Botelho MC, Machado JC, Brindley PJ, Correia da Costa JM. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence. 2011;2(4):267–279. doi: 10.4161/viru.2.4.16734. [DOI] [PubMed] [Google Scholar]
- 60.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett. 2011;305(2):250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 63.Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15(3):329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dyall SD, et al. Presence of a member of the mitochondrial carrier family in hydrogenosomes: Conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol Cell Biol. 2000;20(7):2488–2497. doi: 10.1128/mcb.20.7.2488-2497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delgadillo MG, Liston DR, Niazi K, Johnson PJ. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc Natl Acad Sci USA. 1997;94(9):4716–4720. doi: 10.1073/pnas.94.9.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Twu O, et al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host. parasite interactions. PLoS Pathog. 2013;9(7):e1003482. doi: 10.1371/journal.ppat.1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lustig G, Ryan CM, Secor WE, Johnson PJ. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun. 2013;81(5):1411–1419. doi: 10.1128/IAI.01244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mu Z, Hachem P, Pollack A. Antisense Bcl-2 sensitizes prostate cancer cells to radiation. Prostate. 2005;65(4):331–340. doi: 10.1002/pros.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Addis MF, et al. Identification of Trichomonas vaginalis alpha-actinin as the most common immunogen recognized by sera of women exposed to the parasite. J Infect Dis. 1999;180(5):1727–1730. doi: 10.1086/315095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.