Summary
To identify regulatory drivers of prostate cancer malignancy, we have assembled genome-wide regulatory networks (interactomes) for human and mouse prostate cancer from expression profiles of human tumors and of genetically engineered mouse models, respectively. Cross-species computational analysis of these interactomes has identified FOXM1 and CENPF as synergistic master regulators of prostate cancer malignancy. Experimental validation shows that FOXM1 and CENPF function synergistically to promote tumor growth, by coordinated regulation of target gene expression and activation of key signaling pathways associated with prostate cancer malignancy. Furthermore, co-expression of FOXM1 and CENPF is a robust prognostic indicator of poor survival and metastasis. Thus, genome-wide cross-species interrogation of regulatory networks represents a valuable strategy to identify causal mechanisms of human cancer.
Introduction
It is widely appreciated that cancer is not a single entity but rather a highly individualized spectrum of diseases characterized by a large number of molecular alterations (Hanahan and Weinberg, 2011). Distinguishing those that constitute true drivers of cancer phenotypes from the multitude that are simply de-regulated has proven to be a daunting task, which is further exacerbated by the complexity of elucidating how such drivers interact synergistically to elicit cancer phenotypes. In this regard, prostate cancer is particularly challenging since its notorious heterogeneity, combined with a relative paucity of recurrent gene mutations, has made it especially difficult to identify molecularly distinct subtypes with known clinical outcomes (Baca et al., 2013; Schoenborn et al., 2013; Shen and Abate-Shen, 2010). Additionally, while most early-stage prostate tumors are readily treatable (Cooperberg et al., 2007), advanced prostate cancer frequently progresses to castration-resistance, which is often metastatic and nearly always fatal (Ryan and Tindall, 2011; Scher and Sawyers, 2005). Thus, there is a pressing need to identify bona fide determinants of aggressive prostate cancer as well as prognostic biomarkers of disease outcome.
Analysis of genetically engineered mouse models (GEMMs) can circumvent inherent challenges associated with the intrinsic complexity of more heterogeneous human cancer phenotypes. Indeed, investigations of mouse models of prostate cancer have contributed to characterization of disease-specific pathways, led to the identification of biomarkers of disease progression, and provided useful preclinical models for prevention and therapy (Irshad and Abate-Shen, 2013; Ittmann et al., 2013). Following the description of an initial transgenic model nearly 20 years ago, there are now numerous GEMMs that collectively model key molecular pathways de-regulated in human prostate cancer, and recapitulate the various stages of disease progression including pre-invasive lesions (prostate intraepithelial neoplasia, PIN), adenocarcinoma, castration-resistance, and metastasis (Irshad and Abate-Shen, 2013; Ittmann et al., 2013).
However, inherent species differences often hinder direct comparative analysis of mouse models and human cancer. Indeed, such analysis would greatly benefit from computational approaches that enable accurate cross-species integration of regulatory information from mouse to man. Recent advances in systems biology have led to the reverse engineering of regulatory networks (interactomes) that integrate large-scale datasets encompassing expression profiles, protein-protein interactions, genomic alterations, and epigenetic changes associated with cancer and other diseases (Lefebvre et al., 2012). However, while individual analysis of human and murine interactomes have led to relevant biological discoveries, their cross-species interrogation has not been systematically implemented.
Here, we introduce an approach for accurate cross-species analysis of conserved cancer pathways based on reverse engineering of genome-wide regulatory networks (i.e., interactomes) representing both human and mouse prostate cancer. To accomplish this, we have produced a regulatory network based on in vivo perturbation of a repertoire of mouse cancer models, and implemented comparative analysis with a complementary regulatory network generated from human prostate cancer datasets. Cross-species computational interrogation of these paired interactomes, followed by experimental and clinical validation, elucidated the synergistic interaction of FOXM1 and CENPF as a driver of prostate cancer malignancy. We propose that analysis of genome-wide, cross-species regulatory networks will provide an effective paradigm for elucidating causal mechanisms of human cancer and other complex diseases.
Results
We developed a strategy for genome-wide interrogation of cancer phenotypes based on accurate integration of experimental data from model organisms and human cancer (Figure 1). First, we generated regulatory networks (interactomes) for human and mouse prostate cancer using the Algorithm for the Reconstruction of Accurate Cellular Networks (ARACNe) (Basso et al., 2005; Margolin et al., 2006b). We next evaluated the suitability of these mouse and human interactomes for cross-species interrogation using a computational approach to assess the global conservation of their transcriptional programs. We then used the Master Regulator Inference algorithm (MARINa) (Carro et al., 2010; Lefebvre et al., 2010) to infer candidate master regulators that act individually or synergistically to drive malignant prostate cancer. Finally, we performed experimental studies to validate synergistic interactions of master regulators, to elucidate underlying mechanisms, and to evaluate their clinical relevance.
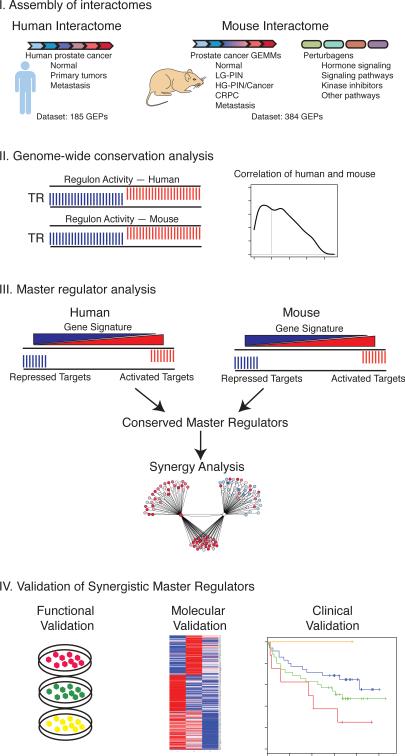
Figure 1. Strategy for genome-wide cross-species analyses of prostate cancer.
Schematic representation of the overall strategy. Step I: Assembly of human and mouse prostate cancer interactomes. Step II: Genome-wide computational analysis of conservation of transcriptional regulon activity in the mouse and human prostate cancer interactomes. Step III: Master regulator analysis for identification of conserved master regulators and prediction of synergy. Step IV: Validation of candidate master regulators using functional, molecular, and clinical analyses.
Assembly of interactomes for human and mouse prostate cancer
ARACNe is an unbiased algorithm that infers direct transcriptional interactions based on the mutual information between each transcriptional regulator and its potential targets. For optimal analysis, ARACNe requires large datasets of gene expression profiles (≥ 100) having significant endogenous (i.e., genetic) and/or exogenous (i.e., perturbation-induced) heterogeneity. To assemble a human prostate cancer interactome, we analyzed the expression profile dataset reported in (Taylor et al., 2010), which is ideally suited for ARACNe because: (i) it is relatively large (n = 185) and diverse, including primary tumors, adjacent normal tissue, metastases, and cell lines; (ii) its primary tumors encompass the full range of pathological Gleason scores and have well-annotated clinical outcome data; and (iii) it displays extensive genetic diversity and tumor heterogeneity, as shown by t-Distributed Stochastic Neighbor Embedding (t-SNE) analysis (Figure 2; Table S1). Notably, interactomes assembled from three alternative human prostate cancer datasets (Table S1) were neither as complete nor as extensive (data not shown).
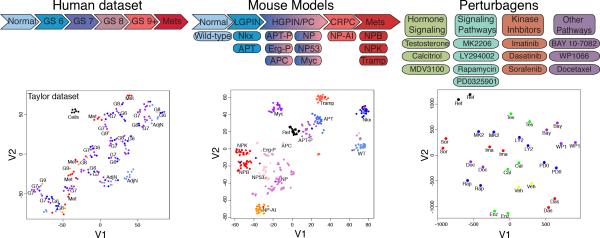
Figure 2. Heterogeneity of human and mouse datasets used for interactome assembly.
t-Distributed Stochastic Neighbor Embedding (t-SNE) analysis of human and mouse datasets used to assemble the prostate cancer interactomes. (Left) t-SNE analysis of the Taylor dataset relative to Gleason score. (Middle) Schematic representation of GEMMs used to assembly the mouse prostate cancer interactome. t-SNE analysis showing relative distribution of the GEMMs. (Right) Schematic diagram depicting perturbagens used to treat the GEMMs. t-SNE analysis showing the relative distribution of perturbagens for a representative GEMM (i.e., the NP mice).
See also Figure S1, Tables S1, and S2.
To assemble a corresponding mouse prostate cancer interactome, it was first necessary to generate an expression profile dataset of appropriate size and representing sufficient expression variability. We selected 13 distinct GEMMs, which together represent the full spectrum of prostate cancer phenotypes, including normal epithelium (wild-type), low-grade PIN (Nkx3.1 and APT), high-grade PIN and adenocarcinoma (APT-P, APC, Myc, NP, Erg-P, and NP53), castration-resistance (NP-AI), and metastatic prostate cancer (NPB, NPK, and TRAMP) (Figure S1A; Table S2). To further increase the variability of the expression profiles, we introduced a controlled set of exogenous perturbations by in vivo administration of 13 small-molecule perturbagens to each GEMM. Perturbagens were selected for their clinical relevance and/or ability to modulate key prostate cancer pathways, including: hormone signaling (testosterone, calcitriol, and enzalutamide); PI3 kinase activity (MK2206, LY294002, and rapamycin); MAP kinase activity (PD035901); tyrosine kinase activity (imatinib, dasatinib, and sorafenib); NFκB signaling (BAY 11-7082); JAK/STAT activity (WP1066); and chemotherapy (docetaxel) (Supplementary Experimental Procedures). Following pilot studies to define appropriate dose and schedule (Figure S1B-D), we adopted a universal treatment schedule of 1 treatment per day for 5 days with dosage determined independently for each perturbagen (Supplementary Experimental Procedures).
The resulting dataset comprises 384 gene expression profiles, corresponding to the 13 GEMMs each treated with the 13 perturbagens or vehicles. t-SNE analysis revealed that the resulting mouse dataset represented an extensive range of expression variability, as required for ARACNe (Figure 2). Specifically, while expression profiles from the same GEMMs and perturbagens clustered together, the diverse GEMMs and perturbagens provided independent and highly effective axes to modulate gene expression variability.
ARACNe was run independently on the human and mouse datasets using a conservative mutual information threshold (p ≤ 1.0×10-9, i.e., p ≤ 0.05 Bonferroni corrected for all candidate interactions). This resulted in highly robust regulatory networks; in particular, the human interactome represented 249,896 interactions between 2,681 transcriptional regulators and their inferred target genes (Figure 3A, Table S3), while the mouse interactome represented 222,787 interactions for 2,072 transcriptional regulators (Figure 3A, Table S4).
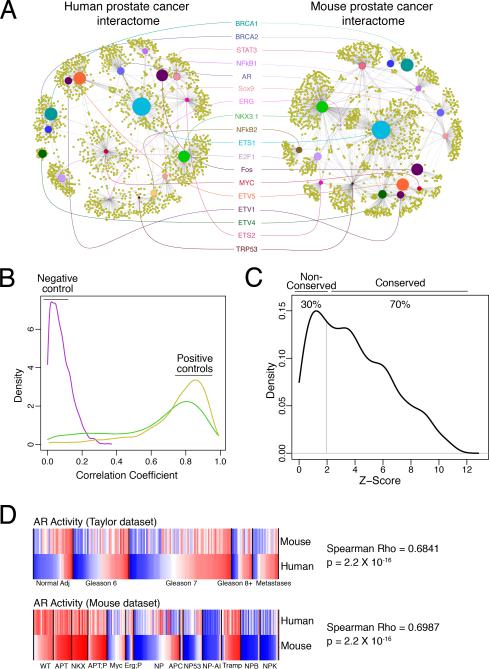
Figure 3. Genome-wide conservation analyses of the human and mouse prostate cancer interactomes.
(A) ARACNe sub-networks from the human and mouse prostate cancer interactomes highlighting selected conserved transcriptional regulators. The scaled size of the transcriptional regulator nodes (colored circles) indicates the degree of conservation while the relative distance between them approximates the strength of their association. (B, C) Histograms (density plots) showing conservation of transcriptional regulator activity between the human and mouse prostate cancer interactomes. (B) Distribution of correlation coefficients of activity profiles of transcriptional regulators for randomized interactomes (negative control; purple line) and the positive control interactomes for human (yellow) and mouse (green) (Supplementary Experimental Procedures). (C) Distribution of Z-scores for conservation of activity profiles between the human and mouse interactomes at p ≤ 0.05. (D) Comparison of the androgen receptor (AR) activity levels in each sample from Taylor et al (top) and the mouse dataset(bottom) showing the Spearman correlation coefficient.
Analysis of genome-wide conservation of transcriptional regulatory pathways
Since it has been previously established that target-by-target analysis may not be optimal to evaluate cross-species interactome conservation (e.g., (Zhang et al., 2012), we developed a quantitative metric to compare conservation of the human and mouse interactomes. In particular, we developed a modification of the MARINa algorithm that allows for single-sample analysis to infer the differential activity of all 2028 transcriptional regulators represented in both interactomes. Analysis was performed on 1009 expression profiles across the 4 human datasets (Table S1) and the mouse dataset (described herein) to determine whether the inferred activities of each regulator were significantly correlated (p ≤ 0.05), indicating that the murine and human regulatory programs were conserved (Supplementary Experimental Procedures). The accuracy of this metric was evident by comparing two equivalent same-species interactomes from the human and mouse datasets (i.e., positive controls), in which virtually all transcriptional regulators were conserved (>90%), in contrast to randomized interactomes (i.e., negative controls), which had virtually no conservation (Figure 3B).
Using this metric, we found that 70% of the transcriptional regulators in the human and mouse prostate cancer interactomes regulate statistically conserved programs (p ≤ 0.05) (Figure 3C; Table S5). Notably, among the conserved transcriptional regulators are many genes important in prostate cancer, such as AR, ETS1, ETV4, ETV5, STAT3, MYC, BRCA1, and NKX3.1 (Shen and Abate-Shen, 2010) (Figure 3A; Table S5). In particular, AR displayed extensive correlation of its transcriptional activity between the human and mouse interactomes (Figure 3D), consistent with its known role as a key regulator of prostate development and tumorigenesis (Ryan and Tindall, 2011; Shen and Abate-Shen, 2010).
Cross-species computational analysis identifies synergistic master regulators of malignant prostate cancer
To identify master regulators (MRs) of malignant prostate cancer (Figure 4), we used the MARINa algorithm, which identifies candidate MRs based on the concerted differential expression of their ARACNe-inferred targets (i.e., their inferred differential activity, DA). Specifically, “activated” MRs have positively regulated and repressed targets significantly enriched among over- and under-expressed genes, respectively, while “repressed” MRs have the converse. We interrogated the human prostate cancer interactome using a gene signature representing prostate cancer malignancy from the Taylor dataset as described in (Aytes et al., 2013), which compares aggressive prostate tumors (Gleason score ≥ 8 with rapid biochemical recurrence; n =10) to indolent ones (Gleason score 6 tumors with no biochemical recurrence; n = 39). This analysis identified 175 candidate MRs, including 49 activated and 126 repressed (p ≤ 0.05) (Figure 4A; Table S6).
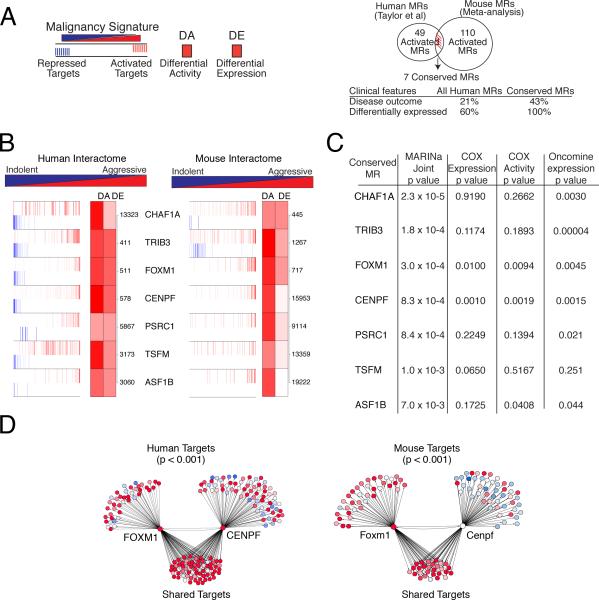
Figure 4. Conserved master regulators of malignant prostate cancer.
(A) (left) Master regulators (MRs) were identified using human or mouse interactomes malignancy signatures; differential activity (DA) is based on enrichment of activated (red) and repressed (blue) targets. DE, differential expression. (Right) Venn diagram showing integration of independent lists of activated MRs from human (49) and mouse (110) with an overlap of 7 conserved MRs. Clinical features of all human MRs versus the conserved MRs showing the percentage associated with disease outcome (using a COX proportional hazard model) and the percentage that are differentially-expressed in advanced prostate cancer (from Oncomine). (B) Conserved activated MRs are shown for the human (left) and mouse (right) malignancy signatures, depicting their positive (activated; red bars) and negative (repressed; blue bars) targets. The ranks of differential activity (DA) and differential expression (DE) are shown by the shaded boxes; the numbers indicate the rank of the DE in the malignancy signature. (C) Summary of conserved MRs showing: joint p value from human and mouse MARINa analysis, calculated using Stouffer's method; p value for COX proportional hazard regression model applied to mRNA expression levels and predicted MR activity; and average p values for differential expression of MRs in metastatic versus non-metastatic primary tumors. (D) Computational synergy analysis depicting FOXM1 and CENPF regulons from the human (left) and mouse (right) interactomes showing shared and non-shared targets. Red corresponds to over-expressed targets and blue to under-expressed targets; the p value for the enrichment of shared targets is shown.
To investigate the robustness of these MRs, we performed MARINa using an independent malignancy signature from the Balk dataset (Table S1) (Stanbrough et al., 2006), which compares castrate-resistant (n=29) with hormone-naïve disease (n=22). The MRs identified from the Balk malignancy signature (Table S6) significantly overlapped with those identified from the Taylor malignancy signature (36 in common; Fisher exact test p < 0.0001; Table S6). Furthermore, MARINa analyses of 15 independent interactomes revealed that the MRs inferred from two independent prostate cancer interactomes significantly overlapped with those inferred from the Taylor prostate cancer interactome (p < 7×10-9 and p < 8×10-20, Fisher exact test), whereas the overlap of those inferred from 13 non-prostate cancer specific interactomes were orders of magnitude less significant (Supplementary Experimental Procedures). Thus, MRs of human prostate cancer malignancy are consistent across independent prostate cancer malignancy signatures, but dependent on a context-specific prostate cancer interactome.
To identify corresponding mouse MRs of malignancy, we performed MARINa on four independent GEMM signatures, which embody the diverse range of prostate cancer phenotypes represented among the GEMMs (Figure S2A; Table S2, and Supplementary Experimental Procedures). Meta-analysis of the resulting MRs from these independent GEMM signatures led to identification of 229 candidate mouse MRs, including 110 activated and 119 repressed MRs (p ≤ 0.001) (Figure 4A; Table S7).
The independent list of human and mouse MRs were then integrated to produce a ranked list of 20 conserved MRs, including 7 activated and 13 repressed (joint p value: p ≤ 0.0074 by Stouffer's method) (Figure 4A,B; Figure S2B). Notably, these conserved MRs were more likely to be associated with disease outcome than the non-conserved ones, as assessed by a univariate COX proportional hazard regression model (p ≤ 0.05), and were also more likely to be differentially expressed in aggressive prostate tumors (Figure 4A,C and Supplementary Experimental Procedures).
We focused our subsequent analysis on the activated conserved MRs, each of which has been associated with cancer-related biological processes: CHAF1A (chromatin activity); TRIB3 (regulation of cell signaling in transcriptional control); FOXM1 (cell cycle progression); CENPF (mitosis); PSRC1 (growth control); TSFM (translational elongation); and ASF1B (regulation of nucleosome assembly) (Figure 4B). We further prioritized these MRs by computationally evaluating their potential synergistic interactions. In particular, any pair of MRs was considered “synergistic” if their co-regulated ARACNe-inferred targets were significantly enriched in the malignancy signature relative to their individual targets (p ≤ 0.001) (Carro et al., 2010; Lefebvre et al., 2010). Among all possible pairs of conserved activated MRs, the only pair that was statistically significant was FOXM1 and CENPF (Figure 4D). Notably, both FOXM1 and CENPF are expressed in aggressive prostate tumors and predicted to be associated with disease outcome (Figure 4C and Supplementary Experimental Procedures). Strikingly, among all activated (rather than conserved) human MRs identified only FOXM1 and CENPF were predicted to be both synergistic and of potential clinical relevance (Supplementary Experimental Procedures). Thus, cross-species analyses of conserved MRs identified a single MR synergy pair of potential clinical relevance.
Co-silencing FOXM1 and CENPF synergistically abrogates prostate tumor growth
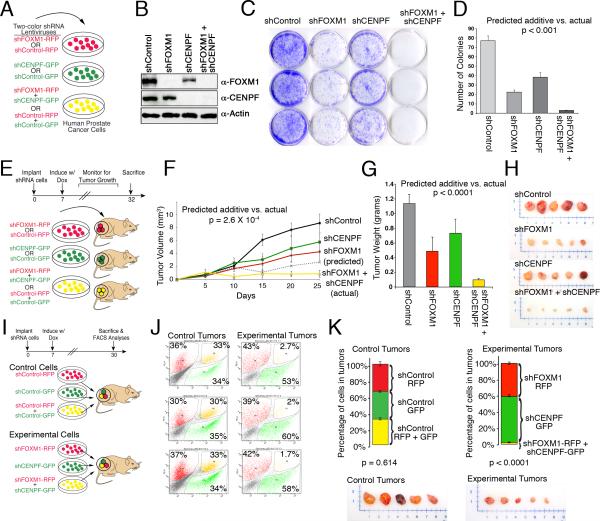
To evaluate their individual and potential synergistic functions in prostate cancer, we silenced FOXM1 and/or CENPF individually or together in four human prostate cell lines, DU145, PC3, LNCaP and 22Rv1, which have differing tumorigenic properties and responses to androgen signaling (Figure 5A, Figure S3A). Notably, each of these cell lines express high levels of FOXM1 and CENPF mRNA; however, LNCaP does not have detectable CENPF protein (Figure 5B, Figure S3B-E), and therefore provides an excellent negative control for synergy analysis. To silence FOXM1 and/or CENPF, we engineered doxycycline-inducible lentiviral vectors expressing shRNAs for FOXM1 or CENPF or a control shRNA, as well as an RFP or GFP reporter (Figure 5A and Supplementary Experimental Procedures); analyses were done using two independent shRNA to minimize concerns about off target effects (Figure S3). We distinguish “synergistic” versus “additive” effects of FOXM1 and CENPF by first extrapolating their “predicted additivity” based on their individual silencing using a log-linear model, and then comparing this predicted value to the “actual” (observed) effect following their co-silencing using a one-sample t-test; if the “actual” is statistically greater than the “predicted additive”, we conclude that FOXM1 and CENPF are synergistic rather than additive (Supplementary Experimental Procedures).
Figure 5. Functional validation of FOXM1 and CENPF.
(A) Human prostate cancer cells were infected with lentiviral silencing vectors expressing shRNA for FOXM1 and/or CENPF (or control) and either an RFP (red) or GFP (green) reporter. Unless otherwise indicated, analyses were done using two independent shRNAs for each gene and in four independent prostate cancer cell lines (DU145, PC3, LNCaP, 22Rv1); in most cases data using shRNA1 are shown. (B) Western blot analysis showing expression of FOXM1 or CENPF proteins in DU145 cells with the indicated shRNAs. (C, D) Colony formation assay. (C) representative analyses of DU145 cells with an shRNA for FOXM1 and/or CENPF (or the control) with colonies visualized using crystal violet. (D) quantification of colonies using ImageJ. (E-H) Analysis of tumor growth in vivo. (E) DU145 cells expressing an shRNA for FOXM1 and/or CENPF, or the control, were implanted subcutaneously into mouse hosts. Beginning on day 7, mice were administered doxycycline to induce shRNA expression and tumor growth was monitored for one month. (F) Tumor growth curves for the indicated shRNA. The dashed line shows the predicted additive effect of co-silencing FOXM1 and/or CENPF. (G) Tumor weights at the time of sacrifice. (H) Representative tumors. In panels D, F, and G the predicted additive was estimated based on the consequences of individual silencing of FOXM1 and CENPF using a log linear model; the p value, calculated using a one-sample t-test, indicates the significance between the predicted additive versus the actual (observed) consequences of co-silencing FOXM1 and CENPF. (I-L) In vivo competition assay. (I) Equal numbers of DU145 cells expressing the control shRNA (control cells), or the experimental shRNA for FOXM1 and/or CENPF (experimental cells) as well as RFP or GFP were implanted into mouse hosts. Beginning on day 7, mice were administered doxycycline to induce shRNA expression and tumor growth was monitored for one month. Following which, tumors were collected and FACS-sorted to quantify the total number of red, green, or yellow cells in individual tumors for control and experimental groups. (J) Representative FACS plots showing the percentage of red, green or yellow cells relative to the total number of fluorescent cells. (K) (Top) Graphs show the average percent of red, green, and yellow cells in the control tumors (n = 4) or experimental tumors (n = 7); p values correspond to a Hotelling's one-sample t-test. (Bottom) Representative tumors. Error bars represent +/- SD.
See also Figure S3.
While individual silencing of FOXM1 and, to a lesser extent, CENPF resulted in reduced cellular proliferation, the actual reduction following their co-silencing was statistically greater (p < 0.01; one-sample t-test) than the predicted additive and therefore synergistic for each cell line that expresses both FOXM1 and CENPF proteins (Figure S3F). Similarly, with respect to colony formation, while individual silencing of FOXM1 or CENPF reduced the number of colonies, their co-silencing resulted in nearly complete abrogation of colony formation in each cell line expressing both FOXM1 and CENPF proteins (p < 0.001; one-sample t-test) (Figure 5C,D; Figure S3G,H). Importantly, co-silencing of FOXM1 and CENPF was not associated with reduced viability, apoptosis, or further cell cycle arrest relative to their individual silencing (Figure S3I-K), suggesting that their observed synergy was not simply due to induction of cell death or secondary to cell cycle arrest.
To investigate their consequences for tumor growth in vivo, we engrafted DU145 cells expressing silencing vectors for FOXM1 and/or CENPF (or controls) into immunodeficient mice and monitored tumor growth (Figure 5E-H). Consistent with the cell culture studies, individual silencing of FOXM1 or CENPF resulted in a modest but statistically significant reduction in tumor growth (2.0 fold, p ≤ 2×10-3 and 1.5 fold, p ≤ 2×10-3, respectively), as well as tumor weight (2.3 fold, p ≤ 7×10-3, and 1.6 fold, p ≤ 1×10-2, respectively) (Figure 5F,G). However, co-silencing of FOXM1 and CENPF resulted in a complete abrogation of tumor growth (10.2 fold reduced, p ≤ 1.3×10-5) and a profound reduction in tumor weight (12.9 fold, p ≤ 1.1×10-5) (Figure 5F,G). Notably, the actual inhibition of tumor growth following co-silencing of FOXM1 and CENPF was significantly greater than the predicted additive (3.3 fold difference, p ≤ 2.6×10-4; one sample t-test) (Figure 5F), supporting the conclusion that FOXM1 and CENPF synergistically regulate tumor growth in vivo.
To further evaluate the synergistic activity of FOXM1 and CENPF for tumor growth, we developed an in vivo competition assay (Figure 5I-K). Specifically, we infected DU145 cells with silencing vectors expressing an FOXM1 shRNA and an RFP reporter (red) or an CENPF shRNA and an GFP reporter (green), or both lentiviruses (yellow) (Figure 5I). As negative controls, we infected DU145 cells with control vectors lacking the FOXM1 or CENPF shRNA but expressing the fluorescent reporters. We then implanted equal numbers of viable red, green, or yellow cells from the experimental or control groups into immunodeficient mice. Following one month of growth in vivo, the resulting tumors were isolated and the percentage of red, green, and yellow cells were quantified by FACS sorting.
Tumors derived from control cells (n = 4) were comprised of equivalent numbers of red (34% ± 0.6%), green (34% ± 2.7%) and yellow (33% ± 1.2%) cells, indicating that the respective lentiviral vectors offer no selective growth advantage (p ≤ 0.614; Hotelling's one-sample T-squared test) (Figure 5J,K). In striking contrast, tumors derived from the experimental cells (n = 7) were comprised primarily of green CENPF-silenced cells (57% ± 3.5%) and red FOXM1-silenced cells (41% ± 2.6%), while there were virtually no yellow, co-silenced cells (2.0% ± 0.3%) (Figure 5J,K). This profound selection against cells co-silenced for FOXM1 and CENPF was highly significant (p ≤ 1×10-4; Hotelling's one-sample T-squared test) (Figure 5K), which further supports the conclusion that FOXM1 and CENPF synergistically regulate tumor growth in vivo.
FOXM1 and CENPF co-regulate gene expression and control tumorigenic signaling pathways in prostate cancer
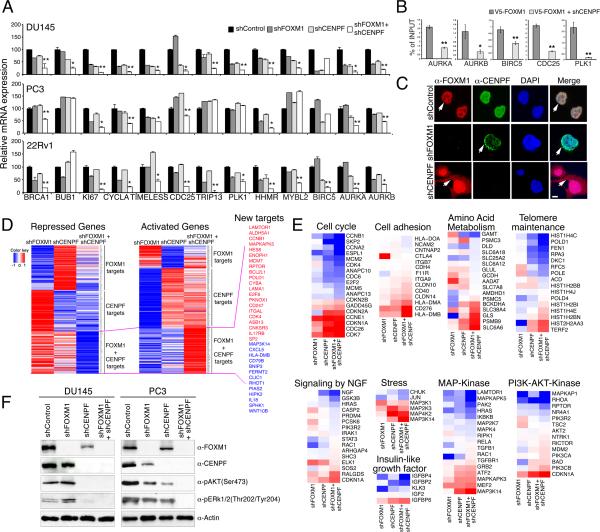
To investigate the mechanism(s) underlying the observed activities of FOXM1 and CENPF, we assessed the consequences of their individual versus co-silencing for expression of their ARACNe-inferred common (shared) target genes (Table S3). Although target gene expression was somewhat reduced by their individual silencing, co-silencing of FOXM1 and CENPF produced a significantly greater reduction for the majority of targets, consistent with co-regulation of target gene expression by FOXM1 and CENPF (Figure 6A, Figure S4). Furthermore, using chromatin immunoprecipitation (ChIP) followed by quantitative PCR, we found that binding by FOXM1 to its known genomic sites on shared target genes was reduced following silencing of CENPF (Figure 6B), suggesting that CENPF is required for appropriate genomic binding by FOXM1. Interestingly, although we did not observe a direct protein-protein interaction of FOXM1 and CENPF in co-immunoprecipitation assays (data not shown), we observed that FOXM1 and CENPF were co-localized in the nucleus of prostate cancer cells and that their subcellular co-localization was mutually dependent (Figure 6C). In particular, silencing of CENPF resulted in the redistribution of FOXM1 to the cytoplasm as well the nucleus, and conversely silencing of FOXM1 resulted in the accumulation of CENPF at the nuclear periphery (Figure 6C). Notably, subcellular co-localization of FOXM1 and CENPF was also observed in human prostate tumors and was associated with disease outcome (see below). Taken together, our findings suggest that FOXM1 and CENPF co-regulate expression of shared target genes in prostate cancer cells, at least in part, through their subcellular co-localization.
Figure 6. FOXM1 and CENPF synergistically regulate gene expression and control tumorigenic signaling pathways in prostate cancer.
(A) Validation of ARACNe-inferred shared targets of FOXM1 and CENPF. The graphs show relative mRNA expression levels, normalized to GADPH, for the indicated genes in the cell lines shown following individual or co-silencing of FOXM1 and CENPF. The p values (indicated by *) show the significance of the predicted additive effect versus actual effect on gene expression calculated using a one-sample t-test (*, p <0.01; **, p <0.001). (B) Chromatin immunoprecipitation (ChIP) followed by qPCR of genomic binding sites of FOXM1. Cells were infected with a lentivirus expressing V5-tagged FOXM1 as well as an shRNA for CENPF (or a control) and ChIP was done using an anti-V5 antibody. Data are expressed as fold enrichment of FOXM1 binding normalized to input. (C) Subcellular localization of FOXM1 and CENPF in prostate cancer cells after silencing. Shown are microphotographs of immunofluorescence staining for FOXM1 or CENPF in the control or silenced cells as indicated. Arrows indicate subcellular localization. Scale bars represent 1 μm. (D-E) Consequences of silencing FOXM1 and/or CENPF for gene expression profiling in DU145 cells. (D) Heatmaps of differentially expressed genes. Colors correspond to levels of differential expression; red corresponds to over-expression and blue to under-expression. Selected genes differentially expressed following co-silencing are indicated. (E) Heatmaps showing leading edge genes of biological pathways enriched by co-silencing of FOXM1 and CENPF as assessed by GSEA. (F) Western blot analyses showing expression of the indicated markers of the PI3-kinase and MAP kinase signaling pathways in DU145 and PC3 prostate cancer cells silenced for FOXM1 and/or CENPF,as indicated. Error bars represent +/- SD.
To elucidate molecular pathways underlying the synergistic interaction of FOXM1 and CENPF for tumor growth, we analyzed expression profiles from prostate cancer cells in which they were individually silenced or co-silenced (Figure 6D, Table S8). The differentially expressed genes following individual silencing of FOXM1 or CENPF included a majority of their ARACNe-inferred targets (p = 0.0028 for enrichment of FOXM1 targets; p ≤ 0.001 for CENPF targets), further confirming the accuracy of the ARACNe analysis (Table S8). Inspection of these differentially expressed genes, as well as gene set enrichment analysis (GSEA) of enriched biological pathways confirmed the known individual functions of FOXM1 and CENPF as regulators of cellular proliferation and/or mitosis (Tables S8 and S9).
However, co-silencing of FOXM1 and CENPF revealed an additional repertoire of significantly differentially expressed genes and enriched biological pathways (Figure 6D,E; Tables S8,S9), including several pathways associated with tumorigenesis: “Cell cycle” (normalized enrichment score (NES) 1.32; p ≤ 0.001), “stress pathway” (NES 1.58; p ≤ 0.01), “regulation of insulin-like growth factor” (NES 1.89; p ≤ 0.001), “signaling by NGF” (NES 1.25; p ≤ 0.001), “Metabolism of amino acids” (NES 1.25; p ≤ 0.01), “PI3-Akt signaling” (NES 1.89; p ≤ 0.001), “MAP kinase pathway” (NES 1.34; p ≤ 0.008), “Telomere maintenance” (NES 1.35; p ≤ 0.01) and “Cell adhesion molecules” (NES 1.32; p ≤ 0.001).
Notable was the enrichment of PI3-kinase and MAP kinase signaling pathways following co-silencing of FOXM1 and CENPF (Figure 6E; Table S9), as these constitute established hallmarks of aggressive prostate cancer (Aytes et al., 2013; Taylor et al., 2010). As evident by Western blot analysis, both pathways are completely abrogated following co-silencing of FOXM1 and CENPF (Figure 6F), suggesting that therapeutic targeting of FOXM1 and CENPF in prostate cancer cells may be effective for inactivation of these signaling pathways.
Co-expression of FOXM1 and CENPF is a prognostic indicator for human prostate cancer
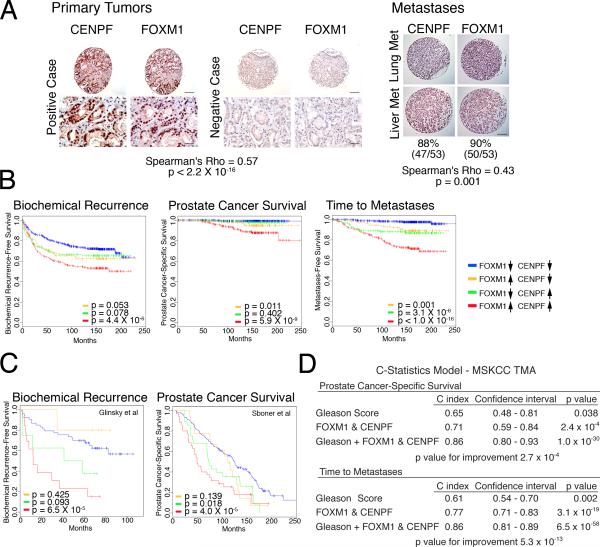
We next asked whether expression of FOXM1 and/or CENPF is associated with cancer progression and/or outcome by analysis of tissue microarrays (TMAs) (Figure 7A; Table S1). In particular, we analyzed a high-density TMA containing primary tumors from a large cohort of patients (n = 916) that had undergone prostatectomy at Memorial Sloan-Kettering Cancer Center from 1985 to 2003 (Donovan et al., 2008). These cases have extensive clinical follow-up data for up to 20 years, including time to biochemical recurrence, prostate cancer specific survival, and time to metastasis (Table S1). We also evaluated a second TMA from the rapid autopsy program at the University of Michigan, which contains prostate cancer metastases (n = 60), including 6 lung, 11 liver, 22 lymph node, and 14 other sites (Shah et al., 2004).
Figure 7. Clinical validation of FOXM1 and CENPF in human prostate cancer.
(A) Analysis of tissue microarrays (TMAs). Representative images from the MSKCC prostatectomy TMA and the Michigan metastasis TMA showing FOXM1 and CENPF protein expression; Spearman correlation of their co-expression with p value is shown. Scale bars represent 10 μm (lower Primary Tumor) or 100 μm (all others). (B) Kaplan-Meier survival analysis based on protein expression levels of FOXM1 and CENPF in MSKCC prostatectomy TMA with respect to time to biochemical recurrence, time to prostate cancer-specific death, or time to metastatic progression. (C) Kaplan-Meier survival analysis based on the ARACNe-inferred activity levels of FOXM1 and CENPF (Supplementary Experimental Procedures) in two independent human prostate cancer datasets using biochemical recurrence-free survival (Glinsky et al., 2004) or prostate cancer-specific survival (Sboner et al., 2010) as disease endpoints. In panels B and C, the p values correspond to a log-rank test and indicate the statistical significance of the association with outcome for each indicated branch compared to control (i.e., patients with low activity levels of both FOXM1 and CENPF, blue line curve). (D) C-statistics analysis, based on the protein levels of FOXM1 and CENPF from the MSKCC TMA, using death due to prostate cancer and time to metastasis as evaluation endpoints.
See also Figure S5
Analysis of the MSKCC prostatectomy TMA revealed that FOXM1 and CENPF were over-expressed in 33% and 37% of all cases, respectively, (n = 821 informative cases) with a trend toward increased expression in tumors with higher Gleason scores (Figure 7A, Figure S5A). Furthermore, analysis of the Michigan metastasis TMA revealed that FOXM1 and CENPF were expressed in most of the prostate cancer metastases (88% and 90%, respectively, n = 53 informative cases) (Figure 7A). Moreover, FOXM1 and CENPF were frequently co-expressed and co-localized in the nucleus in both the MSKCC prostatectomy TMA (Spearman's Rho = 0.57, p ≤ 2×10-16) and the Michigan metastasis TMA (Spearman's Rho = 0.43, p ≤ 1×10-3) (Figure 7A). Additionally, at the mRNA level overexpression of FOXM1 and CENPF were well-correlated in advanced prostate cancer and metastases from independent cohorts of human prostate cancer (Figure S5B).
To determine whether expression of FOXM1 and/or CENPF is associated with disease outcome, we first defined 4 groups of patients from the MSKCC TMA based on their expression levels: (i) low/normal expression of both FOXM1 and CENPF (n = 418); (ii) high expression of FOXM1 and low/normal expression of CENPF (n = 97); (iii) high expression of CENPF and low/normal expression of FOXM1 (n = 133); and (iv) high expression of both FOXM1 and CENPF (n = 173). Kaplan-Meier survival analysis revealed that patients having elevated expression of both FOXM1 and CENPF had the worst outcome for three independent clinical endpoints, namely, time to biochemical-free recurrence (p ≤ 4.4×10-6), death due to prostate cancer (p ≤ 5.9×10-9), and time to metastasis (p ≤ 1.0×10-16) (Figure 7B). Notably, subcellular co-localization of FOXM1 and CENPF in prostate tumors was also associated with the worst outcome for all three independent clinical endpoints (Figure S5A). In contrast, elevated expression of only FOXM1 or CENPF was either not significant or marginally significant forbiochemical recurrence and prostate-specific survival (p ≤ 0.053 and p ≤ 0.011 for FOXM1, respectively; p ≤ 0.078 and p ≤ 0.402 for CENPF, respectively), and was 10 to 13 orders of magnitude less significant for time to metastasis (p ≤ 0.001 for FOXM1 and p ≤ 3.1×10-6 for CENPF) (Figure 7B).
We independently corroborated the association of FOXM1 and CENPF with disease outcome in two independent human prostate cancer datasets that had not been used for training purposes elsewhere in this study; namely, the Glinsky dataset, in which biochemical recurrence is the clinical endpoint (Glinsky et al., 2004), and the Sboner dataset, in which the clinical endpoint is prostate cancer-specific overall survival (Sboner et al., 2010) (Table S1). Using these independent cohorts, we evaluated the mRNA expression levels of FOXM1 and CENPF as well as their MARINa-inferred transcriptional activity (Supplementary Experimental Procedures) (Figures 7C and S5C). We then performed Kaplan-Meier survival analysis comparing 4 patient groups: (i) those with low inferred activity or expression for FOXM1 and CENPF; (ii) those with high inferred activity or expression only for FOXM1; (iii) those with high inferred activity or expression only for CENPF; and (iv) those with high inferred activity or expression for both FOXM1 and CENPF. Similar to our analysis of the TMA, patients with high inferred activity or mRNA expression for both CENPF and FOXM1 were associated with the worst outcome in both cohorts, as measured by biochemical recurrence (p ≤ 6.5×10-5) and prostate cancer-specific survival (p ≤ 4.0×10-5) (Figure 7C and S5C). Notably, these findings reveal that their MARINa-inferred activities are well correlated with the actual protein expression of FOXM1 and CENPF, and further demonstrate the striking association of their co-expression/co-activity with poor disease outcome.
Finally, association of FOXM1 and CENPF protein expression with disease outcome using C-statistics revealed its robust prognostic value for disease-specific survival (C = 0.71; C.I. 0.59-0.84, p ≤ 2.4×10-4), as well as time to metastasis (C = 0.77; C.I. 0.71-0.83, p ≤ 3.0×10-19) (Figure 7D). Notably, co-expression of FOXM1 and CENPF proteins dramatically improves prognosis over Gleason score alone, for both disease-specific survival (C = 0.86; C.I. 0.80-0.93, p ≤ 1.0×10-30; p value for improvement p ≤ 2.0×10-4) and time to metastasis (C = 0.86; C.I. 0.81-0.89, p ≤ 6.5×10-58; p value for improvement, p ≤ 5.3×10-13) (Figure 7D). Taken together, these analyses of independent clinical cohorts and using distinct statistical models demonstrate that co-expression of FOXM1 and CENPF is a robust prognostic indicator of poor disease outcome and metastasis.
Discussion
Recent advances in whole-genome analyses are providing an increasingly high-resolution view of the multitude of genetic, genomic, and epigenetic alterations associated with cancer phenotypes. Given the staggering number of potential interactions, identification of the true causal drivers and essential synergistic interactions represents a considerable challenge. In the current study, we have demonstrated that cross-species interrogation of genome-wide context-specific regulatory networks can address this challenge by dramatically winnowing the candidate gene interactions that implement the regulatory programs underlying cancer phenotypes. In particular, we have introduced a comprehensive systems approach to interrogate complementary regulatory networks for human and mouse prostate cancer to identify conserved causal regulators and to elucidate synergistic interactions among these. These studies have led to the identification of FOXM1 and CENPF as synergistic master regulators of prostate cancer malignancy and robust prognostic biomarkers of aggressive prostate cancer. We propose that this overall approach for genome-wide cross-species analysis will be generally applicable for identifying synergistic interactions that drive physiologic and pathologic phenotypes in cancer and other diseases.
The genome-wide assembly and cross-species interrogation of human and mouse prostate cancer regulatory networks represents a major conceptual advance. A critical requirement was the generation of a mouse prostate cancer interactome from a dataset of appropriate size and expression heterogeneity. We incorporated the diversity afforded by genetically and phenotypically distinct mouse models of prostate cancer obtained through a community effort (see Supplementary Experimental Procedures), in combination with exogenous perturbations administered to each mouse model. This strategy has led to successful construction of a genome-wide, context-specific mouse interactome for the study of prostate cancer, and is generalizable for the generation of interactomes for a wide range of physiologic and pathologic phenotypes.
A second critical requirement was the development of an informative algorithm to determine whether the human and mouse prostate cancer interactomes represented conserved regulatory programs, thus enabling accurate and robust cross-species integrative analysis. Towards this end, we introduced a metric for quantitative assessment of conservation of regulatory networks, which revealed that the large majority of regulatory programs represented by these networks are highly conserved (>70%). Although the current study is focused on prostate cancer interactomes assembled using ARACNe, this general approach for evaluating conservation can be used for cross-species analyses of regulatory networks for other cancers or other diseases and can be readily adapted for analyses of networks inferred using alternative algorithms, such as those based on the Context Likelihood of Relatedness (CLR) and Bayesian-networks algorithms (Akavia et al., 2010; Faith et al., 2007). Indeed, we envision that the ability to quantitatively evaluate conservation of cross-species regulatory programs will be broadly applicable for other physiological and pathological comparisons, and particularly beneficial for accurate integration of pre-clinical findings from genetically engineered mice to human clinical trials.
A third critical requirement for the success of our approach was our ability to effectively mine these cross-species regulatory networks to identify conserved master regulators of cancer malignancy and to identify their synergistic interactions. The MARINa algorithm used for these analyses defines “master regulators” as genes that most significantly regulate the transcriptional program associated with a particular phenotype (in this case, prostate cancer malignancy), and hence are rate-limiting drivers of the phenotype (Carro et al., 2010; Lefebvre et al., 2010). Notably, MARINa also provides an effective computational tool for analyses of synergistic interactions among master regulators (Carro et al., 2010); indeed, our unbiased interrogation of the ~2,000 transcriptional regulators represented in the interactomes led to identification of a single synergy pair, namely FOXM1 and CENPF. The power of this approach suggests that it may be of general value in dissecting polygenic dependencies in cancer and other diseases.
While both FOXM1 and CENPF have been implicated in various cancers, our study has uncovered their unexpected synergistic interaction. FOXM1 encodes a forkhead domain transcription factor that is frequently over-expressed in many different types of cancer, including prostate (Alvarez-Fernandez and Medema, 2013; Halasi and Gartel, 2013a; Kalin et al., 2011; Koo et al., 2012). Many previous studies have established a role for FOXM1 in regulation of cellular proliferation, DNA damage, genomic stability, drug resistance, and metastasis, and have shown that it interacts with other key regulators such as β–Catenin and MYB (Lefebvre et al., 2010; Zhang et al., 2011). In prostate cancer, gain- or loss-of-function of FOXM1 in vivo have been shown to elicit modest effects on tumor growth (Cai et al., 2013; Kalin et al., 2006).
CENPF (also known as mitosin or LEK1 in mouse), a known target of FOXM1, has also been implicated in various cancers, although not previously in prostate, and in some cases has been shown to undergo gene amplification and to be associated with disease outcome (Ma et al., 2006; Varis et al., 2006). However, the actual functional role of CENPF has been more elusive and difficult to reconcile. In particular, while CENPF is named for its association with the centromere-kinetochore protein complex, such association is transient and, in fact, CENPF has other functions, including regulation of mitosis and cellular proliferation (Bomont et al., 2005; Feng et al., 2006; Holt et al., 2005), which are mediated in part by protein interactions (Ma et al., 2006; Varis et al., 2006).
Thus, although the individual functions of FOXM1 and CENPF in cancer had been well-studied, their synergistic interaction could not have been anticipated from previous analyses. Cumulatively, our findings suggest that co-expression of FOXM1 and CENPF in prostate cancer leads to co-regulation of transcriptional programs, which ultimately result in activation of the key signaling pathways associated with prostate cancer malignancy, including the PI3K and MAPK signaling pathways. Since FOXM1 and CENPF can each be targeted pharmacologically (Halasi and Gartel, 2013b; Pan and Yeung, 2005; Radhakrishnan et al., 2006), their inhibition may provide an effective means of treating advanced prostate cancer; indeed, therapeutic targeting of FOXM1 and CENPF may help overcome the complex feedback mechanisms that have hindered therapeutic targeting of PI3K and MAPK signaling pathways.
Furthermore, we envision that by using alternative gene signatures that represent other prostate cancer phenotypes, genome-wide cross-species analysis of master regulators and their potential synergistic interactions may help to define molecular subtypes of prostate cancer, which have thus far been elusive. More broadly, our general approach to elucidate conserved and functionally relevant gene interactions can be applied to many tumor contexts, as well as other human diseases, for which appropriate model systems are available.
Experimental Procedures
Assembly of interactomes and master regulator analyses
Expression profile datasets for human prostate cancer are described in Table S1. Genetically engineered mouse models (GEMMs) are described in Table S2 and their representative histopathology shown in Figure S1A. A description of perturbagen treatments is provided in the Supplementary Experimental Procedures. Human and mouse interactomes were assembled using the ARACNe algorithm (Margolin et al., 2006a). Details of the resulting human and mouse networks are provided in Tables S3 and S4, respectively. Analysis of cross-species network conservation was done using a modification of the MARINa algorithm described in the Supplementary Experimental Procedures. Master regulator analysis and computational synergy analysis were performed using MARINa (Carro et al., 2010; Lefebvre et al., 2010). Master regulators for the human and mouse interactomes are provided in Tables S6 and S7, respectively.
Functional validation
Gene silencing of FOXM1 and CENPF as well as forced expression of FOXM1 were done using lentiviral shRNAs or expression vectors (Open Biosystems and CCSB Human ORFeome Library, respectively). Human cancer cell lines used for functional studies were obtained from ATCC. All experiments using animals were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University Medical Center.
Tissue microarray analyses
All studies involving human subjects were approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center or University of Michigan. The study was conducted with approval from the Human Biospecimen Utilization Committee and Institutional Review Board; consent was obtained from all patients, as required. Analysis of protein expression of FOXM1 and CENPF was performed using a high-density TMA (Donovan et al., 2008) and a metastasis TMA (Shah et al., 2004). Available clinico-pathological features of these TMAs are summarized in Table S1.
Statistical Methods
Statistical analysis was performed with survcomp package using R v2.14.0. Cox proportional hazard model was estimated with the surv and coxph functions. Kaplan-Meier survival analysis was performed using surv, survfit, and survdiff functions. Concordance indexes (c-index) were estimated and compared using coxp and concordance.index (counting ties) and cindex.comp functions. Details of all statistical analyses and all computational procedures are provided in the supplementary experimental procedures as well as in an accompanying executable SWEAVE document.
Accession numbers
Mouse expression profiling data can be accessed at GSE53202. The executable SWEAVE document can be accessed at http://dx.doi.org/10.6084/m9.figshare.928353.
Supplementary Material
Highlights.
Genome-wide cross-species interrogation of disease-specific regulatory networks
Cross-species interrogation identifies conserved master regulators of malignancy
FOXM1 and CENPF interact synergistically to drive malignant prostate cancer
Co-expression of FOXM1 and CENPF is prognostic for prostate cancer outcome
Significance.
Genetically engineered mouse models have been widely used for in vivo analyses of cancer phenotypes as well as for preclinical investigations. However, inherent species differences often hinder the appropriate extrapolation of studies performed in mice to human cancer. Here we introduce a strategy using cross-species computational analysis of context-specific regulatory networks for the effective integration of experimental findings from mouse models and human cancer. This approach enabled the identification of conserved master regulators of malignant prostate cancer, as well as elucidation of their synergistic interactions. This computational paradigm should be broadly applicable for elucidating causal mechanisms of cancer, as well as integrating preclinical analyses from mouse to man.
Acknowledgments
We are indebted to our colleagues who generously provided genetically engineered mouse models for assembly of the mouse prostate cancer interactome, namely Drs. Bart Williams (Van Andel), Terry Van Dyke (NCI-Frederick), Barbara Foster (Roswell Park Cancer Center), Yu Chen (MSKCC) and Charles Sawyers (MSKCC). We are also indebted to Dr. Victor Reuter (MSKCC) for generously sharing the prostatectomy tissue microarray. We thank Jose Silva and members of his laboratory for invaluable help with establishment of the shRNA validation studies, and Drs. Adolfo Ferrando and Riccardo Dalla Favera for critical reading of the manuscript.
This work was supported by grants CA084294 (to CAS, MMS and AC), U54 CA121852 (to AC, CAS, MMS), CA154293 (to MMS and CAS), DK076602 (to MMS), Silico Research Centre of Excellence NCI-caBIG, SAIC 29XS192 (to AC), the Michigan Center for Translational Pathology, SPORE grant P50 CA69568 (to KJP), and an award from the V-Foundation for Cancer Research (to CAS). AA was a recipient of a Marie Curie International Outgoing Fellowship (PIOF-GA-2009-253290), co-sponsored with the Catalan Institute of Oncology-Bellvitge Institute for Biomedical Research, Barcelona, Spain. AM is a recipient of a Prostate Cancer Foundation Young Investigator Award. CAS is an American Cancer Society Research Professor supported in part by a generous gift from the F.M. Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akavia UD, Litvin O, Kim J, Sanchez-Garcia F, Kotliar D, Causton HC, Pochanard P, Mozes E, Garraway LA, Pe'er D. An integrated approach to uncover drivers of cancer. Cell. 2010;143:1005–1017. doi: 10.1016/j.cell.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Fernandez M, Medema RH. Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Frontiers in oncology. 2013;3:30. doi: 10.3389/fonc.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, LeKaye HC, Koutcher JA, Cardiff RD, Califano A, et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci U S A. 2013;110:E3506–3515. doi: 10.1073/pnas.1303558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, Park K, Kitabayashi N, MacDonald TY, Ghandi M, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005;37:382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–3939. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Balli D, Ustiyan V, Fulford LA, Hiller A, Misetic V, Zhang Y, Paluch AM, Waltz SE, Kasper S, Kalin TV. Foxm1 Expression in Prostate Epithelial Cells is Essential for Prostate Carcinogenesis. J Biol Chem. 2013 doi: 10.1074/jbc.M113.455089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. The Journal of urology. 2007;178:S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MJ, Hamann S, Clayton M, Khan FM, Sapir M, Bayer-Zubek V, Fernandez G, Mesa-Tejada R, Teverovskiy M, Reuter VE, et al. Systems pathology approach for the prediction of prostate cancer progression after radical prostatectomy. J Clin Oncol. 2008;26:3923–3929. doi: 10.1200/JCO.2007.15.3155. [DOI] [PubMed] [Google Scholar]
- Faith JJ, Hayete B, Thaden JT, Mogno I, Wierzbowski J, Cottarel G, Kasif S, Collins JJ, Gardner TS. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS Biol. 2007;5:e8. doi: 10.1371/journal.pbio.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Huang H, Yen TJ. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 2006;115:320–329. doi: 10.1007/s00412-006-0049-5. [DOI] [PubMed] [Google Scholar]
- Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL. Gene expression profiling predicts clinical outcome of prostate cancer. J Clin Invest. 2004;113:913–923. doi: 10.1172/JCI20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013a;12:245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochemical pharmacology. 2013b;85:644–652. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Holt SV, Vergnolle MA, Hussein D, Wozniak MJ, Allan VJ, Taylor SS. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci. 2005;118:4889–4900. doi: 10.1242/jcs.02614. [DOI] [PubMed] [Google Scholar]
- Irshad S, Abate-Shen C. Modeling prostate cancer in mice: something old, something new, something premalignant, something metastatic. Cancer metastasis reviews. 2013;32:109–122. doi: 10.1007/s10555-012-9409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, Simons BW, Ward JM, Robinson BD, Chu GC, et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013;73:2718–2736. doi: 10.1158/0008-5472.CAN-12-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle. 2011;10:396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, Lyubimov A, Costa RH. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res. 2006;66:1712–1720. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo CY, Muir KW, Lam EW. FOXM1: From cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Rajbhandari P, Alvarez MJ, Bandaru P, Lim WK, Sato M, Wang K, Sumazin P, Kustagi M, Bisikirska BC, et al. A human B-cell interactome identifies MYB and FOXM1 as master regulators of proliferation in germinal centers. Mol Syst Biol. 2010;6:377. doi: 10.1038/msb.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Rieckhof G, Califano A. Reverse-engineering human regulatory networks. Wiley interdisciplinary reviews Systems biology and medicine. 2012;4:311–325. doi: 10.1002/wsbm.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhao X, Zhu X. Mitosin/CENP-F in mitosis, transcriptional control, and differentiation. Journal of biomedical science. 2006;13:205–213. doi: 10.1007/s11373-005-9057-3. [DOI] [PubMed] [Google Scholar]
- Margolin AA, Nemenman I, Basso K, Wiggins C, Stolovitzky G, Dalla Favera R, Califano A. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7 Suppl. 2006a;1:S7. doi: 10.1186/1471-2105-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin AA, Wang K, Lim WK, Kustagi M, Nemenman I, Califano A. Reverse engineering cellular networks. Nat Protoc. 2006b;1:662–671. doi: 10.1038/nprot.2006.106. [DOI] [PubMed] [Google Scholar]
- Pan J, Yeung SC. Recent advances in understanding the antineoplastic mechanisms of farnesyltransferase inhibitors. Cancer Res. 2005;65:9109–9112. doi: 10.1158/0008-5472.CAN-05-2635. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- Sboner A, Demichelis F, Calza S, Pawitan Y, Setlur SR, Hoshida Y, Perner S, Adami HO, Fall K, Mucci LA, et al. Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med Genomics. 2010;3:8. doi: 10.1186/1755-8794-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- Schoenborn JR, Nelson P, Fang M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin Cancer Res. 2013;19:4058–4066. doi: 10.1158/1078-0432.CCR-12-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varis A, Salmela AL, Kallio MJ. Cenp-F (mitosin) is more than a mitotic marker. Chromosoma. 2006;115:288–295. doi: 10.1007/s00412-005-0046-0. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, et al. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QC, Petrey D, Deng L, Qiang L, Shi Y, Thu CA, Bisikirska B, Lefebvre C, Accili D, Hunter T, et al. Structure-based prediction of protein-protein interactions on a genome-wide scale. Nature. 2012;490:556–560. doi: 10.1038/nature11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.