Abstract
The effects of eight different cations with ionic radii between 69 and 337 pm on the charging of peptides and proteins with electrospray ionization from aqueous acetate salt solutions are reported. Significant adduction occurs for all cations except NH4+, and the average protein charge is lower when formed from solutions containing salts compared to solutions without salts added. Circular dichroism and ion mobility results show the protein conformations are different in pure water compared to salt solutions, which likely affects the extent of charging. The average charge of protein and peptide ions formed from solutions with Li+ and Cs+, which have Gibbs solvation free energies (GSFEs) that differ by 225 kJ/mol, is similar. Lower charge states are typically formed from solutions with tetramethylammonium and tetraethylammonium that have lower GSFE values. Loss of the larger cations that have the lowest GSFEs is facile when adducted protein ions are collisionally activated resulting in the formation of lower analyte charge states. This reaction pathway provides a route to produce abundant singly protonated protein ions under native mass spectrometry conditions. The average protein and peptide charge with NH4+ is nearly the same as that with Rb+ and K+, cations with similar GSFE and ionic radii. This indicates that proton transfer from NH4+ to proteins plays an insignificant role in the extent of protein charging in native mass spectrometry.
Introduction
Electrospray ionization (ESI) is widely used to produce intact, multiply charged gas-phase macromolecular ions directly from solution for analysis by mass spectrometry (MS) [1, 2]. Multiple charging has the advantage that the mass-to-charge (m/z) ratios of the intact molecular ions are typically within the upper range of most mass spectrometers. The extent of charging depends on many factors, including solution-phase conformation [3-5], solvent surface tension [6, 7], basicities of the analyte and solvent [1, 2, 7, 8] instrumental parameters [3-5, 9], and other factors. Broad distributions of high charge states are formed from solutions in which proteins are denatured, whereas more narrow distributions of low charge states are formed from aqueous buffered solutions where proteins have native or native-like conformations. The extent of charging from either denaturing or buffered aqueous solutions can be significantly increased by supercharging [6, 7, 10-19] or decreased by addition of bases either in solution [7] or in the gas phase [20, 21].
Several ESI ion formation mechanisms have been proposed to account for the observed charging, including the charge residue mechanism (CRM) [22], the ion evaporation mechanism (IEM) [23], and the combined charge residue field emission model (CCRFEM) [24], which incorporates aspects of both of the former mechanisms. Highly charged droplets that are produced by ESI can undergo solvent evaporation and Rayleigh fission to form smaller, highly charged droplets that carry away a significant fraction of the original droplet charge [25, 26]. In the IEM, gas-phase ions are produced from the ESI droplet when the electrostatic energy at the surface of the droplet overcomes the Gibbs free energy barrier for the process
where X+ is the ion of interest and n is the number of water molecules [23, 27]. In the CRM, gas-phase ions are produced by solvent evaporation in the late stages of the droplet lifetime [22]. Globular protein and polymer charging is related to the Rayleigh limit charge, ZR, of droplets roughly the same size as the analyte molecule, a result that is consistent with the CRM for ion formation under native conditions [6, 28]. In contrast, much less charge would be expected if these ions were produced by the IEM [6].
Ions, or buffers commonly used in native ESI mass spectrometry, can also affect charging of proteins and peptides, an effect related to the ionic strength as well as charge state of the ions [29-34]. Ammonium acetate is extensively used as a buffer in native ESI mass spectrometry. The extent of protein charging from aqueous ammonium acetate solution is often thought to be determined by proton transfer from NH4+ to the protein [2, 35-49]. It was proposed that “NH4+ ions present at the surface of the shrinking water droplet, which are part of the excess charge on the droplet, are expected to attach themselves to the basic sites and ultimately lead to their protonation” [35]. However, other results indicate that cations typically adduct to acidic sites such as carboxyl groups [50, 51]. In the CCRFEM, small ions that reside at the droplet surface evaporate from the droplet at a rate determined by their solvation energy and the electric field strength at the droplet surface, whereas macromolecules are located in the interior of the droplet and ionize by the CRM [24]. Salts with low solvation energies carry away some of the droplet surface charge resulting in fewer charges available to the macromolecule [24].
Here, the effects of cations with a wide range in Gibbs solvation free energy (GSFE) values in aqueous acetate solutions on protein and peptide charge are investigated. The extent of charging trends with the GSFE, but loss of large cations with low GSFE values readily occurs from adducted molecular ions in the gas phase resulting in formation of lower charge states. This dissociation pathway results in a facile method to produce singly protonated protein ions in native mass spectrometry. These results also show that proton transfer reactions from protonated ammonia to proteins and peptides do not play a significant role in the extent of charging in native mass spectrometry.
Experimental
Data were acquired using either a Waters Q-TOF Premier hybrid mass spectrometer or a Waters Synapt G2 High Definition mass spectrometer (Waters, Milford, MA, USA) equipped with a Z-spray nanoelectrospray (nano-ESI) ion source. Peptide or protein solutions (50 μM) with 25 mM acetate salts (unless otherwise noted) were prepared using Millipore Milli-Q water. The pH of all protein and peptide solutions except those in cesium acetate and rubidium acetate solutions ranges from 6.5 to 6.9. The pH of the protein and peptides in cesium and rubidium acetate solutions is 5.0, likely a result of different suppliers for these salts. Nano-ESI emitters, made by pulling borosilicate glass capillaries (1.0 mm o.d./0.78 mm i.d.; Sutter Instruments, Novato, CA, USA) to a tip i.d. of approximately 1 m with a Flaming/Brown micropipette puller (model P-87; Sutter Instruments), were filled with ~5 μL of sample. A 1.0-1.5 kV potential was applied to a platinum wire (0.127 mm diameter, Sigma, St. Louis, MO, USA) that is in contact with the solution to initiate ion formation by nano-ESI. Gentle source conditions that minimized ion activation were used. For ion mobility experiments, the traveling wave ion mobility cell was operated at a He flow rate of 180 mL/min, an IMS gas (N2) flow rate of 90 mL/min, a traveling wave velocity of 1000 m/s, and a wave height of 40 V.
The average charge was calculated as an abundance weighted sum of all individual charge states divided by the total ion abundance for all charge states. The uncertainties correspond to the standard deviation of three replicate measurements from three separate ESI emitters. Angiotensin II, bradykinin, substance P, bovine ubiquitin, egg white lysozyme, β-lactoglobulin, and ammonium acetate were obtained from Sigma (St. Louis, MO, USA); Substance P (free acid) from GenScript (Piscataway, NJ, USA); Substance P methyl ester from American Peptide Company (Sunnyvale, CA, USA); Lithium acetate, sodium acetate, potassium acetate from Fisher Scientific (Pittsburgh, PA, USA); Tetramethylammonium acetate and tetraethylammonium acetate from MP Biomedicals (Solon, OH, USA); Cesium acetate and rubidium acetate from Alfa Aesar (Ward Hall, MA, USA). All chemicals were used without further purification.
Circular dichroism (CD) measurements were performed using a Jasco 810 spectropolarimeter (JASCO, Inc., Easton, MD, USA). Solutions were stirred with a Teflon stir bar in a 1.0 cm quartz cuvette at room temperature during wavelength scan analysis from 190 to 260 nm.
Results and Discussion
Effects of cations on protein and peptide charge
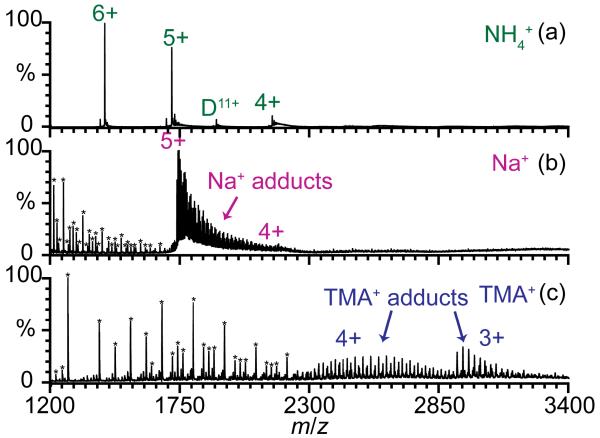
Ions of eight different proteins and peptides were formed by nano-ESI from aqueous solutions containing nine different acetate salt solutions to determine how cations affect the charge states that are produced. Protein and peptide ions formed from these solutions are expected to have native or native-like structures. These biopolymers have pI values between 4.8 and 12.0 [52], and the Gibbs solvation free energies of the different cations span a 475 kJ/mol range [53]. Extensive cation adduction generally occurs for all protein and peptide ions formed from these acetate salt solutions, with the most adduction occurring with sodium and potassium (Supplemental Table 1). Protonated molecular ions with no NH4+ adducts are produced with ammonium acetate, but with the other cations, minimal or no protonated ions are formed. For example, over 30 Na+ adduct to the 5+ charge state of ubiquitin formed from sodium acetate solution, whereas no NH4+ adducts to this same charge state with ammonium acetate (Figure 1). Alkali metal acetate and alkylammonium acetate clusters are also abundant, especially below m/z ~1500 (Figure 1). Ammonium acetate clusters are not observed.
Figure 1.
Nano-ESI mass spectra of 50 μM bovine ubiquitin ions formed from 25 mM aqueous solutions of (a) ammonium acetate, (b) sodium acetate, and (c) tetramethylammonium acetate. (*) indicate (b) sodium acetate or (c) tetramethylammonium acetate clusters, some of which are multiply charged.
The Rayleigh limit charge of a water droplet of the same size as each ion was estimated by approximating the molecules as spheres with densities of 1 mg/mL [6, 28] (ZR,m) and also by using gas-phase collisional cross sections (σ) [20, 54-56] of the lowest charge state reported for the molecular ions to obtain the radius approximating the ion as a sphere (ZR,σ). These values, along with the maximum (Zmax) and average (Zave) charge of these ions formed from water without salts added and aqueous solutions with NH4+ or Rb+ are given in Table 1. The charge of protein and peptide ions formed from NH4+ and Rb+ acetate solutions are compared because the ionic radii of NH4+ and Rb+ are similar (148 pm and 149 pm, respectively) as are their GSFEs (−285 kJ/mol and −275 kJ/mol, respectively; all values are relative to a GSFE of tetraethylammonium (TEA+) which is arbitrarily assigned a value of zero) [53]. ZR,σ is consistently larger than ZR,m by an average of 27% and these values provide an indication of the range of uncertainties in estimating the charging predicted by the CRM. Zmax for protein and peptide ions formed from water is within one charge of ZR,σ except for β-lactoglobulin where Zmax is much lower than ZR,σ. For the biopolymer ions formed from pure water, Zmax is 83% of ZR,σ and 106% of ZR,m, respectively. Lower charge states of biopolymer ions are produced from ammonium acetate and rubidium acetate solutions; Zmax is 75 and 69% of ZR,σ for these respective solutions, and Zmax is 95 and 88% of ZR,m. These results are consistent with those of De la Mora who reported that the Zmax and Zave of protein ions formed from water or ammonium acetate are within 60 to 110% of ZR [28]. Results for dendrimers showed a correlation between ion charging in different solvents and ZR calculated using the surface tension of the different solvents [6]. These results provide support for large globular protein ions being formed by the CRM. In contrast, ions formed by the IEM would have significantly less charge [6].
Table 1.
ZR,σ and ZR,m for water droplet the same size as ion of interest compared to Zmax and Zave for ions formed from aqueous solutions without salts added, or with 25 mM NH4+ or Rb+.
| Water, no salts added |
NH4+ | Rb+ | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein/Peptide | ZR,σ | ZR,m | Zmax | Zave | Zmax | Zave | Zmax | Zave |
|
| ||||||||
| Ubiquitin | 9.5+ | 7.4+ | 10+ | 6.5+ | 6+ | 5.4+ | 5+ | 5.0+ |
| Lysozyme | 12+ | 9.4+ | 11+ | 9.4+ | 9+ | 7.7+ | 9+ | 7.5+ |
| β-lactoglobulin | 14+ | 11+ | 10+ | 8.0+ | 9+ | 7.9+ | 8+ | 7.4+ |
| Angiotensin II | 3.3+ | 2.6+ | 2+ | 1.8+ | 2+ | 1.9+ | 2+ | 1.8+ |
| Bradykinin | 3.2+ | 2.6+ | 3+ | 2.0+ | 3+ | 2.0+ | 2+ | 2.0+ |
| SubPNH2 | 3.7+ | 2.9+ | 3+ | 2.7+ | 3+ | 2.1+ | 3+ | 2.2+ |
| SubPOH | 3.7+ | 2.9+ | 3+ | 2.2+ | 3+ | 2.0+ | 3+ | 2.0+ |
| SubPOMe | 3.7+ | 2.9+ | 3+ | 2.9+ | 3+ | 2.1+ | 3+ | 2.2+ |
The average protein charge as a function of the relative Gibbs solvation free energy of the cations is shown in Figure 2. The dashed lines in the figures correspond to the average charge of these protein ions formed from aqueous solutions without salts added, which are higher than those from solutions containing acetate salts, although the effects of salts are minimal for β-lactoglobulin (Figure 2). The average protein ion charge decreases by 4 ± 1% from Li+ (GSFE is −475 kJ/mol) to Cs+ (−250 kJ/mol), a range of 225 kJ/mol in Gibbs solvation free energy. With tetramethylammonium (TMA+) and TEA+ acetate, which have the lowest Gibbs solvation free energies [53], the average charge is much lower. The most significant change in protein charging occurs between Cs+ and TMA+. Protein ions formed from TMA+ and TEA+ acetate solutions have on average 32 and 43% less charge, respectively, than those formed from Cs+ acetate solutions. The lower charging for protein ions formed from solutions with salts could be a result of cation evaporation from the ESI droplet lowering the overall charge available for the protein as would be the case for the CCRFEM [24].
Figure 2.
Average charge state of 50 μM (a) β-lactoglobulin, (b) bovine ubiquitin, and (c) egg-white lysozyme formed from 25 mM (solid diamond) or 100 mM (solid diamond) acetate salt solutions and water (dashed line) plotted as a function of relative Gibbs solvation free energy of the cation (referenced to TEA+, which is assigned a value of zero).
The relative ion evaporation rates for alkali metal and alkylammonium ions differ significantly and correlate with the Gibbs solvation free energies (GSFEs) of the ions at low concentrations (< 10−5 M), but the differences in ion evaporation rates for the different ions is significantly less at high concentrations (> 10−5 M) and the correlation with the GSFEs of the cations is low [57-59]. The relative rates of ion evaporation for the alkali metals do not depend strongly on GSFE at high concentration, and the rate of evaporation for Li+ is higher than that of Cs+ [27, 58], which is the opposite of the trend reported at low salt concentrations. The lower charging of protein ions formed from Cs+ solutions compared to that from Li+ solutions is inconsistent with the relative ion evaporation rates reported for these alkali metal ions at high concentrations [27, 58] if the protein ions are formed by the CCRFEM. The relative rate of ion evaporation of TEA+ is approximately 5 times that of Cs+ at high concentrations [58], which is consistent with the CCRFEM for the formation of lower protein charge from the alkylammonium acetate versus the alkali metal ion acetate solutions.
Some studies suggest that peptide ions are produced by the IEM [60-62] whereas others indicated the possibility that peptide ions are produced by the CRM [6, 62]. In contrast to proteins, the average charge for peptide ions formed from water without salts added can be lower (three different forms of substance P), higher (angiotensin II) or similar (bradykinin) to the average charge when the alkali metal or ammonium acetate salts are added (Figure 3). The average charge of all peptide ions, except SubPNH2, is lower when these ions are formed from TMA+ and TEA+ acetate solutions compared to the average charge of these ions formed from the other solutions. There is no significant dependence of the average charge of SubPNH2 ions on the GSFE of the cations. The protein and most peptide ions investigated here follow a similar trend, in which similar charge states are formed from ammonium and alkali metal ion acetate solutions and lower charge states are formed from alkylammonium acetate solutions. The higher average charge of angiotensin II ions formed from alkali metal ion and ammonium acetate salt solutions than from pure water suggests that evaporation of small cations does not play a significant role in the charging of these ions.
Figure 3.
Average charge state of 50 μM (a) SubPNH2, (b) SubPOMe, (c) SubPOH, (d) angiotensin II, and (e) bradykinin formed from 25 mM (solid diamond) or 100 mM (solid diamond) acetate salt solutions and water (dashed line) plotted as a function of relative Gibbs solvation free energy of the cation (referenced to TEA+, which is assigned a value of zero).
Effects of protein and peptide structure on charge
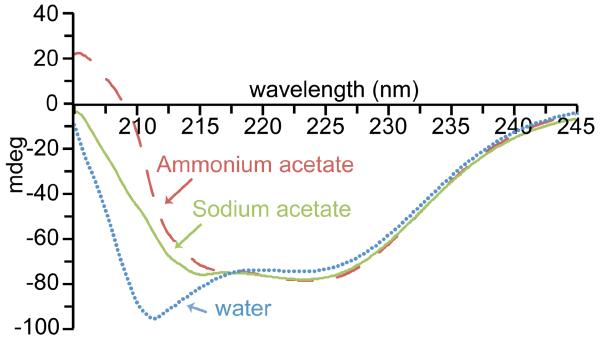
The shapes of molecules in solution can significantly affect the charge of these ions in ESI. The effects of salts on the secondary structure of ubiquitin (Figure 4), β-lactoglobulin and lysozyme (Supplemental Figures 2 and 3) were investigated using circular dichroism (CD) in the far-UV region, which probes α-helical and β-sheet content. The CD spectrum of ubiquitin in water without salts added is distinctly different from the CD spectra of ubiquitin in 25 mM ammonium acetate and 25 mM sodium acetate solutions. There is greater ellipticity at 208 nm in water, indicative of more α-helical structure [63], than when salts are present. There are more subtle differences in the CD spectra of ubiquitin in ammonium acetate and sodium acetate, indicating that the identity of the cation has only a small influence on the secondary structure of ubiquitin in these solutions. CD spectra of β-lactoglobulin and lysozyme (Supplemental Figures 2 and 3) in water without salts added and water with either ammonium acetate or sodium acetate added show similar trends. The CD signal for lysozyme in water has greater positive ellipticity between 225 and 230 nm, indicative of more α-helical structure [63] (Supplemental Figure 3) and the signal for β-lactoglobulin in water is less intense at 217 nm than the corresponding signal of β-lactoglobulin in ammonium acetate and sodium acetate. These CD results show that these proteins have measurable differences in their secondary structure in water without added salts compared to when ammonium acetate or sodium acetate is in the solution and that the identity of the cation also has subtle effects on secondary structure in solution.
Figure 4.
Circular dichroism spectra for 25 μM ubiquitin in 25 mM sodium acetate (green line), 25 mM ammonium acetate (red dashed line), and water (blue dotted line).
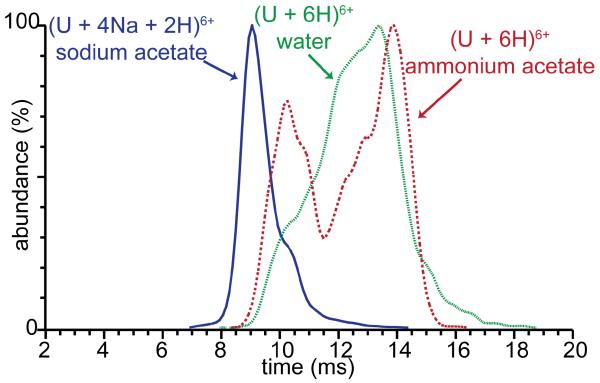
Ion mobility spectrometry (IMS) was also used to determine whether ubiquitin ions formed from water without salts and with ammonium acetate or sodium acetate retain different conformations in the gas phase. Arrival time distributions of (U + 6H)6+ formed from water and from ammonium acetate and for (U + 4Na + 2H)6+ formed from sodium acetate solutions are shown in Figure 5. The arrival time distributions of (U + 6H)6+ formed from ammonium acetate have two broad features centered at 10 and 14 ms, whereas the arrival time distributions for the same ions formed from water has a very broad feature at 13.5 ms with a less abundant undefined shoulder at 10 ms. The arrival time distributions of (U + 6H)6+ formed from these two solutions show a distinct range of conformer families with the more compact conformers most abundant in ammonium acetate solution. These data clearly show that the gas-phase conformations of protonated ubiquitin 6+ formed from water and ammonium acetate solutions are different, and therefore the solution-phase structures of these ions are most likely different as well, a result consistent with the CD measurements. The greater abundance of more compact conformers from ammonium acetate solution is also consistent with the lower charging observed from this solution.
Figure 5.
Ion mobility arrival time distributions of ubiquitin (U + 6H)6+ formed from water (green dotted line), 25 mM ammonium acetate (red solid line) and (U + 4Na + 2H)6+ formed from 25 mM sodium acetate (blue dashed line).
No (U + 6H)6+ is formed from the sodium acetate solution, but the arrival time distribution of (U + 4Na + 2H)6+ has a feature centered at 9 ms with a shoulder at 10.5 ms. This indicates that the partially sodiated ions are even more compact than the compact conformers of the fully protonated ions with the same charge state formed from water or ammonium acetate. Nonspecific cation adduction to gaseous protein ions typically results in compaction of structure compared to the protonated form, even when the ions are formed from the same solution [64]. The CD results show that the protein conformation in aqueous solutions without salts added is different than in ammonium acetate solutions. IMS results show that this conformational difference in solution translates to a difference in the conformation of gas-phase ions. The difference in solution-phase conformation of proteins in solutions without salts versus with salts added likely contributes significantly to the difference in protein charging from these solutions.
CD spectra were also obtained for angiotensin II and the three forms of SubP in water with and without salts added to determine if the salts affect the secondary structure of these peptides. The CD signal for these peptides in water without salts added is the same as that with ammonium acetate or sodium acetate added (Supplemental Figures 1 and 4) indicating that there is not a significant difference in the secondary structure of these peptides with and without salts. The C-termini of the three forms of SubP differ and this affects the relative charging of these peptides from acetate solutions and water. This indicates that the primary structure of the peptide plays a role in the effect of cations on the extent of charging.
Effect of internal energy on protein charge
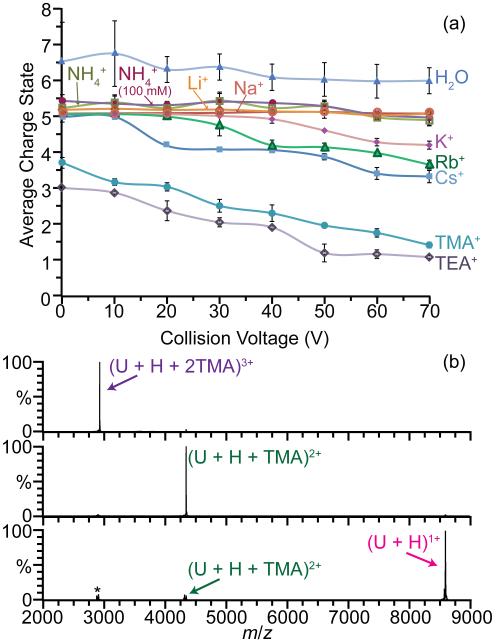
Energetic collision of multiply charged ions with gases can lower the charge states in ESI [65]. The effects of collisional activation on the average charge of ubiquitin ions formed from various acetate salt solutions and from pure water as a function of collision voltage are shown in Figure 6a. For K+, Rb+, Cs+, TMA+, and TEA+ acetate solutions, the average charge decreases with increasing collision voltage. For example, the average charge of ubiquitin ions formed from TEA+ acetate decreases from 2.95 ± 0.02 to 1.03 ± 0.04, at collision voltages of 0 and 70 V, respectively. In contrast, there is a minimal decrease in the average charge of ubiquitin ions formed from water without salts added, and aqueous solutions with Li+, Na+ or NH4+ over the same range of collision voltages. The average charge of ubiquitin ions formed from solutions with TMA+ and TEA+ is lower than with the other ions at 0 V. Based on results at high collision voltage, it is likely that some collisional activation that occurs in the source, even with soft conditions, lowers the charge from that originally produced by ESI.
Figure 6.
(a) Average charge of ubiquitin ions formed from 25 mM (unless otherwise noted) acetate salt solutions from collision voltages 0 to 70 V. (b) Collisional activation of (U + H + 2TMA)3+ at 0 V (top) to form (U + H + TMA)2+ at 40 V (middle) and (U + H)1+ at 80 V (bottom). (*) indicates stable low abundance cluster ions at same m/z as precursor that are also isolated at 0 V.
The decrease in average charge with increasing collision energy can be attributed to two dissociation pathways: loss of adducted cations and preferential fragmentation of the high charge ions. Loss of TEA+ and TMA+ from ubiquitin is facile whereas loss of Li+ or Na+ does not occur prior to backbone fragmentation at higher collision energy. For example, collisional activation of (U + 7Cs − 2H)5+ at 60 V results in the sequential loss of Cs+ to form (U + 6Cs − 2H)4+ and (U + 5Cs − 2H)3+ exclusively whereas collisional activation of (U + 7Na − 2H)5+ at the same voltage results in b2+, b3+, y3+ and y4+ fragments but no loss of Na+. High charge states of ubiquitin ions formed from water, ammonium acetate, sodium acetate and lithium acetate solutions fragment to form b and y ions at collision energies of ~30 V and higher. In contrast, b and y ions from ubiquitin ions generated from the other acetate salt solutions start to form between 50 - 80 V. These results are consistent with the loss of the larger cations taking away internal energy and thus more internal energy deposition is required to produce backbone cleavage.
The binding energies of these cations to gaseous proteins and peptides are not known, but the gas phase binding energies of water molecules to these cations have been reported [66-69]. The binding energies of a water molecule to Li+, Na+, NH4+, K+, Rb+, Cs+, TMA+ and TEA+ are 142, 100, 83, 75, 67, 57, 38 and 29 kJ/mol, respectively [67-69]. This ordering is nearly the same as the GSFE values of these cations. We expect there to be a similar trend in the binding energies of these cations to peptides and proteins. Larger cations should be bound less strongly to the peptide or protein and should be more readily lost. The higher collision voltage necessary to form b and y ions for the large cations can be attributed to the energy lost as a result of breaking the bond between the cation and protein; additional energy must be added to form b and y fragments.
Hogan et al. [24] found that the charge states of several protein ions formed by ESI from triethylammonium bicarbonate solutions are lower than the charge states of the corresponding ions formed from ammonium acetate solutions. Triethylammonium evaporates more rapidly from a droplet than ammonium [24] and could lower droplet charge and the overall charge of the protein. However, TEA+ adducts much less strongly to the protein ion and is easily lost as a result of gas-phase collisional activation, which also lowers the overall charge of the protein ion.
Effects of cation proton transfer reactivity on protein and peptide charge
Adduction of basic molecules, such as diethylamine, in the ESI solution can result in lower charging of proteins [7, 44, 70, 71] as a result of these molecules preferentially carrying away charges as a result of proton transfer from the protein to the basic molecules. Charging of proteins and protein complexes from aqueous ammonium acetate solutions is often attributed to proton transfer from NH4+ to the protein [2, 35-49]. The GSFE of NH4+ (285 kJ/mol) is between that of K+ (295 kJ/mol) and Rb+ (275 kJ/mol) and the ionic radius is nearly the same as Rb+. The average charge of the protein ions formed from NH4+ (25 mM solution) is higher than that of Rb+ and K+ by 5 ± 2% and 5 ± 1%, respectively. Neither Rb+ nor K+ can undergo proton transfer reactions, and this difference in protein charging for solutions containing NH4+ versus Rb+ or K+ is minor. The initial pH of the rubidium acetate solutions is slightly lower than that of the ammonium and potassium solutions, but the similar results from Rb+ and K+ solutions suggest that this difference in initial solution pH has a negligible effect on the protein and peptide ion charge. Higher concentrations of ammonium acetate have little effect on protein or peptide charge. The average charge of proteins and peptides is 2 ± 1% and 1 ± 1% lower, respectively, when these ions are formed from 100 mM ammonium acetate solutions compared to 25 mM. The negligible difference in charging from solutions containing NH4+ versus Rb+ or K+, and the minimal effect of NH4+ concentration on protein charge indicates that proton transfer of NH4+ to the protein ion is insignificant in the extent of protein charging in native mass spectrometry. However, while interaction of NH4+ to deprotonated acidic sites in the protein can reduce the number of cation adducts [35, 38, 72, 73], such as sodium, and proton transfer from NH4+ to the acidic sites may occur, our results indicate that this does not result in a significant change in the overall net charge on the protein ions.
Generating singly charged and neutral proteins
Low charge state ions of proteins and protein complexes are often more compact and more stable to induced conformation changes and gas phase collisional cross sections of low charge states of proteins are often similar to cross sections calculated from crystal structures [20, 74, 75]. The charge states of proteins can be reduced by several different methods, including ion-ion [76], ion-electron [77, 78] or ion-molecule reactions [21, 44]. Low charge state ions can also be readily formed by collisionally activating protein or peptide ions complexed with TMA+ and TEA+. For example, collisional activation of (U + 2TMA + H)3+ results in nearly exclusive formation of (U + TMA + H)2+ and (U + H)1+ at 40 V and 80 V collision energies, respectively (Figure 6b). The (U + H)1+ ion can be formed from (U + TMA + H)2+ with a 96% yield at the latter collision voltage. Similarly, (M + H)1+ of several other protein ions, including cytochrome c, myoglobin, and melittin can be formed from a native ESI solution using this method. Because some of the collisional energy deposited into the ion is required to break the noncovalent interaction of the cation and the protein, this method to produce singly protonated ions may not significantly perturb the structures of large proteins and protein complexes.
The number of TMA+ or TEA+ adducts is often greater than the net ion charge (Figure 1). When the number of adducts is equal to the net charge state of the ion, the protein molecule itself has a net charge of zero. Similarly, the protein molecule has a net negative charge when the number of positively charged adducts exceeds the overall net charge of the adducted protein ion. Isolating and collisionally activating these positively charged ions that are generated by ESI would lead to a negatively charged or neutral beam of protein molecules that is selected for both mass and energy.
Conclusions
The effects of different cations on the charge states of protein and peptide ions produced in electrospray ionization from aqueous acetate solutions were investigated. The charge states of three proteins formed from aqueous solutions without added acetate salts are higher than those formed from solutions with added salts whereas the charge states of peptides can be higher, lower or the same as those formed with added salts. Results from circular dichroism measurements show that the protein secondary structure differs in aqueous solutions without salts added compared to solutions containing either sodium or ammonium acetate. Ion mobility results show that protonated molecular ions with the same charge state formed from water and aqueous ammonium acetate solutions differ, indicating that the solution-phase structures differ as well. These results indicate that the origin of the difference in protein charging with and without salts is an effect of differences in protein conformation in these solutions.
Collisional activation of proteins adducted to the larger cations that have low Gibbs solvation free energies produces facile loss of the cations resulting in intact protein ions with fewer charges. This dissociation pathway may account for the substantially lower protein charging from solutions containing cations such as TMA+ and TEA+. The protein ions formed from TMA+ and TEA+ solutions are less charged than those formed from ammonium or alkali metal ion solutions even at conditions where no intentional collisional activation occurs. This suggests that the CCRFEM may also contribute to the lower charging observed.
Although charging depends on the ionic strength of the aqueous solution, similar extents of protein and peptide charging containing equal concentrations of either ammonium acetate or rubidium acetate indicate that proton transfer from protonated ammonia to proteins and peptides does not play a significant role in the extent of charging in native mass spectrometry.
Formation of singly protonated protein ions from higher charge state ions that are adducted to larger cations is readily achieved by collisional activation to induce loss of the charged cation, leaving the intact singly protonated protein ion. This method can not only produce protein ions with just one charge, but should also provide a route to generating either beams of neutral protein molecules or protein anions that are selected for both mass and kinetic energy from multiply charged positive ions formed by ESI.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health (grant no. R01GM097357) for generous financial support. The authors also thank Dr. Haichuan Liu and Professor Ewa Witkowska of the UCSF Sandler-Moore Mass Spectrometry Core Facility for the use of the Synapt G2 instrument and Professor Bryan A. Krantz for the use of the circular dichroism spectropolarimeter. The authors also thank Catherine A. Cassou for helpful discussions.
References
- 1.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization-principles and practice. Mass Spectrom. Rev. 1990;9:37–70. [Google Scholar]
- 2.Kebarle P, Verkerk UH. Electrospray: from ions in solution to ions in the gas phase, what we know now. Mass Spectrom. Rev. 2009;28:898–917. doi: 10.1002/mas.20247. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury SK, Katta V, Chait BT. Probing conformational changes in proteins by mass spectrometry. J. Am. Chem. Soc. 1990;112:9012–9013. [Google Scholar]
- 4.Dobo A, Kaltashov IA. Detection of multiple protein conformational ensembles in solution via deconvolution of charge-state distributions in ESI MS. Anal. Chem. 2001;73:4763–4773. doi: 10.1021/ac010713f. [DOI] [PubMed] [Google Scholar]
- 5.Loo JA, Loo RRO, Udseth HR, Edmonds CG, Smith RD. Solvent-induced conformational changes of polypeptides probed by electrospray-ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1991;5:101–105. doi: 10.1002/rcm.1290050303. [DOI] [PubMed] [Google Scholar]
- 6.Iavarone AT, Williams ER. Mechanism of Charging and Supercharging Molecules in Electrospray Ionization. J. Am. Chem. Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iavarone AT, Jurchen J, Williams E. Effects of solvent on the maximum charge state and charge state distribution of protein ions produced by electrospray ionization. J. Am. Soc. Mass Spectrom. 2000;11:976–985. doi: 10.1016/S1044-0305(00)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnier PD, Gross DS, Williams ER. Electrostatic forces and dielectric polarizability of multiply protonated gas-phase cytochrome c ions probed by ion/molecule chemistry. J. Am. Chem. Soc. 1995;117:6747–6757. [Google Scholar]
- 9.Thomson BA. Declustering and fragmentation of protein ions from an electrospray ion source. J. Am. Soc. Mass Spectrom. 1997;8:1053–1058. [Google Scholar]
- 10.Iavarone AT, Jurchen JC, Williams ER. Supercharged protein and peptide ions formed by electrospray ionization. Anal. Chem. 2001;73:1455–1460. doi: 10.1021/ac001251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling HJ, Cassou CA, Trnka MJ, Burlingame AL, Krantz BA, Williams ER. The role of conformational flexibility on protein supercharging in native electrospray ionization. Phys. Chem. Chem. Phys. 2011;13:18288–18296. doi: 10.1039/c1cp20277d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterling HJ, Daly MP, Feld GK, Thoren KL, Kintzer AF, Krantz BA, Williams ER. Effects of supercharging reagents on noncovalent complex structure in electrospray ionization from aqueous solutions. J. Am. Soc. Mass Spectrom. 2010;21:1762–1774. doi: 10.1016/j.jasms.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling HJ, Cassou CA, Susa AC, Williams ER. Electrothermal supercharging of proteins in native electrospray ionization. Anal. Chem. 2012;84:3795–3801. doi: 10.1021/ac300468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling HJ, Prell JS, Cassou CA, Williams ER. Protein conformation and supercharging with DMSO from aqueous solution. J. Am. Soc. Mass Spectrom. 2011;22:1178–1186. doi: 10.1007/s13361-011-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassou CA, Sterling HJ, Susa AC, Williams ER. Electrothermal supercharging in mass spectrometry and tandem mass spectrometry of native proteins. Anal. Chem. 2013;85:138–146. doi: 10.1021/ac302256d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling HJ, Kintzer AF, Feld GK, Cassou CA, Krantz BA, Williams ER. Supercharging protein complexes from aqueous solution disrupts their native conformations. J. Am. Soc. Mass Spectrom. 2012;23:191–200. doi: 10.1007/s13361-011-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA. Increasing charge while preserving noncovalent protein complexes for ESI-MS. J. Am. Soc. Mass Spectrom. 2009;20:593–596. doi: 10.1016/j.jasms.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomeli SH, Peng IX, Yin S, Loo RO, Loo JA. New reagents for increasing ESI multiple charging of proteins and protein complexes. J. Am. Soc. Mass Spectrom. 2010 doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall Z, Robinson CV. Do charge state signatures guarantee protein conformations? J. Am. Soc. Mass Spectrom. 2012;23:1161–1168. doi: 10.1007/s13361-012-0393-z. [DOI] [PubMed] [Google Scholar]
- 20.Valentine SJ, Counterman AE, Clemmer DE. Conformer-dependent proton-transfer reactions of ubiquitin ions. J. Am. Soc. Mass Spectrom. 1997;8:954–961. [Google Scholar]
- 21.Williams ER. Proton transfer reactivity of large multiply charged ions. J. Mass. Spectrom. 1996;31:831–842. doi: 10.1002/(SICI)1096-9888(199608)31:8<831::AID-JMS392>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dole M, Mack LL, Hines RL, Mobley RC. Molecular Beams of Macroions. J. Chem. Phys. 1968;49:2240–2449. [Google Scholar]
- 23.Iribarne JV, Thomson BA. On the evaporation of small ions from charged droplets. J. Chem. Phys. 1976;64:2287–2294. [Google Scholar]
- 24.Hogan CJ, Carroll JA, Rohrs HW, Biswas P, Gross ML. Combined Charged Residue-Field Emission Model of macromolecular electrospray ionization. Anal. Chem. 2009;81:369–377. doi: 10.1021/ac8016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duft D, Achtzehn T, Müller R, Huber BA, Leisner T. Coulomb fission: Rayleigh jets from levitated microdroplets. Nature. 2003;421:128. doi: 10.1038/421128a. [DOI] [PubMed] [Google Scholar]
- 26.Smith JN, Flagan RC, Beauchamp JL. Droplet evaporation and discharge dynamics in electrospray ionization. J. Phys. Chem. A. 2002;105:9957–9967. [Google Scholar]
- 27.Thomson BA, Iribarne JV. Field induced ion evaporation from liquid surfaces at atmospheric pressure. J. Chem. Phys. 1979;71:4451–4463. [Google Scholar]
- 28.de la Mora JF. Electrospray ionization of large multiply charged species proceeds via Dole’s charged residue mechanism. Anal. Chim. Acta. 2000;406:93–104. [Google Scholar]
- 29.Wang G, Cole RB. Effect of solution ionic strength on analyte charge state distributions in positive and negative ion electrospray mass spectrometry. Anal. Chem. 1994;66:3702–3708. [Google Scholar]
- 30.Wang G, Cole RB. Effects of solvent and counterion on ion pairing and observed charge states of diquaternary ammonium salts in electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1996;7:1050–1058. doi: 10.1016/1044-0305(96)00051-7. [DOI] [PubMed] [Google Scholar]
- 31.Flick TG, Williams ER. Supercharging with trivalent metal ions in native mass spectrometry. J. Am. Soc. Mass Spectrom. 2012;23:1885–1895. doi: 10.1007/s13361-012-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu MM, Rempel DL, Zhao J, Giblin DE, Gross ML. Probing Ca2+-induced conformational changes in porcine calmodulin by H/D exchange and ESI-MS: Effect of cations and ionic strength. Biochemistry. 2003;42:15388–15397. doi: 10.1021/bi035188o. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Håkansson K. Divalent metal ion-peptide interactions probed by electron capture dissociation of trications. J. Am. Soc. Mass Spectrom. 2006;17:1731–1741. doi: 10.1016/j.jasms.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Ly T, Julian RR. Protein-metal interactions of calmodulin and α-synuclein monitored by selective noncovalent adduct protein probing mass spectrometry. J. Am. Soc. Mass Spectrom. 2008;19:1663–1672. doi: 10.1016/j.jasms.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Felitsyn N, Peschke M, Kebarle P. Origin and number of charges observed on multiply-protonated native proteins produced by ESI. Int. J. Mass Spectrom. 2002;219:39–62. [Google Scholar]
- 36.Kebarle P. A brief overview of the present status of the mechanisms involved in electrospray mass spectrometry. J. Mass. Spectrom. 2000;35:804–817. doi: 10.1002/1096-9888(200007)35:7<804::AID-JMS22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Hautreux M, Hue N, Fou de Kerdaniel, Du A, Zahir A, Malec V, Laprévote O. Under non-denaturing solvent conditions, the mean charge state of a multiply charged protein ion formed by electrospray is linearly correlated with the macromolecular surface. Int. J. Mass Spectrom. 2004;231:131–137. [Google Scholar]
- 38.Prakash H, Kansara BT, Mazumdar S. Effects of salts on the charge-state distribution and the structural basis of the most-intense charge-state of the gaseous protein ions produced by electrospray ionization. Int. J. Mass Spectrom. 2010;289:84–91. [Google Scholar]
- 39.Konermann L, Ahadi E, Rodriguez AD, Vahidi S. Unraveling the mechanism of electrospray ionization. Anal. Chem. 2013;85:2–9. doi: 10.1021/ac302789c. [DOI] [PubMed] [Google Scholar]
- 40.Ho Y, Chen J, Hu T. Elucidating factors manipulated the formation of multiply charged protein homomultimeric complexes by electrospray ionization. J. Chin. Chem. Soc.-Taip. 2007;54:391–400. [Google Scholar]
- 41.Verkerk UH, Peschke M, Kebarle P. Effect of buffer cations and of H3O+ on the charge states of native proteins. Significance to determinations of stability constants of protein complexes. J. Mass. Spectrom. 2003;38:618–631. doi: 10.1002/jms.475. [DOI] [PubMed] [Google Scholar]
- 42.Verkerk UH, Kebarle P. Ion-ion and ion-molecule reactions at the surface of proteins produced by nanospray. Information on the number of acidic residues and control of the number of ionized acidic and basic residues. J. Am. Soc. Mass Spectrom. 2005;16:1325–1341. doi: 10.1016/j.jasms.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Heck AJR, Van Den Heuvel RHH. Investigation of intact protein complexes by mass spectrometry. Mass Spectrom. Rev. 2004;23:368–389. doi: 10.1002/mas.10081. [DOI] [PubMed] [Google Scholar]
- 44.Catalina MI, Van Den Heuvel RHH, van Duijn E, Heck AJR. Decharging of globular proteins and protein complexes in electrospray. Chem. Eur. J. 2005;11:960–968. doi: 10.1002/chem.200400395. [DOI] [PubMed] [Google Scholar]
- 45.Hiraoka K, Asakawa Y, Kawashima Y, Okazaki S, Nakamura M, Yamamoto Y, Takamizawa A. The effect of the presence of foreign salts on the formation of gaseous ions for electrospray and laser spray. Rapid Commun. Mass Spectrom. 2004;18:2437–2442. doi: 10.1002/rcm.1644. [DOI] [PubMed] [Google Scholar]
- 46.Peschke M, Blades A, Kebarle P. Charged states of proteins. Reactions of doubly protonated alkyldiamines with NH3: Solvation or deprotonation. Extension of two proton cases to multiply protonated globular proteins observed in the gas phase. J. Am. Chem. Soc. 2002;124:11519–11530. doi: 10.1021/ja012591e. [DOI] [PubMed] [Google Scholar]
- 47.Peschke M, Verkerk UH, Kebarle P. Features of the ESI mechanism that affect the observation of multiply charged noncovalent protein complexes and the determination of the association constant by the titration method. J. Am. Soc. Mass Spectrom. 2004;15:1424–1434. doi: 10.1016/j.jasms.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Watt SJ, Sheil M, Beck JL, Prosselkov P, Otting G, Dixon NE. Effect of protein stabilization on charge state distribution in positive and negative ion electrospray ionization mass spectra. J. Am. Soc. Mass Spectrom. 2007;18:1605–1611. doi: 10.1016/j.jasms.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Grandori R. Origin of the conformation dependence of protein charge-state distributions in electrospray ionization mass spectrometry. J. Mass. Spectrom. 2003;38:11–15. doi: 10.1002/jms.390. [DOI] [PubMed] [Google Scholar]
- 50.Hu P, Gross ML. Strong interactions of anionic peptides and alkaline earth metal ions: metal-ion-bound peptides in the gas phase. J. Am. Chem. Soc. 1992;114:9153–9160. [Google Scholar]
- 51.Flick TG, Merenbloom SI, Williams ER. Effects of Metal Ion Adduction on the Gas-Phase Conformations of Protein Ions. J. Am. Soc. Mass Spectrom. 2013;24:1654–1662. doi: 10.1007/s13361-013-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.UniProt Consortium Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41:D43–7. doi: 10.1093/nar/gks1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcus Y. Thermodynamics of solvation of ions. Part 5. Gibbs free energy of hydration at 298.15 K. Faraday Trans. 1991;87:2995–2999. [Google Scholar]
- 54.Valentine SJ, Anderson JG, Ellington AD, Clemmer DE. Disulfide-Intact and - Reduced Lysozyme in the Gas Phase: Conformations and Pathways of Folding and Unfolding. J. Phys. Chem. B. 1997;101:3891–3900. [Google Scholar]
- 55.Wyttenbach T, Helden von G., Bowers MT. Gas-phase conformation of biological molecules: Bradykinin. J. Am. Chem. Soc. 1996;118:8355–8364. [Google Scholar]
- 56.Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal. Chem. 2010;82:9557–9565. doi: 10.1021/ac1022953. [DOI] [PubMed] [Google Scholar]
- 57.Kebarle P, Tang L. From ions in solution to ions in the gas phase - the mechanism of electrospray mass spectrometry. Anal. Chem. 1993;65:972–986. [Google Scholar]
- 58.Tang L, Kebarle P. Dependence of ion intensity in electrospray mass spectrometry on the concentration of the analytes in the electrosprayed solution. Anal. Chem. 1993;65:3654–3668. [Google Scholar]
- 59.Wang G, Cole RB. Charged residue versus ion evaporation for formation of alkali metal halide cluster ions in ESI. Anal. Chim. Acta. 2000;406:53–65. [Google Scholar]
- 60.Nguyen S, Fenn JB. Gas-phase ions of solute species from charged droplets of solutions. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1111–1117. doi: 10.1073/pnas.0609969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spencer EAC, Ly T, Julian RR. Formation of the serine octamer: Ion evaporation or charge residue? Int. J. Mass Spectrom. 2008;270:166–172. [Google Scholar]
- 62.Ahadi E, Konermann L. Modeling the behavior of coarse-grained polymer chains in charged water droplets: implications for the mechanism of electrospray ionization. J. Phys. Chem. B. 2012;116:104–112. doi: 10.1021/jp209344z. [DOI] [PubMed] [Google Scholar]
- 63.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. BBA-Proteins and Proteom. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Merenbloom SI, Flick TG, Daly MP, Williams ER. Effects of select anions from the Hofmeister series on the gas-phase conformations of protein ions measured with traveling-wave ion mobility spectrometry/mass spectrometry. J. Am. Soc. Mass Spectrom. 2011;22:1978–1990. doi: 10.1007/s13361-011-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel BJ, Pan P, Reid GE, Wells JM, McLuckey SA. Charge state dependent fragmentation of gaseous protein ions in a quadrupole ion trap: bovine ferri-, ferro-, and apo-cytochrome c. Int. J. Mass Spectrom. 2002;219:171–187. [Google Scholar]
- 66.Meot-Ner Mautner M. Update 1 of: Strong ionic hydrogen bonds. Chem. Rev. 2012;112:PR22–PR103. doi: 10.1021/cr200430n. [DOI] [PubMed] [Google Scholar]
- 67.Dzidic I, Kebarle P. Hydration of the alkali ions in the gas phase. Enthalpies and entropies of reactions M+(H2O)n-1 + H2O = M+(H2O)n. J. Phys. Chem. 1970;74:1466–1474. [Google Scholar]
- 68.Meot-Ner Mautner M. The ionic hydrogen bond and ion solvation. 2. Solvation of onium ions by one to seven water molecules. Relations between monomolecular, specific, and bulk hydrogen. J. Am. Chem. Soc. 1984;106:1265–1272. [Google Scholar]
- 69.Meot-Ner Mautner M, Deakyne CA. Unconventional Ionic Hydrogen Bonds. 1. CH δ+--X. Complexes of Quaternary Ions with n- and pi-Donors. J. Am. Chem. Soc. 1985;107:469–474. [Google Scholar]
- 70.Pagel K, Hyung SJ, Ruotolo BT, Robinson CV. Alternate dissociation pathways identified in charge-reduced protein complex ions. Anal. Chem. 2010;82 doi: 10.1021/ac101121r. [DOI] [PubMed] [Google Scholar]
- 71.Lemaire D, Marie G, Serani L, Laprévote O. Stabilization of gas-phase noncovalent macromolecular complexes in electrospray mass spectrometry using aqueous triethylammonium bicarbonate buffer. Anal. Chem. 2001;73:1699–1706. doi: 10.1021/ac001276s. [DOI] [PubMed] [Google Scholar]
- 72.Flick TG, Cassou CA, Chang TM, Williams ER. Solution additives that desalt protein ions in native mass spectrometry. Anal. Chem. 2012;84:7511–7517. doi: 10.1021/ac301629s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iavarone AT, Udekwu OA, Williams ER. Buffer loading for counteracting metal salt-induced signal suppression in electrospray ionization. Anal. Chem. 2004;76:3944–3950. doi: 10.1021/ac049724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou M, Dagan S, Wysocki VH. Impact of charge state on gas-phase behaviors of noncovalent protein complexes in collision induced dissociation and surface induced dissociation. Analyst. 2013;138:1353–1362. doi: 10.1039/c2an36525a. [DOI] [PubMed] [Google Scholar]
- 75.Clemmer D, Hudgins R. Naked protein conformations: Cytochrome c in the gas phase. J. Am. Soc. Mass Spectrom. 1995;117:10141–10142. [Google Scholar]
- 76.Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Electron transfer ion/ion reactions in a three-dimensional quadrupole ion trap: reactions of doubly and triply protonated peptides with SO2. Anal. Chem. 2005;77:1831–1839. doi: 10.1021/ac0483872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scalf M, Westphall MS, Smith LM. Charge Reduction Electrospray Mass Spectrometry. Anal. Chem. 2000;72:52–60. doi: 10.1021/ac990878c. [DOI] [PubMed] [Google Scholar]
- 78.Horn DM, Breuker K, Frank AJ, McLafferty FW. Kinetic intermediates in the folding of gaseous protein ions characterized by electron capture dissociation mass spectrometry. J. Am. Chem. Soc. 2001;123:9792–9799. doi: 10.1021/ja003143u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.