Abstract
Regulated exocytosis is the main mechanism utilized by specialized secretory cells to deliver molecules to the cell surface by virtue of membranous containers (i.e., secretory vesicles). The process involves a series of highly coordinated and sequential steps, which include the biogenesis of the vesicles, their delivery to the cell periphery, their fusion with the plasma membrane, and the release of their content into the extracellular space. Each of these steps is regulated by the actin cytoskeleton. In this review, we summarize the current knowledge regarding the involvement of actin and its associated molecules during each of the exocytic steps in vertebrates, and suggest that the overall role of the actin cytoskeleton during regulated exocytosis is linked to the architecture and the physiology of the secretory cells under examination. Specifically, in neurons, neuroendocrine, endocrine, and hematopoietic cells, which contain small secretory vesicles that undergo rapid exocytosis (on the order of milliseconds), the actin cytoskeleton plays a role in pre-fusion events, where it acts primarily as a functional barrier and facilitates docking. In exocrine and other secretory cells, which contain large secretory vesicles that undergo slow exocytosis (seconds to minutes), the actin cytoskeleton plays a role in post-fusion events, where it regulates the dynamics of the fusion pore, facilitates the integration of the vesicles into the plasma membrane, provides structural support, and promotes the expulsion of large cargo molecules.
Keywords: Regulated exocytosis, Actin cytoskeleton, Myosin, Secretion
Introduction
Exocytosis is a fundamental process that every cell utilizes to deliver membranes, lipids, and soluble molecules (here referred to as cargo molecules or cargo) to the cell surface and the extracellular space [1]. This process is mediated by membranous carriers of different sizes and shapes (e.g., vesicular, tubular, or pleomorphic; for simplicity here referred to as secretory vesicles), which originate from the biosynthetic or the endocytic pathways [2–4]. Exocytosis can occur either constitutively, when secretory vesicles fuse with the plasma membrane without any stimulation, or in a regulated fashion [1, 5, 6]. Constitutive exocytosis is a constant, on-going process that occurs in every cell type and is necessary for the maintenance of the plasma membrane and several other basic cellular functions. On the other hand, during regulated exocytosis, secretory vesicles are delivered to the cell periphery where they reside until an extracellular stimulus triggers their fusion with the plasma membrane. This process occurs in specialized secretory cells in order to satisfy specific physiological tasks, such as neurotransmission, respiration, digestion, reproduction, the immune response, and many others.
The secretory tissues contain different types of specialized secretory cells that undergo regulated exocytosis and can be divided into four major categories: neuronal, endocrine, exocrine, and hematopoietic [1]. In neurons, neurotransmitters contained in vesicles are secreted from the axon terminals, which are specialized areas of the plasma membrane, and into synaptic clefts where they reach their targets on the juxtaposed dendrites of the following neurons [1, 5, 6]. However, recent evidence suggests that neurotransmitters are exocytosed from dendrites as well [7]. In endocrine and neuroendocrine glands, hormones are secreted into the extracellular space and from there diffuse into the circulatory system to ultimately reach their targets [8–10]. In exocrine glands, macromolecules are contained in large secretory vesicles (or granules) that are secreted from specialized domains of the plasma membrane that are in direct communication with the external environment (e.g., ducts and canaliculi) [11–13]. In hematopoietic cells, such as natural killers (NK) or cytotoxic T-lymphocytes (CTL), molecules are released into the extracellular space from a specific plasma membrane domain (termed the “immunological synapse”) that is formed upon their interaction with target cells [4, 14]. Furthermore, different kinds of secretory cells may coexist within the same organ as occurs in the pancreas in which 1–2 % of its mass is occupied by the islets of Langherans, which are formed by endocrine cells (α, β, δ, PP, and ε cells), and the rest is occupied by exocrine acinar and ductal cells. Finally, there are a few secretory systems such as sperm [15], endothelial cells [16], melanocytes [17], mammary glands [18], and eggs [19] that posses distinct features that will be discussed later.
In the last two decades, the molecular machinery controlling regulated exocytosis has been the subject of extensive investigations, and several factors have been identified as key players in the process, including G proteins-coupled receptors, ion channels, intracellular Ca++ levels, small GTPases, lipid modifying enzymes, and the actin cytoskeleton [20–25]. The actin cytoskeleton, in particular, plays a fundamental role in various membrane trafficking pathways [26–29]. Its remodeling at the surface of intracellular organelles and at the plasma membrane regulates short-range membrane transport and facilitates various processes such as membrane budding, fusion, and fission [27, 30, 31]. In regulated exocytosis, the actin cytoskeleton has been proposed to act either as a positive or negative regulator, depending on the secretory system under examination [9, 19, 28, 31, 32]. These models are based on measuring the amounts of secreted proteins under conditions in which the dynamics of the actin cytoskeleton was impaired by specific pharmacological agents or bacterial toxins. Recently, the use of more specific molecular tools combined with either high-resolution electron microscopy or time-lapse light microscopy have revealed a more complex involvement of the actin cytoskeleton in regulated exocytosis.
The goal of this review is to discuss our current understanding of how actin remodeling controls each step of regulated exocytosis, while comparing the differences and commonalities among the various secretory cells. Although we have tried to cover the subject as extensively as possible, we will not discuss regulated exocytosis in invertebrates and suggest other reviews to the readers interested in this aspect [33–36].
Regulated exocytosis
Secretory vesicles biogenesis and maturation
In most of the secretory cells, newly synthesized molecules destined for regulated exocytosis are transported from the endoplasmic reticulum (ER) to the Golgi apparatus, and are constitutively packaged into secretory vesicles in the trans-Golgi network (TGN) [11–15]. Since the machinery required for the formation of regulated secretory vesicles is present only in specialized tissues, it has been proposed that their biogenesis is regulated by a single “master” regulatory gene. Initial evidence pointed to chromogranin A, a protein expressed in several secretory tissues [37]. Indeed, anti-sense mediated depletion of chromogranin A in both pheochromocytoma cells (PC12) [38] and in mice [39] resulted in a reduction in secretory vesicles that has been attributed to an increased degradation of granular proteins [40]. Further support came from complementary experiments, which showed that over-expression of chromogranin A in non-secretory cells resulted in the formation of secretory vesicles-like structures [38]. On the other hand, deletion of the chromogranin A gene did not affect the synthesis of proteins targeted to the secretory granules or their biogenesis [41] and, in other cell types, such as pancreatic β-cells, insulin vesicle biogenesis was completely independent from both chromogranin A and B [42, 43]. Moreover, chromogranin A ablation resulted in the compensatory expression of other granins. Taken together, this evidence suggests that there are a series of switches that control the overall process of secretory vesicle biogenesis, rather than a single one [44, 45].
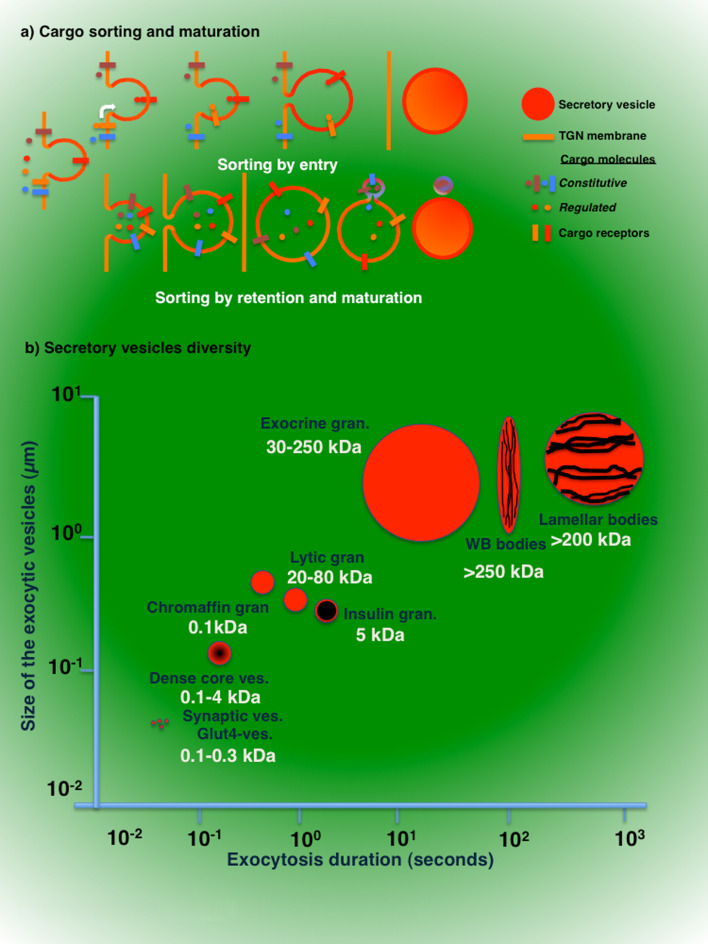
Another aspect that has been extensively investigated is the machinery controlling the sorting of regulated cargo molecules away from the constitutive pathway. Two main mechanisms have been proposed (Fig. 1a). The first, known as “sorting by entry,” [11, 46] involves the binding of cargo molecules to specific receptors that are either proteinaceous [47–50] or lipidic in nature [51–53], and localize or segregate into the nascent secretory vesicles. The second, known as “sorting by retention,” is based on the fact that cargo molecules freely enter newly formed vesicles that are released into the cytoplasm. These “immature” vesicles undergo an extensive “maturation” step, which is mediated by homotypic fusion with other newly formed carriers and/or the removal of specific molecules and membranes via clathrin-coated vesicles [11, 46, 54, 55] (Fig. 1a). The “maturation” step has been proposed for several secretory systems, such as insulin-secreting [10], neuroendocrine [56], salivary [57, 58], endothelial [59], and pancreatic acinar cells [58].
Fig. 1.
Cargo sorting into secretory vesicles and their morphological diversity. a Cargo sorting at the TGN. Molecules destined for secretion are sorted into regulated secretory vesicles at the TGN by two main mechanisms. According to the “sorting by entry” model, cargo molecules bind to specific receptors and are sorted into the nascent secretory vesicles, which detach from the TGN and are transported towards the plasma membrane. According to the “sorting by retention” model, cargo molecules freely enter newly formed vesicles that are released into the cytoplasm. These “immature” vesicles undergo an extensive “maturation” step, which is mediated by the removal of specific molecules and membranes via clathrin-coated vesicles. b Correlation between the sizes of the secretory vesicles and the duration of exocytosis. The sizes of the secretory vesicles ranges from 50 nm up to 2–3 μm in diameter and roughly correlate with the molecular weights of the cargo molecules (from 0.1 kDa up to >200 kDa). The duration of exocytosis, as measured from the opening of the fusion pore to the moment in which the secretory vesicle is completely integrated into the plasma membrane, inversely correlates with vesicles size. Small synaptic vesicles complete exocytosis in a time scale ranging from a microsecond to a few milliseconds. Dense core vesicles, lytic and insulin granules complete exocytosis within a few seconds, whereas large secretory granules in exocrine glands last for several seconds up to a minute, and Weibel–Palade bodies, lamellar bodies, and cortical granules last for several minutes
Secretory vesicles can also originate from the endocytic and the degradative pathways, or from the contribution of multiple organelles. For example, in cytotoxic T-lymphocyte, mature secretory lysosomes are formed from late endosomal compartments upon stimulation with of the T cell receptor. The late endosomes then transition to multivesicular bodies and become progressively enriched in a dense core formed by newly synthesized lytic proteins that are delivered from the TGN [4]. In adipocytes, GLUT4-containing vesicles originate from both the TGN and the recycling endosomal compartment in a process that requires GGA (Golgi-localizing Gamma-adaptin ear domain homology ARF-binding proteins), clathrin, phosphatidylinositol 4-phosphate (PI4P) [60], and GLUT4 ubiquitination [61]. In alveolar type II cells, surfactant phospholipids are arranged in tightly packed membrane sheets (lamellae), and are transported by multivesicular body-derived organelles, called lamellar bodies. [62]. This process is mediated by the hydrophobic SP-B peptide, which is transported via the Golgi apparatus to the lamellar bodies, whereas phospholipids are transported directly from the ER [62]. Finally, in mammary glands, lipid droplets are synthesized in the ER and are transported directly to the plasma membrane where they are secreted by an unusual mechanism [63].
Morphological and content diversity of the secretory vesicles
The diversity in the modality of vesicle biogenesis is reflected in the astonishing diversity of their sizes (ranging from 50 nm up to 2–3 μm in diameter) and in their heterogeneity within the same secretory cell (Fig. 1b), which can be correlated with the molecular nature of the transported molecules, as shown by the following examples. In neurons, synaptic vesicles contain primarily small molecular weight neurotransmitters (such as acetylcholine, epinephrine, GABA, dopamine, glutamine, etc.) and have diameters between 50 and 60 nm, while dense-core vesicles contain slightly larger neuropeptides (such as NPY, Galanin, etc.) and have a diameter of around 100 nm [19]. In neuroendocrine and endocrine glands, the nature of the cargo molecules vary from catecholamines to various hormones (molecular weight up to 5–10 kDa) that are transported either in small vesicles or granules that range from 60 to 300 nm. In addition, their cargo molecules can be loosely associated, form electron-dense aggregates (i.e., dense core vesicles), or assemble in crystalline structures, as shown for insulin [64]. Hematopoietic cells appear to have the most diversified set of secretory vesicles. Neutrophils, for example, contain up to four different kinds of secretory vesicles (azurophil, specific, gelatinase, and non-granular), which contain molecules such as myeloperoxidase (140 kDa) and lactoferrin (80 kDa) and have diameters of 200–250 μm. In CTL and NK cells, lytic granules contain large molecules, such as perforins or granzymes (60 and 85 kDa, respectively) and have diameters of 250–300 μm [65]. Moreover, multiple cargo molecules can be packaged within the same vesicle, as in mast cells where small molecules such as histamine and serotonin are co-transported with large cargo such as heparin and gelatinase. In exocrine glands, zymogens (50–100 kDa) or heavy glycosylated mucins (>200 kDa) are transported in large vesicles (or granules) that have diameters of 1–2 μm [66]. In endothelial or alveolar type II cells, molecules such as the von-Willebrand factor (50–20,000 kDa) and the surfactant are densely packed in cigar shaped Weibel–Palade bodies (0.5 × 1.5 μm) and lamellar bodies, respectively (diameter 1.5–2 μm). Finally, a peculiar case is sperm where a complex mixture of proteins needed for fertilization such as hyaluronidase and acrosin, are released from the acrosome, a large, single, cup-shaped organelle that can reach a few microns in size [67].
In preparation for fusion: reaching the cell periphery, docking, and priming
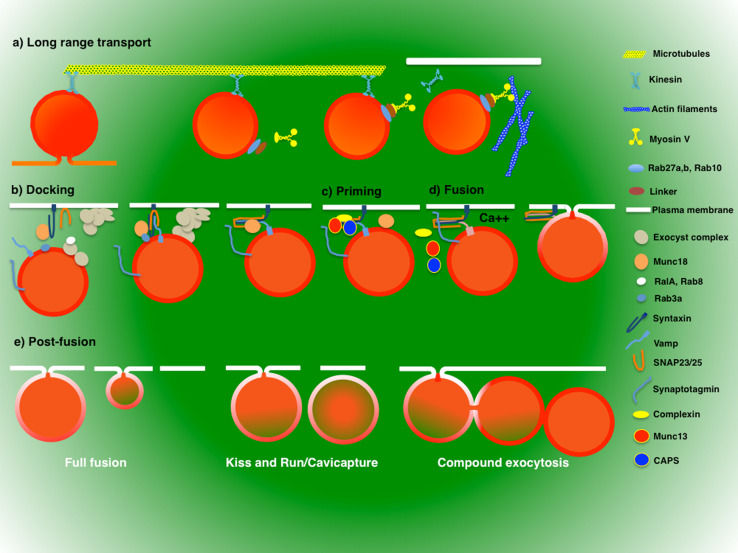
Once assembled, the newly formed secretory vesicles are transported toward the cell periphery. This process is facilitated by microtubules and requires kinesin or dynein motors that are recruited onto the vesicles during their biogenesis [68, 69]. Several studies suggest that microtubules support long-range transport from the sites of biogenesis to the plasma membrane, where the secretory vesicles ultimately associate with the actin cytoskeleton until a stimulus triggers the fusion event [70–72] (Fig. 2a). In neurons, where synaptic vesicles travel for long distances, the disruption of microtubules or interfering with specific microtubule motors result in severe impairment in exocytosis [73–75]. On the other hand, in cells where the secretory vesicles travel for shorter distances, disruption of microtubules leads to delays or slight impairments in exocytosis [68, 76–82].
Fig. 2.
Steps in regulated exocytosis. Newly formed secretory vesicles are transported toward the cell periphery using microtubules and their associated motors kinesin and dynein (a). At the cell periphery, the secretory vesicles associate with the actin cytoskeleton by recruiting and activating myosin motors. Vesicles at the plasma membrane may dock using multiple mechanisms (b). The initial tether may be initiated by the exocyst complex, whereas the docking may be achieved by the interactions between the SNARE complex and Munc18. Both processes are coordinated by small GTPases, such as Rab3a, Rab8, and RalA (b). In order to become fusion-competent, docked secretory vesicles have to undergo priming that is mediated by proteins such as Mun13 and CAPS, which displaces Mun18 from the SNARE complex and facilitates the binding of complexin (c). Upon increase of Ca++ levels of, synaptotagmin, binds to the phospholipid at the plasma membrane, displaces complexin enabling the SNARE complex to promote the fusion of the bilayers (d). After fusion, vesicles can undergo full fusion, kiss and run/cavicapture or compound exocytosis
Once at the cell periphery, secretory vesicles fuse with the plasma membrane and this process requires the proper assembly of the SNARE (SNAP-Receptor) complex, the core component of the fusion machinery that is conserved among several organisms [83, 84]. SNAREs are small proteins (18–42 kDa) containing cytoplasmic amphipathic helices, referred to as SNARE motifs, which engage in coiled-coil interactions with other SNAREs. SNAREs are divided into three families: syntaxins, SNAPs, and VAMPs [85]. VAMPs are present on secretory vesicles (v-SNAREs) whereas syntaxins and SNAPs are present at the plasma membrane (t-SNAREs). Typically, three SNAREs are required to promote fusion, with one SNARE providing two helices (typically a SNAP protein) [85–87]. Individual SNAREs are unfolded, but they can assemble into a stable four-helix bundle, called the “trans-SNARE complex” or “SNARE pin.” The assembly begins from the N-terminus of the SNAREs and zippers them up. This mechanical process forces the membranes to come into close apposition with each other and enables them to overcome the activation energy barrier required for lipid bilayers fusion [84]. In order for the N-termini of the SNAREs to interact, secretory vesicles have to be in close proximity (i.e., docked) to the plasma membrane (Fig. 2b).
Although there are no rigorous criteria to define docked vesicles, they have been operationally identified by either morphological or functional criteria [88]. “Morphological” docking, as defined by using electron microscopy, refers to those vesicles, which appear either apposed to or within less than 10–30 nm from the plasma membrane [88, 89]. “Functional” docking, as defined by using total internal reflection (TIRF) microscopy, refers to those vesicles that exhibit restricted mobility in the evanescent field [3, 88–90]. Docking is controlled by a series of molecules, and among them Munc18 isoforms play a fundamental role [88, 91–93].
In chromaffin cells, modulation of the expression levels of Munc18-1 results in severe disruption of vesicles docking at the plasma membrane, as shown by electron microscopy [94, 95]. In other cells, although docking has not been specifically assessed, manipulation of Munc proteins affects exocytosis. For example, in pancreatic β-cells, overexpression of both Mun18-1 and Munc18-2 increases glucose-stimulated exocytosis of insulin granules [96]. In hematopoietic cells, Munc18-2 seems to be the major regulator of exocytosis. Indeed, in mast cells, increased expression of either Munc18-2 or derived peptides containing an interfering effector loop inhibits IgE-triggered exocytosis [97]. Moreover, in patients affected by familial hemophagocytic lymphohistiocytosis (FHL5), a mutation in Munc18-2 determines the impairment in the release of cytotoxic granules [98]. Finally, PKC-induced phosphorylation of Munc18-3 has been implicated in exocytosis of parotid granules [99].
Although the exact mechanism by which Munc18 proteins promote docking has not been fully elucidated, evidence suggests that their interactions with syntaxins, but not with other SNAREs, play a major role (Fig. 2b) [99–101]. Small GTPases of the Rab family appear to regulate these interactions, as shown in chromaffin cells, where the activation of Rab3a promotes the interaction between Munc18-1 and syntaxin 1 [102, 103]. In addition, Rab27a and Rab27b have been shown to mediate docking in several secretory cells through some of their effectors, such as rabphilin in chromaffin cells, PC12 cells, and neurons [104–106], granuphilin in pancreatic β-cells [107], and exophilin 4 in pancreatic α-cells [108] (Fig. 2b). Interestingly, recent data support the idea that Rab proteins may have intrinsic tethering activity forming interactions in trans [109].
Another set of molecules that regulates docking is the exocyst, an octameric complex initially discovered in a screening for mutants of S. Cerevisiae defective in secretion [110]. Two of the exocyst subunits are associated with the secretory vesicles, whereas six are associated with the plasma membrane (Boyd et al. 2000). The exocyst has been characterized in mammalian cells and shown to be regulated by GTPases, such as RhoA, cdc42, TC10, RalA [111–114], and the scaffolding protein IQGAP1 (Fig. 2b) [115]. Its role in regulated exocytosis has just started to be evaluated. For example, in adipocytes, the Exo70 subunit has been shown to be recruited to the plasma membrane in a TC10-dependent fashion and to regulate the insulin-stimulated exocytosis of Glut4 [111, 116]. In salivary gland cells, antibodies directed against the exocyst subunits sec6 and sec8 inhibited the isoproterenol-stimulated release of amylase [117], and in hippocampal neurons the IGF1-activated release of plasmalemma precursor vesicles was affected by silencing Exo70 and TC10 [118].
In order to become fusion-competent, docked secretory vesicles have to undergo another step called priming (Fig. 2c). The concept of priming was formulated to describe an ATP-dependent process that precedes the fusion step [119]. The first two molecules described to prime secretory vesicles were NSF (N-ethylmaleimide Sensitive Factor) and α-SNAP, whose function is to disassemble the SNARE complex (see below and [120, 121]). The SNARE complex is exceptionally stable and the energy required for its disassembly is provided by the ATPase activity of NSF [122, 123]. In addition, other molecules implicated in ATP-independent priming have been described, and include Munc13 and CAPS proteins [88, 89, 92].
Munc13-1 has been proposed to prime secretory vesicles by binding to syntaxin 1 and displacing Mun18-1, thus preparing for the assembly of the SNARE complex (Fig. 2c) [124]. Although this model has been recently challenged [125, 126], several studies show that down-regulation of Munc13-1 inhibits exocytosis without altering the number of docked vesicles. Munc13-1 regulates priming in neurons [127], chromaffin cells [128], and β-cells [129, 130], and other isoforms have been recently shown to regulate priming in other secretory systems such as mucin granules in airway goblet cells (Munc13-2 [131]), platelets, mast cells, and in LPS-stimulated azurophilic granules in neutrophils (Munc13-4, [132, 133]). A similar function to Munc13 may be performed by CAPS proteins, which contain a Munc13-homology domain. Deletion of CAPS1 and CAPS2 in mice [134, 135] and cell cultures [136] severely impair catecholamine and glucose-stimulated-insulin release. However, the redundancy of CAPS1 and CAPS2 in tissue expression [137, 138] has not allowed researchers to precisely pinpoint their mechanism of action with the exception of the fact that both CAPS proteins bind to phosphatidylinositol 4,5-bisphosphate (PIP2), a phosphoinositide that has been shown to be required for priming [139]. Once the secretory vesicles are primed, a trigger is the only requirement to promote the fusion between the lipid bilayers of the vesicles and the plasma membrane.
Triggering fusion
Generally, fusion is initiated by an extracellular stimulus that is transduced intracellularly through one of several different types of plasma membrane proteins, such as G protein-coupled receptors, tyrosine kinase receptors, or voltage-dependent calcium channels (VDCC) [1, 5, 6]. This stimulus induces the influx or synthesis of second messengers, such as cytosolic Ca++ or cAMP. These second messengers initiate several signaling cascades, which ultimately trigger fusion by affecting the conformation of the SNARE complex.
Increase in cytosolic Ca++ levels above 0.1–1 μM is considered to be the main factor responsible for fusion. The intracellular Ca++ increase can originate from a number of sources, including an extracellular influx that is mediated by a variety of ion channels at the plasma membrane, release from intracellular calcium stores in the endoplasmic reticulum (ER), or both [24, 140]. The most thorough evidence for how an increase in intracellular Ca++ promotes fusion comes from neurons and adrenal chromaffin cells. In neurons, the Ca++ sensor synaptotagmin I (sytI) and its cofactor, complexin, are responsible for vesicle fusion at the presynaptic active zone [5]. SytI is a small transmembrane protein that is localized to the secretory vesicles and possesses two C2 domains that bind Ca++. Complexin is a protein that is bound to the primed SNARE complex in the absence of Ca++, and whose proposed function is to act as a clamp preventing fusion. Upon Ca++ influx, SytI displaces complexin and binds to the phospholipids and to the SNARE complex [141]. By doing so, SytI pulls the vesicle closer to the plasma membrane and into a position favorable for fusion (Fig. 2d) [142]. Furthermore, there is evidence that this mechanism may be universally conserved, since four isoforms have been identified for complexin and sytI belongs to a family of 16 members that are expressed in several secretory tissues [5]. Indeed, SytI has been shown to play a role in the exocytosis of zymogen granules in the pancreas and dense core vesicles in the adrenal medulla [143], whereas sytII, V, VII, and IX have been reported to regulate the exocytosis of Glut 4 vesicles [144, 145] and secretory granules in mast and NK cells [146, 147].
In some cells, an increase in cAMP has been shown to regulate fusion events by either modulating the Ca++ signaling or by acting alone. Indeed, cAMP modulates and enhances Ca++-dependent exocytosis in synapses, adrenal chromaffin cells, melanotrophs, pancreatic β- and acinar cells, and CTL [148]. However, in these cells, elevation of cAMP alone is not sufficient to trigger exocytosis. Interestingly, in the submandibular and parotid salivary glands, cAMP alone is sufficient to elicit exocytosis, even in conditions of low Ca++ concentrations, suggesting that either cAMP reduces Ca++ sensitivity or that in these systems regulated exocytosis is Ca++-independent [149]. Although the precise mechanisms are not well understood, cAMP may act through at least two major pathways: protein kinase A (PKA) and the exchange protein activated by cAMP (EPAC) [148]. Both pathways can modulate calcium signaling through a variety of effectors [148]. Recently, components of the exocytic machinery, including molecules that interact with and regulate SNAREs, such as Syt12 [150], cystein string protein [151], and syntaphilin [152], have been identified as substrates of PKA. In addition, PKA anchoring proteins, such as AKAP5 in the parotid glands and the linker Myrip, have also been shown to participate in the process [153]. As for EPAC, its role in exocytosis has been linked to a few molecules, such as the small GTPases Rap1 and Rab3a [154–156], Rim2, and phospholipase C-ε [157].
The fusion pore and the fate of the secretory vesicles after fusion
The fusion between the lipid bilayers of secretory vesicles and plasma membrane occurs within a few milliseconds leading to the formation of the “fusion pore” [158]. The successive behavior of the fusion pore controls the release of the cargo into the extracellular space and dictates the fate of the secretory vesicles for which multiple scenarios have been described [158, 159]. In the first, the fusion pore progressively expands and leads to the absorption and the integration of the vesicle into the plasma membrane (Fig. 2e). This process, known as “full fusion”, occurs in most of the secretory cells [19, 160–164]. A second possibility is that the fusion pore closes after a fraction of the cargo is secreted, and the vesicle detaches from the plasma membrane (Fig. 2e) [165–168]. This process can be extremely rapid, as observed in neurons (where it is termed “kiss and run”), or it may last for several seconds as shown for neuroendocrine cells (where it is termed “cavicapture”) [169–171]. Finally, the prolonged opening of the fusion pore makes secretory vesicles at the plasma membrane competent to fuse with other secretory vesicles in the cytoplasm, thus generating strings of fused vesicles whose lumens are in continuity with the extracellular space (Fig. 2e). This process, termed compound exocytosis, occurs primarily in exocrine cells but also in insulin-secreting and neuroendocrine cells [165, 172–175].
If we focus on full fusion, the time to complete exocytosis, as measured from the opening of the fusion pore to the moment in which the secretory vesicle is completely integrated into the plasma membrane, varies considerably between secretory cells (Fig. 1b). Small synaptic vesicles complete full fusion in a time scale ranging from μsec to a few milliseconds, as determined by amperometric measurements [176, 177]. On the other hand, dense core vesicles and insulin granules complete exocytosis within a few seconds, whereas large secretory granules in exocrine glands last for several seconds up to a minute, and Weibel–Palade bodies, lamellar bodies, and cortical granules last for several minutes [19] (Fig. 1b). These differences correlate with the kinetics of the fusion pore, which depends on the secretory cell and the size of the secretory vesicle [158, 176, 177]. Indeed, smaller vesicles exhibit a faster pore closure [176, 177], whereas larger vesicles maintain the pore open for longer times, as shown by using imaging approaches such as TIRF in cell cultures [77, 90, 178], two-photon microscopy in explanted organs [179], and confocal microscopy in live animals [164]. At a molecular level, the maintenance of the pore is regulated by several factors, such as syt1 [180] dynamin1, a GTPase regulating fusion and fission of endocytic vesicles [181, 182], and SNAREs [183]. Recently, an intriguing model has been proposed in which two of the SNAREs in the complex promote the fusion of the bilayers, whereas the third one prevents the pore from collapsing [183]. Another family of SNARE interactors, the secretory carrier membrane proteins (SCAMPs), has provided novel insights into this process. In PC12 cells, both SCAMP1 and SCAMP2 regulate the closure of the fusion pore [171, 184], with the latter operating through its interactions with the small GTPase Arf6 and phospholipase D1 (PLD1) [184]. Interestingly, inhibition of PLD1 activity blocked the expansion of the fusion pore during the exocytosis of GLUT4 vesicles in adipocytes [178], thus suggesting a fundamental regulatory role for the lipid composition of the pore [185].
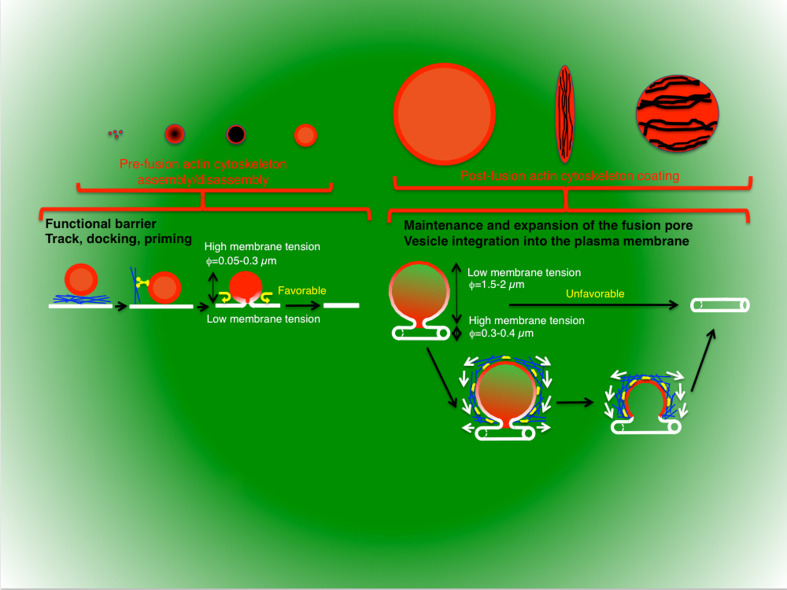
Another factor that may affect the kinetics of full fusion events is the biophysical properties of both vesicles and plasma membrane (e.g., lipid organization, membrane tension, or membrane mobility). Indeed, it has been proposed that upon the establishment of a physical continuity between the two bilayers, membranes spontaneously flow toward the area with the lowest membrane tension [186–189]. According to this model, full fusion would be more energetically favorable when secretory vesicles have a higher membrane tension than the plasma membrane. Based on geometrical considerations, the membrane tension of a secretory vesicle is inversely proportional to its diameter, suggesting that full fusion is a more favorable process for smaller vesicles. Although a precise determination of the membrane tension for the plasma membrane is not so straightforward, it is reasonable to assume that neurons and endocrine cells may have a lower membrane tension than in exocrine cells, where exocytosis occurs on curved surfaces such as the acinar canaliculi (diameters ranging from 0.3–0.4 μm in salivary glands to 1–1.5 μm in the pancreas). As a consequence, a prediction would be that exocytosis in neuron, endocrine, and hematopoietic cells may be more energetically favorable than in exocrine cells or other systems harboring large vesicles. Although this simplistic model does not take in account the nature of the cargo molecules (molecular weight and formation of aggregates) and the fact that fusion may occur in specialized structures such as the porosomes (plasma membrane domains with very specific membrane compositions and morphology) [190–193], it nicely correlates with the experimental data on the kinetics of exocytosis (Fig. 1b).
Role of the actin cytoskeleton in exocytosis
The actin cytoskeleton has been proposed to play multiple and often opposing roles during regulated exocytosis [9, 19, 28, 31, 32, 194]. The two main ideas are that (1) it acts as a physical/functional barrier that is removed during exocytosis, thus allowing the secretory vesicles to access the plasma membrane [9, 28, 31], and (2) it plays a more active role by directing exocytic vesicles to the fusion site, regulating the fusion pore, and providing the driving force to complete fusion [19, 32, 195].
Notably, many studies have provided contradictory findings for the role of the actin cytoskeleton in regulated exocytosis. This is largely due to the use of a single experimental approach and the use of drugs or toxin that induce global changes in the actin cytoskeleton. More recently, specific molecular tools were developed and multiple tools have been integrated. Approaches such as biochemistry, electrophysiology, electron microscopy, and time-lapse imaging have contributed to better define the role of the actin cytoskeleton during exocytosis supporting the view that multiple roles for the actin cytoskeleton are not necessarily mutually exclusive and may coexist within the same secretory cell.
Role of the actin cytoskeleton in pre-fusion steps
Anchoring site for the secretory vesicles and barrier function
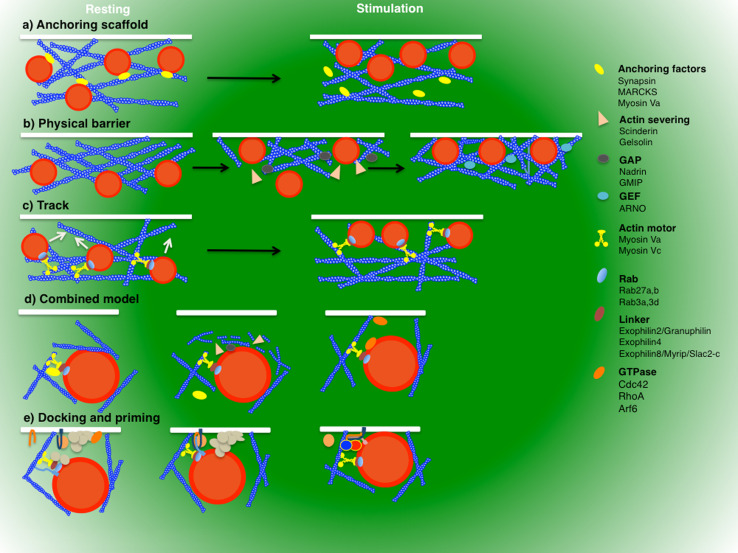
During pre-fusion events, the actin cytoskeleton has been suggested to negatively affect regulated exocytosis in several secretory systems, and at least two models have been proposed so far for this type of regulation. The first model suggests that under resting conditions the actin cytoskeleton provides a scaffold for anchoring secretory vesicles in close proximity to the plasma membrane (Fig. 3a). The second model treats it as a barrier, blocking exocytosis from occurring (Fig. 3b).
Fig. 3.
The actin cytoskeleton in pre-fusion steps. Before fusion, the actin cytoskeleton may provide a scaffold for anchoring secretory vesicles in close proximity to the plasma membrane (a), act as a physical barrier (b), or provide a track to transport the vesicles to the fusion site (c). These models are not mutually exclusive (d). A possible scenario envisions that under resting conditions, secretory vesicles may bind to the cortical actin cytoskeleton via multiple molecules (which include SynapsinI, myosin Va, and MARCKS) and be “functionally” entrapped by actin filaments. Upon stimulation, the small GTPase-mediated disassembly and reassembly of the actin cytoskeleton would transport the vesicles into a position suitable for tethering. Localized actin disassembly permits the vesicles to access the plasma membrane and requires the Ca++-mediated activation of actin severing proteins and the activation of GAPs for specific GTPases. Actin reassembly enables the activation of myosin motors and requires the GEF-mediated activation of selected GTPases and their effectors (d). Myosins and the actin cytoskeleton interact with the exocyst, facilitating tethering, and with the munc18/SNARE complex, regulating docking and priming (e)
The idea behind the first model is based on the observations that secretory vesicles are associated with actin and other cytoskeletal elements and that upon stimulation they detach from the cytoskeleton, increasing their mobility. In neuromuscular and cerebellar synapses, for instance, synaptic vesicles are associated with an intricate network of actin filaments, as shown by using quick-freeze deep-etch electron microscopy [196, 197]. In live pre-synaptic terminal of rat neurons, synaptic vesicles are associated with actin filaments labeled by expression of EGFP-actin [198]. Furthermore, upon stimulation, synaptic vesicles are released from the cytoskeleton through the CAM kinaseII-mediated phosphorylation of the presynaptic marker synapsin I [199, 200]. The mechanism involves Synapsin I binding to actin, and this interaction appears to be modulated by the small GTPase Rab3a [201].
In the pancreas, photonic force microscopy has revealed that secretory vesicles are tethered to filamentous structures in a cytochalasin-dependent manner [202]. Moreover, insulin granules are anchored to actin filaments in resting state via machinery that involves Rab27a, its effector molecule exophilin8 (also known as MyRip or Slac2-c) and the actin motor myosin Va [203], a molecule that plays multiple roles during exocytosis. Myosin Va forms homodimers, through the N-terminal head domain, which contains the ATP binding site and the motor domain. The neck domain contains six calmodulin repeats whereas the C-terminus globular domain contains the organelle binding site [204–206]. Although myosin Va is a processive motor, recent studies have suggested that in resting conditions, it may work as a “dynamic tether” mediating a slow transport along actin cables [207]. Evidence for this was seen in hippocampal neurons, where the expression of a dominant negative tail domain of myosin Va resulted in the release of dense core vesicles from actin and induced their exocytosis [207].
Finally, actin-disrupting agents have been used in some studies in support of the first model. However, although enhanced exocytosis has been consistently observed with the controlled disruption of the actin cytoskeleton with the controlled disruption of the actin cytoskeleton, some groups also reported an increased mobility of secretory vesicles [207, 208], whereas others did not [198, 209]. These contradictory findings lead to the idea that the actin cytoskeleton may act as a storage compartment for molecules that regulate exocytosis [198].
According to the second model, actin acts as a physical barrier between the secretory carriers and the plasma membrane (Fig. 3b). Cortical actin is indeed organized as a thick meshwork of filaments that occasionally present some openings that may allow small secretory vesicles to access the plasma membrane [210, 211]. This model has been first proposed for insulin secretion and is based on the observation that cytochalasin D induces exocytosis of insulin-containing granules in resting pancreatic β-cells [212]. In addition, time-lapse microscopy in neuroendocrine cells has shown that stimulation of exocytosis results in the selective disruption of cortical actin with the creation of actin-free areas that allow the vesicles to dock and fuse at the plasma membrane [213]. Recently, in NK cells, structural illumination microscopy revealed the organization of the immunological synapse, which is characterized by an actin ring delimiting the area of granules fusion. During exocytosis, actin de-polymerizes, forming clear areas of 250 nm where lytic granules dock and fuse with the plasma membrane [214].
A few molecules have been implicated in actin de-polymerization during the stimulation of exocytosis and the most extensively characterized is scinderin (or adseverin), a Ca++-dependent actin severing protein [215, 216]. The N-terminus of scinderin binds actin and PIP2 and both interactions are required during agonist stimulation in chromaffin cells [217, 218]. Although scinderin plays a role in other secretory events, such as mucin secretion in airway cells [219] or insulin secretion in β-cells [220], its expression is limited to few tissues [221], suggesting the involvement of other actin-severing proteins. Indeed, gelsolin has been reported to affect GLUT4 exocytosis in adipocytes, possibly through its interaction with the SNARE protein syntaxin4 [222], enhance the release of amylase in pancreatic acinar cells [194], and control both the acrosomal reaction in the sperm [223] and insulin secretion in β-cells [224]. Interestingly, although other actin de-polymerizing proteins, such as cofilin, are activated during stimulation, they do not have any effect on exocytosis, suggesting that actin de-polymerization has to be spatially targeted [225, 226].
Another molecule implicated in remodeling the actin cytoskeleton during exocytosis is protein kinase C (PKC), which affects the cytoskeleton through myristoylated alanine-rich C-kinase substrate (MARCKS), which is an actin crosslinker whose activity is inhibited by phosphorylation [227]. MARCKS seems to be implicated in controlling several secretory systems, from neurons to airway cells [227–235]. However, PKC can also regulate the activity of phospholipase A2 that has been involved in acrosomal exocytosis [236] most likely by controlling actin dynamics, as suggested by others [237]. Lastly, de-polymerization of the actin cytoskeleton during exocytosis can also be achieved through inhibition of the GTPase-mediated actin assembly via specific GTPase-activating proteins (GAPs) or guanine nucleotide exchange factors (GEF). For example, the expression of Nadrin, a GAP for Rho, Rac, and cdc42, enhances exocytosis in PC12 cells [238] whereas the activation of the Rho-GAP GMIP on the azurophilic granules in neutrophils has been shown to progressively disrupt the cortical actin barrier [239].
Actin cytoskeleton as a track to deliver secretory vesicles to the fusion site
Although the evidence described above suggests that actin performs either a barrier or an anchoring function, a series of other studies indicate that actin plays an active role facilitating the transport of the secretory vesicles toward the fusion sites (Fig. 3c). This movement along actin filaments requires actin-based motors such as members of the myosin V family [205, 240]. Myosin Va is associated with several secretory vesicles and among them are dense core granules [240], melanosomes [241], chromaffin granules [242], insulin granules [243], and synaptic vesicles [244]. Interestingly, its motor activity is activated by increased Ca++ levels, consistent with its role in exocytosis [245]. In chromaffin granules, myosin Va is recruited by Rab27a and the linker myrip [246]. Similar machinery has been proposed for the parotid salivary glands where myosin Va is recruited via Rab27b [99]. Recently, this complex has been shown to be required for the delivery and exocytosis of the Weibel–Palade bodies in endothelial cells, as well [247].
Another myosin motor, myosin II, has been proposed to play a role in vesicular movement along the cytoskeleton. Inhibition of myosin II motor activity with BDM or blebbistatin led to reduction of vesicular mobility in the cortical area [248]. Moreover, a non-phosphorylatable mutant of myosin IIa reduces granule mobility in chromaffin cells [249]. Although non-muscle myosin II is a non-processive motor, its ability to cross-link actin filaments induces contractions that can sustain the movement of small colloidal beads [250].
The apparent discrepancies between the anchoring/physical barrier and the track model are primarily due to the use of pharmacological agents that have different effects depending on their mode of action and dosage. One important point that has been raised is the extent of the disruption of the actin cytoskeleton. Indeed, its partial disassembly stimulates exocytosis and increases the motility of the secretory vesicles [212]. On the other hand, its complete disruption results in a total block of secretion and vesicle movement [194]. This suggests that regulated exocytosis requires the coordinated disassembly and assembly of actin (Fig. 3d). Secretory vesicles may bind to the cortical actin cytoskeleton via multiple molecules and be “functionally” entrapped by actin filaments. In the absence of stimulation (i.e., low Ca++), Myosin Va may serve as an anchor rather than a motor interacting with actin either directly or through MARCKS, as recently reported [251]. Upon stimulation, sequential events may take place including PKC-mediated phosphorylation of MARCKS, which inhibits actin-crosslinking, activation of scinderin (or other actin severing proteins), and GAPs for specific GTPases, which de-polymerize actin (Fig. 3d). These steps are rapidly followed by activation of GTPases, which promote actin assembly, and Myosin Va, which pulls the vesicles close to the plasma membrane (Fig. 3d). This hypothesis is supported by observations that actin initially de-polymerizes and successively re-polymerizes during stimulation of chromaffin cells [210]. This sequential regulation is achieved by coordinating the activities of the two GTPases RhoaA and cdc42. Indeed, activation of RhoA leads to an increase in actin polymerization at the plasma membrane, which impairs exocytosis [252], whereas activation of cdc42 leads to the N-Wasp- and Arp2/3-mediated increase of actin polymerization, which stimulates regulated exocytosis [253]. Other GTPases have been implicated in regulated exocytosis. Arf6 activates both PIP5 K and PLD that leads to the generation of PIP2 and phosphatidic acid, respectively [254]. The important role of these lipids and Arf6 in exocytosis was recently demonstrated in neuroendocrine cells [255, 256]. Furthermore, RalA was shown to interact with Arf6-activated PLD1, suggesting a sequential activation of multiple GTPases in the process [257]. Additional evidence for the role of RalA in exocytosis comes from studies done in pancreatic cells and exocytosis of Weibel–Palade bodies in endothelial cells [258, 259]. Finally, Rap1 and EPAC were linked to regulated exocytosis of Weibel–Palade bodies in endothelial cells [260] and insulin secretion [261].
Actin and regulation of docking and priming
Actin and its motors have been shown to interact or to be functionally linked to some of the molecules involved in vesicle docking and priming (Fig. 3e). Downregulation of munc18-1 affects docking and severely impairs the organization of cortical actin [91, 93]. The PIP2- and cdc42-dependent polymerization of actin promotes docking of secretory vesicles [262] in a process that is also dependent on intersectin-1L, an actin binding protein that binds to SNAREs [263]. Furthermore, F-actin has been shown to directly interact with SNAREs. In primary rat β-cells and in MIN6 β-cells, F-actin interacts directly with the first two N-terminal alpha helical coiled-coil domains of syntaxin 4 and the impairment of this interaction enhances glucose-stimulated insulin release [264]. Defective actin remodeling impairs insulin-dependent fusion of GLUT4 vesicles whereas docking is not affected, suggesting a possible involvement for actin in priming [265]. Among the molecules that may link F-actin, SNAREs, and the lipids at the plasma membrane, we have to mention the members of the Annexin family [266]. Annexins affect membrane domain organization and exocytosis by: (1) binding different phospholipids both on the plasma membrane and on vesicles, in a calcium-dependent manner [267], (2) regulating the delivery of cholesterol and other lipids to plasma membrane microdomains (such as lipid rafts) that may facilitate the assembly of the SNARE complex [268–270], and (3) binding to F-actin [271].
Myosin Va has been found to interact with both syntaxin 1a and VAMP2 and this complex is likely to facilitate tethering at the plasma membrane (Fig. 3e) [244, 272]. The expression of the MyoVa tail in enterochromaffin cells reduces the number of docked granules as assessed by TIRF microscopy [246]. Interestingly, it was recently reported that myo2, the yeast homologue of Myosin Va, interacts with the exocyst complex, suggesting a possible mechanism for the role of myosin Va during docking [273]. Whether Myosin Va plays a role as a processive motor or as a tethering factor may also depend on the linker. Indeed, in β cells, the adaptor exophilin8 regulates the delivery of insulin granules to an actin-rich region, whereas the adaptor granulophilin mediates their docking [203]. The role of myosin Va may also extend to priming since an increase in intracellular Ca++ releases myosin Va from the SNARE complex, facilitating fusion [272]. Myosin Vc is another myosin motor that has been shown to regulate exocytosis primarily in exocrine glands. Myosin Vc has been shown to regulate exocytosis of secretory granules in lacrimal glands [68, 274] and it is expressed in salivary glands, exocrine pancreas [275], and in mucin-containing granules in airway goblet cells [276]. Although the precise step where myosin Vc operates has not been identified, the fact that it does not behave as a processive motor points to a role in docking [277, 278].
Role of the actin cytoskeleton after fusion
Controlling the fusion pore and the dynamics of the membranes after fusion
Although there is no evidence for the recruitment of the cytoskeleton onto the limiting membranes of small secretory vesicles (<500 nm), it has been known for a few decades that F-actin and myosin II coat large secretory vesicles in close proximity to the plasma membrane [279]. However, only recently has it been shown that the recruitment occurs after their fusion with the plasma membrane [164, 280–283]. The observed recruitment of the actomyosin complex occurs with a delay of a few seconds from the opening of the fusion pore, indicating that the fusion step does not require the cytoskeleton [164]. In addition, pharmacological impairment of either actin assembly or myosin motor activity does not affect the fusion of the secretory vesicles with the plasma membrane [164, 281], thus raising several other possibilities about the role of the actomyosin complex.
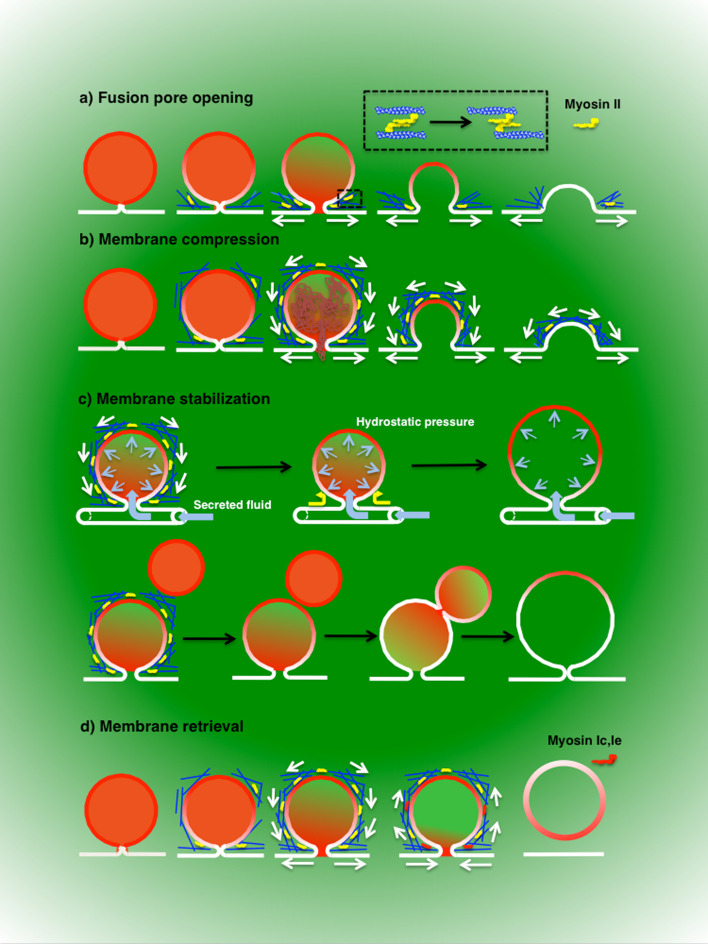
First, it has been proposed that actin regulates the maintenance and closure of the fusion pore, as shown in pancreatic acinar cells, where latrunculin B-mediated actin disruption promotes pore closure [284] (Fig. 4a). In the same system, non-muscle myosin IIa has been localized at the apical plasma membrane and inhibition of its motor activity resulted in closure of the fusion pore [285]. Although myosin II has been localized onto the membranes of large fused secretory vesicles [164, 281], its role in regulating the fusion pore has been mainly characterized in chromaffin cells [249, 286–289]. Inhibitors of myosin light chain kinase [290] and expression of a non-phosphorylatable form of the regulatory light chain of myosin II [249] slows the fusion kinetics of chromaffin granules. Detailed analysis using either amperometry [287, 289] or TIRF microscopy [286] revealed that myosin II contributes to the expansion of the fusion pore. Interestingly, a series of elegant studies have shown that myosin II activity determines whether exocytosis occurs via full collapse or by a “kiss-and-run” mechanism [182, 288, 291]. Indeed, activation of myosin II by phosphorylation results in the complete expansion of the fusion pore leading to the full collapse of dense core vesicles and complete release of catecholamines. On the other hand, impairment of myosin II activity leads to the early closure of the fusion pore, a partial release of the cargo molecules, and the scission of the vesicles from the plasma membrane [288, 291]. At the molecular level, the recruitment of myosin II and other regulators of the pore expansion are controlled by the phosphorylation of dynamin I, revealing a complex coordination between fusion and fission machineries [182].
Fig. 4.
The actin cytoskeleton in post-fusion steps. The contractile activity of the actomyosin complex is required to expand the fusion pore by exerting a force parallel to the plasma membrane (a) and/or to pull the membranes of the secretory vesicles toward the plasma membrane by exerting a force tangential to the surface of the vesicles (b). Both processes favor the expulsion of cargo molecules (a, b). In exocrine glands, the actomyosin complex may also serve as a scaffold to stabilize the secretory vesicles that are fused with the plasma membrane (c). This would prevent the uncontrolled expansion of the vesicles that is caused by either an increase in hydrostatic pressures, as shown to occur in exocrine glands in live animals, or fusion with adjacent secretory vesicles (c). The main myosin motor used for these functions is the non-muscle myosin II. The actomyosin complex may also control the retrieval of the vesicular membranes in order to maintain plasma membrane homeostasis. This process requires myosin II, Ie, and Ic (d)
A second function for the actin cytoskeleton is to provide the force to drive exocytosis to completion via the contractile activity of the actomyosin complex [19, 32, 195] (Fig. 4b). The force generated by the complex can be used to compress the secretory vesicles to facilitate the extrusion of cargo molecules that form large aggregates such as the von-Willebrand factor [281], surfactant [280, 292], or lectin [293–295]. Strikingly, in endothelial cells, actin forms a polarized ring around the fused Weibel–Palade bodies that is perfectly oriented to perform the mechanical squeezing of the cargo molecules [281]. The kinetics of recruitment of both actin and myosin mirror the opening of the fusion pore and the complete emptying of the secretory vesicles. Moreover, disruption of both actin and myosin assembly around the vesicles results in the impairment of cargo release [280, 281, 292]. In other systems in which cargo molecules are more loosely associated, such as salivary glands, pancreas, and lacrimal glands, the contractile activity of the actomyosin complex is required to facilitate the integration of vesicles membranes into the cell surface [164, 248, 296, 297], although with different modalities. In particular, in pancreatic and salivary acini, actin and myosin coat individual granules [164, 297], whereas in lacrimal glands, actin encloses multiple granules [248]. As discussed earlier, in these systems the full collapse of the vesicles is not energetically favorable, and thus the actomyosin complex may be required to provide the necessary energy for the absorption of the membranes. We speculate that the actomyosin complex may act in two different ways by applying: (1) a force tangential to the surface of the vesicles that pull the membranes toward the plasma membrane, and (2) a force parallel to the plasma membrane that expands the fusion pore (Fig. 4b) [296, 298]. In pancreas and lacrimal glands, both forces may be generated by myosin IIa, which is the only isoform of myosin II that is localized onto the secretory vesicles [248, 285]. In salivary glands, in which both myosin IIa an IIb are localized onto the secretory vesicles, it is tempting to speculate that each of the myosins regulates one of the two processes.
The actomyosin complex has also been proposed to stabilize the limiting membranes of the secretory granules during exocytosis in order to prevent changes in their shape or size that may affect either the release of the cargo or their gradual collapse (Fig. 4c). In exocrine pancreas, where compound exocytosis is the main modality for vesicle secretion, impairment in the assembly of the actin cytoskeleton, by using cytochalasin D or agonist overstimulation, increases the rate of compound exocytosis fusion events leading to the formation of large secretory vesicles at the plasma membrane [299]. In lacrimal glands, impairment of F-actin assembly and inhibition of myosin II motor activity resulted in the enhancement of compound fusion revealed by a significant increase in the size of the granules [248]. In explanted salivary glands, cytochalasin D induced the formation of vacuolar structures containing amylase [78], whereas in live animals, the same treatments led to an increase in the size of the secretory vesicles fused with the plasma membrane [164]. A more detailed analysis revealed that this effect is due initially to the hydrostatic pressure generated by the concomitant fluid secretion that occurs in the live animal, suggesting that the actomyosin complex exerts a structural function around the granules (Fig. 4c). However, at later stages, the increase in size was due to compound exocytosis. Especially for secretory systems with slow post-fusion kinetics, the diffusion of proteins and lipid from the plasma membrane into the membranes of the granules make them competent to fuse with other granules [300, 301]. Interestingly, although compound exocytosis has been extensively reported in ex vivo salivary glands [302–305], in live animals it is observed only when actin assembly is impaired [164].
The last function that has been proposed for the actomyosin complex is to control the retrieval of the vesicular membranes (Fig. 4d). Indeed, in order to maintain cell homeostasis, the membranes added to the plasma membrane are retrieved by compensatory endocytosis [166, 298] either via clathrin-mediated [165, 166, 306] or clathrin-independent pathways [307–309]. Both exocytosis and compensatory endocytosis are strictly interdependent, as shown by the fact that they share common machineries [181, 310]. In case of full-fusion events, actin would serve to regulate a de novo endocytic process [165, 307] involving specific motors such as myosin VI [204]. In eggs, where cortical granules are not fully absorbed into the plasma membrane, the retrieval process requires de novo actin polymerization around the granular membranes (a process termed Kiss and Coat [19]) and the motor activity of different myosins such as myosin II, myosin 1c, and 1e [294, 295] (Fig. 4d).
The mechanism of actin recruitment onto the secretory granules has not been fully elucidated. In cortical granules, actin assembly is dependent on the actin nucleation factors Arp2/3 and the small GTPase cdc42 [19, 282, 294, 295]. This process requires the synthesis of PIP2 suggesting a role for a PIP5 kinase. Furthermore, a mixing compartment model has been proposed in which the machinery regulating actin nucleation is split between the secretory vesicles and the plasma membrane and only comes into contact after fusion. This model is consistent with the lag time in actin polymerization detected after fusion of the bilayers [164, 281]. In addition, PI4 kinase, an enzyme upstream to the production of PIP2, has been localized onto the secretory granules and is supposedly involved in the activation of RhoA [311]. In pancreatic acini stimulated by CCK, RhoA and Rac1 activation lead to the recruitment of actin on zymogen granules [12, 312–314], whereas inhibition of RhoA by C3 toxin induces the impairment in actin recruitment and deregulation of compound exocytosis [299].
RhoA activation has also been implicated in regulating actin polymerization during exocytosis of lamellar bodies in a process that requires the actin nucleation factor formin, rather than Arp2/3 [280]. Actin recruitment is also temporally correlated with the loss of Rab3d from the zymogen granules, although the link with actin polymerization is not clear [297]. Finally, other pathways may be implicated in regulating actin. In salivary glands for example, Rap1 is activated by beta-adrenergic stimulation [315, 316] via the cAMP/EPAC pathway [153, 317]. Interestingly, Rap1 has been reported to activate the exchange factors for Rac1, which triggers actin polymerization at the plasma membrane [318].
Conclusions and perspectives
The actin cytoskeleton is clearly implicated in every step of regulated exocytosis in vertebrate cells. The large number of regulatory molecules that temporally and spatially control its dynamic nature and the diversity in the architecture and physiology of the various secretory systems have made it challenging to fully pinpoint its precise role. However, based on our current knowledge, it is reasonable to assign at least two main functions to the actin cytoskeleton in regulated exocytosis: (1) the tight control and coordination of the delivery of the secretory vesicles to their fusion sites at the plasma membrane, and (2) the regulation of the vesicle membranes dynamics after fusion.
The first function appears to be prevalent in cells in which the secretory vesicles have relatively small diameters, exhibit fast rates of fusion, and release small molecular weight cargo (Fig. 5). Typically, these morphological characteristics are associated with neuronal, endocrine, neuroendocrine, and hematopoietic cells, which are designed to elicit fast and highly controlled responses in order to carry out their physiological tasks. From a biophysical point of view, the integration of these small vesicles with the plasma membrane is energetically favorable (Fig. 5) and proceeds very rapidly (milliseconds to seconds). Hence, the actin cytoskeleton may provide an additional layer of regulation to prevent the uncontrolled delivery of secretory vesicles to a fusion competent area of the plasma membrane that may result in severe pathological conditions.
Fig. 5.
Role of the actin cytoskeleton and the morphology of the secretory apparatus. The actin cytoskeleton performs two main functions in regulated exocytosis: (1) the tight control and coordination of the delivery of the secretory vesicles to their fusion sites at the plasma membrane, and (2) the regulation of the vesicle membranes dynamics after fusion. The first function is prevalent in cells in which the secretory vesicles have relatively small diameters, exhibit fast rates of fusion, and release small molecular weight cargo (synaptic vesicles, dense core vesicles, insulin, and lytic granules). From a biophysical point of view, the integration of these small vesicles into the plasma membrane is energetically favorable and proceeds very rapidly. In these systems, the actin cytoskeleton may provide an additional layer of regulation to prevent the uncontrolled delivery of secretory vesicles to fusogenic areas of the plasma membrane that may result in severe pathological conditions. The second function is more prevalent in cells in which secretory vesicles have large diameters and the post-fusion process is kinetically slow. Since the integration of large vesicles that fuse with curved membranes, such as canaliculi and ducts, is not energetically favorable and proceeds slowly (seconds to minutes), the recruitment of a contractile actomyosin complex onto the membranes of the secretory vesicles may provide the energy to facilitate this process. The contractile activity may also facilitate the expulsion of very large cargo, the retrieval of membranes by compensatory endocytosis, and to serve as a scaffold to stabilize them and prevent their disruption, which can be caused by either an increase in hydrostatic pressures fusion with adjacent secretory vesicles. For some small vesicles, such as in neuroendocrine cells, the actomyosin complex regulates the fusion pore and the fate of the vesicles after fusion (full fusion vs. kiss and run) without being recruited onto the membrane of the vesicles
The second function seems to be prevalent in cells in which secretory vesicles have large diameters and the post-fusion process is kinetically slow. These characteristics are associated with exocrine systems and some specialized secretory cells, which are designed to generate slower and more sustained responses. Since the integration of large vesicles that fuse with curved membranes, such as canaliculi and ducts, is not energetically favorable (Fig. 5) and proceeds slowly (seconds to minutes), the recruitment of a contractile actomyosin complex on the membrane of the secretory vesicles may provide the energy to facilitate this process. This can be achieved by enlarging the fusion pore and/or pulling the membranes (Fig. 5). The contractile activity may also facilitate the expulsion of very large cargo that would normally require a longer time to be released into the extracellular space, and the retrieval of membranes by compensatory endocytosis in order to maintain cell homeostasis. Moreover, since the large fused vesicles are held at the plasma membrane for long periods of time, the actomyosin complex may also serve as a scaffold to stabilize them and prevent their disruption, which can be caused by either an increase in hydrostatic pressures, as shown to occur in exocrine glands in live animals, or fusion with adjacent secretory vesicles (Fig. 5). Interestingly, one notable exception to this model is the small vesicles in neuroendocrine cells. Although, the actomyosin complex has not been detected onto the membrane of the secretory vesicles, it regulates closure of the fusion pore dictating the fate of the vesicles after fusion (full fusion vs. kiss and run).
In conclusion, continuous efforts have to be devoted toward elucidating the role of the actin cytoskeleton in regulated exocytosis. The direction to follow is clearly the use of a multi-disciplinary approach that will increase our understanding of these processes. Particularly, more sophisticated imaging technologies, such as TIRF, super-resolution microscopy, and intravital microscopy are very promising, enabling simultaneous imaging of single exocytic events and actin cytoskeleton dynamics. This, in combination with the use of more sophisticated molecular tools and the advancement of structural biology, has the potential to provide novel insights into this process and to define novel paradigms.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research. We sincerely apologize to those whose work could not be cited due to space limitations.
References
- 1.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83(2):581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 2.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9(4):273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 3.Stockli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124(Pt 24):4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17(1):87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4(1):a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudhof TC, Rizo J (2011) Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol 3(12) [DOI] [PMC free article] [PubMed]
- 7.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69(5):856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolensek J, Skelin M, Rupnik MS. Calcium dependencies of regulated exocytosis in different endocrine cells. Physiol Res. 2011;60(Suppl 1):S29–S38. doi: 10.33549/physiolres.932176. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez LM. New insights into the role of the cortical cytoskeleton in exocytosis from neuroendocrine cells. Int Rev Cell Mol Biol. 2012;295:109–137. doi: 10.1016/B978-0-12-394306-4.00009-5. [DOI] [PubMed] [Google Scholar]
- 10.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84(6):500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JA. Regulation of acinar cell function in the pancreas. Curr Opin Gastroenterol. 2010;26(5):478–483. doi: 10.1097/MOG.0b013e32833d11c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu K, et al. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp Eye Res. 2006;83(1):84–96. doi: 10.1016/j.exer.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Jolly C, Sattentau QJ. Regulated secretion from CD4 + T cells. Trends Immunol. 2007;28(11):474–481. doi: 10.1016/j.it.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59(4–5):286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- 16.Valentijn KM, et al. Functional architecture of Weibel–Palade bodies. Blood. 2011;117(19):5033–5043. doi: 10.1182/blood-2010-09-267492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39(5):1191–1196. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- 18.Mather IH, Keenan TW. Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia. 1998;3(3):259–273. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- 19.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17(4):1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Communi D, et al. Advances in signalling by extracellular nucleotides. The role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12(6):351–360. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65(18):2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momboisse F, et al. How important are Rho GTPases in neurosecretion? J Neurochem. 2011;117(4):623–631. doi: 10.1111/j.1471-4159.2011.07241.x. [DOI] [PubMed] [Google Scholar]
- 23.Ory S, Gasman S. Rho GTPases and exocytosis: what are the molecular links? Semin Cell Dev Biol. 2011;22(1):27–32. doi: 10.1016/j.semcdb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sladek CD, Song Z. Diverse roles of G-protein coupled receptors in the regulation of neurohypophyseal hormone secretion. J Neuroendocrinol. 2012;24(4):554–565. doi: 10.1111/j.1365-2826.2011.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19(4):453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2011;14(1):11–19. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- 28.Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763(11):1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Monastyrska I, et al. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc. 2009;84(3):431–448. doi: 10.1111/j.1469-185X.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egea G, Lazaro-Dieguez F, Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol. 2006;18(2):168–178. doi: 10.1016/j.ceb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641(2–3):175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 32.Nightingale TD, Cutler DF, Cramer LP. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 2012;22(6):329–337. doi: 10.1016/j.tcb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Barclay JW, Morgan A, Burgoyne RD. Neurotransmitter release mechanisms studied in Caenorhabditis elegans. Cell Calcium. 2012;52(3–4):289–295. doi: 10.1016/j.ceca.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Levitan ES. Signaling for vesicle mobilization and synaptic plasticity. Mol Neurobiol. 2008;37(1):39–43. doi: 10.1007/s12035-008-8014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18(9):397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerberg J, et al. Sea urchin egg preparations as systems for the study of calcium-triggered exocytosis. J Physiol. 1999;520(Pt 1):15–21. doi: 10.1111/j.1469-7793.1999.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon JP, Aunis D. Biochemistry of the chromogranin A protein family. Biochem J. 1989;262(1):1–13. doi: 10.1042/bj2620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim T, et al. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106(4):499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 39.Kim T, et al. Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci. 2005;25(30):6958–6961. doi: 10.1523/JNEUROSCI.1058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T, Loh YP. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol Biol Cell. 2006;17(2):789–798. doi: 10.1091/mbc.E05-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendy GN, et al. Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol Endocrinol. 2006;20(8):1935–1947. doi: 10.1210/me.2005-0398. [DOI] [PubMed] [Google Scholar]
- 42.Lutherborrow MA, et al. Gene expression profiling of HUH7-ins: lack of a granulogenic function for chromogranin A. Islets. 2009;1(1):62–74. doi: 10.4161/isl.1.1.8994. [DOI] [PubMed] [Google Scholar]
- 43.Obermuller S, et al. Defective secretion of islet hormones in chromogranin-B-deficient mice. PLoS One. 2010;5(1):e8936. doi: 10.1371/journal.pone.0008936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr Opin Cell Biol. 2006;18(4):365–370. doi: 10.1016/j.ceb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Day R, Gorr SU. Secretory granule biogenesis and chromogranin A: master gene, on/off switch or assembly factor? Trends Endocrinol Metab. 2003;14(1):10–13. doi: 10.1016/s1043-2760(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 46.Park JJ, Loh YP. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol. 2008;22(12):2583–2595. doi: 10.1210/me.2008-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cool DR, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88(1):73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 48.Hosaka M, et al. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J Cell Sci. 2005;118(Pt 20):4785–4795. doi: 10.1242/jcs.02608. [DOI] [PubMed] [Google Scholar]
- 49.Lou H, et al. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron. 2005;45(2):245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 50.Prasad P, et al. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181(7):5024–5034. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- 51.Assadi M, et al. The C-terminus of prohormone convertase 2 is sufficient and necessary for Raft association and sorting to the regulated secretory pathway. Biochemistry. 2004;43(24):7798–7807. doi: 10.1021/bi036331g. [DOI] [PubMed] [Google Scholar]
- 52.Milgram SL, Chang EY, Mains RE. Processing and routing of a membrane-anchored form of proneuropeptide Y. Mol Endocrinol. 1996;10(7):837–846. doi: 10.1210/mend.10.7.8813724. [DOI] [PubMed] [Google Scholar]
- 53.Venkatesh SG, et al. Parotid secretory protein binds phosphatidylinositol (3,4) bisphosphate. J Dent Res. 2012;90(9):1085–1090. doi: 10.1177/0022034511410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blazquez M, Shennan KI. Basic mechanisms of secretion: sorting into the regulated secretory pathway. Biochem Cell Biol. 2000;78(3):181–191. [PubMed] [Google Scholar]
- 55.Kuliawat R, et al. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol. 1997;137(3):595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morvan J, Tooze SA. Discovery and progress in our understanding of the regulated secretory pathway in neuroendocrine cells. Histochem Cell Biol. 2008;129(3):243–252. doi: 10.1007/s00418-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castle JD. Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann N Y Acad Sci. 1998;842:115–124. doi: 10.1111/j.1749-6632.1998.tb09639.x. [DOI] [PubMed] [Google Scholar]
- 58.Riedel D, et al. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol. 2002;22(18):6487–6497. doi: 10.1128/MCB.22.18.6487-6497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hannah MJ, et al. Weibel–Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J Cell Sci. 2003;116(Pt 19):3939–3948. doi: 10.1242/jcs.00711. [DOI] [PubMed] [Google Scholar]
- 60.Bogan JS, Kandror KV. Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Curr Opin Cell Biol. 2010;22(4):506–512. doi: 10.1016/j.ceb.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamb CA, et al. Insulin-regulated trafficking of GLUT4 requires ubiquitination. Traffic. 2010;11(11):1445–1454. doi: 10.1111/j.1600-0854.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol. 2002;13(4):263–270. doi: 10.1016/s1084952102000551. [DOI] [PubMed] [Google Scholar]
- 63.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7(5):373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 64.Brange J, Langkjoer L. Insulin structure and stability. Pharm Biotechnol. 1993;5:315–350. doi: 10.1007/978-1-4899-1236-7_11. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths GM, Argon Y. Structure and biogenesis of lytic granules. Curr Top Microbiol Immunol. 1995;198:39–58. doi: 10.1007/978-3-642-79414-8_3. [DOI] [PubMed] [Google Scholar]
- 66.Wolff MS, et al. Development of the rat sublingual gland: a light and electron microscopic immunocytochemical study. Anat Rec. 2002;266(1):30–42. doi: 10.1002/ar.10027. [DOI] [PubMed] [Google Scholar]
- 67.Fukuda M, Morales P, Overstreet JW. Acrosomal function of human spermatozoa with normal and abnormal head morphology. Gamete Res. 1989;24(1):59–65. doi: 10.1002/mrd.1120240109. [DOI] [PubMed] [Google Scholar]
- 68.Chiang L, Karvar S, Hamm-Alvarez SF. Direct imaging of RAB27B-enriched secretory vesicle biogenesis in lacrimal acinar cells reveals origins on a nascent vesicle budding site. PLoS ONE. 2012;7(2):e31789. doi: 10.1371/journal.pone.0031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162(6):1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hume AN, et al. Semi-automated analysis of organelle movement and membrane content: understanding rab-motor complex transport function. Traffic. 2011;12(12):1686–1701. doi: 10.1111/j.1600-0854.2011.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schroeder HW, 3rd, et al. Motor number controls cargo switching at actin-microtubule intersections in vitro. Curr Biol. 2010;20(8):687–696. doi: 10.1016/j.cub.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snider J, et al. Intracellular actin-based transport: how far you go depends on how often you switch. Proc Natl Acad Sci USA. 2004;101(36):13204–13209. doi: 10.1073/pnas.0403092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein AY, Wang X, Schwarz TL. Axonal transport and the delivery of pre-synaptic components. Curr Opin Neurobiol. 2008;18(5):495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonald A, et al. Control of insulin granule dynamics by AMPK dependent KLC1 phosphorylation. Islets. 2009;1(3):198–209. doi: 10.4161/isl.1.3.9608. [DOI] [PubMed] [Google Scholar]
- 75.Zakharenko S, Popov S. Dynamics of axonal microtubules regulate the topology of new membrane insertion into the growing neurites. J Cell Biol. 1998;143(4):1077–1086. doi: 10.1083/jcb.143.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farshori PQ, Goode D. Effects of the microtubule depolymerizing and stabilizing agents Nocodazole and taxol on glucose-induced insulin secretion from hamster islet tumor (HIT) cells. J Submicrosc Cytol Pathol. 1994;26(2):137–146. [PubMed] [Google Scholar]
- 77.Lizunov VA, et al. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169(3):481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nashida T, et al. Presence of cytoskeleton proteins in parotid glands and their roles during secretion. Arch Oral Biol. 2004;49(12):975–982. doi: 10.1016/j.archoralbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Neco P, et al. Differential participation of actin- and tubulin-based vesicle transport systems during secretion in bovine chromaffin cells. Eur J Neurosci. 2003;18(4):733–742. doi: 10.1046/j.1460-9568.2003.02801.x. [DOI] [PubMed] [Google Scholar]
- 80.Schnekenburger J, et al. The role of kinesin, dynein and microtubules in pancreatic secretion. Cell Mol Life Sci. 2009;66(15):2525–2537. doi: 10.1007/s00018-009-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ueda N, et al. Kinesin is involved in regulation of rat pancreatic amylase secretion. Gastroenterology. 2000;119(4):1123–1131. doi: 10.1053/gast.2000.18145. [DOI] [PubMed] [Google Scholar]
- 82.Varadi A, et al. Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J Cell Sci. 2002;115(Pt 21):4177–4189. doi: 10.1242/jcs.00083. [DOI] [PubMed] [Google Scholar]
- 83.Sollner TH. Regulated exocytosis and SNARE function (Review) Mol Membr Biol. 2003;20(3):209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- 84.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hay JC. SNARE complex structure and function. Exp Cell Res. 2001;271(1):10–21. doi: 10.1006/excr.2001.5368. [DOI] [PubMed] [Google Scholar]
- 86.Fasshauer D, et al. Conserved structural features of the synaptic fusion complex: sNARE proteins reclassified as Q- and R-SNAREs . Proc Natl Acad Sci USA. 1998;95(26):15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sutton RB, et al. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395(6700):347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 88.Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9(9):1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 89.Martin TF. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641(2–3):157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 90.Burchfield JG, et al. Exocytotic vesicle behaviour assessed by total internal reflection fluorescence microscopy. Traffic. 2010;11(4):429–439. doi: 10.1111/j.1600-0854.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 91.de Wit H. Morphological docking of secretory vesicles. Histochem Cell Biol. 2010;134(2):103–113. doi: 10.1007/s00418-010-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugita S. Mechanisms of exocytosis. Acta Physiol (Oxf) 2008;192(2):185–193. doi: 10.1111/j.1748-1716.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 93.de Wit H. Molecular mechanism of secretory vesicle docking. Biochem Soc Trans. 2010;38(Pt 1):192–198. doi: 10.1042/BST0380192. [DOI] [PubMed] [Google Scholar]
- 94.Toonen RF, et al. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25(16):3725–3737. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voets T, et al. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31(4):581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 96.Mandic SA, et al. Munc18-1 and Munc18-2 proteins modulate beta-cell Ca2+ sensitivity and kinetics of insulin exocytosis differently. J Biol Chem. 2011;286(32):28026–28040. doi: 10.1074/jbc.M111.235366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin-Verdeaux S, et al. Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J Cell Sci. 2003;116(Pt 2):325–334. doi: 10.1242/jcs.00216. [DOI] [PubMed] [Google Scholar]
- 98.Cote M, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imai A, Nashida T, Shimomura H. Roles of Munc18-3 in amylase release from rat parotid acinar cells. Arch Biochem Biophys. 2004;422(2):175–182. doi: 10.1016/j.abb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 100.de Wit H, et al. Docking of secretory vesicles is syntaxin dependent. PLoS One. 2006;1:e126. doi: 10.1371/journal.pone.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gulyas-Kovacs A, et al. Munc18-1: sequential interactions with the fusion machinery stimulate vesicle docking and priming. J Neurosci. 2007;27(32):8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coppola T, et al. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13(6):1906–1915. doi: 10.1091/mbc.02-02-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graham ME, et al. A gain-of-function mutant of Munc18-1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18-1 with Rab3. Biochem J. 2008;409(2):407–416. doi: 10.1042/BJ20071094. [DOI] [PubMed] [Google Scholar]
- 104.Chung SH, Takai Y, Holz RW. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. Studies in adrenal chromaffin cells. J Biol Chem. 1995;270(28):16714–16718. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- 105.Deak F, et al. Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 2006;25(12):2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuboi T, Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J Biol Chem. 2005;280(47):39253–39259. doi: 10.1074/jbc.M507173200. [DOI] [PubMed] [Google Scholar]
- 107.Gomi H, et al. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol. 2005;171(1):99–109. doi: 10.1083/jcb.200505179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu M, et al. Exophilin4/Slp2-a targets glucagon granules to the plasma membrane through unique Ca2+-inhibitory phospholipid-binding activity of the C2A domain. Mol Biol Cell. 2007;18(2):688–696. doi: 10.1091/mbc.E06-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lo SY, et al. Intrinsic tethering activity of endosomal Rab proteins. Nat Struct Mol Biol. 2011;19(1):40–47. doi: 10.1038/nsmb.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13(7):577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 111.Inoue M, et al. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422(6932):629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 112.Lopez JA, et al. The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta cells. J Biol Chem. 2008;283(26):17939–17945. doi: 10.1074/jbc.M800321200. [DOI] [PubMed] [Google Scholar]
- 113.Moore BA, Robinson HH, Xu Z. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J Mol Biol. 2007;371(2):410–421. doi: 10.1016/j.jmb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu H, et al. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21(3):430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rittmeyer EN, et al. A dual role for IQGAP1 in regulating exocytosis. J Cell Sci. 2008;121(Pt 3):391–403. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- 116.Lizunov VA, et al. Insulin regulates fusion of GLUT4 vesicles independent of Exo70-mediated tethering. J Biol Chem. 2009;284(12):7914–7919. doi: 10.1074/jbc.M806460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Imai A, et al. Exocyst subunits are involved in isoproterenol-induced amylase release from rat parotid acinar cells. Eur J Oral Sci. 2012;120(2):123–131. doi: 10.1111/j.1600-0722.2012.00952.x. [DOI] [PubMed] [Google Scholar]
- 118.Dupraz S, et al. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci. 2009;29(42):13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balch WE, et al. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 120.Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- 121.Weidman PJ, et al. Binding of an N-ethylmaleimide-sensitive fusion protein to Golgi membranes requires both a soluble protein(s) and an integral membrane receptor. J Cell Biol. 1989;108(5):1589–1596. doi: 10.1083/jcb.108.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94(12):6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao C, Smith EC, Whiteheart SW. Requirements for the catalytic cycle of the N-ethylmaleimide-Sensitive Factor (NSF) Biochim Biophys Acta. 2012;1823(1):159–171. doi: 10.1016/j.bbamcr.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sassa T, et al. Regulation of the UNC-18-Caenorhabditis elegans syntaxin complex by UNC-13. J Neurosci. 1999;19(12):4772–4777. doi: 10.1523/JNEUROSCI.19-12-04772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dulubova I, et al. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci USA. 2007;104(8):2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zilly FE, et al. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biol. 2006;4(10):e330. doi: 10.1371/journal.pbio.0040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99(13):9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ashery U, et al. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 2000;19(14):3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang L, et al. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 2006;3(6):463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 130.Sheu L, et al. Regulation of insulin exocytosis by Munc13-1. J Biol Chem. 2003;278(30):27556–27563. doi: 10.1074/jbc.M303203200. [DOI] [PubMed] [Google Scholar]
- 131.Zhu Y, et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586(7):1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boswell KL, et al. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol. 2012;197(2):301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson JL, et al. Munc13-4 restricts motility of Rab27a-expressing vesicles to facilitate lipopolysaccharide-induced priming of exocytosis in neutrophils. J Biol Chem. 2011;286(7):5647–5656. doi: 10.1074/jbc.M110.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, et al. CAPS facilitates filling of the rapidly releasable pool of large dense-core vesicles. J Neurosci. 2008;28(21):5594–5601. doi: 10.1523/JNEUROSCI.5672-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Speidel D, et al. CAPS1 and CAPS2 regulate stability and recruitment of insulin granules in mouse pancreatic beta cells. Cell Metab. 2008;7(1):57–67. doi: 10.1016/j.cmet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Fujita Y, et al. Ca2+-dependent activator protein for secretion 1 is critical for constitutive and regulated exocytosis but not for loading of transmitters into dense core vesicles. J Biol Chem. 2007;282(29):21392–21403. doi: 10.1074/jbc.M703699200. [DOI] [PubMed] [Google Scholar]
- 137.Sadakata T, et al. Differential distributions of the Ca2+-dependent activator protein for secretion family proteins (CAPS2 and CAPS1) in the mouse brain. J Comp Neurol. 2006;495(6):735–753. doi: 10.1002/cne.20947. [DOI] [PubMed] [Google Scholar]
- 138.Sadakata T, et al. Tissue distribution of Ca2+-dependent activator protein for secretion family members CAPS1 and CAPS2 in mice. J Histochem Cytochem. 2007;55(3):301–311. doi: 10.1369/jhc.6A7033.2006. [DOI] [PubMed] [Google Scholar]
- 139.Grishanin RN, et al. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43(4):551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 140.Petersen OH. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium. 2003;33(5–6):337–344. doi: 10.1016/s0143-4160(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 141.Arac D, et al. Close membrane–membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13(3):209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 142.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126(6):1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 143.Falkowski MA, et al. Expression, localization, and functional role for synaptotagmins in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G306–G316. doi: 10.1152/ajpgi.00108.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iezzi M, et al. Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci. 2004;117(Pt 15):3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- 145.Li Y, et al. Regulation of insulin secretion and GLUT4 trafficking by the calcium sensor synaptotagmin VII. Biochem Biophys Res Commun. 2007;362(3):658–664. doi: 10.1016/j.bbrc.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fowler KT, Andrews NW, Huleatt JW. Expression and function of synaptotagmin VII in CTLs. J Immunol. 2007;178(3):1498–1504. doi: 10.4049/jimmunol.178.3.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Melicoff E, et al. Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells. J Biol Chem. 2009;284(29):19445–19451. doi: 10.1074/jbc.M109.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85(4):1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 149.Skelin M, Rupnik M. cAMP increases the sensitivity of exocytosis to Ca(2)+ primarily through protein kinase A in mouse pancreatic beta cells. Cell Calcium. 2011;49(2):89–99. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Maximov A, et al. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176(1):113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Evans GJ, et al. Phosphorylation of cysteine string protein by protein kinase A. Implications for the modulation of exocytosis. J Biol Chem. 2001;276(51):47877–47885. doi: 10.1074/jbc.M108186200. [DOI] [PubMed] [Google Scholar]
- 152.Boczan J, Leenders AG, Sheng ZH. Phosphorylation of syntaphilin by cAMP-dependent protein kinase modulates its interaction with syntaxin-1 and annuls its inhibitory effect on vesicle exocytosis. J Biol Chem. 2004;279(18):18911–18919. doi: 10.1074/jbc.M400496200. [DOI] [PubMed] [Google Scholar]
- 153.Wu CY, et al. The contribution of AKAP5 in amylase secretion from mouse parotid acini. Am J Physiol Cell Physiol. 2010;298(5):C1151–C1158. doi: 10.1152/ajpcell.00382.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Branham MT, et al. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem. 2009;284(37):24825–24839. doi: 10.1074/jbc.M109.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Hooren KW, et al. The Epac-Rap1 pathway controls cAMP-mediated exocytosis of Weibel–Palade bodies in endothelial cells. J Biol Chem. 2012;287(29):24713–24720. doi: 10.1074/jbc.M111.321976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park JH, et al. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic beta-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology. 2012;153(2):574–582. doi: 10.1210/en.2011-0259. [DOI] [PubMed] [Google Scholar]
- 157.Dzhura I, et al. Phospholipase C-epsilon links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets. 2012;3(3):121–128. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sorensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu Rev Cell Dev Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 159.Jesenek D, et al. Exocytotic fusion pore stability and topological defects in the membrane with orientational degree of ordering. Cell Calcium. 2012;52(3–4):277–282. doi: 10.1016/j.ceca.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 160.Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97(6):1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- 161.Kasai H, et al. A new quantitative (two-photon extracellular polar-tracer imaging-based quantification (TEPIQ)) analysis for diameters of exocytic vesicles and its application to mouse pancreatic islets. J Physiol. 2005;568(Pt 3):891–903. doi: 10.1113/jphysiol.2005.093047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kasai H, et al. Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev. 2006;58(7):850–877. doi: 10.1016/j.addr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 163.Martina JA, et al. Imaging of lytic granule exocytosis in CD8(+) cytotoxic T lymphocytes reveals a modified form of full fusion. Cell Immunol. 2011;271(2):267–279. doi: 10.1016/j.cellimm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Masedunskas A, et al. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci USA. 2011;108(33):13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ceridono M, et al. Selective recapture of secretory granule components after full collapse exocytosis in neuroendocrine chromaffin cells. Traffic. 2011;12(1):72–88. doi: 10.1111/j.1600-0854.2010.01125.x. [DOI] [PubMed] [Google Scholar]
- 166.Smith SM, Renden R, von Gersdorff H. Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci. 2008;31(11):559–568. doi: 10.1016/j.tins.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van Kempen GT, et al. Three distinct modes of exocytosis revealed by amperometry in neuroendocrine cells. Biophys J. 2011;100(4):968–977. doi: 10.1016/j.bpj.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xia X, Lessmann V, Martin TF. Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J Cell Sci. 2009;122(Pt 1):75–82. doi: 10.1242/jcs.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taraska JW, et al. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA. 2003;100(4):2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J Biol Chem. 2004;279(45):47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- 171.Zhang J, Castle D. Regulation of fusion pore closure and compound exocytosis in neuroendocrine PC12 cells by SCAMP1. Traffic. 2011;12(5):600–614. doi: 10.1111/j.1600-0854.2011.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nemoto T, et al. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3(3):253–258. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 173.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic. 2006;7(2):109–116. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 174.Thorn P, Gaisano H. Molecular control of compound exocytosis: a key role for VAMP8. Commun Integr Biol. 2012;5(1):61–63. doi: 10.4161/cib.18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Warner JD, et al. Visualizing form and function in organotypic slices of the adult mouse parotid gland. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G629–G640. doi: 10.1152/ajpgi.90217.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jorgacevski J, et al. Fusion pore regulation in peptidergic vesicles. Cell Calcium. 2010;52(3–4):270–276. doi: 10.1016/j.ceca.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 177.Zhang Z, Jackson MB. Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis. Biophys J. 2010;98(11):2524–2534. doi: 10.1016/j.bpj.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu Y, et al. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J Cell Biol. 2011;193(4):643–653. doi: 10.1083/jcb.201008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takahashi N, et al. Two-photon excitation imaging of pancreatic islets with various fluorescent probes. Diabetes. 2002;51(Suppl 1):S25–S28. doi: 10.2337/diabetes.51.2007.s25. [DOI] [PubMed] [Google Scholar]
- 180.Lynch KL, et al. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol Biol Cell. 2008;19(12):5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Anantharam A, et al. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol Biol Cell. 2010;22(11):1907–1918. doi: 10.1091/mbc.E11-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chan SA, Doreian B, Smith C. Dynamin and myosin regulate differential exocytosis from mouse adrenal chromaffin cells. Cell Mol Neurobiol. 2010;30(8):1351–1357. doi: 10.1007/s10571-010-9591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi L, et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335(6074):1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu L, et al. SCAMP2 interacts with Arf6 and phospholipase D1 and links their function to exocytotic fusion pore formation in PC12 cells. Mol Biol Cell. 2005;16(10):4463–4472. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Z, et al. Phosphatidylserine regulation of Ca2+-triggered exocytosis and fusion pores in PC12 cells. Mol Biol Cell. 2009;20(24):5086–5095. doi: 10.1091/mbc.E09-08-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chizmadzhev YA, et al. Lipid flow through fusion pores connecting membranes of different tensions. Biophys J. 1999;76(6):2951–2965. doi: 10.1016/S0006-3495(99)77450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chizmadzhev YA, et al. Dynamics of fusion pores connecting membranes of different tensions. Biophys J. 2000;78(5):2241–2256. doi: 10.1016/S0006-3495(00)76771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Monck JR, Alvarez de Toledo G, Fernandez JM. Tension in secretory granule membranes causes extensive membrane transfer through the exocytotic fusion pore. Proc Natl Acad Sci USA. 1990;87(20):7804–7808. doi: 10.1073/pnas.87.20.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monck JR, Fernandez JM. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8(4):524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 190.Jena BP. Fusion pore or porosome: structure and dynamics. J Endocrinol. 2003;176(2):169–174. doi: 10.1677/joe.0.1760169. [DOI] [PubMed] [Google Scholar]
- 191.Jena BP. Functional organization of the porosome complex and associated structures facilitating cellular secretion. Physiology (Bethesda) 2009;24:367–376. doi: 10.1152/physiol.00021.2009. [DOI] [PubMed] [Google Scholar]
- 192.Jena BP. Porosome: the secretory portal in cells. Biochemistry. 2009;48(19):4009–4018. doi: 10.1021/bi9002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang S, et al. 3D organization and function of the cell: Golgi budding and vesicle biogenesis to docking at the porosome complex. Histochem Cell Biol. 2012;137(6):703–718. doi: 10.1007/s00418-012-0948-x. [DOI] [PubMed] [Google Scholar]
- 194.Muallem S, et al. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128(4):589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Masedunskas A, Porat-Shliom N, Weigert R. Regulated exocytosis: novel insights from intravital microscopy. Traffic. 2012;13(5):627–634. doi: 10.1111/j.1600-0854.2012.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gotow T, Miyaguchi K, Hashimoto PH. Cytoplasmic architecture of the axon terminal: filamentous strands specifically associated with synaptic vesicles. Neuroscience. 1991;40(2):587–598. doi: 10.1016/0306-4522(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 197.Landis DM, et al. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1(3):201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- 198.Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6(2):127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- 199.Ceccaldi PE, et al. Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J Cell Biol. 1995;128(5):905–912. doi: 10.1083/jcb.128.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cesca F, et al. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91(4):313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 201.Giovedi S, et al. Synapsin is a novel Rab3 effector protein on small synaptic vesicles. II. Functional effects of the Rab3A-synapsin I interaction. J Biol Chem. 2004;279(42):43769–43779. doi: 10.1074/jbc.M404168200. [DOI] [PubMed] [Google Scholar]
- 202.Abu-Hamdah R, et al. Secretory vesicles in live cells are not free-floating but tethered to filamentous structures: a study using photonic force microscopy. Ultramicroscopy. 2006;106(8–9):670–673. doi: 10.1016/j.ultramic.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 203.Mizuno K, Ramalho JS, Izumi T. Exophilin8 transiently clusters insulin granules at the actin-rich cell cortex prior to exocytosis. Mol Biol Cell. 2011;22(10):1716–1726. doi: 10.1091/mbc.E10-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bond LM, et al. Myosin motor proteins are involved in the final stages of the secretory pathways. Biochem Soc Trans. 2011;39(5):1115–1119. doi: 10.1042/BST0391115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sellers JR, Knight PJ. Folding and regulation in myosins II and V. J Muscle Res Cell Motil. 2007;28(7–8):363–370. doi: 10.1007/s10974-008-9134-0. [DOI] [PubMed] [Google Scholar]
- 206.Wang F, et al. Regulated conformation of myosin V. J Biol Chem. 2004;279(4):2333–2336. doi: 10.1074/jbc.C300488200. [DOI] [PubMed] [Google Scholar]
- 207.Bittins CM, Eichler TW, Gerdes HH. Expression of the dominant-negative tail of myosin Va enhances exocytosis of large dense core vesicles in neurons. Cell Mol Neurobiol. 2009;29(4):597–608. doi: 10.1007/s10571-009-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miyamoto S. Changes in mobility of synaptic vesicles with assembly and disassembly of actin network. Biochim Biophys Acta. 1995;1244(1):85–91. doi: 10.1016/0304-4165(94)00199-8. [DOI] [PubMed] [Google Scholar]
- 209.Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51(3):317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 210.Giner D, et al. Real-time dynamics of the F-actin cytoskeleton during secretion from chromaffin cells. J Cell Sci. 2005;118(Pt 13):2871–2880. doi: 10.1242/jcs.02419. [DOI] [PubMed] [Google Scholar]
- 211.Nakata T, Hirokawa N. Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. J Neurosci. 1992;12(6):2186–2197. doi: 10.1523/JNEUROSCI.12-06-02186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Orci L, Gabbay KH, Malaisse WJ. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- 213.Giner D, et al. Glycogen synthase kinase 3 activation is essential for the snake phospholipase A2 neurotoxin-induced secretion in chromaffin cells. Eur J Neurosci. 2007;25(8):2341–2348. doi: 10.1111/j.1460-9568.2007.05497.x. [DOI] [PubMed] [Google Scholar]
- 214.Brown AC, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 2011;9(9):e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Trifaro JM, Gasman S, Gutierrez LM. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol (Oxf) 2008;192(2):165–172. doi: 10.1111/j.1748-1716.2007.01808.x. [DOI] [PubMed] [Google Scholar]
- 216.Vitale ML, et al. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol. 1991;113(5):1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dumitrescu Pene T, et al. Expression of various scinderin domains in chromaffin cells indicates that this protein acts as a molecular switch in the control of actin filament dynamics and exocytosis. J Neurochem. 2005;92(4):780–789. doi: 10.1111/j.1471-4159.2004.02907.x. [DOI] [PubMed] [Google Scholar]
- 218.Rodriguez Del Castillo A, et al. Human platelets contain scinderin, a Ca(2+)-dependent actin filament-severing protein. Thromb Haemost. 1992;67(2):248–251. [PubMed] [Google Scholar]
- 219.Ehre C, et al. Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol. 2005;288(1):C46–C56. doi: 10.1152/ajpcell.00397.2004. [DOI] [PubMed] [Google Scholar]
- 220.Bruun TZ, Hoy M, Gromada J. Scinderin-derived actin-binding peptides inhibit Ca(2+)- and GTPgammaS-dependent exocytosis in mouse pancreatic beta-cells. Eur J Pharmacol. 2000;403(3):221–224. doi: 10.1016/s0014-2999(00)00602-6. [DOI] [PubMed] [Google Scholar]
- 221.Tchakarov L, et al. Expression of scinderin, an actin filament-severing protein, in different tissues. FEBS Lett. 1990;268(1):209–212. doi: 10.1016/0014-5793(90)81010-l. [DOI] [PubMed] [Google Scholar]
- 222.Kalwat MA, et al. Gelsolin associates with the N terminus of syntaxin 4 to regulate insulin granule exocytosis. Mol Endocrinol. 2012;26(1):128–141. doi: 10.1210/me.2011-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cabello-Agueros JF, Hernandez-Gonzalez EO, Mujica A. The role of F-actin cytoskeleton-associated gelsolin in the guinea pig capacitation and acrosome reaction. Cell Motil Cytoskeleton. 2003;56(2):94–108. doi: 10.1002/cm.10135. [DOI] [PubMed] [Google Scholar]
- 224.Tomas A, et al. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci. 2006;119(Pt 10):2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 225.Gorlich A, et al. N-cofilin can compensate for the loss of ADF in excitatory synapses. PLoS ONE. 2011;6(10):e26789. doi: 10.1371/journal.pone.0026789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pandey D, et al. Unraveling a novel Rac1-mediated signaling pathway that regulates cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Blood. 2009;114(2):415–424. doi: 10.1182/blood-2008-10-183582. [DOI] [PubMed] [Google Scholar]
- 227.Satoh K, et al. Phosphorylation of myristoylated alanine-rich C kinase substrate is involved in the cAMP-dependent amylase release in parotid acinar cells. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1382–G1390. doi: 10.1152/ajpgi.90536.2008. [DOI] [PubMed] [Google Scholar]
- 228.Coffey ET, et al. Glutamate exocytosis and MARCKS phosphorylation are enhanced by a metabotropic glutamate receptor coupled to a protein kinase C synergistically activated by diacylglycerol and arachidonic acid. J Neurochem. 1994;63(4):1303–1310. doi: 10.1046/j.1471-4159.1994.63041303.x. [DOI] [PubMed] [Google Scholar]
- 229.Coffey ET, et al. Phosphorylation of synapsin I and MARCKS in nerve terminals is mediated by Ca2+ entry via an Aga-GI sensitive Ca2+ channel which is coupled to glutamate exocytosis. FEBS Lett. 1994;353(3):264–268. doi: 10.1016/0014-5793(94)01061-7. [DOI] [PubMed] [Google Scholar]
- 230.Eliyahu E, et al. Association between myristoylated alanin-rich C kinase substrate (MARCKS) translocation and cortical granule exocytosis in rat eggs. Reproduction. 2006;131(2):221–231. doi: 10.1530/rep.1.00794. [DOI] [PubMed] [Google Scholar]
- 231.Elzagallaai A, Rose SD, Trifaro JM. Platelet secretion induced by phorbol esters stimulation is mediated through phosphorylation of MARCKS: a MARCKS-derived peptide blocks MARCKS phosphorylation and serotonin release without affecting pleckstrin phosphorylation. Blood. 2000;95(3):894–902. [PubMed] [Google Scholar]
- 232.Gadi D, et al. Sequestration of phosphoinositides by mutated MARCKS effector domain inhibits stimulated Ca(2+) mobilization and degranulation in mast cells. Mol Biol Cell. 2011;22(24):4908–4917. doi: 10.1091/mbc.E11-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li Y, et al. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276(44):40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 234.Liu JP, et al. Arginine vasopressin (AVP) causes the reversible phosphorylation of the myristoylated alanine-rich C kinase substrate (MARCKS) protein in the ovine anterior pituitary: evidence that MARCKS phosphorylation is associated with adrenocorticotropin (ACTH) secretion. Mol Cell Endocrinol. 1994;101(1–2):247–256. doi: 10.1016/0303-7207(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 235.Park J, Fang S, Adler KB. Regulation of airway mucin secretion by MARCKS protein involves the chaperones heat shock protein 70 and cysteine string protein. Proc Am Thorac Soc. 2006;3(6):493. doi: 10.1513/pats.200603-067MS. [DOI] [PubMed] [Google Scholar]
- 236.Shi QX, et al. Progesterone primes zona pellucida-induced activation of phospholipase A2 during acrosomal exocytosis in guinea pig spermatozoa. J Cell Physiol. 2005;205(3):344–354. doi: 10.1002/jcp.20426. [DOI] [PubMed] [Google Scholar]
- 237.Moes M, Boonstra J, Regan-Klapisz E. Novel role of cPLA(2)alpha in membrane and actin dynamics. Cell Mol Life Sci. 2010;67(9):1547–1557. doi: 10.1007/s00018-010-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Harada A, et al. Nadrin, a novel neuron-specific GTPase-activating protein involved in regulated exocytosis. J Biol Chem. 2000;275(47):36885–36891. doi: 10.1074/jbc.M004069200. [DOI] [PubMed] [Google Scholar]
- 239.Johnson JL, et al. Vesicular trafficking through cortical actin during exocytosis is regulated by the Rab27a effector JFC1/Slp1 and the RhoA-GTPase-activating protein Gem-interacting protein. Mol Biol Cell. 2012;23(10):1902–1916. doi: 10.1091/mbc.E11-12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rudolf R, et al. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J Cell Sci. 2003;116(Pt 7):1339–1348. doi: 10.1242/jcs.00317. [DOI] [PubMed] [Google Scholar]
- 241.Wu XS, et al. Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4(4):271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 242.Rose SD, et al. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J Neurochem. 2003;85(2):287–298. doi: 10.1046/j.1471-4159.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- 243.Yi Z, et al. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22(6):1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Watanabe M, et al. Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol Biol Cell. 2005;16(10):4519–4530. doi: 10.1091/mbc.E05-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sato O, Li XD, Ikebe M. Myosin Va becomes a low duty ratio motor in the inhibited form. J Biol Chem. 2007;282(18):13228–13239. doi: 10.1074/jbc.M610766200. [DOI] [PubMed] [Google Scholar]
- 246.Desnos C, et al. Myosin va mediates docking of secretory granules at the plasma membrane. J Neurosci. 2007;27(39):10636–10645. doi: 10.1523/JNEUROSCI.1228-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rojo Pulido I, et al. Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von-Willebrand factor release from endothelial cells. Traffic. 2011;12(10):1371–1382. doi: 10.1111/j.1600-0854.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 248.Jerdeva GV, et al. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118(Pt 20):4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Neco P, et al. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279(26):27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 250.Mizuno H, et al. Tropomyosin as a regulator of the sliding movement of actin filaments. Biosystems. 2007;90(2):449–455. doi: 10.1016/j.biosystems.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 251.Lin KW, et al. MARCKS and related chaperones bind to unconventional myosin V isoforms in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43(2):131–136. doi: 10.1165/rcmb.2010-0016RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bader MF, et al. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742(1–3):37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 253.Gasman S, et al. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15(2):520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278(43):41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 255.Bader MF, et al. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochimica Et Biophysica Acta-Molecular Cell Research. 2004;1742(1–3):37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 256.Begle A, et al. ARF6 regulates the synthesis of fusogenic lipids for calcium-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2009;284(8):4836–4845. doi: 10.1074/jbc.M806894200. [DOI] [PubMed] [Google Scholar]
- 257.Vitale N, et al. The small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J Biol Chem. 2005;280(33):29921–29928. doi: 10.1074/jbc.M413748200. [DOI] [PubMed] [Google Scholar]
- 258.Lopez JA, et al. The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta cells. J Biol Chem. 2008;283(26):17939–17945. doi: 10.1074/jbc.M800321200. [DOI] [PubMed] [Google Scholar]
- 259.Rondaij MG, et al. Guanine exchange factor RalGDS mediates exocytosis of Weibel–Palade bodies from endothelial cells. Blood. 2008;112(1):56–63. doi: 10.1182/blood-2007-07-099309. [DOI] [PubMed] [Google Scholar]
- 260.van Hooren KWEM, et al. The Epac-Rap1 signaling pathway controls cAMP-mediated exocytosis of Weibel–Palade bodies in endothelial cells. J Biol Chem. 2012;287(29):24713–24720. doi: 10.1074/jbc.M111.321976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Leech CA, Chepurny OG, Holz GG. Epac2-Dependent Rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm. 2010;84:279–302. doi: 10.1016/B978-0-12-381517-0.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wen PJ, et al. Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nat Commun. 2011;2:491. doi: 10.1038/ncomms1500. [DOI] [PubMed] [Google Scholar]
- 263.Momboisse F, et al. Calcium-regulated exocytosis in neuroendocrine cells: intersectin-1L stimulates actin polymerization and exocytosis by activating Cdc42. Ann N Y Acad Sci. 2009;1152:209–214. doi: 10.1111/j.1749-6632.2008.03998.x. [DOI] [PubMed] [Google Scholar]
- 264.Jewell JL, et al. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J Biol Chem. 2008;283(16):10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lopez JA, et al. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20(17):3918–3929. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6(6):449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 267.Creutz CE. The Annexins and Exocytosis. Science. 1992;258(5084):924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- 268.Jahn R, Scheller RH. Snares-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 269.Lang T. SNARE proteins and ‘membrane rafts’. J Physiol-London. 2007;585(3):693–698. doi: 10.1113/jphysiol.2007.134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Reverter M, et al. Cholesterol transport from late endosomes to the Golgi regulates t-SNARE trafficking, assembly, and function. Mol Biol Cell. 2011;22(21):4108–4123. doi: 10.1091/mbc.E11-04-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rescher U, et al. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci. 2004;117(16):3473–3480. doi: 10.1242/jcs.01208. [DOI] [PubMed] [Google Scholar]
- 272.Ohyama A, Komiya Y, Igarashi M. Globular tail of myosin-V is bound to vamp/synaptobrevin. Biochem Biophys Res Commun. 2001;280(4):988–991. doi: 10.1006/bbrc.2001.4236. [DOI] [PubMed] [Google Scholar]
- 273.Jin Y, et al. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21(6):1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Marchelletta RR, et al. The class V myosin motor, myosin 5c, localizes to mature secretory vesicles and facilitates exocytosis in lacrimal acini. Am J Physiol Cell Physiol. 2008;295(1):C13–C28. doi: 10.1152/ajpcell.00330.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jacobs DT, et al. Myosin Vc is a molecular motor that functions in secretory granule trafficking. Mol Biol Cell. 2009;20(21):4471–4488. doi: 10.1091/mbc.E08-08-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Raiford KL, et al. Mucin granule-associated proteins in human bronchial epithelial cells: the airway goblet cell “granulome”. Respir Res. 2011;12:118. doi: 10.1186/1465-9921-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Takagi Y, et al. Human myosin Vc is a low duty ratio, nonprocessive molecular motor. J Biol Chem. 2008;283(13):8527–8537. doi: 10.1074/jbc.M709150200. [DOI] [PubMed] [Google Scholar]
- 278.Watanabe S, et al. Human myosin Vc is a low duty ratio nonprocessive motor. J Biol Chem. 2008;283(16):10581–10592. doi: 10.1074/jbc.M707657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Segawa A, Yamashina S. Roles of microfilaments in exocytosis: a new hypothesis. Cell Struct Funct. 1989;14(5):531–544. doi: 10.1247/csf.14.531. [DOI] [PubMed] [Google Scholar]
- 280.Miklavc P, et al. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion: a role for Rho, formins and myosin II. J Cell Sci. 2012;125(Pt 11):2765–2774. doi: 10.1242/jcs.105262. [DOI] [PubMed] [Google Scholar]
- 281.Nightingale TD, et al. Actomyosin II contractility expels von Willebrand factor from Weibel–Palade bodies during exocytosis. J Cell Biol. 2011;194(4):613–629. doi: 10.1083/jcb.201011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sokac AM, et al. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5(8):727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 283.Turvey MR, Thorn P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflugers Arch. 2004;448(5):552–555. doi: 10.1007/s00424-004-1288-z. [DOI] [PubMed] [Google Scholar]
- 284.Larina O, et al. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell. 2007;18(9):3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bhat P, Thorn P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell. 2009;20(6):1795–1803. doi: 10.1091/mbc.E08-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Aoki R, et al. Duration of fusion pore opening and the amount of hormone released are regulated by myosin II during kiss-and-run exocytosis. Biochem J. 2010;429(3):497–504. doi: 10.1042/BJ20091839. [DOI] [PubMed] [Google Scholar]
- 287.Berberian K, et al. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J Neurosci. 2009;29(3):863–870. doi: 10.1523/JNEUROSCI.2818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Doreian BW, Fulop TG, Smith CB. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J Neurosci. 2008;28(17):4470–4478. doi: 10.1523/JNEUROSCI.0008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Neco P, et al. Myosin II contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008;283(16):10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- 290.Kumakura K, et al. Essential role of myosin light chain kinase in the mechanism for MgATP-dependent priming of exocytosis in adrenal chromaffin cells. J Neurosci. 1994;14(12):7695–7703. doi: 10.1523/JNEUROSCI.14-12-07695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Doreian BW, et al. Cortical F-actin, the exocytic mode, and neuropeptide release in mouse chromaffin cells is regulated by myristoylated alanine-rich C-kinase substrate and myosin II. Mol Biol Cell. 2009;20(13):3142–3154. doi: 10.1091/mbc.E09-03-0197. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 292.Miklavc P, et al. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: a prerequisite for content release or kiss-and-run. Ann N Y Acad Sci. 2009;1152:43–52. doi: 10.1111/j.1749-6632.2008.03989.x. [DOI] [PubMed] [Google Scholar]
- 293.Becker KA, Hart NH. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J Cell Sci. 1999;112(Pt 1):97–110. doi: 10.1242/jcs.112.1.97. [DOI] [PubMed] [Google Scholar]
- 294.Yu HY, Bement WM. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol Biol Cell. 2007;18(10):4096–4105. doi: 10.1091/mbc.E06-11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yu HY, Bement WM. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat Cell Biol. 2007;9(2):149–159. doi: 10.1038/ncb1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Masedunskas A, Porat-Shliom N, Weigert R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Commun Integr Biol. 2012;5(1):84–87. doi: 10.4161/cib.18258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Valentijn JA, et al. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci USA. 2000;97(3):1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Masedunskas A, Sramkova M, Weigert R. Homeostasis of the apical plasma membrane during regulated exocytosis in the salivary glands of live rodents. Bioarchitecture. 2011;1(5):225–229. doi: 10.4161/bioa.18405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nemoto T, et al. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279(36):37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 300.Behrendorff N, et al. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem. 2011;286(34):29627–29634. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280(2):1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- 302.Chen Y, et al. Spatiotemporal analysis of exocytosis in mouse parotid acinar cells. Am J Physiol Cell Physiol. 2005;289(5):C1209–C1219. doi: 10.1152/ajpcell.00159.2005. [DOI] [PubMed] [Google Scholar]
- 303.Kanno T. Compound exocytosis of secretory granules containing salivary chromogranin A in granular duct cells in rat submandibular gland: the last study in collaboration with the late Professor Noboru Yanaihara at Yanaihara Institute. Regul Pept. 2004;123(1–3):3–7. doi: 10.1016/j.regpep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 304.Segawa A, Riva A. Dynamics of salivary secretion studied by confocal laser and scanning electron microscopy. Eur J Morphol. 1996;34(3):215–219. doi: 10.1076/ejom.34.3.215.13023. [DOI] [PubMed] [Google Scholar]
- 305.Segawa A, et al. Exocytosis in living salivary glands: direct visualization by video-enhanced microscopy and confocal laser microscopy. Eur J Cell Biol. 1991;54(2):322–330. [PubMed] [Google Scholar]
- 306.Cardenas AM, Marengo FD. Rapid endocytosis and vesicle recycling in neuroendocrine cells. Cell Mol Neurobiol. 2010;30(8):1365–1370. doi: 10.1007/s10571-010-9579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 2010;29(12):1961–1975. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sramkova M, et al. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. Am J Physiol Cell Physiol. 2009;297(6):C1347–C1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sramkova M, Masedunskas A, Weigert R (2012) Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals. Histochem Cell Biol [DOI] [PMC free article] [PubMed]
- 310.Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137(7):1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gasman S, et al. Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J Biol Chem. 1998;273(27):16913–16920. doi: 10.1074/jbc.273.27.16913. [DOI] [PubMed] [Google Scholar]
- 312.Bi Y, Williams JA. A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am J Physiol Cell Physiol. 2005;289(1):C22–C32. doi: 10.1152/ajpcell.00395.2004. [DOI] [PubMed] [Google Scholar]
- 313.Sabbatini ME, et al. CCK activates RhoA and Rac1 differentially through Galpha13 and Galphaq in mouse pancreatic acini. Am J Physiol Cell Physiol. 2010;298(3):C592–C601. doi: 10.1152/ajpcell.00448.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Williams JA, Chen X, Sabbatini ME. Small G proteins as key regulators of pancreatic digestive enzyme secretion. Am J Physiol Endocrinol Metab. 2009;296(3):E405–E414. doi: 10.1152/ajpendo.90874.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.D’Silva NJ, et al. Immunolocalization of rap1 in the rat parotid gland: detection on secretory granule membranes. J Histochem Cytochem. 1997;45(7):965–973. doi: 10.1177/002215549704500706. [DOI] [PubMed] [Google Scholar]
- 316.D’Silva NJ, et al. Beta-adrenergic-induced cytosolic redistribution of Rap1 in rat parotid acini: role in secretion. Am J Physiol. 1998;274(6 Pt 1):C1667–C1673. doi: 10.1152/ajpcell.1998.274.6.C1667. [DOI] [PubMed] [Google Scholar]
- 317.Shimomura H, Imai A, Nashida T. Evidence for the involvement of cAMP-GEF (Epac) pathway in amylase release from the rat parotid gland. Arch Biochem Biophys. 2004;431(1):124–128. doi: 10.1016/j.abb.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 318.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167(1):111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]