Abstract
The emergence and global spread of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii are of great concern to health services worldwide. These β-lactamases hydrolyse almost all β-lactams, are plasmid-encoded, and are easily transferable among bacterial species. They are mostly of the KPC, VIM, IMP, NDM, and OXA-48 types. Their current extensive spread worldwide in Enterobacteriaceae is an important source of concern. Infections caused by these bacteria have limited treatment options and have been associated with high mortality rates. Carbapenemase producers are mainly identified among Klebsiella pneumoniae, Escherichia coli, and A. baumannii and still mostly in hospital settings and rarely in the community. The Mediterranean region is of interest due to a great diversity and population mixing. The prevalence of carbapenemases is particularly high, with this area constituting one of the most important reservoirs. The types of carbapenemase vary among countries, partially depending on the population exchange relationship between the regions and the possible reservoirs of each carbapenemase. This review described the epidemiology of carbapenemases produced by enterobacteria and A. baumannii in this part of the world highlighting the worrisome situation and the need to screen and detect these enzymes to prevent and control their dissemination.
1. Introduction
Carbapenems are β-lactam group of drugs that are often used as antibiotics of last resort for treating infection due to multidrug-resistant Gram-negative bacilli. They are also stable even in response to extended-spectrum (ESBL) and AmpC β-lactamases. However, this scenario has changed with the emergence in the last few years of carbapenem resistant bacteria both in nonfermenters (Acinetobacter baumannii and Pseudomonas aeruginosa) and in fermenters (Enterobacteriaceae) Gram-negative bacilli [1].
Resistance to carbapenems is mediated mostly by two main mechanisms: (i) production of a β-lactamase (derepressed cephalosporinase or ESBL) with nonsignificant carbapenemase activity combined with decreased permeability due to porin loss or alteration; (ii) production of a carbapenem-hydrolyzing β-lactamase [2].
Carbapenemases have now become a major concern worldwide [3, 4]. They are an increasing concern for global healthcare due to their association with resistance to β-lactam antibiotics and to other classes of antibiotics such as aminoglycosides, fluoroquinolones, and cotrimoxazole [5]. Thus they reduce the possibility of treating infections due to multidrug-resistant strains [6]. The first description of carbapenemase-producing enterobacteria (NmcA) was in 1993 [7]. Since then, large varieties of carbapenemases have been identified belonging to three molecular classes: the Ambler class A, B, and D β-lactamases [8]. They have emerged and diffused in different parts of the world, including Mediterranean countries, in recent years [2–6, 9]. These enzymes are carried either on chromosome or acquired via plasmids [10].
The aim of this review is to describe the epidemiology of the main carbapenemases circulating in the Mediterranean countries, a region of the world with a great diversity and population mixing. This region includes 11 European countries (Albania, Bosnia, and Herzegovina, Croatia, Spain, France, Greece, Italia, Malta, Montenegro, Monaco and Slovenia), 5 Asian countries (Cyprus, Israel, Lebanon, Syria, Turkey) and 5 African countries (Algeria, Egypt, Libya, Morocco, Tunisia).
2. Class A Carbapenemases
2.1. Enterobacteriaceae
A variety of class A carbapenemases have been described: some are chromosome encoded (NmcA, Sme, IMI-1, SFC-1) and others are plasmid encoded (KPC, IMI-2, GES derivatives such as GES-1, GES-2, GES-4, and GES-5) but all effectively hydrolyze carbapenems and are partially inhibited by clavulanic acid [8].
KPCs (acronym for K. pneumoniae carbapenemase) are the most frequently encountered enzymes in this group [2]. Since the first report of this enzyme in 1996 isolated from a clinical Klebsiella pneumonia strain in North Carolina, USA [11], the KPC producers had spread around the world and are becoming a major clinical and public health concern [12].
Several KPC clones are disseminating harboring different multilocus sequence type, β-lactamase content, and plasmids. However the bla KPC genes are flanked by the same transposon Tn4401 located on conjugative plasmids and are horizontally transferred [13]. This gives to this enzyme an extraordinary spreading capacity [14]. They have been detected more often in Klebsiella spp. [2] but have also been reported in other Enterobacteriaceae [15]. Thirteen variants of KPC are known so far; KPC-2 and KPC-3 are the most frequent worldwide variants [16]. The mortality rate due to infection with a KPC producer ranged from 25% to 69% [2, 17].
The first outbreak of KPC-producing K. pneumoniae outside the United States was described in Israel in 2006 [18]. This strain belonged to the pandemic clone ST258, suggesting an importation from the USA [19]. Moreover, a large range of enterobacteria producing these variants was described in Israel [20–26]. Since then, many studies have reported outbreaks of KPC producers in enterobacterial isolates in many Mediterranean countries (Figure 1), in which most cases have been reported so far in Greece, where the situation can be described as endemic [27, 28]. Moreover a recent study showed a wide dissemination of KPC-producing strains to many healthcare institutions in Italy [2, 29]. KPC producers became the most prevalent carbapenemase found in this country [30]. Spain and France have recently described a rapid increase of cases [31, 32]. Single or sporadic hospital outbreaks caused by KPCs isolated from various species were reported [32–34]. KPC-2 is clearly the most prevalent variant in Europe [12, 35]. In most of the cases reported from France, the patients had been transferred from a country where KPC enzymes are endemic (e.g., Israel, Greece, USA, or Italy) [34]. Croatia is another Mediterranean country affected [36].
Figure 1.

Geographic distribution of KPC enzymes in Mediterranean countries. White, no case reported; yellow, single KPC-producing isolates; green, some outbreaks of KPC-producing isolates; orange, several outbreaks of KPC-producing isolates; red, endemicity of KPC-producing isolates.
To date, there is no description of class A carbapenemases from North African countries. However, KPC producers have already been isolated in an E. coli strain in Algeria (N. Djahmi et al., unpublished data).
2.2. Acinetobacter baumannii
Among the class A carbapenemases, KPCs and GES-type have been described in A. baumannii [37]. KPC-2, KPC-3, KPC-4, and KPC-10 variants were identified in 10 A. baumannii clinical isolates collected in 2009 from 17 hospitals in Puerto Rico [38].
In Mediterranean countries, only GES-type carbapenemase was reported. A GES-14-producing A. baumannii clinical strain was isolated in France. This strain was demonstrated to confer resistance to all β-lactams, including carbapenems [39]. Very recently, an emergence of GES-11 was reported from Turkey [40]. Some strains coexpressed both OXA-23 and GES-11. They belonged to ST2, being part of the worldwide distributed clone II group.
3. Class B Carbapenemases
3.1. Enterobacteriaceae
Class B metallo-β-lactamases (MBLs) are mostly of the Verona integron-encoded metallo-β-lactamase (VIM) and IMP types and, more recently, of the New Delhi metallo-β-lactamases-1 (NDM-1) type [8, 41]. MBLs can hydrolyze all β-lactams except monobactam (e.g., aztreonam) [41]. Their activity is inhibited by EDTA but not by clavulanic acid [41].
IMP-1 was the first MBL reported in Serratia marcescens from Japan in 1991 [42]. Since then, MBLs have been observed worldwide [8, 41]. The most commonly found class B carbapenemases are of the VIM type [43], which has been identified in all continents [44]. The death rates associated with MBL producers are high (18% to 67%) [2, 45].
Italy was the first Mediterranean country to report acquired metallo-β-lactamases, with sporadic isolates of VIM-4-producing K. pneumoniae and Enterobacter cloacae [8, 46]. Since then, single or sporadic hospital outbreaks caused by VIM-1 like enzymes were described from various regions in this country [47, 48]. However, such VIM-producing Enterobacteriaceae have not undergone wide dissemination, unlike that observed in Greece during the same period [49]. Endemicity of VIM- and IMP-producing Klebsiella pneumoniae strains has now been noted in Greece [8, 41]. Additionally, outbreaks and single reports of VIM- or IMP-type producers have been reported in several countries of Mediterranean area, such as France [50, 51], Spain [33], Morocco [52], Egypt [53, 54], Algeria [55], and Tunisia [56].
Most recently reported, NDM-1 enzyme is spreading rapidly worldwide [44] notably Central and South America that represented the last zone without description of this enzyme [57, 58]. NDM-1 was initially identified in E. coli and K. pneumoniae in a patient returning to Sweden from India in 2008 [59]. Most of the outbreaks indicated a link with the Indian subcontinent, in some cases with the Balkan countries [60], and the Middle East [61]. Five minor variants of NDM-1 (NDM-2 to NDM-6) have been now identified in enterobacteria and very recently, a novel variant NDM-7 was detected in E. coli in France [62]. Contrarily to other carbapenemase genes, bla NDM-1 is not associated with a single clone. Thus NDM-1 has been identified mostly in nonclonally related E. coli and K. pneumoniae and to a lesser extent in other enterobacterial species [63]. These enzymes are encoded on highly transmissible plasmids that spread rapidly between bacteria, rather than relying on clonal proliferation. The strains harboring NDM are broadly resistant to many other drug classes in addition to β-lactams and carry a diversity of other resistance mechanisms, which leaves few treatment options (tigecycline or colistin) [63, 64]. NDM-1 producers have been reported in the environment and in the community [2, 63]. They have been identified in Enterobacteriaceae species around the world [59] highlighting the ability of this gene to disseminate in bacteria [65]. Moreover NDM-1 has been identified in E. coli ST131, a well-known source of community infections [66, 67].
Single or sporadic hospital outbreaks caused by NDM-1 producing enterobacterial strains were reported from many countries in Mediterranean area (Figure 2): France [68, 69], Italy [70], Lebanon [71], Morocco [52, 72], Spain [33, 73–75], Tunisia [76], and Turkey [77, 78]. Very recently, NDM-5 was identified in E. coli in Algeria (Sassi et al., unpublished data). There are no published data yet from Libya, but a very recent study has reported identification of NDM-1 in K. pneumoniae from patient transferred from Libya to Tunisia [76], indicating the emergence of this enzyme resistance in Mediterranean countries. Finally an emergence of NDM-producing K. pneumoniae was recently reported in Greece [79].
Figure 2.
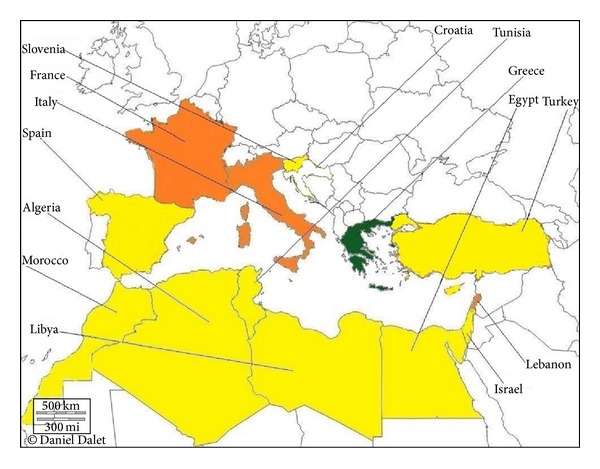
Geographic distribution of NDM type producers in Mediterranean countries. White, no case reported; yellow, sporadic NDM-producing isolates; green, emerging outbreak of NDM-producing isolates; orange, single hospital outbreaks of NDM-producing isolates.
3.2. Acinetobacter baumannii
To date, four groups of MBLs have been identified in A. baumannii: IMP-like, VIM-like, SIM-like, and recently the NDMs [80].
The first MBL identified in A. baumannii strains was IPM-2 reported in 2000 from Italy [81]. Since then, IMP-like, VIM-like, and SIM-like have been sporadically reported in some parts of the world [82], including Mediterranean countries, especially in Greece and Italy [81–85]. Concerning NDM producers, A. baumannii bacteria harboring these enzymes were increasingly observed around the world [86] notably in Mediterranean countries. They were detected in North Africa: Algeria [87, 88] and Libya (isolated from a patient transferred from Libya to Denmark) [89]; in Europa: France [87, 90, 91] and Slovenia [86]; and in Turkey [92]. The isolation of an NDM-1-producing A. baumannii in a Czech patient repatriated in 2011 from Egypt was described [93]. In France, the emergence of imported cases of NDM-1-producing A. baumannii was linked with Algeria [87, 90]. The strains belonged to ST85, the main clone isolated in Mediterranean countries [90, 91]. Finally, another clone NDM variant, NDM-2, was found in A. baumannii isolates in Egypt [94] and Israel [95].
4. Class D Carbapenemases
4.1. Enterobacteriaceae
Class D β-lactamases, also named OXAs for oxacillinases include 232 enzymes with few variants, possessing the same carbapenemase activity [96]. Initially OXA β-lactamases were reported from P. aeruginosa but until now, these carbapenemases have been detected in many other Gram-negative bacteria, including Enterobacteriaceae [16].
OXA-48 represents the main enzyme isolated around the world. This enzyme hydrolyses penicillins but has a weak activity against carbapenems or extended-spectrum cephalosporins (third generation cephalosporin, aztreonam) [2]. However, its frequent association with ESBL (notably CTX-M-15 enzyme) increases the level of resistance to carbapenem. Its activity is not inhibited by EDTA or clavulanic acid [2], tazobactam, and sulbactam, whereas its activity may be inhibited by NaCl in vitro [96, 97]. Its high level of resistance to temocillin is interesting to detect this enzyme [98, 99]. A point mutant analog of OXA-48, namely, OXA-181, with similar carbapenemase activity, has been identified in enterobacterial strains from India [100, 101] and from patients with a link to the Indian subcontinent [100, 102]. Further analysis of the OXA-48-producing isolates demonstrated that this enzyme was not exclusively linked with a single clone, and the bla OXA-48 gene was associated with either transposon Tn1999 or transposon Tn1999.2 within transferable nontypable plasmids of 70 or 150 kb [103]. The death rates associated with OXA-producers are unknown.
OXA-48 was initially identified in K. pneumoniae isolate from Turkey in 2001 [104]. Since then, OXA-48 producing strains have been extensively reported as sources of nosocomial outbreaks in many parts of the world notably in Mediterranean countries [105–110] (Figure 3): Croatia [111], Egypt [54], France [109], Greece [112], Israel [113, 114], Italy [53], Lebanon [71, 115, 116], Libya [117], Slovenia [118], Spain [33, 119], Tunisia [120], and Turkey [106]. Moreover, this enzyme disseminated in various Enterobacteriaceae species [2, 96]. To date OXA-48 represents the most common carbapenemase type circulating in this part of the world notably in Spain [33] and France [109]. The Middle East and North Africa are considered as reservoirs of OXA-48 producers [121]. In the last few years, a nosocomial dissemination of OXA-48-producing Enterobacteriaceae has been reported in different hospitals in Morocco [122]. This problem was exacerbated by the occurrence of this enzyme in community [123] and in environment [124] suggesting that OXA-48 is endemic in this country [122]. More recently, the identification of the bla OXA-48 gene in a K. pneumoniae isolate has been reported in Algeria (N. Djahmi, personal data).
Figure 3.

Geographic distribution of OXA-48 type producers in Mediterranean countries. White, no case reported; yellow, single OXA-48-producing isolates; orange, several outbreaks of OXA-48-producing isolates; red, nationwide distribution of OXA-48-producing isolates.
4.2. Acinetobacter baumannii
The class D carbapenemases (oxacillinases) are by far the most prevalent carbapenemases in A. baumannii [125, 126]. They can be grouped into six subclasses: intrinsic chromosomal OXA-51-like, among which there are over 70 variants and the acquired OXA-23-like, OXA-24/40-like, OXA-58-like, OXA-143-like, and OXA-235-like β-lactamases [97, 127].
The first case of OXA-type enzyme was reported from a clinical A. baumannii isolate detected in Scotland in 1985. It was initially named ARI-1 (Acinetobacter resistant to imipenem) [128] and renamed OXA-23 after sequencing [129]. A. radioresistens was identified as the progenitor of the bla OXA23-like gene [130].
Nosocomial outbreaks or sporadic cases caused by carbapenem-resistant A. baumannii producing these OXA-enzymes have been reported worldwide [80, 131–133]. A. baumannii epidemic strains were assigned to international clonal lineages I or II [134], with recent studies reporting the spread of genetically related epidemic clone of OXA-23-producing A. baumannii and belonging to IC-II within the Mediterranean region [135–137]. The bla OXA-23 gene was either located on the chromosome or on plasmids and was associated with four different genetic structures, with the most frequent being transposons Tn2006 [134].
The emergence and spread of several outbreak or sporadic A. baumannii strains producing OXA-23-like enzymes have been reported around the world [134]. During a long period, the bla OXA-58 carbapenemase gene has been predominated among carbapenem-resistant A. baumannii isolates in various Mediterranean countries [85]. Since 2009, a replacement of bla OXA-58 gene with bla OXA-23 gene has been reported and it became the most prevalent carbapenemase-encoding gene circulating in the Mediterranean region: Algeria [88, 136], Croatia [111], Egypt [138], France [139], Greece [140], Italy [135, 141], Israel [132], Spain [137, 142], Tunisia [143], and Turkey [83, 144]. The replacement of OXA-58 by OXA-23 might be explained by the selective advantage associated with the higher carbapenemase activity of OXA-23 [37, 142] and/or acquisition of carbapenem resistance through horizontal gene transfer [37].
Concerning other OXA-producers, outbreaks of OXA-72-producing A. baumannii were described in Croatia [145] and OXA-69 or OXA-97 in Tunisia [146, 147].
5. Conclusion
In recent years the emergence of carbapenem-resistant Gram-negative bacilli in Mediterranean region is an alarming problem. This part of the world is the cradle of western civilization representing nearly 475 million inhabitants (6.3% of world population). It is the location of a large population mixing explaining the importance of the dissemination of carbapenemase producers. This situation imposes a series of measures as soon as possible. These need the over-the-counter sale of indistinctly antibiotics, improving basic and extended knowledge on hygiene, the reinforcement of infection control measures, and the early and accurate detection, with restriction of the usage of carbapenems, to control the spread of these multidrug resistant organisms.
Conflict of Interests
The authors state that there is no conflict of interests.
References
- 1.Jesudason MV, Kandathil AJ, Balaji V. Comparison of two methods to detect carbapenemase & metallo-β-lactamase production in clinical isolates. Indian Journal of Medical Research. 2005;121(6):780–783. [PubMed] [Google Scholar]
- 2.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase producing Enterobacteriaceae . Emerging Infectious Diseases. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeli Y, Akova M, Cornaglia G, et al. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clinical Microbiology and Infection. 2010;16(2):102–111. doi: 10.1111/j.1469-0691.2009.03115.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams? The Lancet Infectious Diseases. 2011;11(5):381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 5.Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveillance. 2008;13(47) [PubMed] [Google Scholar]
- 6.Giamarellou H, Poulakou G. Multidrug-resistant gram-negative infections: what are the treatment options? Drugs. 2009;69(14):1879–1901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(16):7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clinical Microbiology Reviews. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh TR. Emerging carbapenemases: a global perspective. International Journal of Antimicrobial Agents. 2010;36(3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 10.Nahid F, Khan AA, Rehman S, Zahra R. Prevalence of metallo-β-lactamase NDM-1-producing multi-drug resistantbacteria at two Pakistani hospitals and implications for public health. Journal of Infection and Public Health. 2013;6(6):487–493. doi: 10.1016/j.jiph.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae . Antimicrobial Agents and Chemotherapy. 2001;45(4):1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet Infectious Diseases. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 13.Cuzon G, Naas T, Truong H, et al. Worldwide diversity of Klebsiella pneumoniae that produces β-lactamase blaKPC-2 Gene. Emerging Infectious Diseases. 2010;16(9):1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naas T, Cuzon G, Villegas M-V, Lartigue M-F, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrobial Agents and Chemotherapy. 2008;52(4):1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande LM, Rhomberg PR, Sader HS, Jones RN. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999–2005) Diagnostic Microbiology and Infectious Disease. 2006;56(4):367–372. doi: 10.1016/j.diagmicrobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. International Journal of Medical Microbiology. 2010;300(6):371–379. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Robustillo Rodela A, Díaz-Agero Pérez C, Sanchez Sagrado T, Ruiz-Garbajosa P, Pita López MJ, Monge V. Emergence and outbreak of carbapenemase-producing KPC-3 Klebsiella pneumoniae in Spain, September 2009 to February 2010: control measures. Eurosurveillance. 2012;17(7) [PubMed] [Google Scholar]
- 18.Samra Z, Ofir O, Lishtzinsky Y, Madar-Shapiro L, Bishara J. Outbreak of carbapenem-resistant Klebsiella pneumoniae producing KPC-3 in a tertiary medical centre in Israel. International Journal of Antimicrobial Agents. 2007;30(6):525–529. doi: 10.1016/j.ijantimicag.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrobial Agents and Chemotherapy. 2009;53(8):3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo-Grass C, Warburg G, Temper V, et al. KPC-9, a novel carbapenemase from clinical specimens in Israel. Antimicrob Agents Chemother. 2012;56:6057–6059. doi: 10.1128/AAC.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geffen Y, Adler A, Paikin S, et al. Detection of the plasmid-mediated KPC-2 carbapenem-hydrolysing enzyme in three unusual species of the Enterobacteriaceae family in Israel. Journal of Antimicrobial Chemotherapy. 2013;68:719–720. doi: 10.1093/jac/dks443. [DOI] [PubMed] [Google Scholar]
- 22.Adler A, Paikin S, Sterlin Y, et al. A swordless knight: epidemiology and molecular characteristics of the blaKPC-negative sequence type 258 Klebsiella pneumoniae clone. Journal of Clinical Microbiology. 2012;50:3180–3185. doi: 10.1128/JCM.00987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goren MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. Transfer of Carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerging Infectious Diseases. 2010;16(6):1014–1017. doi: 10.3201/eid1606.091671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC- 3- producing Klebsiella pneumoniae strain. Clinical Microbiology and Infection. 2011;17(1):52–56. doi: 10.1111/j.1469-0691.2010.03209.x. [DOI] [PubMed] [Google Scholar]
- 25.Chmelnitsky I, Hermesh O, Navon-Venezia S, Strahilevitz J, Carmeli Y. Detection of aac(6′)-Ib-cr in KPC-producing Klebsiella pneumoniae isolates from Tel Aviv, Israel. Journal of Antimicrobial Chemotherapy. 2009;64(4):718–722. doi: 10.1093/jac/dkp272. [DOI] [PubMed] [Google Scholar]
- 26.Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrobial Agents and Chemotherapy. 2009;53(8):3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giakkoupi P, Papagiannitsis CC, Miriagou V, et al. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009-10) Journal of Antimicrobial Chemotherapy. 2011;66(7):1510–1513. doi: 10.1093/jac/dkr166. [DOI] [PubMed] [Google Scholar]
- 28.Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to β-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology, and outcomes. Clinical Infectious Diseases. 2010;50(3):364–373. doi: 10.1086/649865. [DOI] [PubMed] [Google Scholar]
- 29.Grundmann H, Livermore DM, Giske CG, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Eurosurveillance. 2010;15(46) doi: 10.2807/ese.15.46.19711-en. [DOI] [PubMed] [Google Scholar]
- 30.Gaibani P, Ambretti S, Berlingeri A, et al. Rapid increase of carbapenemase-producing Klebsiella pneumoniae strains in a large Italian hospital: surveillance period 1 March—30 September 2010. Eurosurveillance. 2011;16(8) [PubMed] [Google Scholar]
- 31.Carbonne A, Thiolet JM, Fournier S, et al. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Eurosurveillance. 2010;15(48) doi: 10.2807/ese.15.48.19734-en. [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Gil MR, Paño-Pardo JR, Romero-Gómez MP, et al. Detection of KPC-2-producing Citrobacter freundii isolates in Spain. Journal of Antimicrobial Chemotherapy. 2010;65(12):2695–2697. doi: 10.1093/jac/dkq352. [DOI] [PubMed] [Google Scholar]
- 33.Oteo J, Saez D, Bautista V, et al. Spanish collaborating group for the antibiotic resistance surveillance program. Carbapenemase-producing enterobacteriaceae in Spain in 2012. Antimicrob Agents Chemother. 2013;57:6344–6347. doi: 10.1128/AAC.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuzon G, Naas T, Demachy M-C, Nordmann P. Nosocomial outbreak of Klebsiella pneumoniae harbouring blaKPC-3 in France subsequent to a patient transfer from Italy. International Journal of Antimicrobial Agents. 2012;39(5):448–449. doi: 10.1016/j.ijantimicag.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Miriagou V, Cornaglia G, Edelstein M, et al. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clinical Microbiology and Infection. 2010;16(2):112–122. doi: 10.1111/j.1469-0691.2009.03116.x. [DOI] [PubMed] [Google Scholar]
- 36.Bedenić B, Mazzariol A, Plečko V, et al. First report of KPC-producing Klebsiella pneumoniae in Croatia. Journal of Chemotherapy. 2012;24(4):237–239. doi: 10.1179/1973947812Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 37.Zarrilli R, Pournaras S, Giannoulia M, Tsakrisc A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. International Journal of Antimicrobial Agents. 2013;41(1):11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Robledo IE, Aquino EE, Santé MI, et al. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrobial Agents and Chemotherapy. 2010;54(3):1354–1357. doi: 10.1128/AAC.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnin RA, Nordmann P, Potron A, Lecuyer H, Zahar J-R, Poirel L. Carbapenem-hydrolyzing GES-type extended-spectrum β-lactamase in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2011;55(1):349–354. doi: 10.1128/AAC.00773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeka AN, Poirel L, Sipahi OR, et al. GES-type and OXA-23 carbapenemase-producing Acinetobacter baumannii in Turkey. Journal of Antimicrobial Chemotherapy. 2013;69(4):1145–1146. doi: 10.1093/jac/dkt465. [DOI] [PubMed] [Google Scholar]
- 41.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clinical Microbiology Reviews. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene bla(IMP) among clinically isolated strains of Serratia marcescens . Antimicrobial Agents and Chemotherapy. 1995;39(4):824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vatopoulos A. High rates of metallo-β-lactamase-producing Klebsiella pneumoniae in Greece—a review of the current evidence. Euro Surveillance. 2008;13(4) [PubMed] [Google Scholar]
- 44.Nordmann P, Poirel L, Toleman MA, Walsh TR. Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? Journal of Antimicrobial Chemotherapy. 2011;66(4):689–692. doi: 10.1093/jac/dkq520. [DOI] [PubMed] [Google Scholar]
- 45.Daikos GL, Petrikkos P, Psichogiou M, et al. Prospective observational study of the impact of VIM-1 metallo-β- lactamase on the outcome of patients with Kebsiella pneumoniae bloodstream infections. Antimicrobial Agents and Chemotherapy. 2009;53(5):1868–1873. doi: 10.1128/AAC.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luzzaro F, Docquier J-D, Colinon C, et al. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-β-lactamase encoded by a conjugative plasmid. Antimicrobial Agents and Chemotherapy. 2004;48(2):648–650. doi: 10.1128/AAC.48.2.648-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aschbacher R, Doumith M, Livermore DM, Larcher C, Woodford N. Linkage of acquired quinolone resistance (qnrS1) and metallo-β-lactamase (blaVIM-1) genes in multiple species of Enterobacteriaceae from Bolzano, Italy. Journal of Antimicrobial Chemotherapy. 2008;61(3):515–523. doi: 10.1093/jac/dkm508. [DOI] [PubMed] [Google Scholar]
- 48.Aschbacher R, Pagani L, Doumith M, et al. Metallo-β-lactamases among Enterobacteriaceae from routine samples in an Italian tertiary-care hospital and long-term care facilities during 2008. Clinical Microbiology and Infection. 2011;17(2):181–189. doi: 10.1111/j.1198-743X.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 49.Cantón R, Akóva M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clinical Microbiology and Infection. 2012;18(5):413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 50.Poirel L, Ros A, Carricajo A, et al. Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrobial Agents and Chemotherapy. 2011;55(1):447–448. doi: 10.1128/AAC.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lartigue M-F, Poirel L, Nordmann P. First detection of a carbapenem-hydrolyzing metalloenzyme in an Enterobacteriaceae isolate in France. Antimicrobial Agents and Chemotherapy. 2004;48(12):4929–4930. doi: 10.1128/AAC.48.12.4929-4930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barguigua A, El Otmani F, El Yaagoubi FL, Talmi M, Zerouali K, Timinouni M. First report of a Klebsiella pneumoniae strain coproducing NDM-1, VIM-1 and OXA-48 carbapenemases isolated in Morocco. APMIS. 2013;121:675–677. doi: 10.1111/apm.12034. [DOI] [PubMed] [Google Scholar]
- 53.Giani T, Marchese A, Coppo E, Kroumova V, Rossolini GM. VIM-1-producing Pseudomonas mosselii isolates in Italy, predating known VIM-producing index strains. Antimicrobial Agents and Chemotherapy. 2012;56(4):2216–2217. doi: 10.1128/AAC.06005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poirel L, Abdelaziz MO, Bernabeu S, Nordmann P. Occurrence of OXA-48 and VIM-1 carbapenemase-producing Enterobacteriaceae in Egypt. International Journal of Antimicrobial Agents. 2013;41:90–91. doi: 10.1016/j.ijantimicag.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Robin F, Aggoune-Khinache N, Delmas J, Naim M, Bonnet R. Novel VIM metallo-β-lactamase variant from clinical isolates of Enterobacteriaceae from Algeria. Antimicrobial Agents and Chemotherapy. 2010;54(1):466–470. doi: 10.1128/AAC.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ktari S, Arlet G, Mnif B, et al. Emergence of multidrug-resistant Klebsiella pneumoniae isolates producing VIM-4 metallo-β-lactamase, CTX-M-15 extended-spectrum β-lactamase, and CMY-4 AmpC β-lactamase in a Tunisian University Hospital. Antimicrobial Agents and Chemotherapy. 2006;50(12):4198–4201. doi: 10.1128/AAC.00663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasteran F, Albornoz E, Faccone D, et al. Emergence of NDM-1-producing Klebsiella pneumoniae in Guatemala. Journal of Antimicrobial Chemotherapy. 2012;67(7):1795–1797. doi: 10.1093/jac/dks101. [DOI] [PubMed] [Google Scholar]
- 58.Pérez JAE, Escobar NMO, Castro-Cardozo B, et al. Outbreak of NDM-1-producing Klebsiella pneumonia in a neonatal unit in Colombia. Antimicrob Agents Chemother. 2013;57(4):1957–1960. doi: 10.1128/AAC.01447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-β-lactamase gene, bla NDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents and Chemotherapy. 2009;53(12):5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? The Lancet Infectious Diseases. 2011;11(3):p. 164. doi: 10.1016/S1473-3099(11)70048-2. [DOI] [PubMed] [Google Scholar]
- 61.Poirel L, Fortineau N, Nordmann P. International transfer of NDM-1-producing Klebsiella pneumoniae from Iraq to France. Antimicrobial Agents and Chemotherapy. 2011;55(4):1821–1822. doi: 10.1128/AAC.01761-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuzon G, Bonnin RA, Nordmann P. First Identification of Novel NDM Carbapenemase, NDM 7, in Escherichia coli in France. PLoS ONE. 2010;8(4) doi: 10.1371/journal.pone.0061322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. The Lancet Infectious Diseases. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muir A, Weinbren MJ. New Delhi metallo-β-lactamase: a cautionary tale. Journal of Hospital Infection. 2010;75(3):239–240. doi: 10.1016/j.jhin.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends in Microbiology. 2011;19(12):588–595. doi: 10.1016/j.tim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Nicolas-Chanoine M-H, Blanco J, Leflon-Guibout V, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. Journal of Antimicrobial Chemotherapy. 2008;61(2):273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 67.Peirano G, Schreckenberger PC, Pitout JDD. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrobial Agents and Chemotherapy. 2011;55(6):2986–2988. doi: 10.1128/AAC.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birgy A, Doit C, Mariani-Kurkdjian P, et al. Early detection of colonization by VIM-1-producing Klebsiella pneumoniae and NDM-1-producing Escherichia coli in two children returning to France. Journal of Clinical Microbiology. 2011;49(8):3085–3087. doi: 10.1128/JCM.00540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denis C, Poirel L, Carricajo A, et al. Nosocomial transmission of NDM-1-producing Escherichia coli within a non-endemic area in France. Clinical Microbiology and Infection. 2012;18(5):E128–E130. doi: 10.1111/j.1469-0691.2012.03761.x. [DOI] [PubMed] [Google Scholar]
- 70.Giani T, Pini B, Arena F, et al. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: results of the first countrywide survey. Eurosurveillance. 2013;18(22) [PubMed] [Google Scholar]
- 71.Baroud M, Dandache I, Araj GF, et al. Underlying mechanisms of carbapenem resistance in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates at a tertiary care centre in Lebanon: role of OXA-48 and NDM-1 carbapenemases. International Journal of Antimicrobial Agents. 2013;41(1):75–79. doi: 10.1016/j.ijantimicag.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 72.Poirel L, Benouda A, Hays C, Nordmann P. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. Journal of Antimicrobial Chemotherapy. 2011;66(12):2781–2783. doi: 10.1093/jac/dkr384. [DOI] [PubMed] [Google Scholar]
- 73.Solé M, Pitart C, Roca I, et al. First description of an Escherichia coli strain producing NDM-1 carbapenemase in Spain. Antimicrobial Agents and Chemotherapy. 2011;55(9):4402–4404. doi: 10.1128/AAC.00642-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gil-Romero Y, Sanz-Rodríguez N, Almagro-Moltó M, Gómez-Garcés JL. New description of a NDM-1 carbapenemase producing Klebsiella pneumoniae carrier in Spain. Enfermedades Infecciosas y Microbiología Clínica. 2013;31(6):418–419. doi: 10.1016/j.eimc.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Oteo J, Domingo-García D, Fernández-Romero S, et al. Abdominal abscess due to NDM-1-producing Klebsiella pneumoniae in Spain. Journal of Medical Microbiology. 2012;61:864–867. doi: 10.1099/jmm.0.043190-0. [DOI] [PubMed] [Google Scholar]
- 76.Nasr AB, Decré D, Compain F, Genel N, Barguellil F, Arlet G. Emergence of NDM-1 in association with OXA-48 in Klebsiella pneumoniae from Tunisia. Antimicrob Agents Chemother. 2013;57(8):4089–4090. doi: 10.1128/AAC.00536-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poirel L, Özdamar M, Ocampo-Sosa AA, Türkoglu S, Ozer UG, Nordmann P. NDM-1-producing Klebsiella pneumoniae now in Turkey. Antimicrobial Agents and Chemotherapy. 2012;56(5):2784–2785. doi: 10.1128/AAC.00150-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alp E, Perçin D, Colakoğlu S, et al. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary university hospital in Turkey. Journal of Hospital Infection. 2013;84:178–180. doi: 10.1016/j.jhin.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Giakkoupi P, Tryfinopoulou K, Kontopidou F, et al. Emergence of NDM-producing Klebsiella pneumonia in Greece. Diagnostic Microbiology and Infectious Disease. 2013;77:382–384. doi: 10.1016/j.diagmicrobio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Kempf M, Rolain J-M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. International Journal of Antimicrobial Agents. 2012;39(2):105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Riccio ML, Franceschini N, Boschi L, et al. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of bla(IMP) allelic variants carried by gene cassettes of different phylogeny. Antimicrobial Agents and Chemotherapy. 2000;44(5):1229–1235. doi: 10.1128/aac.44.5.1229-1235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Figueiredo S, Poirel L, Papa A, Koulourida V, Nordmann P. First identification of VIM-4 metallo-β-lactamase in Acinetobacter spp. Clinical Microbiology and Infection. 2008;14(3):289–290. doi: 10.1111/j.1469-0691.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- 83.Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clinical Microbiology and Infection. 2011;17(2):197–201. doi: 10.1111/j.1469-0691.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 84.Ikonomidis A, Ntokou E, Maniatis AN, Tsakris A, Pournaras S. Hidden VIM-1 metallo-β-lactamase phenotypes among Acinetobacter baumannii clinical isolates. Journal of Clinical Microbiology. 2008;46(1):346–349. doi: 10.1128/JCM.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsakris A, Ikonomidis A, Pournaras S, et al. VIM-1 metallo-β-lactamase in Acinetobacter baumannii . Emerging Infectious Diseases. 2006;12(6):981–983. doi: 10.3201/eid1206.051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonnin RA, Poirel L, Naas T, et al. Dissemination of New Delhi metallo-β-lactamase-1-producing Acinetobacter baumannii in Europe. Clinical Microbiology and Infection. 2012;18(9):E362–E365. doi: 10.1111/j.1469-0691.2012.03928.x. [DOI] [PubMed] [Google Scholar]
- 87.Boulanger A, Naas T, Fortineau N, Figueiredo S, Nordmann P. NDM-1-producing Acinetobacter baumannii from Algeria. Antimicrobial Agents and Chemotherapy. 2012;56(4):2214–2215. doi: 10.1128/AAC.05653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesli E, Berrazeg M, Drissi M, Bekkhoucha SN, Rolain JM. Prevalence of carbapenemase-encoding genes including New Delhi metallo-β-lactamase in Acinetobacter species, Algeria. International Journal of Infectious Diseases. 2013;17(9):739–743. doi: 10.1016/j.ijid.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 89.Hammerum AM, Larsen AR, Hansen F, et al. Patients transferred from Libya to Denmark carried OXA-48-producing Klebsiella pneumoniae, NDM-1-producing Acinetobacter baumannii and meticillin-resistant Staphylococcus aureus . International Journal of Antimicrobial Agents. 2012;40(2):191–192. doi: 10.1016/j.ijantimicag.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Decousser JW, Jansen C, Nordmann P, et al. Outbreak of NDM-1-producing Acinetobacter baumannii in France. Eurosurveillance. 2013;18(31) doi: 10.2807/1560-7917.es2013.18.31.20547. [DOI] [PubMed] [Google Scholar]
- 91.Bonnin RA, Cuzon G, Poirel L, Nordmann P. Multidrug-resistant Acinetobacter baumannii clone, France. Emerging Infectious Diseases Journal. 2013;19(5):822–823. doi: 10.3201/eid1905.121618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cicek AC, Saral A, Iraz M, et al. OXA- and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clinical Microbiology and Infection. 2013 doi: 10.1111/1469-0691.12338. [DOI] [PubMed] [Google Scholar]
- 93.Hrabák J, Stolbová M, Studentová V, Fridrichová M, Chudáčková E, Zemlickova H. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt. Eurosurveillance. 2012;17(7) [PubMed] [Google Scholar]
- 94.Kaase M, Nordmann P, Wichelhaus TA, Gatermann SG, Bonnin RA, Poirel L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. Journal of Antimicrobial Chemotherapy. 2011;66(6):1260–1262. doi: 10.1093/jac/dkr135. [DOI] [PubMed] [Google Scholar]
- 95.Espinal P, Fugazza G, López Y, et al. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli Rehabilitation Center. Antimicrobial Agents and Chemotherapy. 2011;55(11):5396–5398. doi: 10.1128/AAC.00679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends in Molecular Medicine. 2012;18(5):263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrobial Agents and Chemotherapy. 2010;54(1):24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woodford N, Pike R, Meunier D, Loy R, Hill R, Hopkins KL. In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp. and Enterobacter spp., and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. Journal of Antimicrobial Chemotherapy. 2013 doi: 10.1093/jac/dkt383. [DOI] [PubMed] [Google Scholar]
- 99.Hartl R, Widhalm S, Kerschner H, Apfalter P. Temocillin and meropenem to discriminate resistance mechanisms leading to decreased carbapenem susceptibility with focus on OXA-48 in Enterobacteriaceae . Clinical Microbiology and Infection. 2013;19(5):230–232. doi: 10.1111/1469-0691.12146. [DOI] [PubMed] [Google Scholar]
- 100.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrobial Agents and Chemotherapy. 2011;55(3):1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalpoe JS, Al Naiemi N, Poirel L, Nordmann P. Detection of an ambler class D Oxa-48-type β-lactamase in a Klebsiella pneumoniae strain in the netherlands. Journal of Medical Microbiology. 2011;60(5):677–678. doi: 10.1099/jmm.0.028308-0. [DOI] [PubMed] [Google Scholar]
- 102.Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. Characterization of OXA-181, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae . Antimicrobial Agents and Chemotherapy. 2011;55(10):4896–4899. doi: 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Potron A, Kalpoe J, Poirel L, Nordmann P. European dissemination of a single OXA-48-producing Klebsiella pneumoniae clone. Clinical Microbiology and Infection. 2011;17(12):E24–E26. doi: 10.1111/j.1469-0691.2011.03669.x. [DOI] [PubMed] [Google Scholar]
- 104.Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae . Antimicrobial Agents and Chemotherapy. 2004;48(1):15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carrër A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrobial Agents and Chemotherapy. 2008;52(8):2950–2954. doi: 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carrër A, Poirel L, Yilmaz M, et al. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrobial Agents and Chemotherapy. 2010;54(3):1369–1373. doi: 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poirel L, Ros A, Carrër A, et al. Cross-border transmission of OXA-48-producing Enterobacter cloacae from Morocco to France. Journal of Antimicrobial Chemotherapy. 2011;66(5):1181–1182. doi: 10.1093/jac/dkr023. [DOI] [PubMed] [Google Scholar]
- 108.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrobial Agents and Chemotherapy. 2011;55(5):2420–2423. doi: 10.1128/AAC.01452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Potron A, Poirel L, Rondinaud E, Nordmann P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Eurosurveillance. 2013;18(31) doi: 10.2807/1560-7917.es2013.18.31.20549. [DOI] [PubMed] [Google Scholar]
- 110.Benouda A, Touzani O, Khairallah M-T, Araj GF, Matar GM. First detection of oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Morocco. Annals of Tropical Medicine and Parasitology. 2010;104(4):327–330. doi: 10.1179/136485910X12743554760108. [DOI] [PubMed] [Google Scholar]
- 111.Vranić-Ladavac M, Bedenić B, Minandri F, et al. Carbapenem resistance and acquired class D β-lactamases in Acinetobacter baumannii from Croatia 2009–2010. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(3):471–478. doi: 10.1007/s10096-013-1991-9. [DOI] [PubMed] [Google Scholar]
- 112.Voulgari E, Zarkotou O, Ranellou K, et al. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. Journal of Antimicrobial Chemotherapy. 2013;68(1):84–88. doi: 10.1093/jac/dks356. [DOI] [PubMed] [Google Scholar]
- 113.Adler A, Shklyar M, Schwaber MJ, et al. Introduction of OXA-48-producing enterobacteriaceae to israeli hospitals by medical tourism. Journal of Antimicrobial Chemotherapy. 2011;66(12):2763–2766. doi: 10.1093/jac/dkr382. [DOI] [PubMed] [Google Scholar]
- 114.Goren MG, Chmelnitsky I, Carmeli Y, Navon-Venezia S. Plasmid-encoded OXA-48 carbapenemase in Escherichia coli from Israel. Journal of Antimicrobial Chemotherapy. 2011;66(3):672–673. doi: 10.1093/jac/dkq467. [DOI] [PubMed] [Google Scholar]
- 115.Matar GM, Dandache I, Carrër A, et al. Spread of OXA-48-mediated resistance to carbapenems in Lebanese Klebsiella pneumoniae and Escherichia coli that produce extended spectrum β-lactamase. Annals of Tropical Medicine and Parasitology. 2010;104(3):271–274. doi: 10.1179/136485910X12647085215651. [DOI] [PubMed] [Google Scholar]
- 116.Matar GM, Cuzon G, Araj GF, et al. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clinical Microbiology and Infection. 2008;14(9):887–888. doi: 10.1111/j.1469-0691.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 117.Kaase M, Pfennigwerth N, Szabados F, Gatermann S. Abstracts of the Twenty-Second European Congress of Clinical Microbiology and Infectious Diseases. London, UK: 2012. OXA-48, OXA-23 and NDM-1 carbapenemases in Gram-negative bacteria from patients from Libya. [Google Scholar]
- 118.Pirš M, Andlovic A, Cerar T, et al. A case of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a patient transferred to Slovenia from Libya, November 2011. Euro Surveillance. 2011;16(50) [PubMed] [Google Scholar]
- 119.Oteo J, Hernández JM, Espasa M, et al. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. Journal of Antimicrobial Chemotherapy. 2013;68(2):317–321. doi: 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 120.Ktari S, Mnif B, Louati F, et al. Spread of Klebsiella pneumoniae isolates producing OXA-48 β-lactamase in a Tunisian university hospital. Journal of Antimicrobial Chemotherapy. 2011;66(7):1644–1646. doi: 10.1093/jac/dkr181. [DOI] [PubMed] [Google Scholar]
- 121.Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemase: the phantom menace. Journal of Antimicrobial Chemotherapy. 2012;67(7):1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 122.Hays C, Benouda A, Poirel L, Elouennass M, Nordmann P. Nosocomial occurrence of OXA-48-producing enterobacterial isolates in a Moroccan hospital. International Journal of Antimicrobial Agents. 2012;39(6):545–547. doi: 10.1016/j.ijantimicag.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 123.Barguigua A, El Otmani F, Talmi M, Zerouali K, Timinouni M. Emergence of carbapenem-resistant Enterobacteriaceae isolates in the Moroccan community. Diagnostic Microbiology & Infectious Disease. 2012;73(3):290–291. doi: 10.1016/j.diagmicrobio.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Potron A, Poirel L, Bussy F, Nordmann P. Occurrence of the carbapenem-hydrolyzing β-lactamase gene blaOXA-48 in the environment in Morocco. Antimicrobial Agents and Chemotherapy. 2011;55(11):5413–5414. doi: 10.1128/AAC.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Munoz-Price LS, Weinstein RA. Acinetobacter infection. The New England Journal of Medicine. 2008;358(12):1214–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 126.Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. Journal of Antimicrobial Chemotherapy. 2006;57(3):373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 127.Higgins PG, Perez-Llarena FJ, Zander E, Fernández A, Bou G, Seifert H. OXA-235, a novel class D beta-lactamase involved in resistance to carbapenems in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2013;57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paton R, Miles RS, Hood J, Amyes SGB. ARI 1: β-lactamase-mediated imipenem resistance in Acinetobacter baumannii . International Journal of Antimicrobial Agents. 1993;2(2):81–88. doi: 10.1016/0924-8579(93)90045-7. [DOI] [PubMed] [Google Scholar]
- 129.Donald HM, Scaife W, Amyes SGB, Young H-K. Sequence analysis of ARI-1, a novel OXA β-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrobial Agents and Chemotherapy. 2000;44(1):196–199. doi: 10.1128/aac.44.1.196-199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poirel L, Figueiredo S, Cattoir V, Carattoli A, Nordmann P. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrobial Agents and Chemotherapy. 2008;52(4):1252–1256. doi: 10.1128/AAC.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Valenzuela JK, Thomas L, Partridge SR, Van Der Reijden T, Dijkshoorn L, Iredell J. Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii . Journal of Clinical Microbiology. 2007;45(2):453–460. doi: 10.1128/JCM.01971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii . The Journal of Antimicrobial Chemotherapy. 2010;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 133.Walsh TR. Emerging carbapenemases: a global perspective. International Journal of Antimicrobial Agents. 2010;36(3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 134.Mugnier PD, Poirel L, Naas T, Nordmann P. Worldwide dissemination of the blaOXA-23 Carbapenemase gene of Acinetobacter baumannii . Emerging Infectious Diseases. 2010;16(1):35–40. doi: 10.3201/eid1601.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.D’Arezzo S, Principe L, Capone A, Petrosillo N, Petrucca A, Visca P. Changing carbapenemase gene pattern in an epidemic multidrug-resistant Acinetobacter baumannii lineage causing multiple outbreaks in central Italy. Journal of Antimicrobial Chemotherapy. 2011;66(1):54–61. doi: 10.1093/jac/dkq407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Touati M, Diene SM, Racherache A, Dekhil M, Djahoudi A, Rolain JM. Emergence of bla OXA-23 and bla OXA-58 carbapenemase-encoding genes in multidrug-resistant Acinetobacter baumannii isolates from University Hospital of Annaba, Algeria. International Journal of Antimicrobial Agents. 2012;40(1):89–91. doi: 10.1016/j.ijantimicag.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 137.Mosqueda N, Espinal P, Cosgaya C, et al. Globally expanding carbapenemase finally appears in Spain: nosocomial outbreak of Acinetobacter baumannii producing plasmid-encoded OXA-23 in Barcelona, Spain. Antimicrobial Agents and Chemotherapy. 2013;57(10):5155–5157. doi: 10.1128/AAC.01486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vranic-Ladavac M, Bedenic B, Minandri F, et al. Carbapenem resistance and acquired class D β-lactamases in Acinetobacter baumannii from Croatia 2009–2010. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(3):471–478. doi: 10.1007/s10096-013-1991-9. [DOI] [PubMed] [Google Scholar]
- 139.Fouad M, Attia AS, Tawakkol WM, Hashem AM. Emergence of carbapenem-resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. International Journal of Infectious Diseases. 2013;17:1252–1254. doi: 10.1016/j.ijid.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 140.Bourigault C, Corvec S, Bretonnière C, et al. Investigation and management of multidrug-resistant Acinetobacter baumannii spread in a French medical intensive care unit: one outbreak may hide another. American Journal of Infection Control. 2013;41:652–653. doi: 10.1016/j.ajic.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 141.Liakopoulos A, Miriagou V, Katsifas EA, et al. Identification of OXA-23-producing Acinetobacter baumannii in Greece, 2010 to 2011. Eurosurveillance. 2012;17(11) [PubMed] [Google Scholar]
- 142.Mammina C, Palma DM, Bonura C, et al. Epidemiology and clonality of carbapenem-resistant Acinetobacter baumannii from an intensive care unit in Palermo, Italy. BMC Research Notes. 2012;5:p. 365. doi: 10.1186/1756-0500-5-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Espinal P, Macià MD, Roca I, et al. First report of an OXA-23 carbapenemase-producing Acinetobacter baumannii clinical isolate related to Tn2006 in Spain. Antimicrobial Agents and Chemotherapy. 2013;57(1):589–591. doi: 10.1128/AAC.01157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mansour W, Poirel L, Bettaieb D, Bouallegue O, Boujaafar N, Nordmann P. Dissemination of OXA-23-producing and carbapenem-resistant Acinetobacter baumannii in a university hospital in Tunisia. Microbial Drug Resistance. 2008;14(4):289–292. doi: 10.1089/mdr.2008.0838. [DOI] [PubMed] [Google Scholar]
- 145.Ciftci IH, Aşık G, Karakeçe E, et al. Distribution of blaOXA genes in Acinetobacter baumannii strains: a multicenter study. Mikrobiyoloji Bülteni. 2013;47:592–602. doi: 10.5578/mb.6388. [DOI] [PubMed] [Google Scholar]
- 146.Goic-Barisic I, Towner KJ, Kovacic A, et al. Outbreak in Croatia caused by a new carbapenem-resistant clone of Acinetobacter baumannii producing OXA-72 carbapenemase. Journal of Hospital Infection. 2011;77(4):368–369. doi: 10.1016/j.jhin.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 147.Mansour W, Bouallegue O, Dahmen S, Boujaafar N. Characterization of the resistance mechanism to β-lactams in Acinetobacter baumannii strains isolated in the university hospital Sahloul in Tunisia (2005) Pathologie Biologie. 2008;56(3):116–120. doi: 10.1016/j.patbio.2007.10.004. [DOI] [PubMed] [Google Scholar]


