Abstract
Myoclonus dystonia syndrome is a childhood onset hyperkinetic movement disorder characterized by predominant alcohol responsive upper body myoclonus and dystonia. A proportion of cases are due to mutations in the maternally imprinted SGCE gene. Previous studies have suggested that patients with SGCE mutations may have an increased rate of psychiatric disorders. We established a cohort of patients with myoclonus dystonia syndrome and SGCE mutations to determine the extent to which psychiatric disorders form part of the disease phenotype. In all, 89 patients with clinically suspected myoclonus dystonia syndrome were recruited from the UK and Ireland. SGCE was analysed using direct sequencing and for copy number variants. In those patients where no mutation was found TOR1A (GAG deletion), GCH1, THAP1 and NKX2-1 were also sequenced. SGCE mutation positive cases were systematically assessed using standardized psychiatric interviews and questionnaires and compared with a disability-matched control group of patients with alcohol responsive tremor. Nineteen (21%) probands had a SGCE mutation, five of which were novel. Recruitment of family members increased the affected SGCE mutation positive group to 27 of whom 21 (77%) had psychiatric symptoms. Obsessive–compulsive disorder was eight times more likely (P < 0.001) in mutation positive cases, compulsivity being the predominant feature (P < 0.001). Generalized anxiety disorder (P = 0.003) and alcohol dependence (P = 0.02) were five times more likely in mutation positive cases than tremor controls. SGCE mutations are associated with a specific psychiatric phenotype consisting of compulsivity, anxiety and alcoholism in addition to the characteristic motor phenotype. SGCE mutations are likely to have a pleiotropic effect in causing both motor and specific psychiatric symptoms.
Keywords: myoclonus dystonia, SGCE, psychiatric disorders
Introduction
Myoclonus dystonia syndrome is a rare movement disorder with onset in the first two decades of life. The clinical pattern is of alcohol responsive myoclonus of the trunk and upper limbs with cervical dystonia and/or writer’s cramp, although the lower limbs may also be involved (Asmus et al., 2002; Roze et al., 2008). The disorder affects males and females equally (Raymond et al., 2008) and is clinically consistent across ethnicities (Chung et al., 2007; Chen et al., 2008; Nardocci et al., 2008).
Mutations in the sarcoglycan, epsilon gene (SGCE) are responsible for a proportion of these cases (Zimprich et al., 2001). SGCE mutations are inherited in an autosomal dominant manner with variable penetrance due to maternal imprinting (Muller et al., 2002; Grabowski et al., 2003). SGCE encodes the epsilon-sarcoglycan protein, a single pass transmembrane protein forming part of the dystrophin-associated glycoprotein complex in some tissues (Blake et al., 2002; Esapa et al., 2007; Waite et al., 2009). SGCE mutation rates have varied amongst previously reported cohorts, some reporting no mutations (Valente et al., 2003) and others reporting rates from 21 to 80% (Valente et al., 2005; Gerrits et al., 2006; Tezenas du Montcel et al., 2006; Nardocci et al., 2008; Ritz et al., 2009). It is suggested that clinical classification, genetic heterogeneity (Grimes et al., 2001, 2002) and copy number variants (DeBerardinis et al., 2003; Asmus et al., 2005, 2007; Dale et al., 2011) may account for this observed variation in rates of mutation.
Co-morbid psychiatric disorders have been reported in a number of cases with myoclonus dystonia syndrome (Peall et al., 2011), including obsessive–compulsive disorder (OCD) (Marechal et al., 2003), depression (Doheny et al., 2002), suicide (Misbahuddin et al., 2007), psychosis (Dale et al., 2011), anxiety (Nardocci et al., 2008) and alcohol misuse (Saunders-Pullman et al., 2002b). Systematic assessment using standardized questionnaires has supported an excess of OCD and alcohol misuse amongst mutation carriers (Saunders-Pullman et al., 2002a; Hess et al., 2007; Peall et al., 2011), potentially indicating that SGCE has pleiotropic roles. However, previous studies have not separated primary from secondary psychiatric disorders related to disability, and, to date, there has been no systematic comparison with an appropriate control group.
This study uses systematic and standardized methods to examine the rate and type of psychiatric disorders within a large cohort of patients with myoclonus dystonia syndrome caused by SGCE mutations. This is the first study to compare these patients systematically with a disability matched control group. We also performed intra-familial comparisons of mutation and non-mutation carriers.
Materials and methods
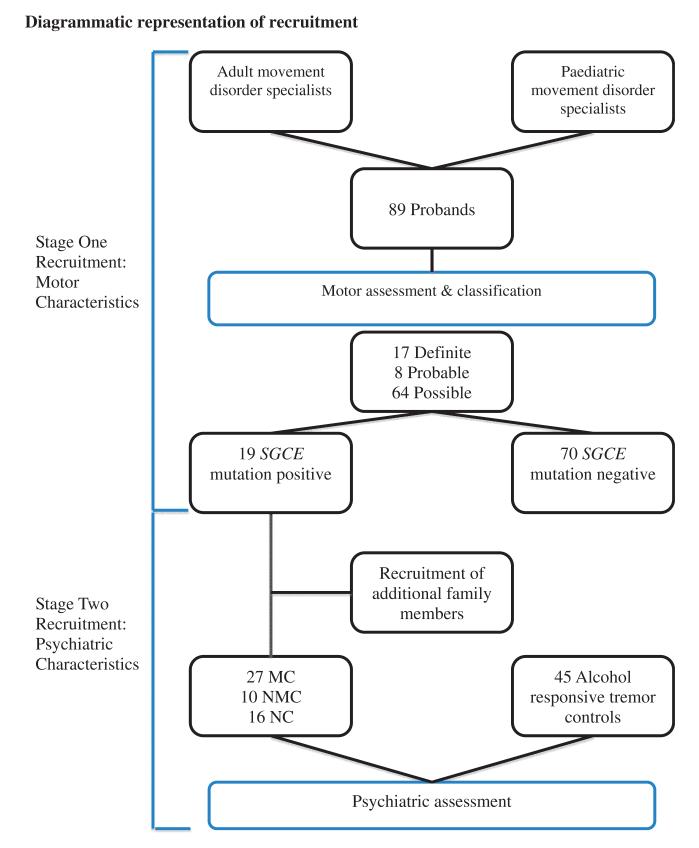
Patients with suspected myoclonus dystonia syndrome, some with previously confirmed SGCE mutations, were referred by adult and paediatric neurology centres throughout the UK and Ireland. The study was approved by the Multi-Centre Research Ethics Committee for Wales (MREC 09/MRE09/56 and 09/MRE09/35). Patients were recruited in two stages (Fig. 1) to allow separate assessment of motor and psychiatric co-morbidity.
Figure 1. Clinical diagnostic criteria used for motor classification (Grunewald et al., 2008).
MC = manifesting carrier; NMC = non-manifesting carrier; NC = non-carrier.
Stage one recruitment
Patients in whom myoclonus dystonia syndrome was considered a potential diagnosis were recruited by movement disorder specialists, and where possible systematically assessed face-to-face by a single investigator. In those for whom this was not possible, a systematic pro forma was used for data collection.
Motor assessment
All cases were assessed using a systematic protocol, including videotaped clinical examination. Patients were classified as ‘definite’, ‘probable’ or ‘possible’ according to previously published clinical criteria (Supplementary Table 1, Grunewald et al., 2008). Severity of motor symptoms in those with SGCE mutations was assessed using modified versions of the Unified Myoclonus Rating Scale (Frucht et al., 2002) and Burke-Fahn Marsden Dystonia Rating Scale (Burke et al., 1985).
Genetic analysis
Blood samples were collected from all cases after obtaining informed consent from the patient or assent from their parent/guardian. DNA was isolated from peripheral blood lymphocytes using standard protocols. All samples underwent direct sequencing of SGCE exons 1–12 (including alternatively spliced 1a and 11b). In those cases where no SGCE mutation was found, multiplex ligation-dependent probe amplification analysis was performed using the commercially available probe set P099B (MRC Holland) according to manufacturer’s instructions. Cases with whole gene deletions were analysed on a custom oligonucleotide Comparative Genomic Hybridization array platform (Roche) with 5900 probes covering chr7:88 000 000–98 000 000 (NCBI36/hg18 genome build). Data were analysed using the segment tool and visualized using SignalMap (Roche). To exclude other potential genetic diagnoses, all remaining samples were sequenced for the TOR1A (Torsin A) GAG deletion and mutations in GCH1 (GTP cyclohydrolase 1), THAP1 (THAP domain containing, apoptosis associated protein 1) and NKX2-1 (NK2 homeobox 1) genes.
Stage two recruitment
Related family members were recruited in SGCE kindreds. These cases were assessed face to face using the same protocol and a blood sample taken for genetic analysis. All SGCE mutation positive patients and family members were classified according to their motor and genetic status into three groups: (i) manifesting carriers: SGCE mutation and movement disorder (n = 27); (ii) non-manifesting carriers: SGCE mutation and no movement disorder (n = 10); and (iii) non-carriers: neither SGCE mutation nor movement disorder (n = 16). Patients with tremor who reported an improvement with alcohol were recruited from general neurology and movement disorder clinics, forming the control group for assessment of psychiatric co-morbidity and were examined using the same protocol. Manifesting carriers and the tremor control groups were matched for the effect of disease on quality of life, assessed by the Short Form Health Survey (SF-36).
Psychiatric and quality of life assessment
Lifetime psychiatric symptoms were assessed in a total of 98 participants (53 SGCE-positive patients and family members; 45 control subjects) using a modified version of the MINI International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998), which allowed symptoms to be classified according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for major depressive episode, manic and hypomanic episode, panic disorder, agoraphobia, social phobia, OCD, alcohol dependence, alcohol abuse, psychotic and mood disorders and generalized anxiety disorder. For those aged <18 years, the M.I.N.I-Kid for Children and Adolescents (Parent version) was completed (nine of the total 27 manifesting carriers were under 18 years and underwent assessment with the MINI Kids). Further assessment was made using the Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001), Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), Yale–Brown Obsessive–Compulsive Scale (YBOCS) (Goodman et al., 1989a, b) and the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993). Quality of life was assessed using the Short Form Health Survey (SF-36) (Ware and Sherbourne, 1992). Population estimates for DSM-IV diagnoses were taken from the North American National Comorbidity Survey (Kessler et al., 1994) and the Dunedin Longitudinal Birth Cohort Study (Douglass et al., 1995), both large comprehensive epidemiological studies.
Statistical analysis
Results were analysed using the ‘R’ statistical software package. Chi-squared testing, Pearson correlation coefficient and binomial stepwise multiple logistic regression methods of analysis were used where appropriate. No correction for multiple testing was performed.
Results
Stage one
Eighty-nine probands were recruited to this study, 50 males and 39 females with a median age at onset of the movement disorder of 5 years (range 0–48 years).
SGCE-positive patients
Nineteen (21%) of the 89 probands were found to have an SGCE mutation with a slight female predominance (8 male, 11 female). Median age at onset was 3 years (range 1.5–18 years). Eight related affected family members of those with SGCE mutations were also assessed, increasing the number of clinically affected SGCE mutation positive patients to 27.
Motor symptoms
All probands had myoclonus and dystonia with 15/19 (79%) meeting criteria for clinically definite myoclonus dystonia syndrome. With the eight additional affected family members, upper limb myoclonus was evident in all 27 cases with head (66.7%) and truncal (66.7%) involvement also prominent. Dystonia was more widely distributed, although still predominantly affecting the upper body i.e. upper limbs (85.2%) and neck (77.8%).
Genetics
Seventy-nine per cent (15/19) of the mutation positive probands had a positive family history of myoclonus dystonia syndrome with mutated SGCE known to be paternally inherited in 74% of cases (14/19). In the remaining cases, we were unable to contact additional family members to confirm the presence or absence of motor symptoms and genetic mutations. Thirteen different types of mutation were identified, of which five are novel mutations. Four were nonsense, one missense, three splice-site mutations, three intra-exonic deletions, one single exon deletion (exon 5) and five whole gene deletions (Supplementary Table 2). The most prevalent was nonsense mutation c.289C>T p.Arg97X in exon 3, occurring in four unrelated Caucasian families.
SGCE-negative patients
Seventy of 89 (78.7%) unrelated patients did not have an identified SGCE mutation with a male predominance (42 male, 28 female). No mutations were detected in the GCH1 and THAP1 genes, nor the GAG deletion in the TOR1A gene. Two cases were found to have NKX2-1 mutations both having a low amplitude upper body tremor.
Stage two
Psychiatric analysis
Data were collected from 27 cases with myoclonus dystonia syndrome (manifesting carriers) from 11 families and 45 control cases with alcohol responsive tremor. A further 26 family members were recruited from the myoclonus dystonia syndrome families, 10 non-manifesting carriers and 16 non-carriers, the latter group including 10 married-in spouses. Manifesting carriers and tremor groups were matched for disability based on median Short Form Health Survey (SF-36) scores, sex, disease duration and alcohol use, although they differed significantly in age at onset and age at examination (P < 0.001) (Table 1).
Table 1. Demographics and analysis of variables.
| Demographics | MC | MC (>18 years) | NMC | NC | Tremor | MC versus tremor (total population) | MC versus tremor (adult only population) |
|---|---|---|---|---|---|---|---|
| Total (male:female) | 27 (10:7) | 18 (6:12) | 10 (6:4) | 16 (5:11) | 45 (14:31) | 0.62a | 0.59a |
| Median age at examination (range) | 28 (3–74) | 42.5 (18–74) | 38 (29–73) | 40 (16–71) | 62 (19–88) | <0.001b | <0.001b |
| Median age at onset of movement disorder (range) | 3 (1.5–18) | 4.5 (1.5–18) | 26.5 (3–76) | <0.001b | <0.001b | ||
| Mean duration of movement disorder | 25.57 | 31.93 | 30.78 | 0.27b | 0.79b | ||
| Alcohol consumption (%)c | 78 | 78 | 90 | 59 | 71 | 0.78a | 0.78a |
| Median SF-36 scores (range) | 92 (38–111) | 92 (38–111) | 104 (97–106) | 101 (95–118) | 99 (64–125) | 0.09a | 0.09a |
Calculated using chi-squared analysis.
Calculated using Student’s t-test.
Alcohol consumption refers to whether the participant drinks any alcohol at all.
MC = manifesting carrier; NMC = non-manifesting carrier; NC = non-carrier.
Results of psychiatric data analysis are shown in Tables 2 and 3 (M.I.N.I and M.I.N.I–Kids) and Table 4 (YBOCS, AUDIT, PHQ-9 and MADRS). All cases completed the M.I.N.I. questionnaires with the exception of two adult patients and one patient aged <18 years, where assent was declined. Both adult patients had recently been seen by consultant psychiatrists confirming diagnoses of depression and anxiety in one and schizophrenia in the other.
Table 2. Rates of psychiatric disease in manifesting carriers and tremor cohorts.
| Lifetime disorder DSM-IV | MC n (%) (total = 27) | Tremor n (%) (total = 45) | Population estimates (%) |
|---|---|---|---|
| Any DSM-IV disorder | 21 (77.8) | 28 (62.2) | 48a |
| Major depressive disorder | 12 (44.4) | 20 (44.4) | 17.1a |
| Mania and hypomania | 0 (0) | 3 (6.7) | 1.6a |
| Panic disorder | 9 (33.3) | 17 (37.8) | 3.5a |
| Agoraphobia | 6 (22.2) | 7 (15.6) | 5.3a |
| Social phobia | 12 (44.4) | 8 (17.8) | 13.3a |
| OCD—overall | 16 (59.2) | 8 (17.8) | 4b |
| Obsessions | 4 (14.8) | 8 (17.8) | |
| Compulsions | 14 (51.9) | 6 (13.3) | |
| Alcohol dependence/abuse | 8 (29.6) | 6 (13.3) | 14.1a |
| Psychotic and mood disorders | 1 (3.7) | 9 (20) | 0.7a |
| Generalized anxiety disorder | 13 (48.1) | 3 (6.7) | 5.1a |
Values taken from National Comorbidity Survey (Kessler et al., 1994).
Values from Dunedin Longitudinal Birth Cohort Study (Douglass et al., 1995).
MC = manifesting carrier; NMC = non-manifesting carrier; NC = non-carrier.
Table 3. Comparison of rates of psychiatric disease between manifesting carrier, non-manifesting carrier and tremor groups.
| Psychiatric diagnosis (DSM-IV) | MC versus tremor | NMC versus tremor | MC versus NMC |
|---|---|---|---|
| Any | 0.20 (2.13; 0.64, 7.31) | 0.29 (0.41; 0.08, 1.96) | 0.05 (5.25; 0.88, 34.12) |
| Major depressive disorder | 1.0 (1.0; 0.34, 2.91) | 1.0 (0.83; 0.17, 4.02) | 1.0 (1.2; 0.22, 6.70) |
| Mania and hypomania | 0.29 (0; 0, 3.8) | 1.0 (0; 0, 11.37) | 1.0 (unable to calculate) |
| Panic disorder | 0.8 (0.82; 0.27, 2.51) | 0.14 (0.18; 0.01, 1.67) | 0.23 (4.5; 0.44, 109.94) |
| Agoraphobia | 0.54 (1.55; 0.39, 6.08) | 0.33 (0; 0, 3.63) | 0.16 (∞; 0.40, ∞) |
| Social phobia | 0.03 (3.7; 1.12, 12.56) | 0.33 (0; 0, 3.03) | 0.02 (∞; 1.23, ∞) |
| OCD | 0.001 (6.7; 2.02, 23.27) | 0.33 (0; 0, 3.03) | 0.002 (∞; 2.20, ∞) |
| Alcohol dependence/abusea | 0.02 (4.33; 1.08, 18.06) | 0.58 (0; 0, 4.46) | 0.03 (∞; 0.94, ∞) |
| Psychotic and mood disorders | 1.00 (0.54; 0.02, 6.36) | 1.00 (0; 0, 11.37) | 1 (∞; 0.02, ∞) |
| Generalized anxiety disorder | 0.02 (3.71; 1.16, 12.22) | 0.67 (0.44; 0.02, 4.36) | 0.06 (8.36; 0.84, 201.48) |
Results analysed using chi-squared testing.
Results expressed as: P-value (odds ratio, 95% confidence interval).
Statistically significant results highlighted in bold.
Only participants aged >18 years included in analysis.
MC = manifesting carrier; NMC = non-manifesting carrier; OCD = obsessive-compulsive disorder.
Table 4. Analysis of YBOCS, AUDIT, PHQ-9 and MADRS questionnaires.
| Questionnaire | Median score (range) | P-value | |||
|---|---|---|---|---|---|
| MC | NMC | NC | Tremor | ||
| YBOCS | |||||
| Total score | 9 (0–26) | 0 (0) | 0 (0–8) | 0 (0–33) | <0.001 |
| Obsessions | 0 (0–15) | 0 (0) | 0 (0–8) | 0 (0–17) | 0.16 |
| Compulsions | 7 (0–17) | 0 (0) | 0 (0) | 0 (0–16) | <0.001 |
| AUDIT | |||||
| Total score | 4 (0–34) | 4.5 (1–9) | 1 (0–4) | 2 (0–18) | <0.001 |
| PHQ-9 | |||||
| Total score | 6 (0–18) | 2 (0–12) | 2 (0–12) | 4 (0–25) | 0.08 |
| MADRS | |||||
| Total score | 15 (0–32) | 1 (0–22) | 1 (0–22) | 6 (0–30) | 0.01 |
Statistical analysis using binomial stepwise multiple logistic regression.
MC = manifesting carrier; NMC = non-manifesting carrier; NC = non-carrier.
Overall rates of psychiatric disorders were higher in manifesting carriers and tremor cohorts than population estimates (77.8 and 62.2 versus 48%, respectively). The largest differences were seen in rates of OCD and generalized anxiety disorder, OCD being three times higher in the manifesting carriers group than tremor controls, the largest contribution coming from compulsive symptoms, which were four times higher in the manifesting carriers group. Rates of generalized anxiety disorder were almost seven times greater amongst manifesting carrier patients with smaller excesses seen in social phobia (two times higher) and alcohol dependence/abuse (two times higher).
Comparison of manifesting carriers and tremor groups found no overall excess of psychiatric disorders amongst manifesting carriers patients [odds ratio (OR) 2.13, 95% confidence interval (CI) 0.64–7.31, P = 0.20]. However, large differences were seen in rates of OCD (OR 6.7, 95% CI 2.02–23.27, P = 0.001), generalized anxiety disorder (OR 3.71, 95% CI 1.16–12.22, P = 0.02) and social phobia (OR 3.7, 95% CI 1.12–12.56, P = 0.03). Alcohol excess/dependence was also more common amongst adult manifesting carriers (OR 4.3, 95% CI 1.08–18.06, P = 0.02). Attempts to discern a gene effect were made by comparing non-manifesting carriers cases with tremor and manifesting carrier cohorts. No overall or disorder-specific difference was seen between non-manifesting carrier and tremor groups. The same pattern of increased psychiatric morbidity was seen when the manifesting carrier group was compared with the non-manifesting carrier group, as had been seen when the manifesting carrier group had been compared with the tremor cohort (Table 3), although when the manifesting carrier and non-manifesting carrier groups were compared, the overall rate of psychiatric disorders was higher in the former (OR 5.25, 95% CI 0.88–34.12, P = 0.05).
Comparison of the manifesting carriers group with each of the other groups found a significantly higher total YBOCS score (P < 0.001). This largely reflected a significantly increased compulsivity score (P < 0.001) with no difference in obsessionality (P = 0.16). Alcohol use over the preceding year, measured with the AUDIT questionnaire, was higher amongst the manifesting carrier group compared with all other groups. Depression self-assessment in the form of the PHQ-9 found no statistical difference between the groups. However, clinician scoring of depressive symptoms using the MADRS found statistically significant differences between the manifesting carrier group and all other groups.
No association between presence of overall psychiatric disorders and motor severity scores was observed (P = 0.08). There was an association between motor severity and MADRS scores (P = 0.05, r = 0.58), but no link with overall YBOCS scores (P = 0.83, r = 0.06), obsessions (P = 0.73, r = 0.095), compulsions (P = 0.98, r = 0.008) and AUDIT scores (P = 0.60, r = 0.14). Stepwise multivariable logistic regression found duration of motor symptoms (P = 0.04) to impact on the incidence of psychiatric disorders, but with no effect when controlling for age at onset (P = 0.20). Results for OCD alone found no significant impact when controlling for age at onset (P = 0.23) or motor disease duration (P = 0.05).
Discussion
This study represents the largest single, multi-family cohort of patients with myoclonus dystonia syndrome systematically assessed using validated scales for rate and type of psychiatric disorders, and the first to compare psychopathology with a disability matched control group. We have confirmed the hypothesis that patients with manifesting SGCE mutations have significantly higher rates of psychiatric illness as compared with control subjects with a significant movement disorder. Our findings point to a specific preponderance of OCD, generalized anxiety disorder and alcohol dependence and reveal that the association with OCD is specific to the compulsivity rather than the obsessional component of the disorder.
Although manifesting carrier and tremor groups are matched for disability, movement disorder duration, sex and alcohol use, a significant difference in age at onset and age at examination (P < 0.001) exists between the two groups (Table 1). However, multivariate analysis found neither the incidence of overall psychiatric pathology nor, more specifically, OCD to be influenced by age at onset of the motor disorder, and OCD alone appeared independent of the duration of movement disorder symptoms. In addition, our psychiatric interview involved lifetime assessment for each of these disorders rather than relating to a restricted time frame. These analyses suggest that the specific pattern of psychiatric morbidity seen in manifesting carriers compared with tremor controls was not due to differences in age at onset or age at examination between the two groups. In addition, alcohol-responsive tremor was considered the best disease match for a chronic disabling disorder that benefits therapeutically from alcohol in an attempt to separate therapeutic and addictive traits. Finally, we have not included a dystonia control group, and therefore we cannot exclude the possibility that the specific pattern of psychiatric morbidity seen in myoclonus dystonia syndrome is also associated with dystonia more widely.
The frequency of SGCE mutations within the primary cohort (21%) is in keeping with previously reported studies (Tezenas du Montcel et al., 2006; Asmus et al., 2009; Ritz et al., 2009). A positive family history was an important factor when determining whether cases were ‘definite’, ‘probable’ or ‘possible’, being present in 95% of cases and paternally inherited in all cases where this information was available. Within this cohort, there were 17 definite, of which 15 had a SGCE mutation (88%), the highest reported rate to date. Eight ‘probable’ cases were identified, four of which had a SGCE mutation and had not been included in the ‘definite’ category owing to a lack of family history. This is likely due to maternal imprinting ‘silencing’ the mutation for several generations and therefore limiting the current diagnostic criteria.
Psychiatric assessment using the M.I.N.I. questionnaire found overall higher rates of psychiatric disorders amongst the manifesting carrier group compared with the tremor controls (77.8 versus 62.2%) and significantly higher than estimated within the general population (48%). Population estimates were taken from large cohort studies in Western populations similar to the one studied in this cohort (Kessler et al., 1994; Douglass et al., 1995). In addition, no association was seen between psychiatric disorders and motor severity, suggesting that the psychiatric phenotype may be independent of the motor disorder.
Obsessive-compulsive disorder
OCD was the most common disorder in manifesting carrier patients (59%), almost seven times more likely to occur compared with control subjects. OCD is generally not recognized as a secondary psychiatric response to chronic disease (Fullana et al., 2009), and therefore is an interesting finding in a chronically disabling and disfiguring disorder. Similar results were seen with the adult only manifesting carriers cohort (OR 5.65, 95% CI 1.53–21.69, P = 0.006) suggesting that despite population estimates that rates of OCD are higher amongst children (Douglass et al., 1995), a significant difference remains between these groups. There were no differences in OCD rates between non-manifesting carrier and tremor groups and a significant difference between manifesting carrier and non-manifesting carrier cohorts. These results argue against a direct gene effect, although this is based on a small number of patients. YBOCS questionnaire scores further strengthened this association with the manifesting carriers median score being nine times higher and significantly different to all other groups. This effect was overwhelmingly owing to compulsivity scores, seven times greater than obsessive traits and did not relate to severity of the motor phenotype.
Rates of OCD have been assessed in other forms of dystonia with conflicting results. Mixed groups of focal dystonias have been compared with groups of other disfiguring disorders (Broocks et al., 1998; Barahona-Correa et al., 2011) and healthy individuals (Cavallaro et al., 2002), finding increased rates of OCD or obsessive–compulsive symptoms amongst the dystonic group, although unable to relate this to a specific form of dystonia. Similarly elevated rates of OCD have been noted amongst first degree relatives of those with dystonia/OCD compared with those with dystonia alone. In contrast, others have found no association either amongst mixed dystonia types (Fabbrini et al., 2010) or genetically defined DYT1 cohorts (Heiman et al., 2007).
Alcohol use disorders
Alcohol excess has also been frequently observed amongst DYT11 cohorts (Asmus et al., 2002; Saunders-Pullman et al., 2002b; Tezenas du Montcel et al., 2006; Hess et al., 2007; Misbahuddin et al., 2007). Amongst the adult manifesting carriers cohort, alcohol excess was more than four times more likely than the tremor control group together with a significant difference in total AUDIT scores between the manifesting carriers and all other groups (P < 0.001). The median AUDIT score of non-manifesting carriers was also higher than that of the tremor participants, suggesting that alcohol use is higher amongst SGCE mutation carriers, irrespective of motor symptoms. This may reflect a functional role of the normally imprinted mutated maternal allele in certain brain regions (Guettard et al., 2008; Beukers et al., 2011a), causing failure of expression of the epsilon-sarcoglycan protein at the cell surface membrane.
Previous literature has suggested a link between those traits that result in excess alcohol consumption and ritualistic OCD behaviour (Caetano, 1985). Assessment using the YBOCS questionnaire of a population diagnosed with alcohol dependence/abuse noted a positive correlation between alcohol craving and both individual obsession and compulsion scores (Modell et al., 1992). Similarly, techniques traditionally used in the treatment of OCD have been found to reduce the desire to drink and to improve alcohol resistance. Collectively, this suggests that excess alcohol consumption in SGCE-positive individuals may not simply be a secondary therapeutic response as hitherto assumed, but rather that it is related to the compulsivity that we have demonstrated and forms part of the phenotype of the disorder.
Anxiety disorders and depressive symptoms
Other psychiatric disturbances that appeared to be influenced by genetic and motor status were social phobia and generalized anxiety disorder. Anxiety related co-morbidity has been noted during intrafamilial comparisons of myoclonus dystonia syndrome cohorts (Foncke et al., 2009) and standardized testing of patients with cervical dystonia compared with the general population (Wenzel et al., 1998; Gündel et al., 2001). These features were also consistent when a similar cohort was compared with a control group of patients with alopecia areata, highlighting a potential dystonia-specific feature (Gündel et al., 2003). Psychiatric features have also been known to predate the onset of dystonic symptoms, again suggesting that this psychopathology is a primary, rather than a secondary, reactive response (Lencer et al., 2009).
Mood disorders differed between the groups when analysed using the self-completed PHQ-9 and assessor-completed MADRS, although under-reporting of symptoms with self-rated questionnaires is a well-recognized feature (Scheidt et al., 1999). Despite this excess of affective symptoms likely being a secondary effect due to a chronic, disabling disorder, other genetically defined groups of dystonias have found an excess of depression (Duane, 2005), again suggesting a general increase of psychiatric co-morbidity amongst this group of disorders.
Other psychiatric disorders
Subtle differences were also observed when comparing psychiatric illness in the whole gene deletion cases to those with point mutations. The four cases with larger mutations (1.9–2.3 Mb) all had symptoms of OCD, depression and anxiety-related disorders similar to the population identified by Sanger sequencing. In contrast, the fifth whole gene deletion case initially presented to adult medical services with symptoms of schizophrenia requiring multiple inpatient admissions. Psychiatric features have not been observed in the majority of previous whole gene deletion case reports, the attention instead being focused on global cognitive impairment and learning difficulties (Asmus et al., 2007; Saugier-Veber et al., 2010). An Australian family was also reported to have symptoms of psychosis and, as in the case in this study, a much smaller deletion than those described above (Dale et al., 2011). With the exception of a single member of a myoclonus dystonia syndrome family being reported to have schizoaffective disorder (Wong et al., 2010), psychosis has not been reported previously in those with SGCE mutations, and none of the genes involved in these deletions (PEG10, SGCE, CASD1 and COL1A2) are believed to contribute to the pathogenesis of psychosis.
Implications for the pathogenesis of dystonia and compulsivity
Comorbid OCD, dystonia and SGCE mutations reaffirm the likely role of the basal ganglia in the underlying pathogenesis of these disorders. PET and functional MRI studies of dystonias (Playford et al., 1998) and OCD (Breiter and Rauch, 1996) have shown abnormal activation of the basal ganglia, thalamus, frontal and cingulate cortices, whereas an association has also been identified between dystonia severity and putaminal grey matter volume (Beukers et al., 2011b). More recent functional MRI studies have also shown altered patterns of activation in the sensorimotor cortex and cerebellum, suggesting that additional brain structures may also contribute (Beukers et al., 2011a). Neurophysiological studies showing impaired saccadic adaptation in SGCE mutation positive cases also support this, suggesting involvement of the posterior cerebellum potentially in the generation of subcortical myoclonus (Hubsch et al., 2011). Use of deep brain stimulation in the treatment of myoclonus dystonia syndrome has shown improvement of myoclonus and dystonia when stimulating the globus pallidus internus (Azoulay-Zyss et al., 2011), compared with thalamic stiumulation (Gruber et al., 2010), while lesions within the striatal-pallidal pathways are also associated with the development of OCD (Lauterbach et al., 1994).
Conclusion
We have demonstrated an excess of specific psychiatric disorders among affected SGCE mutation carriers when compared with an external control group and to unaffected family members. OCD is the most strongly associated psychopathology, and this reflects compulsive rather than obsessive symptoms. Affected SGCE mutation carriers showed evidence of excess alcohol consumption even when compared with a control group with alcohol responsive tremor. Excess consumption in myoclonus dystonia syndrome is often attributed to the therapeutic effects of alcohol, but our findings suggest that this might have a more direct relationship to pathogenesis and may arise as a consequence of a primary disturbance of compulsive behaviour.
In conclusion, this study shows that psychiatric co-morbidity forms a significant part of the clinical phenotype of myoclonus dystonia syndrome due to SGCE mutations. Clinicians need to be aware of this and of the need for psychiatric symptoms to be treated effectively and early. Further work is required to define and delineate the relationship between motor and psychiatric symptoms, which will enhance our understanding of the aetiology and pathophsyiology of both motor and psychiatric disorders.
Supplementary Material
Acknowledgements
The authors would like to thank the patients and their relatives for participating in this study and also to Dr Daniel Lumsden for his assistance with data collection. The authors would also like to thank the British Neurological Surveillance Unit (BNSU) for their assistance with notification of cases.
Funding: The IPSEN fund (AJ11311001 to K.P.). K.P. is also a Welsh Clinical Academic Track (WCAT) trainee.
Abbreviations
- AUDIT
Alcohol Use Disorders Identification Test
- MADRS
Montgomery–Asberg Depression Rating Scale
- M.I.N.I.
MINI International Neuorpsychiatric Interview
- OCD
obsessive–compulsive disorder
- PHQ-9
Patient Health Questionnaire 9
- YBOCS
Yale–Brown Obsessive–Compulsive Scale
References
- Asmus F, Zimprich A, Tezenas Du Montcel S, Kabus C, Deuschl G, Kupsch A, et al. Myoclonus-dystonia syndrome: epsilon-sarcoglycan mutations and phenotype. Ann Neurol. 2002;52:489–92. doi: 10.1002/ana.10325. [DOI] [PubMed] [Google Scholar]
- Asmus F, Salih F, Hjermind LE, Ostergaard K, Munz M, Kühn AA, et al. Myoclonus-dystonia due to genomic deletions in the epsilon-sarcoglycan gene. Ann Neurol. 2005;58:792–7. doi: 10.1002/ana.20661. [DOI] [PubMed] [Google Scholar]
- Asmus F, Hjermind LE, Dupont E, Wagenstaller J, Haberlandt E, Munz M, et al. Genomic deletion size at the epsilon-sarcoglycan locus determines the clinical phenotype. Brain. 2007;130:2736–45. doi: 10.1093/brain/awm209. [DOI] [PubMed] [Google Scholar]
- Asmus F, Langseth A, Doherty E, Nestor T, Munz M, Gasser T, et al. “Jerky” dystonia in children: spectrum of phenotypes and genetic testing. Mov Disord. 2009;24:702–9. doi: 10.1002/mds.22426. [DOI] [PubMed] [Google Scholar]
- Azoulay-Zyss J, Roze E, Welter ML, Navarro S, Yelnik J, Clot F, et al. Bilateral deep brain stimulation of the pallidum for myoclonus-dystonia due to epsilon-sarcoglycan mutations: a pilot study. Arch Neurol. 2011;68:94–8. doi: 10.1001/archneurol.2010.338. [DOI] [PubMed] [Google Scholar]
- Barahona-Correa B, Bugalho P, Guimarães J, Xavier M. Obsessive-compulsive symptoms in primary focal dystonia: a controlled study. Mov Disord. 2011;26:2274–8. doi: 10.1002/mds.23906. [DOI] [PubMed] [Google Scholar]
- Beukers RJ, Foncke EM, van der Meer JN, Veltman DJ, Tijssen MA. Functional magnetic resonance imaging evidence of incomplete maternal imprinting in myoclonus-dystonia. Arch Neurol. 2011a;68:802–5. doi: 10.1001/archneurol.2011.23. [DOI] [PubMed] [Google Scholar]
- Beukers RJ, van der Meer JN, van der Salm SM, Foncke EM, Veltman DJ, Tijssen MA. Severity of dystonia is correlated with putaminal gray matter changes in myoclonus-dystonia. Eur J Neurol. 2011b;18:906–12. doi: 10.1111/j.1468-1331.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL. Functional MRI and the study of OCD: from symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage. 1996;4:S127–38. doi: 10.1006/nimg.1996.0063. [DOI] [PubMed] [Google Scholar]
- Broocks A, Thiel A, Angerstein D, Dressler D. Higher prevalence of obsessive-compulsive symptoms in patients with blepharospasm than in patients with hemifacial spasm. Am J Psychiatry. 1998;155:555–7. doi: 10.1176/ajp.155.4.555. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- Caetano R. Alcohol dependence and the need to drink: a compulsion? Psychol Med. 1985;15:463–9. doi: 10.1017/s0033291700031342. [DOI] [PubMed] [Google Scholar]
- Cavallaro R, Galardi G, Cavallini MC, Henin M, Amodio S, Bellodi L, et al. Obsessive compulsive disorder among idiopathic focal dystonia patients: an epidemiological and family study. Biol Psychiatry. 2002;52:356–61. doi: 10.1016/s0006-3223(02)01332-x. [DOI] [PubMed] [Google Scholar]
- Chen XP, Zhang YW, Zhang SS, Chen Q, Burgunder JM, Wu SH, et al. A novel mutation of the epsilon-sarcoglycan gene in a Chinese family with myoclonus-dystonia syndrome. Mov Disord. 2008;23:1472–5. doi: 10.1002/mds.22008. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Lee WY, Kim JY, Kim JH, Kim GM, Ki CS, et al. Novel SGCE gene mutation in a Korean patient with myoclonus-dystonia with unique phenotype mimicking Moya-Moya disease. Mov Disord. 2007;22:1206–7. doi: 10.1002/mds.21093. [DOI] [PubMed] [Google Scholar]
- Dale RC, Nasti JJ, Peters GB. Familial 7q21.3 microdeletion involving epsilon-sarcoglycan causing myoclonus dystonia, cognitive impairment, and psychosis. Mov Disord. 2011;26:1774–5. doi: 10.1002/mds.23639. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Conforto D, Russell K, Kaplan J, Kollros PR, Zackai EH, et al. Myoclonus in a patient with a deletion of the epsilon-sarcoglycan locus on chromosome 7q21. Am J Med Genet A. 2003;121A:31–6. doi: 10.1002/ajmg.a.20162. [DOI] [PubMed] [Google Scholar]
- Doheny DO, Brin MF, Morrison CE, Smith CJ, Walker RH, Abbasi S, et al. Phenotypic features of myoclonus-dystonia in three kindreds. Neurology. 2002;59:1187–96. doi: 10.1212/wnl.59.8.1187. [DOI] [PubMed] [Google Scholar]
- Douglass HM, Moffitt TE, Dar R, McGee R, Silva P. Obsessive-compulsive disorder in a birth cohort of 18-year-olds: prevalence and predictors. J Am Acad Child Adolesc Psychiatry. 1995;34:1424–31. doi: 10.1097/00004583-199511000-00008. [DOI] [PubMed] [Google Scholar]
- Duane DD. Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology. 2005;64:1821–2. doi: 10.1212/wnl.64.10.1821-a. author reply 1821–2. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RA, et al. SGCE missense mutations that cause myoclonus-dystonia syndrome impair epsilon-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–42. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Berardelli I, Moretti G, Pasquini M, Bloise M, Colosimo C, et al. Psychiatric disorders in adult-onset focal dystonia: a case-control study. Mov Disord. 2010;25:459–65. doi: 10.1002/mds.22983. [DOI] [PubMed] [Google Scholar]
- Foncke EM, Cath D, Zwinderman K, Smit J, Schmand B, Tijssen M. Is psychopathology part of the phenotypic spectrum of myoclonusdystonia?: a study of a large Dutch M-D family. Cogn Behav Neurol. 2009;22:127–33. doi: 10.1097/WNN.0b013e3181a7228f. [DOI] [PubMed] [Google Scholar]
- Frucht SJ, Leurgans SE, Hallett M, Fahn S. The unified myoclonus rating scale. Adv Neurol. 2002;89:361–76. [PubMed] [Google Scholar]
- Fullana MA, Mataix-Cols D, Caspi A, Harrington H, Grisham JR, Moffitt TE, et al. Obsessions and compulsions in the community: prevalence, interference, help-seeking, developmental stability, and co-occurring psychiatric conditions. Am J Psychiatry. 2009;166:329–36. doi: 10.1176/appi.ajp.2008.08071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MC, Foncke EM, de Haan R, Hedrich K, van de Leemput YL, Baas F, et al. Phenotype-genotype correlation in Dutch patients with myoclonus-dystonia. Neurology. 2006;66:759–61. doi: 10.1212/01.wnl.0000201192.66467.a3. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989a;46:1012–16. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989b;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Zimprich A, Lorenz-Depiereux B, Kalscheuer V, Asmus F, Gasser T, et al. The epsilon-sarcoglycan gene (SGCE), mutated in myoclonus-dystonia syndrome, is maternally imprinted. Eur J Hum Genet. 2003;11:138–44. doi: 10.1038/sj.ejhg.5200938. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Bulman D, George-Hyslop PS, Lang AE. Inherited myoclonus-dystonia: evidence supporting genetic heterogeneity. Mov Disord. 2001;16:106–10. doi: 10.1002/1531-8257(200101)16:1<106::aid-mds1022>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Han F, Lang AE, St George-Hyssop P, Racacho L, Bulman DE. A novel locus for inherited myoclonus-dystonia on 18p11. Neurology. 2002;59:1183–6. doi: 10.1212/wnl.59.8.1183. [DOI] [PubMed] [Google Scholar]
- Gruber D, Kühn AA, Schoenecker T, Kivi A, Trottenberg T, Hoffmann KT, et al. Pallidal and thalamic deep brain stimulation in myoclonus-dystonia. Mov Disord. 2010;25:1733–43. doi: 10.1002/mds.23312. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Djarmati A, Lohmann-Hedrich K, Farrell K, Zeller JA, Allert N, et al. Myoclonus-dystonia: significance of large SGCE deletions. Hum Mutat. 2008;29:331–2. doi: 10.1002/humu.9521. [DOI] [PubMed] [Google Scholar]
- Guettard E, Portnoi MF, Lohmann-Hedrich K, Keren B, Rossignol S, Winkler S, et al. Myoclonus-dystonia due to maternal uniparental disomy. Arch Neurol. 2008;65:1380–5. doi: 10.1001/archneur.65.10.1380. [DOI] [PubMed] [Google Scholar]
- Gündel H, Wolf A, Xidara V, Busch R, Ceballos-Baumann AO. Social phobia in spasmodic torticollis. J Neurol Neurosurg Psychiatry. 2001;71:499–504. doi: 10.1136/jnnp.71.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündel H, Wolf A, Xidara V, Busch R, Ladwig KH, Jacobi F, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191:465–73. doi: 10.1097/01.NMD.0000081667.02656.21. [DOI] [PubMed] [Google Scholar]
- Heiman GA, Ottman R, Saunders-Pullman RJ, Ozelius LJ, Risch NJ, Bressman SB. Obsessive-compulsive disorder is not a clinical manifestation of the DYT1 dystonia gene. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:361–4. doi: 10.1002/ajmg.b.30431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Raymond D, Aguiar Pde C, Frucht S, Shriberg J, Heiman GA, et al. Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology. 2007;68:522–4. doi: 10.1212/01.wnl.0000253188.76092.06. [DOI] [PubMed] [Google Scholar]
- Hubsch C, Vidailhet M, Rivaud-Péchoux S, Pouget P, Brochard V, Degos B, et al. Impaired saccadic adaptation in DYT11 dystonia. J Neurol Neurosurg Psychiatry. 2011;82:1103–6. doi: 10.1136/jnnp.2010.232793. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach EC, Spears TE, Prewett MJ, Price ST, Jackson JG, Kirsh AD. Neuropsychiatric disorders, myoclonus, and dystonia in calcification of basal ganglia pathways. Biol Psychiatry. 1994;35:345–51. doi: 10.1016/0006-3223(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Lencer R, Steinlechner S, Stahlberg J, Rehling H, Orth M, Baeumer T, et al. Primary focal dystonia: evidence for distinct neuropsychiatric and personality profiles. J Neurol Neurosurg Psychiatry. 2009;80:1176–9. doi: 10.1136/jnnp.2008.170191. [DOI] [PubMed] [Google Scholar]
- Marechal L, Raux G, Dumanchin C, Lefebvre G, Deslandre E, Girard C, et al. Severe myoclonus-dystonia syndrome associated with a novel epsilon-sarcoglycan gene truncating mutation. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:114–17. doi: 10.1002/ajmg.b.10062. [DOI] [PubMed] [Google Scholar]
- Misbahuddin A, Placzek M, Lennox G, Taanman JW, Warner TT. Myoclonus-dystonia syndrome with severe depression is caused by an exon-skipping mutation in the epsilon-sarcoglycan gene. Mov Disord. 2007;22:1173–5. doi: 10.1002/mds.21297. [DOI] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Cyr L, Mountz JM. Obsessive and compulsive characteristics of craving for alcohol in alcohol abuse and dependence. Alcohol Clin Exp Res. 1992;16:272–4. doi: 10.1111/j.1530-0277.1992.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muller B, Hedrich K, Kock N, Dragasevic N, Svetel M, Garrels J, et al. Evidence that paternal expression of the epsilon-sarcoglycan gene accounts for reduced penetrance in myoclonus-dystonia. Am J Hum Genet. 2002;71:1303–11. doi: 10.1086/344531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardocci N, Zorzi G, Barzaghi C, Zibordi F, Ciano C, Ghezzi D, et al. Myoclonus-dystonia syndrome: clinical presentation, disease course, and genetic features in 11 families. Mov Disord. 2008;23:28–34. doi: 10.1002/mds.21715. [DOI] [PubMed] [Google Scholar]
- Peall KJ, Waite AJ, Blake DJ, Owen MJ, Morris HR. Psychiatric disorders, myoclonus dystonia, and the epsilon-sarcoglycan gene: a systematic review. Mov Disord. 2011;26:1939–42. doi: 10.1002/mds.23791. [DOI] [PubMed] [Google Scholar]
- Playford ED, Passingham RE, Marsden CD, Brooks DJ. Increased activation of frontal areas during arm movement in idiopathic torsion dystonia. Mov Disord. 1998;13:309–18. doi: 10.1002/mds.870130218. [DOI] [PubMed] [Google Scholar]
- Raymond D, Saunders-Pullman R, de Carvalho Aguiar P, Schule B, Kock N, Friedman J, et al. Phenotypic spectrum and sex effects in eleven myoclonus-dystonia families with epsilon-sarcoglycan mutations. Mov Disord. 2008;23:588–92. doi: 10.1002/mds.21785. [DOI] [PubMed] [Google Scholar]
- Ritz K, Gerrits MC, Foncke EM, van Ruissen F, van der Linden C, Vergouwen MD, et al. Myoclonus-dystonia: clinical and genetic evaluation of a large cohort. J Neurol Neurosurg Psychiatry. 2009;80:653–8. doi: 10.1136/jnnp.2008.162099. [DOI] [PubMed] [Google Scholar]
- Roze E, Apartis E, Clot F, Dorison N, Thobois S, Guyant-Marechal L, Tranchant C, et al. Myoclonus-dystonia: clinical and electro-physiologic pattern related to SGCE mutations. Neurology. 2008;70:1010–16. doi: 10.1212/01.wnl.0000297516.98574.c0. [DOI] [PubMed] [Google Scholar]
- Saugier-Veber P, Doummar D, Barthez MA, Czernecki V, Drouot N, Apartis E, et al. Myoclonus dystonia plus syndrome due to a novel 7q21 microdeletion. Am J Med Genet A. 2010;152A:1244–9. doi: 10.1002/ajmg.a.33369. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Ozelius L, Bressman SB. Inherited myoclonusdystonia. Adv Neurol. 2002a;89:185–91. [PubMed] [Google Scholar]
- Saunders-Pullman R, Shriberg J, Heiman G, Raymond D, Wendt K, Kramer P, et al. Myoclonus dystonia: possible association with obsessive-compulsive disorder and alcohol dependence. Neurology. 2002b;58:242–5. doi: 10.1212/wnl.58.2.242. [DOI] [PubMed] [Google Scholar]
- Scheidt CE, Waller E, Schnock C, Becker-Stoll F, Zimmermann P, Lücking CH, et al. Alexithymia and attachment representation in idiopathic spasmodic torticollis. J Nerv Ment Dis. 1999;187:47–52. doi: 10.1097/00005053-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Tezenas du Montcel S, Clot F, Vidailhet M, Roze E, Damier P, Jedynak CP, et al. Epsilon sarcoglycan mutations and phenotype in French patients with myoclonic syndromes. J Med Genet. 2006;43:394–400. doi: 10.1136/jmg.2005.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Misbahuddin A, Brancati F, Placzek MR, Garavaglia B, Salvi S, et al. Analysis of the epsilon-sarcoglycan gene in familial and sporadic myoclonus-dystonia: evidence for genetic heterogeneity. Mov Disord. 2003;18:1047–51. doi: 10.1002/mds.10476. [DOI] [PubMed] [Google Scholar]
- Valente EM, Edwards MJ, Mir P, DiGiorgio A, Salvi S, Davis M, et al. The epsilon-sarcoglycan gene in myoclonic syndromes. Neurology. 2005;64:737–9. doi: 10.1212/01.WNL.0000151979.68010.9B. [DOI] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Ann Med. 2009;41:344–59. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Wenzel T, Schnider P, Wimmer A, Steinhoff N, Moraru E, Auff E. Psychiatric comorbidity in patients with spasmodic torticollis. J Psychosom Res. 1998;44:687–90. doi: 10.1016/s0022-3999(97)00229-8. [DOI] [PubMed] [Google Scholar]
- Wong SH, Steiger MJ, Larner AJ, Fletcher NA. Hereditary myoclonus dystonia (DYT11): a novel SGCE gene mutation with intrafamilial phenotypic heterogeneity. Mov Disord. 2010;25:956–7. doi: 10.1002/mds.23037. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66–9. doi: 10.1038/ng709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.