SUMMARY
Repeated cocaine exposure causes persistent, maladaptive alterations in brain and behavior, and hope for effective therapeutics lies in understanding these processes. We describe here an essential role for fragile X mental retardation protein (FMRP), an RNA-binding protein and regulator of dendritic protein synthesis, in cocaine conditioned place preference, behavioral sensitization and motor stereotypy. Cocaine reward deficits in FMRP-deficient mice stem from elevated mGluR5 function, similar to a subset of Fragile X symptoms, and do not extend to natural reward. We find that FMRP functions in the adult nucleus accumbens (NAc), a critical addiction-related brain region, to mediate behavioral sensitization, but not cocaine reward. FMRP-deficient mice also exhibit several abnormalities in NAc medium spiny neurons, including reduced presynaptic function and premature changes in dendritic morphology and glutamatergic neurotransmission following repeated cocaine treatment. Together, our findings reveal FMRP as a novel mediator of cocaine-induced behavioral and synaptic plasticity.
INTRODUCTION
Recent evidence suggests that exposure to drugs of abuse, as well as other experiences (e.g. motor learning, novel environment and whisker trimming), alter synaptic connectivity by influencing both the strengthening and formation, as well as the weakening and elimination, of glutamatergic synapses. Drug-induced alterations in connectivity are likely important because they can be long-lasting and may underlie aspects of addiction-related behaviors (Lee et al., 2006; Li et al., 2004). In rodent models of drug use, increased dendritic spine density (Lee et al., 2006; Li et al., 2004; Norrholm et al., 2003; Robinson and Kolb, 1999) and synaptic strength (Kourrich et al., 2007) of dopaminoreceptive medium spiny neurons (MSNs) in the nucleus accumbens (NAc) have been widely reported. The presence, degree and longevity of these synaptic changes appear to depend upon several factors such as drug dose, length of exposure and withdrawal, MSN subtype and NAc subregion. However, factors controlling drug-induced synaptic plasticity remain elusive. Here we identify a new role for the RNA-binding protein, fragile X mental retardation protein (FMRP), as a key regulator of synaptic and behavioral alterations following exposure to cocaine.
The molecular and cellular mechanisms that control excitatory synapse number remain poorly understood. We and others have reported that the myocyte enhancer factor 2 (MEF2) family of transcription factors negatively regulates glutamatergic synapse and dendritic spine number in hippocampal neurons (Flavell et al., 2006). We extended these findings to the adult NAc in vivo, showing that MEF2 regulates the dendritic spine density of NAc MSNs basally, and that chronic cocaine administration can increase NAc MSN spine density, at least in part, by inhibition of MEF2 (Pulipparacharuvil et al., 2008; Zhang et al., 2012). Most recently, we reported that MEF2-induced synapse elimination in hippocampal neurons requires the RNA-binding capabilities of FMRP (Pfeiffer et al., 2010) and a complex molecular mechanism involving the MEF2 target gene, Pcdh10, and targeted degradation of the synaptic scaffold protein, PSD-95 (Tsai et al., 2012).
FMRP regulates the transport, stability, and/or translation rate of more than 850 brain mRNAs, many of which are linked to synaptic function (Darnell et al., 2011). Loss of FMRP expression in humans, as occurs in Fragile X syndrome (FXS), results in a variety of neurological symptoms, including sensory hypersensitivity, intellectual disability, and often, autism-associated behaviors and/or epilepsy (for review, see Hagerman, 2006). In dendrites, FMRP regulates local protein synthesis of associated mRNAs, at least in part, by stalling translation on polyribosomes (Ceman et al., 2003; Darnell et al., 2011; Stefani et al., 2004). Glutamate-dependent activation of group I metabotropic glutamate receptors (mGluR1 & 5) increases local protein synthesis through transient dephosphorylation and degradation of FMRP (Ceman et al., 2003; Nalavadi et al., 2012; Narayanan et al., 2007), so in part, FMRP serves as a critical gate to processes downstream of mGluR5, ensuring their activation occurs under appropriate conditions. Several studies have reported an important role for FMRP in regulating mGluR5-dependent synaptic weakening (long-term depression; LTD) (Huber et al., 2002; Narayanan et al., 2007; Weiler and Greenough, 1999), for example, and proper mGluR5 function is required for several cocaine-related behaviors (Chiamulera et al., 2001; Olsen et al., 2010). As such, we sought to test whether FMRP regulates cocaine-induced behavioral and synaptic plasticity in vivo.
RESULTS
FMRP is required for the development of cocaine-induced behavioral sensitization
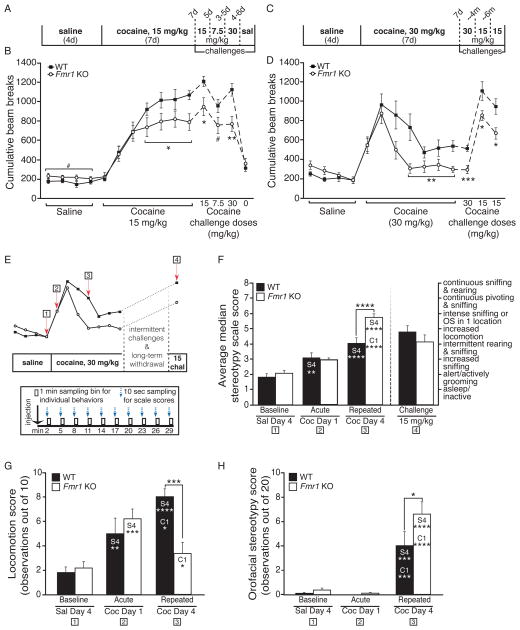
To test the role of FMRP in behavioral responses to repeated cocaine administration, we compared behavioral sensitization in Fmr1 knockout (KO) mice to wild-type (WT) littermates. Behavioral sensitization results from enduring and functional neuroadaptations in the NAc and reward-related circuitry that are hypothesized to underlie the transition to compulsive drug seeking and taking behavior in addiction (Koob and Volkow, 2010). Using either moderate or high doses of cocaine (daily i.p. injections at 15 or 30 mg/kg for seven days), we observed that, while both groups showed sensitization to cocaine, WT mice achieved significantly higher levels of behavioral sensitization than Fmr1 KO mice (Fig. 1A–D; see statistics listed in Table S1). Interestingly, cocaine injections on the first few days elicited similar locomotor responses in both WT and Fmr1 KO mice, suggesting that FMRP is not required for initial cocaine-induced activity or early sensitization, but is required for the mechanisms controlling maximal motor sensitization after repeated exposures. Once developed, reduced sensitized responses in Fmr1 KO mice were also observed after three to seven days of withdrawal, at several different cocaine challenge doses (7.5, 15 & 30 mg/kg; Fig. 1B & 1D), and at four and ten total months of withdrawal (Fig. 1D). In contrast, neither the sensitized locomotion to context alone (SAL; “0” challenge, Fig. 1B) nor the activity during non-injection trials (“habituation”; Fig. S1A & S1B) were different between genotypes.
Fig. 1. Impaired cocaine sensitization in Fmr1 knockout mice is dose-dependently associated with reduced locomotion and enhanced stereotypical behaviors.
(A) Timeline for 15 mg/kg COC sensitization. (B)Fmr1 KO mice show a significant deficit in sensitized locomotion to COC (15 mg/kg) compared to WT littermates (n=13 WT, 11 KO), while activity following the first (acute) exposure does not differ. Deficits are also present at all COC challenge doses: 15, 7.5, and 30 mg/kg, but not at SAL challenge. Fmr1 KO mice also show a trend towards significantly enhanced locomotor activity following SAL injections. (C) Timeline for 30 mg/kg sensitization. (D) Fmr1 KO mice show deficits in locomotor sensitization to 30 mg/kg COC that are not present acutely and that extend to challenges at different time points: 30 mg/kg at 1-wk withdrawal, and 15 mg/kg at 4- and 10-mos cumulative withdrawal (n=12 WT, 12 KO). Cumulative beam breaks for the first 20 min of each daily trial following injection are shown. (E) Composite illustration demonstrating time points (red arrows) for stereotypy analysis following injection (top). Blue arrows and black boxes (bottom) illustrate example time sampling method (see Experimental Procedures). (F) Average median stereotypy score for WT and Fmr1 KO littermates using a modified scale (Spangler et al., 1997) (OS=orofacial stereotypy). Fmr1 KO mice show a significantly higher score after repeated high-dose COC, but not after a lower dose challenge following long-term withdrawal (n=12 KO, 12 WT). (G) Hand-scored locomotor activity during stereotypy analysis. (H) The increased stereotypy observed in Fmr1 KO mice after repeated COC is largely made up of orofacial behaviors (composite score: paw-to-mouth movements + taffy pulling). Asterisks in F, G & H refer to significant follow-up Bonferroni post-hoc tests; column abbreviations denote within-group differences of the labeled bar compared to noted day (S4=SAL day 4, C1=COC day 1, C4=COC day 4). (# p<0.10, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001; data shown are mean±S.E.M.; see also Table S1 & Fig. S1.)
Since the observed group differences could result from hypersensitivity to cocaine-induced stereotypy in Fmr1 KO mice, we employed a time-sampling approach (Kelley, 1998) to evaluate saline- or cocaine-induced stereotypy in Fmr1 KO and WT littermates, scoring behaviors both individually and on a published scale (Spangler et al., 1997). While WT and Fmr1 KO littermates did not differ after repeated saline or acute, high-dose cocaine (30 mg/kg) on any stereotypy-related assessments, Fmr1 KO mice showed significantly greater stereotypy after repeated high-dose cocaine (Fig. 1F, “repeated”; for matching locomotion, see Fig. 1G & S1E). More detailed analysis showed that the heightened stereotypy involved primarily orofacial behaviors (i.e., taffy pulling and paws-to-mouth; Fig. 1H), but not grooming- or sniffing-related activities (Fig. S1F & S1G). In contrast, stereotypy was not heightened in Fmr1 KO mice following a lower dose challenge (15 mg/kg; Fig. 1F, “challenge”), even though locomotor activity remained significantly impaired (Fig. 1D, last challenge). To determine whether the increase in stereotypy at 30 mg/kg could reflect a general hypersensitivity to cocaine in Fmr1 KO mice, we also assessed sensitization using two lower doses of cocaine (5 and 10 mg/kg). While both genotypes sensitized to each dose, there were no locomotor differences between the groups (Fig. S1C & S1D), indicating that Fmr1 KO mice are not hypersensitive to cocaine, in general. Together, these data suggest that loss of FMRP impairs behavioral sensitization at moderate cocaine doses, but enhances the development of stereotypy at higher cocaine doses.
Deficits in the cyclic-adenosine monophosphate (cAMP) signaling cascade have previously been shown in Fmr1 KO mice (Wang et al., 2008), which could be relevant to our observations regarding sensitization. To address this possibility, we examined phosphorylation of the serine-845 site of GluA1 (glutamate receptor 1), a downstream target of this cascade, in Fmr1 KO and WT littermates after a cocaine dosing regimen similar to that used for sensitization testing. We observed a similar induction of P-S845 levels in WT and KO mice striata after acute or chronic cocaine (Fig. S1H), suggesting that a deficit in cocaine-induced activation of cAMP/PKA-dependent signaling in the striatum is unlikely to account for the behavioral alterations that we observed.
FMRP is required for the development of cocaine-related reward learning
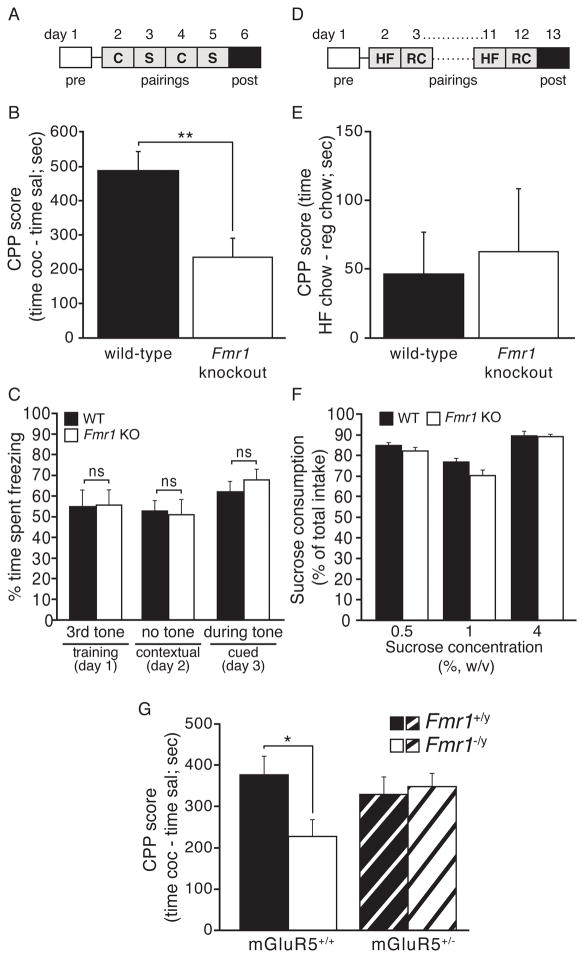
To test whether FMRP influences other types of cocaine-induced behaviors, we examined cocaine reward in a naïve cohort of WT and Fmr1 KO mice using the cocaine conditioned place preference (CPP) assay. In this assay, mice are injected with either cocaine (7.5 mg/kg) or saline and placed in one of two contextually distinct chambers on conditioning days. On pre- and post-test days, mice are placed into the center chamber and allowed to freely explore all three chambers for 20 minutes, and time spent in the cocaine-paired chamber minus the saline-paired chamber is defined as cocaine preference. Similar to the locomotor sensitization results, we observed a significant reduction in preference for the cocaine-paired chamber in Fmr1 KO mice (Fig. 2B), suggesting that FMRP is required for cocaine reward and/or learning and memory of context-drug associations. Similar to the behavioral sensitization findings, the cocaine reward deficit disappeared at a lower dose (5 mg/kg), where both WT and KO mice showed reduced, but comparable, preference for the drug-paired chamber (Fig. S2A). Consistent with demonstration of normal reward learning at lower doses of cocaine, we observed that Fmr1 KO mice showed normal context- and cue-induced freezing at 24 hrs after receiving three mild, aversive foot shocks in the standard fear conditioning assay (Fig. 2C, S2B), suggesting that Fmr1 KO mice are able to remember a salient stimulus-context association in a time-frame similar to that used in the cocaine CPP assay.
Fig. 2. FMRP-deficient mice have cocaine reward deficits that relate to mGluR5 hyperactivity and not to general deficits in natural reward processes or learning/memory.
(A) Timeline for CPP (C=COC, S=SAL). (B) Fmr1 KO mice show significantly decreased preference for a COC-paired context (7.5 mg/kg; n=11 WT, 12 KO). (C) Assessment of learning and memory in Fmr1 KO and WT mice using fear conditioning shows no differences in training, contextual or cued testing (n=11 KO, 12 WT). (D) Timeline for food CPP (HF=high fat, RC=regular chow). (E) Fmr1 KO and WT littermates show comparable preference for high fat chow (n=16 KO, 15 WT). (F) They also do not differ in two-bottle choice preference for sucrose (n=10 KO, 10 WT). (G) Compound mutant littermates show that impaired CPP in total Fmr1KO mice is rescued by a reduction in the mGluR5 receptor (n=29 WT/WT, 27 HET/WT, 22 WT/KO, 27 HET/KO). Asterisk in G indicates significant follow-up Bonferroni post-hoc test. (* p<0.05, ** p<0.01, *** p<0.001; data shown are mean±S.E.M.; see also Table S1 & Fig. S2.)
Given that Fmr1 KO mice model a clinical condition, FXS, we next asked whether deficits in cocaine reward behaviors would extend to natural rewards, which would have important implications for this patient population. To assess this possibility, we used the same cocaine CPP chambers to test WT and Fmr1 KO mice for palatable food-based CPP. Both genotypes showed equivalent preference for high-fat chow compared to regular chow (Fig. 2D & 2E). Similarly, we observed no genotype differences in the preference for sucrose, at multiple concentrations (Fig. 2F & S2C) in the standard 2-bottle choice test. Together these results suggest that FMRP is not required for all reward-related behaviors, and may be more specific to drug reward behavior.
A number of FXS-related phenotypes have now been attributed to hyperactivity or hypersensitivity of mGluR5 (Dölen et al., 2007), and manipulating mGluR5 activity has been shown to alter drug reward behaviors (Olive, 2009; Rutten et al., 2010). Therefore, we used a gene dosage approach to test whether mGluR5 hyperfunction might contribute to the observed deficit in cocaine reward behavior in Fmr1 KO mice. While mice lacking one copy of the mGluR5 gene (mGluR5+/−) had normal cocaine reward learning behavior, reducing mGluR5 levels did rescue the Fmr1 KO deficit (Fig. 2G), suggesting that Fmr1 KO impairments in cocaine CPP stem from mGluR5 hyperactivity. Taken together these results suggest that mice lacking FMRP have reduced sensitivity to the rewarding impact of higher-dose cocaine.
FMRP is required in the adult NAc for behavioral sensitization, but not drug reward
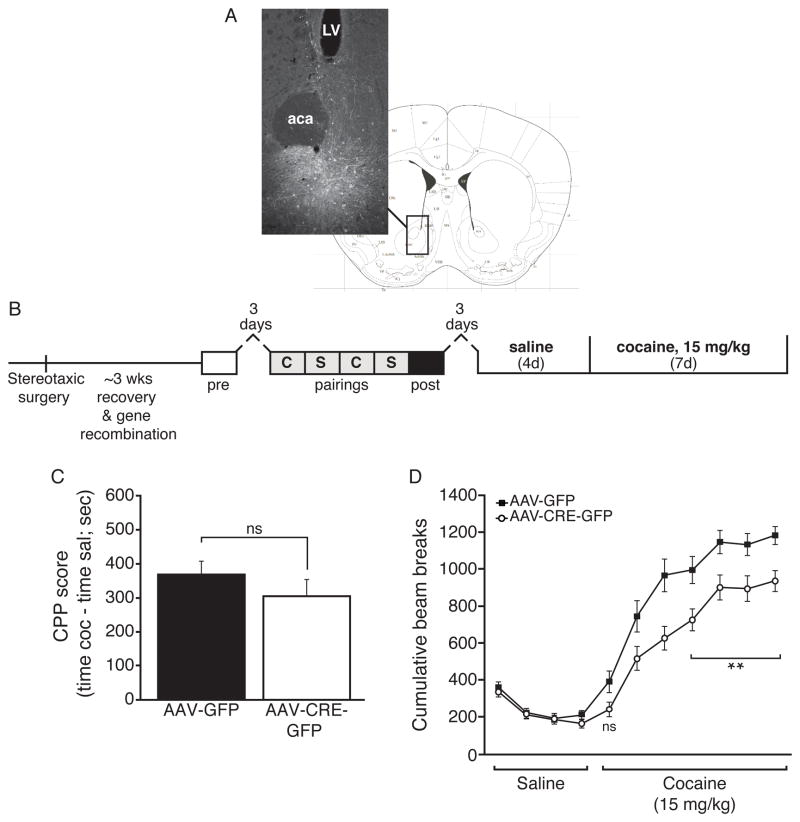
In the adult brain, the NAc is a critical brain region for the development and expression of cocaine reward and behavioral sensitization. Fmr1/FMRP is expressed in the adult NAc (see Allen Mouse Brain Atlas and Fig. S3C & S3D). To test whether FMRP in the adult NAc is required for the rewarding and behavioral effects of repeated cocaine administration, we performed bilateral injections of AAV2-Cre-GFP or AAV2-GFP control viruses in the NAc of adult floxed Fmr1 mutant mice (Mientjes et al., 2006) to generate conditional knockout of Fmr1 (NAc cKO) in the adult brain (Fig. 3A). The Fmr1 NAc cKO and control mice were then tested for cocaine reward and behavioral sensitization, as indicated (Fig. 3B). Neither conditional NAc deletion of Fmr1 (Fig. 3C), nor conditional deletion of Fmr1 in dopaminergic neurons (DAT-Cre:Fmr1fl/y, Fig. S3A), caused a significant deficit in cocaine reward behavior, suggesting that the observed cocaine reward deficit in Fmr1 KO mice is due to a role for FMRP in additional brain region(s) and/or to a developmental role for FMRP. In contrast, the Fmr1 NAc cKO mice showed a significant reduction in cocaine-induced behavioral sensitization (Fig. 3D & S3B) reminiscent of the effect observed in Fmr1 total KO mice after repeated cocaine exposure, suggesting strongly that FMRP plays an active role in the adult NAc to mediate the plasticity underlying behavioral sensitization.
Fig. 3. FMRP in the adult nucleus accumbens is required for normal behavioral sensitization to cocaine.
(A) Example intra-NAc stereotaxic injection with respect to the mouse brain atlas (Bregma 0.98 mm) (Paxinos and Franklin, 2001). (B) Timeline for viral stereotaxic surgery and testing. (C) Loss of NAc FMRP does not recapitulate the reduction in CPP observed in total Fmr1 KO mice (7.5 mg/kg; n=18 CRE, 22 GFP). (D) Floxed-Fmr1 conditional KO mice injected with AAV-CRE-GFP show a significant deficit in COC sensitization (15 mg/kg) compared to those injected with AAV-GFP, which is not significant at acute exposure (n=18 CRE, 22 GFP). Cumulative beam breaks for the first 20 min of each daily trial following injection are shown. (LV=lateral ventricle, aca=anterior commissure; ns=not significant; * p<0.05; data shown are mean±S.E.M.; see also Table S1 & Fig. S3.)
In contrast to other factors implicated in cocaine-induced behavioral plasticity that appear to be regulated by cocaine (e.g., ΔFosB), we did not observe any evidence that FMRP mRNA or protein levels were altered by repeated cocaine administration at 24 hours after the last injection of either 15 or 30 mg/kg (Fig. S3C & S3D). These findings indicate that FMRP’s function in mediating the normal development of behavioral sensitization does not likely involve changes in its basal levels. Taken together, our combined data suggest that FMRP functions in the adult NAc to mediate behavioral plasticity induced by repeated, high-dose cocaine, but its role in cocaine reward behavior is more complex.
FMRP limits cocaine-induced increases in structural alterations in the NAc
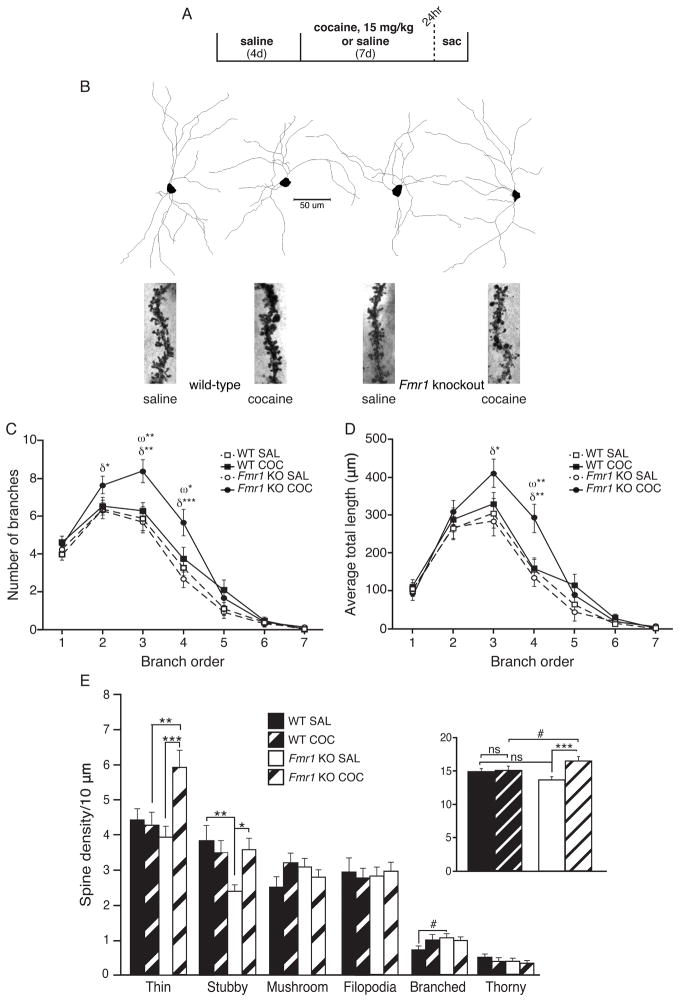
Numerous studies have reported that repeated, high-dose cocaine exposure induces structural and synaptic plasticity in MSNs of the NAc, and some of these changes have been proposed to support or antagonize cocaine behaviors. FMRP is known to regulate synapse weakening and to promote dendritic spine and excitatory synapse elimination (Pfeiffer et al., 2010; Weiler and Greenough, 1999), as well as to control proper dendritic branch extension and pruning (Thomas et al., 2008). To explore FMRP’s role in repeated cocaine-induced structural plasticity in the NAc, we injected WT and Fmr1 KO mice with repeated, daily cocaine or saline in the same manner as previously (i.e., 15 mg/kg daily COC injections × 7 days; Fig. 4A). We analyzed MSNs 24-hr after the last injection since: (1) maximal behavioral sensitization has been fully established by this time, (2) behavioral sensitization differences between WT and Fmr1 KO mice are established and persistent through short- or long-term withdrawal and (3) any relevant structural or functional changes in the NAc that regulate behavioral sensitization must also be present. Using a well-established Golgi staining procedure, we used stereomicroscopy to reconstruct MSNs from the NAc core or shell regions (Fig. 4B). In the core sub-region of Fmr1 KO mice, we observed that repeated cocaine induced a significant increase in the number and total length of dendritic branches induced by cocaine (Fig. 4C & 4D). In contrast, no significant changes were observed in shell MSN dendritic morphology for WT or Fmr1 KO mice after repeated cocaine exposure (Fig. S4A & S4B).
Fig. 4. FMRP opposes cocaine-induced changes in dendritic branch morphology and structural synapse number.
(A) Timeline for NAc shell and core MSN dendritic analyses. (B) Example MSN tracings and focused image stacks of spines from each group. (C) COC treatment significantly increased core MSN 2nd, 3rd and 4th order branches inFmr1 KO mice, but not WT littermates. Also, KO MSNs had significantly more 3rd and 4th order branches than WT MSNs after COC, but not SAL (n, cells=22 WT SAL; 23 WT COC; 22 KO SAL; 22 KO COC). (D) In keeping, the total length of 3rd and 4th order core MSN branches was increased by COC in Fmr1 KO, but not WT mice, and 4th order branch length was increased in KO compared to WT MSNs following COC, but not SAL. (E)An increase in overall spine density of NAc shell MSNs was seen in Fmr1KO, but not WT mice, following COC (inset). Analysis by spine type revealed a basal decrease in KO SAL stubby spine density compared to WT SAL, accompanied by a strong trend towards a significant increase in branched spines in KO compared to WT SAL-treated mice. COC treatment increased both stubby and thin spine densities in KO mice compared to SAL treatment. While COC treatment “rescued” a low basal density of stubby spines in Fmr1 KO mice to no different than either WT group, thin spine density was significantly elevated above that of WT COC. Symbols indicate significant simple main effects of genotype (ω) and treatment (δ). In E, asterisks refer to significant follow-up Univ ANOVAs. Focused images were created using an ImageJ Extended Depth of Field plug-in (Aguet et al., 2008) (http://imagej.nih.gov/ij/). (Scale bar=50 um; # p<0.10, * p<0.05, ** p<0.01, *** p<0.001, ns=not significant; data shown are mean±S.E.M.; see also Table S1 & Fig. S4.)
We next analyzed dendritic spine density in core and shell MSNs in both WT and Fmr1 KO mice. In the core, we observed no alterations in dendritic spine density in WT or KO mice after repeated cocaine administration; however, in the Fmr1 KO mice, there was a modest basal increase in core MSN overall spine density (Fig. S4C, inset), which reached significance for the thin spine category. Similar to the core, we observed no cocaine-induced changes in overall net dendritic spine density in shell MSNs from WT mice (Fig. 4E, inset). While others, including our group, have observed cocaine-induced changes in this region, we note that most published studies have used higher doses of cocaine (20–30 mg/kg COC) and longer administrations. In contrast to the WT littermates, repeated cocaine did significantly increase overall shell MSN spine density in Fmr1 KO mice (Fig. 4E, inset). Closer examination revealed that thin and stubby spine subtypes were increased following cocaine treatment in Fmr1 KO mice, whereas no spine subtypes were altered by cocaine in WT mice. Together, these findings suggest that FMRP normally functions to antagonize the formation of NAc dendritic spines and branching in response to cocaine exposure, which is consistent with its known role as a mediator of dendritic pruning and spine elimination (Pfeiffer et al., 2010; Weiler and Greenough, 1999). The findings reveal that an overall increase in NAc core or shell MSN spine density is not strictly required for the development and short-term expression of behavioral sensitization. Moreover, the precocious increase in dendritic spine density in NAc shell in the Fmr1 KO mice after repeated cocaine correlates with a decrease in sensitized locomotion after short-term withdrawal, similar to other several other reports (LaPlant et al., 2010; Norrholm et al., 2003; Pulipparacharuvil et al., 2008).
FMRP limits cocaine-induced increases in synaptic function in the NAc shell
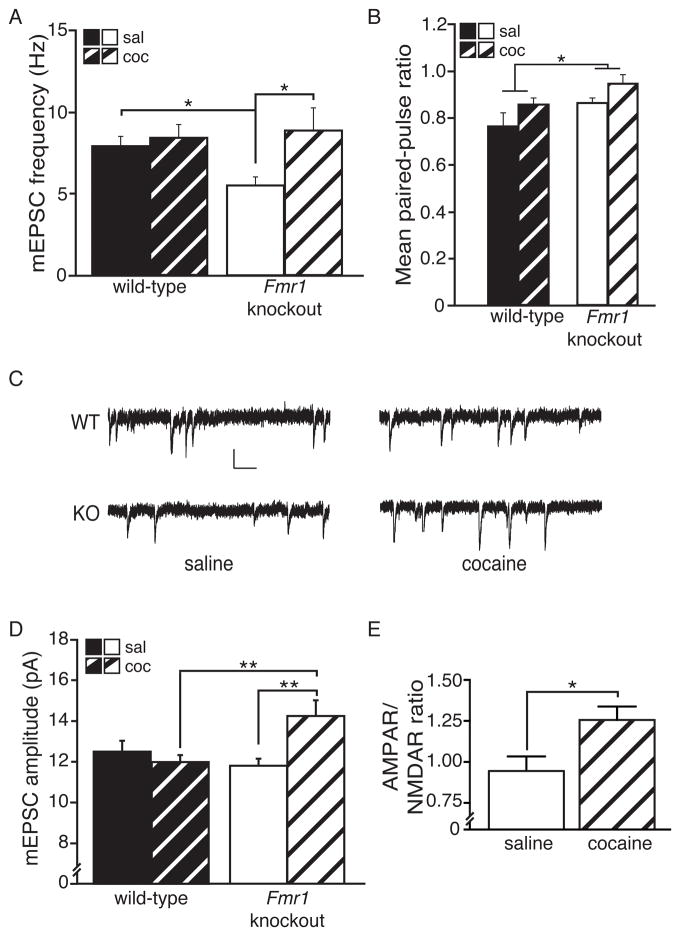
We next investigated the role of FMRP in regulating synaptic transmission in NAc shell MSNs by measuring miniature excitatory postsynaptic currents (mEPSCs) under the same conditions used to analyze behavioral sensitization and dendritic spine density. Consistent with our shell spine analysis (Fig. 4E), we failed to observe an effect of repeated cocaine on the mEPSC frequency in WT mice. However, similar to the dendritic spine observations, we found that repeated cocaine administration significantly increased mEPSC frequency in Fmr1 KO mice (Fig. 5A). Paired-pulse ratio (PPR) in Fmr1 KO mice was not significantly altered by repeated cocaine (Fig. 5B), suggesting that the observed increase in mEPSC frequency is likely due to an increase in dendritic spine synapses. Interestingly, we also observed that the mEPSC frequency was reduced in saline-treated Fmr1 KO mice when compared to saline-treated WT mice, suggesting a basal decrease in functional synapse number (e.g., as noted for stubby spine density, Fig. 4E) and/or a deficit in presynaptic function in the Fmr1 KO mice. Indeed, when we measured PPRs from WT and Fmr1 KO mice (Fig. 5B, S5A & S5B), Fmr1 KO mice showed an increased ratio, indicating a deficit in presynaptic release onto NAc shell MSNs compared to WT mice (Fig. 5B).
Fig. 5. FMRP opposes cocaine-induced changes in functional synapse number and synaptic strength.
(A) Similar to observations for overall spine density, the frequency of mEPSC events was observed to be uniquely susceptible to COC treatment (5 d, 15 mg/kg + 24 hrs) in Fmr1KO mice (n, cells=11 KO SAL, 16 KO COC, 13 WT SAL, 15 WT COC). Additionally, a significant basal decrease in mEPSC frequency was present in Fmr1 KO compared to WT mice (planned comparison). (B) Increased paired-pulse ratio (PPR) (i.e., decreased presynaptic release) was seen in Fmr1 KO compared to WT mice (inter-stimulus interval=100 msec; n, cells=9 KO SAL, 14 KO COC, 7 WT SAL, 11 WT COC). A trend was also present for COC to decrease presynaptic release. (C) Representative traces of mEPSCs from each group. (D) An overall significant interaction was observed for mEPSC amplitude, which was uniquely increased by COC treatment in Fmr1 KO mice and, among COC-treated mice only, KO had higher amplitude than WT. (E) COC also increased the AMPAR/NMDAR ratio in Fmr1 KO MSNs (n, cells=7 SAL, 11 COC). Asterisks in A & D indicate significant follow-up Bonferroni post-hoc tests, except planned comparison (Ind. Samples T-test). (Scale bar=10 pA/100 msec; * p<0.05; data shown are mean±S.E.M.; see also Table S1 & Fig. S5.)
Recent evidence also demonstrates that repeated cocaine administration alters post-synaptic function by increasing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) surface expression. To assess the effects of cocaine on post-synaptic AMPAR function in WT and Fmr1 KO mice, we measured and analyzed mEPSC amplitudes (Fig. 5D). Interestingly, chronic cocaine significantly increased mEPSC amplitude in the Fmr1 KO MSNs, but had no effect on mEPSC amplitude in WT MSNs at the 24-hr withdrawal time point. To gain additional confidence regarding the role of AMPAR-mediated transmission in the cocaine-induced increase in mEPSC amplitude in Fmr1 KO mice, we examined the ratio of AMPARs to N-methyl-D-aspartate receptors (NMDARs) in KO shell MSNs at this same time point (Fig. 5E). As expected, cocaine treatment increased the AMPAR/NMDAR ratio in Fmr1 KO mice. These findings suggest that FMRP antagonizes both spine density and AMPAR-mediated synaptic transmission in the NAc shell in response to chronic cocaine, and that in WT mice, cellular mechanisms that promote synapse strengthening and synaptogenesis are equally balanced, at least at this cocaine dose and time point, by synapse weakening and elimination processes that require FMRP.
DISCUSSION
Chronic exposure to drugs of abuse induces changes in synapse structure and function that are thought to underlie addiction-related behavioral plasticity. However, the cellular and molecular mechanisms that mediate behaviorally relevant plasticity in brain reward circuitry remain poorly understood. We show here that fragile X mental retardation protein (FMRP), an RNA binding protein that regulates dendritic pruning and synapse weakening/elimination, regulates these processes in NAc core and shell MSNs following cocaine exposure and is critically involved in the development of multiple cocaine-induced behaviors. Specifically, loss of FMRP impairs both cocaine-induced reward and behavioral sensitization, apparently via its function in multiple, possibly distinct brain regions. While FMRP is required acutely in the adult NAc for the development of behavioral sensitization, its absence either in the NAc or in dopaminergic neurons is not sufficient to impair cocaine reward. Instead, deficiencies in drug-induced reward appear to relate to general hyperactivity of mGluR5 function in Fmr1 KO mice, since genetic reduction of mGluR5 expression rescues this deficit. In FMRP-deficient mice, a sensitizing regimen of cocaine induces significant changes in NAc shell and core MSNs that are observable 24 hrs following the last exposure. In Fmr1 KO mice, repeated cocaine exposure increases the number of structural and functional synapses and synaptic strength of NAc shell MSNs, while core MSNs undergo cocaine-induced increases in dendritic branching and length. In contrast, despite robust behavioral sensitization, cocaine-induced changes in net NAc spine density and synaptic strength are not observed in WT mice at this time point and cocaine dose, suggesting that these changes, at least on the global NAc MSN population, are not required for the development and short-term expression of behavioral sensitization. In the process of describing FMRP’s novel role in cocaine-induced behavioral and synaptic plasticity, we also add to a small, but growing, literature defining striatal abnormalities in FXS, and reveal, in a unique setting, how experience-dependent synaptic plasticity goes awry in its absence—observations that may have relevance to FXS-associated motor, learning and behavioral abnormalities.
Our findings with regard to cocaine reward in Fmr1 KO mice are intriguing for several reasons. First, our mGluR5 genetic rescue study suggests that the reduced cocaine reward behavior in the Fmr1 KO mice is a result, at least in part, of elevated mGluR5 levels or function, a well-described feature of FXS (Bear et al., 2004; Osterweil et al., 2010). Genetic/pharmaceutical reduction of mGluR5 expression/activity reverses most, but not all, of the observed morphological, behavioral and functional plasticity deficits in FXS (Dölen et al., 2007; Pop et al., 2012); here we find that it also rescues cocaine reward dysfunction in Fmr1KO mice. Of note, mGluR5 antagonists are also being explored as potential therapeutics for addiction, based on their ability to reduce drug-motivated behaviors, such as CPP (for review, see Olive, 2009), although it is not yet clear whether they achieve this effect by reducing reward sensitivity or via reward substitution (Rutten et al., 2010). Our results that reducing mGluR5 levels in the Fmr1KO restores normal cocaine reward behavior suggest that mGluR5’s regulation of cocaine reward is non-linear, such that too much, too little or constitutive mGluR5 activity might alter drug reward sensitivity.
Given that FXS is associated with intellectual impairment in human patients and mice, we tested whether the cocaine reward deficit was a result of reduced learning/memory. However, Fmr1 KO mice made normal contextual associations with both mildly aversive and naturally rewarding stimuli, as well as with a lower dose of cocaine (i.e., 5 mg/kg). These findings are in general agreement with several other studies showing little or no learning impairments in Fmr1 KO mice on the C57BL/6 background (Dobkin et al., 2000; Paradee et al., 1999), and suggest that deficits in cocaine reward are not due to general defects in contextual learning. A second implication from our findings is the role of FMRP in brain reward function and plasticity. We did not observe evidence for reduced natural reward in Fmr1 KO mice, at least with respect to food-based incentives; however, analysis of other types of reward deficits that have relevance to FXS, such as social reward, will be important to explore in the future.
Our close examination of behavioral sensitization in Fmr1 KO mice highlights the complex inter-relationship between cocaine-induced locomotor sensitization and motor stereotypies. Since deficits in sensitized locomotion only became apparent in Fmr1 KO mice at higher doses (15 and 30 mg/kg) of cocaine and only after repeated exposures, we suspected that the reduced activity in Fmr1 KO mice could be a result of enhanced stereotypy behavior competing with locomotion. Indeed, the decrease in locomotion seen over daily repeated high-dose cocaine (30 mg/kg) in both genotypes is almost certainly a result of competing stereotypy that develops over several days of drug exposure, and at this high dose, Fmr1 KO mice develop enhanced stereotypy behavior more rapidly and/or to a greater extent than WT mice. However, increased stereotypy in Fmr1 KO mice was only observed at the highest dose (30 mg/kg), suggesting that the reduction in locomotion observed at 15 mg/kg is not a result of potentiated stereotypy. Furthermore, at lower doses of cocaine (5 and 10 mg/kg), Fmr1 KO mice show a similar development of behavioral sensitization to WT mice, arguing against a general hypersensitivity to cocaine that reduces locomotion at the 15 mg/kg cocaine dose. Together, these findings suggest that FMRP contributes to the normal development of both sensitized locomotion and stereotypy behaviors, but in a dose-sensitive manner. Previous studies have primarily attributed locomotor activation to the ventral striatum (including the NAc) and the development of stereotypy to the dorsal striatum (Joyce and Iversen, 1984); therefore, it seems likely that FMRP is regulating the development of sensitized locomotion in the NAc, consistent with our conditional Fmr1 KO data (Fig. 3D), but regulating the development of motor stereotypies in the dorsal striatum (or other regions). Notably, heightened repetitive or stereotypical behaviors are a central feature of FXS, and more broadly, of autism spectrum disorders. While we did not observe cocaine-independent differences in stereotypy between WT and KO mice, it is interesting to speculate that the enhanced cocaine-induced stereotypy behavior in Fmr1 KO mice results from subtle dysfunction in the striatal circuit that regulates repetitive motor behaviors. In the future, it will be interesting to examine how the role and regulation of FMRP in striatal sub-regions affects motor stereotypies.
Abnormalities in dendritic morphology have been repeatedly observed in both patients and animal models of FXS, including increased basal densities of (especially immature) dendritic spines (Hinton et al., 1991; Irwin et al., 2000; 2002; 2001; Kooy, 2003; Liu et al., 2011), and altered dendritic branch number and length (Guo et al., 2011; Lee et al., 2003; Restivo et al., 2005). However, basal spine density in FXS has, thus far, largely been studied in the cerebral cortex and hippocampus. We report here that FMRP-deficient MSNs, which are GABAergic projection neurons, show no basal spine density alterations in the NAc shell, and only a very small increase in the NAc core. While FMRP’s spine-regulating function in MSNs could be distinct from its role in pyramidal glutamatergic neurons, we also reason that basal spine density may be altered by resting-state activity in different brain regions. Many of FMRP’s functions are activity-dependent, including dendritic transport (Dictenberg et al., 2008), spine/synapse elimination (Pfeiffer et al., 2010) and LTD (Huber et al., 2002), and NAc MSNs have a highly negative resting membrane potential and require strong afferent activity to reach spike threshold (Pennartz et al., 1994). Despite the lack of strong basal differences in WT and Fmr1 KO MSNs, when a moderate, sensitizing regimen of cocaine is given, we see that FMRP-deficient mice produce, what appear to be, precocious net increases in branching and structural and functional synapse number, as well as synaptic strength. Specifically, we observed increases in thin and stubby spine subtypes in the NAc shell of Fmr1 KO mice at this short, 24-hr withdrawal time point, the same spine subtypes observed to be increased in WT rats after two weeks of withdrawal from similar cocaine exposure (Wang et al., 2013). Even though dendritic spine density did not change in the NAc core MSNs after cocaine, we note that the increase in branch complexity and length may translate to an overall increase in core dendritic spine number, as well. Similarly, the increases in synaptic strength (i.e., mEPSC amplitude and AMPA/NMDA receptor ratio) that were observed in NAc shell MSNs of Fmr1 KO mice following cocaine exposure are highly reminiscent of those typically observed in WT rodents after longer term (i.e., 7–21 day) cocaine withdrawal (Boudreau and Wolf, 2005; Kourrich et al., 2007).
The functional relevance of these precocious synapse-related changes in Fmr1 KO mice is intriguing since the WT, littermate control mice did not increase structural or functional synapses in the NAc shell (or structural in core), despite developing robust behavioral sensitization. Whether and how cocaine-induced spine density, in particular, in the NAc is related to drug-induced behaviors, especially behavioral sensitization and cocaine reward, remain long-standing and largely unresolved questions in the addiction field (Rothenfluh and Cowan, 2013; Russo et al., 2010). A number of previous studies, including one from our own lab, report molecular or genetic manipulations that regulate cocaine-induced dendritic spine density and behavioral sensitization. While some inversely alter (LaPlant et al., 2010; Norrholm et al., 2003; Taylor et al., 2007; Waselus et al., 2013), or even dissociate the two (Kiraly et al., 2010; Pulipparacharuvil et al., 2008; Wang et al., 2013), other manipulations positively regulate both spines and behavior (Dietz et al., 2012; Kelz et al., 1999; Maze et al., 2010). Similar to the Dnmt3a KO mice (LaPlant et al., 2010), Fmr1 KO mice show a selective increase in “thin” dendritic spines following cocaine treatment compared to WT mice, and in both studies this increase is associated with reduced behavioral sensitization. However, in all of the studies on this subject, the primary manipulation is specifically on the molecule/gene, which can have many cellular functions that influence behavioral sensitization independent of cocaine-induced changes in dendritic spines. Similar to our current findings, a recent report suggests that the dendritic spine density increase is not required for behavioral sensitization, but does underlie incentive sensitization (Wang et al., 2013), a process whereby cocaine experience increases later sensitivity to cocaine reward. A relationship between cocaine-induced alterations in NAc spine density and CPP has also been proposed (Dietz et al., 2012; Marie et al., 2012; Maze et al., 2010; Russo et al., 2009). While we did not specifically look at dendritic spine density changes in WT or Fmr1 KO mice under cocaine CPP conditions (e.g., 7.5 mg/kg cocaine × 2 injections), we suspect that changes in WT mice would not be observed under these low dose conditions; however, in the future, it will be important to test this idea more directly.
The lack of change in the AMPAR-mediated synaptic transmission of our WT mice treated with cocaine is consistent with previous studies showing that repeated daily cocaine administration does not increase overall NAc glutamatergic synaptic strength (synaptic AMPAR surface expression or AMPA/NMDA receptor ratio) in WT mice after short-term (i.e., 1 day) withdrawal (Boudreau and Wolf, 2005; Kourrich et al., 2007), and supports the idea that global changes in overall NAc glutamatergic synapse strength are not required for the short-term expression of behavioral sensitization (Boudreau et al., 2007). Instead, time-dependent increases in synaptic potentiation have been suggested to promote withdrawal-associated behaviors, such as incubation of drug craving (Conrad et al., 2008), as well as withdrawal-dependent locomotor sensitization following a single previous exposure (Pascoli et al., 2012); therefore, it may be important to investigate such behaviors in the Fmr1 KO mice in the future. Also, recent studies report that repeated cocaine triggers the generation of transient, silent synapses in the NAc after short-term withdrawal (Huang et al., 2009; Koya et al., 2012). It will be interesting to determine if FMRP contributes to the generation or duration of silent synapses in the future.
One interesting finding in our study is basal reduction in presynaptic function of glutamatergic inputs onto NAc shell MSNs in Fmr1 KO mice. While its presynaptic role is not well understood, FMRP is present in axons, including in presynaptic compartments, particularly in neurons undergoing high levels of activity-dependent plasticity, such as that seen during brain development, re-innervation following injury and adult neurogenesis (Akins et al., 2009; Christie et al., 2009). FMRP binds approximately one-third of the mRNAs known to encode proteins in the mouse presynaptic proteome (Darnell et al., 2011). Cocaine CPP was normal when FMRP was selectively deleted from either the adult NAc or in all DAT-expressing, dopaminergic neurons, suggesting that loss of presynaptic FMRP in NAc afferents, either during development or adulthood, might contribute to the cocaine reward deficit in the Fmr1 KO mouse. However, it is not yet clear whether or how the observed presynaptic function deficit in naïve Fmr1 KO mice impacts any of the observed behavioral or synaptic plasticity changes seen after cocaine administration. It will be interesting to investigate the potential contribution of presynaptic alterations in FMRP-deficient mice in the cocaine-induced behavioral and synaptic changes in future studies.
Overall, our data reveal a novel role for FMRP in the adult NAc to facilitate behavioral plasticity to repeated cocaine, possibly via its negative regulation of MSN structural and functional synapse number and strength during repeated cocaine exposure. Through this process, we help to elucidate FMRP’s role in the ventral striatum and how its absence alters experience-driven plasticity, which has implications for understanding the abnormal brain function and plasticity that contribute to symptoms of FXS. Our findings suggest that FMRP-dependent synapse weakening and elimination are important for the development of repeated cocaine-induced behavioral plasticity, and reveal an important role for FMRP, or one of its regulated processes (e.g., local protein synthesis, dendritic RNA trafficking, AMPAR endocytosis and LTD, MEF2-dependent synapse elimination, etc.) in the establishment of stable drug-induced behaviors. As such, FMRP, or one of its associated and regulated mRNAs, may be desirable therapeutic targets for the treatment of drug addiction.
Experimental Procedures
Detailed methods are given in the Supplemental Experimental Procedures.
Animals and drugs
Experimental procedures were approved by Institutional Animal Care and Use Committees at the University of TX Southwestern Medical Center, McLean Hospital or the University of MN. Cocaine HCl was dissolved in 0.9% NaCl (vehicle).
Locomotor sensitization
Activity was collected via photobeam arrays (San Diego Instruments, San Diego, CA) prior to and after injection. Mice received SAL for 3–4 days, and COC (5, 10, 15 or 30 mg/kg) for the next seven days. After one (10, 15 and 30 mg/kg) or two wks (5 mg/kg), a same-dose COC challenge was given, followed by additional challenges (see timelines & supplemental experimental procedures). Data shown are the sum of total beam breaks over the first 20 or 30 min of each trial.
Stereotypy analysis
During the indicated locomotor trials, mouse behavior was scored at regular intervals for 30 min following injection using a published scale (Spangler et al., 1997), and for specific behaviors (e.g., taffy pulling). Median scores (scale) or individual behavior sums for each mouse across the trial were used to calculate group averages.
Cocaine conditioned place preference
CPP (unbiased) consisted of a pretest, four alternating COC or SAL conditioning sessions, and a posttest, conducted on separate days. Food CPP was similar, except there were 12 alternating high fat or regular chow conditioning sessions. Data are expressed as time in the reward-paired chamber minus time in the control-paired chamber during the posttest.
Fear conditioning
Fear conditioning consisted of three 30-sec tones that co-terminated with a two-sec (0.5 mA) shock (training). On subsequent days, mice were re-exposed to the same context (contextual test) and to the tone in a new context (cued test). Freezing was hand-scored by an observer blind to genotype.
Sucrose preference
After acclimation, individually housed mice were given four 24-hr periods of access to water and 1% sucrose in identical sipper tubes (test). Additional tests (4% & 0.5%) were performed after short periods of normal water access.
Stereotaxic surgery
Anesthetized floxed-Fmr1 mice received either AAV2-GFP or AAV2-CRE-GFP (1 uL/hemisphere; Vector Biolabs, Philadelphia, PA) bilaterally to the NAc (D/L −4.4, M/L +1.5, A/P +1.6). CPP began 20–21 days after surgery (Fig. 3B).
Tissue collection and processing
Bilateral NAc and dorsal striata were individually dissected from coronal sections and processed by hemisphere for RT-PCR (Hale et al., 2011) or Western blotting. For Golgi staining, brains were processed according to the manufacturer (FD Neurotechnologies, Catonsville, MD), sliced (150 um) and mounted. For immunohistochemistry, anaesthetized mice were perfused intracardially with PBS and 4% PFA. Brains were postfixed, cryoprotected and sectioned (30 um).
cAMP signaling
Fmr1 KO and WT male mice were given five (1X/day; i.p.) injections. Exposure was considered acute (SAL on days 1–4 & SAL or COC on day 5; 20 mg/kg) or chronic (SAL or COC on days 1–5; 20 mg/kg). Mice were sacrificed 20 min after the last dose.
Western blotting
Equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes, which were blocked, rinsed and incubated with primary and HRP-conjugated secondary antibodies. Membranes were washed and developed using standard ECL and X-ray film.
Quantitative real-time PCR
RNA isolation, reverse transcription, and quantitative real-time PCR were carried out, as previously described (Hale et al., 2011). Fmr1 expression was determined by running reactions of 5 ng of cDNA and is expressed as fold change relative to GAPDH.
Dendritic analyses
Unobstructed MSNs of the NAc core or shell (2–6/subregion/mouse) were traced bilaterally (40X) using Neurolucida software (MBF Bioscience, Williston, VT). Spines were counted (100X) from a single 3rd-order or higher, complete terminal tip (≥20 uM) and categorized by type. Quantitative assessment was performed using NeuroExplorer software (MBF Bioscience).
Immunohistochemistry/injection placement
Sections were blocked and incubated with primary and secondary antibodies. Sections were counterstained, mounted and assessed for placement. Mice with GFP staining outside the NAc or with little/no GFP staining in the NAc were removed.
Electrophysiology
Mice received daily injections (2 SAL+5 SAL or COC; 15 mg/kg). 24 hrs after the last dose, sagittal slices containing the NAc shell were prepared as described (Thomas et al., 2001). Briefly, MSNs were identified by typical morphology and voltage clamped at −80 mV. Recordings were performed in the presence of picrotoxin (100um) to block GABAA-mediated IPSCs. To assess excitatory synaptic neurotransmission, mEPSCs (amplitudes & frequencies) were collected in the presence of 0.7 mM lidocaine. For AMPA/NMDA ratio measurements, NAc afferents were stimulated to evoke AMPAR- and NMDAR-EPSCs in MSNs voltage clamped at +40mv. The peak amplitude of AMPAR-mediated EPSCs was divided by the peak amplitude of NMDAR-mediated EPSCs. For PPR, two EPSC amplitudes were generated at −80 mV (inter-stimulus intervals: 20, 50, 100, & 200 msec). The peak amplitude of the 2nd EPSC (P2) was divided by the peak of the 1st (P1) to generate the PPR ratio (P2/P1).
Data analysis
Statistical analyses/results are listed in Table S1. Sensitization, stereotypy and dendritic spine density data were initially analyzed by Two- or Three-Way Repeated Measures (RM) ANOVAs for each experiment/NAc subregion, as appropriate. Within-subjects variables were Day and Spine Type, respectively, and between-subjects variables were Genotype alone or Genotype x Treatment. Dendritic branch number and length were analyzed by separate Three-Way RM ANOVAs for each NAc subregion, with Genotype and Treatment as between-subjects variables and Branch Order as the within-subjects variable. CPP scores were analyzed by One- or Two-Way ANOVAs, as appropriate. Overall spine density and P-Ser845 data were each analyzed by Two-Way ANOVAs. Significant interactions were followed by post hoc analyses or Multivariate (MV)/Univariate ANOVAs, as noted, to determine simple main effects. Locomotor challenge time points were analyzed by MV ANOVAs. All statistics were performed using either SPSS or GraphPad Prism software, remaining consistent within dataset. Significance was set at alpha=0.05. The Greenhouse-Geisser correction was applied whenever the data violated the sphericity assumption.
Supplementary Material
Acknowledgments
The authors thank Marissa Baumgardner, Lindsey Williams, Julia Wilkerson, Sarah Jenevein, Seth Hays and Katie Schaukowitch for technical assistance, Ami Pettersen and Lauren Peca for behavioral assistance, and Dr. David Nelson for generously sharing the floxed-Fmr1 conditional KO mouse line. We thank Rachel Penrod for insightful comments on the written manuscript. L.N.S. was supported by fellowships from NIDA (T32 DA07290 and F32 DA027265) and FRAXA Research Foundation. We also acknowledge the generous support of the Simons Foundation (SFARI grant to C.W.C. and K.M.H.), NIDA (DA008277, DA027664 and DA030590 to C.W.C. and DA019666, R21DA033457 and K02DA035459 to M.J.T.) and NINDS (NS062158 to M.J.T.).
Footnotes
Author Contribution Statement: Experiments were performed by L.N.S., J.P.J., M.R.F., C.F.H., F.S.T., K.C.D., M.T., B.C.Z., & S.G.B. Statistical analyses were performed by L.N.S. & J.P.J. Experimental designs were conceived primarily by C.W.C. & L.N.S., with input from S.G.B., J.P.J., K.C.D. & M.J.T. The manuscript was written by C.W.C., L.N.S. & J.P.J. with input from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura N. Smith, Email: lsmith@mclean.harvard.edu.
Jakub P. Jedynak, Email: jjedynak@mclean.harvard.edu.
Miles R. Fontenot, Email: miles.fontenot@utsouthwestern.edu.
Carly F. Hale, Email: carly.hale@utsouthwestern.edu.
Karen C. Dietz, Email: kcdietz@hotmail.com.
Makoto Taniguchi, Email: mtaniguchi@mclean.harvard.edu.
Feba S. Thomas, Email: fthomas77@gmail.com.
Benjamin C. Zirlin, Email: bzirlin@mclean.harvard.edu.
Shari G. Birnbaum, Email: shari.birnbaum@utsouthwestern.edu.
Kimberly M. Huber, Email: kimberly.huber@utsouthwestern.edu.
Mark J. Thomas, Email: tmhomas@umn.edu.
References
- Aguet F, Van De Ville D, Unser M. Model-based 2.5-d deconvolution for extended depth of field in brightfield microscopy. IEEE Trans Image Process. 2008;17:1144–1153. doi: 10.1109/TIP.2008.924393. [DOI] [PubMed] [Google Scholar]
- Akins MR, Berk-Rauch HE, Fallon JR. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits. 2009;3:17. doi: 10.3389/neuro.04.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29:1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, Idrissi ElA, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra Ben A, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hale CF, Dietz KC, Varela JA, Wood CB, Zirlin BC, Leverich LS, Greene RW, Cowan CW. Essential role for vav Guanine nucleotide exchange factors in brain-derived neurotrophic factor-induced dendritic spine growth and synapse plasticity. J Neurosci. 2011;31:12426–12436. doi: 10.1523/JNEUROSCI.0685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. Dissociable effects of 6-OHDA-induced lesions of neostriatum on anorexia, locomotor activity and stereotypy: the role of behavioural competition. Psychopharmacologia. 1984;83:363–366. doi: 10.1007/BF00428546. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Measurement of rodent stereotyped behavior. Current Protocols in Neuroscience. 1998;Chap. 8:8.8.1–8.8.13. doi: 10.1002/0471142301.ns0808s04. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy RF. Of mice and the fragile X syndrome. Trends Genet. 2003;19:148–154. doi: 10.1016/s0168-9525(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2011;14:618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie N, Canestrelli C, Noble F. Transfer of neuroplasticity from nucleus accumbens core to shell is required for cocaine reward. PLoS One. 2012;7:e30241. doi: 10.1371/journal.pone.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE3, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J Neurosci. 2012;32:2582–2587. doi: 10.1523/JNEUROSCI.5057-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Childs DS, Stanwood GD, Winder DG. Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5) PLoS One. 2010;5:e15085. doi: 10.1371/journal.pone.0015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop AS, Levenga J, de Esch CEF, Buijsen RAM, Nieuwenhuizen IM, Li T, Isaacs A, Gasparini F, Oostra BA, Willemsen R. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2947-y. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci USA. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin’? Curr. Opin Neurobiol. 2013;23:507–512. doi: 10.1016/j.conb.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, et al. Nuclear Factor B Signaling Regulates Neuronal Morphology and Cocaine Reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Van Der Kam EL, De Vry J, Bruckmann W, Tzschentke TM. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates conditioned place preference induced by various addictive and non-addictive drugs in rats. Addict Biol. 2010;16:108–115. doi: 10.1111/j.1369-1600.2010.00235.x. [DOI] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Behavioral stereotypies induced by “binge’ cocaine administration are independent of drug-induced increases in corticosterone levels. Behav Brain Res. 1997;86:201–204. doi: 10.1016/s0166-4328(96)02257-7. [DOI] [PubMed] [Google Scholar]
- Stefani G, Fraser CE, Darnell JC, Darnell RB. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J Neurosci. 2004;24:7272–7276. doi: 10.1523/JNEUROSCI.2306-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci USA. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CC, Combe CL, Dyar KA, Inglis FM. Modest alterations in patterns of motor neuron dendrite morphology in the Fmr1 knockout mouse model for fragile X. Int J Dev Neurosci. 2008;26:805–811. doi: 10.1016/j.ijdevneu.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature Neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Tsai NP, Wilkerson JR, Guo W, Maksimova MA, Demartino GN, Cowan CW, Huber KM. Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95. Cell. 2012;151:1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Martínez-Degollado M, González-Burgos I. Morphological development of dendritic spines on rat cerebellar Purkinje cells. Int J Dev Neurosci. 2011;29:515–520. doi: 10.1016/j.ijdevneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJS, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, et al. FMRP Acts as a Key Messenger for Dopamine Modulation in the Forebrain. Neuron. 2008;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, Loweth JA, Marinelli M, Russo SJ, Penzes P, et al. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. J Neurosci. 2013;33:11012–11022. doi: 10.1523/JNEUROSCI.1097-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Flagel SB, Jedynak JP, Akil H, Robinson TE, Watson SJ. Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats. Neuroscience. 2013;248C:571–584. doi: 10.1016/j.neuroscience.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Liu N, Wang B, Gu J, Zhang M, Zhou Z, Jiang Y, Zhang L, Zhang L. Signaling via dopamine D1 and D3 receptors oppositely regulates cocaine-induced structural remodeling of dendrites and spines. Neurosignals. 2012;20:15–34. doi: 10.1159/000330743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.