Abstract
A major controversy in memory research concerns whether recognition is subdivided into distinct cognitive mechanisms of recollection and familiarity that are supported by different neural substrates. Here we developed a new associative recognition protocol for rats that enabled us to show that recollection is reduced, whereas familiarity is increased following hippocampal damage. These results provide strong evidence that these processes are qualitatively different and that the hippocampus supports recollection and not familiarity.
Some of the most compelling data on recognition memory and hippocampal function involve the use of signal detection analyses. In these analyses, subjects are initially presented with a stimulus list and are then required to identify test stimuli as the same (old) items or different (new) stimuli across a range of confidence levels or response biases. In normal human subjects, the receiver operating characteristic (ROC) function for lists of single items is typically asymmetrical (featuring an above-zero y intercept), interpreted by some to reflect a threshold for recollection, and has a curvilinear shape, reflecting the strength of familiarity (the dual process model1). A major alternative view is that recognition is supported by qualitatively similar memory signals, wherein for each the degree of curvilinearity reflects the sum of the strengths of memory components and the asymmetry reflects greater variability in strength for old than for new items (the unequal variance model2). According to this latter view, familiarity and recollection differ only in sensitivity, such that familiarity reflects the detection of weaker memories, whereas recollection is experienced when memories are stronger or involve more information.
There is also compelling evidence indicating that recollection and familiarity may have distinct neural substrates, but the question of whether specific brain areas make qualitatively different contributions to recognition memory remains controversial1,3. Evidence from studies on amnesia in humans have contributed to, but not resolved, these controversies. Amnesia consequent to transient hypoxia associated with hippocampal damage results in a decrease in the asymmetry of the ROC function, reflecting a deficit in recollection, but the curvilinear shape is relatively spared, indicating that there is no effect on familiarity, whereas damage that reaches into the parahippocampal region results in deficits in both recollection and familiarity4,5. Our own ROC analyses, using an animal model where we definitively limited the damage to the hippocampus, resulted in a selective deficit in the same index of recollection (loss of asymmetry) and no impairment in familiarity (retained curvilinearity)6. However, deficits in both the asymmetry and curvilinearity of the ROC are also reported in amnesic patients with damage that is described as being limited to the hippocampus7. A similar controversy exists over the findings from functional imaging studies. Different studies have shown either recollection-specific activation of the hippocampus8 or activation that occurs more generally in medial temporal lobe areas associated with the strength of memory9.
A major difficulty in resolving the controversies concerning whether recollection and familiarity are qualitatively different processes and whether the hippocampus has a selective role is that the critical comparisons in the studies on both humans and animals rely on quantitative differences in memory performance or neural activation. Recent experiments, however, have suggested a way in which recollection and familiarity might be put into competition and, consequently, could be affected in opposite directions by hippocampal damage10. These studies focus on associative recognition, an experimental protocol in which the subjects are initially presented with a list of stimulus pairs and must distinguish the previously experienced (old) stimulus pairings from rearranged (new) pairs of the same stimulus elements. When the pairs are processed as separate stimulus elements, performance may depend largely on recollection of the acquired associations, as old and new pairs cannot be distinguished on the basis of differential familiarity for the individual elements3. Alternatively, when the elements of a pair are readily ‘unitized’ into a single configuration, such as when the elements are features of a face or parts of a compound word, familiarity can support memory for stimulus pairings just as it does for single stimuli11. Both kinds of processing can contribute to recognition, and here we asked whether selective experimental damage to the hippocampus would decrease the contribution of recollection, consistent with a diminished ability to associate the elements, and conversely increase the contribution of familiarity, consistent with an uncovering of the ability of other brain areas to unitize the stimuli.
We developed a version of the associative recognition protocol for rats, using stimulus pairs composed of combinations of an ordinary household odor (for example, lemon, thyme and cumin) mixed into a digging medium (for example, wood chips, beads and sand) contained in a cup (Fig. 1a). Rats can readily learn to separately attend to odors and media as distinct stimulus dimensions12, so we expected that substantial experience during initial training and testing with many combinations of the same stimulus elements would encourage the rats to distinguish these elements and rely on recollection of their associations (for example, lemon is associated with wood chips). Alternatively, odors and media could readily be unitized into scented medium configurations (for example, lemon-smelling wood chips), allowing the use of familiarity to make recognition judgments.
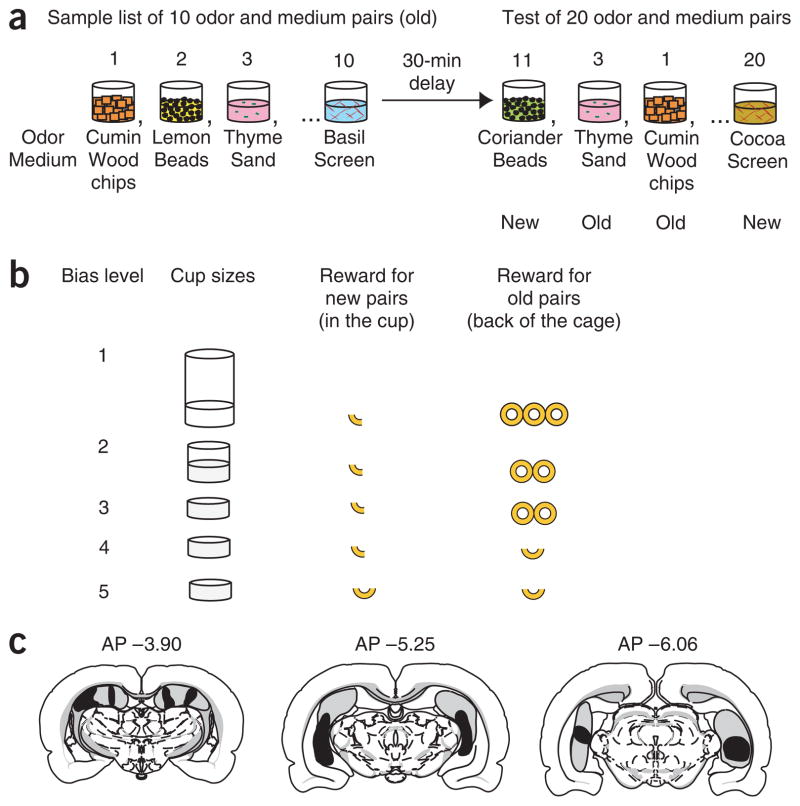
Figure 1.
Testing associative recognition in rats. (a) Associative recognition protocol with examples of combinations of odors and media used in the sample and test phases of the task. (b) Five levels of response bias were generated by varying the cup sizes and the amount of reward. (c) Extent of the hippocampal lesion. Dark gray, smallest lesion; light gray, largest lesion. Lesions were carried out under isoflurane anesthesia (1%) by radiofrequency (7–11 mA, 1 min, Radionics RFG-4A) using a 100-um nichrome electrode (0.7-mm noninsulated tip) that was lowered in the brain at 12 sites bilaterally. Area measurements revealed that animals lost 38 ± 5% of the hippocampus. Two hippocampal animals had slight damage to the medial geniculate nucleus. Control subjects were given a sham operation in which the electrode was lowered only into the cortex directly above the hippocampus. All procedures were approved by the Boston University Institutional Animal Use Committee.
On each daily training session, a series of 10 odor-medium pairings was initially presented (Fig. 1a). Following a 30-min delay, rats then distinguished between the 10 original (old) pairs and 10 rearranged (new) pairings of the same odors and media presented one at a time in a target cup. According to a ‘nonmatching’ rule, when the rat recognizes an old pair, it must refrain from digging in the target cup and can obtain a reward from an alternate cup at the opposite end of the chamber (a ‘hit’). Conversely, when a new pairing is presented, the animal can dig in the target cup for a reward. A false alarm is scored when the rat incorrectly responds as if the stimulus pair was old. On each session, the response bias was manipulated using one of five combinations of target cup size and ratio of rewards that encouraged choosing the target or alternate cup (Fig. 1b). The ratio of hits and false alarms was averaged across five repetitions of each bias level to determine the ROC function for each of seven sham-operated rats and eight rats with selective hippocampal damage (see Fig. 1c and Supplementary Methods online).
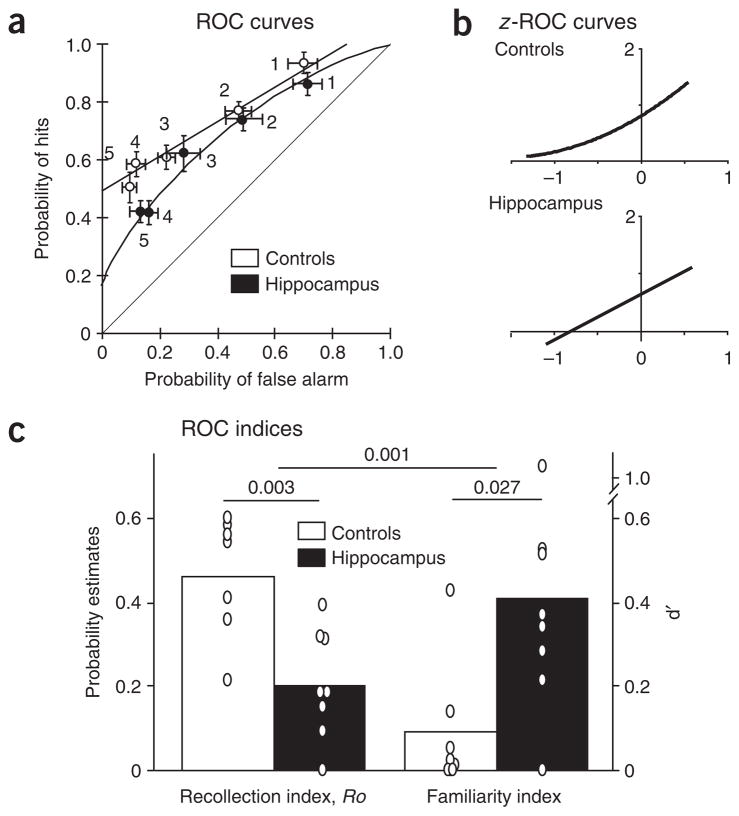
Analyses of the behavioral data were carried out using the dual process model, the unequal variance model and simple regression analyses that are independent of both models. Our primary aim was to determine whether the dual process model would be confirmed by observing qualitatively distinct effects of selective hippocampal damage on recollection and familiarity, or whether the single process model would be supported by observing qualitatively similar effects on both components of the recognition performance. First, we examined whether the predictions of the dual process model would be confirmed by observing performance largely on the basis of recollection in control rats and diminished recollection with enhanced familiarity in rats with selective hippocampal damage. The model analysis indicated that control rats relied mainly on recollection to solve tasks, as shown by a positive index of recollection Ro (y intercept = 0.49, Fig. 2a) and the absence of curvilinearity (familiarity index d′ = 0.0, Fig. 2a), indicating that there was no contribution from familiarity. Characterization of the ROC function as linear was confirmed by observation of a curvilinear function when the data were z-transformed (z-ROC quadratic coefficient significantly different from 0; t6 = 3.792, P = 0.009, Fig. 2b). Hippocampal damage reduced performance on the basis of recollection, reflected in a significant reduction of the recollection index Ro (y intercept = 0.17, t13 = 4.47, P = 0.003; Fig. 2a,c). In addition, and in marked contrast to control subjects, the ROC function for animals with hippocampal damage was curvilinear, as confirmed by an above-zero familiarity index (d′ = 0.55, Fig. 2a,c) and a linear z-ROC function (quadratic coefficient not different from 0; t7 = 1.385, P = 0.209, Fig. 2b). Furthermore, a direct between-group comparison of d′ and Ro scores (see Supplementary Methods) confirmed that damage to the hippocampus had statistically significant opposite effects on familiarity and recollection (interaction: F1, 26 = 25.92, P = 0.001; post hoc d′, P = 0.027; Ro, P = 0.003). Taken together, these results indicate that hippocampal damage produces a deficit in recollection and a complementary enhancement of familiarity. These findings, indicating qualitatively different recognition strategies, are all the more notable considering that overall performance, measured by the percent correct across all bias levels, did not significantly differ between the groups (sham 68 ± 2% and hippocampal 62 ± 1%, t13 = 1.14, P = 0.28).
Figure 2.
ROC functions for associative recognition. (a) Control subjects employed recollection primarily, as indicated by an asymmetrical and linear ROC function. Rats with hippocampal damage were impaired in recollection and showed enhanced familiarity, reflected in a decrease in the y intercept and the appearance of a curvilinear ROC function (± s.e.m.). (b) The linearity of the ROC function for control subjects was confirmed by observation of a curvilinear function in z-space. Conversely, the curvilinearity of the ROC function for rats with hippocampal damage was confirmed by a linear z-ROC function. (c) Hippocampal damage had opposite effects on indices of recollection (Ro) and familiarity (d′). Bars represent means; 0 = individual score; P values for the main effects and interaction are indicated across indices. Controls, sham-operated animals; hippocampus, rats with hippocampal damage.
We then assessed the extent to which the unequal variance model could also fit the data, and we compared the predictions of both models using a model-independent regression analysis. Consistent with a large body of literature2,3, a goodness of fit analysis showed that the unequal variance model fit the behavioral data well (χ2control = 0.29 and χ2hippocampus = 0.44, where P < 0.05 requires χ2[4] ≥ 9.49), and indeed revealed a deficit in rats with hippocampal damage (t13 = 3.01, P = 0.010). Notably though, the dual process model provided a slightly better fit (χ2control = 0.19 and χ2hippocampus = 0.34). Moreover, direct comparisons using model-independent linear and quadratic regression analyses supported the predictions of the dual process model and not those of the unequal variance model. The ROC function of control subjects was linear (no significant alteration of the curve by adding a quadratic component, R2quadr compared with R2lin, t6 = 1.36, P = 0.225; Supplementary Fig. 1 online). In contrast, the ROC function of rats with hippocampal damage was curvilinear, as confirmed by a significant alteration of the function by adding a quadratic component to the equation (R2quadr compared with R2lin, t7 = 3.05, P = 0.019; Supplementary Fig. 1). Furthermore, the y intercept of the regressions was lower in the rats with hippocampal damage than in the controls, regardless of whether the ROCs are fit with linear or quadratic models (all P’s < 0.040; Supplementary Fig. 1). The observation of a linear ROC is inconsistent with the unequal variance model. Furthermore, this model cannot account for the opposite effects of hippocampal damage on the curvilinearity (higher than controls) and y intercept (lower) of the regressions. In contrast, this combination of findings is fully consistent with the predictions of the dual process model2,3.
These results provide the first evidence that rats, like humans, can rely primarily on recollection in associative recognition. The findings also show that control rats and rats with hippocampal damage can perform at a similar overall level on an associative-recognition task by using different strategies, with the control subjects relying mainly on recollection and rats with hippocampal damage relying principally on familiarity. The present findings do not directly show that the observed decrease in recollection and increase in familiarity in rats with hippocampal damage is a result of an enhanced tendency to unitize the stimulus elements. However, other recent studies have reported that manipulations that encourage unitization of the stimulus elements can facilitate the use of familiarity, and consequently reduce the deficit in amnesic patients that is normally observed in associative recognition13. Also, previous studies have shown that hippocampal damage increases the tendency to unitize stimulus elements into configural stimuli14. For example, rats with hippocampal damage tend to unitize pairs of odor stimuli that are presented in close juxtaposition in simultaneous discrimination problems, and they subsequently perform poorly when required to identify individual stimuli selected from different pairs. Also, in monkeys, the explicit learning of visual stimulus configurations is facilitated over that of normal animals by damage limited to the hippocampus, whereas configural representation is severely impaired following damage to the perirhinal cortex15. Consistent with these findings, we suggest that rats with hippocampal damage and preserved perirhinal function have an increased tendency to unitize the elements of stimulus pairs, allowing them to employ familiarity as a compensatory strategy for distinguishing new and old pairs.
The present results provide the first evidence that recollection and familiarity are qualitatively dissociable and distinctively affected by hippocampal damage. The pattern of opposite effects on recollection and familiarity cannot be explained by models in which recollection and familiarity involve qualitatively similar processes contributing to a continuous memory strength signal2,9. Furthermore, these results are inconsistent with the view that the hippocampus supports both recollection and familiarity. Instead, these findings provide compelling evidence that the hippocampus and other areas (such as the perirhinal cortex) make distinct and complementary contributions to memory.
Supplementary Material
Acknowledgments
We thank C. Ergorul, L. Devito and N. Simuro for help with behavioral testing, and L. Ho and S. Hattori for assistance with histological processing. This work was supported by US National Institute of Mental Health grants MH52090 and MH71702.
Footnotes
AUTHOR CONTRIBUTIONS
M.M.S. designed and conducted the experiment and data analyses, and wrote the manuscript. N.J.F. consulted on data analyses, and A.P.Y. consulted on data analyses and manuscript preparation. C.B.O. participated in conducting the experiment. H.E. supervised the project and participated in writing the manuscript.
Reprints and permissions information is available online at http://npg.nature.com/reprints and permissions
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Yonelinas AP. Phil Trans R Soc Lond B. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wixted JT. Psychol Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- 3.Parks CM, Yonelinas AP. Psychol Rev. 2007;114:188–202. doi: 10.1037/0033-295X.114.1.188. [DOI] [PubMed] [Google Scholar]
- 4.Yonelinas AP, et al. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 5.Aggleton JP, et al. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Fortin NJ, Wright SP, Eichenbaum H. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wais PE, Wixted JT, Hopkins RO, Squire LR. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H, Yonelinas AR, Ranganath C. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squire LR, Wixted JT, Clark RE. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovanello KS, Keane MM, Verfaellie M. Neuropsychologia. 2006;44:1859–1865. doi: 10.1016/j.neuropsychologia.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quamme JR, Yonelinas AP, Norman KA. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- 12.Birrell JM, Brown VJ. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinitz MT, Alexander R. Mem Cognit. 1996;24:129–135. doi: 10.3758/bf03200875. [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H. In: Memory Systems 1994. Schacter DL, Tulving E, editors. MIT Press; Cambridge, Massachusetts: 1994. pp. 147–202. [Google Scholar]
- 15.Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. Cereb Cortex. 2007;17:108–115. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.