Abstract
Recent research has found that individuals with posttraumatic stress disorder (PTSD) exhibit an impaired memory of fear extinction compounded by deficient functional activation of key nodes of the fear network including the amygdala, hippocampus, ventromedial prefrontal cortex (vmPFC) and dorsal anterior cingulate cortex (dACC). Research has shown these regions are sexually dimorphic and activate differentially in healthy men and women during fear learning tasks. To explore biological markers of sex differences following exposure to psychological trauma, we used a fear learning and extinction paradigm together with functional magnetic resonance imaging (fMRI) and skin conductance response (SCR) to assess 31 individuals with PTSD (18 women; 13 men) and 25 matched trauma-exposed healthy control subjects (13 women; 12 men). Whereas no sex differences appeared within the trauma-exposed healthy control group, both psychophysiological and neural activation patterns within the PTSD group indicated deficient recall of extinction memory among men and not among women. Men with PTSD exhibited increased activation in the left rostral dACC during extinction recall compared with women with PTSD. These findings highlight the importance of tracking sex differences in fear extinction when characterizing the underlying neurobiological mechanisms of PTSD psychopathology.
Keywords: PTSD, Trauma, Gender, Sex, Fear, Extinction, Recall, dACC
1. Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that develops following exposure to psychological trauma (American Psychiatric Association [APA], 2013), with women facing higher risk for developing PTSD than men (Breslau & Anthony, 2007; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Olff, Langeland, Draijer, & Gersons, 2007; Tolin & Foa, 2006). Although the mechanisms underlying increased PTSD risk in women are not fully understood, research has identified the potential role of sexually dimorphic neurobiology in the stress systems of animals (Dalla & Shors, 2009; Graham & Milad, 2013; Milad, Igoe, Lebron-Milad, & Novales, 2009; Zeidan et al., 2011) and humans (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Goldstein, Jerram, Abbs, Whitfield-Gabrieli, & Makris, 2010; Goldstein et al., 2001; Graham & Milad, 2013; Lebron-Milad et al., 2012; Milad et al., 2006, 2010; Stevens et al., 2013). Recent work suggests that varying levels of female gonadal hormones, particularly estrogen, may influence fear extinction in women with PTSD (Glover et al., 2012; Lebron-Milad, Graham, & Milad, 2012). Specifically, women with PTSD who have low estradiol levels exhibit impaired extinction learning (Glover et al., 2012). No study has yet applied neuroimaging and psychophysiology to examine sex differences in fear conditioning, extinction, and recall in PTSD.
Fear conditioning and extinction paradigms have proven extremely valuable in revealing underlying circuitries of anxiety pathology and for understanding the development and maintenance of PTSD (Pitman et al., 2012; Shvil, Rusch, Sullivan, & Neria, 2013). Recent evidence specifically associates PTSD with impaired capacity to recall extinction memory, demonstrated by increased skin conductance levels to previously extinguished conditioned stimuli (Milad, Pitman, et al., 2009). Neuroimaging studies of PTSD using fear conditioning and extinction paradigms have reported functional and structural irregularities in neural regions demonstrated in the preclinical literature to mediate conditioned fear and extinction, including the ventromedial prefrontal cortex (vmPFC) and hippocampus (Milad & Quirk, 2012). During extinction recall, patients with PTSD show reduced activation in the hippocampus and vmPFC compared with trauma-exposed healthy controls, whereas dorsal anterior cingulate (dACC) and amygdala activity are greater (Milad & Quirk, 2012).
Importantly, activity in key fear network regions appears to be sexually dimorphic, with distinctive neural activations in these regions between healthy men and women when under acute stress (Goldstein et al., 2010) and during fear learning tasks (Cahill et al., 2004; Glover et al., 2012; Goldstein et al., 2001; Zeidan et al., 2011). For example, Lebron-Milad et al. (2012) found greater right rostral anterior cingulate (rACC) activity differences in healthy women than healthy men during extinction recall. However, the two groups did not differ significantly in skin conductance response (SCR) during extinction recall (Lebron-Milad et al., 2012). Among PTSD patients, Inslicht et al. (2013) recently reported significantly greater acquisition of conditioned fear (higher SCR) in women with PTSD compared to men with PTSD.
To clarify the potential role of sexually dimorphic neurobiology and psychophysiology in PTSD, the present pilot study explored sex differences during conditioning, extinction learning, and extinction recall using event-related fMRI and SCR in PTSD patients and matched trauma-exposed healthy control participants.
Based on previous studies, it was predicted that across the trauma-exposed healthy controls (TE-HC) and PTSD groups, men would exhibit better extinction recall than women as measured by SCR, with top-down control manifested by greater activation in the vmPFC and hippocampus, as well as diminished activation in the amygdala and dACC compared with women during extinction recall.
2. Material and methods
2.1. Participants
PTSD patients (18 females and 13 males) and TE-HCs (13 females and 12 males) subjects were recruited via advertisement and fliers. All participants met PTSD criterion A1 for adult traumatic events, including vehicular accidents, sexual or physical assaults, and witnessing serious injuries or deaths. Medical history, review of systems, physical examination, and laboratory tests determined participant health status. Raters with reliability training in psychometric assessments administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995) and the Clinician-Administered PTSD Scale (CAPS; Weathers, Keane, & Davidson, 2001) to assess PTSD diagnosis and clinical severity. For PTSD subjects, exclusion criteria included substance/alcohol dependence within the past six months or abuse within past two months, use of any psychotropic medication 4 weeks prior to participation (6 weeks for fluoxetine), a Hamilton Depression Rating Scale (HAM-D-17; Hamilton, 1960) score greater than 24, or a CAPS score less than 50. Exclusion criteria for TE-HC subjects were current or past Axis I disorders including substance use disorders, and CAPS scores greater than 19. The New York State Psychiatric Institute Institutional Review Board approved all procedures, and all participants provided written informed consent.
2.2. Task procedures
The protocol employed an established two-day fMRI fear conditioning and extinction paradigm (See Fig. 1; Milad, Orr, Pitman, & Rauch, 2005; Milad et al., 2007). On Day 1, subjects participated in the conditioning and extinction phases of the paradigm. On the following day (Day 2), subjects were tested for level of extinction recall. Digital images of two different rooms served as the visual contexts (CXs) within which the conditioned stimuli (CSs) were presented. A lamp, which turned on and off, served as the cue and CSs were differentiated by the color of the light (e.g., red, blue, and yellow). The unconditioned stimulus (US) was a 500 ms shock delivered via electrodes attached to the second and third fingers of the dominant hand.
Fig. 1.
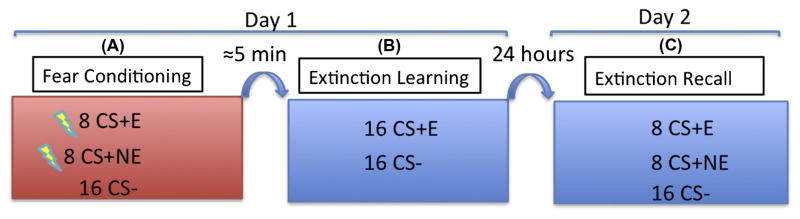
Schematic diagram of the experimental design. (A) Fear conditioning phase, two CSs (e.g., red light and blue light) were paired with a shock (CS+E, CS+NE) at a partial reinforcement rate of 60%. A third CS (e.g., a yellow light) was never paired with a shock (CS−). This phase consisted of 32 trials: 8 CS+E, 8 CS+NE, and 16 CS−. (B) Extinction learning phase, the CS+E was presented in absence of a shock along with the CS− for 32 trials: 16 CS+E and 16 CS. The CS+NE was not presented in this phase. On Day 2, the (C) Extinction Recall phase 32 trials: 8 CS+E, 8 CS+NE, and 16 CS− were presented in the absence of a shock. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The US intensity was determined by a calibration procedure for each participant, after announcing that a mild electric stimulus would be used and reminding the participant that he or she could terminate the experiment at any time. Participants were instructed: “For this experiment, you will set your own level of electric stimulation. You should choose a level that is highly annoying, but not painful. I will start the stimulation at a very low level and gradually increase the level until you say ‘stop.’ The level that you set will then be used throughout the remainder of the experiment.” The technician then recorded the participant-selected intensity level, ranging from 0.8 to 4.0 mA. A habituation phase followed the US calibration, consisting of 12 CS presentation trials, presenting the two to-be CS+s and the to-be CS− (4 of each of the three CS types) in counterbalanced trials.
The conditioning phase paired each of the two CS+s with the US at a partial reinforcement rate of 60% in the conditioning CX. One of the two CS+s was then extinguished during the subsequent extinction phase (CS+E), whereas the other was not extinguished (CS+NE). A third CS presented during the conditioning phase was never paired with the US (CS−). As in Milad et al. (2007), the study presented CSs in the following order: eight trials of the first CS+ were intermixed with 8 trials of the CS−, followed by eight trials of the second CS+ intermixed with 8 additional trials of the CS−. The shock (US) in the reinforced trials was delivered immediately following the CS+ offset, with no delay between CS offset and US onset. The shock electrodes remained attached to the subject’s fingers throughout subsequent phases of the experiment, and subjects were told throughout (except during the habituation phase), “You may or may not receive an electric shock.” However, shocks were only delivered during the conditioning phase.
The extinction-learning phase began approximately five minutes after the conditioning phase ended. 16 CS+E and 16 CS− trials were presented in intermixed fashion, without any shock, in a novel extinction CX. On Day 2, in the extinction recall phase, participants were presented with intermixed trials of the 8 CS+E, 8 CS+NE, and 16 CS− in the extinction (safe) CX. Within each of the three phases (conditioning, extinction learning, and extinction recall), each of the 32 trials involved presentation of the context picture for 9 s: 3 s alone, followed by 6 s in combination with the CS+E, CS+NE, or CS−. The mean inter-trial interval was about 15 s (range: 12–18 s).
2.3. fMRI acquisition
Images were acquired on a 1.5 TGE Twin SpeedMRScanner operating on the Excite 3 12.0 M4 HD platform using a 1-channel head coil. An Integrated Functional Imaging System (IFIS, MRI Devices Corp.) synchronized the behavioral paradigm with scanning and conditioned stimuli presentation. A high-resolution T1-weighted 3D MPRAGE sequence (TR/TE/Flip angle = 7.25 ms/3 ms/7°; 1 × 1 mm in plane × 1.3 mm) followed for spatial normalization and anatomical localization. Functional MRI images (i.e., blood oxygenation level dependent, BOLD) were acquired using gradient echo T2*-weighted sequence (TR/TE/Flip angle = 3 s/30 ms/90°; Kwong et al., 1992). The T1, T2, and gradient-echo functional images were collected in the same plane (45 coronal oblique slices parallel to the anterior-posterior commissure line, tilted 30° anterior, 3 × 3 × 3 mm voxels). Identical scanning procedures were conducted on Day 1 and Day 2.
3. Data analysis
3.1. Psychophysiological assessment
The SCR scores were preprocessed as previously described by Milad and colleagues (Milad, Pitman, et al., 2009). Briefly, SCR for each CS trial was calculated by subtracting the mean skin conductance level (SCL) during the 2-s before CS onset (while the context alone was presented) from the highest SCL during the 6-s CS duration (Milad, Pitman, et al., 2009). Thus, SCRs to the different CSs reflect changes in SCL beyond any change in response to the context. SCRs were square root transformed prior to analysis. Unless otherwise specified, all data are presented as means ± standard error (SE). The contrast for within-group differential fear conditioning (CS+ > CS−) was calculated by comparing the mean of the combined first four CS+ presentations during the conditioning phase to the mean of the first four presentations of the CS−. During the extinction-learning phase, the contrast was calculated by comparing the mean of the last 12 trials of the CS+E to the last 12 trials of the CS−. During the Day 2 extinction recall phase, the contrast was calculated by comparing the means of the first four CS+E and the first four of the CS+NE trials. The magnitude of extinction recall, or extinction recall magnitude (ERM), was quantified by subtracting the mean SCR of the first four CS+NE from the mean SCR for the first four CS+E during extinction recall. As mean of the CS+E is expected to be lower than the mean of the CS+NE, successful extinction recall is represented by the ERM as a negative number, and the better the extinction recall, the greater the negative value. The ERM was used when comparing within-group sex differences on magnitude of extinction recall and represents the same contrast as used in the BOLD analysis (CS+E > CS+NE).
For comparison with prior research in extinction recall in PTSD (Milad, Pitman, et al., 2009), the extinction recall index (ERI) was also calculated to assess levels of extinction recall that are normalized for each participant’s SCR to the level each exhibited during the conditioning phase. The ERI is calculated by dividing each subject’s mean SCR to the first four CS+ trials of the extinction recall phase by their highest SCR to a CS+ trial during the conditioning phase and multiplying by 100, yielding a percentage of maximal conditioned responding. This in turn is subtracted from 100% to yield the “extinction index” or ERI (Milad, Pitman, et al., 2009).
3.2. fMRI analysis
Prior to analyses, all fMRI images were preprocessed using standard procedures in SPM8. All images were subjected to slice timing and motion correction before being coregistered to the MNI canonical brain atlas. The coregistered images were warped into MNI space and smoothed with an 8 mm full-width half-max kernel. All analyses were performed on the smoothed images.
A general linear model (GLM) was fit within each subject using SPM’s canonical double-gamma hemodynamic response function. Each subject-specific GLM included regressors to control for head motion (24 per run, including 6 motion regressors derived from realignment parameter estimates, squared motion estimates, and their derivatives) as well as dummy regressors to account for outlier images identified as having a significantly greater Mahalanobis distance compared to the other images at FDR-corrected p < .05 in a χ2 test. Within-subject contrasts were generated for each subject separately for the conditioning, extinction learning, and extinction recall phases (first four CS+ vs. first four CS−, last 12 CS+E vs. last 12 CS−, and first four CS+E vs. first four CS+NE, respectively). These contrasts parallel those used in the SCR analyses.
3.2.1. Whole brain analysis
The voxel-wise difference in activation between men and women (PTSD & TE-HC) was calculated using robust regression (Wager, Keller, Lacey, & Jonides, 2005). We used a robust regression toolkit written in-house that uses SPM as its base and performs voxel-wise robust regression across subject groups. Robust regression has the benefit of being less prone to errors when assumptions of error distribution are violated.
The results were corrected for multiple comparisons to a corrected False Discovery Rate (FDR; Genovese, Lazar, & Nichols, 2001) threshold of p < .05. An additional voxel-wise analysis was performed at a threshold of p < .001 uncorrected to compare our findings to previously reported differences between PTSD patients and TE-HC (Milad, Pitman, et al., 2009).
3.2.2. A priori ROI analysis
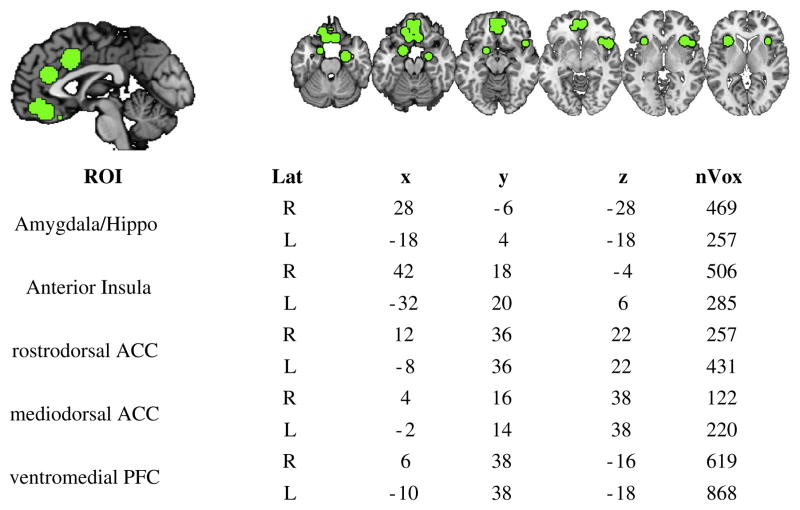
The difference in activation between PTSD and TE-HC men and women was evaluated within regions of interest (ROIs) previously associated with fear conditioning and extinction: amygdala, hippocampus, dorsal ACC, and vmPFC. ROIs were generated by drawing spheres of 8 mm around BOLD activations drawn from meta-analyses of fear conditioning (Diekhof, Geier, Falkai, & Gruber, 2011; Mechias, Etkin, & Kalisch, 2010) and studies of extinction recall (Milad, Pitman, et al., 2009). These spheres were then merged and divided into separate ROIs based on overlap. Cortical ROIs on the sagittal midline (dACC and vmPFC) were further divided into left and right lateral sections. In total, ten ROIs were generated from reported studies (See Fig. 2). Contrast-values within each subject were averaged within each ROI, and those averaged values were compared between subjects.
Fig. 2.
A priori regions of interest (ROIs). Lat = laterality; x, y, z = coordinates in mm; nVox = number of voxels within the ROI.
3.3. Statistics
Continuous demographic and clinical variables, such as selected shock level, CAPS and HAM-D scores, age at primary trauma, and chronological age, were compared between the male and female groups with two-sample t-tests.
For the main analyses, 2 × 2 × 2 repeated-measures ANOVA were conducted with the within-subjects factor Stimulus Type (CS+ vs. CS− for the conditioning and extinction learning phases; CS+E vs. CS+NE for the extinction recall phase) and between-subjects factors of Group (PTSD vs. TE-HC) and Sex (males vs. females). ANOVA analyses were repeated, controlling for variables having significant group differences such as years of education and time since trauma. Post-hoc t-tests and ANOVA were conducted as appropriate.
4. Results
4.1. Clinical and demographical variables
Preliminary analyses revealed that the TE-HC had significantly more years of education than the PTSD group. Furthermore, in both diagnostic groups, women reported more sexual assaults than men, and men more physical assaults than women.
No significant demographic or clinical (i.e., CAPS and HAM-D scores) differences between men and women within the PTSD and the TE-HC groups were found (see Table 1).
Table 1.
Demographic, trauma exposure, and clinical characteristics of the study sample.
| Total
|
PTSD
|
TE-HC
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PTSD (n = 31) | TE-HC (n = 25) | Statistics | Men (n = 13) | Women (n = 18) | Statistics | Men (n = 12) | Women (n = 13) | Statistics | |
|
| |||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | |||||||
|
|
|
|
|||||||
| Gender (Females) | 18 | 13 | Equation p = 0.250 | ||||||
| Age | 37.5 (9.9) | 36.4 (10.7) | t54 = 0.35, p = 0.73 | 40.8 (9.9) | 35.8 (9.7) | t29 = 1.4, p = 0.18 | 40.1 (9.9) | 33.1 (10.6) | t23 = 1.7, p = 0.16 |
| Using oral contraceptives (n) b | 2 | 2 | N/A | 2 | N/A | 2 | |||
| Years of education | 14.4 (1.9) | 15.9 (1.8) | t54 = −2.8, p = 0.006 | 13.3 (2.1) | 14.5 (2.3) | t29 = −1.9, p = 0.07 | 15.9 (1.6) | 15.9 (2.2) | t23 = −0.01, p = 0.99 |
| Age during the trauma | 33.8 (9.9) | 31.8 (9.4) | t54 = 0.72, p = 0.48 | 36.8 (10.8) | 32.2 (9.5) | t29 = 1.1, p = 0.25 | 34.4 (8.7) | 29.7 (9.7) | t23 = 1.2, p = 0.25 |
| Years since the trauma | 3.4 (3.1) | 5.3 (5.1) | t54 = −1.40, p = 0.17 | 4.9 (4.6) | 2.6 (2.8) | t29 = 1.2, p = 0.24 | 7 (6.9) | 4 (2.7) | t23 = 1.4, p = 0.18 |
| Type of trauma exposure (n) | |||||||||
| Motor vehicle accident | 2 | 2 | 1 | 1 | 1 | 1 | |||
| Sexual assault | 7 | 8 | 2 | 5 | 3 | 5 | |||
| Physical assault | 13 | 9 | 7 | 6 | 6 | 3 | |||
| Childhood trauma | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Combat exposure | 2 | 0 | 1 | 1 | 0 | 0 | |||
| Terrorism | 3 | 2 | 1 | 2 | 1 | 1 | |||
| Natural disaster | 1 | 2 | 0 | 1 | 1 | 1 | |||
| Witnessed a trauma | 3 | 2 | 1 | 2 | 0 | 2 | |||
| Selected shock level (mA)a Mean (SD) | 2.7 (1.8) | 2.3 (1.1) | t54 = 1.3, p = 0.20 | 3.0 (1.1) | 2.6 (1.2) | t29 = 0.95, p = 0.35 | 2.7 (1.2) | 2.0 (.93) | t23 = −0.53, p = 0.61 |
| CAPS Mean (SD) | 83.0 (16.7) | 5.4 (7.0) | t54 = 20.01, p < 0.005 | 86.6 (12.2) | 81.1 (17.9) | t29 = 0.92, p = 0.36 | 4.3 (8.5) | 6.2 (6.0) | t23 = 30.51, p = 0.60 |
| HAM-D Mean (SD) | 16.7 (5.7) | 2.4 (2.6) | t54 = −12.03, p < 0.005 | 18.1 (5.1) | 15.8 (6.0) | t29 = 0.14, p = 0.73 | 2.4 (2.1) | 2.4 (2.3) | t23 = −0.94, p = 0.36 |
PTSD, Posttraumatic stress disorder; TE-HC, Trauma exposed healthy control, CAPS, Clinician-Administered PTSD Scale. HAM-D, Hamilton Rating Scale for Depression.
Shock level units are from 0.8 to 4.0 mA.
No information was collected from one participant in the control group.
4.2. Behavioral and BOLD responses
4.2.1. Day 1: Fear conditioning
The main analysis (mean of first four trials of the combined CS+1 and CS+2 trials vs. mean of first four trials of the CS−) revealed a significant effect of Stimulus (F(1,54) = 27.64, p < 0.001, η2 = 1.2), with greater SCRs to the CS+ than to CS− across PTSD and TE-HC groups. There was no significant effect of Group (PTSD vs. TE-HC; F(1,54) = 0.31, p = 0.41, η2 = 0.51), nor a Stimulus × Group interaction (F(1,54) = 0.19, p = 0.16, η2 = 0.07). There was a significant effect of Sex (F(1,54) = 5.8, p < 0.02, η2 = 1.4) with men having a higher mean SCR then women, but no Stimulus × Sex interaction (F(1,54) = 1.13, p = 0.19, η2 = 0.08). Post hoc t-test demonstrated a significantly higher response to the CS+ than to the CS− in each sex for both PTSD and TE-HC groups (Fig. 3A). fMRI analyses indicated lower BOLD activation in the right vmPFC for the CS+ contrast with the CS− (F(1,4) = 6.01, p < 0.02), which did not change with Group or Sex (Fig. 3B). When using a less stringent threshold of p < 0.001 uncorrected, previous findings were replicated that showed decreased activation for CS+ compared to CS− in the vmPFC and increased activation in the dACC and insula (Fig. 3C). Whole-brain analysis revealed no other significant results.
Fig. 3.
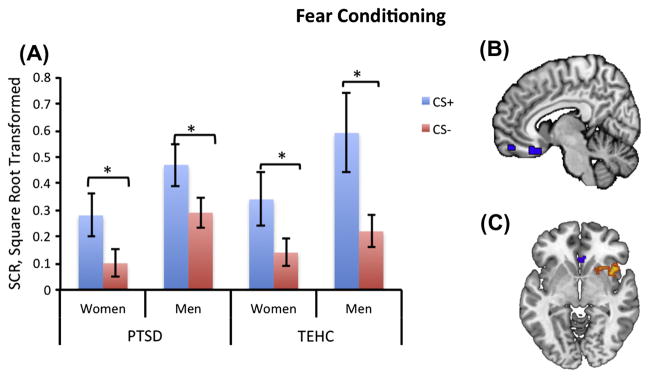
PTSD: Posttraumatic stress disorder; TE-HC: Trauma exposed healthy control; CS+: mean SCR of the first four trials for the light paired with a shock; CS−: mean SCR of the first four trials for the light that was never paired with a shock. (A) PTSD: both men and women show significant differences between CS+ and CS− during the conditioning phase (men: t12 = 3.1, p < 0.006; women: t17 = 3.1, p < 0.008). TE-HC: both men and women show significant differences between CS+ and CS− during the conditioning phase (men: t11 = 2.7, p < 0.02; women: t12 = 2.8, p < 0.019). Error bars denote standard errors of mean. (B) Voxel-wise activation within the right ventromedial prefrontal cortex (vmPFC) ROI was reduced for the CS+ compared to the CS− in both the PTSD and TE-HC groups. Regions of activation shown are significant to p < 0.05. The uncorrected voxel-wise threshold was used for visualization of spatial activation within vmPFC and it does not correspond to the statistical test performed to determine significance. (C) Activation in dACC and right insula was greater for CS+ presentations compared to CS− presentations during conditioning p < 0.001 uncorrected, extent shown to p < 0.01).
4.2.2. Day 1: Extinction learning
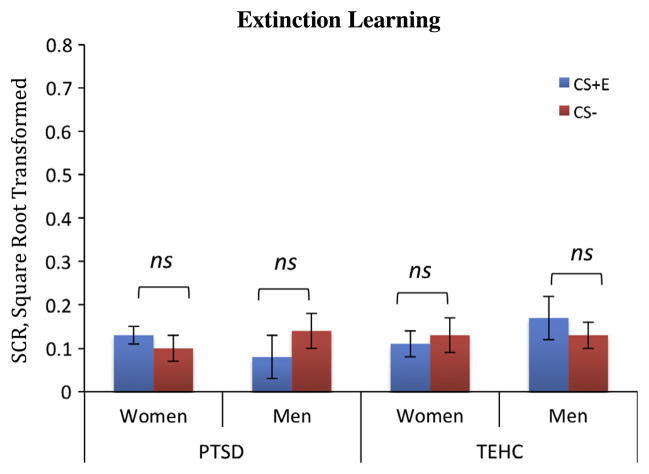
An ANOVA for the late extinction SCR data (last 12 CS+E vs. last 12 CS− trials) revealed no significant main effect of Stimulus (F(1,54) = 0.81, p = 0.42, η2 = 0.10), Group (F(1,52) = 0.47, p = 0.45, η2 = 0.04), or Stimulus × Group interaction (F(1,54) = 0.01, p = 0.91, η2 = 0.001). Nor were there significant main effects of Sex (F(1,54) = 0.22, p = 0.69, η2 = 0.005) or Stimulus × Sex interaction (F(1,54) = 3.1, p = 0.09, η2 = 0.001), suggesting that both groups and sexes achieved comparable extinction learning (Fig. 4). BOLD results for the last 12 trials showed no difference in activation for the extinguished CS+(CS+E) vs. the CS−, nor differences based on Group or Sex. Whole-brain analysis revealed no other significant results.
Fig. 4.
PTSD: Posttraumatic stress disorder; TEHC: Trauma exposed healthy control; CS+E: mean SCR of the last 12 trials for the light that was paired with a shock and later extinguished. CS−: mean SCR of the last 12 trials for the light that was never paired with the shock. PTSD: both men and women show no significant differences between CS+E and CS− during extinction the learning phase (men: t12 = −1.12, p > 0.32; women: t17 = 0.69, p > 0.5) TEHC: both men and women show no significant differences between.
4.2.3. Day 2, Extinction recall magnitude: Behavioral and BOLD responses
A 2 × 2 × 2 repeated-measures ANOVA for early extinction recall SCR data (mean of first 4 CS+E vs. first 4 CS+NE) revealed no significant effect of Stimulus (F(1,54) = 0.63, p = 0.53, η2 = 0.04) or Group (F(1,54) = 0.08, p = 0.90, η2 = 0.005), and a trend level Stimulus × Group interaction (F(1,54) = 1.25, p = 0.06, η2 = 0.07). Consistent with this trend, there was a trend for poorer extinction retention on the ERI in the PTSD group (31% for the PTSD group and 60% for the TE-HC group t54 = 0.9, p = 0.07, one-tailed).
Importantly, ANOVA for early extinction recall showed a significant effect of Sex (F(1,54) = 7.2, p = 0.014, η2 = 1.87), with men showing less difference in SCR level between the CS+E and CS+NE than women. There were no significant Stimulus × Sex (F(1,54) = 0.22, p = 0.70, η2 = 0.01) or Group × Sex (F(1,54) = 0.40, p = 0.90, η2 = 0.07) interactions.
To further assess sex influence on extinction recall magnitude, we applied ANOVA with factors group and sex on the ERM. Results showed a significant effect for Sex (F(1,54) = 4.7, p < 0.05, η2 = 1.33), with women having a better extinction recall magnitude (lower SCR difference between CS+E and CS+NE) than men, but no significant Group (F(1,54) = 1.18, p = 0.28, η2 = 1.44) or Sex × Group interaction (F(1,54) = 1.7, p = 0.21, η2 = 0.20).
Post hoc testing with independent samples t-test revealed significantly greater ERM among the women with PTSD than the men with PTSD (−0.09 ± 0.03 μS for women vs. 0.09 ± 0.03 μS for men, t30 = 2.67, p < 0.03). No significant ERM level differences emerged between TE-HC men and women (−0.05 ± 0.07 μS for the women vs. −0.12 ± 0.18 μS for men, t24 = −0.43, p = 0.67) (Fig. 5A). Additional analyses within the PTSD group women and TE-HC group women revealed no significant differences in ERM level between those reporting use of hormonal contraceptives and those who reported no use (PTSD: −0.09 ± 0.04 μS for women not using vs. 0.07 ± 0.05 μS for women using hormonal contraceptives, t16 = 0.30 p = 0.6; TE-HC: 0.04 ± 0.04 μS for the women not using vs. −0.26 ± 0.24 μS for women using hormonal contraceptives, t10 = −1.8, p = 0.14).
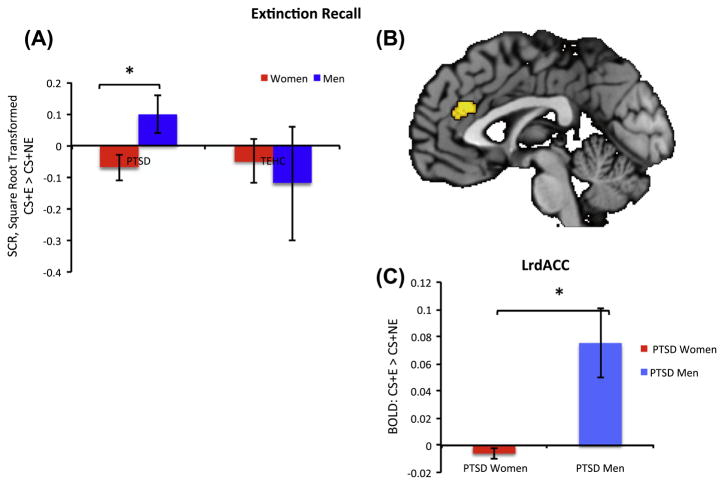
Fig. 5.
Differential Extinction recall on Day 2 of the experiment was calculated by subtracting mean SCR for the first four CS+NE from first four CS+E acquired during the extinction recall phase. The lower the SCR the better the recall magnitude. See detailed explanation in Section 3.1. (A) PTSD: There were significant differences between men and women t30 = 2.42, p < 0.03. TE-HC: No significant differences were found between men and women t24 = −0.43, p > 0.67. Error bars denote standard errors of mean. (B) PTSD: Voxel-wise activation within the left rostrodorsal anterior cingulate cortex (rdACC) ROI was increased for CS+E compared to CS+NE specifically in men. Regions of activation shown are significant to p < 0.05, the uncorrected voxel-wise threshold was used for visualization of spatial activation within the dACC and it does not correspond to the statistical test performed to determine significance. (C) PTSD: Mean SCR contrast values for CS+E vs. CS+NE within the entire left rdACC ROI.
To test for potentially confounding effects of variance of fear conditioning levels on the finding of sex differences during extinction recall, an ANCOVA was conducted examining the ERM, covarying for levels of fear conditioning (using the mean of the first four trials to the combined CS+s). The effect of Sex on the ERM level remained when controlling for the level of SCR during conditioning (F(1,27) = 5.75, p < 0.03). The TE-HC group again showed no significant effect of Sex on ERM level when controlling for level of conditioning (F(1,21) = 3.75, p = 0.39). Thus, while no sex differences appeared in extinction retention within the TE-HC group, men with PTSD had significantly poorer extinction recall than women with PTSD.
Across both groups, BOLD response for the CS+E compared with CS+NE during extinction recall was lower in the left rostral dACC (F(1,54) = 4.16, p < 0.05). Consistent with previous findings (Milad, Pitman, et al., 2009), subjects with PTSD had greater BOLD response in the right rostral dACC (F(1,54) = 6.6, p < 0.01) than TE-HCs. However, the deactivation of vmPFC reported in prior work was not found (Milad, Pitman, et al., 2009). Within the PTSD group, men showed significantly greater BOLD activation in left rostral dACC than women (F(1,30) = 4.37, p < 0.04) and a trend for greater activation in right rostral dACC (F(1,30) = 3.43, p = 0.07) (See Fig. 5B and C). Whole-brain analysis found no other significant between-group differences.
5. Discussion
The current study investigated sex differences in SCR and BOLD activation during conditioning, extinction learning, and extinction recall in PTSD patients and matched TE-HC participants. During fear conditioning on Day 1, men exhibited higher SCR than women across groups, with no significant differences in BOLD responses based on group or sex. No significant group or sex differences in SCR or BOLD activity were observed during extinction learning. Importantly, during extinction recall on Day 2, SCR response to the CS+E vs. CS+NE was greater for men than women with PTSD (i.e., men had poorer extinction recall). Furthermore, fMRI activity during extinction recall was greater in the rostral dACC in men vs. women with PTSD, paralleling a positive association between rostral dACC activation and threat cues across studies (Lane & Wager, 2009; Phelps, Delgado, Nearing, & LeDoux, 2004; Roy, Shohamy, & Wager, 2012). No sex differences were evident within the TE-HC group on either SCR or fMRI BOLD activity during the extinction recall phase.
Why women in the general population have twice the lifetime risk of PTSD as men remains unclear (Breslau & Anthony, 2007; Kessler et al., 1995; Olff et al., 2007; Tolin & Foa, 2006; Weissman et al., 2005). As sex differences in the fear neurocircuitry implicated in vulnerability to PTSD could potentially underlie this increased risk in women, we explored sex effects in psychophysiological and in regional brain BOLD activity during conditioning, extinction learning, and extinction recall. Surprisingly, males with PTSD exhibited poorer extinction recall and greater left rostral dACC BOLD activity, suggesting that sexually dimorphic responding within the fear extinction circuitry is unlikely to underlie greater lifetime risk of PTSD in women. To the contrary, extinction recall deficits in males with PTSD may specifically contribute to the failure of post trauma recovery involving extinction circuitry, which, if functioning better, might facilitate resilience in men post trauma.
Our data corroborate previous work indicating greater activation in the dACC among PTSD patients in comparison to trauma-exposed healthy controls regardless of sex during extinction recall (Milad, Pitman, et al., 2009), further implicating this brain region in the pathophysiology of PTSD. Our findings suggest for the first time that relative to women with PTSD, men with PTSD tend to increase activation in the dACC – a key fear network region. The dACC has been implicated in diverse cognitive and emotional processes including fear expression, appraisal of emotionally salient stimuli, and pain (Bush et al., 2002; Etkin, Egner, & Kalisch, 2011; Milad et al., 2007; Roy et al., 2012; Shackman et al., 2011; Vogt, 2005). Further, dACC may be involved in fear extinction (Graham & Milad, 2011; Quirk & Mueller, 2008), and dACC activity has been shown to positively correlate with PTSD hyperarousal symptoms and symptom severity (Shin et al., 2009). Although we did not find sex differences in PTSD hyperarousal symptoms, the elevated dACC activation during extinction recall in men and not in women with PTSD may indicate that men with PTSD have generally greater hyperarousal than women during extinction recall.
While the present analyses focused on exploring sex effects in conditioning and extinction in trauma, we report similar group results, albeit at trend levels, as Milad, Pitman, et al. (2009), showing a Stimulus × Group interaction on the SCR during the recall phase, indicating deficient extinction recall within the PTSD group, and BOLD results showing dACC hyperactivation. We did not find significant deactivation during extinction recall in the vmPFC in the PTSD group, in contrast with Milad, Pitman, et al. (2009). There are several potential reasons for the lack of vmPFC differences between groups in the present study. Our sample, both patients and healthy controls, contained more women than men, and a higher proportion of Hispanics than that studied by Milad, Pitman, et al. (2009). Differences in ethnic composition or gender ratio of the two cohorts could have contributed to this apparent discrepancy. Milad, Pitman, et al. (2009) conducted their study using a 3 T fMRI scanner with a 12 channel head-coil whereas our study employed a 1.5 T scanner with a 1 channel head-coil; our signal-to-noise ratio may have been less robust, resulting in less sensitivity to detect vmPFC deactivation in PTSD.
A key limitation of this study is the lack of control for hormonal/cycle effects among female subjects. However, while it was recently reported that the level of fear extinction in women with PTSD was significantly influenced by estrogen level (Glover et al., 2012; Lebron-Milad, Graham, & Milad, 2012), several reports demonstrate extinction recall impairment in healthy women without controlling for hormonal effects (Lebron-Milad et al. 2012, Inslicht et al., 2013). Furthermore, our finding that women who used or did not use hormonal contraceptives during the study did not differ in extinction recall deficits argues against a significant impact of hormonal contraceptives or hormone replacement therapies. Moreover, as our findings suggest better extinction recall among PTSD women than PTSD men, regardless of differences in estrogen levels between women, we may speculate that data on estradiol levels would not change the direction of the findings.
Another potential limitation is the different rates of particular trauma types between the females and males across groups. Specifically, women in the study had a greater rate of sexual assaults as the index trauma, while men reported more physical assaults. Our sample size does not permit controlling for this level of discrimination between trauma types, so it should be considered that these trauma type differences might confound the between-sex findings in the PTSD group.
In conclusion, while previous studies have pointed to deficient extinction retention memory in PTSD as compared to trauma-exposed healthy controls, the current pilot study is the first to identify that men with PTSD exhibit higher levels of deficient recall of extinction memory, coupled with greater dACC activation, than do women with PTSD.
If supported by future research on underlying mechanisms of sex differences in trauma related pathology, our findings would contribute to a better understanding of sex differences in PTSD pathophysiology and maintenance, and might assist in developing enhanced, more personalized treatments to address sex-specific neurobiological targets. Future studies should examine whether trauma-focused treatments such as prolonged exposure can normalize extinction deficits in men with PTSD.
Acknowledgments
This study is supported by NIMH grants MH015144-34 and MH072833 (Y. Neria).
We would like to thank Heather L. Rusch, of the National Institutes of Health, for her helpful advice and editing contributions.
References
- American Psychiatric Association. Diagnostic criteria from DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Breslau N, Anthony JC. Gender differences in the sensitivity to posttraumatic stress disorder: An epidemiological study of urban young adults. Journal of Abnormal Psychology. 2007;116(3):607–611. doi: 10.1037/0021-843X.116.3.607. http://dx.doi.org/10.1037/0021-843X.116.3.607. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences USA. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. http://dx.doi.org/10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An FMRI investigation. Learning and Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. http://dx.doi.org/10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors T. Sex differences in learning processes of classical and operant conditioning. Physiology and Behavior. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. http://dx.doi.org/10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. http://dx.doi.org/10.1016/J.Neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. http://dx.doi.org/10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. New York, New York: The New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- Genovese C, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Department of Statistics; 2001. Paper 204. [DOI] [PubMed] [Google Scholar]
- Glover E, Jovanovic T, Mercer K, Kerley K, Bradley B, Ressler K, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. http://dx.doi.org/10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. http://dx.doi.org/10.1523/jneurosci.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. http://dx.doi.org/10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: Implications for anxiety disorders. American Journal of Psychiatry. 2011;168:10. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological Psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. http://dx.doi.org/10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, et al. Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(1):64–71. doi: 10.1016/j.jpsychires.2012.08.027. http://dx.doi.org/10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. http://dx.doi.org/10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences USA. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R, Wager T. The new field of brain-body medicine: What have we learned and where are we headed? Neuroimage. 2009;47(3):1135–1140. doi: 10.1016/j.neuroimage.2009.06.013. http://dx.doi.org/10.1016/j.neuroimage.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Graham B, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):6–7. doi: 10.1016/j.biopsych.2012.04.029. http://dx.doi.org/10.1016/j.biopsych.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bücking A, Zeidan M, et al. Sex differences in the neurobiology of fear conditioning and extinction: A preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biology of Mood & Anxiety Disorders. 2012;2(1):2–7. doi: 10.1186/2045-5380-2-7. http://dx.doi.org/10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. http://dx.doi.org/10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein J, Orr S, Wedig M, Klibanski A, Pitman R, et al. Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. http://dx.doi.org/10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe S, Lebron-Milad K, Novales J. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. http://dx.doi.org/10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. doi: 10.1111/j.1469-8986.2005.00302.x. http://dx.doi.org/10.1111/J.1469-8986.2005.00302.X. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman R, Ellis C, Gold A, Shin L, Lasko N, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. http://dx.doi.org/10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk G. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. http://dx.doi.org/10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk G, Pitman R, Orr S, Fischl B, Rauch S. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. http://dx.doi.org/10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan M, Contero A, Pitman R, Klibanski A, Rauch S, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. http://dx.doi.org/10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons B. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. http://dx.doi.org/10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Phelps E, Delgado M, Nearing K, LeDoux J. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. http://dx.doi.org/10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. http://dx.doi.org/10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. http://dx.doi.org/10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager T. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. http://dx.doi.org/10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A, Salomons T, Slagter H, Fox A, Winter J, Davidson R. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. http://dx.doi.org/10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Archives of General Psychiatry. 2009;66(10):1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. http://dx.doi.org/10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvil E, Rusch H, Sullivan GM, Neria Y. Neural, psychophysiological, and behavioral markers of fear processing in PTSD: A review of the literature. Current Psychiatry Reports. 2013;15(5):358. doi: 10.1007/s11920-013-0358-3. http://dx.doi.org/10.1007/s11920-013-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Jovanovic T, Fani N, Ely T, Glover E, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. http://dx.doi.org/10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin D, Foa E. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin. 2006;132(6):959–992. doi: 10.1037/0033-2909.132.6.959. http://dx.doi.org/10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6(7):533–544. doi: 10.1038/nrn1704. http://dx.doi.org/10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Keller M, Lacey S, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. http://dx.doi.org/10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weissman M, Neria Y, Das A, Feder A, Blanco C, Olfson M. Gender differences in posttraumatic stress disorder among primary care patients after the World Trade Center attack of September 11, 2001. Gender Medicine. 2005;2(2):76–87. doi: 10.1016/s1550-8579(05)80014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan M, Igoe S, Linnman C, Vitalo A, Levine J, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. http://dx.doi.org/10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]