Abstract
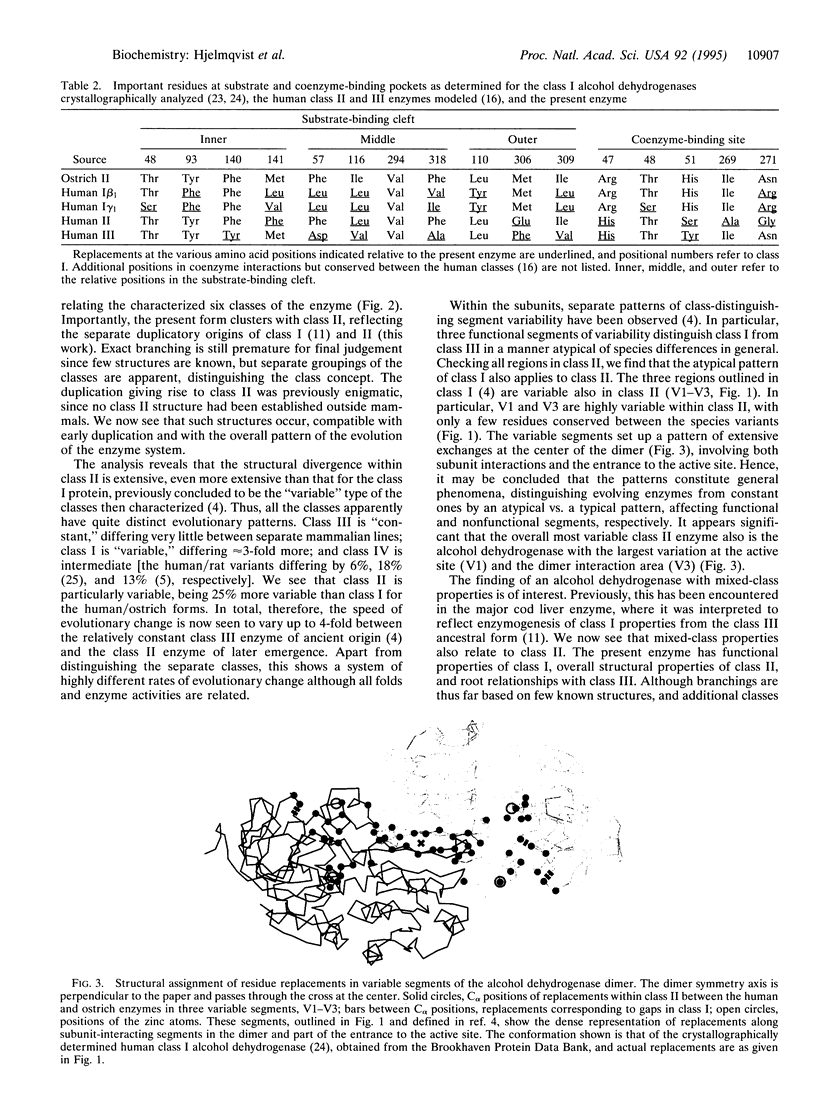
A mixed-class alcohol dehydrogenase has been characterized from avian liver. Its functional properties resemble the classical class I type enzyme in livers of humans and animals by exhibiting low Km and kcat values with alcohols (Km = 0.7 mM with ethanol) and low Ki values with 4-methylpyrazole (4 microM). These values are markedly different from corresponding parameters of class II and III enzymes. In contrast, the primary structure of this avian liver alcohol dehydrogenase reveals an overall relationship closer to class II and to some extent class III (69 and 65% residue identities, respectively) than to class I or the other classes of the human alcohol dehydrogenases (52-61%), the presence of an insertion (four positions in a segment close to position 120) as in class II but in no other class of the human enzymes, and the presence of several active site residues considered typical of the class II enzyme. Hence, the avian enzyme has mixed-class properties, being functionally similar to class I, yet structurally similar to class II, with which it also clusters in phylogenetic trees of characterized vertebrate alcohol dehydrogenases. Comparisons reveal that the class II enzyme is approximately 25% more variable than the "variable" class I enzyme, which itself is more variable than the "constant" class III enzyme. The overall extreme, and the unusual chromatographic behavior may explain why the class II enzyme has previously not been found outside mammals. The properties define a consistent pattern with apparently repeated generation of novel enzyme activities after separate gene duplications.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cederlund E., Peralba J. M., Parés X., Jörnvall H. Amphibian alcohol dehydrogenase, the major frog liver enzyme. Relationships to other forms and assessment of an early gene duplication separating vertebrate class I and class III alcohol dehydrogenases. Biochemistry. 1991 Mar 19;30(11):2811–2816. doi: 10.1021/bi00225a011. [DOI] [PubMed] [Google Scholar]
- Danielsson O., Atrian S., Luque T., Hjelmqvist L., Gonzàlez-Duarte R., Jörnvall H. Fundamental molecular differences between alcohol dehydrogenase classes. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4980–4984. doi: 10.1073/pnas.91.11.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson O., Eklund H., Jörnvall H. The major piscine liver alcohol dehydrogenase has class-mixed properties in relation to mammalian alcohol dehydrogenases of classes I and III. Biochemistry. 1992 Apr 21;31(15):3751–3759. doi: 10.1021/bi00130a004. [DOI] [PubMed] [Google Scholar]
- Danielsson O., Jörnvall H. "Enzymogenesis": classical liver alcohol dehydrogenase origin from the glutathione-dependent formaldehyde dehydrogenase line. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9247–9251. doi: 10.1073/pnas.89.19.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlow C. C., Holmquist B., Morelock M. M., Vallee B. L. Physical and enzymatic properties of a class II alcohol dehydrogenase isozyme of human liver: pi-ADH. Biochemistry. 1984 Dec 18;23(26):6363–6368. doi: 10.1021/bi00321a012. [DOI] [PubMed] [Google Scholar]
- Eklund H., Müller-Wille P., Horjales E., Futer O., Holmquist B., Vallee B. L., Hög J. O., Kaiser R., Jörnvall H. Comparison of three classes of human liver alcohol dehydrogenase. Emphasis on different substrate binding pockets. Eur J Biochem. 1990 Oct 24;193(2):303–310. doi: 10.1111/j.1432-1033.1990.tb19337.x. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Estonius M., Hjelmqvist L., Jörnvall H. Diversity of vertebrate class I alcohol dehydrogenase. Mammalian and non-mammalian enzyme functions correlated through the structure of a ratite enzyme. Eur J Biochem. 1994 Sep 1;224(2):373–378. doi: 10.1111/j.1432-1033.1994.00373.x. [DOI] [PubMed] [Google Scholar]
- Estonius M., Hög J. O., Danielsson O., Jörnvall H. Residues specific for class III alcohol dehydrogenase. Site-directed mutagenesis of the human enzyme. Biochemistry. 1994 Dec 20;33(50):15080–15085. doi: 10.1021/bi00254a017. [DOI] [PubMed] [Google Scholar]
- Farrés J., Moreno A., Crosas B., Peralba J. M., Allali-Hassani A., Hjelmqvist L., Jörnvall H., Parés X. Alcohol dehydrogenase of class IV (sigma sigma-ADH) from human stomach. cDNA sequence and structure/function relationships. Eur J Biochem. 1994 Sep 1;224(2):549–557. doi: 10.1111/j.1432-1033.1994.00549.x. [DOI] [PubMed] [Google Scholar]
- Gutheil W. G., Holmquist B., Vallee B. L. Purification, characterization, and partial sequence of the glutathione-dependent formaldehyde dehydrogenase from Escherichia coli: a class III alcohol dehydrogenase. Biochemistry. 1992 Jan 21;31(2):475–481. doi: 10.1021/bi00117a025. [DOI] [PubMed] [Google Scholar]
- Hjelmqvist L., Hackett M., Shafqat J., Danielsson O., Iida J., Hendrickson R. C., Michel H., Shabanowitz J., Hunt D. F., Jörnvall H. Multiplicity of N-terminal structures of medium-chain alcohol dehydrogenases. Mass-spectrometric analysis of plant, lower vertebrate and higher vertebrate class I, II, and III forms of the enzyme. FEBS Lett. 1995 Jul 3;367(3):237–240. doi: 10.1016/0014-5793(95)00572-q. [DOI] [PubMed] [Google Scholar]
- Hurley T. D., Bosron W. F., Hamilton J. A., Amzel L. M. Structure of human beta 1 beta 1 alcohol dehydrogenase: catalytic effects of non-active-site substitutions. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8149–8153. doi: 10.1073/pnas.88.18.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hög J. O. Cloning and characterization of a novel rat alcohol dehydrogenase of class II type. FEBS Lett. 1995 Jul 24;368(3):445–448. doi: 10.1016/0014-5793(95)00707-g. [DOI] [PubMed] [Google Scholar]
- Hög J. O., von Bahr-Lindström H., Hedén L. O., Holmquist B., Larsson K., Hempel J., Vallee B. L., Jörnvall H. Structure of the class II enzyme of human liver alcohol dehydrogenase: combined cDNA and protein sequence determination of the pi subunit. Biochemistry. 1987 Apr 7;26(7):1926–1932. doi: 10.1021/bi00381a021. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Hög J. O. Nomenclature of alcohol dehydrogenases. Alcohol Alcohol. 1995 Mar;30(2):153–161. [PubMed] [Google Scholar]
- Koivusalo M., Baumann M., Uotila L. Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 1989 Oct 23;257(1):105–109. doi: 10.1016/0014-5793(89)81797-1. [DOI] [PubMed] [Google Scholar]
- Lutz R. A., Bull C., Rodbard D. Computer analysis of enzyme-substrate-inhibitor kinetic data with automatic model selection using IBM-PC compatible microcomputers. Enzyme. 1986;36(3):197–206. doi: 10.1159/000469292. [DOI] [PubMed] [Google Scholar]
- Moreno A., Parés X. Purification and characterization of a new alcohol dehydrogenase from human stomach. J Biol Chem. 1991 Jan 15;266(2):1128–1133. [PubMed] [Google Scholar]
- Parés X., Cederlund E., Moreno A., Hjelmqvist L., Farrés J., Jörnvall H. Mammalian class IV alcohol dehydrogenase (stomach alcohol dehydrogenase): structure, origin, and correlation with enzymology. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1893–1897. doi: 10.1073/pnas.91.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Bazzone T. J. Isozymes of human liver alcohol dehydrogenase. Isozymes Curr Top Biol Med Res. 1983;8:219–244. [PubMed] [Google Scholar]
- Yin S. J., Vagelopoulos N., Wang S. L., Jörnvall H. Structural features of stomach aldehyde dehydrogenase distinguish dimeric aldehyde dehydrogenase as a 'variable' enzyme. 'Variable' and 'constant' enzymes within the alcohol and aldehyde dehydrogenase families. FEBS Lett. 1991 May 20;283(1):85–88. doi: 10.1016/0014-5793(91)80559-l. [DOI] [PubMed] [Google Scholar]
- Zheng Y. W., Bey M., Liu H., Felder M. R. Molecular basis of the alcohol dehydrogenase-negative deer mouse. Evidence for deletion of the gene for class I enzyme and identification of a possible new enzyme class. J Biol Chem. 1993 Nov 25;268(33):24933–24939. [PubMed] [Google Scholar]



