Abstract
Objective:
The objective of this study is to test the antimicrobial effect of zinc oxide when incorporated into an orthodontic bonding material and to check the effect of addition of zinc oxide on the shear bond strength of the bonding material.
Materials and Methods:
Zinc oxide was added to a resin modified light cure glass ionomer cement (GIC) (Fuji Ortho LC GC America, Alsip, Ill) to make modified bonding agent containing 13% and 23.1% ZnO and the antimicrobial assay was done using agar disc diffusion method. Discs of the modified bonding agent were prepared and a culture of Streptococcus mutans mixed with soft agar was poured over it and incubated at 38°C for 48 h and zones of inhibition were measured. The test was repeated after a month to check the antimicrobial effect. In addition shear bond strength of the brackets bonded with the modified bonding agent was tested.
Results:
The agar disc showed zones of inhibition around the modified bonding agent and the antimicrobial activity was more when the concentration of ZnO was increased. The antimicrobial effect was present even after a month. The shear bond strength decreased as the concentration of ZnO increased.
Conclusion:
The incorporation of ZnO into a resin modified light cure GIC (Fuji Ortho LC GC America, Alsip, Ill) added antimicrobial property to the original compound.
Keywords: Antimicrobial effect, bond strength, decalcification, white spot lesion, zinc oxide, zone of inhibition
INTRODUCTION
Enamel decalcification or white spot formation is the most common sequel of orthodontic treatment. It can occur whenever the bacterial plaque is retained on enamel surface for a prolonged period.[1] Decalcification is defined as loss of calcified tooth substance and it occurs when the pH of the oral environment favors dissolution of calcium and phosphate ions out of the enamel. The early lesion is an opaque white spot known as the white spot lesion.[2]
Both orthodontic attachments and bonding materials may retain plaque. The presence of archwires complicates cleaning and makes access to plaque retaining areas difficult, especially when multiple loops, auxillary archwires and different types of elastics are used. As a consequence of this, new sites susceptible to caries will form next to the bands and brackets during orthodontic treatment with fixed appliance.[3] There is a significant increase in salivary and plaque levels of Streptococcus mutans in patients undergoing fixed appliance treatment, concomitant with an elevated risk to dental caries.[4]
Since Newman published his first clinical report on directly bonding orthodontic brackets to teeth, banding has replaced the earlier method of bonding. Composite resins have become the material of choice for bonding brackets. However, composite resins require a completely dry environment and enamel etching with 35-40% of phosphoric acid to dissolve the superficial layer of the enamel (8-15 μm). Enamel loss occurs not only during etching but also during debonding procedures.[5] Recently, interest has developed in the use of glass ionomer cements (GICs) as orthodontic bonding agents.
The ability of fluoride to reduce demineralization and enhance remineralization is well-established. Although fluoride traditionally has been thought to affect caries through its physiochemical interaction with enamel, evidence exists that fluoride also alters bacterial metabolism at low concentrations and is bactericidal at higher concentrations.[6] While fluoride varnishes, fluoride mouth rinses and oral hygiene instructions have been employed they rely on patient compliance and provide only intermittent protection against decalcification. When bonding with GIC there is no need for pretreatment of enamel with acid as there is chemical bonding of GIC with the tooth and it also releases fluoride into the oral cavity for several months.[7] However the fluoride release by the bonding agents have very little demineralization inhibiting effect and hence it is important to add an antimicrobial agent along with fluoride to have a cariostatic effect.[8]
However, some in vitro and in vivo studies have reported that GICs have a weaker bond strength, with higher bond failure rates, when compared with composite resins; thus they are not recommended for routine clinical orthodontic bracket bonding. To increase the mechanical properties of GICs and their bond strengths, resin-modified glass ionomer cements (RMGICs) have been proposed.[5]
Zinc oxide has an antimicrobial effect and it has been used in various ways, including pharmaceutical creams or ointments for the treatment of leg ulcers, traumatic wounds and burns. Dental materials such as endodontic sealers and fixed restoration cements have been utilized for this same reason. Zinc, which serves as an activator of enzymes that can be toxic to microbes at concentrations as low as 0.5 ppm, has been shown to inhibit the growth of plaque at concentrations of 4, 6 and 16 ppm.[9]
Hence the aims of the present study were to:
Test the antimicrobial effect of zinc oxide when incorporated into an orthodontic bonding material
Check the duration of antimicrobial effect if present
Check the effect of addition of zinc oxide on the shear bond strength of the bonding material.
MATERIALS AND METHODS
The study was divided into 2 parts: The antimicrobial testing and the shear bond testing.
Four groups of bonding material were made:
Group 1: Control group. Bonding with conventional light cure composite (Transbond XT)
Group 2: Control group. Bonding with RMGIC (Fuji Ortho LC GC America, Alsip, Ill)
Group 3: Experimental group. Bonding with RMGIC (Fuji Ortho LC GC America, Alsip, Ill) mixed with 13% zinc oxide powder
Group 4: Experimental group. Bonding with RMGIC (Fuji Ortho LC GC America, Alsip, Ill) mixed with 23.1% of zinc oxide powder.
Bonding material preparation
The bonding material for Group 3 and 4 was prepared as follows. For Group 3 the bonding material was prepared by mixing 13 g of zinc oxide powder to 100 g of RMGIC (Fuji Ortho LC GC America, Alsip, Ill). For Group 4 bonding material was prepared using 23.1 g of zinc oxide powder to 100 g of RMGIC (Fuji Ortho LC GC America, Alsip, Ill). Each mixture was placed in a separate test tube and mixed for 1 minute using a vibrator to create a uniform powder.
Antimicrobial testing
For the antimicrobial testing test discs of all the four boding material were prepared (one disc per group). The discs were made from empty insulin syringes (1 mL). The syringes were sliced using a micromotor at 3200 rpm creating discs that were approximately 2 mm × 2 mm. Discs were allowed to air dry and the bonding material was loaded onto the discs and light cured for 40 s. The discs were stored in airtight containers.
Agar plate preparation
Two different brain heart infusion (BHI) plates were prepared for antimicrobial testing using a decreased concentration of nutrient material, 2.31 g/L BHI, and 16 g/L of bacto-agar (hi media). One disc from each mixture was placed on each plate. A soft agar overlay was prepared with 2.3 g/L BHI and 7.0 g/L agar. An overnight S. mutans culture (200 μL) grown in BHI at 37°C was mixed with 3.5 mL of soft agar and poured evenly over the plates, surrounding the discs. Following solidification of the overlay, the plates were then incubated at 37°C for 48 h. The zones of inhibition were measured after 48 h using a zone of inhibition measuring scale.
After initial data collection, the test discs were removed from the plates, rinsed, and placed in distilled water. The discs were rinsed using fresh, distilled water each day for approximately 20 s. After 1 month, the previously described antimicrobial assay and evaluations were performed.
Shear bond testing
For the shear bond testing 48 freshly extracted human premolar teeth were collected and stored in a solution of 0.2% (weight/volume) thymol. The teeth were embedded in acrylic resin placed in cuboidal aluminum blocks. After mounting the teeth were cleaned and polished with pumice. Metallic first premolar brackets (Gemini 3M Unitek Monrovia, Calif) were used. The samples were divided into the above mentioned four groups each containing 12 teeth.
Bonding procedure
Each tooth was cleaned with pumice and water for 5 s, rinsed for 10 s and air dried to avoid desiccation. Teeth were then etched with 37% of phosphoric acid gel for 20 s and rinsed for 10 s. The bonding material was mixed using one scoop of powder with one drop of liquid for the control group. For mixtures containing zinc oxide, two drops of liquid had to be used in order to prepare a material with the appropriate viscosity. The RMGIC material was incorporated into the base of the premolar bracket using a cement spatula and the bracket was placed on the enamel surface. Excess material was removed from around the bracket and the remaining adhesive was light cured for 40 s (10 from each side) using the 3 M Unitek Ortholux LED curing light.
For bonding with light cure composite the teeth were etched with 37% of phosphoric acid for 30 s, rinsed and air dried using moisture free air. A thin layer of Transbond XT primer (3 M Unitek) was applied on the enamel surface and light cured for 10 s. Brackets were bonded onto the centre of the buccal surface of the teeth with Transbond XT (3 M Unitek), a light cured composite adhesive. Before curing, the excess resin was removed with a sharp scaler without disturbing the bracket position; the adhesive was light cured for a total of 40 s.
Bonded teeth were stored in water at room temperature for 24 h before testing bond strength.
Shear bond testing
Shear bond strength test was done with JJ Lloyd Universal testing machine having a capacity of 20,000 N (2040 kg) with a cross head speed of 1 mm/min. The aluminum block with mounted premolar and its bonded bracket were positioned in the jig, so that the labial surface parallel to the force during the shear strength tests. A steel rod with a flattened end was used to apply load at the bracket.
Statistical analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS) version 16. Descriptive analysis was used for finding the mean and standard deviations of the samples tested for shear bond strength and antimicrobial assay. A paired student t-test was done for comparison of the various groups used for the shear bond testing and a post hoc test and t-test was done for the comparison of groups used for antimicrobial testing.
RESULTS
Disc diffusion assay
The RMGI (Fuji Ortho LC GC America, Alsip, Ill) without zinc oxide and Transbond XT showed no zones of inhibition. Antimicrobial activity, as measured by the zones of inhibition, increased as the concentration of ZnO increased. The 23.1% of zinc oxide mixture showed a greater zone of inhibition than the 13% of zinc oxide mixture [Table 1 and Figure 1] and the difference in the antimicrobial activity was statistically significant [Table 2] (P < 0.01).
Table 1.
Descriptive analysis (mean and standard deviation of the samples of antimicrobial testing)
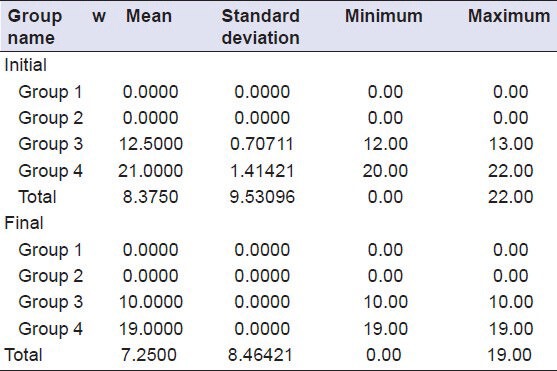
Figure 1.
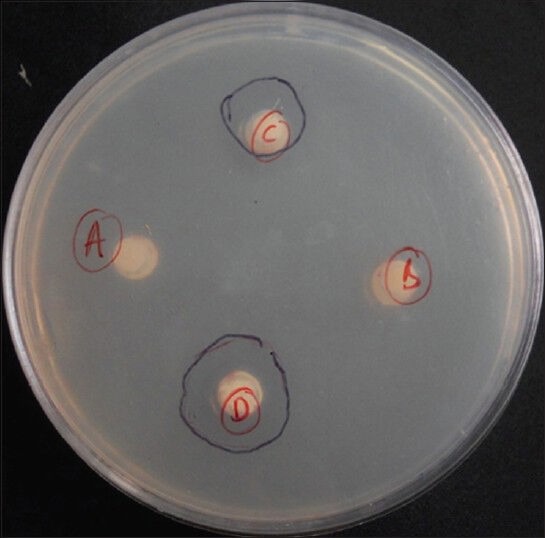
Agar plate showing zones of inhibition around Groups 3 and 4
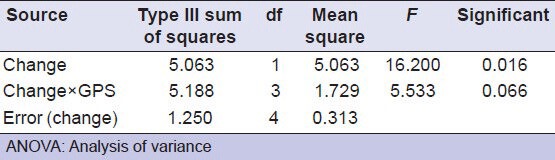
Table 2.
Post hoc ANOVA showing comparision of the groups used for antimicrobial testing (measure: measure 1)
After daily rinsing for the 1 month, both the 13% ZnO and 23.1% ZnO mixtures showed antimicrobial activity, whereas there were no zones of inhibition around discs of Groups 1 and 2 [Figure 2]. There was no statistically significant difference between the antimicrobial activity of Group 3 or 4 compared to the previous month (P > 0.01) [Tables 3 and 4].
Figure 2.
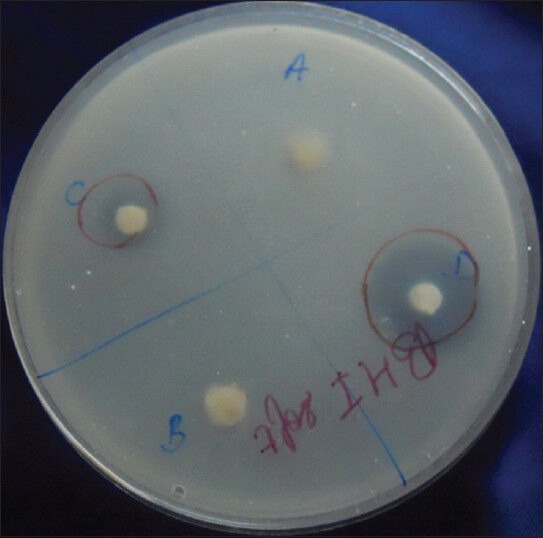
Agar plate showing zones of inhibition around Groups 3 and 4 (testing after 1 month)
Table 3.
T test: Paired samples test to compare the antimicrobial activity of Group 3 (initial and after 1 month)
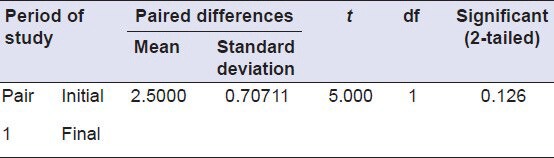
Table 4.
T test: Paired samples test to compare the antimicrobial activity of Group 4 (initial and after 1 month)
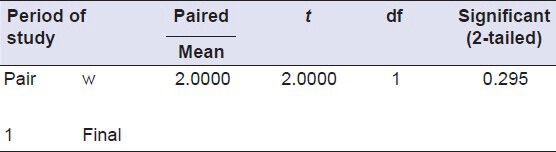
Shear bond testing
The data obtained is tabulated and the mean values for the shear bond strength with their standard deviations are determined. The mean value of shear bond strength of the composite is measured to be around 12.1708 ± 0.588, RMGIC 10.2683 ± 0.192, RMGIC + 13% ZnO is 8.9042 ± 0.13767, RMGIC + 23.1% ZnO is 7.6783 ± 0.07791 [Table 5]. The independent samples t-test showed that there was a significant difference between the shear bond strength of different groups [Table 6]. The mean shear bond strengths of all the groups are represented in the Figure 3.
Table 5.
Mean and standard deviation of the shear bond strength
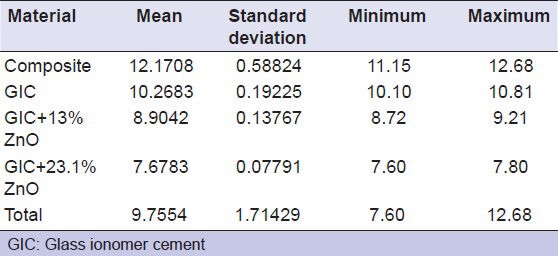
Table 6.
ANOVA to compare the difference in shear bond strength between the different groups
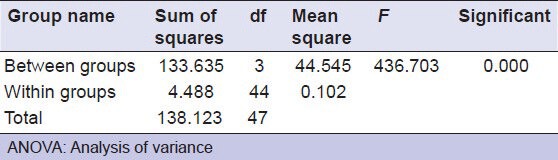
Figure 3.
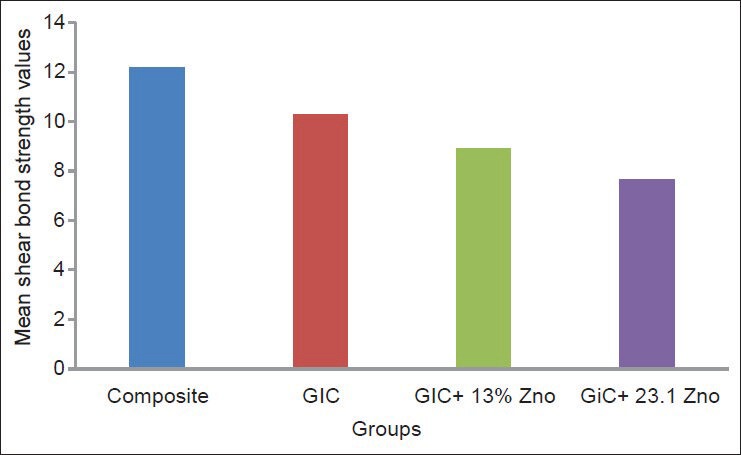
Shear bond strength of different samples
DISCUSSION
Zinc oxide showed significant antimicrobial effects on the disc diffusion test, as demonstrated by visible zones of inhibition. Zones of inhibition were formed on both the agar plates, which were tested. Since the addition of zinc oxide was the only change made to the RMGIC (Fuji Ortho LC GC America, Alsip, Ill), it can be assumed that the antimicrobial property is attributed to the addition of zinc oxide. This is in accordance with the other studies conducted on the antimicrobial activity of zinc oxide.[10,11] In this study, the antibacterial properties of ZnO were investigated against S. mutans which is the most common microorganisms which causes dental caries.[12] Most of the studies reported have evaluated the initial microbial inhibition only, but it seems equally important to determine the effect over a longer time interval. In the study the antimicrobial effect of zinc oxide was tested over a 1 month period.
It is necessary to understand the mechanism of action of ZnO against bacteria, but to date, the process underlying their antibacterial effect is not clear. However, a few studies have suggested that the primary cause of the antibacterial function might be from the disruption of the cell membrane activity. Another possibility could be the induction of intercellular reactive oxygen species, including hydrogen peroxide (H2O2), a strong oxidizing agent harmful to bacterial cells.[10,13]
The antimicrobial property of zinc oxide was concentration dependent with an increase in the concentration of zinc oxide (23.1%) there was an increase in the antimicrobial property. These findings agree with other studies, which show that the antimicrobial properties are concentration dependent.[4,14]
Moorer and Genet[15] proposed that antimicrobial action from zinc oxide may be a result of a “reservoir” effect. Including larger amounts of zinc into the bonding material would, therefore, make more zinc ions available for mobilization.
The shear bond strength of the brackets bonded with light cure composite was greater than the shear bond strength of the brackets bonded with RMGIC and RMGIC with 13% and 23.1% zinc oxide. Shammaa et al.[16] in 1999 concluded that the bracket debonding force of RMGIC (Fuji Ortho LC GC America, Alsip, Ill) in wet and dry conditions was found to be significantly lower than the conventional resins. The shear bond strength of the RMGIC group was found to be 10.2683 MPa (±0.192), which was in the range of shear bond strength recommended for RMGIC.[5,16,17,18,19,20]
There are no clear guidelines as to the best method for enamel treatment prior to bonding with conventional GICs. Simple prophylaxis and drying the tooth with a cotton pellet has been shown to produce a better bond than treating the enamel surface with an enamel cleanser (40% polyacrylic acid) while the effect of etching enamel with phosphoric acid is disputed some authors finding it produced a significantly poorer bond strength while others found an increase in mean bond strength although this was not significantly greater than to un-etched enamel.[21,22,23]
The variation in the bond strength in the study might be attributed to the method used for measuring the shear bond strength. The blade used in the study applied the force closer to the tie wing rather than the base. Klocke and Kahl-Nieke[24] demonstrated that variation of the direction of debonding force significantly influences shear bond-strength measurements. Changes in orientation of the shearing force by as little as 15° can decrease bond strength values by 27.4%.
Mean bond strength of GIC mixed zinc oxide in this study falls in the lower range of shear bond strength recommended for GIC. It might be partly attributed to the fact that simple means of incorporating the zinc oxide were employed, and a larger than normal liquid portion of the RMGIC was used, which could have weakened the material.
Future studies should evaluate more refined methods of adding zinc oxide in order to have less of an impact on the physical properties of the bonding agent.
CONCLUSION
The present study has shown that zinc oxide powder when added to GIC produces antimicrobial effect, which increases as the concentration of zinc oxide is increased.
The antimicrobial effect of zinc oxide lasts at least for 1 month.
The shear bond strength decreases as the concentration of ZnO increases.
ACKNOWLEDGMENT
We would like to thank Dr. G. Vijay Kumar, MD Professor and Head, Department of microbiology, Dr. Raghavendra, MD Associate Professor and the post graduate students of Department of microbiology J.S.S. Medical College and Hospital, Mysore, for their valuable help and cooperation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–8. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell L. Decalcification during orthodontic treatment with fixed appliances-An overview. Br J Orthod. 1992;19:199–205. doi: 10.1179/bjo.19.3.199. [DOI] [PubMed] [Google Scholar]
- 3.Artun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod. 1986;8:229–34. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
- 4.Al-Musallam TA, Evans CA, Drummond JL, Matasa C, Wu CD. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofacial Orthop. 2006;129:245–51. doi: 10.1016/j.ajodo.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Hitmi L, Muller C, Mujajic M, Attal JP. An 18-month clinical study of bond failures with resin-modified glass ionomer cement in orthodontic practice. Am J Orthod Dentofacial Orthop. 2001;120:406–15. doi: 10.1067/mod.2001.115931. [DOI] [PubMed] [Google Scholar]
- 6.Tinanoff N, Klock B, Camosci DA, Manwell MA. Microbiologic effects of SnF2 and NaF mouthrinses in subjects with high caries activity: Results after one year. J Dent Res. 1983;62:907–11. doi: 10.1177/00220345830620081201. [DOI] [PubMed] [Google Scholar]
- 7.Marcusson A, Norevall LI, Persson M. White spot reduction when using glass ionomer cement for bonding in orthodontics: A longitudinal and comparative study. Eur J Orthod. 1997;19:233–42. doi: 10.1093/ejo/19.3.233. [DOI] [PubMed] [Google Scholar]
- 8.Uysal T, Amasyali M, Ozcan S, Koyuturk AE, Sagdic D. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: An in-vivo study. Am J Orthod Dentofacial Orthop. 2011;139:650–6. doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Spencer CG, Campbell PM, Buschang PH, Cai J, Honeyman AL. Antimicrobial effects of zinc oxide in an orthodontic bonding agent. Angle Orthod. 2009;79:317–22. doi: 10.2319/011408-19.1. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77:2325–31. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha S, Samadi F, Jaiswal JN, Ghoshal U. Antimicrobial activity of different endodontic sealers: An in vitro evaluation. J Indian Soc Pedod Prev Dent. 2010;28:251–7. doi: 10.4103/0970-4388.76151. [DOI] [PubMed] [Google Scholar]
- 12.Alves PV, Alviano WS, Bolognese AM, Nojima LI. Treatment protocol to control Streptococcus mutans level in an orthodontic patient with high caries risk. Am J Orthod Dentofacial Orthop. 2008;133:91–4. doi: 10.1016/j.ajodo.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Atmaca S, Gul K, Cicek R. The effect of zinc on microbial growth. Turk J Med Sci. 1998;28:595–7. [Google Scholar]
- 14.Othman HF, Wu CD, Evans CA, Drummond JL, Matasa CG. Evaluation of antimicrobial properties of orthodontic composite resins combined with benzalkonium chloride. Am J Orthod Dentofacial Orthop. 2002;122:288–94. doi: 10.1067/mod.2002.123947. [DOI] [PubMed] [Google Scholar]
- 15.Moorer WR, Genet JM. Antibacterial activity of gutta-percha cones attributed to the zinc oxide component. Oral Surg Oral Med Oral Pathol. 1982;53:508–17. doi: 10.1016/0030-4220(82)90468-6. [DOI] [PubMed] [Google Scholar]
- 16.Shammaa I, Ngan P, Kim H, Kao E, Gladwin M, Gunel E, et al. Comparison of bracket debonding force between two conventional resin adhesives and a resin-reinforced glass ionomer cement: An in vitro and in vivo study. Angle Orthod. 1999;69:463–9. doi: 10.1043/0003-3219(1999)069<0463:COBDFB>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Bishara SE, Vonwald L, Laffoon JF, Jakobsen JR. Effect of altering the type of enamel conditioner on the shear bond strength of a resin-reinforced glass ionomer adhesive. Am J Orthod Dentofacial Orthop. 2000;118:288–94. doi: 10.1067/mod.2000.104903. [DOI] [PubMed] [Google Scholar]
- 18.Sfondrini MF, Cacciafesta V, Pistorio A, Sfondrini G. Effects of conventional and high-intensity light-curing on enamel shear bond strength of composite resin and resin-modified glass-ionomer. Am J Orthod Dentofacial Orthop. 2001;119:30–5. doi: 10.1067/mod.2001.111399. [DOI] [PubMed] [Google Scholar]
- 19.Summers A, Kao E, Gilmore J, Gunel E, Ngan P. Comparison of bond strength between a conventional resin adhesive and a resin-modified glass ionomer adhesive: An in vitro and in vivo study. Am J Orthod Dentofacial Orthop. 2004;126:200–6. doi: 10.1016/j.ajodo.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Movahhed HZ, Ogaard B, Syverud M. An in vitro comparison of the shear bond strength of a resin-reinforced glass ionomer cement and a composite adhesive for bonding orthodontic brackets. Eur J Orthod. 2005;27:477–83. doi: 10.1093/ejo/cji051. [DOI] [PubMed] [Google Scholar]
- 21.Millett DT, McCabe JF. Orthodontic bonding with glass ionomer cement - A review. Eur J Orthod. 1996;18:385–99. doi: 10.1093/ejo/18.4.385. [DOI] [PubMed] [Google Scholar]
- 22.Ewoldsen N, Beatty MW, Erickson L, Feely D. Effects of enamel conditioning on bond strength with a restorative light-cured glass ionomer. J Clin Orthod. 1995;29:621–4. [PubMed] [Google Scholar]
- 23.Gaworski M, Weinstein M, Borislow AJ, Braitman LE. Decalcification and bond failure: A comparison of a glass ionomer and a composite resin bonding system in vivo. Am J Orthod Dentofacial Orthop. 1999;116:518–21. doi: 10.1016/s0889-5406(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 24.Klocke A, Kahl-Nieke B. Effect of debonding force direction on orthodontic shear bond strength. Am J Orthod Dentofacial Orthop. 2006;129:261–5. doi: 10.1016/j.ajodo.2004.07.048. [DOI] [PubMed] [Google Scholar]


