Abstract
The ubiquitous presence of long noncoding RNAs (lncRNAs) in eukaryotes points to the importance of understanding how their sequences impact function. As many lncRNAs regulate nuclear events and thus must localize to nuclei, we analyzed the sequence requirements for nuclear localization in an intergenic lncRNA named BORG (BMP2-OP1-responsive gene), which is both spliced and polyadenylated but is strictly localized in nuclei. Subcellular localization of BORG was not dependent on the context or level of its expression or decay but rather depended on the sequence of the mature, spliced transcript. Mutational analyses indicated that nuclear localization of BORG was mediated through a novel RNA motif consisting of the pentamer sequence AGCCC with sequence restrictions at positions −8 (T or A) and −3 (G or C) relative to the first nucleotide of the pentamer. Mutation of the motif to a scrambled sequence resulted in complete loss of nuclear localization, while addition of even a single copy of the motif to a cytoplasmically localized RNA was sufficient to impart nuclear localization. Further, the presence of this motif in other cellular RNAs showed a direct correlation with nuclear localization, suggesting that the motif may act as a general nuclear localization signal for cellular RNAs.
INTRODUCTION
Discovery of the nearly ubiquitous presence of long non-protein-coding RNAs (lncRNAs) in higher eukaryotes has been one of the most fundamental advances in understanding the function of eukaryotic genomes in recent years (1–6). Increasing evidence suggests that many lncRNAs are tissue specific and developmentally regulated and play important functional roles in diverse aspects of cellular function, with regulation of chromatin states and other nuclear events emerging as a major function of this class of transcripts (2, 7–10). However, due to their relatively recent discovery, fundamental aspects of lncRNAs' mechanisms of action have remained almost entirely unknown. This is particularly true in the case of long intergenic lncRNAs (11). Many intergenic lncRNAs act in trans, and thus, by necessity, their function must be mediated by the RNA molecule itself rather than by the mere act of transcription, which seems to be the mode of function of many cis-acting RNAs (12). Data from the study of a small number of intergenic lncRNAs suggest that, at least in some cases, they modulate the functions of proteins with which they interact through poorly understood mechanisms that may involve the juxtaposition of two or more bound macromolecules in a functionally conducive manner and recognition of particular RNA or DNA sequences through base pairing (2, 13–15). However, it is not clear how the ability of an RNA molecule to interact with other RNAs, proteins, or DNA sequences can result in a particular functional outcome. While it is possible that many lncRNAs act by increasing the local concentration of a certain protein through binding and “recruiting” it to a certain cellular domain (2, 13, 14, 16), it is likely that, at least in some cases, the RNAs play a role beyond serving as a simple recruitment platform (9, 15).
The functions of proteins, which in most cases also depend on their interactions with other proteins and RNA and DNA sequences, largely hinge on the presence of one or more discrete functional motifs from a finite repertoire. In many cases, the specific context and spatial orientation of the motif within the protein molecule enables a particular mode of interaction with other biological macromolecules, leading to function (17). While many structural and chemical properties of RNA molecules are distinct from those of proteins, it is possible to envision that at least some lncRNAs also contain shared RNA functional motifs that fall into distinct classes and impart a related functional property to the RNAs that contain them. At the other extreme of the spectrum, the functions of lncRNAs may be mediated through unique functional motifs and strategies that are specific to each RNA, and thus, each lncRNA may be mechanistically unique. Unlike proteins, most lncRNAs seem to have arisen de novo rather than via gene duplication and thus are unlikely to share similar motifs due to common evolutionary descent. However, significant fractions of the sequences of many lncRNAs are derived from transposable elements (18, 19), leading to the existence of shared sequences among many lncRNAs. It is possible that through evolutionary adaptation some transposon-derived sequences have acquired functional roles in the context of lncRNAs, forming the basis for the evolution of related sets of RNA functional motifs (18–20). Further, analysis of other classes of functional RNAs, such as ribozymes and riboswitches, has pointed to the possibility of convergent evolution, especially in the generation of small RNA motifs (21, 22). Thus, it is possible to envision a scenario in which lncRNAs for the most part use highly diverse strategies to perform their functions yet some share RNA motifs that have convergently evolved in different RNAs in response to the evolutionary pressure to perform very similar functions. The discovery of such motifs will not only provide critical insight into the functions of individual lncRNAs (23, 24) but will also shed light on the fundamental functional strategies used by this diverse and abundant class of transcripts.
As a first step toward understanding the functional architecture of the lncRNAs, we focused on a commonly observed property of these transcripts, namely, their localization to cell nuclei (25, 26). As mentioned above, many lncRNAs are involved in regulation of nuclear events, and consistently, a large fraction of them are predominantly or even strictly localized in the nucleus. Further, the nuclear localization of these RNAs likely plays a crucial functional role by preventing them from interacting with the translation machinery and coding for potentially harmful short peptides. Studies on subcellular localization of RNAs have mostly focused on the nuclear export of protein-coding RNAs, in which 5′ capping, splicing, and deposition of the exon junction complex and polyadenylation play the major roles, although the involvement of some RNA sequence motifs has also been documented (27–31). In intronless RNAs, polyadenylation seems to play the dominant role in export of RNAs to the cytoplasm (32–35). In contrast, the mechanism of nuclear retention and import of RNAs is poorly understood, except in the case of a few highly abundant classes of housekeeping RNAs. The majority of such studies have focused on the nuclear localization of snRNAs and snoRNAs and have revealed the involvement of short, shared RNA elements, namely, the U-rich sm motifs and H/ACA and C/D boxes (36–38). These nucleate the assembly of protein complexes (e.g., the Sm ring in the case of snRNAs) that in turn mediate the nuclear import of the resulting RNA-protein complex from the cytoplasm (37). In the case of U6 snRNA, which unlike other snRNAs is an RNA polymerase III (Pol III) transcript and is never exported to the cytoplasm, the nuclear retention is mediated through the interaction of a short U-rich sequence at its 3′ end with the Pol III-specific La protein (Sjögren syndrome antigen B [SSB]) and later assembly of an LSm ring (37, 39). Thus, the nuclear import and retention mechanisms acting on small housekeeping RNAs depend on the presence of short, shared RNA motifs that nucleate RNA-protein interactions that lead to the nuclear localization of the RNA, and, at least in one case, seem to be influenced by the promoter from which the RNA is transcribed. Although the presence of a significant population of longer, fully mature polyadenylated transcripts that are predominantly localized in the nucleus has been known for well over a decade (25, 40), the mechanism of nuclear localization of these RNAs, the majority of which are lncRNAs, has remained largely unstudied.
While many lncRNAs function as nascent transcripts and are rapidly degraded after the completion of their transcription, a significant fraction of the nucleus-localized lncRNAs are stable transcripts and are spliced and polyadenylated (25, 26, 41). This is somewhat surprising, since both splicing and polyadenylation are known to strongly stimulate nuclear export in protein-coding transcripts (27, 28). Thus, the nuclear localization of fully processed lncRNAs points to the presence of additional, hitherto-unknown subcellular localization mechanisms and, potentially, novel RNA motifs that govern the functionally required nuclear retention or import of these transcripts.
To gain insight into the identity of the signal(s) that mediates nuclear localization of RNAs, we chose a trans-acting, spliced and polyadenylated, nucleus-localized intergenic lncRNA named BORG (BMP2-OP1-responsive gene) (42) as a study model. We could show that BORG was strictly nuclear and that its nuclear localization was not affected by the cellular state or its genomic expression locus but rather by sequences or structures within the mature, spliced BORG transcript. Using an extensive mutagenesis approach, we succeeded in identifying a short RNA motif in BORG that mediated the nuclear localization of the RNA. Mutation of the motif to a scrambled sequence resulted in loss of nuclear localization, while addition of a single copy of the motif to a cytoplasmically localized RNA led to nuclear localization. To determine whether this motif was functional only in the context of BORG RNA or had a more global role, we analyzed the subcellular localization of several protein-coding and noncoding cellular transcripts. Interestingly, the presence and copy number of this motif, which consists of the pentamer sequence AGCCC with sequence restrictions at positions −8 (T or A) and −3 (G or C) relative to the first nucleotide of the pentamer, showed a direct correlation with the extent of nuclear localization of each transcript. These results provide the first glimpse into the identity and organization of functional motifs that govern the cellular activity of lncRNAs and also point to the presence of shared functional motifs between lncRNAs and protein-coding RNAs.
MATERIALS AND METHODS
Vector construction and stable cell line derivation.
The full-length mouse BORG overexpression vectors were constructed by cloning the sequence of the spliced BORG transcript, followed by its native polyadenylation signal, into mammalian expression vectors pCMV-Script A2 (Stratagene; for lower expression levels) or pLntx (for achieving higher expression levels). The sequence of BORG was cloned in a way that the transcription start site of the vector corresponded to the first nucleotide of BORG; thus, the resulting BORG transcript did not contain any sequences not present in the endogenous BORG RNA. Both vectors contained a neomycin/G418 resistance gene. The expression vectors for various truncation mutants and fragment constructs were made in the same fashion, using the pCMV-Script A2 vector. All constructs contained the desired BORG-derived sequence cloned directly at the transcription start site of the vector, followed by the native polyadenylation signal of BORG. For the Mutant-1 to -6 and Frag1 to -6 series, we chose the boundaries of deleted regions/fragments based on the predicted secondary structure of the RNA and the level of primary sequence conservation, so that secondary structures and conserved regions remained intact and within a single fragment. All constructs (listed in Table S1 in the supplemental material) were sequenced to ensure the accuracy of the cloned sequences. All transfections were carried out on ∼40% confluent cells grown in a monolayer, using Fugene 6 (Roche). Since transiently transfected cells may have compromised subcellular RNA localization, we derived stable cell lines for every construct tested in this study. To derive stable cell lines in C2C12 mouse myoblasts, newly transfected cells received 1 mg/ml of G418 for selection of stably transfected cells. The cell culture medium was changed every 3 days, and the emerging, well-separated colonies were isolated using cloning disks 2 weeks after transfection. At least four colonies were analyzed for each transfected construct to ensure reproducibility. The cells were cultured in medium supplemented with 500 μg/ml G418 to maintain the expression of the transfected genes. The expression of all BORG-derived constructs was verified by both quantitative and radioactive reverse transcription-PCR (RT-PCR).
Cell culture.
The C2C12 cells were a kind gift from Nikki Harter (early-passage C2C12 stock). An independent C2C12 stock, originating from ATCC, was also used. 4T1 and NMuMg cells were kind gifts from William Schiemann. HeLa and HEK293 cells were the kind gift of Maria Hatzoglou. All cells were cultured in Dulbecco's modified essential media (DMEM) (Invitrogen) supplemented with 15% fetal bovine serum (Invitrogen), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere containing 5% CO2. The cells were seeded at a density of 1.0 × 104/cm2 and passaged at ∼55% confluence 48 h before harvesting. The cells were harvested at a confluence of 80%.
Preparation of nuclear and cytoplasmic fractions.
The nuclear and cytoplasmic fractions were prepared as described previously (43). Briefly, C2C12 cells were cultured in 150-mm dishes for 48 h to reach 80% confluence. For harvesting, the cells were washed with cold phosphate-buffered saline (PBS) and scraped into a 15-ml conical tube. Next, the cells were centrifuged at 1,000 × g for 5 min. The pellet was resuspended in prechilled cell disruption buffer containing 1.5 mM MgCl2, 10 mM KCl, 20 mM Tris-Cl, and 1 mM dithiothreitol (DTT) and incubated on ice for 10 min. After the incubation period was over, the cell membrane was disrupted using a Dounce homogenizer. The homogenate was visually inspected under a microscope to ensure that ≥85% of the cells had broken cellular membranes, and every effort was made to ensure that the nuclei remained intact. This was done to minimize the contamination of the cytoplasmic fraction with nuclear fraction, allowing better detection of strictly nucleus-localized RNAs. The homogenate was transferred to a new centrifuge tube, and Triton X-100 was added to a final concentration of 0.1%. The tubes were inverted several times to mix. The nuclear and cytoplasmic portions were separated by centrifuging the homogenate at 1,500 × g for 5 min. The nuclear and cytoplasmic RNAs were extracted using Tri-Reagent according to the manufacturer's instructions (Molecular Research Center; TRI 118).
RT-PCR.
For the quantitative RT-PCRs (RT-qPCRs), cDNA was synthesized in reverse transcription reactions using Moloney murine leukemia virus (MMLV) reverse transcriptase (United States Biochemicals) according to the manufacturer's instructions, using primers specific to each amplicon (the reverse primer used in each PCR) except when noted. The cDNA obtained was added to mixtures for quantitative PCRs, which were performed using IQ SYBR green supermix from Bio-Rad on a Mastercycler ep realplex detection system (Eppendorf). For the radiolabeled PCRs, all the primer sets were optimized to determine the linear range and the uniqueness of the resulting amplicon. The forward primers were 5′ 32P labeled using Optikinase (United States Biochemicals). The reactions were performed using the One-Step RT-PCR mix (United States Biochemicals), and the products were loaded on a 5% native PAGE, along with a radiolabeled size marker. To determine the polyadenylation state of an RNA, the reverse transcription reaction was performed using oligo(dT) primers, and the PCR was performed using a primer set that specified an amplicon close to the 3′ end of each RNA construct. Mock reactions that lacked the oligo(dT) were set up to ensure that the observed amplicon came from cDNAs resulting from oligo(dT) reverse transcription. To ensure that the observed PCR product did not result from oligo(dT) binding to an A-rich sequence within the BORG RNA, the sequence of each mutant RNA was analyzed for the presence of such sequences, and the PCR primers were designed in a way that there would not be any A-rich sequences between the 3′ end of the RNA and the PCR-amplified region. The gels were exposed using a PhosphorImager, and the bands corresponding to the desired amplicon were quantitated using ImageQuant software. Each experiment was repeated at least three times, with at least two biological repeats. The average value from the different repeats was graphed, with error bars showing 2 standard deviations. The averaged value of the control sample in each experiment was set to an arbitrary value of 1, and the value of the rest of the samples was normalized against it. The PCR primers used in this study are listed in Table S3 in the supplemental material.
In situ hybridization.
C2C12 cells were plated in a Labtek II slide chamber (Nalgene Nunc International). After reaching 70% confluence, the cells were washed in 1× PBS (pH 7.4) and fixed in freshly prepared 4% paraformaldehyde (Electron Microscopy Science) in PBS for 20 min at room temperature. The fixed cells were rinsed twice for 5 min each time in PBS and were permeabilized by incubation in a solution of 0.3% Triton X-100 in PBS for 10 min at room temperature, followed by acetylation in freshly prepared 0.25% acetic acid anhydride in 0.1 M triethanolamine (TEA) for 10 min at room temperature. The cells were rinsed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 10 min at 45°C and air dried for 5 min on a slide warmer at 60°C and were used immediately for hybridization. Locked nucleic acid (LNA) hybridization probes containing a fluorescein isothiocyanate (FITC) label at the 5′ end were purchased from Exiqon. The probes were designed to minimize off-target binding and were tested against short hairpin RNA (shRNA)-mediated BORG knockdown cells in which the level of BORG was over 90% reduced based on RT-PCR assays in order to prove the specificity of the signal. The LNA probes were diluted with hybridization buffer (see below) to a concentration of 100 nM and denatured at 80°C for 5 min. Hybridization was performed overnight in a humid chamber at 44°C in 300 μl of hybridization buffer containing 50% (vol/vol) formamide, 10 mM Tris-HCl, pH 7.5, 5 mM EDTA, pH 8.0, 2× SSC, 1× Denhardt's solution, 100 μg/ml salmon sperm DNA, 10% dextran sulfate, and 100 nM denatured LNA RNA probes. Nonspecific RNA hybridization was eliminated by washing with 2× SSC, 0.1% Tween 20 at 44°C for 10 min, followed by 3 washes with 0.1× SSC at 65°C. The slides were dehydrated by 5 min of sequential incubation in 50%, 75%, 90%, and 100% ethanol at room temperature and were then air dried and mounted with ProLong Gold Antifade reagent (Invitrogen) containing DAPI (4′,6-diamidino-2-phenylindole) for nuclear counterstaining. The LNA RNA probe was complementary to nucleotides 2403 to 2424 of mouse BORG RNA (5′-CCTTTAATATTCCCATTAACCT-3′). Images were obtained with a Zeiss Axioskop 2 Plus fluorescence microscope at the indicated exposure times.
Heat shock, BMP2, DRB, and cycloheximide treatments.
C2C12 cells were seeded at a density of 1.0 × 104 cells/cm2 48 h before treatment to allow them to reach a confluence of 80% to 90%. For heat shock treatment, the cells were incubated at 44°C in a humidified atmosphere containing 5% CO2 for 1 h and then harvested for fractionation as described above. For cycloheximide treatments, cells were incubated in the presence of 20 μg/ml of cycloheximide for 1 to 12 h, followed by harvest of the cells for fractionation. For BMP2 treatment, cells were plated 24 h before the addition of BMP2 at a concentration of 300 ng/ml, followed by the harvest of cells for fractionation at the indicated time points. For analysis of the impact of cycloheximide on BMP2-treated cells, cells received BMP2 as described above, and cycloheximide was added after 4 h, followed by harvest of the cellular RNA after an additional 2 h. For DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole) treatments, cells were plated at 15% density 48 h prior to the experiment. Once at 80% confluence, DRB was added to the medium at a 20 μM final concentration, and cells were harvested at the indicated time points.
Analysis of the pentamer motifs.
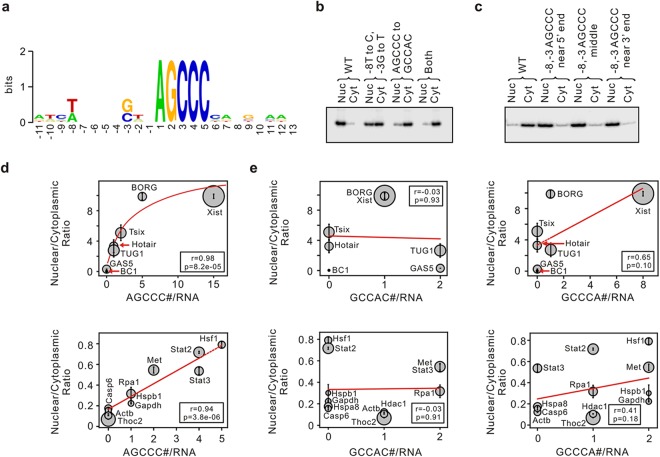
The sequences in BORG that harbored the putative nuclear localization signal were analyzed by BLAST-based algorithms and Weblogo (44) to determine the presence of a potential shared motif, with the two methods yielding identical motifs (see Fig. 6a). The sequence and annotation data for all mouse protein-coding and noncoding genes in the Ensembl database (45) were downloaded from the Ensembl website. To ensure our data were not significantly affected by the annotation discrepancy between different genomic databases, we also obtained sequence information from the University of California—Santa Cruz (UCSC) database (46). Determination of the pentamer copy number, analysis of the data, and preparation of the graphs were performed using scripts written in Python and employed SciPy, NumPy, and Matplotlib libraries on a Linux platform. From the list of protein-coding RNAs, five subgroups containing 0 to 5 copies of the −8 (A/T), −3 (C/G), AGCCC motif were made, and several candidates were randomly chosen from each subgroup for RT-qPCR analysis. From these candidate RNAs, those with multiple splicing isoforms that harbored different copy numbers of the pentamer motifs were discarded. The two commonly used housekeeping genes, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes, were also included. From the functionally studied lncRNAs, six candidates were chosen. The levels of cytoplasmic and nucleus-localized transcripts for each RNA species were determined using RT-qPCR analysis on at least two independently prepared fractionated extracts from C2C12 cells with at least two technical repeats per extract. The RT-PCR primers were chosen so that they detected all isoforms of an RNA, if it had any, and also, when possible, the primers flanked an exon-exon junction (see Table S3 in the supplemental material) to ensure that mature, rather than nascent, transcripts were being detected. For the RNAs that showed detectable expression in the RT-qPCR assays on C2C12 extracts (12 protein-coding RNAs and seven lncRNAs, including BORG [see Table S2 in the supplemental material]), the ratio of nucleus- to cytoplasmically localized transcripts for each RNA was calculated and graphed against the copy number of pentamer motifs. To choose scrambled pentamers for comparison with the AGCCC motif, we examined all 19 possible scrambled motifs. We eliminated those that strongly resembled the AGCCC motif (CAGCC and CCAGC) and the ones that were not present (zero copies) in over 3/4 of our tested RNAs and thus did not provide analyzable data. The copy numbers of the remaining two pentamers (along with sequence restrictions at positions −8 and −3 identical to those in Fig. 6a) were determined for each RNA and graphed against the ratio of nuclear to cytoplasmic RT-qPCR signals. The statistical tests used to analyze the significance of the data were chosen in consultation with Case Western Reserve University's Statistical Sciences Core Facility. Trendlines, Spearman's correlation coefficient, and two-tailed P values were drawn and calculated using NumPy, SciPy, and the Pyplot library of Matplotlib.
FIG 6.
A short RNA motif mediates the localization of RNAs to the nucleus. (a) Information content of the nucleotide positions within and around the AGCCC motif. (b) Mutation of the AGCCC sequence or the nucleotides at positions −3 and −8 leads to loss of nuclear localization of Frag2 RNA. The identity of the mutation tested is shown above each set of samples. Nuc and Cyt refer to nuclear and cytoplasmic fractions, respectively. WT, unmodified Frag2 transcript. (c) Insertion of a single copy of the motif near the 5′ or 3′ end or the middle of the Frag5-Reverse+complement RNA results in nuclear localization. WT, unmodified Frag5-Reverse+complement RNA. (d) The copy number of −8 (A/T), −3 (G/C), AGCCC motif per transcript shows a direct correlation with nuclear localization in both lncRNAs (top) and mRNAs (bottom). The correlation coefficient (r) and two-tailed P values (p) for the log (top) and linear (bottom) fits are shown. The size of the circle marking each transcript is proportional to the length of the RNA. The center of each circle corresponds to the average value of three independent experiments, with vertical lines (error bars) corresponding two standard deviations. The identity of each RNA is shown. (e) Analysis similar to that in panel d with two scrambled pentamers replacing AGCCC indicating the specificity of the correlation observed with AGCCC.
RESULTS
The spliced, polyadenylated BORG lncRNA has a strict nuclear localization.
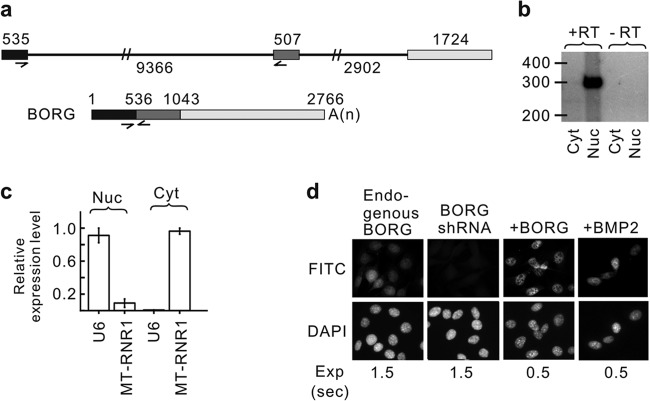
As the first step toward understanding the organization of functional RNA domains in lncRNAs, we focused on the mechanisms regulating the subcellular localization of these transcripts. Since a large fraction of lncRNAs localize to the nucleus, it is likely that many of them contain a sequence motif(s) that mediates their localization, and thus, looking for such motifs was likely to be a fruitful starting point. As a study model, we chose the lncRNA BORG, which was discovered as a gene upregulated in response to BMP2 in mouse C2C12 myoblasts (42). We analyzed the genomic locus of BORG on chromosome 15qB3.1 in the mouse, which showed that it did not overlap any known transcripts and thus fell into the category of intergenic, potentially trans-acting lncRNAs. The mature, 2,766-nucleotide-long BORG transcript is generated through splicing of two introns from an ∼15-kb-long primary transcript (Fig. 1a) and contains a canonical polyadenylation signal and downstream 3′ processing elements (see Fig. S1 in the supplemental material). We confirmed that BORG is indeed spliced and polyadenylated (Fig. 1b; see Fig. S2a to f in the supplemental material). To determine the subcellular localization of BORG, we performed nuclear/cytoplasmic fractionation on C2C12 cells, followed by both RT-qPCR and sensitive RT-PCR assays using radiolabeled primers, which indicated that BORG could be detected only in the nuclear fraction (Fig. 1b; see Fig. S2a in the supplemental material). In these experiments, we used a pair of PCR primers that flanked the first intron of BORG (Fig. 1a) and thus ensured that the detected RT-PCR signal originated from the mature BORG transcript and not from the nascent transcript or possible genomic-DNA contamination in the nuclear fraction. We confirmed the purity of the nuclear and cytoplasmic fractions using U6 snRNA and U99 snoRNA as nuclear markers and the mitochondrial rRNA MT-RNR1 and 18S rRNA as cytoplasmic markers (Fig. 1c; see Fig. S2g and h in the supplemental material). We further confirmed these results by in situ hybridization assays, which indicated a diffuse nuclear localization pattern for BORG RNA (Fig. 1d). In addition to confirming the nuclear localization of BORG, the diffuse pattern of distribution of BORG in the nucleus indicates that the detected BORG RNAs are not nascent transcripts made and rapidly degraded at the site of transcription but rather are mature transcripts distributed throughout the nucleus. Analysis of the subcellular localization of BORG in other cell types, including mouse 4T1 breast cancer cells and human HEK293 and HeLa cells, indicated that BORG is a strictly nuclear RNA and that its subcellular localization is conserved between mouse and human (see Fig. S3a to e in the supplemental material).
FIG 1.
BORG lncRNA is a nuclear transcript. (a) Genomic architecture of the mouse BORG gene. The numbers indicate the lengths of the three exons (shown as rectangles) and two introns (shown as thin lines). The half-arrows mark the positions of PCR primers used in the majority of the experiments in this study. The processed, mature BORG transcript is shown at the bottom, with the numbers indicating the position of the first nucleotide of each exon from the 5′ end. A(n) represents the position of the poly(A) tail. The slash marks on the introns indicate that they have not been drawn to scale. (b) RT-PCRs on the nuclear (Nuc) and cytoplasmic (Cyt) fractions obtained from C2C12 cells using radiolabeled BORG primers shown in panel a that flank an exon-exon junction. The lanes marked −RT are control reactions in which the reverse transcriptase was omitted. The numbers mark the relative positions of double-stranded DNA of the indicated sizes (in nucleotides). The size of the amplicon corresponds to the expected size in the spliced BORG RNA. (c) Analysis of the levels of U6 (nuclear marker) and MT-RNR1 (cytoplasmic marker) in the nuclear and cytoplasmic fractionation using quantitative RT-PCR. The error bars represent the standard deviations calculated based on at least three independent experiments. (d) In situ hybridization assays using a FITC-conjugated BORG probe on wild-type C2C12 cells (endogenous BORG), C2C12 cells transfected with an shRNA against BORG to show specificity (BORG shRNA), C12C12 cells transfected with a full-length BORG overexpression cassette (+BORG), and BMP2-stimulated C2C12 cells (+BMP2). The exposure time (Exp) for each FITC image is shown at the bottom in seconds.
Since BORG was originally described as a BMP2-induced transcript (42), we asked whether the induction of BORG by BMP2 leads to a change in its subcellular localization. As can be seen in Fig. 2a, despite the expected rise in the level of BORG in BMP2-treated cells, BORG remained in the nucleus through the time course of the experiment. Analysis of MT-RNR1 and the snoRNA U99 as cytoplasmic and nuclear markers, respectively, showed that the small amount of BORG found in the cytoplasmic fraction results from leakage of nuclear fraction into the cytoplasm, as a small amount of U99 was also found in the cytoplasmic fraction at the same time points (see Fig. S3f in the supplemental material). Thus, even in the physiological context of its function (42), BORG remains strictly nuclear.
FIG 2.
The subcellular localization of BORG is independent of its expression level and cellular state. (a) BORG remains nuclear after BMP2 treatment. The average values of the relative expression levels of BORG from at least three independent experimental repeats are graphed with 2 standard deviations as error bars. (b) Transgene-expressed BORG transcripts are nucleus localized. The lanes marked Nuc and Cyt contain the results of RT-PCR assays on nuclear and cytoplasmic fractions, respectively. +BORG, cells stably transfected with the BORG transgene expressed from a low-expression-level promoter. (c) Quantitative RT-PCR assays indicating the extent of expression of the BORG transgene in BORG-overexpressing clones 1 and 2 compared to the endogenous copy. Ctrl, untransfected controls. (d and e) BORG remains in the nucleus after heat shock (d) and cycloheximide treatment (e). (f) Expression of BORG from a transgene with a highly active promoter (+++BORG) can result in over 50-fold overexpression of BORG compared to the endogenous levels. The expression level of the transgene used in panels b and c (+BORG) is shown for comparison. (g) BORG transcripts remain undetectable in the cytoplasmic fraction from cells stably transfected with the high-expression transgene (+++BORG) that express BORG at levels ∼50-fold higher than the endogenous level. The lane marked +BORG indicates the level of BORG in the nuclear fraction of +BORG cells. (h) RT-qPCR assays on total cellular RNA from untransfected NMuMG mammary epithelial cells (Control) and from NMuMG cells transfected with the low-expression transgene (+BORG) to detect the expression of BORG. (i) The transgene-expressed BORG in NMuMG cells is localized to the nucleus.
Sequence elements within the mature BORG transcript mediate its nuclear localization.
To gain more insight into the mechanism of nuclear localization of BORG, we asked whether, similar to the case of proteins, BORG itself contains a discrete nuclear localization signal or, if it is dependent on its promoter, genomic locus, or sequences within its introns for its subcellular localization. We constructed BORG expression cassettes that contained a nonendogenous cytomegalovirus (CMV)-based promoter, followed by the sequence of mature BORG transcript completely lacking the intronic sequences. To enable the resulting intronless transcript to interact with the nuclear export machinery, we included the genomic sequences ∼180 nucleotides downstream of the cleavage site of BORG RNA, which contained the GU-rich sequence required for efficient polyadenylation, as it has been shown that polyadenylation enables the efficient export of intronless transcripts (32–35). In addition, the presence of the native polyadenylation signal, cleavage site, and downstream elements ensured that the 3′-end processing of the transgene-derived BORG transcripts would be similar to that of the endogenous BORG. We further ensured that the transgene-expressed BORG would be identical in sequence to the endogenous BORG without any additional, vector-derived sequences by placing the first nucleotide of the BORG RNA at the transcription start site of the CMV promoter. We made stably transfected cell lines that were each derived from a single transfected cell and thus had distinct integration sites that would allow us to determine whether the genomic locus of the transcript had any impact on its localization. Importantly, in situ hybridization and RT-PCR analysis of the nuclear and cytoplasmic fractions derived from the stably transfected cells indicated that the transgene-expressed BORG remained strictly nuclear (Fig. 1d and 2b and c; see Fig. S3g in the supplemental material). Since it has been shown that expression of some cDNA constructs (such as β-globin cDNA) results in unstable, nonpolyadenylated transcripts that are degraded in the nucleus and are not efficiently exported to the cytoplasm (34, 47, 48), we compared the stability of the endogenous spliced BORG with that of the transgene-expressed RNAs. RT-PCR analysis of cellular RNA obtained at several time points following treatment with DRB, which inhibits RNA polymerase II, indicated that the endogenous BORG and transgene-expressed RNAs have closely similar half-lives of 8 to 9 h (see Fig. S3h and i in the supplemental material). Thus, both the endogenous and the transgene-expressed BORG RNAs are stable transcripts, and their lack of cytoplasmic localization is not due to rapid degradation in the nucleus. We also looked for the presence of the expression and nuclear retention element (ENE), which has been shown to mediate the nuclear stabilization of lncRNAs PAN, MALAT1, and MENβ, but could not find a similar motif in BORG RNA (49–51). We also looked for the presence of A-to-I-edited nucleotides in BORG, which has been previously shown to result in nuclear retention (52, 53). Reverse transcription reactions followed by PCR and dideoxy sequencing did not show any evidence of mixed A/G peaks (data not shown) (54), indicating the absence of detectable A-to-I editing in BORG.
Another potential mechanism to explain the observed nuclear localization is the rapid degradation of the cytoplasmically localized transcripts by nonsense-mediated decay (NMD) pathways. As the majority of lncRNAs contain multiple small open reading frames (ORFs) that can potentially trigger NMD (55), the depletion of cytoplasmically localized transcripts can lead to the dominant presence of intact transcripts in the nucleus, as was proposed to be the case for GAS5 lncRNA (56, 57). To determine if the subcellular localization of BORG was regulated by NMD, we treated the cells with heat shock (Fig. 2d; see Fig. S4a and d in the supplemental material) and cycloheximide (Fig. 2e; see Fig. S4b and e in the supplemental material), both of which block translation and thus prevent NMD-mediated RNA degradation. Analysis of the nuclear and cytoplasmic fractions of the treated cells indicated that, whereas the level of GAS5 transcript in the cytoplasm of cycloheximide-treated cells showed a significant increase (see Fig. S4c in the supplemental material), BORG remained strictly nuclear (Fig. 2d and e; see Fig. S4a and b in the supplemental material). Cycloheximide treatment of BMP2-stimulated cells indicated that the nuclear localization of BMP2-induced BORG transcripts was similarly not regulated through NMD (see Fig. S4f in the supplemental material). Since nuclear import through the nuclear pore complex is inhibited during heat shock (58, 59), the complete absence of BORG accumulation in the cytoplasm after heat shock indicates that BORG is not exported to the cytoplasm. Thus, the nuclear localization of BORG is likely to be the result of nuclear retention of the transcript.
We also considered the possibility that the low copy number of BORG transcripts (∼50 copies/cell) prevented us from detecting the cytoplasmically localized BORG RNAs even with our sensitive RT-PCR assays performed with radiolabeled primers, especially if the cytoplasmic portion constitutes only a small fraction of the total cellular BORG transcripts. To address this possibility, we expressed BORG from a highly active promoter, which raised the level of BORG about 10-fold above the previously tested BORG-overexpressing cells and over 50-fold above the endogenous level of BORG (Fig. 2f). However, even at this expression level, we could not detect BORG in the cytoplasmic fraction (Fig. 2g). We also tested the effect of overexpression of BORG in NMuMg mouse mammary epithelial cells, which have an almost undetectable level of endogenous BORG (Fig. 2h), and found that, even in these cells, the transgene-expressed BORG localized to the nuclei (Fig. 2i). Taken together, the above-mentioned results indicate that the nuclear localization of BORG is not a passive result of cellular-compartment-specific degradation or an experimental artifact but rather is an intrinsic property of the mature BORG transcript.
BORG RNA contains multiple copies of an RNA nuclear localization motif.
To determine how the sequence of mature BORG RNA mediates its nuclear localization, we attempted to find the region of BORG that contained the sequence elements necessary for its localization to the nuclei. We generated a series of BORG constructs that lacked fragments of BORG RNA (Fig. 3a, Mutant-1 to -6; see Table S1 in the supplemental material). To ensure that any observed effects resulted from the loss of a fragment of BORG rather than destabilization of the transcript due to loss of polyadenylation, we included the last ∼150 nucleotides of BORG RNA, which contain the polyadenylation signal, in all constructs. We confirmed the expression of the mutant transcripts using RT-PCR assays on total cellular RNA from cells stably transfected with each construct, using primers that flanked the deleted regions and thus could distinguish the mutant RNAs from the endogenous BORG (Fig. 3b, lane Mut-Specific). RT-PCRs on cDNAs made with oligo(dT) primers showed that the mutant transcripts were indeed polyadenylated (Fig. 3b, lane 3′ BORG) (see Materials and Methods). We also made constructs with larger deletions in which entire exons were removed and which thus had no shared sequences beyond the ∼150-nucleotide-long sequence at the 3′ end of BORG (Fig. 3a, Exon2, Exon3, and Exons1+3 constructs; see Table S1 in the supplemental material). Surprisingly, RT-PCR assays on nuclear and cytoplasmic fractions from cells stably expressing the above-mentioned constructs indicated that BORG RNAs from all mutant constructs were strictly localized to the nuclei (Fig. 3c; see Fig. S5a to d in the supplemental material).
FIG 3.
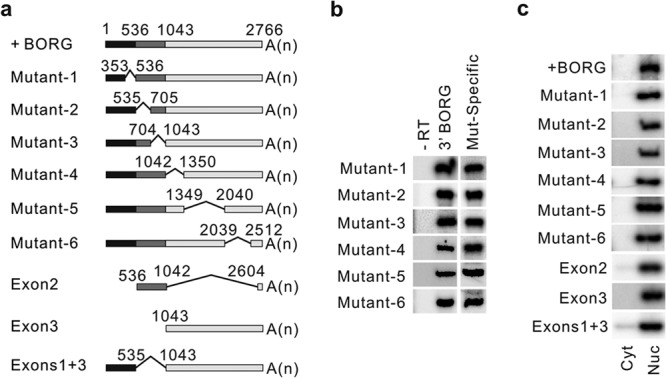
All BORG truncation mutant transcripts are nuclear. (a) Schematic representation of the BORG truncation mutants. The full-length BORG (+BORG) is shown on top, with the numbers indicating the position of the first nucleotide of each exon from the 5′ end. The sequences derived from each of the three exons are shown by distinct shades of gray. A(n) represents the position of the poly(A) tail. The region deleted in internal truncation mutant constructs is marked by thin lines connecting the ends of the included sequences, with the positions of the nucleotides at the boundaries of the deleted regions shown. (b) PCR assays on cDNAs generated with oligo(dT) from total cellular RNA of cells transfected with each mutant. Lane Mut-Specific shows results of PCRs performed with primers that flank the location of the deletion in each mutant. The results of PCR assays using a fixed set of primers that amplify a region close to the 3′ end of BORG are shown in lane 3′ BORG. Lane −RT contains the results of mock RT reaction mixtures lacking the oligo(dT) primer. (c) RT-PCR assays using radiolabeled primers indicating that all BORG truncation mutant transcripts are strictly localized to the nucleus. Nuc and Cyt refer to nuclear and cytoplasmic fractions, respectively. The PCRs were performed with primers that flanked the region deleted in each construct (see Table S3 in the supplemental material) to enable us to distinguish the transgene-expressed transcript from the endogenous BORG RNA (shown in Fig. S5a in the supplemental material) based on size (see Fig. S5d in the supplemental material).
These unexpected results suggested either that the sequences within the last 150 nucleotides of BORG mediated the nuclear localization or that BORG-derived transcripts lacked a nuclear localization signal but were passively retained in the nucleus by being unable to interact with the nuclear export machinery. Alternatively, it was possible that there were multiple copies of “nuclear localization signals” throughout the sequence of BORG. As a first step toward distinguishing between these possibilities, we generated a set of constructs that each contained a small fragment of the BORG RNA (Fig. 4a, Frag1 to -6; see Table S1 in the supplemental material). The sequences included in constructs Frag1 to -6 were identical to the sequences deleted in the constructs Mutant-1 to -6, with the sequences deleted in a Mutant construct being the sequence expressed in the Frag construct with the same number (Fig. 4a). In an effort to ensure that the constructs were comparable in terms of efficiency of transcription and stability, we included the first 120 and last 150 nucleotides of full-length BORG transcript in all Frag constructs (Fig. 4a). Next, we made stable cell lines that expressed each of the Frag constructs, followed by fractionation and RT-PCR analysis. Intriguingly, while four of the Frag RNAs were still strictly nuclear, two were detected in both nuclear and cytoplasmic compartments (Fig. 4b; see Fig. S6a and b in the supplemental material). These results were consistent with the presence of multiple nuclear localization signals in the BORG RNA sequence and, further, indicated that the common sequences at the 3′ ends of the Frag constructs, which were also present in the Mutant and Exon constructs (Fig. 3a), were not mediating the nuclear localization of the transcripts containing them. To further determine if these sequences had any impact on localization of the transcripts, we generated another construct by keeping the common sequences at the 5′ and 3′ ends of Frag5 intact and substituted the reverse complement of the sequences in between (nucleotides 1350 to 2039 of BORG) (Fig. 4a, Frag5-Reverse+complement). Analysis of the localization of the resulting transcript indicated that it mainly localized to the cytoplasm, while the original Frag5 RNA was strictly nuclear (Fig. 4b; see Fig. S6c and d in the supplemental material). Thus, the common sequences at the ends of the Frag and Mutant series of constructs were unlikely to play a dominant role in determining their localization. In addition, the above-mentioned results indicated that at least some fragments of BORG RNA (sequences contained in Frag3 and -4) could be exported to the cytoplasm, and thus, passive retention in the nucleus due to inability of the RNA to interact with the export machinery was not likely to be responsible for the observed nuclear localization of BORG RNA.
FIG 4.

BORG RNA contains multiple nuclear localization signals. (a) Schematic representation of expression constructs containing fragments of BORG. The full-length BORG is shown on top, with numbers indicating the position of the first nucleotide of each exon from the 5′ end. Sequences derived from each of the three exons are shown by distinct shades of gray. A(n) represents the position of the poly(A) tail. The sequences deleted in each Frag construct are marked by thin lines connecting the ends of the included sequences, with the positions of the nucleotides at the boundaries of the deleted regions shown. WT, wild type. (b) Localization of transcripts resulting from expression of Frag constructs determined by RT-PCR assays using radiolabeled primers. Lanes Nuc and Cyt contain the results of RT-PCR assays on nuclear and cytoplasmic fractions, respectively.
Interestingly, while Frag2 localized to the nuclei and Frag3 was found in both compartments, the Exon2 construct, which contained the sequences of these two fragments joined together, was strictly nuclear (Fig. 3c; see Fig. S5b in the supplemental material). This suggested that the sequences within the Frag2 portion of Exon2, which mediate nuclear localization, act dominantly in defining the localization of the entire Exon2 RNA. Similarly, the Exon3 construct contains sequences in Frag4, -5, and -6 joined together (Fig. 3a), and sequences that mediate nuclear localization in Frag5 and -6 likewise seem to act dominantly in defining the localization of the entire transcript. To identify these “nuclear localization signal” sequences, we generated a series of progressively truncated mutants of Exon2 and Exon3 constructs (Fig. 5a and b). The constructs were progressively shortened from the side containing the sequences of nucleus-localized fragments toward the side containing the fragment that localized to both nuclei and cytoplasm (Fig. 5a and b). Analysis of nuclear and cytoplasmic fractions from stable cell lines expressing the resulting Exon2 mutants by RT-PCR indicated that while removal of the first 105 nucleotides of Exon2 did not affect the nuclear localization of the resulting transcripts, deletion of an additional 40-nucleotide-long sequence led to a strong shift in the localization of the transcript toward the cytoplasm (Fig. 5a; see Fig. S6e to g in the supplemental material). Similarly, in the case of the much longer Exon3 construct, the removal of the first 724 nucleotides did not alter the localization, but deleting an additional 150 nucleotides in the Ex3-Mut4 construct led to an almost equal distribution of the resulting transcript between the nuclear and cytoplasmic compartments (Fig. 5b; see Fig. S6e to g in the supplemental material). Deletion of an additional 190 nucleotides did not change the subcellular distribution of the resulting RNA; however, removal of another 110 nucleotides in Ex3-Mut7 caused the transcript to become dominantly cytoplasmic (Fig. 5b; see Fig. S6e to g in the supplemental material). These results pointed to the presence of three “nuclear localization signal” elements, one in Exon2 between nucleotides 631 and 670 and two others in the Exon3 construct, located between nucleotides 1730 and 1880 and between nucleotides 1430 and 1540.
FIG 5.
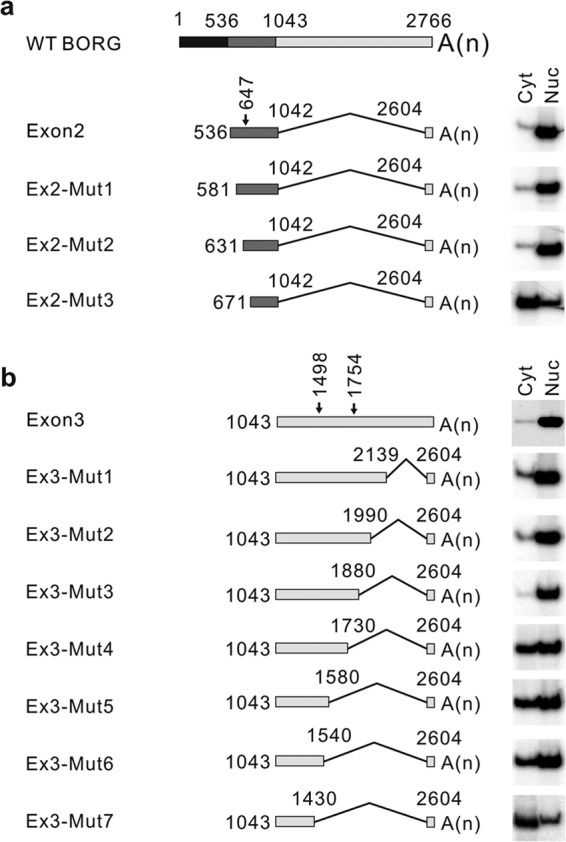
Defining the locations of the nuclear localization signals of BORG. The full-length BORG RNA is shown on top, with the progressively truncated mutants of Exon2 (a) and Exon3 (b) shown below. The thin lines connect regions of BORG RNA that are included in each construct. The numbers indicate the beginning and end of the included sequences relative to the 5′ end of full-length BORG. A(n) denotes the location of the poly(A) tail of each RNA. The arrows show the position of the AGCCC motif(s) in each exon, with the numbers next to the arrows denoting the position of each motif from the 5′ end of full-length BORG. The cellular localization of each transcript is determined by RT-PCR assays using radiolabeled primers and shown to the right of the corresponding construct. Nuc and Cyt refer to nuclear and cytoplasmic fractions, respectively.
Analysis of the sequences of these three regions using BLAST-based algorithms indicated the presence of a shared sequence motif containing an invariant AGCCC sequence with additional sequence restrictions at positions −8 (A or T) and −3 (G or C) relative to the first nucleotide of the pentamer (Fig. 6a). To determine if the presence of this sequence correlated with nuclear localization, we analyzed the sequences of all constructs used in this study for the presence of the AGCCC motif. Interestingly, all strictly nucleus-localized constructs contained at least one copy of the motif, including both the AGCCC pentamer and the sequence restrictions at positions −8 and −3 (see Table S1 in the supplemental material). The constructs that generated transcripts that were dominantly cytoplasmic, on the other hand, did not contain the motif (see Table S1 in the supplemental material). These results pointed to this RNA motif as the nuclear localization signal in BORG RNA.
To confirm that the motif was indeed responsible for nuclear localization of the RNAs containing it, we took a mutagenesis approach. Mutation of the AGCCC sequence in the Frag2 construct to a scrambled sequence (GCCAC) resulted in almost complete loss of nuclear localization (Fig. 6b; see Fig. S7a in the supplemental material). To determine if the sequence restrictions at positions −3 (G or C) and −8 (A or T) had any functional roles, we mutated the nucleotides at these two positions to C (at position −8) and T (at position −3) without any changes to the AGCCC motif. Interestingly, changes in the −3 and −8 positions led to nearly equal distribution of the resulting RNA between the nucleus and cytoplasm, indicating the functional importance of the nucleotides at these two positions (Fig. 6b; see Fig. S7a in the supplemental material). Combining the two mutations led to nearly exclusive localization of the RNA to the cytoplasm, indicating that, indeed, this motif is necessary for nuclear localization of the Frag2 RNA (Fig. 6b; see Fig. S7a in the supplemental material).
To determine if the presence of the motif is also sufficient for nuclear localization, we used the Frag5-Reverse+complement construct, which we have shown to generate an almost exclusively cytoplasmic RNA and which lacks an AGCCC motif (Fig. 4b and 6c). To determine if the position of the AGCCC motif within an RNA has any functional impact, we inserted the motif, including the restrictions at the −3 and −8 positions, close to the 5′ or 3′ end or in the middle of the RNA. Analysis of the localization of the resulting transcripts in stably transfected cells showed that all three AGCCC-containing constructs were almost exclusively nuclear, indicating that regardless of its position, even a single copy of the motif is sufficient to impart nuclear localization to an otherwise cytoplasmic RNA (Fig. 6c; see Fig. S7b in the supplemental material).
We next asked whether this motif may play a role in the subcellular localization of other cellular RNAs. To this end, we analyzed the sequences of 2,421 intergenic lncRNAs, 2,007 antisense noncoding RNAs, and 30,767 protein-coding RNAs from the mouse Ensembl database (45). The vast majority of the examined transcripts had 0 to 2 copies of the motif per transcript, with median copy numbers of 0 and 1 for lncRNAs and protein-coding RNAs, respectively (see Fig. S8a in the supplemental material). Once corrected for the length of the transcript or the number of exons (see Fig. S8b and c in the supplemental material), the lncRNAs and protein-coding RNAs had essentially similar distributions of the motif (see Fig. S8d and e in the supplemental material). To determine whether the presence of the motif correlated with subcellular localization in other cellular RNAs beyond BORG, we randomly chose a number of protein-coding and noncoding RNAs (see Materials and Methods) and determined their levels in nuclear and cytoplasmic RNA extracts using RT-qPCR assays. Interestingly, the presence and copy number of the motif showed a direct correlation with the fraction of nucleus-localized RNAs in both protein-coding and noncoding RNAs (Fig. 6d). Neither the length nor the number of exons per RNA showed a relationship to the nuclear/cytoplasmic localization of the analyzed transcripts (see Fig. S8f to i in the supplemental material). In the absence of the sequence restrictions at positions −3 and −8, the AGCCC motif showed a weaker correlation with the level of nucleus-localized fraction (correlation coefficients of 0.87 versus 0.94 and P values of 1.7e−04 versus 3.8e−06) (compare Fig. S8j in the supplemental material and Fig. 6d), consistent with our mutational analysis results (Fig. 6b). As our data indicated that the human BORG was also nucleus localized, we analyzed the sequences of human BORG and other lncRNAs studied in Fig. 6d for the presence of the AGCCC motif. Interestingly, the lncRNAs that contained the motif in the mouse also had one or more copies of the motif in humans, whereas GAS5 RNA did not have a copy of the motif in either species (see Table S4 in the supplemental material). In order to define the specificity of these observations, we repeated the analysis shown in Fig. 6d with two other motifs of the same length and nucleotide composition (GCCAC and GCCCA), with sequence restrictions at positions −3 and −8 identical to those in the AGCCC motif (Fig. 6A) (see Materials and Methods). The presence and copy number of neither motif showed a correlation with the pattern of subcellular localization of the analyzed transcripts (Fig. 6e). Together, these results suggest that the presence and copy number of the AGCCC motif has a direct and specific correlation with nuclear localization of RNAs.
DISCUSSION
To gain insight into the mechanism of nuclear localization of cellular RNAs, we analyzed the sequence requirements for the strict nuclear localization of the intergenic lncRNA BORG and showed that multiple copies of a small RNA nuclear localization motif mediate its nuclear localization. The mechanism behind subcellular localization of RNAs is likely to be highly complex, with the balance between the actions of various RNA motifs and RNA-bound proteins influencing the transport of RNAs between nuclei and cytoplasm. For example, the presence of splicing enhancers and the binding of splicing factors have been shown to favor nuclear retention, but after splicing occurs, the deposited export signals shift the balance toward export (60, 61). In the case of BORG and other spliced and polyadenylated but nucleus-localized RNAs, the strong proexport action of splicing and polyadenylation must be counterbalanced by the presence of stronger nuclear localization signals. Our data suggest that, at least in the case of BORG, this nuclear localization signal is a short, novel RNA motif consisting of the pentamer AGCCC with two sequence restrictions at positions −8 and −3 relative to the start of the pentamer.
The mechanism by which the presence of the AGCCC motif leads to nuclear localization remains to be determined. Existing studies on nuclear localization of lncRNAs are limited to the analysis of a very small number of RNAs, which were shown to be localized to specific subnuclear bodies through RNA-protein interactions (61, 62). In the case of Gomafu/MIAT/RNCR2 RNA, the localization was mediated through the interaction of SF1 with tandem repeats of a short sequence that resembles the intronic branch site consensus sequence, resulting in localization of the RNA to discrete nuclear subdomains (61). Similarly, MALAT-1/NEAT2 is localized to the nuclear speckles through binding to the nuclear speckle protein RNPS1 (62). While the interaction partner of the AGCCC motif is currently unknown, it is conceivable that the motif may interact with an abundant, nucleus-restricted protein or protein complex, such as transcriptional complexes, or even other nucleus-localized RNAs or chromatin-associated RNA-protein complexes (63) that in turn anchor the RNA containing the motif within the nucleus. Alternatively, the motif may interact with factors that interfere with the formation of export complexes, resulting in retention of the RNA in the nucleus. Finally, we cannot formally rule out the possibility that the motif interacts with a protein that induces the rapid nuclear import of any cytoplasmically localized transcripts in a manner that does not involve the canonical nuclear pore complex-mediated import mechanisms and is not inhibited by heat shock. However, studies on two other nucleus-restricted RNAs, Xist and MALAT-1, suggest that, at least under normal conditions, they do not shuttle between nuclear and cytoplasmic compartments and are retained in the nuclei (62, 64). Interestingly, even after the interaction of Xist and the X chromosome is disrupted, the mislocalized Xist remains entirely nuclear (65), pointing to the presence of sequence motifs such as AGCCC within the RNA (Fig. 6d) that prevent even the mislocalized transcripts from being exported to the cytoplasm.
Analysis of the sequences of other cellular RNAs indicated that the AGCCC motif is found in other lncRNAs and also in protein-coding RNAs. Interestingly, at least for the small number of randomly selected protein-coding RNAs and lncRNAs tested in this study, the copy number of the motif showed strong correlation with nuclear localization. Despite the small number of the analyzed RNAs, these results suggest that the AGCCC motif may play a role in nuclear localization of additional lncRNAs and protein-coding RNAs. It is known that a significant fraction of the transcript population of many protein-coding RNAs is nucleus-localized (66, 67), and at least in some cases, nuclear retention is used as a means for regulation of the level of translation (68, 69). In addition, increasing evidence suggests that the protein-coding RNAs have functions beyond merely coding for proteins, and indeed, many of them may regulate cellular processes as functional RNAs (70). Thus, nuclear localization of protein-coding transcripts may be necessary for their cellular role as functional RNAs, justifying the presence of shared RNA nuclear localization motifs between lncRNAs and protein-coding RNAs.
While a large number of RNA structural motifs have been reported in the literature, only a few RNA functional sequence motifs have been described. Data from well-studied functional RNAs, such as snRNAs, snoRNAs, and ribozymes, suggest that although many functional aspects of cellular RNAs are mediated through their ability to form simple or complex secondary and tertiary structures, RNA sequence motifs play an equally crucial role in mediating the functions or cellular properties of RNAs (23, 24, 36–38, 71, 72). Discovery of additional RNA sequence motifs, such as the motif described in this work, will not only provide clues to the architectural organization and mechanism of function of an RNA but also may make it possible, at least in a limited sense, to predict the function or cellular properties of an RNA based on the analysis of its primary sequence.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Hatzoglou, William Schiemann, Edward Stavnezer, and M. L. Nikki Harter for the kind gifts of the pLntx plasmid and HeLa, HEK293, NMuMg, 4T1, and C2C12 cells and Jo Ann Wise, Hua Lou, and Ahmad Khalil for critical reading of the manuscript. We also thank Penny Benchek for advice on statistical analyses and Mahshid Malakootian for help in literature search.
This work was supported by grants from the ALS Therapy Alliance and National Center for Regenerative Medicine and Tech 09-071 from the Ohio Third Frontier Program.
B.Z. and S.V. designed the experiments. B.Z. performed the majority of the experiments and analyzed the data. F.N. performed the computational analyses. L.G. participated in analysis of fractionated mutant RNAs. F.J. performed the initial characterization of BORG and the in situ hybridization assays, cloned BORG RNA, and participated in making the mutant constructs and stable cell lines. B.Z. and S.V. prepared the manuscript. S.V. performed the statistical analysis.
We declare that we do not have any competing financial interests.
ADDENDUM IN PROOF
While this paper was in press, analysis of high-throughput sequencing results on RNAs obtained from nuclear and cytoplasmic fractions in mouse cell lines confirmed that the AGCCC motif was enriched in transcripts with a high nuclear/cytoplasmic ratio at a transcriptome-wide level (B. Tian, Rutgers University, personal communication).
Footnotes
Published ahead of print 14 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01673-13.
REFERENCES
- 1.Clark MB, Mattick JS. 2011. Long noncoding RNAs in cell biology. Semin. Cell Dev. Biol. 22:366–376. 10.1016/j.semcdb.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Rinn JL, Chang HY. 2012. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81:145–166. 10.1146/annurev-biochem-051410-092902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Project Consortium ENCODE, Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kung JTY, Colognori D, Lee JT. 2013. Long noncoding RNAs: past, present, and future. Genetics 193:651–669. 10.1534/genetics.112.146704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasanth KV, Spector DL. 2007. Eukaryotic regulatory RNAs: an answer to the “genome complexity” conundrum. Genes Dev. 21:11–42. 10.1101/gad.1484207 [DOI] [PubMed] [Google Scholar]
- 6.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest ARR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SPT, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schönbach C, Sekiguchi K, Semple CAM, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y. 2005. The transcriptional landscape of the mammalian genome. Science 309:1559–1563. 10.1126/science.1112014 [DOI] [PubMed] [Google Scholar]
- 7.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. 2009. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 106:11667–11672. 10.1073/pnas.0904715106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JT. 2012. Epigenetic regulation by long noncoding RNAs. Science 338:1435–1439. 10.1126/science.1231776 [DOI] [PubMed] [Google Scholar]
- 9.Mercer TR, Mattick JS. 2013. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 20:300–307. 10.1038/nsmb.2480 [DOI] [PubMed] [Google Scholar]
- 10.Chen L-L, Carmichael GG. 2010. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell Biol. 22:357–364. 10.1016/j.ceb.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulitsky I, Bartel DP. 2013. lincRNAs: genomics, evolution, and mechanisms. Cell 154:26–46. 10.1016/j.cell.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guil S, Esteller M. 2012. Cis-acting noncoding RNAs: friends and foes. Nat. Struct. Mol. Biol. 19:1068–1075. 10.1038/nsmb.2428 [DOI] [PubMed] [Google Scholar]
- 13.Wang KC, Chang HY. 2011. Molecular mechanisms of long noncoding RNAs. Mol. Cell 43:904–914. 10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer TR, Dinger ME, Mattick JS. 2009. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10:155–159. 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 15.Guttman M, Rinn JL. 2012. Modular regulatory principles of large non-coding RNAs. Nature 482:339–346. 10.1038/nature10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batista PJ, Chang HY. 2013. Long noncoding RNAs: cellular address codes in development and disease. Cell 152:1298–1307. 10.1016/j.cell.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleanthous C. 2000. Protein-protein recognition. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 18.Kapusta A, Kronenberg Z, Lynch VJ, Zhuo X, Ramsay L, Bourque G, Yandell M, Feschotte C. 2013. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 9:e1003470. 10.1371/journal.pgen.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley D, Rinn J. 2012. Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol. 13:R107. 10.1186/gb-2012-13-11-r107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnsson P, Ackley A, Vidarsdottir L, Lui W-O, Corcoran M, Grandér D, Morris KV. 2013. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat. Struct. Mol. Biol. 20:440–446. 10.1038/nsmb.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perreault J, Weinberg Z, Roth A, Popescu O, Chartrand P, Ferbeyre G, Breaker RR. 2011. Identification of hammerhead ribozymes in all domains of life reveals novel structural variations. PLoS Comput. Biol.. 7:e1002031. 10.1371/journal.pcbi.1002031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi-Ashtiani K, Szostak JW. 2001. In vitro evolution suggests multiple origins for the hammerhead ribozyme. Nature 414:82–84. 10.1038/35102081 [DOI] [PubMed] [Google Scholar]
- 23.Ackley A, Lenox A, Stapleton K, Knowling S, Lu T, Sabir KS, Vogt PK, Morris KV. 2013. An algorithm for generating small RNAs capable of epigenetically modulating transcriptional gene silencing and activation in human cells. Mol. Ther. Nucleic Acids 2:e104. 10.1038/mtna.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C, Yang M, Tian J, Wang X, Li Z. 2011. MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int. J. Oncol. 39:169–175. 10.3892/ijo.2011.1007 [DOI] [PubMed] [Google Scholar]
- 25.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. 2007. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316:1484–1488. 10.1126/science.1138341 [DOI] [PubMed] [Google Scholar]
- 26.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. 2012. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22:1775–1789. 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grünwald D, Singer RH, Rout M. 2011. Nuclear export dynamics of RNA-protein complexes. Nature 475:333–341. 10.1038/nature10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natalizio BJ, Wente SR. 2013. Postage for the messenger: designating routes for nuclear mRNA export. Trends Cell Biol. 23:365–373. 10.1016/j.tcb.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei H, Zhai B, Yin S, Gygi S, Reed R. 2013. Evidence that a consensus element found in naturally intronless mRNAs promotes mRNA export. Nucleic Acids Res. 41:2517–2525. 10.1093/nar/gks1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno M. 2012. Size matters in RNA export. RNA Biol. 9:1413–1417. 10.4161/rna.22569 [DOI] [PubMed] [Google Scholar]
- 31.Lei EP, Silver PA. 2002. Intron status and 3′-end formation control cotranscriptional export of mRNA. Genes Dev. 16:2761–2766. 10.1101/gad.1032902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuke H, Ohno M. 2008. Role of poly(A) tail as an identity element for mRNA nuclear export. Nucleic Acids Res. 36:1037–1049. 10.1093/nar/gkm1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei H, Dias AP, Reed R. 2011. Export and stability of naturally intronless mRNAs require specific coding region sequences and the TREX mRNA export complex. Proc. Natl. Acad. Sci. U. S. A. 108:17985–17990. 10.1073/pnas.1113076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Wimler KM, Carmichael GG. 1999. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 18:1642–1652. 10.1093/emboj/18.6.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Carmichael GG. 1996. Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol. Cell. Biol. 16:1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speckmann W, Narayanan A, Terns R, Terns MP. 1999. Nuclear retention elements of U3 small nucleolar RNA. Mol. Cell. Biol. 19:8412–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SB, Bellini M. 2008. The assembly of a spliceosomal small nuclear ribonucleoprotein particle. Nucleic Acids Res. 36:6482–6493. 10.1093/nar/gkn658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiss T, Fayet E, Jády BE, Richard P, Weber M. 2006. Biogenesis and intranuclear trafficking of human box C/D and H/ACA RNPs. Cold Spring Harb. Symp. Quant. Biol. 71:407–417. 10.1101/sqb.2006.71.025 [DOI] [PubMed] [Google Scholar]
- 39.Boelens WC, Palacios I, Mattaj IW. 1995. Nuclear retention of RNA as a mechanism for localization. RNA 1:273–283 [PMC free article] [PubMed] [Google Scholar]
- 40.Huang S, Deerinck TJ, Ellisman MH, Spector DL. 1994. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J. Cell Biol. 126:877–899. 10.1083/jcb.126.4.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P, Dinger ME, Mattick JS. 2012. Genome-wide analysis of long noncoding RNA stability. Genome Res. 22:885–898. 10.1101/gr.131037.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda K, Ichijo H, Fujii M, Mochida Y, Saitoh M, Nishitoh H, Sampath TK, Miyazono K. 1998. Identification of a novel bone morphogenetic protein-responsive gene that may function as a noncoding RNA. J. Biol. Chem. 273:17079–17085. 10.1074/jbc.273.27.17079 [DOI] [PubMed] [Google Scholar]
- 43.Rio DC, Ares M, Jr, Hannon GJ, Nilsen TW. 2010. Preparation of cytoplasmic and nuclear RNA from tissue culture cells. Cold Spring Harb. Protoc. 2010 :pdb.prot5441. 10.1101/pdb.prot5441 [DOI] [PubMed] [Google Scholar]
- 44.Crooks GE, Hon G, Chandonia J-M, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188–1190. 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flicek P, Ahmed I, Amode MR, Barrell D, Beal K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fairley S, Fitzgerald S, Gil L, Garcia-Giron C, Gordon L, Hourlier T, Hunt S, Juettemann T, Kahari AK, Keenan S, Komorowska M, Kulesha E, Longden I, Maurel T, McLaren WM, Muffato M, Nag R, Overduin B, Pignatelli M, Pritchard B, Pritchard E, Riat HS, Ritchie GRS, Ruffier M, Schuster M, Sheppard D, Sobral D, Taylor K, Thormann A, Trevanion S, White S, Wilder SP, Aken BL, Birney E, Cunningham F, Dunham I, Harrow J, Herrero J, Hubbard TJP, Johnson N, Kinsella R, Parker A, Spudich G, Yates A, Zadissa A, Searle SMJ. 2013. Ensembl 2013. Nucleic Acids Res. 41:D48–D55. 10.1093/nar/gks1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Raney BJ, Pohl A, Malladi VS, Li CH, Lee BT, Learned K, Kirkup V, Hsu F, Heitner S, Harte RA, Haeussler M, Guruvadoo L, Goldman M, Giardine BM, Fujita PA, Dreszer TR, Diekhans M, Cline MS, Clawson H, Barber GP, Haussler D, Kent WJ. 2013. The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res. 41:D64–D69. 10.1093/nar/gks1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson JJ, Foresman MD, Wilson N, McIvor RS. 1992. Intron requirement for expression of the human purine nucleoside phosphorylase gene. Nucleic Acids Res. 20:3191–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu WS, Mertz JE. 1989. Simian virus 40 late transcripts lacking excisable intervening sequences are defective in both stability in the nucleus and transport to the cytoplasm. J. Virol. 63:4386–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JA, Valenstein ML, Yario TA, Tycowski KT, Steitz JA. 2012. Formation of triple-helical structures by the 3′-end sequences of MALAT1 and MENβ noncoding RNAs. Proc. Natl. Acad. Sci. U. S. A. 109:19202–19207. 10.1073/pnas.1217338109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tycowski KT, Shu M-D, Borah S, Shi M, Steitz JA. 2012. Conservation of a triple-helix-forming RNA stability element in noncoding and genomic RNAs of diverse viruses. Cell Rep. 2:26–32. 10.1016/j.celrep.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conrad NK, Shu M-D, Uyhazi KE, Steitz JA. 2007. Mutational analysis of a viral RNA element that counteracts rapid RNA decay by interaction with the polyadenylate tail. Proc. Natl. Acad. Sci. U. S. A. 104:10412–10417. 10.1073/pnas.0704187104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L-L, Carmichael GG. 2008. Gene regulation by SINES and inosines: biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle 7:3294–3301. 10.4161/cc.7.21.6927 [DOI] [PubMed] [Google Scholar]
- 53.DeCerbo J, Carmichael GG. 2005. Retention and repression: fates of hyperedited RNAs in the nucleus. Curr. Opin. Cell Biol. 17:302–308. 10.1016/j.ceb.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Carmichael GG. 2004. Methods for the analysis of adenosine-to-inosine editing in RNA, p 75–83 In Schoenberg DR. (ed), mRNA processing and metabolism. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 55.Niazi F, Valadkhan S. 2012. Computational analysis of functional long noncoding RNAs reveals lack of peptide-coding capacity and parallels with 3′ UTRs. RNA 18:825–843. 10.1261/rna.029520.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tani H, Torimura M, Akimitsu N. 2013. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One 8:e55684. 10.1371/journal.pone.0055684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L, Sorrentino V. 1992. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biol. 12:3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stutz F, Rosbash M. 1998. Nuclear RNA export. Genes Dev. 12:3303–3319 [DOI] [PubMed] [Google Scholar]
- 59.Vainberg IE, Dower K, Rosbash M. 2000. Nuclear export of heat shock and non-heat-shock mRNA occurs via similar pathways. Mol. Cell. Biol. 20:3996–4005. 10.1128/MCB.20.11.3996-4005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taniguchi I, Masuyama K, Ohno M. 2007. Role of purine-rich exonic splicing enhancers in nuclear retention of pre-mRNAs. Proc. Natl. Acad. Sci. U. S. A. 104:13684–13689. 10.1073/pnas.0704922104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuiji H, Yoshimoto R, Hasegawa Y, Furuno M, Yoshida M, Nakagawa S. 2011. Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes Cells 16:479–490. 10.1111/j.1365-2443.2011.01502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyagawa R, Tano K, Mizuno R, Nakamura Y, Ijiri K, Rakwal R, Shibato J, Masuo Y, Mayeda A, Hirose T, Akimitsu N. 2012. Identification of cis- and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA 18:738–751. 10.1261/rna.028639.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. 2011. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 44:667–678. 10.1016/j.molcel.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen HR, Panning B. 2007. XIST RNA exhibits nuclear retention and exhibits reduced association with the export factor TAP/NXF1. Chromosoma 116:373–383. 10.1007/s00412-007-0100-1 [DOI] [PubMed] [Google Scholar]
- 65.Sarma K, Levasseur P, Aristarkhov A, Lee JT. 2010. Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc. Natl. Acad. Sci. U. S. A. 107:22196–22201. 10.1073/pnas.1009785107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trask HW, Cowper-Sal-lari R, Sartor MA, Gui J, Heath CV, Renuka J, Higgins A-J, Andrews P, Korc M, Moore JH, Tomlinson CR. 2009. Microarray analysis of cytoplasmic versus whole cell RNA reveals a considerable number of missed and false positive mRNAs. RNA 15:1917–1928. 10.1261/rna.1677409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solnestam BW, Stranneheim H, Hällman J, Käller M, Lundberg E, Lundeberg J, Akan P. 2012. Comparison of total and cytoplasmic mRNA reveals global regulation by nuclear retention and miRNAs. BMC Genomics 13:574. 10.1186/1471-2164-13-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L-L, Carmichael GG. 2009. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol. Cell 35:467–478. 10.1016/j.molcel.2009.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. 2005. Regulating gene expression through RNA nuclear retention. Cell 123:249–263. 10.1016/j.cell.2005.08.033 [DOI] [PubMed] [Google Scholar]
- 70.Ulveling D, Francastel C, Hubé F. 2011. When one is better than two: RNA with dual functions. Biochimie 93:633–644. 10.1016/j.biochi.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 71.Lange TS, Borovjagin A, Maxwell ES, Gerbi SA. 1998. Conserved boxes C and D are essential nucleolar localization elements of U14 and U8 snoRNAs. EMBO J. 17:3176–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pyle AM. 2010. The tertiary structure of group II introns: implications for biological function and evolution. Crit. Rev. Biochem. Mol. Biol. 45:215–232. 10.3109/10409231003796523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.