ABSTRACT
Latency-associated nuclear antigen (LANA), a multifunctional protein expressed by the Kaposi sarcoma-associated herpesvirus (KSHV) in latently infected cells, is required for stable maintenance of the viral episome. This is mediated by two interactions: LANA binds to specific sequences (LBS1 and LBS2) on viral DNA and also engages host histones, tethering the viral genome to host chromosomes in mitosis. LANA has also been suggested to affect host gene expression, but both the mechanism(s) and role of this dysregulation in KSHV biology remain unclear. Here, we have examined LANA interactions with host chromatin on a genome-wide scale using chromatin immunoprecipitation with high-throughput sequencing (ChIP-seq) and show that LANA predominantly targets human genes near their transcriptional start sites (TSSs). These host LANA-binding sites are generally found within transcriptionally active promoters and display striking overrepresentation of a consensus DNA sequence virtually identical to the LANA-binding site 1 (LBS1) motif in KSHV DNA. Comparison of the ChIP-seq profile with whole-transcriptome (high-throughput sequencing of RNA transcripts [RNA-seq]) data reveals that few of the genes that are differentially regulated in latent infection are occupied by LANA at their promoters. This suggests that direct LANA binding to promoters is not the prime determinant of altered host transcription in KSHV-infected cells. Most surprisingly, the association of LANA to both host and viral DNA is strongly disrupted during the lytic cycle of KSHV. This disruption can be prevented by the inhibition of viral DNA synthesis, suggesting the existence of novel and potent regulatory mechanisms linked to either viral DNA replication or late gene expression.
IMPORTANCE Here, we employ complementary genome-wide analyses to evaluate the distribution of the highly abundant latency-associated nuclear antigen, LANA, on the host genome and its impact on host gene expression during KSHV latent infection. Combined, ChIP-seq and RNA-seq reveal that LANA accumulates at active gene promoters that harbor specific short DNA sequences that are highly reminiscent of its cognate binding sites in the virus genome. Unexpectedly, we found that such association does not lead to remodeling of global host transcription during latency. We also report for the first time that LANA's ability to bind host and viral chromatin is highly dynamic and is disrupted in cells undergoing an extensive lytic reactivation. This therefore suggests that the association of LANA to chromatin during a productive infection cycle is controlled by a new regulatory mechanism.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is the causative agent of Kaposi's sarcoma (KS), the most frequent malignancy associated with HIV/AIDS (1, 2). KSHV is also tightly associated with two lymphoproliferative disorders: primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (3, 4). Like all herpesviruses, KSHV displays two alternative transcriptional programs, latency and lytic replication. Latency is a state in which viral gene expression is tightly restricted, with only a few genes being expressed. The major viral antigen detected during a latent infection is the latency-associated nuclear antigen (LANA), a large nuclear protein (222 to 234 kDa) essential for the maintenance of the viral latent state and the propagation of the KSHV episome (5–8). LANA mediates latent viral DNA replication from the terminal repeats (TRs) and tethers viral episomes to the host chromatin to allow their proper segregation during cell division (8–11). These functions require direct binding of LANA to DNA; in vitro, LANA recognizes two 16-bp DNA sequences located within the TR units called LANA-binding site 1 and 2 (LBS1/2), with LBS1 being essential for LANA binding in vivo (12, 13).
In addition to binding to the viral TRs, LANA interacts directly with nucleosomes via histones H2A and H2B (H2A/B) and uses them as a docking station to tether viral episomes to cellular chromatin and mitotic chromosomes (14). Recent reports have also evidenced a direct interaction of LANA with H2AX, an isoform of H2A shown to be enriched at KSHV TRs that may contribute to the accumulation of LANA in this region (15). Besides the H2A/B dimer, LANA can associate with both the linker histone H1 and the core histone H3 (16–18). Interestingly, histone methylation on lysine 9 of H3 has been shown to be detrimental for its interaction with LANA, thus arguing that covalent modifications of histones may impact the binding of LANA to the host chromatin (17). Further evidence supporting the LANA-nucleosome connection comes from several studies establishing a link between LANA and histone modifiers, including the histone methyltransferase SUV39H1, the mSin3-containing histone deacetylase (HDAC) complex, and the histone demethylase KDM3A (17, 19, 20). LANA has also been proposed to be linked to DNA methylation through its association with the DNA methyltransferase Dnmt3a (21).
Despite clear evidence for the physical interaction of LANA with host chromatin, its role in cellular and viral gene expression regulation is still not clear. Among viral genes, LANA has been associated with the repression of the major lytic switch protein RTA (replication and transcription activator; ORF50) and the auto-activation of its own promoter expression (22–27). Experiments in transiently transfected cells show that LANA can repress reporter genes bearing natural or artificial LANA binding sites (12, 20, 28, 29). Transcriptional profiling of cells ectopically expressing LANA shows evidence of both upregulation and downregulation of host genes (21, 26, 30, 31). However, the mechanisms underlying these effects remain unclear. Moreover, there are only a few confirmed examples of host gene promoters that are directly bound by LANA (32, 33). A popular notion is that LANA mediates host gene regulation indirectly through interactions with host partners, and indeed many interactions between LANA and host proteins have been identified. For instance, LANA has been reported to associate with Brd2/4, RB, p53, RBP-Jκ, MeCP2, ATF4/CREB2 complex, CBP, and glycogen synthase kinase 3β (GSK-3β), potentially leading to a wide range of transcriptional outcomes (20, 23, 34–39). Similarly, LANA expression can disrupt the association of Sp1 and Daxx with DNA, which concomitantly upregulates the expression of their target genes human telomerase reverse transcriptase (hTERT), BIRC5 (survivin), and vascular endothelial growth factor (VEGF) (40–42). Conversely, LANA has been reported to promote the c-Jun-Fos heterodimer formation leading to downstream transactivation of the interleukin-6 (IL-6) promoter (43).
Although a close relationship between LANA and host chromatin is beyond dispute, the effects of these interactions on host gene expression and their functional consequences in a bona fide viral infection remain uncertain. Part of the uncertainty emanates from the fact that most studies have employed isolated LANA overexpression in heterologous cell types or focused on individual host genes or artificial reporter constructs. In this study, we investigated the genomic positioning of LANA on host chromatin in cells infected by replication-competent KSHV, both in latency and during lytic reactivation. We studied cells of both epithelial and endothelial lineages, using the method of genome-wide chromatin immunoprecipitation with high-throughput sequencing (ChIP-seq). LANA-enriched sites were examined for recursive features such as common DNA motifs, overrepresented gene pathways, and patterns of chromatin modifications. We also identified host transcripts that are differentially regulated during KSHV infection using the technology of high-throughput sequencing of RNA transcripts (RNA-seq). By merging the results from these large-scale approaches, we have been able to examine for the first time the global relationship between LANA localization on the host genome and KSHV-driven host gene regulation.
MATERIALS AND METHODS
Cell culture, infection, and reagents.
iSLK, iSLK-219, and SLK cells were maintained in Dulbecco's modified Eagle's medium (DMEM), whereas BJAB, BJAB-219, and BCBL-1 cells were grown in RPMI medium at 37°C under a 5% CO2 atmosphere. Both culture media were supplemented with 10% fetal bovine serum, l-glutamine (2 mM; Invitrogen), penicillin (100 IU/ml; Gibco), and streptomycin (100 μg/ml; Gibco). Primary blood endothelial cells (BECs) and primary lymphatic endothelial cells (LECs) were purchased from Lonza and cultured in EBM-2 medium supplemented with the EGM-2 MV kit for microvascular cells (cc3203; Lonza). Recombinant KSHV.219 (rKSHV.219) stocks were prepared from the iSLK-219 cell line, and stable infections of BECs and LECs (BEC-219s and LEC-219s, respectively) were established as described before (44). Culture medium was supplemented with 10 and 0.25 μg/ml puromycin (Invivogen) in order to maintain selection for the viral episome in iSLK-219 and LEC-219s, respectively. Lytic reactivation of iSLK-219 was triggered using 1 μg/ml of doxycycline (DOX; Sigma-Aldrich). To prevent viral replication, DOX treatments were done in the presence of 500 μM phosphonoformic acid (PFA; Sigma-Aldrich). SLK cells were transfected using the FuGene HD transfection reagent (Promega) according to the manufacturer's instructions.
ChIP.
ChIP assays were performed as described before (45) with minor modifications. Briefly, 1 × 107 cells were fixed with 1% formaldehyde 5 min prior to harvest. Nucleus preparations were done using a truChIP High Cell Chromatin Shearing Kit with SDS from Covaris, and chromatin was sheared with a Covaris E220 Ultrasonicator. The average size of chromatin fragments was either ∼200 bp or ∼600 bp for ChIP-seq or ChIP with quantitative PCR (ChIP-qPCR) assays, respectively. Antibodies for ChIP were coupled to Dynabeads (Invitrogen) and incubated with chromatin extracts for 4 h at 4°C in cold radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 150 mM NaCl, 5% glycerol, 0.1% Na deoxycholate, 0.1% SDS, 1% Triton X-100, protease inhibitors [Complete Mini; Roche]). The following antibodies were used for ChIP: rat anti-LANA (13-210-100; Advanced Biotechnologies), anti-histone H3 trimethylated at K4 (H3K4me3) (ab8580; Abcam), anti-H3K27me3 (07-449; Millipore), anti-H3K36me3 (ab9050; Abcam), and anti-polymerase II (Pol II) (sc-899X; Santa Cruz Biotechnology). The immunoprecipitated chromatin was sequentially washed in high-salt RIPA buffer (500 mM NaCl) and LiCl wash buffer (50 mM Tris, pH 8.0, 1 mM EDTA, 250 mM LiCl, 1% NP-40, 0.5% Na deoxycholate). Chromatin cross-links were reversed by incubating samples overnight at 65°C in the presence of proteinase K (200 μg/ml; Invitrogen). DNA was recovered using AMPure XP beads (Beckman Coulter, Inc.). For ChIP-seq, Illumina-compatible libraries were generated using a TruSeq ChIP Sample Preparation Kit (15034288; Illumina). Each library was then subjected to a 51-cycle single-read sequencing run on a HiSeq 2500 platform (FC-401-3002; Illumina). Two independent replicates of LANA ChIP-seq were performed in iSLK-219s and LEC-219s, two technical replicates in the BCBL-1 cell line, and single experiments in BEC-219s and BJAB-219s. Quantitative PCR analysis of ChIP samples (ChIP-qPCR) was performed in triplicate using the Fast SYBR green master mix (Applied Biosystems), 500 nM primers (listed in Table S1 in the supplemental material), 4% of the immunoprecipitated DNA (or 1% of the input material), and an ABI 7500 real-time PCR instrument (Applied Biosystems). ChIP-qPCR enrichment values were calculated using the percent input method. ChIP-qPCR results are representative of independent experiments done in triplicate.
ChIP-seq analysis.
All programs were run using their default parameters unless otherwise stated. Raw sequencing files (bcl format) were converted to Fastq sequence files using CASAVA, version 1.8.2 (Illumina). DNA sequences (51-nucleotide [nt] reads) were then aligned to human (human genome build 19 [hg19]) and KSHV (GQ994935.1) genomes with Bowtie 2 (46). Details for the ChIP-seq data generated, including the percentage of aligned reads and the number of replicates performed, are available in Table S2 in the supplemental material. SAM (sequence alignment/map) files generated by Bowtie 2 were processed by the MACS program (model-based analysis of ChIP-seq; version 1.4.2) to identify statistically significant ChIP-seq peaks compared to negative-control samples (47). We used MACS default parameters with the following exceptions: (i) the size of chromatin fragments was manually adjusted to fit experimentally verified values (options --nomodel and --shiftsize); (ii) for the analysis of the viral episome, the effective genome size was set at 1.37 × 105 (long unique region [LUR]), and the option global lambda (--nolambda) was activated; (iii) P value cutoffs were set at 10−6 for BEC-219s and at 10−3 for BCBL-1 cells. Negative-control data sets for LANA peak calling on the host genome of rKSHV.219-infected cells were generated by performing ChIP-seq with the anti-LANA antibody in isotypical, uninfected cell lines. Otherwise, negative-control data sets were based on ChIP-seq experiments done with IgG alone. Extensive sequencing of H3K4me3 ChIP fragments from both iSLK and iSLK-219 cells led to very deep sequencing coverage and high levels of read redundancy (∼30%) during the peak calling process by MACS. To address this issue, both SAM files were downsized by randomly selecting of 1 out of 7 (iSLK) and 1 out of 5 (iSLK-219) reads, which significantly reduced the redundancy level (<10%). H3K4me3 peaks with a false-discovery rate over 0.25% were discarded. The initially hg18-aligned sequencing reads from the Pol II ChIP-seq data set (eland export format) were converted to hg19-aligned reads using the UCSC (University of California, Santa Cruz) liftOver tool (http://genome.ucsc.edu/cgi-bin/hgLiftOver) (48).
Output data from MACS were further analyzed using an in-house Python-based pipeline. Peaks were annotated according to their closest transcriptional start site (TSS) using the UCSC Hg19 annotation databases at http://hgdownload.soe.ucsc.edu/goldenPath/hg19/database (49). The annotation process considered TSSs from both open reading frames and noncoding RNA genes. Reproducible peaks were identified by examining the overlap between samples. Two LANA peaks were considered reproducible if the distance between their summits was not greater than 250 bp. LANA peaks and the wide H3K4me3-enriched regions were considered overlapping if they shared at least 1 bp. To find overrepresented DNA motifs at LANA-binding sites, genomic DNA sequences spanning (±250 bp) all reproducible LANA peak summits were retrieved and submitted to the MEME-ChIP motif discovery pipeline (50). The FIMO tool was used to identify individual occurrences of LBS1-like sequences on the human genome (51). Both programs are part of the MEME suite (version 4.9.0 [http://meme.nbcr.net]) (52). The PscanChip algorithm was used to calculate the overrepresentation of known transcription factor DNA-binding sequence using the promoter background model and the Jaspar database as descriptor (http://www.beaconlab.it/pscan_chip_dev) (53). ChIP-seq densities at LANA peaks or at TSSs were assessed using the SeqMiner program (54). Density arrays were generated by k-means clustering, and intensities were further adjusted by the linear normalization method.
RNA-seq.
RNA extractions from KSHV-infected LEC-219s and uninfected LECs were performed as described previously (55). Libraries for RNA-seq were prepared using the Ribo-Zero magnetic and the ART-seq kits according to the manufacturer recommendations (MRZH11124 and RPHMR12126 kits, respectively; Epicentre). Fastq files were generated with CASAVA, version 1.8.2 (Illumina), and the reads were then aligned to the KSHV (GQ994935.1) and human (hg19) genomes using TopHat2 (56). The downstream analysis of aligned reads was performed with the Cufflinks package (57). Briefly, the relative abundance of all host transcripts was calculated by Cufflinks (version 2.1.1) using a reference annotation file (-GTF option) generated by the UCSC table browser (58). We then generated a combined GTF file from all samples and replicates using Cuffcompare. Differential expression of host genes was finally determined by Cuffdiff 2 using the merged GTF file produced by Cuffcompare (59). Box plots were generated by the Spotfire software (Tibco). For comparisons with random sets of genes, genomic locations within proximal promoters were randomly selected by a custom script. These positions were then annotated and compared to the Cuffdiff data as we did with our ChIP-seq data.
Western blotting.
Cells were washed with phosphate-buffered saline (PBS) and harvested, and pellets were thoroughly resuspended in RIPA buffer supplemented with both protease and phosphatase inhibitor cocktails (Complete and PhosSTOP; Roche). Samples were clarified for 15 min at top speed at 4°C, and the supernatants were collected. Total protein concentration was determined by a detergent-compatible (DC) assay (Bio-Rad). Equal amounts of total protein were migrated in a polyacrylamide gel and transferred onto a nitrocellulose membrane. Membranes were blotted with primary antibodies against LANA (custom), RTA (custom), and β-actin (Sigma) and with goat horseradish peroxidase (HRP)-conjugated secondary antibodies against mouse IgG and rabbit IgG (Bio-Rad).
High-throughput sequencing data accession number.
Chip-seq and RNA-seq data have been submitted to the NCBI Gene Expression Omnibus (GEO [http://www.ncbi.nlm.nih.gov/geo]) under accession number GSE56144.
RESULTS
KSHV LANA is enriched at host promoters.
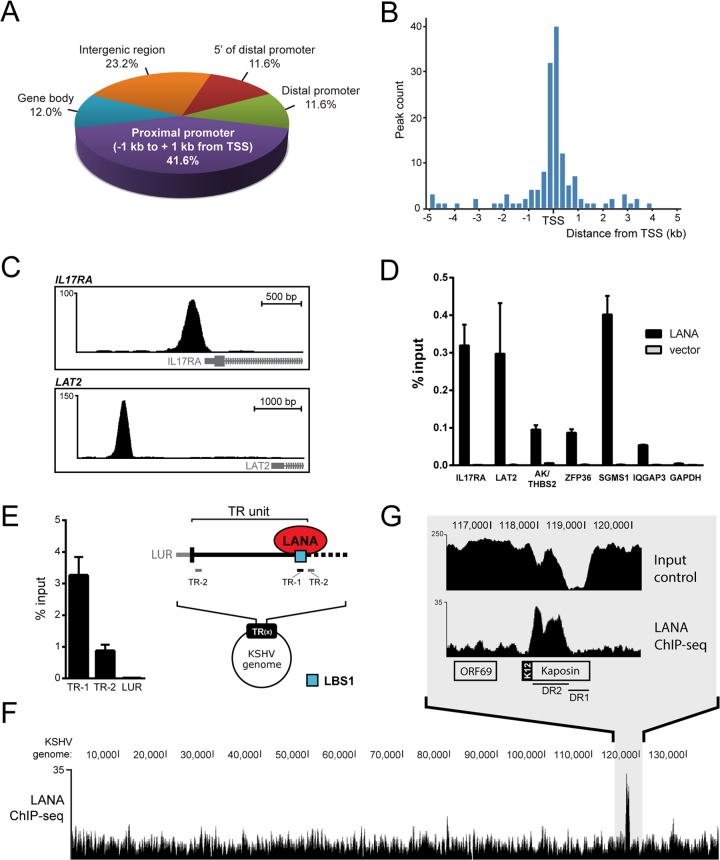
To better understand LANA's binding preferences toward host chromatin, we performed chromatin immunoprecipitation of LANA, followed by massive parallel sequencing (ChIP-seq) in the well-characterized KSHV-infected epithelial cell line iSLK-219 (60). The sequencing reads obtained were aligned to the human genome (hg19) using the Bowtie 2 software (46). Specific details about the alignment of the ChIP-seq data (e.g., percentage of reads aligned) are available in Table S2 in the supplemental material. The main advantages of this cell line are its tight latency and the virtual absence of lytic induction, as well as the availability of an isogenic uninfected cell line (iSLK). We performed in parallel a negative-control ChIP-seq in uninfected iSLK cells, which lack the LANA antigen, using the same anti-LANA antibody. With this strategy, the false-positive peaks called from DNA fragments that were nonspecifically immunoprecipitated by the anti-LANA antibody in iSLK-219 were ultimately rejected by the peak calling program MACS (47). LANA peaks were considered reproducible if their summits in both replicates were detected within a range of 250 bp. By using these parameters, we identified a total of 267 reproducible LANA-binding sites on the host genome of latent iSLK-219 cells. These 267 sites were annotated according to their closest transcriptional start site (TSS) with an in-house pipeline that uses UCSC annotation databases for both open reading frames and noncoding RNAs (see Data Set S1 in the supplemental material) (49). We then calculated the distance between each LANA peak and its closest TSS. According to this analysis, 41.8% of LANA peaks were located within 1 kb of a TSS (Fig. 1A, proximal promoter). Most of these peaks were in fact concentrated within 250 bp of their closest TSS (Fig. 1B). In comparison, only 2.8% of genomic sites that were randomly selected in silico were found in such close range to TSSs (data not shown). The position analysis of LANA binding sites also mapped 11.6% of the LANA peaks between −1 kb and −10 kb of a TSS (Fig. 1A, distal promoter). Other genomic regions occupied by LANA were between 10 and 50 kb upstream of a TSS (11.6% at sites 5′ to distal promoter), within a gene body (12.0%), and at intergenic regions (23.2% of all annotated peaks). Figure 1C shows examples of LANA peaks detected in the promoters of IL17RA (proximal) and LAT2 (distal).
FIG 1.
LANA accumulates at host promoters in iSLK-219 cells. (A) Genomic distribution of LANA peaks in iSLK-219 cells. Indicated ranges from TSS are as follows: distal promoter, −1 kb to −10 kb; 5′ of distal promoter, −10 kb to −50 kb. (B) Distribution of LANA peak summit distances from TSSs in bins of 250 bp. (C) Screen shots from the UCSC browser (hg19) depicting examples of LANA peaks detected within the promoter regions of IL17RA (top) and LAT2 (bottom) (http://genome.ucsc.edu) (98). (D) ChIP-qPCR of LANA in SLK cells transfected with a plasmid expressing LANA or an empty vector. LANA enrichment at the indicated annotated peaks on the host genome is shown as percentage of input. (E) ChIP-qPCR of LANA that targets indicated viral sites (TR-1/TR-2) on viral TR units. TR-1 encompasses the LANA-binding site 1 (LBS1, blue square), which is localized at the junction of TR units. (F) Screen shot from the UCSC browser showing the nonredundant distribution of sequencing reads from the ChIP-seq of LANA that aligned to the KSHV genome long unique region (LUR). (G) Close-up view of the LANA-enriched region spanning the DR2 segment of the Kaposin locus. Input material was also sequenced and is shown as a sequencing control of this GC-rich DNA segment.
Latently infected cells express a few other viral gene products in addition to LANA that could influence the binding of LANA to its targets. To investigate the potential contribution of other viral components to LANA's ability to bind host DNA, we performed LANA ChIP coupled to quantitative PCR (ChIP-qPCR) analysis in an infection-free context. Uninfected parental SLK cells were transiently transfected with a vector expressing LANA or an empty vector. Chromatin was collected at 48 h posttransfection and immunoprecipitated with an anti-LANA antibody. For the qPCR analysis, we targeted newly identified locations within the proximal promoters of IL-17 receptor A (IL-17RA) and zinc finger protein 36 (ZFP36), in the distal promoter of LAT2, and in the intergenic region between AK055570, and thrombospondin 2 (AK/THBS2). The promoters of SGMS1 and IQGAP3 were assayed as positive controls considering that LANA has been previously detected at these loci (33). A DNA fragment located in the coding region of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as a negative control. As shown in Fig. 1D, all four host LANA-binding regions, as well as both positive controls, were significantly enriched compared to the negative control (GAPDH). The absence of amplification in samples from cells transfected with the vector alone confirmed that the detection of these DNA fragments in the material immunoprecipitated from iSLK-219 cells was dependent on the expression of LANA. As expected from our ChIP-seq data, we also detected the enrichment of LANA at these six genomic sites in KSHV-infected iSLK-219 by ChIP-qPCR (data not shown). The enrichment of LANA on host DNA from uninfected SLK cells that ectopically express LANA indicates that the association of LANA with host chromatin can occur independently of KSHV infection and the presence of other viral factors.
We also examined the distribution of LANA on the KSHV genome in iSLK-219 cells. The viral episome contains a long unique region (LUR) joined at both ends by 35 to 45 GC-rich terminal repeat (TR) units of 801 bp, which harbor the well-characterized LANA-binding site LBS1 (12, 13). Owing to technical difficulties with the sequencing of highly repetitive GC-rich segments within the TR region, we confirmed the association of LANA to this region of the viral episome by immunoprecipitating LANA and evaluating its binding to the LBS1 motif by conventional ChIP-qPCR (Fig. 1E). In this assay, we targeted two sites within the TRs: one located directly at the LBS1 site (TR-1) and a second one adjacent to this site (TR-2) (Fig. 1E). Indeed, we detected the highest enrichment of LANA at the LBS1 motif (TR-1) and a more modest one at the LBS1-adjacent site (TR-2). This pattern of LANA enrichment at the TRs is reminiscent of what was observed in BCBL-1 cells by Lu et al. within the same regions (33). Our ChIP-seq results further revealed that LANA could potentially accumulate to one additional location on the viral episome of iSLK-219 cells, identified by MACS within the DR2 domain of the Kaposin locus (Fig. 1F). Sequence analysis did not reveal LBS1/2-like DNA elements in the DR2 coding region (data not shown). Taken together, these results indicate that LANA preferentially targets host chromatin at gene promoters in latent iSLK-219, whereas its association to viral DNA appears limited to the TR region and the Kaposin locus.
The viral LBS1 motif is found at host LANA-binding sites.
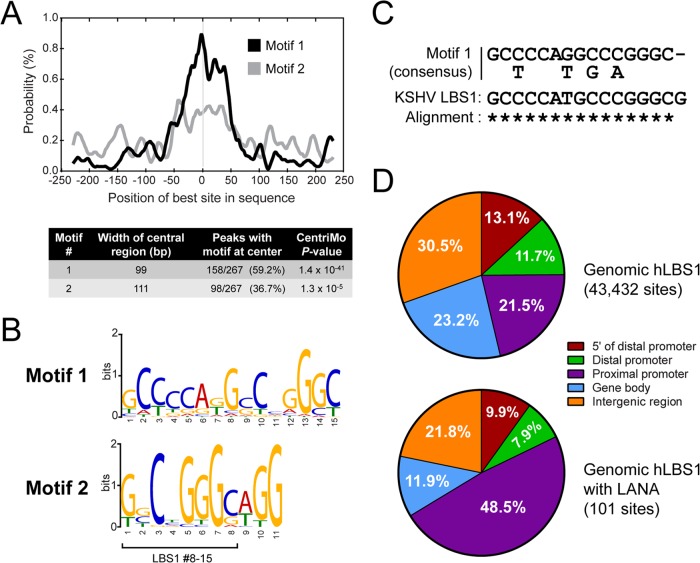
To identify the DNA motif(s) driving the association of LANA to the host genome, we retrieved the host genomic sequences flanking ±250 bp from the center of all the LANA peaks and submitted them to the MEME-ChIP pipeline (50). MEME-ChIP is a motif-based sequence analysis tool that combines the power of motif discovery algorithms, such as MEME and TOMTOM (61, 62), with a central motif enrichment analysis (CMEA) performed by the CentriMo program (63). As illustrated by the site probability curve shown in Fig. 2A (black line), the CentriMo analysis determined that one particular motif (motif 1) had a strong central enrichment among the DNA sequences bound by LANA. Motif 1 had a very low E value of 1.4 × 10−41 compared to the E value of 1.3 × 10−5 given to the second-best motif (motif 2). CentriMo mapped motif 1 to a 99-bp central region in 157 of the 267 DNA sequences (58.8%) retrieved from host LANA-binding sites. Motif 1 and motif 2 are presented in Fig. 2B in a LOGO format. Remarkably, the most represented nucleotides at each position of motif 1 were all identical to the viral LBS1 (Fig. 2C). Taking into consideration the central distribution of this LBS1-like motif at host LANA-binding sites and the observation that LANA has been shown to bind the viral LBS1 motifs (12, 13), our results strongly suggest that LANA binds the LBS1 motifs on host DNA in latent iSLK-219 cells.
FIG 2.
LANA binds host sequences that are reminiscent of the viral LBS1 motif. (A) CentriMo analysis showing the density of the best occurrence of motif 1 and motif 2 within ±250 bp of host LANA peaks (graph) and the analysis results (table). (B) LOGO representations of the most centrally enriched DNA motifs identified by CentriMo. (C) Alignment of the 15-bp degenerated consensus sequence of motif 1 (defined by MEME) with the 16-bp core sequence of the viral LBS1 motif (13). Asterisks denote matching nucleotides. (D) Genomic distribution of all host LBS1 motifs (motif 1, hLBS1) identified by the FIMO algorithm with a P value cutoff of 5 × 10−7 (top) and the genomic distribution of the 101 sites within that group that were experimentally confirmed to be bound by LANA in iSLK-219 cells by ChIP-seq (bottom). Indicated ranges from TSS are as follows: proximal promoter, −1 kb to + 1 kb; distal promoter, −1 kb to −10 kb; 5′ of distal promoter, −10 kb to −50 kb.
In the human genome, GC-rich DNA sequences are mostly found in the vicinity of genes, with the highest GC-content found at TSSs (64). Considering the very high GC-rich composition of the host-derived LBS1 consensus motif (motif 1, hLBS1) and its overrepresentation among LANA-binding sites, one could propose that the enrichment of LANA peaks in the vicinity of TSSs is inherent to the genomic localization profile of hLBS1 sequences. We therefore determined the positions of potential hLBS1 motifs on the human genome and examined their genomic locations. To do so, we scanned the human genome using the FIMO motif search tool (51) to which we supplied the hLBS1 position-specific probability matrix (PWM) calculated by the MEME algorithm from the iSLK-219 data (see Table S3 in the supplemental material). FIMO found on the human genome a total of 4.3 × 104 occurrences of hLBS1 sequences (P value cutoff of 5 × 10−7). Figure 2D shows that 21.5% of these hLBS1 sites were found within 1 kb of a TSS (proximal promoter). The majority of other hLBS1 sites are localized in intergenic regions (30.5%) and gene bodies (23.2%). It is noteworthy that from the 4.3 × 104 hLBS1 genomic locations detected by FIMO, only 101 (∼0.2%) were experimentally identified as LANA-binding sites in our ChIP-seq experiments. Interestingly, 48.5% of the 101 sites occupied by LANA were detected within proximal promoters (Fig. 2D, lower panel), indicating that the localization bias of LANA at proximal promoters is not simply driven by the genomic localization of hLBS1 motifs. Notably, we did not observe a correlation between P value scores of hLBS1 sequences found by FIMO and the experimental detection of LANA at those sites by ChIP-seq, suggesting that a highly conserved hLBS1 motif is not a sufficient predictor of LANA-binding to host chromatin (data not shown). Overall, our findings indicate that despite a clear enrichment of DNA sequences highly reminiscent of the viral LBS1 motif at the center of several LANA-binding sites, other factors rather than DNA sequence alone may drive the association of LANA with host chromatin.
LANA associates with H3K4me3-decorated promoters.
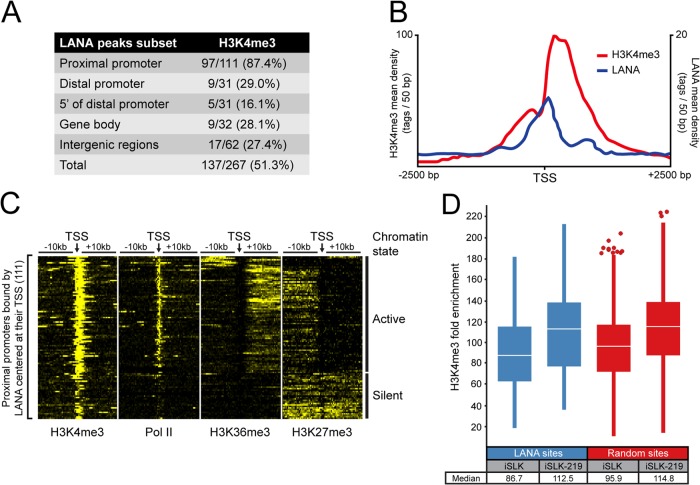
The preferential enrichment of LANA at TSSs led us to explore the chromatin environment surrounding the proximal promoters occupied by LANA. We first verified if LANA-bound TSSs bear the signature of active promoters by performing a ChIP-seq of histone 3 trimethylated at lysine 4 (H3K4me3) in iSLK-219 cells. Indeed, our results showed the accumulation of H3K4me3 at cellular TSSs in KSHV-infected iSLK cells (data not shown). To assess the colocalization of H3K4me3 and LANA-binding sites on the host genome, we considered both features cooccurring if their enriched regions, as defined by the MACS program, shared at least 1 bp. This analysis showed that LANA- and H3K4me3-enriched areas were highly congruent within proximal promoters, with 87.4% of LANA peaks overlapping with H3K4me3-marked regions (Fig. 3A). The significance of this coenrichment is highlighted by a global analysis of proximal promoter sequences in iSLK-219s, which indicates that only ∼50% of these regions are marked with H3K4me3 (data not shown). Interestingly, the majority of LANA peaks found at proximal promoters accumulated between two distinct H3K4me3 high-density regions flanking the TSSs, corresponding to the nucleosome-depleted region (NDR) (Fig. 3B). Such positioning is highly reminiscent of the localization of most transcription factors at NDR-containing promoters (65–67).
FIG 3.
Most promoters occupied by LANA are marked with H3K4 trimethylation. (A) The overlap of LANA- and H3K4me3-enriched regions according to their genomic location in iSLK-219s. Indicated ranges from TSS are as follows: proximal promoter, −1 kb to + 1 kb; distal promoter, −1 kb to −10 kb; 5′ of distal promoter, −10 kb to −50 kb. (B) Distribution of LANA and H3K4me3 within 2.5 kb of TSSs from proximal promoters occupied by LANA. (C) Density of H3K4me3, H3K27me3, H3K36me3, and Pol II within 10-kb of TSSs from proximal promoters bound by LANA. The heat map shows normalized tag counts clustered by the SeqMiner program. Contrast level was set at 25. According to their chromatin features, LANA-binding sites were clustered in two groups: active and silent chromatin. (D) Box plot analysis of H3K4me3 levels (fold enrichment above background determined by the MACS software) at LANA-binding sites or at a random set of genomic H3K4me3-enriched sites. Data are from proximal promoters of uninfected iSLKs and KSHV-infected iSLKs (iSLK-219). Outliers are shown as circles, and the median is indicated by a white line.
Our results highlight the cooccurrence of LANA and H3K4me3 at proximal promoters and suggest the possibility of LANA associating with active chromatin regions. To evaluate the activity of the LANA-bound promoters, we examined by ChIP-seq the accumulation of Pol II at these regions, as well as the deposition of two histone modifications, H3K36me3 and H3K27me3. While Pol II marks active or paused promoters and H3K36me3 marks transcriptionally active loci, H3K27me3 is associated with silent and bivalent genomic loci (68, 69). The accumulation of these chromatin features was determined within 10 kb on either side of the 111 TSSs occupied by LANA using the SeqMiner program (54). As shown in Fig. 3C, most LANA-associated TSSs harbored features reminiscent of transcriptionally active promoters, with a high density of H3K4me3, accumulation of Pol II at the TSS, and propagation of H3K36me3. Conversely, a small group of LANA-enriched regions was populated by features characteristic of silent chromatin, with lower accumulation of H3K4me3, a relative absence of Pol II and H3K36me3 signals, and higher levels of the repressive mark H3K27me3. We also sought to determine if the presence of LANA on proximal promoters correlates with the extent of H3K4me3 accumulation at these sites and if LANA binding modulates the deposition of H3K4me3 during a latent infection. We therefore compared the levels of H3K4me3 enrichment at proximal promoters occupied by LANA in latently infected iSLK-219 and uninfected iSLK cells. As illustrated by the box plot analysis shown in Fig. 3D, the median fold enrichment value of H3K4me3 in the vicinity of LANA peaks in latently infected iSLKs (112.5-fold) was slightly higher than in uninfected cells (86.7-fold). However, this 1.3-fold increase could not be linked to the accumulation of LANA at those promoters as a 1.2-fold variation in H3K4me3 levels was observed across all proximal promoters where this mark was detected (Fig. 3D). Taken together, these results showed that LANA mostly associates with active promoters but can also target loci bearing the signature of silent chromatin. They also indicate that while most LANA-binding sites at TSSs are decorated with H3K4me3 marks, it is unlikely that LANA locally modulates the deposition of this modification during infection.
LANA binds to a vast array of promoters in KSHV-infected lymphatic endothelial cells.
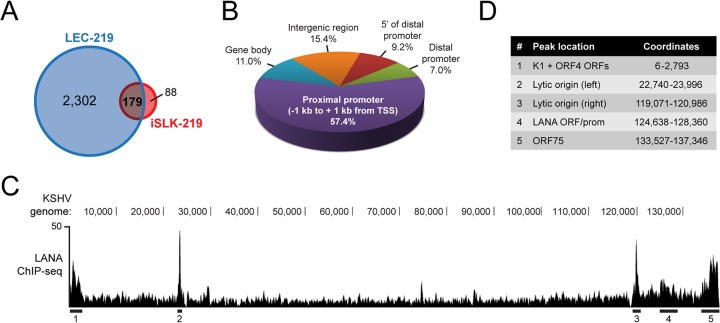
iSLK-219 cells represent a useful tool to study latency as they exhibit almost no spontaneous lytic reactivation. It was, however, recently unveiled that SLK cells, which were long thought to be of endothelial origin, are instead derived from an epithelial cell lineage (70). This prompted us to make additional analyses in a cellular environment closer to the context of KS. Lymphatic endothelial cells (LECs) have long been suspected of playing a role in the histogenesis of KS, and recent data reveal that they display a striking permissiveness to widespread, deregulated KSHV gene expression (44, 71–73). We thus sought to investigate the genomic positioning of LANA in KSHV-infected primary LECs (LEC-219s). As shown in Fig. 4A, data from two independent ChIP-seq experiments revealed that LANA associated with host chromatin at 2,481 locations in LEC-219s. Out of the 267 LANA peaks identified in iSLK-219 cells, 179 (67.0%) were also detected in LEC-219s (Fig. 4A). The annotation of all 2,481 LANA-binding sites from LEC-219 cells revealed an even stronger bias for LANA positioning at TSSs than observed in iSLK-219 cells, with 57.4% of LANA peaks detected within 1 kb of any given TSS (Fig. 4B). Other LANA-binding sites in LEC-219 cells were distributed along the host genome in a fashion similar to that found in iSLK-219 cells (Fig. 4B), indicating that, while the pattern of recognized sites is similar in the two lineages, many more sites are occupied in infected LECs. In addition to the host genome, we also studied the association of LANA with the KSHV episome in the LEC-219 cell line. Figure 4C shows the MACS-generated pile-up view of the LANA ChIP-seq tags that uniquely mapped to the LUR region of the KSHV genome. MACS identified five LANA-enriched regions within the viral episome (LUR), which are underlined in Fig. 4C and annotated in Fig. 4D. Strikingly, the highest enrichment of LANA (regions 2 and 3) was located at both origins of lytic replication. Other LANA-enriched locations on the viral LUR spanned the K1 and ORF4 genes (region 1), the LANA locus (4), and the K15 open reading frame (5). Together, our results show that the distribution of LANA on the viral and host genomes of iSLK-219 and LEC-219 cell lines follows a distinct pattern.
FIG 4.
LANA binds host gene promoters and KSHV lytic origins of replication in LEC-219 cells. (A) Venn diagram illustrating the overlap between data sets from our ChIP-seq of LANA in iSLK-219 and LEC-219 cells. (B) Distribution of LANA peaks on the genome of LEC-219s. Indicated ranges from the TSS are as follows: proximal promoter, −1 kb to + 1 kb; distal promoter, −1 kb to −10 kb; 5′ of distal promoter, −10 kb to −50 kb. (C) Screen shot from the UCSC browser showing the nonredundant sequencing tags from the ChIP-seq of LANA in LEC-219s that align to the KSHV genome LUR. LANA-enriched regions considered statistically significant by the MACS program and common to two independent replicates are underlined. (D) Annotation of LANA-enriched regions indicated in C with their genomic coordinates. ORF, open reading frame.
To further explore the contribution of cell lineage to the distribution of LANA on host chromatin, we performed LANA ChIP-seq experiments using chromatin purified from primary blood endothelial cells (BECs) and human Burkitt lymphoma B cells (BJAB) infected with rKSHV-219 and from the KSHV-infected PEL cell line BCBL-1. We then mapped the LANA-enriched regions on host chromatin in those cell lines and identified reproducible LANA peaks between the different data sets. Our analyses revealed that half the sites bound by LANA in BEC-219s (250/483) and BJAB-219s (248/479) were also identified in iSLK-219s and/or LEC-219s (data not shown). Furthermore, our data indicate that only 57 LANA-binding sites are common to the iSLK-219, LEC-219, BEC-219, and BJAB-219 cell lines (see Table S4 in the supplemental material), strongly suggesting positional bias that is dependent on the host cell lineage. In line with this assessment, we observed a strikingly different landscape of LANA in BCBL-1 cells. Only 29 LANA-bound sites were detected in these cells (see Data Set S1 in the supplemental material), a very limited number of peaks considering the use of a low P value cutoff (10−3) for peak calling and the use of the IgG control for peak calling normalization, which may lead to potentially larger sets of false-positive and false-negative peaks. Nevertheless, of these 29 LANA-binding sites, 19 (65.5%) were also detected in one of the other cell lines (iSLK-219, LEC-219, BEC-219, or BJAB-219), but only four genomic locations (gene promoters of SGMS1, SBF2, IQGAP3, and NIPAL2) were bound by LANA in all five cell lines. These findings indicate that while LANA preferentially targets a few loci on host DNA, its global genomic localization pattern is significantly altered by cell lineage-specific determinants.
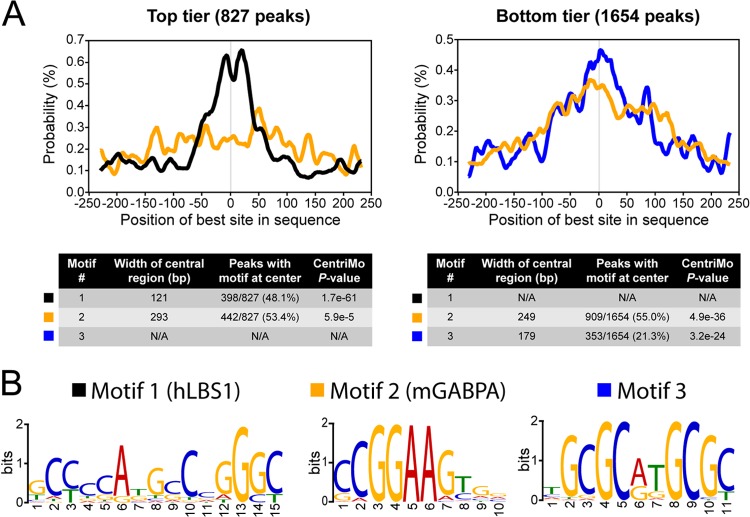
Considering that most LANA-binding sites mapped on the genome of iSLK-219 harbored a central hLBS1 motif, we investigated if this motif was also found in the majority of the 2,481 DNA sequences bound by LANA in LEC-219 cells. Surprisingly, no enrichment of the hLBS1 motif was detected at LANA-binding sites in LEC-219 cells when the whole data set was analyzed as previously done with the iSLK-219 data (data not shown). We then hypothesized that there could be a correlation between the presence of an hLBS1 motif on host DNA and the level of enrichment of LANA. The MEME-ChIP pipeline was rerun in order to separately analyze two groups of LANA peaks: one that consisted of the top third of LANA-binding sites in terms of fold enrichment above background as calculated by MACS (top tier, 827 peaks) and a second subset composed of all the other LANA-enriched genomic regions (bottom tier, 1,654 peaks). Strikingly, the CentriMo analysis of the top tier subset detected a consensus motif positioned at the center of LANA peaks (E value, 1.70 × 10−61) that was virtually identical to the hLBS1 sequence identified at LANA-binding sites in iSLK-219 (Fig. 5A, left panel, and B). This hLBS1 motif (motif 1) was detected within a 121-bp central segment of 398 of the 827 LANA peaks (48.1%). As a comparison, the second-best motif (motif 2) mapped by CentriMo had a P value of 5.90 × 10−5, which is indicative of a limited central enrichment. In contrast, no hLBS1 motif was detected by CentriMo in the bottom tier subset. The two most centrally enriched sequences in the bottom tier (motif 2 and motif 3) are depicted in Fig. 5A (right panel) and B. These motifs were, respectively, detected at the center of 909 and 777 of the 1,654 LANA-binding sites analyzed in this group. With respective widths of 249 and 179 bp, their large centrally enriched regions were suggestive of an indirect association with chromatin rather than of direct DNA binding (63). Interestingly, motif 2 is the consensus DNA-binding sequence of the murine transcription factor GA-binding protein, alpha subunit (GABPA), a member of the ETS family of transcription factors. In fact, the top five human motifs detected by CentriMo in the bottom tier of LANA-binding sites were DNA sequences known to interact with ETS proteins such as ELK-4, GABPA, SPI1, FEV, and ELK-1 (see Data Set S2 in the supplemental material). Considering that ETS sequences are commonly found at human promoters (74), we used the PscanChIP program to confirm that their enrichment at LANA-binding sites was indeed overrepresented in such a genomic context. Confirming the CentriMo findings, PscanChIP determined that the overrepresentation of ETS sequences such as GABPA (murine), ELK-4, ELK-1, FEV, ETS1, and SPI1 was largely beyond what is statistically expected from their typical occurrence at gene promoters (see Data Set S2). Analyses of DNA sequence enrichment conducted with LANA ChIP-seq data from four other cell lines (iSLK-219, BEC-219, BJAB-219, and BCBL-1) did not reveal any consensus DNA sequences bound by ETS proteins in the vicinity of host LANA-binding sites (data not shown). On the other hand, hLBS1 motifs enriched at the center of LANA peaks were readily detected in all of those data sets (data not shown). Overall, these results indicate that most LANA-binding sites on the host genome of LEC-219 cells are found at gene promoters and that LANA preferentially accumulates at genomic locations that contain an hLBS1 motif. Furthermore, they also revealed that most LANA-binding regions in this cell line are enriched with DNA sequences recognized by ETS transcription factors and that this feature is specific to KSHV-infected LECs.
FIG 5.
Host LANA-binding sites in LEC-219s are enriched in LBS1 and ETS sequences. (A) CentriMo analysis of the three best-scoring centrally enriched DNA sequences at LANA-binding regions (±250 bp from peaks summits) on the host genome of LEC-219s. LANA peaks were analyzed in two separate groups: the top tier group (left) composed of the top third peaks as a function of their fold enrichment and the bottom tier group (right) containing all of the other LANA peaks. Data are shown as site probability curves illustrating the density of the indicated motifs (best occurrence) within 250 bp of LANA peak summits. Motif 1 is shown in black, motif 2 is in yellow, and motif 3 is in blue. Tables contain details of the CentriMo analyses. (B) LOGO representations of the best motifs identified by CentriMo. Motifs are annotated according to their predicted binding protein.
LANA binding to host gene promoters does not impact their transcriptional activity in LECs.
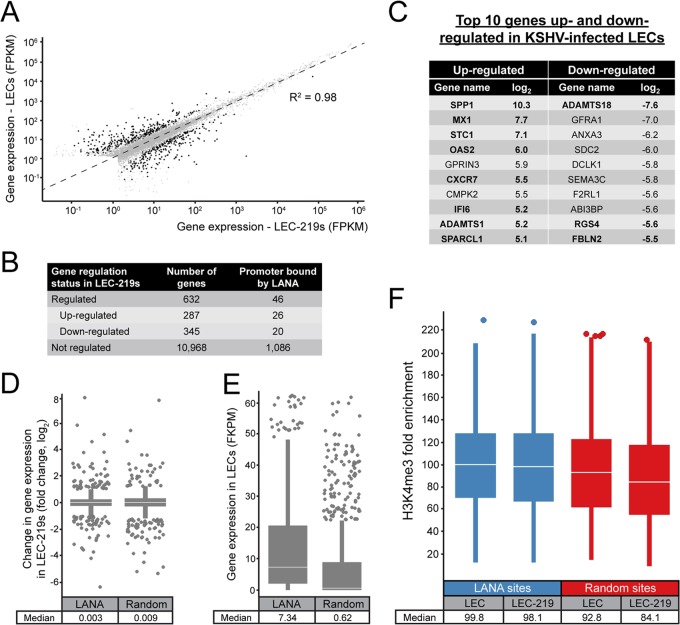
Our results in both latent iSLK-219s and LEC-219s have shown that the association of LANA to host chromatin is highly biased toward gene promoters and TSSs (Fig. 1A and 4A). This localization profile could be indicative of a role in host gene regulation during KSHV infection. If, indeed, LANA binding modulates host gene expression, we should be able to detect changes in mRNA levels of genes whose promoters are targeted by LANA following KSHV infection. To test this hypothesis, we initially identified all host genes that are differentially regulated in KSHV-infected LECs by analyzing the transcriptome of these cells using a global and unbiased approach (RNA-seq). The transcriptomes of LEC and LEC-219 were assembled from two independent experiments using the TopHat2 program (56), and gene expression was determined using the Cufflinks software package (57). Figure 6A shows a dot plot of the gene expression values in fragments per kilobase of exon per million fragments mapped (FPKMs), observed in uninfected LECs and KSHV-infected LEC-219s. A linear regression analysis of the expression values gave an R2 of 0.98, underscoring the robustness of our RNA-seq data. The 10,968 gray dots in the plot represent genes for which mRNA levels did not change significantly in KSHV-infected LEC-219s in comparison to uninfected LECs, as determined by the Cuffdiff algorithm (59). Cuffdiff classifies a gene as significantly regulated if it has good sequencing coverage, a minimal log2 value of 1, and a false-discovery rate no greater than 5%. The black dots in Fig. 6A depict the 632 host genes that were modulated by KSHV infection in LEC-219 cells. Of these genes, 287 were found induced, whereas 345 were negatively regulated in this KSHV-infected cell line (Fig. 6B). Figure 6C shows a succinct list of the most regulated genes in LEC-219s identified by our RNA-seq approach. The host genes previously reported to be modulated in KSHV-infected endothelial cells are depicted in bold (72, 75–79). The full list of KSHV-regulated genes is available in Data Set S3 in the supplemental material.
FIG 6.
Most differentially regulated genes in KSHV-infected LECs are not transcriptionally regulated by LANA. (A) Dot plot showing the expression levels of host genes in LECs as a function of their levels in LEC-219 cells. FPKM values were calculated by the Cuffdiff 2 algorithm, taking into account two independent replicates for each cell line. Black and gray dots depict genes that were differentially regulated or not, respectively, according to the Cuffdiff 2 program. The linear regression coefficient (R2) is indicated. (B) Table showing the number of genes that were upregulated, downregulated, or not regulated in KSHV-infected LECs (LEC-219) compared to noninfected LECs. The number of these genes that had their proximal promoters occupied by LANA according to our ChIP-seq experiments is also shown. Genes that did not have sufficient sequencing coverage for the Cuffdiff 2 analysis were filtered out. (C) Top 10 genes that were the most induced or repressed in LEC-219 cells compared to uninfected LECs (log2). Genes previously reported to be up- or downregulated in KSHV-infected endothelial cells are in bold. (D) Box plot analysis comparing expression changes (log2) of genes that have their promoter bound by LANA to a random set of genes. (E) Expression levels (FPKM) of genes which have their promoter targeted by LANA in uninfected LECs compared to a random set of genes. Outliers with FPKM values over 65 are not shown. (F) Box plot analysis of H3K4me3 levels (fold enrichment) at LANA-binding sites or at a random set of genomic H3K4me3-enriched sites from proximal promoters of uninfected LECs and KSHV-infected LECs. For box plots, outliers are shown as circles, and the median is indicated by a white line.
Next, to investigate if the differential expression of genes in LEC-219 cells is associated with the presence of LANA at their promoters, we compared the list of genes that have their proximal promoters occupied by LANA (Fig. 4B) to the transcriptome analysis data. According to our ChIP-seq experiments, 46 of the 632 genes differentially regulated in LEC-219s (i.e., 7.3% of the regulated genes) have their promoters bound by LANA, with 26 of them being upregulated and 20 downregulated (Fig. 6B; see also Data Set S3 in the supplemental material). LANA could potentially influence the induction or repression of these genes, which may play important roles in infection; however, LANA binding accounts for only a small fraction of the host transcriptional changes in these cells. The limited regulatory role of LANA on host gene expression is further supported by the analysis of the transcriptional changes from all promoters occupied by LANA. The box plot analysis in Fig. 6D shows that the median log2 value associated with these promoters was null and that there were no observable differences between promoters occupied by LANA and a random set of genes in terms of changes in gene expression. Moreover, DNA motif analyses showed that these 46 LANA-binding sites do not harbor the central enrichment of hLBS1 sequences, motif 2 (ETS), or motif 3 characteristic of the LEC-219 data set (data not shown). Taken together, these observations indicate that the transcriptional activity of most promoters bound by LANA does not vary significantly in KSHV-infected LECs.
In the absence of a clear connection between the binding of LANA to host genomic DNA and host gene regulation during KSHV infection, we sought to determine if LANA exhibits a clear preference for binding to active or silent promoters. Accordingly, we examined host gene expression in uninfected LECs and analyzed the subset of genes shown to have LANA bound at their promoter sites in LEC-219s. By doing a box plot analysis of gene expression in uninfected LECs, we found that the median FPKM value of genes whose promoters are occupied by LANA (7.34) was much higher than the median FPKM value from a random set of promoters (0.62) (Fig. 6E). This observation strongly suggests that LANA preferentially associates with active promoters in KSHV-infected LECs. Such bias is reminiscent of the preferential binding of LANA to promoters decorated with the activating histone modification H3K4me3 in iSLK-219 cells.
In line with our observations in iSLK-219 cells, the levels of H3K4me3 at genomic sites targeted by LANA in LECs did not change upon KSHV infection. Figure 6F shows that the median value of H3K4me3 fold enrichment at LANA-bound promoters in LEC-219s (98.1-fold) was almost identical to its value in uninfected LECs (99.8-fold). This is similar to what was observed in a random set of H3K4me3-marked promoters, where the median value of H3K4me3 enrichment changed by a factor of only 1.1-fold in KSHV-infected versus uninfected LECs. Overall, our data indicate that while KSHV infection perturbs the expression of a subset of host genes in LECs, LANA does not play a direct transcriptional regulatory role in this process. Furthermore, our results also show that LANA preferentially associates with active promoters and that the recruitment of this viral factor does not modulate/alter the deposition of H3K4me3 at those sites.
LANA disengages from chromatin during lytic reactivation.
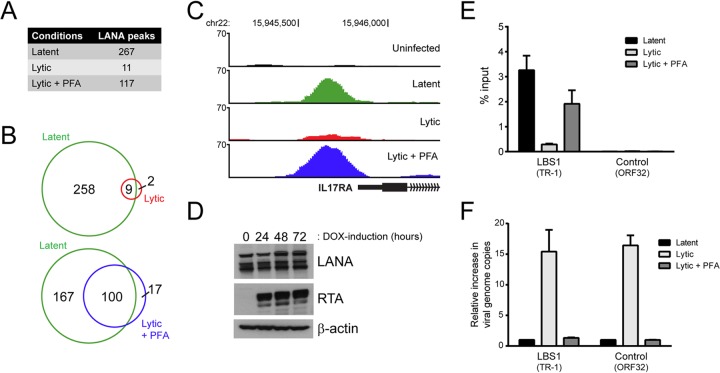
KSHV-infected iSLK-219 cells have the property of being tightly latent in the absence of exogenous stimuli (<0.05% spontaneous reactivation) while being efficiently reactivated into a productive lytic cycle by the addition of doxycycline (DOX) to the culture medium (60). We investigated the chromatin-binding profile of LANA during the late stage of lytic reactivation by performing a ChIP-seq of LANA in iSLK-219s treated with DOX for 72 h. To examine the contribution of late lytic gene expression to any phenotypes identified in those cells, we induced iSLK-219s in the absence or presence of the viral DNA replication inhibitor phosphonoformic acid (PFA). Notably, the results from two independent ChIP-seq experiments showed that the amount of reproducible LANA peaks identified by the MACS program is drastically reduced in lytic cells (11 peaks) compared to latent cells (267 peaks) (Fig. 7A). The disappearance of LANA-binding sites in reactivated iSLK-219s was less pronounced in the presence of PFA, with 117 LANA peaks detected. We then compared the sites enriched for LANA in the lytic and lytic/PFA data sets with the latent LANA-binding sites. In total, 9 of the 11 LANA peaks in lytic cells were also detected in latent iSLK-219s (Fig. 7B). These nine sites were among the most LANA-enriched regions during latency (data not shown), and their detection could therefore be attributed to a residual fraction of latent iSLK-219 cells that were not efficiently reactivated by the DOX treatment. In cells induced in the presence of PFA, 100 out of the 117 LANA-binding sites overlapped with latent LANA-binding sites (Fig. 7B). This observation indicates that the binding of LANA to host chromatin is severely impaired during the late stage of the KSHV lytic cycle and that this phenotype is dependent on viral DNA replication and possibly on late-lytic viral gene expression. Figure 7C shows the promoter of the IL-17RA gene as a representative example of the differential pattern of LANA-binding to host chromatin during the lytic cycle in iSLK-219 cells.
FIG 7.
The association of LANA with host DNA and viral TRs is impaired in lytically reactivated iSLK-219s. (A) Number of reproducible LANA peaks detected in latent, lytic, and PFA-treated lytic cells from two independent ChIP-seq experiments. (B) Venn diagrams illustrating the overlap between LANA-enriched regions detected in lytic and PFA-treated lytic cells compared to LANA-binding sites mapped in latent cells. (C) Screenshots from the UCSC browser (hg19) illustrating the loss of LANA enrichment within the promoter region of IL-17RA in lytically reactivated iSLK-219 cells. (D) The expression of LANA was examined by Western blotting in iSLK-219 cells that were mock induced or DOX induced for 24, 48, or 72 h. RTA and β-actin levels were assayed as controls of lytic reactivation and protein loading, respectively. (E) The enrichment of LANA at the viral LBS1 motif (TR-1) and at a control viral site (ORF32) in latent, lytic, and PFA-treated lytic cells was assessed by ChIP-qPCR. (F) The increase in viral genome copy number was determined by ChIP-qPCR using the input material from the ChIP experiment described in panel E. The amount of DNA fragment mapping to the indicated viral sites was compared to a control region on the host genome (GAPDH), and the results were normalized to those obtained in latent cells.
One possible explanation for the disengagement of LANA from host chromatin in lytic cells could be that LANA protein levels decline under lytic conditions. However, this hypothesis is not supported by our Western blot analysis, where constant levels of the LANA protein were detected throughout a 72-h time course of viral reactivation (Fig. 7D). Alternatively, and as suggested by the results in PFA-treated cells, the phenotype observed could be linked to viral DNA replication. For example, it is possible that LANA molecules that were previously bound to host chromatin are being recruited to the high-affinity LBS1 motif present in the TRs of the viral genomes being synthesized in reactivated iSLK-219s. To clarify this point, we monitored the enrichment of LANA at this viral location following lytic reactivation by ChIP-qPCR. Figure 7E shows that the enrichment of LANA at the viral LBS1 decreased by 11.2-fold following the lytic reactivation of iSLK-219 cells. To compare this phenotype with the amount of viral DNA replication taking place in lytic cells, we measured the increase in viral episome copies during lytic reactivation by qPCR. This analysis revealed that the number of viral genomes increased by ∼15-fold in iSLK-219 cells after 72 h of reactivation (Fig. 7F). Thus, the fact that we observed an 11.2-fold decrease in LANA binding at the LBS1 motif in lytic cells indicates that the majority of newly replicated viral TRs were not bound by LANA. This result argues against the idea that LANA is displaced from host LANA-binding sites because of its massive recruitment to newly synthesized viral LBS1 motifs. As shown in Fig. 7E, the association of LANA with viral LBS1 motifs was mostly unaffected in PFA-treated cells, which is reminiscent of what we observed at host LANA-binding sites (Fig. 7E). Figure 7F shows that the inhibition of viral DNA replication by PFA treatment was efficient as no significant increase in viral genome copy number was detected in cells reactivated in the presence of that compound. Taken together, these results indicate that the ability of LANA to bind both host DNA and viral LBS1 sites is impaired by the lytic reactivation of KSHV in iSLK-219 cells and that this phenotype is prominently dependent on a molecular mechanism that mostly occurs downstream of viral DNA replication.
DISCUSSION
The main latency gene product expressed by KSHV, the multifunctional protein LANA, has been the subject of numerous studies over the years. Its association with transcription factors and chromatin remodelers is well documented and has been proposed to reflect a regulatory role for LANA at the host chromatin level. Accordingly, previous studies have assessed the impact of LANA on host gene transcription using ectopically expressed LANA and identified several potential (direct or indirect) target genes of LANA. We therefore sought to clarify the role of LANA toward host chromatin and host gene expression through the use of global and unbiased approaches. For this purpose, we used ChIP-seq to identify LANA-binding sites on host chromatin and to investigate the chromatin context at those locations and RNA-seq to explore the whole cellular transcriptome of KSHV-infected cells. The combination of these approaches allowed us to study the association of LANA with host DNA and its impact on both host gene expression and the host chromatin landscape.
To examine the impact of the cellular context on LANA binding to host chromatin, we performed our analysis using two well-characterized cell lines that are known targets of KSHV: SLKs and primary LECs. The KSHV-infected iSLK-219 epithelial cell line is an interesting model due to its tight latency program and its highly inducible lytic cycle (60), while the KSHV-infected endothelial LECs are an attractive model due to their phenotypical similarities with KS tumor cells (44, 71–73). Our ChIP-seq of LANA revealed 267 reproducible host LANA-binding sites in iSLK-219 cells and 2,481 in LEC-219s. While 67.0% of the sites mapped in iSLK-219s (179 peaks) were also detected in KSHV-infected LECs, most of the sites identified in LEC-219s were unique to that cell line. The larger repertoire of LANA-enriched sites in LEC-219s could not be correlated with a higher occurrence of hLBS1 motifs or the density of these motifs at trimethylated H3K4 loci (data not shown). Additional ChIP-seq of LANA in rKSHV.219-infected blood endothelial cells (BECs) and Burkitt lymphoma B cells (BJAB cells) confirmed that the location of LANA's accumulation on host chromatin varies from one cell type to the other. However, they also showed that many LANA-binding sites are shared by divergent cell lines, with a subset of 57 being common to each of the four aforementioned cell lines. Surprisingly, only four of the shared LANA-enriched loci from rKSHV.219-infected cells were also detected in BCBL-1 cells. Such difference could be linked to the nature of the virus strains as variations in viral gene sequences, including LANA itself, could ultimately lead to radical changes in LANA's ability to bind host chromatin. It is also possible that LANA binds to distinct partners or is subjected to different posttranslational modifications in BCBL-1 cells. Additionally, only 8 out of 29 (27.6%) host LANA-binding sites we identified in BCBL-1 cells were also reported by Lu et al. (33) in the same cell line. This indicates that factors other than cell lineage, such as divergent experimental conditions and/or analytical processes, can deeply influence the identification of the host genomic regions occupied by LANA.
Our ChIP-seq data also revealed differences between cell types regarding the binding of LANA to the viral episome. For instance, only one potential LANA-enriched region was detected in latent iSLK-219s in addition to the TR LBS1 motif, whereas a few LANA-binding sites were mapped on the KSHV episome of LEC-219s. However, despite this apparent cell type specificity, LANA-enriched regions that were detected on the viral genome of LEC-219s were also reported in B cells by various groups using large-scale approaches (33, 80, 81). Many studies showed that these viral loci harbor histone marks reminiscent of open chromatin (82–84). Interestingly, the two most enriched sites in LEC-219s were located at the lytic origins of replication (OriLyts), within the DNA segment that harbors four palindromic pairs of the C/EBP binding motif (85, 86). The binding of LANA to these two regions was previously associated with the repression of lytic DNA replication (87). However, since the tightly latent iSLK-219 cells do not display LANA at the OriLyts, this binding cannot be essential to silencing these origins during latency, at least in this cellular context.
Our ChIP-seq data were further analyzed to identify specific DNA sequences recognized by LANA on the host genome. Strikingly, a consensus DNA sequence (hLBS1) that aligns perfectly with the viral TR LBS1 motif was uncovered at the sites of LANA binding in both iSLK-219 and LEC-219 cells. However, the fact that only a minority of hLBS1 sites are bound by LANA indicates that this motif alone is not sufficient to specify a LANA binding site in host chromatin. Intriguingly, the site probability curve of the host LBS1 consensus in iSLK-219s and LEC-219s did not show a clear unimodal pattern, as would have been expected from a DNA motif directly bound by LANA (63). Instead, the curve derived from the top tier LANA-binding sites of iSLK-219s and LEC-219s revealed a distinct dip at the center of LANA-bound sequences (Fig. 5A; also data not shown). Such a feature is likely indicative of additional context-specific features determining the association of LANA with host DNA beyond sequence information alone. For instance, the association of LANA with juxtaposed DNA- or chromatin-binding proteins (e.g., transcription factors and chromatin-modifying enzymes) or the effect of adjacent nucleosomes on chromatin shearing could interfere with the positioning of hLBS1 motifs within LANA-bound DNA fragments. Alternatively, the attachment of LANA to host DNA could extend beyond the hLBS1 motif and involve the dimerization of LANA in a way that is reminiscent of its association to LBS1 and its adjacent LBS2 motif on the viral episome (13). In line with this hypothesis, an analysis of LANA-binding sites in the LEC-219 top tier subset with the Motif Alignment and Search Tool (MAST) (88) revealed that many host LBS1 motifs are juxtaposed to a second hLBS1 motif (data not shown). The site probability curve of such genomic loci was indeed quite distinct from that of LANA-bound fragments harboring a single occurrence of the hLBS1 motif. Thus, further studies should determine how the association of LANA to host DNA is influenced by its dimerization and by the pairing of hLBS1 sequences on the human genome.
Interestingly, a significant number of LANA-enriched regions on the genome of LEC-219 cells did not harbor LBS1-like sequences but were instead populated with DNA sequences that are recognized by ETS proteins (Fig. 5B). The most overrepresented ETS sequence at LANA-binding sites was initially identified as a binding site for the murine GABPA protein; however, a recent study indicates that in human cells this site can be occupied by ELK-4 (also known as SAP-1) (89). While this suggests that the most overrepresented ETS sequence at LANA-binding sites is potentially bound by ELK-4, it should be noted that the 28 members of the ETS family of proteins show substantial redundancy in terms of promoter occupancy (90). For instance, ChIP-seq of ELK-4 and ELK-1 revealed an extensive overlap in terms of enriched genomic sequences (89, 91). This ETS binding redundancy is also reflected by the diversity of ETS motifs that were identified in our CentriMo analysis. Interestingly, no ETS DNA sequences were detected in the vicinity of genomic regions occupied by LANA in iSLK-219s, BEC-219s, BJAB-219s, or BCBL-1, indicating that the ETS sequence enrichment at LANA-binding sites in LEC-219s is specific to this cell type. One interpretation of this result is that LANA can bind directly to ETS family members in this cell context. We are currently pursuing this hypothesis using a plethora of biochemical approaches. However, it is also possible that the LANA/ETS connection involves a common binding partner. For instance, LANA and ELK-1 are both known to associate with the transcriptional regulators CBP/p300, SRF, mSin3A, and HDAC-1 (20, 38, 92–96). Our data suggest that the functional interaction between LANA and members of the ETS family of transcriptional regulators is complex, with specificities that lie beyond sequence information, chromatin context, and cell lineage. These interactions indubitably warrant further investigations.
To further study the determinants of the association of LANA with host DNA, we examined the chromatin status at LANA-binding sites. We found that most promoters targeted by LANA are transcriptionally active and frequently enriched with the activating trimethylated H3K4 mark. Our results indicate that the binding of LANA at proximal promoters does not have a major impact on the deposition of H3K4Me3 or the transcriptional activity of the promoters it occupies in infected cells. Even so, the accumulation of LANA at TSSs and, more precisely, between the −1 and +1 nucleosome, is reminiscent of the positioning of transcriptional factors at NDR-containing promoters (65–67). Thus, one could envisage that in a different cellular context, LANA could play a regulatory role on host gene expression. By performing a transcriptome analysis of KSHV-infected and uninfected LECs, we surprisingly found that only 3.7% of genes with their promoter occupied by LANA were differentially regulated in infected cells. In addition to this apparent lack of global, direct LANA-driven gene regulation, only two of these genes, PTPRG and NUDT4, were previously reported to be differentially expressed in cells ectopically overexpressing LANA (30, 31). Nonetheless, LANA could modulate the expression of a small number of key regulatory genes in that group (e.g., MECOM and PKN2), which in turn could have a larger impact on the transcriptome of KSHV-infected LECs. Alternatively, LANA could be poised and awaiting a particular cellular stimuli before perturbing host gene expression. In line with this hypothesis, Lu et al. demonstrated that the induction by gamma interferon of TAP1, PSMB9, and PARL, three genes under the control of promoters occupied by LANA, is impaired by the ectopic expression of LANA in a B-cell line (33). Overall, our results suggest that LANA's association to host gene promoters in KSHV-infected cells is not sufficient to directly impact host gene expression. Nevertheless, it remains possible that LANA may act as a permissive factor that allows participation in higher orders of gene regulation dependent upon downstream stimuli.
Finally, our ChIP-seq results showed that the association of LANA with the host genome is dramatically impaired in the late stage of the lytic cycle in reactivated iSLK-219 cells, a phenotype that was prevented by the inhibition of viral DNA replication (PFA treatment). Correspondingly, a decrease in the occupancy of viral LBS1 motifs by LANA in lytic cells was also observed. Since the protein levels of LANA remain abundant, even at the late stage of the lytic cycle, the phenotype observed could rely on a posttranslational regulatory event (e.g., posttranslational modification and trans-acting factor). Since LANA binds mostly to host DNA at sites that contain an LBS1-like motif in iSLK-219s, such a mechanism would simultaneously affect its association to both the host and viral genome. It is also possible that host chromatin is generally perturbed during the late phase of KSHV reactivation (e.g., host shutoff and induction of apoptosis), which could lead to a widespread dissociation of DNA-binding factors from the host genome along with LANA. Interestingly, the association of LANA with host chromatin and the viral genome did not appear to be impaired by the atypical lytic-like program expressed by LEC-219 cells (44), supporting the notion that LANA disengages from LBS1 motifs only at the late stage of lytic reactivation. Considering that TR units are chromatinized during viral latency and that histones appear to dissociate, at least in part, from KSHV genomes during lytic replication (19, 83, 97), it is plausible that the physical remodeling of chromatin architecture at specific loci negatively impacts the binding of LANA to newly replicated episomes. The absence of LANA at new TR units could be beneficial for the late stage of the KSHV lytic cycle, as the potential tethering of newly replicated viral genomes to host chromosomes could impair their packaging into virions. How newly replicated genomes are denuded of LANA remains unknown. This might be the result of the action of one or more viral late gene products, or it could be an indirect consequence of the subnuclear compartmentalization of viral DNA replication to a locale devoid of LANA. We are currently exploring these scenarios, and we surmise that our ongoing studies will shed light on the aforementioned events, leading to a better understanding of the viral life cycle.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Novartis Institutes for BioMedical Research.
We thank JinJong Myoung for providing the iSLK-219 and the BJAB-219 cell lines and Jeffrey Vieira (University of Washington) for the rKSHV.219 virus. We thank Audrey Parent, Diego Acosta-Alvear, and Philipp Tropberger for their critical readings of the manuscript.
Footnotes
Published ahead of print 2 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00268-14.
REFERENCES
- 1.Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willig JH, Mugavero MJ, Saag MS. 2011. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS 25:691–700. 10.1097/QAD.0b013e3283437f77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dezube BJ. 1996. Clinical presentation and natural history of AIDS-related Kaposi's sarcoma. Hematol. Oncol. Clin. North Am. 10:1023–1029. 10.1016/S0889-8588(05)70382-8 [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, D'Agay MF, Clauvel JP, Raphael M, Degos L. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191. 10.1056/NEJM199505043321802 [DOI] [PubMed] [Google Scholar]
- 5.Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore PS. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233–241. 10.1056/NEJM199607253350403 [DOI] [PubMed] [Google Scholar]
- 6.Kedes DH, Lagunoff M, Renne R, Ganem D. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Invest. 100:2606–2610. 10.1172/JCI119804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballestas ME, Chatis PA, Kaye KM. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644. 10.1126/science.284.5414.641 [DOI] [PubMed] [Google Scholar]
- 9.Ballestas ME, Kaye KM. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250–3258. 10.1128/JVI.75.7.3250-3258.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Garber A. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677–11687. 10.1128/JVI.76.22.11677-11687.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Renne R. 2005. Characterization of the minimal replicator of Kaposi's sarcoma-associated herpesvirus latent origin. J. Virol. 79:2637–2642. 10.1128/JVI.79.4.2637-2642.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber AC, Shu MA, Hu J, Renne R. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882. 10.1128/JVI.75.17.7882-7892.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garber AC, Hu J, Renne R. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401–27411. 10.1074/jbc.M203489200 [DOI] [PubMed] [Google Scholar]
- 14.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, Kaye KM. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856–861. 10.1126/science.1120541 [DOI] [PubMed] [Google Scholar]
- 15.Jha HC, Upadhyay SK, Prasad AJM, Lu J, Cai Q, Saha A, Robertson ES. 2013. H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence. J. Virol. 87:5255–5269. 10.1128/JVI.03575-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter MA, Robertson ES. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254–264. 10.1006/viro.1999.9999 [DOI] [PubMed] [Google Scholar]
- 17.Kim KY, Huerta SB, Izumiya C, Wang D-H, Martinez A, Shevchenko B, Kung H-J, Campbell M, Izumiya Y. 2013. Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen regulates the KSHV epigenome by association with the histone demethylase KDM3A. J. Virol. 87:6782–6793. 10.1128/JVI.00011-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma SC, Cai Q, Kreider E, Lu J, Robertson ES. 2013. Comprehensive analysis of LANA interacting proteins essential for viral genome tethering and persistence. PLoS One 8:e74662. 10.1371/journal.pone.0074662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakakibara S, Ueda K, Nishimura K, Do E, Ohsaki E, Okuno T, Yamanishi K. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78:7299–7310. 10.1128/JVI.78.14.7299-7310.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596–11604. 10.1128/JVI.76.22.11596-11604.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamay M, Krithivas A, Zhang J, Hayward SD. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. U. S. A. 103:14554–14559. 10.1073/pnas.0604469103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye F, Zhou F, Yoo SM, Xie J, Browning PJ, Gao S. 2004. Disruption of Kaposi's sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J. Virol. 78:11121–11129. 10.1128/JVI.78.20.11121-11129.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan K, Kuppers DA, Robertson ES. 2005. Kaposi's sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jκ, the major downstream effector of the Notch signaling pathway. J. Virol. 79:3468–3478. 10.1128/JVI.79.6.3468-3478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu F, Day L, Gao S-J, Lieberman PM. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 80:5273–5282. 10.1128/JVI.02541-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong J, Papin J. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J. Virol. 75:1798–1807. 10.1128/JVI.75.4.1798-1807.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renne R, Barry C, Dittmer D, Compitello N, Brown PO, Ganem D. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458–468. 10.1128/JVI.75.1.458-468.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong JH, Orvis J, Kim JW, McMurtrey CP, Renne R, Dittmer DP. 2004. Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 279:16822–16831. 10.1074/jbc.M312801200 [DOI] [PubMed] [Google Scholar]
- 28.Schwam DR, Luciano RL, Mahajan SS, Wong L, Wilson AC. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532–8540. 10.1128/JVI.74.18.8532-8540.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma SC, Lan K, Choudhuri T, Robertson ES. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen modulates K1 expression through its cis-acting elements within the terminal repeats. J. Virol. 80:3445–3458. 10.1128/JVI.80.7.3445-3458.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe T, Sugaya M, Atkins AM, Aquilino EA, Yang A, Borris DL, Brady J, Blauvelt A. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 77:6188–6196. 10.1128/JVI.77.11.6188-6196.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An F-Q, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862–3874. 10.1074/jbc.M407435200 [DOI] [PubMed] [Google Scholar]
- 32.Su L, Liao Q, Wu Y, Chen X. 2011. Kaposi's sarcoma-associated herpesvirus-encoded LANA down-regulates IL-22R1 expression through a cis-acting element within the promoter region. PLoS One 6:e19106. 10.1371/journal.pone.0019106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu F, Tsai K, Chen H-S, Wikramasinghe P, Davuluri RV, Showe L, Domsic J, Marmorstein R, Lieberman PM. 2012. Identification of host-chromosome binding sites and candidate gene targets for Kaposi's sarcoma-associated herpesvirus LANA. J. Virol. 86:5752–5762. 10.1128/JVI.07216-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottinger M, Christalla T, Nathan K, Brinkmann MM, Viejo-Borbolla A, Schulz TF. 2006. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80:10772–10786. 10.1128/JVI.00804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radkov SA, Kellam P, Boshoff C. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121–1127. 10.1038/80459 [DOI] [PubMed] [Google Scholar]
- 36.Friborg J, Kong W, Hottiger MO, Nabel GJ. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889–894 [DOI] [PubMed] [Google Scholar]
- 37.Lim C, Sohn H, Gwack Y, Choe J. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645–2652 [DOI] [PubMed] [Google Scholar]
- 38.Lim C, Gwack Y, Hwang S, Kim S, Choe J. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016–31022. 10.1074/jbc.M102431200 [DOI] [PubMed] [Google Scholar]
- 39.Fujimuro M, Wu FY, ApRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300–306. 10.1038/nm829 [DOI] [PubMed] [Google Scholar]
- 40.Verma SC, Borah S, Robertson ES. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 78:10348–10359. 10.1128/JVI.78.19.10348-10359.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J, Verma SC, Murakami M, Cai Q, Kumar P, Xiao B, Robertson ES. 2009. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-Associated B-lymphoma cells and contributes to their proliferation. J. Virol. 83:7129–7141. 10.1128/JVI.00397-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami Y, Yamagoe S, Noguchi K, Takebe Y, Takahashi N, Uehara Y, Fukazawa H. 2006. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem. 281:28113–28121. 10.1074/jbc.M602026200 [DOI] [PubMed] [Google Scholar]
- 43.An J, Sun Y, Rettig MB. 2004. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood 103:222–228. 10.1182/blood-2003-05-1538 [DOI] [PubMed] [Google Scholar]
- 44.Chang HH, Ganem D. 2013. A unique herpesviral transcriptional program in KSHV-infected lymphatic endothelial cells leads to mTORC1 activation and rapamycin sensitivity. Cell Host Microbe 13:429–440. 10.1016/j.chom.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. 2007. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27:53–66. 10.1016/j.molcel.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 46.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9:357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9:R137. 10.1186/gb-2008-9-9-r137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinrichs AS, Karolchik D, Baertsch R, Barber GP, Bejerano G, Clawson H, Diekhans M, Furey TS, Harte RA, Hsu F, Hillman-Jackson J, Kuhn RM, Pedersen JS, Pohl A, Raney BJ, Rosenbloom KR, Siepel A, Smith KE, Sugnet CW, Sultan-Qurraie A, Thomas DJ, Trumbower H, Weber RJ, Weirauch M, Zweig a, Haussler SD, Kent WJ. 2006. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 34:D590–D598. 10.1093/nar/gkj144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Raney BJ, Pohl A, Malladi VS, Li CH, Lee BT, Learned K, Kirkup V, Hsu F, Heitner S, Harte RA, Haeussler M, Guruvadoo L, Goldman M, Giardine BM, Fujita PA, Dreszer TR, Diekhans M, Cline MS, Clawson H, Barber GP, Haussler D, Kent WJ. 2013. The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res. 41:D64–9. 10.1093/nar/gks1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machanick P, Bailey TL. 2011. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27:1696–1697. 10.1093/bioinformatics/btr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant CE, Bailey TL, Noble WS. 2011. FIMO: scanning for occurrences of a given motif. Bioinformatics 27:1017–1018. 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37:W202–W208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zambelli F, Pesole G, Pavesi G. 2013. PscanChIP: Finding over-represented transcription factor-binding site motifs and their correlations in sequences from ChIP-Seq experiments. Nucleic Acids Res. 41:W535–43. 10.1093/nar/gkt448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye T, Krebs AR, Choukrallah MA, Keime C, Plewniak F, Davidson I, Tora L. 2011. seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res. 39:e35–e35. 10.1093/nar/gkq1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Madrid AS, Bellare P, Holdorf M, Weissman JS, Ganem D. 2014. KSHV 2.0: A comprehensive annotation of the Kaposi's sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLoS Pathog. 10:e1003847. 10.1371/journal.ppat.1003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14:R36. 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28:511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karolchik D, Hinrichs AS, Furey TS, Roskin KM, Sugnet CW, Haussler D, Kent WJ. 2004. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 32:D493–6. 10.1093/nar/gkh103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31:46–53. 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myoung J, Ganem D. 2011. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods 174:12–21. 10.1016/j.jviromet.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey TL, Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2:28–36 [PubMed] [Google Scholar]
- 62.Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. 2007. Quantifying similarity between motifs. Genome Biol. 8:R24. 10.1186/gb-2007-8-2-r24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey TL, Machanick P. 2012. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res. 40:e128. 10.1093/nar/gks433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Kasif S, Cantor CR, Broude NE. 2004. GC/AT-content spikes as genomic punctuation marks. Proc. Natl. Acad. Sci. U. S. A. 101:16855–16860. 10.1073/pnas.0407821101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morse RH. 2007. Transcription factor access to promoter elements. J. Cell. Biochem. 102:560–570. 10.1002/jcb.21493 [DOI] [PubMed] [Google Scholar]
- 66.Tirosh I, Barkai N. 2008. Two strategies for gene regulation by promoter nucleosomes. Genome Res. 18:1084–1091. 10.1101/gr.076059.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell O, Tiwari VK, Thomä NH, Schübeler D. 2011. Determinants and dynamics of genome accessibility. Nat. Rev. Genet. 12:554–564. 10.1038/nrg3017 [DOI] [PubMed] [Google Scholar]
- 68.Adelman K, Lis JT. 2012. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13:720–731. 10.1038/nrg3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura H. 2013. Histone modifications for human epigenome analysis. J. Hum. Genet. 58:439–445. 10.1038/jhg.2013.66 [DOI] [PubMed] [Google Scholar]
- 70.Stürzl M, Gaus D, Dirks WG, Ganem D, Jochmann R. 2013. Kaposi's sarcoma-derived cell line SLK is not of endothelial origin, but is a contaminant from a known renal carcinoma cell line. Int. J. Cancer 132:1954–1958. 10.1002/ijc.27849 [DOI] [PubMed] [Google Scholar]
- 71.Ganem D. 2010. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J. Clin. Invest. 120:939–949. 10.1172/JCI40567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng F, Pekkonen P, Laurinavicius S, Sugiyama N, Henderson S, Günther T, Rantanen V, Kaivanto E, Aavikko M, Sarek G, Hautaniemi S, Biberfeld P, Aaltonen L, Grundhoff A, Boshoff C, Alitalo K, Lehti K, Ojala PM. 2011. KSHV-initiated notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe 10:577–590. 10.1016/j.chom.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 73.Cancian L, Hansen A, Boshoff C. 2013. Cellular origin of Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. 23:421–432. 10.1016/j.tcb.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 74.FitzGerald PC, Shlyakhtenko A, Mir AA, Vinson C. 2004. Clustering of DNA sequences in human promoters. Genome Res. 14:1562–1574. 10.1101/gr.1953904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72–84. 10.1158/0008-5472.CAN-03-2767 [DOI] [PubMed] [Google Scholar]
- 76.Raggo C, Ruhl R, McAllister S, Koon H, Dezube BJ, Früh K, Moses AV. 2005. Novel cellular genes essential for transformation of endothelial cells by Kaposi's sarcoma-associated herpesvirus. Cancer Res. 65:5084–5095. 10.1158/0008-5472.CAN-04-2822 [DOI] [PubMed] [Google Scholar]
- 77.Aguilar B, Choi I, Choi D, Chung HK, Lee S, Yoo J, Lee YS, Maeng YS, Lee HN, Park E, Kim KE, Kim NY, Baik JM, Jung JU, Koh CJ, Hong Y-K. 2012. Lymphatic reprogramming by Kaposi sarcoma herpes virus promotes the oncogenic activity of the virus-encoded G-protein-coupled receptor. Cancer Res. 72:5833–5842. 10.1158/0008-5472.CAN-12-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcendor DJ, Knobel S, Desai P, Zhu WQ, Hayward GS. 2011. KSHV regulation of fibulin-2 in Kaposi's sarcoma: implications for tumorigenesis. Am. J. Pathol. 179:1443–1454. 10.1016/j.ajpath.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poole LJ, Yu Y, Kim PS, Zheng Q-Z, Pevsner J, Hayward GS. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395–3420. 10.1128/JVI.76.7.3395-3420.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell M, Chang P, Huerta S, Izumiya C, Davis R, Tepper CG, Kim KY, Shevchenko B, Wang D-H, Jung JU, Luciw PA, Kung H, Izumiya Y. 2012. Protein arginine methyltransferase 1-directed methylation of Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Biol. Chem. 287:5806–5818. 10.1074/jbc.M111.289496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilton IB, Simon JM, Lieb JD, Davis IJ, Damania B, Dittmer DP. 2013. The open chromatin landscape of Kaposi's sarcoma-associated herpesvirus. J. Virol. 87:11831–11842. 10.1128/JVI.01685-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Günther T, Grundhoff A. 2010. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 6:e1000935. 10.1371/journal.ppat.1000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toth Z, Maglinte DT, Lee SH, Lee H, Wong L, Brulois KF, Lee S, Buckley JD, Laird PW, Marquez VE, Jung JU. 2010. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog. 6:e1001013. 10.1371/journal.ppat.1001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toth Z, Brulois K, Lee H-R, Izumiya Y, Tepper C, Kung H, Jung JU. 2013. Biphasic euchromatin-to-heterochromatin transition on the KSHV genome following de novo infection. PLoS Pathog. 9:e1003813. 10.1371/journal.ppat.1003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin CL, Li H, Wang Y, Zhu FX, Kudchodkar S, Yuan Y. 2003. Kaposi's sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: identification of the ori-Lyt and association of K8 bZip protein with the origin. J. Virol. 77:5578–5588. 10.1128/JVI.77.10.5578-5588.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Li H, Chan MY, Zhu FX, Lukac DM, Yuan Y. 2004. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: cis-acting requirements for replication and ori-Lyt-associated RNA transcription. J. Virol. 78:8615–8629. 10.1128/JVI.78.16.8615-8629.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rossetto C, Yamboliev I, Pari GS. 2009. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 K-bZIP modulates latency-associated nuclear protein-mediated suppression of lytic origin-dependent DNA synthesis. J. Virol. 83:8492–8501. 10.1128/JVI.00922-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailey TL, Gribskov M. 1998. Combining evidence using P values: application to sequence homology searches. Bioinformatics 14:48–54. 10.1093/bioinformatics/14.1.48 [DOI] [PubMed] [Google Scholar]
- 89.O'Geen H, Lin Y-H, Xu X, Echipare L, Komashko VM, He D, Frietze S, Tanabe O, Shi L, Sartor M a, Engel JD, Farnham PJ. 2010. Genome-wide binding of the orphan nuclear receptor TR4 suggests its general role in fundamental biological processes. BMC Genomics 11:689. 10.1186/1471-2164-11-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hollenhorst PC, McIntosh LP, Graves BJ. 2011. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80:437–471. 10.1146/annurev.biochem.79.081507.103945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boros J, Donaldson IJ, O'Donnell A, Odrowaz ZA, Zeef L, Lupien M, Meyer CA, Liu XS, Brown M, Sharrocks AD. 2009. Elucidation of the ELK1 target gene network reveals a role in the coordinate regulation of core components of the gene regulation machinery. Genome Res. 19:1963–1973. 10.1101/gr.093047.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roupelieva M, Griffiths SJ, Kremmer E, Meisterernst M, Viejo-Borbolla A, Schulz T, Haas J. 2010. Kaposi's sarcoma-associated herpesvirus Lana-1 is a major activator of the serum response element and mitogen-activated protein kinase pathways via interactions with the Mediator complex. J. Gen. Virol. 91:1138–1149. 10.1099/vir.0.017715-0 [DOI] [PubMed] [Google Scholar]
- 93.Sarek G, Järviluoma Moore AHM, Tojkander S, Vartia S, Biberfeld P, Laiho M, Ojala PM. 2010. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. PLoS Pathog. 6:e1000818. 10.1371/journal.ppat.1000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nissen LJ, Gelly JC, Hipskind RA. 2001. Induction-independent recruitment of CREB-binding protein to the c-fos serum response element through interactions between the bromodomain and Elk-1. J. Biol. Chem. 276:5213–5221. 10.1074/jbc.M007824200 [DOI] [PubMed] [Google Scholar]
- 95.Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A. 1991. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 354:531–534. 10.1038/354531a0 [DOI] [PubMed] [Google Scholar]
- 96.Yang SH, Vickers E, Brehm A, Kouzarides T, Sharrocks AD. 2001. Temporal recruitment of the mSin3A-histone deacetylase corepressor complex to the ETS domain transcription factor Elk-1. Mol. Cell. Biol. 21:2802–2814. 10.1128/MCB.21.8.2802-2814.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stedman W, Deng Z, Lu F, Lieberman PM. 2004. ORC, MCM, and histone hyperacetylation at the Kaposi's sarcoma-associated herpesvirus latent replication origin. J. Virol. 78:12566–12575. 10.1128/JVI.78.22.12566-12575.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler a D. 2002. The Human Genome Browser at UCSC. Genome Res. 12:996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.