ABSTRACT
The segmented nature of the influenza virus genome allows reassortment between coinfecting viruses. This process of genetic exchange vastly increases the diversity of circulating influenza viruses. The importance of reassortment to public health is clear from its role in the emergence of a number of epidemiologically important viruses, including novel pandemic and epidemic strains. To gauge its impact on within-host genomic variation, we tracked reassortment in coinfected guinea pigs over time and given matched or discordant doses of coinfecting viruses. To ensure unbiased detection of reassortants, we used parental viruses of equivalent fitness that differ only by noncoding nucleotide changes. These viruses were based on the isolate A/Panama/2007/1999 (H3N2). At a dose of 2 × 102 PFU, one parental virus was absent from each guinea pig throughout the time course, indicating the presence of a bottleneck. With an intermediate dose of 2 × 103 PFU, genomic diversity present in nasal lavage samples increased from 1 to 3 days postinfection (dpi) and then declined by 6 dpi. With a high dose of 2 × 106 PFU, however, reassortment levels were high (avg. 59%) at 1 dpi and remained stable. Even late in the course of infection, parental viruses were not eclipsed by reassortants, suggesting that a uniformly high multiplicity of infection was not achieved in vivo. Inoculation with ∼10-fold discordant doses did not reduce reassortment relative to equivalent inputs but markedly changed the spectrum of genotypes produced. Our data reveal the potential for reassortment to contribute to intrahost diversity in mixed influenza virus infection.
IMPORTANCE Influenza virus reassortment is prevalent in nature and is a major contributor to the diversity of influenza viruses circulating in avian, swine, human and other host species. This diversity, in turn, increases the potential for influenza viruses to evade selective pressures or adapt to new host environments. As examples, reassortment was key to the emergence of the 1957, 1968, and 2009 pandemics; the unusually severe influenza epidemics of 2003, 1951, and 1947; and the rise in adamantane resistance among currently circulating human H3N2 viruses. We reveal here the diversity of viral genotypes generated over time in a host coinfected with two influenza viruses. We found that intrahost diversity driven by reassortment is dynamic and dependent on the amount of each virus initiating infection. Our results demonstrate the readiness with which reassortant influenza viruses arise, offering new insight into this important mechanism of influenza virus evolution.
INTRODUCTION
The influenza A virus genome comprises eight segments of negative-sense RNA (1). Segmentation of the genome allows for ready genetic exchange between differing influenza viruses, when these viruses meet within a coinfected cell. This process is termed reassortment and, together with genetic drift, it is a major mechanism underlying the rapid evolution of influenza viruses (2, 3). Reassortment among influenza A viruses circulating in humans and those adapted to avian and/or swine hosts played a critical role in the emergence of the last three influenza pandemics (4, 5). The possibility for reassortment among viruses currently circulating in humans and poultry to generate strains with pandemic potential has also been demonstrated in the laboratory (6–8).
In addition, large-scale, population-based analyses of influenza virus evolution have underlined the importance of reassortment to the epidemiology of seasonal influenza. Human seasonal influenza A viruses of both the H3N2 and H1N1 subtypes comprise multiple divergent clades cocirculating on a small spatial-temporal scale (9–15). Cocirculating lineages reassort with high frequency, generating significant genomic diversity and epidemiologically important variants, including those that triggered the unusually severe epidemics of 1947, 1951, and 2003, and allowed adamantane resistance to become widespread among current human H3N2 viruses (10–13, 16).
To better understand how novel strains detected at a population level emerge initially, a few recent studies have focused on viral genomic variation within an individual host. Thus, human surveillance samples previously used to obtain only consensus viral sequences are being reanalyzed with the aim of describing a greater complexity of viral genomic variation within the host (17, 18). Experimental studies in pigs and other natural hosts are also being used to track changes in genomic diversity over time (19–22). These efforts have revealed high levels of mixed infection arising from the cotransmission of related viral variants or superinfection with distinct influenza viruses. The spectrum of genotypes present in an individual host was also found to be highly dynamic, with a high turnover of mutations observed during the course of infection and different genotypes predominating on different days (19–21). These efforts have given valuable insight into the time scale on which drift mutations arise and become lost, fixed, or passed on to another host. However, in these studies, sequencing was performed on individual gene segments cloned from the mixed population, precluding the identification of reassortant viruses. As a result, a significant gap remains in our understanding of how viral diversity develops over time within an infected individual.
We have recently developed a well-defined coinfection system designed to allow ready identification of reassortant influenza viruses (23). Here, we exploit this system to track reassortment within individual guinea pigs and thereby describe—to our knowledge for the first time—the intrahost dynamics of reassortment. Central to our approach is the use of two parental viruses that differ only by silent nucleotide changes introduced into each segment. By studying reassortment between these well-matched wild-type (wt) and silently mutated variant (var) viruses, we eliminate the confounding effects of fitness differences among parental and reassortant progeny viruses. In this way, we were able to study the process of reassortment itself rather than the genetic compatibility of a particular pair of influenza viruses. At the same time, the silent differences between wt and var gene segments allow them to be differentiated using high-resolution melt analysis (23, 24); thus, reassortants can be detected without full or partial sequencing of all eight gene segments. Using this system, we now describe the change in reassortment levels over time after infection and the impact on reassortment frequency of using discordant doses of wt and var viruses for inoculation.
MATERIALS AND METHODS
Cells.
Madin-Darby canine kidney cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum and penicillin-streptomycin.
Viruses.
Recombinant A/Panama/2007/1999 (H3N2) (rPan/99wt or wt) and rPan/99var6 (var) viruses were described previously (23, 25). Briefly, these viruses were generated by reverse genetics and propagated in embryonated hens' eggs for two (var) or three (wt) passages. rPan/99var6 virus contains the following silent mutations relative to rPan/99wt virus (nucleotide numbering is from the 5′ end of the cRNA): NS C329T, C335T, and A341G; M C413T, C415G and A418C; NA C418G, T421A, and A424C; NP C537T, T538A, and C539G; HA T308C, C311A, C314T, A464T, C467G, and T470A; PA A342G and G333A; PB1 C288T and T297C; and PB2 C354T and C360T. Collectively, these mutations were shown not to attenuate the growth of rPan/99var6 virus relative to rPan/99wt virus in guinea pigs and to allow distinction of wt and var gene segments using high-resolution melt analysis (23).
Guinea pigs.
Animal work was performed in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Emory Institutional Animal Use and Care Committee under protocol number DAR-2000719-051614GA. Female, Hartley strain, guinea pigs weighing 300 to 350 g were obtained from Charles River Laboratories. Prior to intranasal inoculation, nasal lavage, or CO2 euthanasia, guinea pigs were sedated with a mixture of ketamine and xylazine (30 and 4 mg/kg, respectively). Inoculation and nasal lavage were performed as described previously (26), with phosphate-buffered saline (PBS) as the diluent/collection fluid in each case.
Preparation of mixed inocula.
To ensure the accuracy of the overall virus titer and of the relative amounts of wt and var viruses in mixtures used to infect guinea pigs, the following procedure was followed. High titer virus stocks of wt and var viruses were combined in the appropriate proportions (1:1 or 1:10 ratios of var PFU to wt PFU) and mixed. A portion of each mixture was divided into aliquots and stored at −80°C. A second portion was diluted serially in PBS to concentrations approximating those needed for the desired inoculation doses to be used in guinea pigs. The diluted virus mixtures were then divided into aliquots and stored at −80°C.
The var/wt ratios of the concentrated virus mixtures were verified by determining the genotypes of PB2 and NA segments of 36 to 40 plaque isolates from each mixture. Two, rather than eight, segments were screened since only intact wt and var viruses would be present in the mixed virus stocks.
Total virus titers of the wt-var mixtures were determined with at least three replicate plaque assays. Diluted mixtures with titers nearest to those needed for the desired inoculation doses were selected. A fresh aliquot of each mixture was thawed, diluted slightly where necessary to achieve the desired dose, and used to inoculate guinea pigs intranasally.
Genotyping of viral isolates.
Virus genotypes were determined by high-resolution melt analysis essentially as described previously (23). Briefly, the following steps were performed. (i) Plaque isolates were obtained by plaque assay of guinea pig nasal wash fluids. (ii) RNA was extracted from agar plugs using the Qiagen QiaAmp viral RNA kit, with the following modifications to the manufacturer's protocol: carrier RNA was not used, and 40 μl of water was used for the elution step. (iii) Twelve microliters of RNA was reverse transcribed using Maxima reverse transcriptase (Fermentas) according to the manufacturer's instructions. (iv) cDNA was used as the template in qPCRs with the appropriate primers (23) and Precision Melt Supermix (Bio-Rad) in wells of a white, thin-wall, 384-well plate (Bio-Rad). Quantitative PCR and melt analyses were carried out in a CFX384 real-time PCR detection system, in accorance with the instructions provided with the Precision Melt Supermix. The data were analyzed using Precision Melt Analysis software (Bio-Rad). Viruses were scored as reassortant if the genome comprised a mixture of wt and var gene segments in any proportion. Infrequently, unclear results were obtained for one or more gene segments. Isolates with one unclear segment were genotyped based on the remaining seven segments; isolates with >1 unclear segment were discarded from the analysis.
RESULTS
Variation in viral genomic diversity with time after coinfection.
The diversity of viral genomes shed from coinfected guinea pigs was determined by genotyping viral isolates derived from nasal washings collected on days 1, 2, 3, and 6 postinfection. Groups of five guinea pigs were used, and three inoculation doses were evaluated. Animals 1 to 5 received a mixture comprising 1 × 102 PFU wt virus plus 1 × 102 PFU var virus for a total of 2 × 102 PFU, animals 6 to 10 received 1 × 103 PFU of each virus, and animals 11 to 15 received 1 × 106 PFU of each virus. Virus titers and genomic diversity present in nasal washings over the course of infection are presented in Fig. 1.
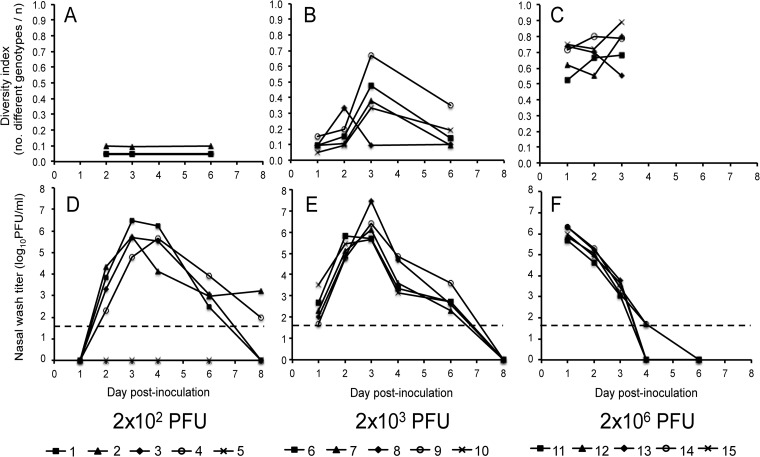
FIG 1.
The kinetics of viral growth and reassortment in guinea pigs are dose dependent. In the top panels (A, B, and C), viral genomic diversity, calculated as the number of different genotypes identified divided by the number of viral clones analyzed, is plotted as a function of day postinoculation. The bottom panels (D, E, and F) show virus titers present in nasal washings collected 1, 2, 3, 4, 6, and 8 days after inoculation. Dashed lines indicate the limit of detection (50 PFU/ml). The data are plotted for each individual guinea pig, and the symbols used for each animal are indicated under the corresponding graphs. Guinea pigs 1 to 5 were inoculated with 1 × 102 PFU wt and 1 × 102 PFU var virus (A and D), guinea pigs 6 to 10 received 1 × 103 PFU wt and 1 × 103 PFU var virus (B and E), and guinea pigs 11 to 15 received 1 × 106 PFU wt and 1 × 106 PFU var virus (C and F).
In the 2 × 102 PFU group, one of the five guinea pigs did not become productively infected, most likely because the dose used was near to the 50% infectious dose of Pan/99 virus in guinea pigs (25–27). The nasal washes of the remaining four animals in this group were negative by plaque assay at 1 dpi but positive at 2 to 6 dpi (Fig. 1D). Genotyping of viruses derived from these nasal washes indicated that only one parental virus was present in each case. Guinea pigs 1, 3, and 4 shed only wt virus throughout the time course, whereas guinea pig 2 shed predominantly var virus, with a reassortant containing one or two wt segments detected at each time point (Table 1 and Fig. 2). This highly limited viral diversity indicated that, although a total of 2 × 102 PFU was administered intranasally, only a small number of viruses initiated infection in each guinea pig. Thus, inoculation at a low dose revealed a bottleneck effect or a stochastic loss of diversity.
TABLE 1.
Variation in the frequency of reassortant progeny over time after infection
| Guinea pig no. | Inoculum dose (PFU)a | Genotypes of virus isolates (%)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 3 |
Day 6 |
||||||||||
| R | wt | var | R | wt | var | R | wt | var | R | wt | var | ||
| 1 | 2 × 102 | ND | ND | ND | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| 2 | 2 × 102 | ND | ND | ND | 5 | 0 | 95 | 5 | 0 | 95 | 5 | 0 | 95 |
| 3 | 2 × 102 | ND | ND | ND | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| 4 | 2 × 102 | ND | ND | ND | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| 5 | 2 × 102 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 2 × 103 | 0 | 5 | 95 | 5 | 20 | 75 | 38 | 33 | 29 | 5 | 90 | 5 |
| 7 | 2 × 103 | 0 | 29 | 71 | 0 | 5 | 95 | 28 | 24 | 48 | 0 | 14 | 86 |
| 8 | 2 × 103 | 0 | 81 | 19 | 24 | 43 | 33 | 5 | 0 | 95 | 5 | 0 | 95 |
| 9 | 2 × 103 | 5 | 10 | 85 | 10 | 40 | 50 | 57 | 33 | 10 | 35 | 65 | 0 |
| 10 | 2 × 103 | 0 | 0 | 100 | 0 | 5 | 95 | 24 | 71 | 5 | 24 | 38 | 38 |
| 11 | 2 × 106 | 48 | 33 | 19 | 67 | 14 | 19 | 63 | 37 | 0 | ND | ND | ND |
| 12 | 2 × 106 | 57 | 43 | 0 | 50 | 15 | 35 | 67 | 33 | 0 | ND | ND | ND |
| 13 | 2 × 106 | 63 | 32 | 5 | 65 | 35 | 0 | 45 | 35 | 20 | ND | ND | ND |
| 14 | 2 × 106 | 62 | 33 | 5 | 70 | 20 | 10 | 74 | 16 | 10 | ND | ND | ND |
| 15 | 2 × 106 | 65 | 25 | 10 | 61 | 17 | 22 | 78 | 17 | 5 | ND | ND | ND |
Inocula contained 50% wt and 50% var viruses (n = 36). The total dose for wt + var is indicated.
n = 18 to 21. R, any reassortant genotype. ND, virus was not detected in nasal washings at these time points (limit of detection = 50 PFU/ml).
FIG 2.
Viral genotypes detected in coinfected guinea pigs over the course of infection. Viral gene segments are represented schematically by eight colored bars arranged horizontally: PB2, PB1, PA, HA, NP, NA, M, and NS segments are shown from left to right. Red indicates wt, turquoise represents var, and white bars signify segments that could not be typed. The 18 to 21 viral isolates genotyped from each nasal lavage sample are grouped together. The guinea pig from which viruses were collected is indicated at the left (numbers 1 to 15). Note that guinea pig 5 is not included, since virus was not detected in this animal throughout the time course. The day postinfection on which nasal lavage samples were collected is shown at the top. The doses of wt and var virus administered to the guinea pigs are shown at the right of the figure. The phrase “virus not detected” indicates nasal wash samples for which the virus titer was below the limit of detection of the plaque assay (50 PFU/ml).
In the 2 × 103 PFU group, all animals were infected productively and peak virus titers were observed at 3 dpi (Fig. 1E). Genomic diversity was low 1 day after infection, with most guinea pigs shedding only parental viruses (Table 1 and Fig. 2). A small increase in the frequency of reassortant genotypes was seen at 2 dpi, and a significant increase in diversity was seen by 3 dpi (P = 0.015, Student t test comparing diversity on d1 and d3) (Fig. 1B). The number of different genotypes present in these animals declined as the virus was cleared, such that wt, var, and/or certain reassortant genotypes were over-represented at 6 dpi (Table 1 and Fig. 2). Thus, when the inoculation dose was sufficient to overcome the bottleneck and initiate infection with both wt and var viruses, genomic diversity generated through reassortment first increased and then decreased with viral spread and clearance, respectively.
Guinea pigs coinfected with 2 × 106 PFU shed high virus titers already at 1 dpi and cleared the infection rapidly, by 4 or 6 dpi (Fig. 1F). This sharp decline in shedding titers was in marked contrast to the more prolonged course seen with 2 × 102 or 2 × 103 PFU infections and allowed the analysis of genomic diversity only at 1, 2, and 3 dpi (Fig. 1C). The proportion of reassortant viruses present in nasal washes collected at 1 to 3 dpi was high, ranging from 59 to 65% (Table 1). In addition, the reassortant genotypes identified were not frequently detected multiple times; thus, viral genomic diversity was high throughout the course of shedding in this group of guinea pigs (Fig. 1C and Fig. 2). Nevertheless, in each guinea pig, either the wt or var parental genotype was the predominant genotype detected at all three time points and the proportion of viruses carrying an intact parental gene constellation did not decline over the time course (Table 1). These data demonstrate that a 2 × 106 PFU dose is sufficient to achieve coinfection of an appreciable proportion of cells early after infection. Conversely, the persistence of intact parental genotypes indicates that uniformly high multiplicity conditions are not achieved in vivo, even following multiple cycles of replication.
Reassortment remains efficient following coinoculation with discordant doses.
To test the importance to reassortment efficiency of the relative doses of two incoming viruses, we measured reassortment levels following coinfection of guinea pigs with mixtures containing ∼10-fold more var virus than wt virus. Because we anticipated a reduction in reassortment relative to that seen with 1:1 inocula, we chose to perform this experiment with high total doses of 2 × 106 or 1 × 105 PFU. The genotypes of viruses isolated at 2 dpi were determined and are reported in Fig. 3. In contrast to our expectation, discordant doses of wt and var viruses did not decrease the overall level of reassortment in guinea pigs infected with 2 × 106 PFU (P = 0.09 by t test comparing day 2 results of guinea pigs 11 to 15 in Table 1 to guinea pigs 6 to 10 in Table 2). Also, at the lower dose of 1 × 105 PFU, reassortants were detected in all guinea pigs. Two clear effects of discordant doses did emerge: (i) among the parental viruses identified, var outnumbered wt in each animal, and (ii) when all reassortant genotypes identified in a given animal were considered together, var genome segments outnumbered wt genome segments in all but one animal (Table 3). We assessed whether these two measures revealed significant differences between viruses shed from the 1:7 discordantly infected guinea pigs compared to those shed from animals infected at the same dose with a 1:1 mixture of wt and var viruses. As reported in Table 3, the differences were highly significant for both parameters, indicating that inoculation with a 7-fold greater proportion of var than wt virus resulted in overrepresentation of var parental progeny and enrichment of var gene segments in reassortant viruses. Thus, while the administration of discordant doses of wt and var did not significantly reduce the proportion of progeny that carried a reassortant genome, it did have a marked effect on the type of parental and reassortant genomes formed.
FIG 3.
Viral genotypes detected in guinea pigs coinfected with ∼10-fold discordant doses of two influenza A viruses. Viral gene segments are represented schematically by eight colored bars arranged horizontally: PB2, PB1, PA, HA, NP, NA, M, and NS segments are shown from left to right. Red indicates wt, turquoise represents var, and white bars signify segments that could not be typed. The 20 to 21 viral isolates genotyped from each nasal lavage sample are grouped together. The guinea pig from which viruses were collected is indicated at the left (numbers 1 to 10). All nasal lavage samples were collected at 2 dpi. The total dose of wt and var virus administered to the guinea pigs is shown at the bottom of the figure. (A) Guinea pigs 1 to 5 were inoculated with a total dose of 1 × 105 PFU, of which 92.5% comprised var virus and 7.5% comprised wt virus. (B) Guinea pigs 6 to 10 were inoculated with a total dose of 2 × 106 PFU, of which 87.2% comprised var virus and 12.8% comprised wt virus.
TABLE 2.
Frequency of reassortment in guinea pigs administered discordant doses of two influenza A viruses
| Guinea pig no. | Inoculum dose (PFU)a | Input mixtureb |
Genotypes of virus isolates (%)c at day 2 |
|||
|---|---|---|---|---|---|---|
| % wt | % var | R | wt | var | ||
| 1 | 1 × 105 | 7.5 | 92.5 | 9.5 | 9.5 | 81 |
| 2 | 1 × 105 | 7.5 | 92.5 | 33 | 0 | 67 |
| 3 | 1 × 105 | 7.5 | 92.5 | 10 | 0 | 90 |
| 4 | 1 × 105 | 7.5 | 92.5 | 35 | 10 | 55 |
| 5 | 1 × 105 | 7.5 | 92.5 | 25 | 25 | 50 |
| 6 | 2 × 106 | 12.8 | 87.2 | 55 | 5 | 40 |
| 7 | 2 × 106 | 12.8 | 87.2 | 55 | 0 | 45 |
| 8 | 2 × 106 | 12.8 | 87.2 | 43 | 0 | 57 |
| 9 | 2 × 106 | 12.8 | 87.2 | 38 | 0 | 62 |
| 10 | 2 × 106 | 12.8 | 87.2 | 65 | 0 | 35 |
Total for both wt and var viruses.
n = 39 to 40.
n = 20 to 21.
TABLE 3.
Relative inoculation doses of wt and var viruses impact which genotypes emerge following coinfectiona
| Ratio (wt/var), inoculum (PFU) | No. of var parental/total parental isolatesb (P = 0.0026) | No. of var segments/total segments in reassortant isolatesc (P = 0.0005) |
|---|---|---|
| 1:12.5, 1 × 105 | 0.89 | 0.50 |
| 1.00 | 0.84 | |
| 1.00 | 0.63 | |
| 0.85 | 0.66 | |
| 0.67 | 0.67 | |
| Avg | 0.88 | 0.66 |
| 1:7, 2 × 106 | 0.89 | 0.68 |
| 1.00 | 0.76 | |
| 1.00 | 0.68 | |
| 1.00 | 0.63 | |
| 1.00 | 0.78 | |
| Avg | 0.98 | 0.71 |
| 1:1, 2 × 106 | 0.57 | 0.43 |
| 0.70 | 0.56 | |
| 0.00 | 0.52 | |
| 0.33 | 0.48 | |
| 0.57 | 0.46 | |
| Avg | 0.44 | 0.49 |
Data are derived from day 2 nasal washes of guinea pigs 11 to 15 shown in Fig. 2 (1:1 group) and the guinea pigs shown in Fig. 3. A Student t test was used to compare guinea pigs inoculated with 1:1 and 1:7 mixtures at the 2 × 106 PFU dose. The P values are indicated in the respective column headings.
The number of var parental isolates, divided by the sum of wt + var parental isolates, is shown.
The number of var gene segments included in reassortant genomes, divided by the sum of wt + var gene segments in reassortant genomes, is shown.
DISCUSSION
Our data indicate that the dynamics of influenza virus reassortment are very much dependent on the dose of the coinfecting viruses.
At a relatively low dose, a population bottleneck was observed. Such a bottleneck could arise due to innate host defenses, including mucus shielding target epithelia and the action of mucociliary clearance. Population bottlenecks are known to be potent factors in determining viral genetic diversity (28, 29). Our data underscore the potential of bottlenecks to limit influenza virus reassortment: clearly, if one or more variant viruses present in a mixed inoculum are lost upon transmission, these variants will not contribute to reassortment in the newly infected individual. Such tight bottlenecks may not, however, be the norm for influenza viruses in nature. For equine and swine influenza viruses transmitting among their natural hosts, bottlenecks have been found to be relatively loose, allowing the transfer between animals of several viral variants (20, 21, 30). The relatively tight bottleneck observed in our experiment is likely a function of dose: the 2 × 102 PFU used in our study group was near to the 50% infectious dose for guinea pigs. It is unclear what amount of infectious virus is typically transferred between horses or pigs and whether these doses may significantly exceed the minimal infectious dose. Infection studies in humans furthermore suggest that viral infectivity and therefore bottleneck severity may differ with the route of inoculation (or, by extension, the mode of transmission). The human 50% infectious dose of A/Bethesda/10/1963 (H2N2) virus was approximately 0.6 to 3 50% tissue culture infective doses when individuals were exposed to infectious aerosols, but ∼10-fold higher when standard intranasal inoculation was used (33, 34). Another consideration is that looser bottlenecks may occur when an influenza virus is paired with its natural host. However, multiple variants of influenza A virus were found to transmit among guinea pigs, indicating that this species supports a loose bottleneck under conditions of both respiratory droplet and contact transmission (31, 32).
When an intermediate dose of 2 × 103 PFU was used for coinfection, reassortment was low early after infection, presumably due to a low incidence of coinfected cells. Genomic diversity and shedding titers peaked at the same time point, 3 days after inoculation. Clearance of virus then coincided with a decline in genetic diversity, suggesting a nonhomogenous model of clearance in which the residual virus sampled at 6 days after infection is derived from a small number of infected cells rather than many cells producing a small amount of virus. Importantly, these data suggest that genomic diversity is highest at times when the host is shedding large amounts of virus, and therefore most likely to transmit to contacts (35, 36).
When a high dose of 2 × 106 PFU of wt and var viruses was administered, reassortant viruses were recovered at high levels throughout the course of shedding. In this group the duration of shedding was brief, however, with titers at or below the limit of detection at 4 dpi. More rapid cessation of shedding, relative to that seen with low-dose inoculations, may be due to more immediate induction of innate immune responses or exhaustion of target cells in the presence of a higher viral load. Under these conditions of rapid clearance, a decline in genomic diversity among residual viruses was not detected. This difference relative to the 2 × 103 PFU group may be due to a more concerted clearance of virus from the respiratory tract following high dose infection or a lack of sampling at the appropriate time point due to the accelerated course of the 2 × 106 PFU infections.
Coinfections occurring in nature frequently involve virus mixtures comprising major and minor subpopulations (17, 20). We therefore determined the efficiency of reassortment following coinfection with discordant doses of wt and var viruses. Given a total dose of 2 × 106 PFU, we found that reassortment was similarly efficient when wt and var viruses were combined in a 1:1 ratio or a 1:7 ratio. Indeed, reassortant viruses were detected in all guinea pigs infected with discordant mixtures of var and wt viruses, both with the 2 × 106 PFU and the 1 × 105 PFU doses. However, when discordant doses were used, reassortant progeny viruses comprised a majority of segments from the predominant parental strain, whereas more random mixing was seen with 1:1 coinfections. Thus, at least when coinfecting viruses are of equivalent fitness, an ∼10-fold difference in abundance does not prevent their reassortment in vivo but does alter the range of genotypes produced.
Our data demonstrate the vast potential for influenza virus reassortment to generate diversity within a single host and indicate that the extent of diversity varies with total dose, relative dose, and time after infection.
ACKNOWLEDGMENTS
This study was funded by R01 AI099000 from the NIH (to A.C.L.). Support for the purchase of equipment came from the Georgia Research Alliance (to J.S. and A.C.L.).
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Palese P, Shaw ML. 2006. Orthomyxoviridae: the viruses and their replication, p 1647–1690 In Knipe DM, Howley PM. (ed), Fields virology. Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 2.Scholtissek C. 1995. Molecular evolution of influenza viruses. Virus Genes 11:209–215. 10.1007/BF01728660 [DOI] [PubMed] [Google Scholar]
- 3.Worobey M, Han GZ, Rambaut A. 2014. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature 508:254–257. 10.1038/nature13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilbourne ED. 2006. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 12:9–14. 10.3201/eid1201.051254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. 10.1126/science.1176225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimble JB, Sorrell E, Shao H, Martin PL, Perez DR. 2011. Compatibility of H9N2 avian influenza surface genes and 2009 pandemic H1N1 internal genes for transmission in the ferret model. Proc. Natl. Acad. Sci. U. S. A. 108:12084–12088. 10.1073/pnas.1108058108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorrell EM, Wan H, Araya Y, Song H, Perez DR. 2009. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 106:7565–7570. 10.1073/pnas.0900877106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. 10.1038/nature10831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghedin E, Sengamalay NA, Shumway M, Zaborsky J, Feldblyum T, Subbu V, Spiro DJ, Sitz J, Koo H, Bolotov P, Dernovoy D, Tatusova T, Bao Y, St George K, Taylor J, Lipman DJ, Fraser CM, Taubenberger JK, Salzberg SL. 2005. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature 437:1162–1166. 10.1038/nature04239 [DOI] [PubMed] [Google Scholar]
- 10.Nelson MI, Simonsen L, Viboud C, Miller MA, Taylor J, George KS, Griesemer SB, Ghedin E, Sengamalay NA, Spiro DJ, Volkov I, Grenfell BT, Lipman DJ, Taubenberger JK, Holmes EC. 2006. Stochastic processes are key determinants of short-term evolution in influenza a virus. PLoS Pathog. 2:e125. 10.1371/journal.ppat.0020125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes EC, Ghedin E, Miller N, Taylor J, Bao Y, St George K, Grenfell BT, Salzberg SL, Fraser CM, Lipman DJ, Taubenberger JK. 2005. Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses. PLoS Biol. 3:e300. 10.1371/journal.pbio.0030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. 2008. The genomic and epidemiological dynamics of human influenza A virus. Nature 453:615–619. 10.1038/nature06945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson MI, Edelman L, Spiro DJ, Boyne AR, Bera J, Halpin R, Sengamalay N, Ghedin E, Miller MA, Simonsen L, Viboud C, Holmes EC. 2008. Molecular epidemiology of A/H3N2 and A/H1N1 influenza virus during a single epidemic season in the United States. PLoS Pathog. 4:e1000133. 10.1371/journal.ppat.1000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westgeest KB, Russell CA, Lin X, Spronken MIJ, Bestebroer TM, Bahl J, van Beek R, Skepner E, Halpin RA, de Jong JC, Rimmelzwaan GF, Osterhaus ADME, Smith DJ, Wentworth DE, Fouchier RAM, de Graaf M. 2014. Genome-wide analysis of reassortment and evolution of human influenza A(H3N2) viruses circulating between 1968 and 2011. J. Virol. 88:2844–2857. 10.1128/JVI.02163-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westgeest KB, de Graaf M, Fourment M, Bestebroer TM, van Beek R, Spronken MI, de Jong JC, Rimmelzwaan GF, Russell CA, Osterhaus AD, Smith GJ, Smith DJ, Fouchier RA. 2012. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J. Gen. Virol. 93:1996–2007. 10.1099/vir.0.043059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson MI, Viboud C, Simonsen L, Bennett RT, Griesemer SB, St George K, Taylor J, Spiro DJ, Sengamalay NA, Ghedin E, Taubenberger JK, Holmes EC. 2008. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog. 4:e1000012. 10.1371/journal.ppat.1000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghedin E, Fitch A, Boyne A, Griesemer S, DePasse J, Bera J, Zhang X, Halpin RA, Smit M, Jennings L, St George K, Holmes EC, Spiro DJ. 2009. Mixed infection and the genesis of influenza virus diversity. J. Virol. 83:8832–8841. 10.1128/JVI.00773-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, Stockwell T, Angus B, Cozzi-Lepri A, Delfino M, Dugan V, Dwyer DE, Freiberg M, Horban A, Losso M, Lynfield R, Wentworth DN, Holmes EC, Davey R, Wentworth DE, Ghedin E. 2013. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J. Virol. 87:8064–8074. 10.1128/JVI.00240-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelzer K, Murcia PR, Baillie GJ, Wood JL, Metzger SM, Osterrieder N, Dubovi EJ, Holmes EC, Parrish CR. 2010. Intrahost evolutionary dynamics of canine influenza virus in naive and partially immune dogs. J. Virol. 84:5329–5335. 10.1128/JVI.02469-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murcia PR, Baillie GJ, Daly J, Elton D, Jervis C, Mumford JA, Newton R, Parrish CR, Hoelzer K, Dougan G, Parkhill J, Lennard N, Ormond D, Moule S, Whitwham A, McCauley JW, McKinley TJ, Holmes EC, Grenfell BT, Wood JL. 2010. Intra- and interhost evolutionary dynamics of equine influenza virus. J. Virol. 84:6943–6954. 10.1128/JVI.00112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murcia PR, Hughes J, Battista P, Lloyd L, Baillie GJ, Ramirez-Gonzalez RH, Ormond D, Oliver K, Elton D, Mumford JA, Caccamo M, Kellam P, Grenfell BT, Holmes EC, Wood JL. 2012. Evolution of an Eurasian avian-like influenza virus in naive and vaccinated pigs. PLoS Pathog. 8:e1002730. 10.1371/journal.ppat.1002730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murcia PR, Baillie GJ, Stack JC, Jervis C, Elton D, Mumford JA, Daly J, Kellam P, Grenfell BT, Holmes EC, Wood JL. 2013. Evolution of equine influenza virus in vaccinated horses. J. Virol. 87:4768–4771. 10.1128/JVI.03379-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall N, Priyamvada L, Ende Z, Steel J, Lowen AC. 2013. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog. 9:e1003421. 10.1371/journal.ppat.1003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ. 2003. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin. Chem. 49:853–860. 10.1373/49.6.853 [DOI] [PubMed] [Google Scholar]
- 25.Steel J, Lowen AC, Mubareka S, Palese P. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5:e1000252. 10.1371/journal.ppat.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 103:9988–9992. 10.1073/pnas.0604157103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvier NM, Lowen AC, Palese P. 2008. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J. Virol. 82:10052–10058. 10.1128/JVI.01226-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergstrom CT, McElhany P, Real LA. 1999. Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens. Proc. Natl. Acad. Sci. U. S. A. 96:5095–5100. 10.1073/pnas.96.9.5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeras DI, Hraber PT, Hurlston M, Evans-Strickfaden T, Bhattacharya T, Giorgi EE, Mulenga J, Karita E, Korber BT, Allen S, Hart CE, Derdeyn CA, Hunter E. 2011. Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proc. Natl. Acad. Sci. U. S. A. 108:E1156–E1163. 10.1073/pnas.1103764108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes J, Allen RC, Baguelin M, Hampson K, Baillie GJ, Elton D, Newton JR, Kellam P, Wood JL, Holmes EC, Murcia PR. 2012. Transmission of equine influenza virus during an outbreak is characterized by frequent mixed infections and loose transmission bottlenecks. PLoS Pathog. 8:e1003081. 10.1371/journal.ppat.1003081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ince WL, Gueye-Mbaye A, Bennink JR, Yewdell JW. 2013. Reassortment complements spontaneous mutation in influenza A virus NP and M1 genes to accelerate adaptation to a new host. J. Virol. 87:4330–4338. 10.1128/JVI.02749-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seibert CW, Kaminski M, Philipp J, Rubbenstroth D, Albrecht RA, Schwalm F, Stertz S, Medina RA, Kochs G, Garcia-Sastre A, Staeheli P, Palese P. 2010. Oseltamivir-resistant variants of the 2009 pandemic H1N1 influenza A virus are not attenuated in the guinea pig and ferret transmission models. J. Virol. 84:11219–11226. 10.1128/JVI.01424-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alford RH, Kasel JA, Gerone PJ, Knight V. 1966. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 122:800–804. 10.3181/00379727-122-31255 [DOI] [PubMed] [Google Scholar]
- 34.Jao RL, Wheelock EF, Jackson GG. 1970. Production of interferon in volunteers infected with Asian influenza. J. Infect. Dis. 121:419–426. 10.1093/infdis/121.4.419 [DOI] [PubMed] [Google Scholar]
- 35.Lowen AC, Mubareka S, Steel J, Palese P. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 3:1470–1476. 10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowen AC, Steel J, Mubareka S, Carnero E, Garcia-Sastre A, Palese P. 2009. Blocking interhost transmission of influenza virus by vaccination in the guinea pig model. J. Virol. 83:2803–2818. 10.1128/JVI.02424-08 [DOI] [PMC free article] [PubMed] [Google Scholar]