Abstract
Hepatic CD1d-restricted and natural killer T cell populations are heterogeneous. Classical ‘Type 1’ α-galactosylceramide-reactive CD1d-restricted T cells express ‘invariant’ TCRα (‘iNKT’). iNKT dominating rodent liver are implicated in inflammation, including in hepatitis models. Low levels of iNKT are detected in human liver, decreased in subjects with chronic hepatitis C (CHC). However, high levels of human hepatic CD161±CD56± non-invariant pro-inflammatory CD1d-restricted ‘Type 2’ T cells have been identified in vitro. Unlike rodents, healthy human hepatocytes only express trace and intracellular CD1d. Total hepatic CD1d appears to be increased in CHC and primary biliary cirrhosis.
Direct ex vivo analysis of human intra-hepatic lymphocytes (IHL), including matched ex vivo versus in vitro expanded IHL, demonstrated detectable non-invariant CD1d-reactivity in substantial proportions of HCV-positive livers and significant fractions of HCV-negative livers. However, α-galactosylceramide-reactive iNKT were detected only relatively rarely. Liver CD1d-restricted IHL produced IFNγ, variable levels of IL-10, and modest levels of Th2 cytokines IL-4 and IL-13 ex vivo. In a novel FACS assay, a major fraction (10–20%) of hepatic T cells rapidly produced IFNγ and up-regulated activation marker CD69 in response to CD1d. As previously only shown with murine iNKT, non-invariant human CD1d-specific responses were augmented by IL-12. Interestingly, CD1d was also found selectively expressed on the surface of hepatocytes in CHC, but not those CHC subjects with history of alcohol usage or resolved CHC. In contrast to hepatic iNKT, non-invariant IFNγ-producing Type 2 CD1d-reactive NKT cells are commonly detected in CHC, together with cognate ligand CD1d, implicating them in CHC liver damage.
Keywords: CD1, chronic, human, inflammation, NKT
Introduction
Chronic hepatitis C (CHC) virus (HCV)-infection involves immune-mediated liver destruction, although immunity also controls viral replication (1–8). HCV profoundly influences immunity (1–10). ~30% of human intra-hepatic lymphocytes (IHL) co-expressing T and natural killer (NK) cell proteins are frequently activated/memory and MHC-restricted (1–5;8,9;11–16). However, a major fraction of in vitro-cultured IHL is CD1d-restricted (5,8,9;16–22). Increased hepatic CD1d and CD1d-reactivity were reported in CHC (5,8,9;16–22). Increased Th2/pro-fibrotic CD1d-reactive cytokines are found in cirrhosis (20,21). However, most functional studies were in vitro, well-established for conventional T cells, but un-validated for CD1d-reactivity.
MHC-like non-polymorphic CD1d is constitutively expressed by myeloid and B cells as well as in the gut (23–25). Low level CD1d is apparently expressed inside healthy human hepatocytes (26), up-regulated in CHC and primary biliary cirrhosis (PBC) (21,22,27). CD1d is constitutively expressed on rodent hepatocytes (23,24,28). Possibly related to CD1d differences, high prevalence of rodent hepatic invariant TCR+ ‘NKT’ subset (iNKT; ‘Type 1’) compares to rare human liver iNKT, which are further reduced in CHC (5,8,9,16;20–22;29–32). While rare in human blood (~0.1%), iNKT are less frequent in matched liver (18). Also, human in vitro-cultured healthy hepatic CD1d-reactive NKT cells are Th1-biased (19,21,22), whereas rodent iNKT are Th1/Th2 (5,8,9;29–32). Hence, caution is needed in extrapolating the rodent CD1 system to humans.
Potent CD1d-restricted NKT cell ability to promote Th1 responses and/or CD1d-specific cytotoxicity contribute to resistance against certain infections and tumors through dendritic cell/macrophage maturation and IL-12, leading to activation of NK, B, and T cells (5,8,9,19;29–39). CD1d-reactive NKT rapidly secrete large amounts of Th1, Th2, Treg, and/or Th17 cytokines, depending on origin and health status (5,8,9;19–22;29–32).
iNKT cells express invariant Valpha, limited Vbetas, and NK receptors (29–33). iNKT specifically respond to CD1d-presented alpha-galactosylceramide (αGalCer) (29–32). CD1d responses are not always protective. Several bacterial genera make αGalCer-related iNKT-ligands, including Koch’s postulate-like demonstration of iNKT recognition of Novosphingobium lipid in PBC (27,34,35). Although functionally similar to iNKT, ‘non-invariant’ CD1d-restricted T cells (‘Type 2 NKT’) use diverse TCR. Indeed, recognition of up-regulated CD1d by murine Vγ4+ T cells causes viral myocarditis, an autoimmune sequela of otherwise successful picornaviral immunity (40,41). Murine iNKT can cause acute hepatitis (42–45). However, αGalCer suppresses viral replication and phenotypically NKT are activated in HBV models (46,47).
CD1d is expressed on human liver mononuclear cells and unlike other CD1s, CD1d-reactivity is high in uninvolved liver of wedge biopsies (22). Using surgical specimens, we now report low level iNKT activity, but a high proportions of hepatic CD1d-reactivity demonstrated ex vivo from CHC subjects and from a proportion of controls.. CD1d recognition by IHL from HCV± donors produced prototype inflammatory IFNγ, variable IL-10, and detectable Th2 cytokines. Interestingly, hepatocyte surface CD1d was also markedly elevated, specifically in CHC. Results suggest that resident hepatic non-invariant CD1d-restricted NKT respond to increased hepatocyte CD1d in CHC, with potentially pathologic consequences.
Material & Methods
Study Subjects
Discarded liver tissue surplus to pathology were obtained from patients with ESLD/liver failure due to amyloidosis, autoimmune or viral hepatitis, primary sclerosing cholangitis, and/or alcohol abuse (Table 1). Cirrhotic transplant recipient ESLD/FHF subjects reflected this demographic (21–62 yo,; mostly US Veteran males, late 40s–mid-50s). Non-ESLD control liver samples were from similar subjects with primary HCC or metastatic (primarily documented or presumed colonic) tumors obtained from Cooperative Human Tissue Network or National Disease Resource Interchange. Studies were approved by the institutional Committee on Clinical Investigations.
Table 1. Subject Status and Relative Hepatic IFNγ Production ex vivo.
Subject clinical and other status and % CD1d-specific responses relative to mitogen ± SEM. EtOH: documented history of alcoholism. Unk: unknown cadaver donor.
| ID | Virus | Status | %rel.CD1d | ± % | |
|---|---|---|---|---|---|
| Group 1 | HR67 | HCV | ESLD chronic | 7.2 | 4.0 |
| ESLD with Viral Hepatitis | HR206 | HCV | ESLD chronic | 0 | 0 |
| HR398 | HCV | ESLD chronic | 19.9 | 19.3 | |
| HR1206 | HCV | ESLD chronic | 0 | 0.1 | |
| 2F-IHL | HCV | ESLD chronic | 58.7 | 12.1 | |
| HR1724 | HCV/EtOH | ESLD chronic | 0 | 50.7 | |
| HR1789 | HCV/EtOH | ESLD chronic | 0 | 0 | |
| HR2018 | HCV/EtOH | ESLD chronic | 0 | 62.8 | |
| HR381 | HCV/EtOH | ESLD chronic/active | 7.4 | 0.9 | |
| HR1791 | HCV/EtOH | HCC | 0 | 74.5 | |
| HR1292 | HBV | ESLD | 0 | 10.0 | |
| HR879 | HBV | FHF | 1.2 | 3.0 | |
| HR1386 | HBV/EtOH | ESLD; ex-HCV | 1 | 1.0 | |
| HR650 | HAV/EtOH | ESLD acute | 0 | 0.9 | |
| Group 2 | HR1300 | - | ESLD FHF | 0 | - |
| Non-viral Controls | HR708 | - | ESLD Amyloidosis | 4.7 | 2.0 |
| HR291 | - | ESLD AIH | 3.3 | 14.0 | |
| HR1624 | - | ESLD EtOH | 0 | 0 | |
| HR419 | - | ESLD PSC/alloRej't | 0 | 14.6 | |
| KY9 | - | ESLD EtOH | 19.3 | 3.0 | |
| KY1 | - | tumor resection | 161.2 | 36.6 | |
| KY2 | - | tumor resection | 0 | 0 | |
| KY3 | - | tumor resection | 2.1 | 1.1 | |
| KY4 | - | tumor resection | 34.3 | 13.7 | |
| KY6 | - | tumor resection | 0 | 1.0 | |
| KY10 | - | colonic met.resection | 3.8 | 1.9 | |
| KY11 | - | HCC | 4.8 | 10.1 | |
| KY16 | - | bladder met.resection | 209 | 8.5 | |
| KY21 | - | colonic met.resection | 59.1 | 1.9 | |
| iNKT | iNKT5/4 | - | healthy | 30.5 | 12.9 |
| iNKT3/24 | - | healthy | 71.2 | 10.0 | |
| Controls | iNKT124 | - | healthy | 110 | 7.4 |
Reagents
Antibodies, including CD1d-specific mAbs, and blood-derived iNKT controls, and human mock and CD1d transfectants were described (21,22,25,36, 24,28,33). mAb were from eBioScience, Inc., except the cytokine capture reagents from Miltenyi Bio., Inc. (Table 2).
Table 2. Reagents & Source.
Reagents used and source.
| Reagent | Catalogue # | Source |
|---|---|---|
| Isotype mAb | 12-4732 | eBioScience, Inc. |
| CD1d | 12-0016 | eBioScience, Inc. |
| CD8 | 25-0088 | eBioScience, Inc. |
| CD69 | 12-0699 | eBioScience, Inc. |
| IFNγ Capture bi-mAb | 130-054-202 | Miltenyi BioSciences, Inc. |
Isolation of intra-Hepatic lymphocytes (IHL), FACS, & CD1d Functional Assays
To obtain IHL, surgical samples were minced to ~2mm, passed through 70µM sieve and subjected to standard Percoll gradient centrifugation. Where noted, small fractions of IHL were expanded in vitro, as previously (19,21), to directly compare to ex vivo. Media: RPMI-1640, 10% fetal bovine serum, antibiotics, 20IU/mL IL-2 (NIH AIDS Reagent Resource). Briefly, CD1d-reactive proportions were determined as previously (19,21,22,33,36,48,49) by incubating IHL or iNKT with CD1d+ or Mock C1R transfectants at 1:1 ratio (50,000/well) with phorbol myristic acid (PMA; 1ng.mL−1; ‘Total CD1d’=CD1d - Mock), or IL-12 (10ng.mL−1) (50). Cytokines were measured by ELISA (Endogen, Inc.), limit 1ng.mL−1. Standard error of means shown. Cytokine capture FACS was performed after 4hr. stimulation and with CD8, CD69 and IFNγ mAb (Table 2), gating on lymphocytes using FC500 (Beckman-Coulter), as described (19,21). FACS analysis was gated on lymphocytes (Fig. 3) or large hepatocytes (Fig. 4) from the same liver samples.
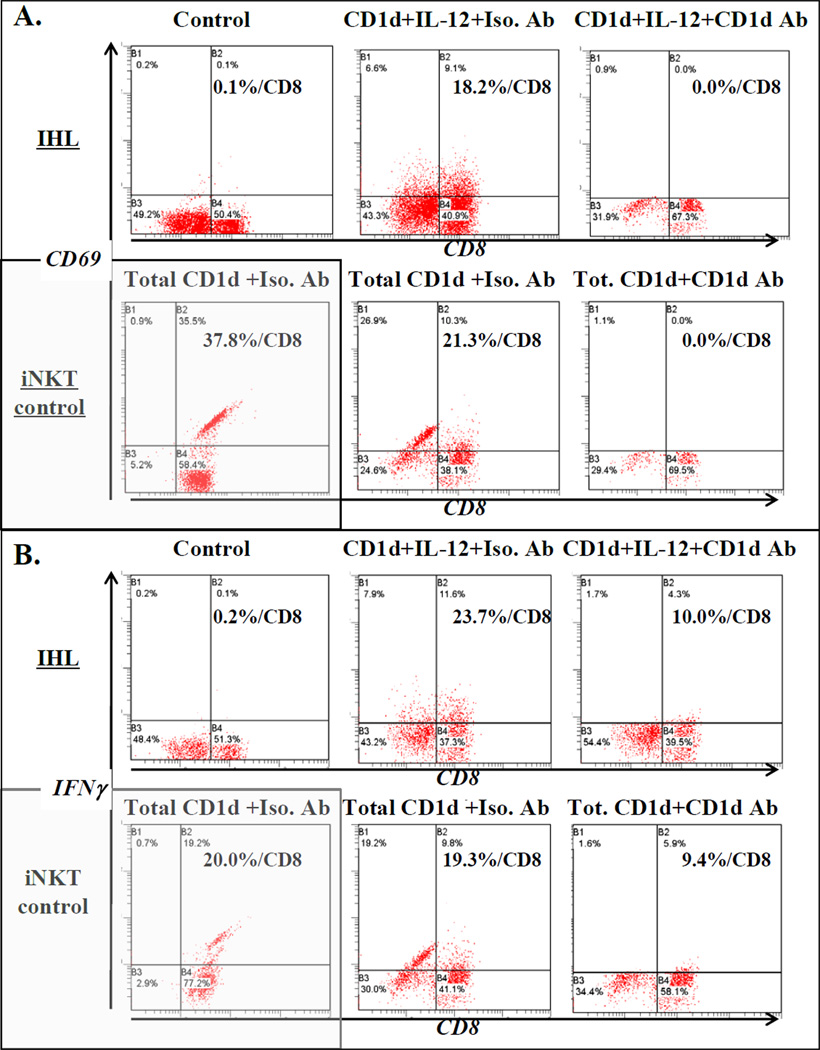
Figure 3. Identification of IFNγ-producing hepatic CD1d-reactive T cells ex vivo.
Ex vivo IHL and iNKT control lines were co-incubated with C1R CD1d in the presence or absence (‘control’) of IL-12 or PMA (‘total’ CD1d-specific). Activated cells were identified by FACS of CD69 up-regulation (A.) and production of IFNγ (B.). CD1d mAb or isotype control were included to determine specific CD1d-dependency.
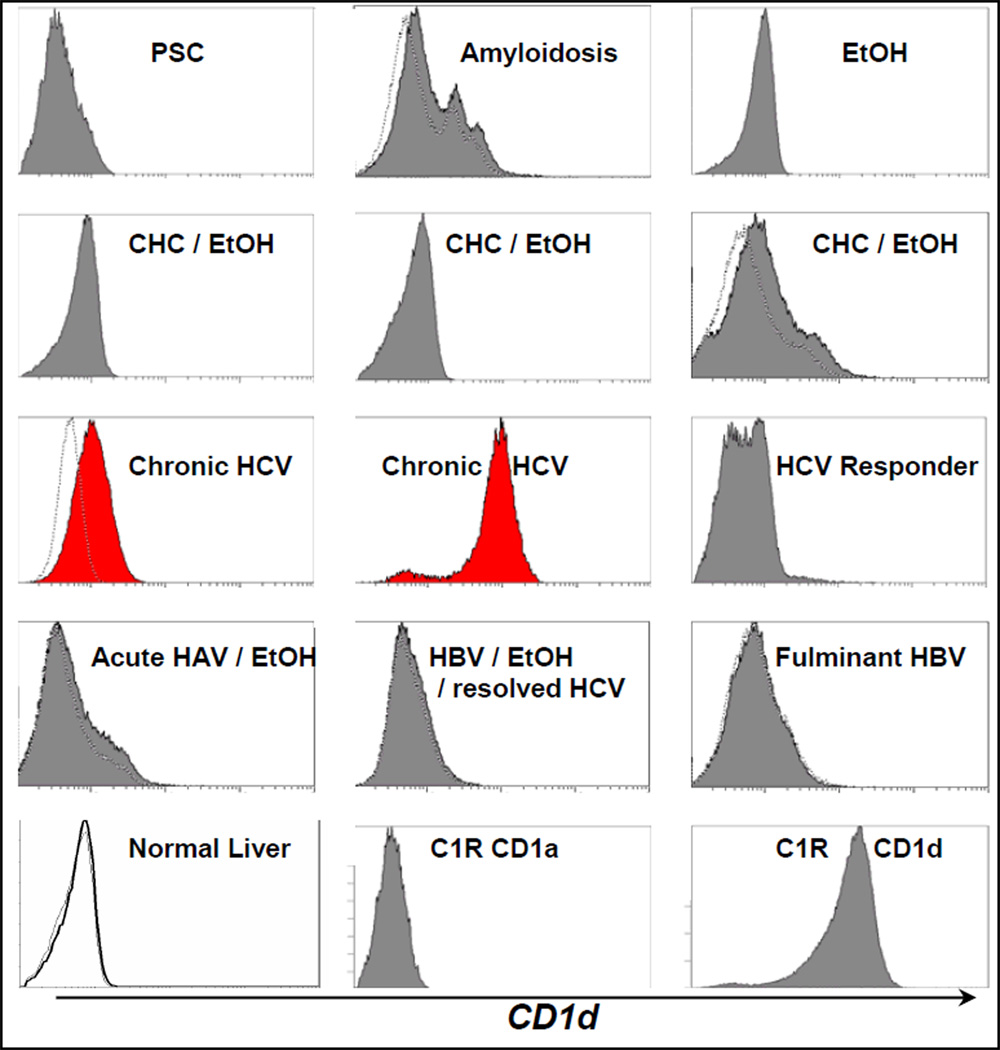
Figure 4. CD1d expression by hepatocytes from HCV-infected subjects ex vivo.
FACS of hepatocytes ex vivo from control and HCV± subjects were stained with CD1a or isotype control mAb (dotted tracing) or CD1d mAb (solid curve, except normal liver, bold line). Samples were ESLD Caucasian or African American males in upper 40s–50s. CD1d+ hepatocytes in CHC shown. ESLD PSC, chronic HCV with documented chronic alcohol use, alcohol alone, HAV, and/or HBV infections had little or no detectable CD1d expression. Minimal CD1d expression by a fraction of hepatocytes in ESLD amyloidosis shown. Control CD1d and CD1a C1R transfectants also shown. Similar results were found with other CD1d mAb to distinct epitopes.
Results
Comparison of hepatic CD1d-reactive T cells assayed ex vivo versus after in vitro expansion
CD1d-reactivity (predominantly IFNγ) is detectable in the majority of human liver biopsy samples assayed after in vitro expansion, from wedge biopsy lymphocytes assayed from healthy liver transplant donors, and from uninvolved tissue of tumor resections ex vivo (19,21,22). To test the validity of these findings, IHL from a range of donors were directly tested ex vivo compared to responses of similar liver samples after expansion in vitro (Figure 1A,B). A range of modest to strong (>100pg.mL−1) net CD1d-specific (CD1d+–Mock C1R) IFNγ responses were detected from directly ex vivo-assayed IHL (Figure 1A), which, when normalized, represented 5–10% of quality control mitogen (PHA) responses for the majority of positive IHL (Figure 1B). CD1d responses of IHL ex vivo were comparable to levels obtained with in vitro expanded IHL, although as expected, mostly less than from anonymous leukopak-derived pure iNKT cell line controls (19,21,22) assayed at the same cell numbers (Figure 1A–E).
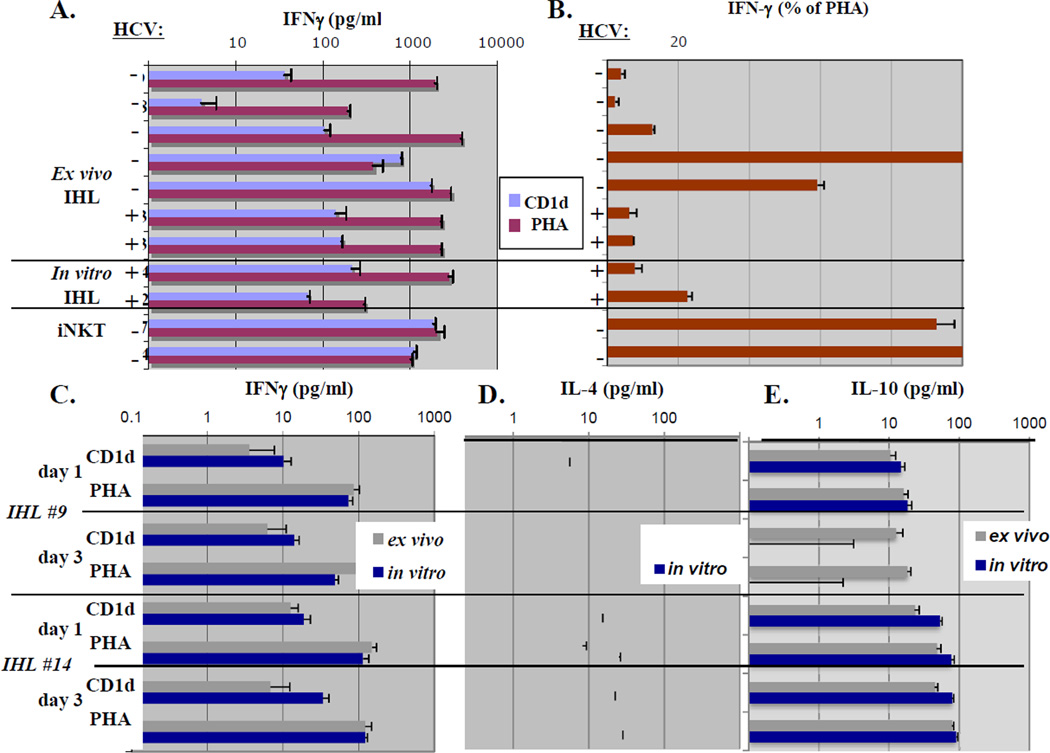
Figure 1. Comparison of hepatic CD1d-reactive T cells assayed directly ex vivo versus after in vitro expansion: cytokine profile of hepatic CD1d-reactive T cells ex vivo.
- Ex vivo CD1d-specific and mitogen IFNγ responses of IHL from HCV± subjects, compared to in vitro-expanded IHL.
- % CD1d-specific IFNγ responses of IHL ex vivo relative to mitogen, compared to HCV± donor in vitro-expanded IHL. There were no significant IHL responses to invariant NKT-specific ligand αGalcer (not shown), although pure blood iNKT served as positive controls.
- Total (net) CD1d-specific and mitogen IFNγ responses of IHL from subjects without HCV were assayed ex vivo compared to matched donor in vitro-expanded IHL and pure iNKT controls.
- CD1d-specific and mitogen IL-4 responses of the same matched IHL as in 1C. No CD1d-specific IL-4 was detected from these IHL.
- CD1d-specific and mitogen IL-10 responses of the same matched IHL as in 1C.
Given these results, IHL were directly tested ex vivo compared to responses obtained from matched liver samples after expansion in vitro. Again, although responses were somewhat lower on a per cell basis than from matched in vitro expanded IHL, direct ex vivo assayed material contained clear CD1d reactivity (Figure 1C–E).
We further analyzed cytokines known to be produced by blood iNKT (33) as well as some CD1d-restricted IHL lines (19,21,22). Most IHL produced little or no IL-4 to CD1d ex vivo, although significant amounts in vitro, as previously (20,21) and to mitogen (limit of detection 1ng.mL−1) (Figure 1D,E). Variable, but significant levels of CD1d-dependent IL-10 were produced (Figure 1A–C). Interestingly, unlike other cytokines, CD1d IL-10 levels, while variable, were comparable to mitogen (Figure 1E), suggesting a large proportion of human liver IL-10-producing cells were CD1d-reactive.
Non-invariant-type hepatic CD1d-reactive T cells are frequently detectable from HCV-infected and negative subjects ex vivo
To determine the specificity of net CD1d responses observed ex vivo, control or CD1d mAb was included in assays. As shown in Figure 2A, 2->10-fold of IHL and iNKT CD1d reactivity was specifically inhibited by CD1d mAb, similar to previous in vitro results of IHL and other CD1d-reactive NKT (19,21,22,33).
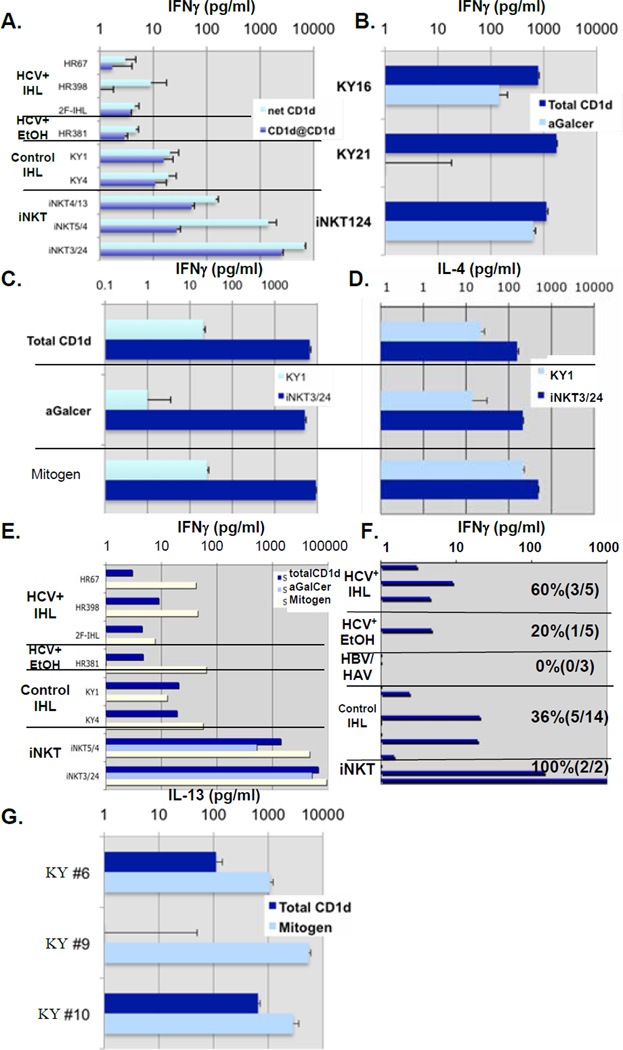
Figure 2. Functional characterization of hepatic CD1d-reactive T cells ex vivo.
- CD1d-specificity was determined by measuring CD1d-reactivity ex vivo in the presence of neutralizing CD1d or control mAb.
- CD1d-specific, αGalCer iNKT, and mitogen IFNγ responses of 2 representative IHL from subjects without HCV assayed ex vivo.
- CD1d-specific, αGalCer iNKT, and mitogen IFNγ responses of IHL from a third representative subject assayed ex vivo.
- CD1d-specific, αGalCer iNKT, and mitogen IL-4 responses of IHL from the same subject as in Fig. 2C assayed ex vivo.
- Total CD1d-specific, αGalCer, and mitogen IFNγ responses of IHL from 3 representative HCV+ subjects, an HCV+ subject with documented chronic alcoholism, and 2 HCV-negative subjects assayed ex vivo compared to iNKT lines.
- Summary of representative CD1d-specific IFNγ reactivity data. Subjects: 5 chronic HCV+ subjects, 5 HCV+ subjects with documented history of alcohol use, 2 chronic HBV+ subjects, 1 acute HAV+ subject, and 14 patients with other non-viral liver diseases were analyzed. iNKT controls also shown.
- CD1d-specific and mitogen IL-13 responses of IHL from 3 representative subjects (2/3 were IFNγ CD1d-reactive) assayed ex vivo.
We next determined whether the presence of Th1-like hepatic CD1d-reactive T cells assayed directly ex vivo or as matched cell lines represented αGalCer-specific iNKT. Only 3/28 IHL showed significant αGalCer-specific iNKT IFNγ production, compared to 9/28 total CD1d-reactive and 1/10 αGalCer-reactive HCV+ subjects, compared to 4/10 total CD1d-reactive (Figures 2B,C,E,F). As expected, control iNKT total IFNγ CD1d-reactivity was comparable to αGalCer responses (Figure 2B,C). Since IHL IFNγ responses to αGalCer were less frequent than total CD1d-reactivity, such reactivity was not mainly due to iNKT.
iNKT produce large amounts of IL-4 (29–33). Ex vivo IHL IL-4 CD1d reactivity was relatively rarely detected, only 2/26 samples tested producing detectable CD1d-specific IL-4 (>1pg.mL−1), although mitogen demonstrated potential of some liver T cells to produce IL-4 (Figures 1D,2D). This reflects overall Th1 bias of human hepatic T cells (1–9,17). IHL IL-4 total CD1d-reactivity appeared to be more closely αGalCer-induced and iNKT-related, since where produced, these were of a similar fraction to each other (both ~10% of mitogen; Figure 2D). Control iNKT cell lines derived from healthy subject blood produced >100pg.mL−1 IL-4 in response to CD1d, αGalCer, and to mitogen (Figure 2D).
To further evaluate levels of non-invariant-type hepatic CD1d-reactive T cells ex vivo, we examined HCV± IHL directly ex vivo from healthy liver or with a range of diseases (transplant recipients, viral/non-viral fibrosis/cirrhosis, tumor-bearing, etc.; Table 1; Figure 2). Although there were relatively few inflammatory cells obtained from healthy controls and from cirrhotics, IHL were obtained using available larger samples. Such IHL frequently contained readily detectable CD1d-reactivity ex vivo, whether HCV+ or HCV-negative (Table 1; Figures 1,2).
Overall, 32% (9/28) of liver samples tested ex vivo demonstrated CD1d-reactivity. 5/14 HBV/HCV-negative and 0/3 HBV+ subjects produced significant levels of CD1d-specific IFNγ. 1/5 IHL from HCV+ subjects with documented history of alcohol abuse and 3/5 other HCV+ IHL produced readily detectable CD1d IFNγ responses (Figure 2E,F; Table 1). Measurable CD1d-reactivity of HCV+ IHL was 7, 20, and 59% of mitogen IFNγ responses (Table 1), comparable to HCV-negative subjects (median=34% of mitogen; range: undetectable- comparable to mitogen). Finally, significant IL-13 could be detected in response to CD1d from some subjects ex vivo (Figure 2G), consistent with modest levels detected from in vitro IHL cultures (19).
In summary, ex vivo results were consistent with our previous results of a substantial population of largely non-invariant Th1-biased human hepatic CD1d-reactive T cells with or without HCV infection, most readily detectable in CHC (19,21,22). Apparently, human hepatic iNKT activity was relatively rare. Non-invariant CD1d responses were somewhat less readily detectable directly ex vivo than in vitro from both HCV+ and HCV-negative subjects. CD1d-specific IFNγ was most consistently detected compared to other cytokines tested.
Proportion of hepatic CD1d-reactive T cells ex vivo
Next, we addressed the fraction of IHL capable of responding to CD1d ex vivo. IHL were co-incubated with C1R CD1d or controls in the presence or absence of different stimuli and activation determined by FACS measurement of up-regulation of CD69 and IFNγ production (Figure 3). A substantial fraction of control highly-enriched iNKT line cells responded to CD1d (Figure 3A,B). As expected given their low frequency in human IHL, iNKT-specific ligand αGalCer did not stimulate many IHL ex vivo (not shown), although iNKT stimulation is well known to rapidly lead to activation of first iNKT and then NK cells (both CD69 up-regulation and IFNγ production), followed by other immune cells downstream (9;29–32). However, 2 co-stimuli known to be active with CD1d for at least murine iNKT (IL-12) (50) and for all types of CD1d-reactive T cells (19,21,22,33,48) (‘Total’=PMA), IL-12 and PMA, each produced comparable and substantial proportions of CD1d-responsive IHL (Figure 3A,B). IL-12 has not previously been shown to co-stimulate CD1d-specific non-invariant NKT responses, so this provides an alternative to PMA. Importantly, CD1d mAb specifically reduced the proportion of CD69+ and IFNγ-producing IHL, demonstrating CD1d-dependency of these responses (Figure 3A,B), as previously for IHL and other NKT cell populations (19,21,22,33,48). Therefore, a substantial fraction of human IHL, larger than the typical proportion of antigen-specific T cells (e.g. 1–9;17), is directly CD1d-reactive ex vivo.
Selective hepatocyte cell surface CD1d up-regulation in active CHC without history of alcohol
To date, only limited CD1d expression has been shown in human liver. These are at trace levels inside normal hepatocytes (26,27), increased expression by biliary epithelia in PBC (27) and in HCV infection (21), by unidentified cells adjacent to hepatic stellate cells in HCV cirrhosis (20), and on hepatic mononuclear cell surface in normal liver (22).
Figure 4 shows hepatocyte CD1d surface expression compared to both related CD1a and isotype control antibody staining ex vivo. Uninfected livers expressed little if any hepatocyte cell surface CD1d, with at most, limited expression in ESLD amyloidosis (Figure 4). Samples with non-HCV ESLD hepatitis (fulminant HBV; acute HAV and HBV, both chronic alcohol users) also did not show detectable hepatocyte CD1d (Figure 4). However, CD1d was specifically up-regulated on most hepatocytes in simple active CHC (Figure 4). Interestingly, where alcohol was known to be involved, no significant increase in hepatocyte CD1d was detected alone or in the presence of HCV, HBV or HAV (Figure 4). Similarly, resolved HCV infection and HCV treatment responders lacked hepatocyte CD1d up-regulation (Figure 4). Results were confirmed with CD1d-specific mAb (not shown) reactive with distinct epitopes (25). This selective up-regulation of hepatocyte surface CD1d in CHC extends previous data showing increased hepatic CD1d protein expression by immunoprecipitation/western blotting (21) or immuno-histochemistry (20,21). Together with enhanced detection of CD1d-reactive T cells ex vivo in HCV infection, this provides supportive evidence that HCV-mediated CD1d up-regulation on hepatocytes makes them a target for destruction by the large CD1d-reactive NKT population.
Discussion
Here we report high fractions of mostly non-invariant hepatic CD1d-reactive T cells producing IFNγ, some IL-10, and detectable but variable levels of IL-4 and IL-13 ex vivo, readily detected from chronic HCV-infected subjects and somewhat less frequently from other liver diseases. Furthermore, we found surface CD1d specifically up-regulated by hepatocytes in CHC. These results extend previous data on relatively Th1-biased CD1d-reactivity of in vitro cultured human IHL (19,21), except in cirrhosis, where Th2 cytokine levels were higher (20,21), ex vivo HCV-negative subjects (22), and on hepatic CD1d (20–22). We detected CD1d-reactivity from >50% of HCV-negative and >75% HCV+ subjects in vitro (19,21) (Figure 1). Therefore, in vitro culture may enhance measurement of CD1d-reactive IHL, but Th1 bias. Human resident hepatic non-invariant CD1d-reactive NKT are evidently more like rodent Th1/Th2 iNKT (5,8,9;29–32).
CD1d can be up-regulated (20,21;40,41) or down-regulated (29–32) by infection. Therefore, apparently, certain pathogens have adopted countermeasures toward anti-microbial CD1d-reactive NKT (20,21;29–32;40,41), consistent with findings of selective defects of CD1d–reactive NKT in immunodeficiencies with viral sensitivity (29–32,38). Tissue CD1d up-regulation presumably alerts local CD1d–reactive NKT of potential infection. However, this strategy may be exploited by HCV and other infections (20,21,40,41), supported by our finding of lack of CD1d in resolved CHC. Such induced expression could be on HCV-infected or neighboring cells. Lack of CD1d in CHC with history of alcohol may reflect a further net immuno-suppressive effect over CHC alone.
Selectively increased hepatocyte cell surface CD1d expression in simple active CHC, but apparently not resolved CHC or other hepatotropic viral infections, together with enhanced detection of hepatic CD1d-reactivity, specifically implicates the CD1d:NKT axis in hepatitis C immuno-pathology. High level hepatic CD1d-reactivity has implications for therapeutic applications of NKT subsets (51,52).
Acknowledgements
We are particularly indebted to Drs. Abrignani, Brenner, Galli. We acknowledge tissue from NIH-supported Cooperative Human Tissue Network and National Disease Research Interchange (5U42-RR006042). This study was funded in part by DK066917(ME), CA143748(ME), AI066313(DS,ME); AI053481, BMS ‘Freedom to Discover’ Award (MJK); VA Merit Award; U19 AI 1066328 (HRR).
Abbreviations
- IHL
intrahepatic lymphocyte
- NKT
natural killer T cell
Footnotes
Disclosure
Authors declare no competing interests.
References
- 1.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 2.Mengshol JA, Golden-Mason L, Rosen HR. Mechanisms of Disease: HCV-induced liver injury. Nat Clin Pract Gastroenterol Hepatol. 2007;4:622–634. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 3.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 4.Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:675–687. doi: 10.1111/j.1365-2893.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 5.Rehermann B. HCV versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alatrakchi N, Koziel M. Treg and viral liver disease. J Viral Hepat. 2009;16:223–229. doi: 10.1111/j.1365-2893.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 7.Burke KP, Cox AL. HCV evasion of adaptive immune responses: a model for viral persistence. Immunol Res. 2010;47:216–227. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2011 Aug 28; doi: 10.1136/gutjnl-2011-300620. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Exley MA, Koziel MJ. To be or not to be NKT: NKT cells in the liver. Hepatology. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin Liver Dis. 2008;12:675–692. doi: 10.1016/j.cld.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuti S, Rosa D, Valiante N, Saletti G, Caratozzolo M, Dellabona P, et al. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Va24+ T cells & rapid elimination of effector cells by apoptosis. Eur J Immunol. 1998;28:3448–3455. doi: 10.1002/(SICI)1521-4141(199811)28:11<3448::AID-IMMU3448>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Norris S, Collins C, Doherty D, Smith F, McEntee G, Traynor O, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, Ishikawa Y, et al. CD8+NKR-P1A+ T cells preferentially accumulate in human liver. Eur J Immunol. 1999;29:2406–2413. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike KT, Habu Y, et al. Decrease of CD56+ T cells and NK cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deignan T, Curry MP, Doherty D, Golden-Mason L, Volkov Y, Norris S, et al. Decrease in hepatic CD56+ T cells and Valpha24+ NKT cells in chronic HCV infection. J. Hepatol. 2002;37:101–108. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 16.Kenna T, Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O'Farrelly C, et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 17.Crispe IN. Liver as lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 18.Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc.Natl.Acad.Sci.(USA) 2001;98:3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, et al. Compartmentalization of Th1-like non-invariant CD1d-reactive T cells in HCV-infected liver. J Immunol. 168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. 220. [DOI] [PubMed] [Google Scholar]
- 20.de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, et al. Production of pro-fibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J. Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- 21.Durante-Mangoni E, Wang R, Shaulov A, He Q, Afdhal Nasser N, et al. Hepatic CD1d expression in HCV infection and recognition by resident pro-inflammatory CD1d-reactive T cells. J. Immunol. 2004;173:2159–2166. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 22.Kenna T, O'Brien M, Hogan AE, Exley MA, Porcelli SA, Hegarty JE, et al. CD1 expression and CD1-restricted T cell activity in normal and tumour-bearing human liver. Cancer Immunol Immunother. 2007;56:563–572. doi: 10.1007/s00262-006-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL, et al. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997;159:1216–1224. [PubMed] [Google Scholar]
- 24.Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, Castano AR, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 25.Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561–565. [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin M, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28:620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 28.Trobonjaca Z, Leithuser F, Muller P, Schirmbeck R, Reimann J. Activating immunity in the liver. I. Liver dendritic cells (but not hepatocytes) are potent activators of IFN-gamma release by liver NKT cells. J Immunol. 2001;167:1413–1422. doi: 10.4049/jimmunol.167.3.1413. [DOI] [PubMed] [Google Scholar]
- 29.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diana J, Lehuen A. NKT cells: friend or foe during viral infections? Eur J Immunol. 2009;39:3283–3291. doi: 10.1002/eji.200939800. [DOI] [PubMed] [Google Scholar]
- 31.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv.Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 32.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 33.Exley M, Garcia J, Balk S, Porcelli S. Requirements for CD1d recognition by human invariant Va24+CD4−CD8− T cells. J. Exp. Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, et al. Quantitation and phenotypic analysis of NKT cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 35.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, et al. Liver autoimmunity triggered by microbial activation of NKT cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 costimulation of CD1d-dependent activation of human T cells expressing invariant Va24JaQ. J. Exp. Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metelitsa L, Naidenko O, Kant A, Wu H, Loza M, Perussia B, et al. Human NKT mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J. Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 38.Levy O, Orange J, Hibberd P, Steinberg S, LaRussa P, Weinberg A, et al. Disseminated Varicella infection due to vaccine strain Varicella Zoster virus in a patient with a novel deficiency in NKT cells. J. Infect. Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- 39.Tahir M, Cheng O, Shaulov A, Koezuka Y, Bubley G, Wilson SB, et al. Loss of IFNγ production by invariant NKT of advanced cancer patients. J. Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 40.Huber SA, Sartini D, Exley M. Vγ4+ T cells promote autoimmune CD8+ cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4+ Th1 cells. J. Virol. 2002;76:10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J. Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 42.Ishigami M, Nishimura H, Naiki Y, Yoshioka K, Kawano T, Tanaka Y, et al. The roles of intrahepatic Va14+NK1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–1808. doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Hayakawa T, VanKaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver NKT cells to a murine model of hepatitis. Proc.Natl.Acad.Sci.(USA) 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osman Y, Kawamura T, Naito T, Takeda K, VanKaer L, Okumura K, et al. Activation of hepatic NKT and subsequent liver injury following administration of alpha-galactosylceramide. Eur. J. Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, et al. NKT cell dysfunction in CD39/Entpd1−/− mice protects against ConA hepatitis. Hepatology. 2008;48:841–845. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakimi K, Guidotti L, Koezuka Y, Chisari F. NKT cell activation inhibits HBV replication in vivo. J. Exp. Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of HBV infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 48.Exley M, Tahir S, Cheng, Shaulov A, Joyce R, Avigan D, et al. A major fraction of human bone marrow lymphocytes are Th2-Like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J. Immunol. 2001;167:5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 49.Exley MA, Wilson SB, Balk SP. Isolation and functional use of human NKT cells. In: Coligan, et al., editors. Current Protocols Immunol. Unit 14.11. Wiley & Sons; 2010. [DOI] [PubMed] [Google Scholar]
- 50.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted NKT cell activation during microbial infection. Nat. Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 51.Ohira M, Ishiyama K, Tanaka Y, Doskali M, Igarashi Y, Tashiro H, et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J. Clin. Invest. 2009;119:3226–3235. doi: 10.1172/JCI38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneiders FL, Scheper RJ, vonBlomberg BM, Woltman AM, Janssen HL, van den Eertwegh, et al. Clinical experience with α-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–141. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]