Abstract
Breast cancer incidence rates have declined among older but not younger women; the latter are more likely to be diagnosed with breast cancers carrying a poor prognosis. Epidemiological evidence supports an increase in breast cancer incidence following pregnancy with risk elevated as much as 10 years postpartum. We investigated the association between years since last full-term pregnancy at the time of diagnosis (≤10 or >10 years) and breast tumor subtype in a case series of premenopausal Hispanic women (n = 627). Participants were recruited in the United States, Mexico, and Spain. Cases with known estrogen receptor (ER), progesterone receptor (PR), and HER2 status, with one or more full-term pregnancies ≥1 year prior to diagnosis were eligible for this analysis. Cases were classified into three tumor subtypes according to hormone receptor (HR+ = ER+ and/or PR+; HR− = ER− and PR−) expression and HER2 status: HR+/HER2−, HER2+ (regardless of HR), and triple negative breast cancer (TNBC). Case-only odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for HER2+ tumors in reference to HR+/HER2− tumors. Participants were pooled in a mixed-effects logistic regression model with years since pregnancy as a fixed effect and study site as a random effect. When compared to HR+/HER2− cases, women with HER2+ tumors were more likely be diagnosed in the postpartum period of ≤10 years (OR=1.68; 95% CI, 1.12–2.52). The effect was present across all source populations and independent of the HR status of the HER2+ tumor. Adjusting for age at diagnosis (≤45 or >45 years) did not materially alter our results (OR=1.78; 95% CI, 1.08–2.93). These findings support the novel hypothesis that factors associated with the postpartum breast, possibly hormonal, are involved in the development of HER2+ tumors.
Keywords: breast cancer, breast tumor subtypes, etiologic heterogeneity, HER2, Hispanic, parity
Introduction
Although incidence rates of invasive breast cancer in the U.S. have decreased slightly over time, this trend has not been observed among younger women, in whom there is evidence for substantial racial/ethnic disparities [1,2]. While the overall proportion of breast cancer occurring in women age <40 years is only 5.6%, it is higher for U.S. Hispanics (10.3%) [1], for whom age at diagnosis has been shown to be as much as ten years earlier than non-Hispanic Whites (NHWs) [3–5]. Furthermore, despite lower incidence rates than in the U.S., the average age at diagnosis in Mexico is 50.1 years [6], approximately six years younger than for U.S. Hispanics [7,3]. In Mexico, it is estimated that 45% of cases are diagnosed in women age <50 years [6], while this proportion is 36% for U.S. Hispanics and 21% for NHWs [6,1].
Clinically, breast cancer represents a heterogeneous disease that is grouped based on its hormone receptor (HR) status (estrogen receptor [ER] or progesterone receptor [PR] positivity) and amplification of the ERBB2 gene (hereafter referred to as HER2+) [8]. Genomic profiling has identified at least two major lineages (i.e., luminal and basaloid) that split predominantly on their HR status [9,10]. These breast tumor subtypes differ by age at diagnosis, race/ethnicity, reproductive patterns, lifestyle factors, stage at diagnosis, and survival [11–14]. Recent epidemiologic and genetic studies support the concept of underlying etiologic heterogeneity of breast tumor subtypes [15]. For example, breast cancer risk differs by age at onset and menopausal status [16,17], as well as timing and number of births [18,19], depending on its HR status.
Findings from large, prospective studies provide strong evidence for a “dual” effect of pregnancy, where a transient postpartum increase in breast cancer risk is followed by long-term reduction in risk, relative to nulliparous women [20,18]. Depending on factors such as number of births and age at last birth, the estimated peak in breast cancer risk in the postpartum period ranges from 3–7 years, persisting for 10 or more years [21,20,22]. Further, it has been suggested that two forms of cancer are associated with pregnancy [23–26]. One, referred to as pregnancy associated breast cancer (PABC), is thought to involve those tumors that are diagnosed during or within the first year after a pregnancy; the other consists of tumors diagnosed in the postpartum period (more than one year after birth). The distinction is based on observed differences in mortality risk according to the time of diagnosis relative to pregnancy. Cases diagnosed in the postpartum period experience a higher risk of mortality than those diagnosed during pregnancy, after taking stage and other prognostic markers into account [25]. However, these observations have considered breast cancer as a single entity, and little is known about how, or if, this increase in risk differs by tumor subtype.
With the recognition of what appear to be distinct etiological paths to breast tumorigenesis, the complex role of pregnancy as a risk factor for breast cancer in younger women has gained a renewed interest. In vitro and in vivo studies provide evidence of critical cross-talk between progesterone/PR and estrogen/ER, possibly ER, and heregulin/HER2 signaling transduction pathways and a contribution of hormones or hormone conditioning of the breast in the activation of HER2 [27–29]. There are no known risk factors for the development of HER2+ tumors, and few epidemiological studies have assessed HER2+ tumors as a separate subgroup. Kwan et al. [30], showed that the odds of HER2-overexpressing tumors, defined as HR−/HER2+, were significantly greater in younger women, Asian and Hispanic women, and cases with 3 or more children who never breastfed when compared to cases with luminal A (HR+/HER2−) tumors. Analyses for HER2+ tumors independent of HR status were not considered separately.
Data from our previous genomic study of early-stage breast tumors show that HER2+ tumors are genetically more similar to each other than to HR+/HER2− or triple negative breast cancer (TNBC) [31]. We therefore speculate that, at least at the DNA level, HER2+ tumors may represent a subset of breast cancers that arise under distinct etiological influences, regardless of HR status. Here, we hypothesize that hormone exposure during pregnancy or lactation/involution confers a selective pressure for the outgrowth of cells harboring disturbances in HER2 signaling, independent of their hormone receptor status. Given the younger age at diagnosis and higher fertility rates in Hispanics compared with NHWs [32], we explored associations between pregnancy-related factors and HER2+ breast cancer in a case series of Hispanic women. Specifically, we investigated the association between the number of years since the last full-term pregnancy at the time of diagnosis and HER2+ tumors.
Materials and Methods
Study population
We pooled data from two Hispanic case series: the Ella Binational Breast Cancer study (Ella) and the Breast Oncology Galician Network (BREOGAN) study. Ella comprises 1,515 patients diagnosed with invasive breast cancer in the previous 24 months. Participants were recruited between 2007–2010; the present analysis includes data available by July 11, 2011. This multi-center study includes two sites in the U.S. [the Arizona Cancer Center (AZCC) in Tucson, Arizona and the M.D. Anderson Cancer Center (MDACC) in Houston, Texas] and three sites in Mexico [the Universidad de Sonora (UNISON) in Hermosillo, Sonora; the Instituto Tecnológico de Sonora (ITSON) in Ciudad Obregón, Sonora; and the Universidad de Guadalajara (UG) in Guadalajara, Jalisco]. All recruitment sites used a predominately clinic-based recruitment strategy. A detailed description of the organizational structure and methods of the study has been previously described [33]. Women were eligible to participate if they were diagnosed with incident invasive breast cancer, were age 18 years or older, and self-identified as Mexican or Mexican-American. Risk factor characteristics, including a detailed pregnancy history and menopausal status at the time of diagnosis, were collected via an interviewer-administered questionnaire [33]. Age at diagnosis was abstracted from medical records. All participants provided written informed consent. The Institutional Review Board (IRB) from each participating institution approved the study protocol.
The BREOGAN study is a population-based study conducted in the cities of Vigo and Santiago de Compostela, Spain. A total of 979 invasive and in situ breast cancer cases diagnosed between 1997–2012 were recruited at the Clinical University Hospital of Vigo and the Clinical University Hospital of Santiago de Compostela. Risk factor information was abstracted from patient medical records using the Ella risk factor questionnaire format for consistency between U.S. and Mexico datasets. Age at diagnosis, pregnancy history, and other reproductive factors are routinely obtained as part of the comprehensive patient medical record system in Spain. All participants provided written informed consent. The study was conducted in accordance to the Helsinki Principles of 1975, as revised in 1983. The Galician Ethics and Research Committee (CEIC, Comité Ético de Investigación Clínica de Galicia), responsible for the oversight of both university hospitals, approved the study protocol.
Tumor marker classification
In both studies, trained physicians abstracted tumor marker data from medical records. In the Ella study, ER and PR were classified as positive or negative according to the most recent guidelines [34]. In the abstraction, priority was given to a numeric value for the percent of cells staining. Any positive staining (≥1% of cells) resulted in ER/PR classified as positive. In 13.1% of cases, no specific number for percent of cells staining was available. We therefore used the interpretation value (“negative”, “positive”, or “low positive”) with any positive staining interpreted as positive. For the BREOGAN study, the cut-point for ER/PR was set at <10% as negative and ≥ 10% as positive. Had Ella used the higher cut-point, an additional 2% of cases would have been classified as ER/PR negative. For HER2 status, priority was given to determination by fluorescent in situ hybridization over immunohistochemistry (IHC). IHC values of 0 and 1+ were considered negative, 2+ equivocal, and 3+ positive [35].
Statistical methods
Analyses were restricted to premenopausal women with a known diagnosis of invasive breast cancer (n=898). We limited our focus to early-onset, premenopausal cases to minimize heterogeneity introduced by menopause on breast cancer risk and to assess the effect of pregnancy during reproductive years. Time (years) since last full-term pregnancy was calculated using age at diagnosis and age at last full-term pregnancy, where a full-term pregnancy was defined as one lasting >5 months, regardless of outcome. Cases with equivocal HER2 status not confirmed by FISH (n=31), missing tumor marker information (n=122), or zero full-term pregnancies (n=106) were excluded. We further excluded participants with missing information on age at first and/or last pregnancy (n=2) and those with a full-term pregnancy <1 year prior to diagnosis (n=10). After applying inclusion and exclusion criteria, 627 cases were available for analysis (414 from Ella and 213 from BREOGAN).
We dichotomized time since last full-term pregnancy as ≤10 or >10 years, based on evidence supporting a window of increased risk and poor survival in the postpartum period [36,37]. Surrogates of tumor subtypes were approximated according to joint ER, PR, and HER2 status. Breast tumors were classified as HR+ if they were ER+ and/or PR+ and HR-negative if ER− and PR−. Tumors were grouped into the following subtypes: HR+/HER2−, HER2+ (independent of HR), and TNBC (HR− and HER2−). We classified HER2+ tumors independent of their HR status under the a priori hypothesis that ERBB2 amplification arises as a consequence of an unknown, but distinct, etiological event. Our analysis is based on the working assumption that the aforementioned tumor subtypes represent three different forms of breast cancer.
All statistical analyses were performed using Stata 12.1 (StataCorp, College Station, TX). Descriptive statistics (mean ± SD and proportions) for reproductive and clinical characteristics were calculated separately by country of recruitment (USA, Mexico, or Spain). Potential associations between each risk factor and HER2+ status were tested using t-tests (continuous variables) or Fisher’s exact tests (categorical variables) in each country separately. The crude association between time since last full-term pregnancy (≤ 10 vs. > 10 y) and HER2+ status was tested using logistic regression, with either HR+/HER2− tumors or TNBC as the reference group.
Participants from all 3 countries were pooled in a mixed-effects logistic regression model, with time since last full-term pregnancy and age at diagnosis (≤ 45 or > 45 y) included as fixed effects and study site as a random effect. This approach is advantageous as it allows us to combine smaller study groups into a larger dataset and model distributions of exposure variables across sites. Importantly, the pooled approach is defensible in this context because BREOGAN used the same risk factor instrument to abstract pregnancy history as Ella. In addition, to examine the robustness of our findings and to test for heterogeneity of effects across study sites, we also conducted a 2-stage random effects meta-analysis where site-specific effects were calculated in the first stage, and site-specific estimates were aggregated using a random effects model in the second stage [38]. In order to assess heterogeneity of effect by study site, we calculated the I2 and χ2 (Q) statistics using the metan command in Stata [39].
Reported associations between HER2+ tumors and reproductive factors include age at menarche [40,41], parity [30,42], age at first birth [12,30,43], lifetime duration of breastfeeding [12], and ever using oral contraceptives [30]. We considered all of these factors plus additional characteristics listed in Table 1 as potential confounders in our analysis. We did not find any variables associated with both HER2+ tumors and time since last full-term pregnancy. Previous studies have reported an association between HER2+ tumors and age at diagnosis [12,30]. Although age at diagnosis was not associated with HER2+ in our data, we report age-adjusted pooled estimates as a sensitivity analysis. Additionally, in order to assess whether categorization of HER2+ tumors independent of HR status affected our estimates, we compared HER2+/HR+ and HER2+/HR− cases separately to the referent category. We considered a two-sided p-value of < 0.05 to be statistically significant.
Table 1.
Reproductive and clinical characteristics of premenopausal women, by country
| Characteristic | USA (n = 269) |
Mexico (n = 145) |
Spain (n = 213) |
|---|---|---|---|
| Time since last full-term pregnancy (y), n (%) | |||
| > 10 | 163 (60.6) | 96 (66.2) | 153 (71.8) |
| ≤ 10 | 106 (39.4) | 49 (33.8) | 60 (28.2) |
| Mean ± SD | 13.1 ± 7.8 | 13.8 ± 7.3 | 15.5 ± 8.1 |
| Total full-term pregnancies, n (%) | |||
| 1–2 | 119 (44.2) | 56 (38.6) | 177 (83.1) |
| 3+ | 150 (55.8) | 89 (61.4) | 36 (16.9) |
| Mean ± SD | 2.8 ± 1.3 | 3.0 ± 1.4 | 1.9 ± 0.7 |
| Age at first full-term pregnancy (y), n (%) | |||
| ≤ 23 | 169 (62.8) | 83 (57.2) | 97 (45.5) |
| > 23 | 100 (37.2) | 62 (42.8) | 116 (54.5) |
| Mean ± SD | 22.5 ± 5.4 | 23.0 ± 5.3 | 25.1 ± 5.4 |
| Age at last full-term pregnancy (y), n (%) | |||
| ≤ 30 | 149 (55.4) | 68 (46.9) | 122 (57.3) |
| > 30 | 120 (44.6) | 77 (53.1) | 91 (42.7) |
| Mean ± SD | 29.7 ± 5.7 | 30.5 ± 5.2 | 29.6 ± 5.9 |
| Interval between first and last pregnancy (y) | |||
| Mean ± SD (if parity > 1) | 8.5 ± 5.5 | 8.5 ± 5.3 | 6.4 ± 4.7 |
| Lifetime breastfeedinga (mo), n (%) | |||
| Never | 79 (29.4) | 16 (11.0) | 69 (32.6) |
| ≤ 12 | 115 (42.8) | 60 (41.4) | 113 (53.3) |
| > 12 | 75 (27.9) | 69 (47.6) | 30 (14.2) |
| Mean ± SD | 11.5 ± 16.2 | 21.5 ± 29.2 | 6.4 ± 9.8 |
| Age at menarche (y) | |||
| Mean ± SD | 12.6 ± 1.7 | 12.8 ± 1.5 | 12.8 ± 1.6 |
| Hormone contraceptive use, n (%) | |||
| Never | 84 (31.2) | 45 (31.0) | 82 (38.5) |
| Ever | 185 (68.8) | 100 (69.0) | 131 (61.5) |
| Age at diagnosis (y), n (%) | |||
| ≤ 45 | 163 (60.6) | 69 (47.6) | 101 (47.4) |
| > 45 | 106 (39.4) | 76 (52.4) | 112 (52.6) |
| Mean ± SD | 42.8 ± 6.4 | 44.3 ± 7.0 | 44.9 ± 5.1 |
| Family history of breast cancerb, n (%) | |||
| No | 225 (84.6) | 127 (88.8) | 182 (85.9) |
| Yes | 41 (15.4) | 16 (11.2) | 30 (14.2) |
| Tumor subtype, n (%) | |||
| HR+/HER2− | 164 (61.0) | 80 (55.2) | 149 (70.0) |
| HER2+ | 59 (21.9) | 33 (22.8) | 40 (18.8) |
| TNBC | 46 (17.1) | 32 (22.1) | 24 (11.3) |
| Tumor markers, n (%) | |||
| ER+ | 185 (68.8) | 82 (56.6) | 175 (82.2) |
| PR+ | 173 (64.3) | 85 (58.6) | 155 (72.8) |
| HER2+ | 59 (21.9) | 33 (22.8) | 40 (18.8) |
Missing data for lifetime breastfeeding (Spain, n = 1)
Family history of breast cancer in a first-degree relative; missing data (USA, n = 3; Mexico, n = 2; Spain, n = 1)
Results
Descriptive characteristics of the study populations by country
Table 1 presents reproductive and clinical characteristics by country of recruitment. The mean interval of time between last full-term pregnancy and breast cancer diagnosis for the U.S., Mexico, and Spain was 13.1, 13.8, and 15.5 years, respectively. Approximately one-third of cases (range 28.2% to 39.4%) were diagnosed ≤10 years after their last full-term pregnancy. U.S. cases were younger at diagnosis and had the youngest age at first full-term pregnancy compared with cases recruited in Mexico or Spain. On average, Mexican participants had a greater number of full-term pregnancies (3.0), followed by U.S. and Spanish women. The majority of women in all countries reported breastfeeding their children, with prevalence ranging from 89% in Mexico to 67.4% in Spain. Not considering number of births, Spanish women had a shorter mean interval between the first and last pregnancy than those in the U.S. or Mexico. The majority of cases in all three countries were classified as HR+/HER2−: 70.0% in Spain, 61.0% in the U.S., and 55.2% in Mexico; TNBCs were the least common subtype: 17.1%, 22.1%, and 11.3% in U.S., Mexican, and Spanish women, respectively. Relatively little variation was observed for HER2+ tumors, which made up 22.8%, 21.9%, and 18.8% of cases in Mexico, the U.S. and Spain, respectively.
Reproductive and clinical characteristics and HER2+ tumors
The characteristics of HR+/ HER2− and HER2+ cases by country are presented in Table 2. No statistically significant differences were observed in reproductive characteristics between HR+/HER2− and HER2+ cases, with the exception of time since last full-term pregnancy (≤10 vs. >10 y) in Spain. When we evaluated the interval between last full-term pregnancy and breast cancer diagnosis, we observed no difference in the prevalence of HER2+ tumors for women whose diagnosis occurred within 5 years following a pregnancy (24.1%) and those occurring in 5–10 years (26.9%). This was our justification for selecting 10 years after pregnancy as the interval to define postpartum cases. Furthermore, we excluded cases within one year of pregnancy or pregnant at diagnosis to differentiate post-partum breast cancers from PABC. Participants with HER2+ tumors were more likely to be diagnosed ≤10 years since their last full-term pregnancy than HR+/HER2− tumors. The proportion of HER2+ tumors diagnosed ≤10 years was 42.5% in Spain, 36.4% in Mexico, and 45.8% in the U.S., while the respective proportions for HR+/HER2− tumors were 22.8%, 26.3%, and 39.6%. Although not reaching statistical significance at the 5% level, HER2+ cases in Mexico were more likely to be younger at first and last pregnancies than HR+/ HER2− cases.
Table 2.
Bivariate associations between reproductive/clinical characteristics and HER2+ status (excluding TNBC cases), by country
| Characteristic | USA | Mexico | Spain | |||
|---|---|---|---|---|---|---|
| HR+/HER2− (n = 164) |
HER2+ (n = 59) |
HR+/HER2− (n = 80) |
HER2+ (n = 33) |
HR+/HER2− (n = 149) |
HER2+ (n = 40) |
|
| Time since last full-term pregnancy (y), n (%) | ||||||
| > 10 | 99 (60.4) | 32 (54.2) | 59 (73.8) | 21 (63.6) | 115 (77.2) | 23 (57.5)a |
| ≤ 10 | 65 (39.6) | 27 (45.8) | 21 (26.3) | 12 (36.4) | 34 (22.8) | 17 (42.5)a |
| Mean ± SD | 13.0 ± 7.8 | 12.9 ± 8.1 | 14.8 ± 6.6 | 14.9 ± 8.0 | 16.0 ± 7.6 | 13.8 ± 8.4 |
| Total full-term pregnancies, n (%) | ||||||
| 1–2 | 72 (43.9) | 30 (50.9) | 33 (41.3) | 13 (39.4) | 123 (82.6) | 34 (85.0) |
| 3+ | 92 (56.1) | 29 (49.2) | 47 (58.8) | 20 (60.6) | 26 (17.5) | 6 (15.0) |
| Mean ± SD | 2.7 ± 1.2 | 2.8 ± 1.7 | 3.0 ± 1.4 | 3.2 ± 1.7 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| Age at first full-term pregnancy (y), n (%) | ||||||
| ≤ 23 | 99 (60.4) | 33 (55.9) | 43 (53.8) | 19 (57.6) | 71 (47.7) | 17 (42.5) |
| > 23 | 65 (39.6) | 26 (44.1) | 37 (46.3) | 14 (42.4) | 78 (52.4) | 23 (57.5) |
| Mean ± SD | 22.9 ± 5.5 | 23.3 ± 5.7 | 23.5 ± 5.7 | 22.6 ± 5.1 | 24.6 ± 4.9 | 25.6 ± 6.0 |
| Age at last full-term pregnancy (y), n (%) | ||||||
| ≤ 30 | 92 (56.1) | 28 (47.5) | 34 (42.5) | 20 (60.6) | 89 (59.7) | 19 (47.5) |
| > 30 | 72 (43.9) | 31 (52.5) | 46 (57.5) | 13 (39.4) | 60 (40.3) | 21 (52.5) |
| Mean ± SD | 29.7 ± 5.6 | 30.1 ± 5.8 | 30.8 ± 5.3 | 30.2 ± 5.2 | 29.2 ± 5.7 | 30.5 ± 6.3 |
| Interval between first and last pregnancy (y) | ||||||
| Mean ± SD (if parity > 1) | 8.0 ± 5.1 | 8.3 ± 6.2 | 8.4 ± 5.6 | 8.3 ± 4.6 | 6.4 ± 4.4 | 7.9 ± 6.1 |
| Lifetime breastfeedingb (mo), n (%) | ||||||
| Never | 47 (28.7) | 19 (32.2) | 10 (12.5) | 5 (15.2) | 45 (30.2) | 12 (30.8) |
| ≤ 12 | 71 (43.3) | 26 (44.1) | 35 (43.8) | 12 (36.4) | 83 (55.7) | 20 (51.3) |
| > 12 | 46 (28.1) | 14 (23.7) | 35 (43.8) | 16 (48.5) | 21 (14.1) | 7 (18.0) |
| Mean ± SD | 11.5 ± 16.9 | 10.7 ± 15.7 | 22.3 ± 32.1 | 24.5 ± 33.1 | 6.9 ± 10.4 | 6.8 ± 9.0 |
| Age at menarche (y) | ||||||
| Mean ± SD | 12.6 ± 1.6 | 12.3 ± 1.8 | 12.8 ± 1.5 | 12.9 ± 1.3 | 12.8 ± 1.6 | 12.7 ± 1.5 |
| Hormone contraceptive use, n (%) | ||||||
| Never | 54 (32.9) | 17 (28.8) | 27 (33.8) | 6 (18.2) | 61 (40.9) | 12 (30.0) |
| Ever | 110 (67.1) | 42 (71.2) | 53 (66.3) | 27 (81.8) | 88 (59.1) | 28 (70.0) |
| Age at diagnosis (y) | ||||||
| ≤ 45 | 98 (59.8) | 37 (62.7) | 30 (37.5) | 13 (39.4) | 66 (44.3) | 22 (55.0) |
| > 45 | 66 (40.2) | 22 (37.3) | 50 (62.5) | 20 (60.6) | 83 (55.7) | 18 (45.0) |
| Mean ± SD | 42.6 ± 6.8 | 43.0 ± 6.0 | 45.6 ± 6.6 | 45.1 ± 7.4 | 45.2 ± 5.1 | 44.3 ± 4.9 |
| Family history of breast cancerc, n (%) | ||||||
| No | 143 (88.3) | 51 (86.4) | 71 (88.8) | 31 (93.9) | 124 (83.8) | 38 (95.0) |
| Yes | 19 (11.7) | 8 (13.6) | 9 (11.3) | 2 (6.06) | 24 (16.2) | 2 (5.00) |
Significantly different from HR+ (Fisher’s exact test, p = 0.016)
Missing data for lifetime breastfeeding (Spain, n = 1)
Family history of breast cancer in a first-degree relative; missing data (USA, n = 3; Mexico, n = 2; Spain, n = 1)
Time since last full-term pregnancy and HER2+ tumors
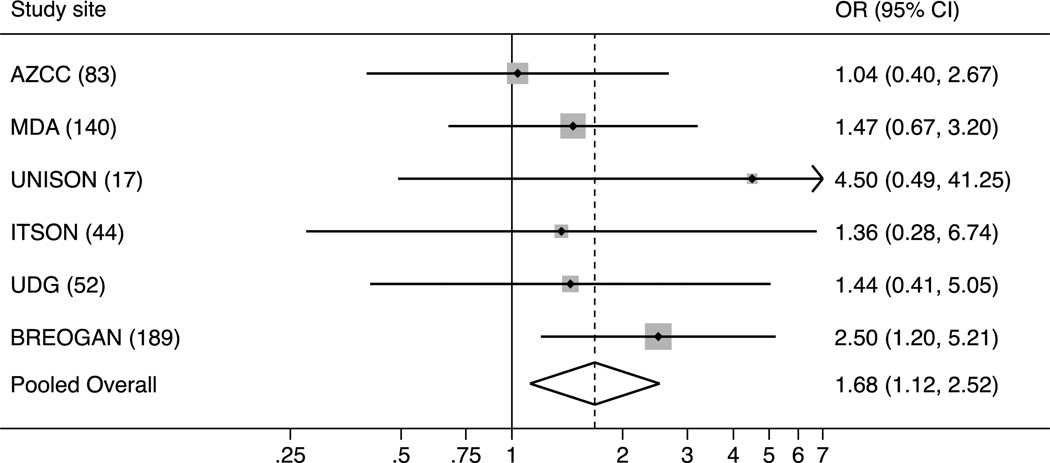
Table 3 presents country-specific and pooled odds ratios (ORs) for the association between time since last full-term pregnancy (≤ 10 vs. > 10 years) and tumor subtype (HER2+ vs. HR+/HER2−). Country-specific ORs varied in magnitude but not in direction of effect. ORs (95% confidence intervals) for each study site ranged from 1.04 (0.40–2.67) for AZCC to 4.50 (0.49–41.25) for UNISON (Figure 1). The pooled point estimate calculated using a random effects model of HER2+ versus HR+/HER2− tumors was OR=1.68 (1.12–2.52). The 2-stage meta-analytic point estimate of the OR was also 1.68 with heterogeneity statistics I2 = 0.0% and χ2 (Q) =3.13 (p=0.68). Adjusting for age at diagnosis (≤45 or >45 y) only marginally affected the estimate (OR=1.78; 95% CI, 1.08–2.93). Results remained significant in analyses treating age at diagnosis as a continuous variable (OR=2.15; 95% CI, 1.26–3.67).
Table 3.
Association between time since last full-term pregnancy and HER2+ status, by country
| Country | Subtype | n | OR (95% CI) |
|---|---|---|---|
| USA | |||
| HR+/HER2− | 164 | 1.00 | |
| HER2+ | 59 | 1.29 (0.71–2.34) | |
| Mexico | |||
| HR+/HER2− | 80 | 1.00 | |
| HER2+ | 33 | 1.61 (0.67–3.82) | |
| Spain | |||
| HR+/HER2− | 149 | 1.00 | |
| HER2+ | 40 | 2.50 (1.20–5.21) | |
| Pooleda | |||
| HR+/HER2− | 393 | 1.00 | |
| HER2+ | 132 | 1.68 (1.12–2.52) | |
| Pooledb | |||
| HR+/HER2− | 393 | 1.00 | |
| HER2+ | 132 | 1.78 (1.08–2.93) |
Study site included as a random effect in logistic regression model
Adjusted for age at diagnosis (≤ 45 or > 45 y); study site included as a random effect in logistic regression model
Fig. 1. Forest plot of the association between time since last full-term pregnancy (≤10 vs. 10 years) and HER2+ tumors versus HR+/HER2− tumors.
Study sites: AZCC (Arizona Cancer Center, USA), MDA (M.D. Anderson Cancer Center, USA), UNISON (Universidad de Sonora, Mexico), ITSON (Instituto Technológico de Sonora, Mexico), UDG (Universidad de Guadalajara, Mexico), and BREOGAN (Breast Oncology Galician Network, Spain).
Further, when we considered the HR status of HER2+ tumors in the association between time since last full-term pregnancy and HER2 status, we found no evidence of a difference by HR status. The OR (95% CI) for HR+/HER2+ was 1.71 (1.05–2.82) and that for HR−/HER2+ was 1.54 (0.84–2.80). Lastly, when we compared the odds of having HER2+ tumors compared to TNBCs for time since last full-term pregnancy, a positive, albeit non-significant association was observed. Country-specific positive associations between time since last full-term pregnancy and HER2+ compared to TNBCs were observed for the U.S. (OR=1.93; 95% CI, 0.86–4.34) and Spain (OR=1.31; 95% CI, 0.47–3.68) but not Mexico (OR=0.57; 95% CI 0.21–1.54). The pooled OR (95% CI) was 1.21 (0.71–2.05), and the age-adjusted pooled OR (95% CI) was 1.84 (0.97–3.50).
Discussion
To our knowledge, this is the first case-only report examining the distribution of tumor subtypes by time since last full-term pregnancy in a large sample of premenopausal women with high parity. Our results indicate that HER2+ tumors have higher odds of being diagnosed in the 10-year period following a full-term pregnancy than HER2− tumors, an increase in odds that appears to be independent of HR status. The results were consistent across all three countries, increasing the validity of these findings.
Several groups have investigated the association between reproductive factors and tumor subtypes [12,44,41,43,42,45,46,40,30,14], but results for HER2+ tumors have been inconclusive. Few studies have reported on the association between a recent pregnancy and HER2+ tumors [12,47,48]. Two case-control studies [47,48] reported no association between time since pregnancy and HER2+ tumors. In a third, a case-only analysis [12] using HR+/HER2− tumors as the reference, HR−/HER2+ tumors were more prevalent in cases diagnosed ≤5 years (OR= 5.05; 95% CI, 1.43–17.86) and >5 years (OR=2.14; 95% CI, 0.86, 5.34) since last pregnancy than in nulliparous women. However, no association was observed with HR+/HER2+ tumors. Only one of the previous studies [47] excluded pregnant cases and no exclusions were made based on time since pregnancy in any other study. All three studies categorized time since last pregnancy into shorter intervals of 2 [48,47] or 5 years [12], and all three were consistent in finding increased odds of HR- or TNBC tumors during this time period. Also consistent across studies, the association with recent pregnancy and TNBC or HR- tumors disappeared after the first couple of years postpartum.
There are numerous reasons for inconsistency between these and our findings, including choice of reference group, sample size and case-control or case-only study design. A limitation of our study is the lack of a nulliparous referent group. The small proportion of nulliparous cases in our study precluded their inclusion in our analyses. Other considerations include the differences in HER2 classification. In most epidemiological studies conducted to date, HER2+ tumors have been separated by their HR status, with HR+/HER2+ classified as luminal B [11,49,30,12,14,40,45,42]. This hierarchical-based classification of breast tumors is not entirely consistent with gene expression studies [10], and the appropriateness of such grouping for etiological studies has been debated [50]. Our results indicate that the positive association between time since last full-term pregnancy and HER2+ tumors (relative to HR+/HER2− tumors) is independent of HR status. Confirmation of our findings would suggest that HER2+ tumors may derive from a distinct set of etiological factors than either HR+/HER2− or TNBC that would be more consistent with the genomic character of the disease at the genome level [31].
While our study provides evidence for higher odds of HER2+ tumors in the postpartum period relative to HR+/HER2− tumors, future larger studies would be valuable to assess finer postpartum intervals, as well as the effect of other reproductive characteristics (e.g., age at first birth, number of births, interval between births, and breast feeding) as potential modifiers of the associated risk. Replication of our findings of differential risk by time since last pregnancy in the context of parity may provide a partial explanation for the reported disparities observed among certain populations that have a higher rate of parity (i.e., African American and Hispanic women) when compared to those that exhibit lower parity rates (NHWs).
While novel, our findings need confirmation in studies with different control populations (i.e. non-diseased, nulliparous), particularly because ours can address heterogeneity in the association by tumor subtype only [15]. It is possible our results are due to selection bias. The exclusion of cases with incomplete tumor markers and difference in tumor marker classification might be a source of confounding that is difficult to address. By country, the proportion of otherwise-eligible participants missing tumor markers was 2% (n=7) in the U.S., 23% (n=50) in Mexico, and 14% (n=34) in Spain. Although the direction of effect is consistent across all studies, it is important to note that there is variability in the site-specific ORs (Figure 1: AZCC OR=1.04 versus UNISON OR=4.5). An advantage of our meta-analytic approach is that it provides an estimate derived as a weighted average of results across study sites. The pooled OR of 1.68 reflects the greater weight given to larger sites such as Arizona relative to smaller sites such as UNISON. Furthermore, the consistency of our findings across the three countries is striking, especially as it is highly likely that a number of sources of residual confounding are present in our data.
These findings extend previous observations [11,30] that the relative proportion of specific breast tumor subtypes in a population may arise from differences in reproductive factors to include consideration of time since last pregnancy. In addition, our results support the possibility that hormonal influences related to pregnancy may contribute to the development of HER2+ tumors. These results are consistent with recent mechanistic studies demonstrating cross-talk between HER2 and certain pregnancy-associated hormones. While a hypothesis, we believe these findings are significant and warrant additional study since HER2+ tumors have no recognized risk factor(s).
Acknowledgements
This work was supported by a grant from the National Cancer Institute (UO1CA153086); a Susan G. Komen for the Cure® Post-baccalaureate Training in Disparities Research Grant (KG090934); the Avon Foundation; a supplement to the Arizona Cancer Center Core Grant from the National Cancer Institute (CA-023074-2953); a supplement to the M.D. Anderson Cancer Center SPORE in Breast Cancer (P50 CA116199-02S1); FIS Intrasalud (PS09/02368); Programa Grupos Emergentes EMER ISCIII, Instituto de Salud Carlos III, Servicio Galego Saúde (SERGAS), Oncology and Genetics Unit. Complejo Hospitalario Universitario de Vigo, Spain; and the Botin Foundation. We are indebted to Ana Lilia Amador, Leticia Cordova, Carole Kepler and Fang Wang for their contribution.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100(22):1643–1648. doi: 10.1093/jnci/djn344. doi:djn344 [pii] 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 20(5):733–739. doi: 10.1158/1055-9965.EPI-11-0061. doi:1055-9965.EPI-11-0061 [pii] 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 3.Martinez ME, Nielson CM, Nagle R, Lopez AM, Kim C, Thompson P. Breast cancer among Hispanic and non-Hispanic White women in Arizona. J Health Care Poor Underserved. 2007;18(4 Suppl):130–145. doi: 10.1353/hpu.2007.0112. doi:S1548686907401309 [pii] 10.1353/hpu.2007.0112. [DOI] [PubMed] [Google Scholar]
- 4.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 5.Hedeen AN, White E. Breast cancer size and stage in Hispanic American women, by birthplace: 1992–1995. Am J Public Health. 2001;91(1):122–125. doi: 10.2105/ajph.91.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Cuevas S, Macias CG, Franceschi D, Labastida S. Breast carcinoma presents a decade earlier in Mexican women than in women in the United States or European countries. Cancer. 2001;91(4):863–868. doi:10.1002/1097-0142(20010215)91:4<863::AID-CNCR1074>3.0.CO;2-Y [pii] [PubMed] [Google Scholar]
- 7.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. doi:ioi10945 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Sorlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40(18):2667–2675. doi: 10.1016/j.ejca.2004.08.021. doi:S0959-8049(04)00709-9 [pii] 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 10.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. doi:295/21/2492 [pii] 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 12.Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, Eley JW. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15(6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. doi:TBJ822 [pii] 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 14.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez ME, Cruz GI, Brewster AM, Bondy ML, Thompson PA. What can we learn about disease etiology from case-case analyses? Lessons from breast cancer. Cancer Epidemiol Biomarkers Prev. 19(11):2710–2714. doi: 10.1158/1055-9965.EPI-10-0742. doi:1055-9965.EPI-10-0742 [pii] 10.1158/1055-9965.EPI-10-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. doi:13/10/1558 [pii] [PubMed] [Google Scholar]
- 17.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–727. doi: 10.1038/sj.bjc.6600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer JR, Wise LA, Horton NJ, Adams-Campbell LL, Rosenberg L. Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst. 2003;95(6):478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 19.Pathak DR, Osuch JR, He J. Breast carcinoma etiology: current knowledge and new insights into the effects of reproductive and hormonal risk factors in black and white populations. Cancer. 2000;88(5 Suppl):1230–1238. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1230::aid-cncr9>3.0.co;2-f. doi:10.1002/(SICI)1097-0142(20000301)88:5+<1230::AID-CNCR9>l3.0.CO;2-F [pii] [DOI] [PubMed] [Google Scholar]
- 20.Albrektsen G, Heuch I, Kvale G. The short-term and long-term effect of a pregnancy on breast cancer risk: a prospective study of 802,457 parous Norwegian women. Br J Cancer. 1995;72:480–484. doi: 10.1038/bjc.1995.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. doi: 10.1038/sj.bjc.6602302. doi:6602302 [pii] 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13(4):299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 23.Lethaby AE, O'Neill MA, Mason BH, Holdaway IM, Harvey VJ. Overall survival from breast cancer in women pregnant or lactating at or after diagnosis. Auckland Breast Cancer Study Group. Int J Cancer. 1996;67(6):751–755. doi: 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q. doi:10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q [pii] 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Kroman N, Mouridsen HT. Prognostic influence of pregnancy before, around, and after diagnosis of breast cancer. Breast. 2003;12(6):516–521. doi: 10.1016/s0960-9776(03)00159-0. doi:S0960977603001590 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14(2):87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges VF, Schedin PJ. Pregnancy-associated breast cancer: An entity needing refinement of the definition. Cancer. 2012;118(13):3226–3228. doi: 10.1002/cncr.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proietti CJ, Rosemblit C, Beguelin W, Rivas MA, Diaz Flaque MC, Charreau EH, Schillaci R, Elizalde PV. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29(5):1249–1265. doi: 10.1128/MCB.00853-08. doi:MCB.00853-08 [pii] 10.1128/MCB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273(47):31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Ordonez-Ercan D, Fan Z, Huang X, Edgerton SM, Yang X, Thor AD. Estrogenic Promotion of ErbB2 Tyrosine Kinase Activity in Mammary Tumor Cells Requires Activation of ErbB3 Signaling. Molecular Cancer Research. 2009;7(11):1882–1892. doi: 10.1158/1541-7786.MCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 30.Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, Lee MM, Ambrosone CB, Caan BJ. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. doi: 10.1186/bcr2261. doi:bcr2261 [pii] 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson PA, Brewster AM, Kim-Anh D, Baladandayuthapani V, Broom BM, Edgerton ME, Hahn KM, Murray JL, Sahin A, Tsavachidis S, Wang Y, Zhang L, Hortobagyi GN, Mills GB, Bondy ML. Selective genomic copy number imbalances and probability of recurrence in early-stage breast cancer. PLoS One. 2011;6(8):e23543. doi: 10.1371/journal.pone.0023543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton P, Hamilton BE, Mathews TJ. NCHS Data Brief No. 60. Hyattsville, MD: National Center for Health Statistics; 2011. Recent decline in births in the United States, 2007–2009. [PubMed] [Google Scholar]
- 33.Martinez ME, et al. Comparative Study of Breast Cancer in Mexican and Mexican-American Women. Health. 2010;2(9):1040–1048. [Google Scholar]
- 34.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. Journal of Clinical Oncology. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Journal of Clinical Oncology. 2006;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 36.Bladstrom A, Anderson H, Olsson H. Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clin Breast Cancer. 2003;4(4):280–285. doi: 10.3816/cbc.2003.n.033. [DOI] [PubMed] [Google Scholar]
- 37.Olson SH, Zauber AG, Tang J, Harlap S. Relation of time since last birth and parity to survival of young women with breast cancer. Epidemiology. 1998;9(6):669–671. doi:00001648-199811000-00015 [pii] [PubMed] [Google Scholar]
- 38.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychological methods. 1998;3(4):486–504. [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521–1526. doi: 10.1002/cncr.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing P, Li J, Jin F. A case-control study of reproductive factors associated with subtypes of breast cancer in Northeast China. Med Oncol. 27(3):926–931. doi: 10.1007/s12032-009-9308-7. [DOI] [PubMed] [Google Scholar]
- 43.Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, Li CI. Reproductive history and risk of three breast cancer subtypes defined by three biomarkers. Cancer Causes Control. 22(3):399–405. doi: 10.1007/s10552-010-9709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma H, Wang Y, Sullivan-Halley J, Weiss L, Marchbanks PA, Spirtas R, Ursin G, Burkman RT, Simon MS, Malone KE, Strom BL, McDonald JA, Press MF, Bernstein L. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women's contraceptive and reproductive experiences study. Cancer Res. 70(2):575–587. doi: 10.1158/0008-5472.CAN-09-3460. doi:0008-5472.CAN-09-3460 [pii] 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, Garcia-Closas M. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–443. doi: 10.1158/1055-9965.EPI-06-0806. doi:16/3/439 [pii] 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 46.Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, Marotti J, Connolly JL, Schnitt SJ, Collins LC. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–167. doi: 10.1007/s10549-011-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daling JR, Malone KE, Doody DR, Anderson BO, Porter PL. The relation of reproductive factors to mortality from breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(3):235–241. [PubMed] [Google Scholar]
- 48.Pilewskie M, Gorodinsky P, Fought A, Hansen N, Bethke K, Jeruss J, Scholtens D, Khan SA. Association between recency of last pregnancy and biologic subtype of breast cancer. Annals of surgical oncology. 2012;19(4):1167–1173. doi: 10.1245/s10434-011-2104-6. [DOI] [PubMed] [Google Scholar]
- 49.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10(4):R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhargava R, Dabbs DJ. Luminal B breast tumors are not HER2 positive. Breast Cancer Res. 2008;10(5):404. doi: 10.1186/bcr2134. author reply 405. [DOI] [PMC free article] [PubMed] [Google Scholar]