Abstract
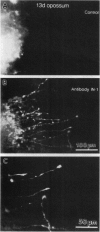
Neurite outgrowth across spinal cord lesions in vitro is rapid in preparations isolated from the neonatal opossum Monodelphis domestica up to the age of 12 days. At this age oligodendrocytes, myelin, and astrocytes develop and regeneration ceases to occur. The role of myelin-associated neurite growth-inhibitory proteins, which increase in concentration at 10-13 days, was investigated in culture by applying the antibody IN-1, which blocks their effects. In the presence of IN-1, 22 out of 39 preparations from animals aged 13-17 days showed clear outgrowth of processes into crushes. When 34 preparations from 13-day-old animals were crushed and cultured without antibody, no axons grew into the lesion. The success rate with IN-1 was comparable to that seen in younger animals but the outgrowth was less profuse. IN-1 was shown by immunocytochemistry to penetrate the spinal cord. Other antibodies which penetrated the 13-day cord failed to promote fiber outgrowth. To distinguish between regeneration by cut neurites and outgrowth by developing uncut neurites, fibers in the ventral fasciculus were prelabeled with carbocyanine dyes and subsequently injured. The presence of labeled fibers in the lesion indicated that IN-1 promoted regeneration. These results show that the development of myelin-associated growth-inhibitory proteins contributes to the loss of regeneration as the mammalian central nervous system matures. The definition of a critical period for regeneration, coupled with the ability to apply trophic as well as inhibitory molecules to the culture, can permit quantitative assessment of molecular interactions that promote spinal cord regeneration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein-Goral H., Bregman B. S. Spinal cord transplants support the regeneration of axotomized neurons after spinal cord lesions at birth: a quantitative double-labeling study. Exp Neurol. 1993 Sep;123(1):118–132. doi: 10.1006/exnr.1993.1145. [DOI] [PubMed] [Google Scholar]
- Bregman B. S., Goldberger M. E. Infant lesion effect: I. Development of motor behavior following neonatal spinal cord damage in cats. Brain Res. 1983 Aug;285(2):103–117. doi: 10.1016/0165-3806(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Bregman B. S., Kunkel-Bagden E., Reier P. J., Dai H. N., McAtee M., Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993 Sep;123(1):3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- Caroni P., Schwab M. E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988 Mar;1(1):85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Caroni P., Schwab M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988 Apr;106(4):1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. A., Bray G. M., Aguayo A. J. Long-term growth and remodeling of regenerated retino-collicular connections in adult hamsters. J Neurosci. 1994 Feb;14(2):590–598. doi: 10.1523/JNEUROSCI.14-02-00590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Aguayo A. J. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Ghooray G. T., Martin G. F. The development of myelin in the spinal cord of the North American opossum and its possible role in loss of rubrospinal plasticity. A study using myelin basic protein and galactocerebroside immuno-histochemistry. Brain Res Dev Brain Res. 1993 Mar 19;72(1):67–74. doi: 10.1016/0165-3806(93)90160-c. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989 Sep;12(9):333-5, 340-1. [PubMed] [Google Scholar]
- Iwashita Y., Kawaguchi S., Murata M. Restoration of function by replacement of spinal cord segments in the rat. Nature. 1994 Jan 13;367(6459):167–170. doi: 10.1038/367167a0. [DOI] [PubMed] [Google Scholar]
- Keifer J., Kalil K. Effects of infant versus adult pyramidal tract lesions on locomotor behavior in hamsters. Exp Neurol. 1991 Jan;111(1):98–105. doi: 10.1016/0014-4886(91)90055-h. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E., Dai H. N., Bregman B. S. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp Neurol. 1992 Apr;116(1):40–51. doi: 10.1016/0014-4886(92)90174-o. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S., Clarke D. B., Wang Y. C., Bray G. M., Aguayo A. J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L., David S., Jackson D. L., Kottis V., Dunn R. J., Braun P. E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994 Oct;13(4):805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G., Doherty P., Walsh F. S., Crocker P. R., Filbin M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994 Sep;13(3):757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Møllgård K., Balslev Y., Janas M. S., Treherne J. M., Saunders N. R., Nichols J. G. Development of spinal cord in the isolated CNS of a neonatal mammal (the opossum Monodelphis domestica) maintained in longterm culture. J Neurocytol. 1994 Mar;23(3):151–165. doi: 10.1007/BF01181557. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Stewart R. R., Erulkar S. D., Saunders N. R. Reflexes, fictive respiration and cell division in the brain and spinal cord of the newborn opossum, Monodelphis domestica, isolated and maintained in vitro. J Exp Biol. 1990 Sep;152:1–15. doi: 10.1242/jeb.152.1.1. [DOI] [PubMed] [Google Scholar]
- Paíno C. L., Fernandez-Valle C., Bates M. L., Bunge M. B. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J Neurocytol. 1994 Jul;23(7):433–452. doi: 10.1007/BF01207115. [DOI] [PubMed] [Google Scholar]
- Savio T., Schwab M. E. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4130–4133. doi: 10.1073/pnas.87.11.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio T., Schwab M. E. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989 Apr;9(4):1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L., Schneider R., Kolbeck R., Barde Y. A., Schwab M. E. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994 Jan 13;367(6459):170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Schnell L., Schwab M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990 Jan 18;343(6255):269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Stewart R. R., Zou D. J., Treherne J. M., Møllgård K., Saunders N. R., Nicholls J. G. The intact central nervous system of the newborn opossum in long-term culture: fine structure and GABA-mediated inhibition of electrical activity. J Exp Biol. 1991 Nov;161:25–41. doi: 10.1242/jeb.161.1.25. [DOI] [PubMed] [Google Scholar]
- Treherne J. M., Woodward S. K., Varga Z. M., Ritchie J. M., Nicholls J. G. Restoration of conduction and growth of axons through injured spinal cord of neonatal opossum in culture. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):431–434. doi: 10.1073/pnas.89.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Qin Y. Q., Xu X. M., Martin G. F. Developmental plasticity of reticulospinal and vestibulospinal axons in the north American opossum, Didelphis virginiana. J Comp Neurol. 1994 Nov 8;349(2):288–302. doi: 10.1002/cne.903490210. [DOI] [PubMed] [Google Scholar]
- Woodward S. K., Treherne J. M., Knott G. W., Fernandez J., Varga Z. M., Nicholls J. G. Development of connections by axons growing through injured spinal cord of neonatal opossum in culture. J Exp Biol. 1993 Mar;176:77–88. doi: 10.1242/jeb.176.1.77. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Guénard V., Kleitman N., Bunge M. B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995 Jan 2;351(1):145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Martin G. F. Evidence for new growth and regeneration of cut axons in developmental plasticity of the rubrospinal tract in the North American opossum. J Comp Neurol. 1991 Nov 1;313(1):103–112. doi: 10.1002/cne.903130108. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Martin G. F. The response of rubrospinal neurons to axotomy at different stages of development in the North American opossum. J Neurotrauma. 1992 Summer;9(2):93–105. doi: 10.1089/neu.1992.9.93. [DOI] [PubMed] [Google Scholar]