Abstract
Caloric restriction (CR) is the most reliable intervention to extend lifespan and prevent age-related disorders in various species from yeast to rodents. Short- and long-term CR confers cardio protection against ischaemia/reperfusion injury in young and even in aged rodents. A few human trials suggest that CR has the potential to mediate improvement of cardiac or vascular function and induce retardation of cardiac senescence also in humans. The underlying mechanisms are diverse and have not yet been clearly defined. Among the known mediators for the benefits of CR are NO, the AMP-activated PK, sirtuins and adiponectin. Mitochondria, which play a central role in such complex processes within the cell as apoptosis, ATP-production or oxidative stress, are centrally involved in many aspects of CR-induced protection against ischaemic injury. Here, we discuss the relevant literature regarding the protection against myocardial ischaemia/reperfusion injury conferred by CR. Furthermore, we will discuss drug targets to mimic CR and the possible role of calorie restriction in preserving cardiovascular function in humans.
Keywords: ROS, mitophagy, AMPK, sirtuin, NO, CR mimetic, mitobiogenesis
Caloric restriction (CR): an introduction
The level of oxidative stress increases with age. Subsequently, oxidative damage to DNA, proteins or lipids accumulates with age, and as a consequence, an impairment of cellular functions may occur. Mitochondria are a major source of reactive oxygen species (ROS) as a by-product of the normal functioning of the respiratory chain. In cardiomyocytes, which possess a high energy demand, but a relatively poor repair capacity, a chronic state of oxidative stress exists. Life-long CR has long been known to attenuate this age-associated increase in mitochondrial ROS production (Sohal et al., 1994; Barja, 2002), in lipid peroxidation (Matsuo et al., 1993), in protein oxidation (Leeuwenburgh et al., 1997), and in oxidative damage of mitochondrial and nuclear DNA (Gredilla et al., 2001). All these ROS-induced alterations, sensitive to modulation by CR, are considered as manifestations of the free radical theory of ageing (Harman, 1956).
In 1935, Clive McCay and colleagues reported for the first time that decreasing food intake extends the lifespan of rats (McCay et al., 1935). By now, long-term CR is known to retard the ageing process in a number of organisms from yeast to smaller, short-lived mammals, but the detailed mechanisms of its efficacy remain unclear and its actions in different organs are remarkably heterogeneous (Masoro, 2000). The so-called Hormesis Hypothesis (Masoro, 1998) tries to unify various theories of ageing and longevity as merely specific examples of hormetic processes. Hormesis refers to a beneficial action resulting from the response of an organism to a low-intensity stressor otherwise detrimental when administered at higher concentrations or intensities (Masoro, 1998; Sinclair, 2005; Rattan, 2008; Calabrese et al., 2012). The hormesis hypothesis of CR predicts that reduction in calorie intake is a mild stress that provokes a survival response resulting in increased cellular defences, partial repair of age-associated damage, attenuation of stress-induced cell death and altered metabolism.
Beneficial effects of CR were reported using various regimens. The key factor for the efficacy of CR seems to be an overall reduction in energy intake (Masoro, 1988), although a restriction of dietary methionine replicates some of the effects of CR, such as an increase in lifespan in rats (Orentreich et al., 1993) and mice (Miller et al., 2005) or a decrease in mitochondrial ROS production and damage (Sanz et al., 2006). A common CR approach is to measure the ad libitum food intake of an individual animal and then reduce the food by a certain percentage. This approach works well in adult animals, but not in young or senescent animals as food intake is not constant across the lifespan. However, CR animals fed a certain percentage of their own baseline intake will not only experience reduced levels of calories but also a reduction of all micronutrients. Thus, this type of approach should be called dietary restriction instead of CR (Masoro, 2009).
Common, commercially available CR diets provide reduced levels of calories but micronutrients adjusted to the levels of ad libitum fed controls and hence do not lead to malnutrition. The magnitude of CR applied in most rodent studies varies between severe restriction with a reduction in calories of 40–50%, a moderate restriction with a reduction in calories of 20–25%, and a mild restriction with a reduction in calories of 5–10%. The latter protocol is sometimes also used for control groups by restricting food intake in order to prevent obesity as some mouse or rat strains overeat and become quite obese when given free access to food (ad libitum feeding). The maximum lifespan-extending effect of CR is achieved with a 40–45% CR, while a stronger CR reduces lifespan (review in (Speakman and Mitchell, 2011). Another diet regimen is alternate day fasting (also called ‘every-other-day feeding’), in which animals alternate between days where they are fed ad libitum and days of fasting (Goodrick et al., 1990; Anson et al., 2003). Interestingly, these animals also experience enhanced longevity (Goodrick et al., 1990).
Other factors known to influence CR efficacy are the total duration of CR and the time of initiation. Besides life-long CR, short-term CR (3 months) is commonly applied. Some of the metabolic effects of CR have even been reproduced using overnight starvation or prolonged starvation (48 h). Depending on the scientific focus, CR is initiated at birth, in adulthood or late in life. Although there are many beneficial effects on age-related diseases in various organs when CR is initiated late in life (Rae, 2004), the greatest gains are achieved when a strong CR is initiated relatively early in life and sustained to late age. Indeed, there is a linear relationship between the extent of CR and the extent to which lifespan is increased (Weindruch, 1996). CR started late in life results in a lesser extension of lifespan compared with the effect observed when CR is started at weaning (Weindruch and Walford, 1982). Similarly, alternate day fasting results in an increased lifespan only when initiated at the age of 1–6 months in mice, but not when introduced at 10 months of age (Goodrick et al., 1990). However, a recent meta-analysis evaluating laboratory experiments that have investigated life-extending effects of CR in rats and mice suggests significant differences in CR efficacy and even failure to increase lifespan in some species and strains (Swindell, 2012).
To address the question whether CR is also beneficial in long-lived animal species, two independent studies using rhesus monkeys were initiated in the late 1980s. The first report published in 2009 demonstrated that CR extends lifespan in rhesus monkeys (Colman et al., 2009). However, most recently, such beneficial effect of CR in rhesus monkeys was questioned again as CR had no effect on longevity (Mattison et al., 2012). Differences in study design, husbandry and diet composition were alleged to have caused this divergent effect on the life extension by CR. Controlled trials on the effects of long-term CR on longevity and/or cardiac function in humans are lacking for obvious reasons, including unresolved safety issues or difficulties in lifelong observation of participants. One small-scale study described protective effects on diastolic dysfunction in humans practising self-imposed CR for 3–15 years (n = 25; Meyer et al., 2006). The few studies suggesting that CR affects ageing in humans are controversial (Dirks and Leeuwenburgh, 2006; Shanley and Kirkwood, 2006; Morley et al., 2010). However, many human studies have been conducted in obese individuals over short durations and are therefore not comparable with long-term CR performed in normal-weight animals. The Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy (CALERIE) trials systematically investigate the effects of CR in healthy, non-obese human beings (Rochon et al., 2011). Phase 1 of CALERIE used short-term CR (6–12 months), while phase 2 of CALERIE is a randomized, multicentre study that uses dietary and behavioural interventions to achieve 25% CR for 2 years (Rochon et al., 2011). Similar to the data obtained from animal studies, biomarkers of longevity such as fasting insulin level or body temperature are decreased (Heilbronn et al., 2006), and major risk factors for coronary heart disease are substantially improved in humans after 6–12 months of CR (Fontana et al., 2007). The results of phase 2 are expected to be released in late 2013.
Mitochondria in cardiac physiology and pathophysiology: focus on effects during ischaemia/reperfusion
Energetic and ionic homeostasis
Under physiological conditions, mitochondria consume large amounts of oxygen to produce ATP at complex V of the respiratory chain. When the heart becomes hypoperfused and oxygen is lacking, the electron flow along the respiratory chain complexes is inhibited and mitochondrial oxygen consumption as well as ATP production decreases (Lesnefsky et al., 1997; Paradies et al., 2004; Boengler et al., 2007). During ischaemia, glycolysis becomes the major source of ATP production and AMPK activation promotes glycolysis by increasing glucose uptake (Russell et al., 2004) and phosphorylation of phosphofructokinase-2 (Marsin et al., 2000). Mitochondria, instead of producing myocardial ATP, now consume ATP to maintain their inner membrane potential and inhibition of such reversed mode of F1Fo-ATPase protects cardiomyocytes from irreversible injury. During reperfusion, AMPK activation can stimulate fatty acid oxidation (FAO) by inhibiting acetyl-CoA carboxylase and reducing the production of malonyl-CoA, an inhibitor of the mitochondrial transporter of long-chain fatty acids carnitine palmitoyltransferase-1. Restoration of ATP production contributes to the re-establishment of cellular ion homeostasis, but at the same time may paradoxically contribute to irreversible reperfusion injury. During ischaemia, cardiomyocytes become calcium overloaded, which at reperfusion, when ATP is available, is rapidly taken up into the sarcoplasmic reticulum (SR). However, once the SR is calcium overloaded, it again releases calcium resulting in high cytosolic calcium concentrations. The repetition of this process at early reperfusion has been termed ‘calcium oscillation’. High cytosolic calcium concentrations in the presence of ATP result in hypercontracture of cardiomyocytes, membrane disruption and subsequent necrosis (for review, see Piper et al., 2006). Mitochondria also take up calcium in the presence of high cytosolic calcium concentrations and thus become calcium overloaded (Maack and O'Rourke, 2007). Here, the tight intracellular communication between the SR and mitochondria seems to be involved. Specific domains for direct interaction with mitochondria known as the mitochondria-associated membrane exist (Raturi and Simmen, 2013). As has been recently reviewed, the mitochondria-associated membrane tethers the SR/ER (endoplasmic reticulum) to mitochondria and is enriched with proteins relevant to calcium and lipid metabolism, such as mitofusins (Mfn, especially Mfn 2) (de Brito and Scorrano, 2008; Ruiz-Meana et al., 2010).
ROS formation and mitochondrial permeability transition pore (MPTP) opening
Apart from ATP, mitochondria also generate ROS (Droge, 2002; Balaban et al., 2005; Murphy, 2009). ROS within mitochondria originate from different sources, one of them is the respiratory chain, mainly complexes I and III. Some cytosolic proteins that are important under physiological (like connexin 43) or pathophysiological (like the signal transducer and activator of transcription 3, STAT-3) conditions interact either directly or indirectly with the respiratory chain to modify respiration and/or ROS formation (Boengler et al., 2011; 2012; 2013). ROS are also produced by MAOs located at the outer mitochondrial membrane, which transfer electrons from amine compounds to oxygen leading to hydrogen peroxide formation. Especially under pathophysiological conditions MAO-induced ROS formation contributes to heart failure development secondary to pressure overload or ischaemia/reperfusion injury (see later) (Kaludercic et al., 2010; 2011; 2014). Furthermore, the cytosolic protein p66Shc becomes phosphorylated under stress conditions and shuttles into the mitochondrial intermembrane space, where it oxidizes reduced cytochrome c resulting in peroxide formation (Giorgio et al., 2005; Carpi et al., 2009) (for further review also see Di Lisa et al., 2009). During hypoperfusion, ROS formation increases, and a further burst of ROS occurs in early reperfusion (Vanden Hoek et al., 1997; Becker et al., 1999; Paradies et al., 2004). Increased ROS formation during reperfusion aggravates cell death (Turrens, 2003; Adlam et al., 2005), and accordingly, hearts from p66Shc knockout (KO) mice are protected from ischaemia/reperfusion injury in vitro, and inhibition of MAO reduces infarct size following ischaemia/reperfusion injury in vivo (Bianchi et al., 2005; Carpi et al., 2009; Di Lisa et al., 2009).
High concentrations of mitochondrial ROS – together with other factors – facilitate opening of the MPTP, a large conductance pore in the inner mitochondrial membrane, most likely build up by dimerization of the mitochondrial ATP synthase (Giorgio et al., 2013). MPTP opening at reperfusion enhances the inner mitochondrial membrane permeability to solutes with molecular weights up to 1.5 kDa and therefore leads to mitochondrial depolarization and subsequently to ATP depletion. Mitochondrial matrix volume increases and induces rupture of the outer mitochondrial membrane, inhibition of electron flow along the electron transport chain (ETC) and initiation of apoptotic cell death (Zou et al., 1999; Di Lisa et al., 2011; Griffiths, 2012). While prolonged opening of MPTP is detrimental, flickering of MPTP appears to be essential for putting the heart into a protected state and controlling mitochondrial matrix calcium concentration. Indeed, long-term blockade of MPTP opening facilitates the development of heart failure in mice upon increased stress (Hausenloy et al., 2004; Saotome et al., 2009; Elrod et al., 2010; Korge et al., 2011; Wong et al., 2012).
There are two subpopulations of mitochondria located in different regions of the cell, the subsarcolemmal mitochondria (SSM) and the interfibrillar mitochondria (IFM), which differ in their respiratory and calcium retention capacity (Fannin et al., 1999; Judge et al., 2005; Bugger et al., 2006; Hofer et al., 2009). Age-related declines in oxidative phosphorylation rates in rat heart mitochondria occur mainly in the IFM (Fannin et al., 1999; Judge et al., 2005), while the increase in ROS-production during ischaemia and reperfusion (I/R) is comparable in SSM and IFM from rat hearts (Chen et al., 2008). The depletion of cardiolipin and the accompanying cytochrome c loss in SSM but not in IFM during ischaemia results in an amplified ROS production and contributes substantially to the mitochondrial damage and the myocardial I/R injury (Chen and Lesnefsky, 2006; Chen et al., 2010b). Ischaemia was also reported to cause a decreased recovery of citrate synthase in isolates of SSM but not of IFM (Bugger et al., 2006). Besides, loss of mitochondrial connexin 43, which is located at the inner membrane of SSM but not of IFM, abolishes the cardioprotection by ischaemic preconditioning (IPC), suggesting that SSM and IFM also differ in their function and signal transduction during endogenous cardioprotection (Boengler et al., 2009b).
Mitochondrial fusion and fission
Many cardiovascular diseases affect mitochondrial function, but even more affect mitochondrial morphology and the inter-mitochondrial network, that is modify fusion and fission of mitochondria. Mitochondrial fusion proteins include the outer membrane proteins Mfn 1 and 2 and the inner membrane protein optic atrophy protein 1 (Opa1). Mitochondrial fission in mammalian cells is mediated by a large GTPase, the dynamin-related protein 1 (Drp1), along with other proteins such as the outer mitochondrial fission protein 1 (Fis1) and the mainly cytoplasmic endophilin B1. Mitochondrial fusion and fission are constant ongoing processes in many cell types and are essential for the maintenance of normal mitochondrial function. However, in the adult heart where mitochondrial movements are restricted by their tightly packed distribution along myofibrils or beneath the subsarcolemma, the relevance of mitochondrial dynamics is less obvious (Ong et al., 2013). Mitochondrial fusion serves as a pro-survival mechanism and content/protein exchange might help to overcome local functional deficiencies such as mitochondrial DNA (mtDNA) mutations within the mitochondrial network (Nakada et al., 2001; Ono et al., 2001; Chen et al., 2010a). Accordingly, inhibition of fusion results in an accumulation of mtDNA mutations triggering mitochondrial dysfunction, the loss of the mitochondrial genome and finally organ dysfunction (Chen et al., 2010a).
Pathophysiological conditions also affect mitochondrial fusion and fission. Ischaemia reduces Opa1 protein levels in H9c2 cells and increases apoptosis and fragmentation of the mitochondria. Also studies in heart failure suggested a possible reduction in mitochondrial fusion (Chen and Knowlton, 2011). In turn, Opa1 overexpression preserves mitochondrial morphology and protects cells against apoptosis (Chen et al., 2009b). Similarly, overexpression of Mfn-1 or Mfn-2 decreases MPTP sensitivity and protects from I/R injury in the cardiac muscle cell line HL-1 (Ong et al., 2010). However, Mfn-2 deletion was also demonstrated to induce a better cardiac recovery following I/R (Papanicolaou et al., 2011). Cells lacking human Fis1 undergo senescence-associated phenotypic changes associated with extensive mitochondrial elongation and increased ROS production (Lee et al., 2007). Mutation of dynamin-1-like protein, which is also critical for mitochondrial fission, induces mitochondrial dysfunction and cardiomyopathy in mice (Ashrafian et al., 2010). Decreasing mitochondrial fission is cardioprotective, as a Drp1 inhibitor reduces cardiomyocyte death following I/R both in vitro and in vivo (Ong et al., 2010). There are also rare and generally overlooked examples of cardiomyopathies linked either to naturally occurring mutations or to experimentally induced mutagenesis of mitochondrial fusion/fission genes (Dorn, 2013).
Mitophagy
Accumulation of damaged and dysfunctional mitochondria can lead to a cellular energy deficit, increased ROS production and the release of pro-apoptotic factors (Gottlieb et al., 2009). Interestingly, cells have developed a defence mechanism against aberrant mitochondria that can cause harm, in that selective sequestration and subsequent degradation of the dysfunctional mitochondria are initiated before they cause activation of the cell death machinery (Kubli and Gustafsson, 2012). Components required for this organelle-specific type of autophagy include the mitochondrial kinase PTEN-induced novel kinase 1 (PINK1), the E3 ubiquitin ligase Parkin, the atypical B-cell lymphoma 2 homology domain 3-only (BH3-only) proteins BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3) and Nip-like protein X, and several autophagy-related genes (Kanki et al., 2011). The elimination of damaged mitochondria by mitophagy is cardioprotective (Gottlieb and Carreira, 2010; Gottlieb and Mentzer, 2010; Sheng et al., 2010) and endogenous cardioprotective interventions such as preconditioning rely on autophagy/mitophagy (Gottlieb and Gustafsson, 2011). Preconditioning results in an up-regulation of autophagy in association with the pro-survival molecule BCL2-associated athanogene 1 protein, while inhibition of autophagy abolishes the cardioprotective effects of preconditioning (Gurusamy et al., 2009). Furthermore, the E3 ubiquitin ligase Parkin mediates cardioprotection by IPC through increased mitophagy (Huang et al., 2011). Bnip3 is activated following I/R and induces fragmentation of the mitochondrial network and increased mitophagy (Hamacher-Brady et al., 2006b; 2007), which was suggested to function as a cytoprotective pathway to oppose I/R-related apoptosis. Bnip3 also functions as a redox sensor during I/R (Kubli et al., 2008). Homodimerization and activation of Bnip3 is observed after I/R (Kubli et al., 2008). Bnip3 induces mitochondrial dysfunction through permeabilization of the mitochondrial membranes, disassembly of Opa1 mitochondrial fusion complexes and their release from the mitochondria (Quinsay et al., 2010). The Bnip3-triggered autophagy and mitochondrial turnover appears to be independent of ROS generation or MPTP opening (Quinsay et al., 2010). The p53-dependent up-regulation of TP53-induced glycolysis and apoptosis regulator (TIGAR) reduces Bnip3 activation and mitophagy in cardiomyocytes, resulting in an accumulation of damaged mitochondria and subsequent apoptosis in ischaemic myocardium (Hoshino et al., 2012). Enhancement of the autophagic flux following I/R through overexpression of Beclin 1 significantly reduces pro-apoptotic activation (Hamacher-Brady et al., 2006a).
Mitobiogenesis
If depletion of damaged mitochondria as described in the previous paragraph is not followed by an appropriate induction of mitochondrial biogenesis, this will have detrimental consequences for the heart as well. Mitochondrial biogenesis requires the coordination of the nuclear and the mitochondrial genome and involves changes in the expression of more than 1000 genes (review in Lopez-Lluch et al., 2008). Transcription factors involved in this process include the nuclear respiratory factors (NRF-1/NRF-2), the mitochondrial transcription factor A (Tfam), the oestrogen-related receptors ERα and ERγ (for nomenclature see Alexander et al., 2013a), ubiquitous transcription factors and the transcriptional coactivators PPARγ coactivator-1α (PGC-1α)or PGC-1-related co-activator (PRC; Goffart and Wiesner, 2003). Many of the pathways regulating mitochondrial biogenesis seem to converge at the transcriptional co-activator PGC-1α,which has been shown to directly dock on some of these transcription factors and modulate their activity (Schreiber et al., 2004; Gleyzer et al., 2005; Zhu et al., 2010; Safdar et al., 2011). Overexpression of PGC-1α in cultured myoblasts increases mitochondrial biogenesis and oxidative respiration (Wu et al., 1999), but PGC-1α also plays a key role in coordinating metabolic flux (Rodgers et al., 2005; Dominy et al., 2010) and regulates processes such as gluconeogenesis or FAO (Handschin and Spiegelman, 2006). The hearts of PGC-1α KO mice (Arany et al., 2005; Leone et al., 2005) demonstrate reduced oxidative capacity and mitochondrial gene expression, but normal mitochondrial volume density, suggesting additional mechanisms controlling cardiac mitochondrial biogenesis. Although cardiac dysfunction under basal conditions is moderate in PGC-1α KO mice (Arany et al., 2005; Leone et al., 2005), aortic constriction leads to accelerated heart failure (Arany et al., 2006). In contrast, PGC-1α overexpression causes uncontrolled mitochondrial proliferation and loss of sarcomeric structure, finally leading to dilated cardiomyopathy and premature death (Lehman et al., 2000). Thus, a well-balanced and tightly controlled change in the expression of PGC-1α appears to be necessary to maintain optimal mitochondrial and cardiac function. With ageing or under pathophysiological conditions (obesity, diabetes mellitus, I/R) major disturbances in mitochondrial biogenesis and function occur in various tissues including the heart (Semple et al., 2004; Sparks et al., 2005; Zahn et al., 2006; Linford et al., 2007; Niemann et al., 2011).
Protection against myocardial ischaemia/reperfusion injury conferred by CR
Ischaemia/reperfusion injury
Ischaemic tolerance decreases with age (Frolkis et al., 1991; Ataka et al., 1992; Tani et al., 1997) and many cardioprotective interventions including IPC and postconditioning are less effective in aged individuals (Abete et al., 1996; Schulman et al., 2001; Lee et al., 2002; Boengler et al., 2008; 2009a). Short- and long-term CR improves ischaemic tolerance in young and in old animals (Shinmura et al., 2005; 2008; Edwards et al., 2010), and IPC reduces post-ischaemic dysfunction in isolated, perfused hearts from food-restricted senescent rats, but not in hearts from ad libitum-fed senescent rats (Abete et al., 2002). Combining CR with exercise training completely restores the protection afforded by IPC in senescent hearts when compared with young hearts (Abete et al., 2005). Apart from ageing, cardioprotective interventions are affected by a number of co-morbidities (for a review, see: Ferdinandy et al., 2007; Ovize et al., 2010; Hausenloy et al., 2013). Indeed, ischaemic postconditioning is less effective in obese or diabetic animals (Bouhidel et al., 2008) (for a review, see Przyklenk, 2011; Oosterlinck et al., 2013).
Only a few data exist on the influence of CR on the protective effects of ischaemic postconditioning. Ischaemic postconditioning improves contractile recovery and cell viability in fed, but attenuates them in fasted hearts. Furthermore, ATP content and ATP synthesis increases while oxidative stress decreases in hearts of fasted rats following ischaemic postconditioning (Marina Prendes et al., 2011). The same group also reported improved functional recovery and increased resistance to MPTP opening after ischaemic postconditioning (Hermann et al., 2012).
Energetic homeostasis
The increased resistance to severe ischaemia and better functional recovery of hearts after CR is strongly related to changes in mitochondrial respiration (Broderick et al., 2002). Mitochondria isolated from CR hearts after I/R demonstrate an increased state 3 respiration, increased respiratory control ratios as a sign of well-coupled mitochondria and a higher efficiency of mitochondrial energy production (Broderick et al., 2002), indicating the involvement of metabolic changes in the mitochondrial compartment as a basis for improved left ventricular (LV) function. A cause and effect relationship between cardiac functional improvement and increased mitochondrial metabolism was, however, not determined in that study (Broderick et al., 2002). In addition to these mitochondrial changes, activation of glucose uptake and glycolysis, improved insulin sensitivity and increased antioxidative defence appear also to be involved in CR-induced protection during I/R (Russell et al., 2004; Mitchell et al., 2010; Wan et al., 2010; Yamagishi et al., 2010).
ROS formation
ROS balance is lost at the extremes of reduction or oxidation of the redox couples involved in electron transport or ROS scavenging. Isolated mitochondria display increased oxidative stress at high reduction potentials, while intact cardiac cells experience oxidative stress when mitochondria are uncoupled or maximally reduced as in ischaemia (Aon et al., 2010). The ‘uncoupling to survive’ hypothesis states that the attenuation of ROS by partial dissipation of the mitochondrial membrane potential while maintaining sufficient ATP production is a potential mechanism for delaying cellular senescence (Papa and Skulachev, 1997; Brand, 2000). Indeed, mild uncoupling has been shown to be associated with longevity in various experimental models (Barros et al., 2004; Speakman et al., 2004; Padalko, 2005; Caldeira da Silva et al., 2008; Lemire et al., 2009), suggesting that the beneficial effects of CR on mitochondrial respiration and lifespan can be mimicked by uncoupling agents. The induction of a mitochondrial biogenic response by uncoupling agents has been suggested to be involved in these beneficial effects as well. Accordingly, the authors of a comparative analysis in mice reported recently that systemic mild uncoupling by dinitrophenol and 40% CR for 6 months similarly increased activities of Akt and the Akt downstream target endothelial NOS (eNOS), leading to mitochondrial biogenesis in adipose tissue and skeletal muscle (Cerqueira et al., 2011).
Mitochondrial fusion and fission
CR increases Mfn-2 protein expression in various organs, suggesting a role for CR in the control of mitochondrial morphology and dynamics (Cerqueira et al., 2011). During nutrient deprivation, PKA activation phosphorylates the pro-fission protein Drp1, which is therefore retained in the cytoplasm, leading to unopposed mitochondrial fusion (Gomes et al., 2011). This mitochondrial hyperfusion is required to sustain cellular ATP levels and allow survival of starving cells (Gomes et al., 2011). Blocking fusion and thus elongation results in dysfunctional mitochondria, reduced membrane potential and starvation-induced cell death in vitro (Gomes et al., 2011). How these mechanisms operate in vivo during CR has not yet been investigated.
Mitophagy and mitobiogenesis
Starvation or reduced insulin signalling are strong inducers of autophagy (review in Levine and Kroemer, 2008), and inhibition of autophagy prevents the beneficial effects of CR in all species investigated so far (Rubinsztein et al., 2011). However, the efficiency of this process declines with age (Brunk and Terman, 2002), but this age-related impairment of autophagy was also attenuated by mild, life-long CR in the skeletal muscle of rats (Wohlgemuth et al., 2010).
In vitro starvation results in mitochondrial depletion (Carreira et al., 2010). This mitochondrial depletion may contribute to the observed myocardial dysfunction after food restriction (Pinotti et al., 2010). If depletion of mitochondria is not followed by an induction of mitochondrial biogenesis (Hancock et al., 2011), this will be detrimental for the heart. However in many studies, CR increases the number of functional mitochondria and promotes changes in mitochondrial dynamics (Lopez-Lluch et al., 2006; Civitarese et al., 2007), favouring tighter coupling between beta-oxidation and the tricarboxylic acid (TCA) cycle, and may concomitantly improve insulin sensitivity (Lopez-Lluch et al., 2006).
Mechanisms and mediators of cardioprotection conferred by CR
Mitochondrial quality control
Over their lifespan, cardiomyocytes experience repetitive exposure to ROS resulting in damage to macromolecules and cell organelles, which cannot be diluted via cell division. Stressful conditions such as I/R induce an increase in mitochondrial damage requiring an adequate removal by mitophagy, followed by an increase in cardiac mitochondrial biogenesis to meet the high energetic demand of the heart. Interestingly, CR has a major impact on most processes of mitochondrial quality control from mitochondrial biogenesis to mitochondrial fusion and fission or removal of damaged mitochondria by mitophagy.
Various endogenous and exogenous factors regulate the activity of the transcriptional coregulator of mitochondrial biogenesis PGC-1α, including NO (Leary and Shoubridge, 2003; Nisoli et al., 2003), cAMP response element-binding protein (CREB; Shaw et al., 2005; Than et al., 2011), AMPK (Jager et al., 2007; Canto et al., 2009), Akt or p38 MAPK (Puigserver et al., 2001; Fan et al., 2004), many of which have also been shown to be regulated in response to CR. CR increases the expression of eNOS, accompanied by increased mitochondrial biogenesis, mitochondrial respiration and ATP production (Nisoli et al., 2005; Nisoli and Carruba, 2006). The eNOS-induced mitochondrial biogenic response involves an Akt-dependent phosphorylation of eNOS (Dimmeler et al., 1999; Nisoli et al., 2003) and can be mimicked with serum from CR rats, an effect putatively mediated by adiponectin (Cerqueira et al., 2012a). However, Akt2 also phosphorylates PGC-1α at Ser570 preventing its recruitment to the promoter of PGC-1α target genes (Li et al., 2007b), possibly reducing mitochondrial biogenesis. The reversible acetylation and inhibition of PGC-1α by the histone acetyltransferase generally controls non-repressed protein 5 (acetyltransferase), but deacetylation and activation by sirtuin 1 (SIRT1) is another key way to alter PGC-1α activity. PGC-1α contains multiple distinct acetylation sites and PGC-1α deacetylation has been demonstrated to occur via SIRT1 in vitro as well as in vivo during fasting (Rodgers et al., 2005; Gerhart-Hines et al., 2007) and can be mimicked by resveratrol (Lagouge et al., 2006). Recently, a reduced PGC-1α acetylation and increased mitochondrial biogenesis in skeletal muscle was demonstrated following exercise (Canto et al., 2009; Li et al., 2011).
In a screen for upstream regulators of PGC-1α gene transcription, transducer of regulated cAMP-regulated element-binding protein 1 (TORC1) was identified as the most potent activator of PGC-1α gene transcription and thus mitochondrial biogenesis (Wu et al., 2006). Furthermore, it has been shown that phosphorylation of CREB at Ser133 activates the promoter of PGC-1α, increases PGC-1α expression and induces mitochondrial biogenesis (Chowanadisai et al., 2010). Accordingly, CREB-deficient mice are only poorly responsive to CR because of a reduction of the CREB-target genes PGC-1α and neuronal NOS in the brain (Fusco et al., 2012). Indirect evidence also suggests a role for p38 MAPK-mediated changes in PGC-1α activity in response to diet. The p38 MAPK phosphorylates PGC-1α at three residues (Thr262; Ser265; Thr298), resulting in a more active and stable protein (Puigserver et al., 2001). Insulin has been demonstrated to inhibit the p38 MAPK-mediated increase in PGC-1α expression (Hong et al., 2011) and prolonged exposure to insulin decreases mitochondrial mass, cellular ATP content, and oxygen consumption in hepatocytes (Liu et al., 2009). Under physiological conditions, insulin is increased in the blood in the fed state but falls during CR (Niemann et al., 2008; 2010). Reduced insulin levels in CR-treated animals may thus contribute to increased PGC-1α expression via altered p38 MAPK activation.
Reduced insulin signalling is also a strong inducer of autophagy (review in Levine and Kroemer, 2008) and autophagy seems to have positive effects on longevity. Life-long CR increases the occurrence of autophagy in the hearts of rats (Wohlgemuth et al., 2007) and extends lifespan, at least in part, by increasing expression or activity of the NAD-dependent deacetylase SIRT1 in various species including mammals (Chen et al., 2005; Bordone et al., 2007; Haigis and Sinclair, 2010). Indeed, SIRT1 appears to be necessary for the induction of starvation-induced autophagy (Lee et al., 2008; Morselli et al., 2010) and the protective effects of CR. Lack of SIRT1 inhibits autophagy in vivo, leads to an elevated acetylation of proteins required for autophagy, accumulation of damaged mitochondria and impaired metabolism (Lee et al., 2008). Down-regulation of the nicotinamide phosphoribosyltransferase (Nampt), which is the rate-limiting enzyme in the NAD+ salvage pathway and thus influences the SIRT activity, impairs autophagic flux, suggesting that endogenous Nampt positively regulates autophagy (Hsu et al., 2009).
Other pathways involved in CR-mediated effects on autophagy include established modulators of mitochondrial biogenesis such as Akt or AMPK. Signalling pathways converging at the activation of Akt inhibit the negative regulator of mammalian target of rapamycin kinase (mTOR) mTOR (see Alexander et al., 2013b) inhibiting proteins (TSC1/TSC2). The resulting activation of mTOR inhibits autophagy, while the opposite occurs when nutrients are depleted or Akt activation is reduced. Likewise, inhibition of phosphatidylinositide 3-kinase (PI3K), which generates lipid second messengers essential for the translocation and subsequent activation of Akt, preserves cardiac function in aged mice, attenuates cardiac signs of senescence and enhances autophagy (Inuzuka et al., 2009). Accordingly, long-term CR results in a decreased cardiac Akt phosphorylation and increased autophagy associated with preserved cardiac contractile function in mice (Han et al., 2012). In addition, CR also induces autophagy via AMPK activation (Egan et al., 2011; Kim et al., 2011) or through the inhibition of insulin/insulin-like growth factor (IGF) signalling (Kenyon, 2010). However, the fact that nutritional stress induced by serum/glucose deprivation in vitro or starvation in vivo strongly induces autophagy and cell death, which can be inhibited by IGF-1 (Troncoso et al., 2012), also points to the importance of IGF-1 in maintaining cardiac adaptation to nutritional insults. Furthermore, it also suggests that autophagy has the potential to induce harmful effects in response to nutritional challenges.
Role of micro RNAs (miRs)
A new emerging field is now unravelling the role of miRs in cardiac pathophysiology and their regulation in response to diet. Hypercholesterolaemia induces a down-regulation of cardiac miR-25, resulting in increased NADPH oxidase (NOX) 4 expression and consequently increased oxidative stress in the heart (Varga et al., 2013). Recently, it was shown that ageing is associated with a down-regulation of the miR processing enzyme Dicer and multiple miRs in adipose tissue in mice and humans (Mori et al., 2012). Interestingly, these effects in murine adipose tissue are largely prevented by CR in vivo and by nutrient deprivation, AMPK activation or mTOR inhibition in vitro. Oxidative stress, in contrast, induces a prematurely aged phenotype with low Dicer expression in vitro (Mori et al., 2012). Age-associated changes in miR expression in the skeletal muscle of rhesus monkeys and the brain of mice have also been reported to be partially modified by CR (Khanna et al., 2011; Mercken et al., 2013). Recently, increasing levels of circulating miRs, sensitive to modulation by CR, have been implicated in the ageing process in addition to tissue miRs and may thus convey cell–cell communications or influence the function of distant organs (Dhahbi et al., 2013). MiR-80 deletion in Caenorhabditis elegans results in extended lifespan and positive effects on the quality of cardiac-like muscle ageing, effects involving the transcription factor daf-16/FOXO, an important modulator of longevity through insulin signalling, and the CREB-binding protein CBP-1 (Vora et al., 2013). Interestingly, deletion of miR-80, which is highly expressed under ad libitum feeding but low under CR, exhibits these beneficial CR effects regardless of food availability. MiR-80 may thus represent a core regulator of metabolism and provide a novel point of application for CR mimetics (CRM) under normal calorie intake (Vora et al., 2013).
In addition to their role in response to ageing and diet, miRs have been shown to be involved in mitochondrial biology. So far, miR-499, miR-484 and the miR-30 family have been implicated in targeting the mitochondrial fusion or fission machinery in cardiomyocytes. miR-499 expression is reduced in the area at risk following myocardial ischaemia and in myocytes after anoxia (Wang et al., 2011). miR-499 inhibits cardiomyocyte apoptosis induced by anoxia. miR-499 transgenic mice show less apoptosis, reduced infarct size and improved LV function following I/R, while knockdown of miR-499 aggravates cardiac injury (Wang et al., 2011). The catalytic subunit of the serine and threonine protein phosphatase calcineurin was identified as a target of miR-499. Calcineurin dephosphorylates the mitochondrial fission protein Drp1 at Ser656 and promotes its translocation to mitochondria, which initiates the fission programme in cardiomyocytes. miR-499 mediates its anti-apoptotic effects through the suppression of calcineurin-mediated dephosphorylation of Drp1 and inhibition of mitochondrial fission (Wang et al., 2011). The promoter region of miR-499 contains p53 binding sites and p53 knockdown results in increased miR-499 levels and attenuation of mitochondrial fission and cell death, an effect that can also be mimicked in vivo (Wang et al., 2011). Recently, it was also reported that FOXO3a-mediated activation of miR-484 suppresses the translation of Fis1, resulting in an inhibition of Fis1-mediated fission and apoptosis in cardiomyocytes. Accordingly, FOXO3a KO mice exhibit low levels of miR-484 and an enhanced mitochondrial fission, apoptosis and myocardial infarction (Wang et al., 2012). Finally, members of the miR-30 family (miR-30a, miR-30b, miR-30d) are able to inhibit mitochondrial fission and apoptosis (Li et al., 2010). Low levels of miR-30 result in a p53-induced transcriptional activation of the mitochondrial fission protein Drp1 in cardiomyocytes in response to oxidative stress and subsequently to apoptosis (Li et al., 2010). Although we are just beginning to unravel the role of miRs in mitochondrial fusion or fission, the modulation of miRs may present a future therapeutic approach to treat apoptosis-related cardiac diseases including ischaemic heart diseases.
Role of SIRTs
SIRTs are a highly conserved group of NAD+-dependent class III histone deacetylases and/or ADP-ribosyltransferases, which are localized in different cellular compartments and deacetylate histones and a number of non-histone proteins. The non-histone targets of SIRTs include p53, the FOXO family of transcription factors, PGC-1α, hypoxia-inducible factor 1α (HIF-1α), NF-κB, the apoptosis inhibitor survivin, liver kinase B1 (LKB1), β-catenin, eNOS, Tfam, the mitochondrial acetyl CoA synthetase 2, glutamate dehydrogenase, isocitrate dehydrogenase 2 (IDH2), and others. Among the deacetylated proteins in CR hearts are components of complex I (NDUFS1) and complex III (Rieske subunit) of the respiratory chain. Deacetylation of these ETC components and reduced ROS production can be mimicked in cardiomyocytes treated with the SIRT activator resveratrol and attenuated by SIRT inhibition with nicotinamide (Shinmura et al., 2011b), suggesting that deacetylation of these complex I and complex III subunits is closely associated with reduced mitochondrial ROS production during I/R in CR hearts (Shinmura et al., 2011b). At least seven silent information regulator 2 (Sir2) homologues, SIRTs 1 to 7, have been identified in mammals. Three SIRTs, SIRT3, SIRT4 and SIRT5, localize to mitochondria. Data obtained from yeast, worms and rodents suggest that some SIRTs are conserved mediators of many benefits of CR. The dependence on NAD+ availability couples their activation to the cellular energy status. Nampt catalyses the conversion of nicotinamide to nicotinamide mononucleotide and is the rate-limiting enzyme in the NAD+ salvage pathway (Revollo et al., 2004; Garten et al., 2009). While intracellular Nampt is part of the NAD salvage pathway, extracellular Nampt acts as a pro-inflammatory cytokine and induces cardiac hypertrophy or adverse ventricular remodelling (Pillai et al., 2013). Intracellular Nampt is up-regulated by fasting (Yang et al., 2007b) or in vitro glucose restriction (Fulco et al., 2008), but reduced in the heart by I/R or pressure overload (Hsu et al., 2009). Cardiac-specific overexpression of Nampt reduces the infarct size after I/R, while down-regulation of Nampt significantly decreases NAD+ and ATP levels, increases apoptotic activation and reduces the autophagic flux in cardiomyocytes (Hsu et al., 2009). Thus, the molecular mechanisms by which Nampt protects the heart from ischaemic injury seem to involve a variety of signalling pathways in addition to SIRTs.
SIRT1
CR activates Sir2 by decreasing NADH levels in yeast, resulting in an increased lifespan (Lin et al., 2004). Similar results have been obtained in rodents undergoing CR, where higher NAD+ levels increased expression and activity of SIRT1, the mammalian Sir2 homologue (Rodgers et al., 2005). Increased expression of Sir2 in yeast extends lifespan, while Sir2 deletion shortens lifespan (Kaeberlein et al., 1999; Lin et al., 2000). Interestingly, CR does not extend lifespan of SIRT1 KO mice (Boily et al., 2008). Loss of SIRT1 activity leads to dilated cardiomyopathy in adult hearts associated with altered acetylation of different isoforms of myocyte enhancer factor 2 transcription factors and with major mitochondrial morphological and functional disturbances, where the latter may actually occur before the onset of dilated cardiomyopathy (Planavila et al., 2012). Furthermore, SIRT1 is necessary for the induction of starvation-induced autophagy (Lee et al., 2008; Morselli et al., 2010) and required for the lifespan-prolonging effects of CR and resveratrol, a pharmacological activator of SIRT1 (Morselli et al., 2010). SIRT1 is also involved in the CR-mediated increase in mitochondrial biogenesis, as PGC-1α deacetylation occurs via SIRT1 in vitro as well as in vivo during fasting (Rodgers et al., 2005; Gerhart-Hines et al., 2007).
Shinmura et al. (2008) provided evidence that the increase in nuclear SIRT1 is critically involved in the cardioprotection against ex vivo I/R injury conferred by 6 months of CR in middle-aged rats. The nuclear SIRT1 increase is NO-dependent and chronic NOS inhibition prevents not only the SIRT1 translocation but also the CR-induced cardioprotection (Shinmura et al., 2008). Accordingly, myocyte-specific SIRT1 overexpression inhibits I/R injury while cardiac loss of SIRT1 enhances I/R injury (Hsu et al., 2010). The authors suggested that cardioprotection against I/R injury occurs mainly via long-term transcriptional effects on cardioprotective or apoptotic genes. Mild to moderate cardiac overexpression of SIRT1 (2.2–7.5-fold) attenuates age-dependent increases in hypertrophy, apoptosis/fibrosis, cardiac dysfunction, retards cardiac ageing and protects against oxidative stress through FOXO-dependent catalase expression, elevated cellular ATP levels, and increased mitochondrial citrate synthase activity (Alcendor et al., 2007). However, high levels of SIRT1 (12.5-fold overexpression) result in the development of cardiomyopathy (Alcendor et al., 2007). Another group also generated mice with different levels of cardiac SIRT1 overexpression (Kawashima et al., 2011). They reported that moderate, 6.8-fold, overexpression of SIRT1 results in impaired diastolic function, but preserved systolic function in young mice. Interestingly, major metabolic changes were observed in these hearts with reduced fatty acid uptake, but increased glucose uptake, degenerated mitochondria, reduced mitochondrial respiration, mitochondrial gene expression and reduced mitochondrial ROS production (Kawashima et al., 2011). However, even mild overexpression of SIRT1 (3.2-fold) caused early cardiac dysfunction after pressure overload. All the observed cardiac changes demonstrate a clear dependency on the gene dose of SIRT1. Furthermore, it appears that modulation of SIRT1 activity in heart is context-dependent. An increase in lysine deacetylation has been observed following in vitro and in vivo IPC in cellular models and in mouse hearts, which occurred concurrent with an increase in SIRT1 activity (Nadtochiy et al., 2011a). Direct inhibition of SIRT1 as well as the reduction of NAD+ levels reverse lysine deacetylation and inhibit IPC-induced cardioprotection. SIRT1(+/−) hearts cannot be preconditioned and exhibit higher cytosolic lysine acetylation (Nadtochiy et al., 2011b). However, infusion of the SIRT1 activator SRT1720 alone does not elicit cardioprotection, suggesting that SIRT1 is necessary, but not sufficient for the cardioprotective effects of IPC (Nadtochiy et al., 2011a). Pharmacological inhibition or down-regulation of SIRT1 in cardiomyocytes/.cardiomyoblasts induces apoptosis because of overactivation of p53 (Alcendor et al., 2004; Passariello et al., 2011). Furthermore, a signalling cascade involving p53, the farnesoid X receptor and miR34a participates in the regulation of SIRT1 in age- and obesity-related diseases (Lee and Kemper, 2010).
SIRT3
CR increases the expression of SIRT3 (Shi et al., 2005; Palacios et al., 2009) and mitochondrial NAD+ levels (Nakagawa et al., 2009), it decreases the level of acetylation of mitochondrial proteins and increases mitochondrial SIRT activity, associated with an increased post-ischaemic state 3 respiration and reduced post-ischaemic ROS production (Shinmura et al., 2011b). Interestingly, the protective effects of CR on oxidative damage are diminished in SIRT3 KO mice, an effect involving deacetylation of critical lysine residues on manganese superoxide dismutase (MnSOD) as shown by two independent groups (Qiu et al., 2010; Tao et al., 2010). Therefore, SIRT3 up-regulation during CR (Shi et al., 2005; Palacios et al., 2009) or through interventions leading to a higher SIRT3 expression and activity may promote this cardioprotective potential. Indeed, among the different SIRTs, SIRT3 is the only protein whose increased expression has been directly linked to an increased lifespan in humans. Increased expression of SIRT3 due to a polymorphism in the promoter is associated with longevity in humans, suggesting that SIRT3 is involved in genetic control of lifespan (Bellizzi et al., 2005). However, a more recent larger study did not show a strong association between SIRT3 polymorphism and longevity (Lescai et al., 2009).
SIRT3 expression in the heart, which utilizes more ATP than many other organs, is high and the hearts of SIRT3-deficient mice show a strong reduction in ATP levels, suggesting that SIRT3 is an important regulator of cellular ATP content (Ahn et al., 2008). KO of SIRT3 results in elevated levels of acetylation of mitochondrial proteins in various tissues (Lombard et al., 2007; Ahn et al., 2008). SIRT3 influences a variety of proteins involved in oxidative phosphorylation. In particular, components of complex I of the ETC demonstrate increased acetylation in SIRT3 KO mice and SIRT3 physically interacts with the complex I subunit NDUFA9, increasing its activity in vitro (Ahn et al., 2008). The complex II subunit SdhA (succinate dehydrogenase complex, subunit A) is also a substrate for SIRT3 and deacetylation results in increased complex II activity (Cimen et al., 2010; Finley et al., 2011). Finally, SIRT3 may influence respiratory chain activity via deacetylation of cyclophilin D (CypD) at lysine 145. Subsequently, the reduced peptidyl-prolyl cis-trans isomerase activity of CypD induces dissociation from ANT1 (adenine nucleotide translocator) and thereafter promotes the dissociation of hexokinase II from the mitochondria, resulting in an increase in oxidative phosphorylation (Shulga et al., 2010). SIRT3 also reduces oxidative damage by modulating the activity of the mitochondrial IDH2, which acts as a source of electrons for cellular antioxidants, through deacetylation (Someya et al., 2010). This IDH2 deacetylation occurs in response to CR in various organs, but cardiac tissue or mitochondria were not investigated so far (Someya et al., 2010). SIRT3 also acts as an endogenous negative regulator of cardiac hypertrophy by activating MnSOD and catalase, thereby supressing cellular ROS levels and downstream signals involved in cardiac hypertrophic response (Sundaresan et al., 2009).
SIRT3 deacetylates CypD also on lysine 166, adjacent to the binding site of the CypD inhibitor cyclosporine A, thus reducing mPTP opening and flux through the pore (Hafner et al., 2010). SIRT3 KO mice exhibit an age-dependent increase in mitochondrial swelling, because of increased mPTP opening, and signs of accelerated cardiac ageing (Hafner et al., 2010). Interestingly, SIRT3 does not seem to influence the structurally related cyclophilins A and B, which is an important secondary finding and may be valuable for any treatment of cardiac diseases involving SIRT3 without affecting immune functions. Furthermore, SIRT3 was demonstrated to deacetylate and activate LKB1, resulting in an AMPK-meditated inhibition of cardiac hypertrophy in vitro as well as in vivo (Pillai et al., 2010). The authors also show that pathological cardiac hypertrophy is characterized by low cellular NAD+ levels. Exogenous supplementation of NAD+ normalizes these levels and blocks pathological hypertrophy through activation of SIRT3 but not SIRT1 (Pillai et al., 2010). SIRT3 is also directly involved in the regulation of apoptotic cell death in cardiomyocytes. The pro-apoptotic protein Bcl2-associated X protein (Bax) translocates from the cytosol to the mitochondrial membranes during the early phase of apoptosis, a process prevented through complex formation of Bax with Ku70. Acetylation of Ku70 results in the release of Bax, thus enabling mitochondrial translocation. In cardiomyocytes, SIRT3 effectively deacetylates Ku70, resulting in anti-apoptotic effects (Sundaresan et al., 2008). Many of these studies clearly suggest that SIRT3 represents a survival factor for cardiomyocytes under stress conditions.
SIRT2, 4, 5, 6, 7
Much less data are available on the role of other SIRTs in the heart as well as in response to CR. A CR-induced up-regulation of SIRT2 in adipose tissue and kidney has been demonstrated in mice (Wang et al., 2007). SIRT2 reduces the acetylation of FOXO3a and increases the expression of FOXO target genes such as MnSOD, enabling a better ROS defence in vitro (Wang et al., 2007). In contrast to SIRT3, no mitochondrial hyperacetylation was detectable in mice lacking the two other mitochondrial SIRTs, SIRT4 and SIRT5 (Lombard et al., 2007). SIRT4 has no identified substrate so far and may only show ADP-ribosyltransferase activity. SIRT4 binds ANT (Ahuja et al., 2007) and SIRT5 can deacetylate cytochrome c (Schlicker et al., 2008), although the biological significance of these interactions is as yet not known. In contrast to SIRT1, which stimulates the resveratrol-induced, glucose-stimulated insulin secretion (Vetterli et al., 2011), SIRT4 represses insulin secretion (Haigis et al., 2006; Ahuja et al., 2007). Knockdown of SIRT4 results in increased FAO, expression of genes involved in FAO and cellular respiration (Nasrin et al., 2010). SIRT4 may thus counteract some of the SIRT1 or SIRT3 metabolic effects and putatively also oppose some of the metabolic effects of CR.
SIRT6, which is highly expressed in the heart and brain (Mostoslavsky et al., 2006) and reduced in human failing hearts (Sundaresan et al., 2012), was first shown to be a nuclear, chromatin-associated protein that is important for DNA repair, genomic instability and chromatin silencing at telomeres (Mostoslavsky et al., 2006; Tennen et al., 2011). Although there are no data on the effects of CR on cardiac SIRT6 expression or function, the expression of SIRT6 increases in the ovary after moderate CR, which may have an impact on the reserve of germ cells (Luo et al., 2012). In the CVS, SIRT6 delays vascular ageing through protection from telomere and genomic DNA damage (Cardus et al., 2013) and to inhibit angiotensin II-induced hypertrophy in cardiomyocytes (Cai et al., 2012; Yu et al., 2013). Loss of SIRT6 leads to accelerated ageing, ageing-associated degenerative abnormalities and premature death in mice (Mostoslavsky et al., 2006). Furthermore, SIRT6-deficient mice more rapidly develop heart failure, whereas SIRT6 transgenic mice are protected from hypertrophic stimuli through attenuation of the IGF-Akt signalling pathway (Sundaresan et al., 2012). Recently, it was shown that SIRT6 overexpression extends lifespan only in male transgenic mice (Kanfi et al., 2012).
SIRT7 KO mice demonstrate reduced lifespan and develop cardiac hypertrophy with increased inflammation, apoptosis, lipofuscin accumulation and fibrosis, characteristics also known for the ageing heart (Vakhrusheva et al., 2008). Interestingly, cardiac SIRT7 levels indeed decrease with age (Vakhrusheva et al., 2008). SIRT7 deacetylates p53 in vitro (Vakhrusheva et al., 2008). Accordingly, myocytes from SIRT7 KO mice show a hyperacetylation of p53 and increased rate of apoptosis (Vakhrusheva et al., 2008). SIRT7 myocytes are more susceptible to oxidative stress (Vakhrusheva et al., 2008), which is suggestive for a role of this SIRT also in I/R, although this has not yet been investigated. There seem to be overlapping functions of SIRT1 and SIRT7 in the heart. Both, SIRT7 and SIRT1, protect cardiomyocytes from apoptosis through p53 deacetylation (Alcendor et al., 2004; Vakhrusheva et al., 2008) and higher levels of acetylated p53 have been shown to occur in diseased human myocardium together with reduced activity of SIRT7 and SIRT1 (Alcendor et al., 2004; Pillai et al., 2005; Vakhrusheva et al., 2008).
Role of NO
CR for either 3 or 12 months induces NOS expression and cGMP formation in various tissues of male mice (Nisoli et al., 2005; Cerqueira et al., 2011; 2012b). This is accompanied by mitochondrial biogenesis, with increased oxygen consumption and ATP production, and an enhanced expression of SIRT1 (Nisoli et al., 2005). SIRT1 in turn plays a fundamental role in regulating endothelial NO and endothelium-dependent vascular tone by deacetylating eNOS and thus increasing NOS activity (Mattagajasingh et al., 2007). CR reverses obesity-induced endothelial dysfunction and reduces uncoupling of eNOS and thereby oxidative stress (Ketonen et al., 2010). Short-term CR also reverses age-associated vascular endothelial dysfunction by restoring NO bioavailability, reducing oxidative stress (via reduced NOX-mediated superoxide production and stimulation of anti-oxidant enzyme activity) (Rippe et al., 2010), and normalizing the inducible NOS (iNOS)/eNOS ratio (Zanetti et al., 2010). Short-term CR alters BP in spontaneously hypertensive rat (SHR) via stimulation of an adiponectin/AMPK/eNOS signalling axis and may serve as an effective non-pharmacological treatment of hypertension (Dolinsky et al., 2010). Similarly, CR improves endothelial function in obese, hypertensive patients and enhances the response of forearm blood flow to ACh. The i.a. infusion of NG-monomethyl-L-arginine (L-NAME, 8 mmol·min−1), a NOS inhibitor, decreases the enhanced ACh-induced blood flow response induced by CR, again pointing to the involvement of NOS (Sasaki et al., 2002).
In addition to direct vascular effects, NO might also contribute to angiogenesis. In wild-type mice CR improves revascularization of ischaemic limbs and stimulates the phosphorylation of eNOS in the ischaemic limbs. Administration of the AMPK inhibitor compound C abolishes the CR-induced increase in limb perfusion and eNOS phosphorylation in wild-type mice (Kondo et al., 2009). CR also reduces infarct size following I/R, an effect completely blocked by NOS inhibition using L-NAME (Shinmura et al., 2008). Similar to CR, the adipocytokine adiponectin protects from myocardial contractile dysfunction and limits infarct size following I/R by a mechanism involving activation of AMPK and production of NO (Tao et al., 2007; Gonon et al., 2008).
Role of AMP-activated PK
AMPK is a heterotrimeric complex (see Alexander et al., 2013c), which consists of the catalytic α-subunit with a phosphorylation site at Thr172 and the two regulatory subunits β and γ. Upstream kinases, which can phosphorylate and thus activate AMPK, include LKB1, calcium/calmodulin-dependent PK kinase (CAMKKβ) and possibly also TGF-β-activating kinase 1 (TAK1). In mammalian cells, the activity of AMPK depends on the ratio between AMP and ATP and a very small rise in AMP can induce an allosteric activation of AMPK (Hardie, 2004). AMPK senses any circumstances that cause energy deficiency including starvation, hypoxia, ischaemia, glucose deprivation, metabolic poisons, oxidative and hyperosmotic stress. Whenever AMPK is activated, ATP-generating catabolic pathways such as FAO and glycolysis are switched on, while ATP-consuming anabolic pathways such as fatty acid synthesis, protein synthesis and cholesterol synthesis are turned off.
It is conceivable that adaptive responses to CR can be triggered by mechanisms that are able to sense and respond to changes in nutrient availability. AMPK acts as such a key nutrient sensor and is therefore thought to be a major regulator of the beneficial effects of CR (Hardie, 2004). The most conclusive genetic evidence supporting an important role for AMPK activation in the effects of CR on longevity is derived from studies performed in C. elegans (Apfeld et al., 2004; Greer et al., 2007a). Among the potential mediators of the protective effects of AMPK and thus CR are SIRT1, the FOXO transcription factors, PGC-1α and mTOR. AMPK activation promotes an increase in cellular NAD+ levels, thus increasing the activity of SIRT1 (Fulco et al., 2008; Canto et al., 2009). Furthermore, AMPK phosphorylates PGC-1α, increasing its activity and enabling deacetylation by SIRT1 (Canto et al., 2009). The CR-induced, AMPK-mediated phosphorylation of FOXO3a and consecutively increased transcriptional activity appears to be important for the regulation of genes involved in the control of energy balance and stress resistance (Greer et al., 2007b). Genetic reduction of the nutrient-responsive mTOR signalling results in increased lifespan, resistance to age-associated pathologies and gene expression patterns observed after CR or pharmacological AMPK activation (Selman et al., 2009). In addition, the complex network of AMPK, SIRT1, FOXO and mTOR is also involved in the control of autophagy/mitophagy (Lee et al., 2008; Sengupta et al., 2009; Canto et al., 2010; Hariharan et al., 2010; Egan et al., 2011; Kim et al., 2011) as described earlier. Previous studies suggest a strong activation of AMPK in the heart after long-term CR (Edwards et al., 2010; Niemann et al., 2010) and after short-term CR in mice and rats (Shinmura et al., 2005; 2008), while obesity or high nutrient supply induce reduced AMPK activation and downstream signalling (Niemann et al., 2011; 2013). Short-term CR results in a chronic AMPK activation before ischaemia and in reduced infarct size and improved LV function following ex vivo I/R. These protective effects are abolished by AMPK inhibition in aged hearts (Edwards et al., 2010). However, others have also reported that 24 h of fasting or 4 months of CR did not result in a significant increase in AMPK activation in various mouse organs including the heart (Gonzalez et al., 2004). Similarly, while Edwards et al. reported that AMPK plays a major role in protecting the aged heart from I/R injury after long-term CR (Edwards et al., 2010), others (Shinmura et al., 2008) provided evidence that long-term CR-induced cardioprotection occurs without changes in AMPK activation (Shinmura et al., 2008).
Whether or not cardiac AMPK activation during I/R is indeed beneficial has been explored in different mouse models of AMPK deficiency. Studies in isolated hearts have demonstrated that AMPK deficiency results in a more severe injury after I/R (Xing et al., 2003; Russell et al., 2004; Carvajal et al., 2007) and experimental data even suggest that a stronger AMPK activation in acute ischaemia results in a stronger reduction in infarct size (Nishino et al., 2004). Transgenic mice expressing a kinase dead (KD) form of AMPKα2 fail to augment glucose uptake and glycolysis during ischaemia and FAO during reperfusion. Furthermore, these hearts demonstrate significantly impaired contractile recovery during reperfusion and increased cardiac injury compared with wild-type hearts (Russell et al., 2004). AMPK was shown to be responsible for activation of glucose uptake and glycolysis and to limit cardiac damage by reducing apoptotic activity during low-flow ischaemia and reperfusion in these experiments (Russell et al., 2004). Another study using mice overexpressing a dominant negative AMPKα2 found no impairment in energetic status or cardiac recovery during reperfusion in these hearts in the presence of fatty acids (Folmes et al., 2009). Furthermore, in the presence of insulin, which inhibits AMPK activation and FAO, these hearts demonstrate a better functional recovery than wild-type mice, suggesting that AMPK inhibition is actually beneficial (Folmes et al., 2009). However, the KD mutation almost completely abolishes AMPKα2 activity and also substantially blunts AMPKα1 activity even under baseline conditions (Russell et al., 2004), while the dominant negative AMPKα2 mice demonstrate a more moderate reduction in AMPK activity (Folmes et al., 2009). In contrast, AMPKα2 KO mice are characterized by a higher sensitivity to ischaemic contracture, but their post-ischaemic contractility is similar to wild-type mice in the absence of fatty acids although delayed in the presence of oleate (Carvajal et al., 2007). The latter conditions may represent a setting more closely resembling the in vivo situation, which is also in agreement with deleterious effect of free fatty acids during reperfusion (Lopaschuk et al., 1993; Liu et al., 2002). The higher susceptibility to development of ischaemic contracture in the AMPKα2 KO hearts is most likely related to their low glycogen stores and decreased glucose uptake (Carvajal et al., 2007). In general, these studies using three different mouse models of AMPK deficiency suggest that the role of AMPK during I/R is also dependent on substrate availability and the possible opposing effects of AMPK on fatty acid and glucose metabolism.
Ischaemic tolerance decreases with age (Frolkis et al., 1991; Ataka et al., 1992; Tani et al., 1997), and many cardioprotective interventions including ischaemic pre- and postconditioning are less effective in aged animals and in elderly patients (Abete et al., 1996; Schulman et al., 2001; Lee et al., 2002; Boengler et al., 2008; 2009a). Interestingly, short- and long-term CR improves ischaemic tolerance even in aged animals (Shinmura et al., 2005; 2008; Edwards et al., 2010). In general, ischaemic AMPK activation is significantly lower in aged myocardium (Ma et al., 2010). AMPK activation by IPC not only contributes to reduced infarct size, but also to a better metabolic adaptation of the post-ischaemic myocardium, possibly via increased glucose uptake during reperfusion (Baldwin et al., 2002; Kristiansen et al., 2009), and mechanisms involving PKC-dependent translocation of the glucose transporter type 4 to the plasma membrane (Nishino et al., 2004). A recent study shows that CR significantly improves cardiac recovery following ischaemia, which is associated with a higher rate of glucose oxidation during reperfusion compared with controls (Sung et al., 2011). This improved energy supply also results in higher ATP content and lower AMPK activation at the end of reperfusion. While AMPK activation is decreased, CR results in a higher phosphorylation of Akt and ERK1/2 at the end of reperfusion, suggesting an involvement of the reperfusion injury salvage kinase (RISK) pathway (Sung et al., 2011). Both signalling pathways have previously been shown to be necessary in preconditioning-induced cardioprotection (Hausenloy et al., 2005). A concise summary on mechanisms and mediators of cardioprotection conferred by CR is presented in Figure 1.
Figure 1.
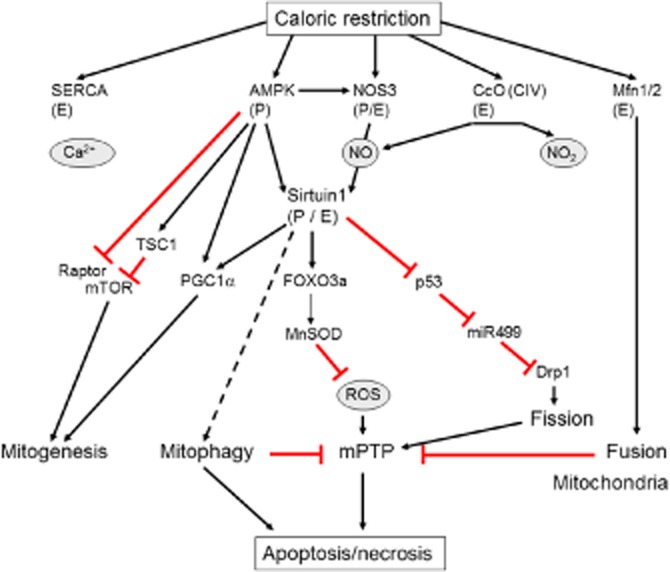
Mechanisms and mediators of cardioprotection conferred by CR because of altered expression (E) or phosphorylation (P) resulting in inhibition ( ) or activation (→) of downstream targets.
) or activation (→) of downstream targets.
Role of adiponectin and the adiponectin paralogues
Many investigations try to address the question about physiological AMPK activators during I/R and the cardioprotection conferred by CR. Among the candidates are adipocytokines such as adiponectin, which has repeatedly been shown to be increased after CR (Shinmura et al., 2007; Zhu et al., 2007; Niemann et al., 2008; 2010; Wan et al., 2010; Ding et al., 2012). The protective effects of short-term CR appear to be mediated by a marked increase in circulating high molecular weight complexes of adiponectin. Accordingly, recombinant adiponectin is able to restore the CR-induced improvement of myocardial ischaemic tolerance in adiponectin-deficient mice (Shinmura et al., 2007). However, inhibition of AMPK completely prevents the CR-induced cardioprotection as deduced from the recovery of LV-developed pressure and reduced infarct size in wild-type mice (Shinmura et al., 2007). While IPC reduces infarct size and results in higher adiponectin plasma levels, also after 6 months of CR, myocardial AMPK phosphorylation is not altered (Shinmura et al., 2008). However, this does not exclude the possibility that an initial AMPK activation is necessary for long-term cardioprotective CR effects. Three months of intermittent fasting, which has been suggested to be as effective as continuous, steady CR, also result in increased circulating adiponectin and cardioprotection against ischaemic injury in rats (Wan et al., 2010).
Adiponectin activates AMPK, resulting in a stimulation of glucose utilization and FAO (Yamauchi et al., 2002). Activation of the AMPK pathway by adiponectin mediates antihypertrophic effects in cardiomyocytes (Chan et al., 2004; Shibata et al., 2004a) and also plays an important role in the improved functional LV recovery and reduction of infarct size after I/R in mice (Shibata et al., 2005; Shinmura et al., 2007). Adiponectin-deficient mice, in contrast, show a more pronounced I/R injury, while exogenous adiponectin results in cardioprotection (Shibata et al., 2005; Tao et al., 2007). Adiponectin deficiency increases TNF-α production, iNOS expression, ROS release by NOX and formation of peroxynitrite in the I/R myocardium (Tao et al., 2007). Adiponectin-mediated activation of AMPK results mainly in an inhibition of apoptosis while the inhibitory effects on TNF-α production appeared to be AMPK-independent in one of these studies (Shibata et al., 2005). The protective effects of adiponectin during I/R do not only involve the myocardium and cardiomyocytes, but also the vasculature and endothelial cells. Angiogenic repair of ischaemic hind limbs is severely impaired in adiponectin-KO mice (Shibata et al., 2004b). Adiponectin promotes angiogenesis mainly through AMPK-dependent mechanisms (Shibata et al., 2004b; 2005). Adiponectin deficiency accelerates the transition from cardiac hypertrophy to heart failure following pressure overload, a mechanism induced through inhibition of AMPK-dependent VEGF induction and microvessel formation (Shimano et al., 2010). Furthermore, the CR-induced stimulation of revascularization in response to ischaemia critically involves the adiponectin-induced, AMPK-mediated activation of eNOS as shown in adiponectin- and eNOS-deficient mice (Kondo et al., 2009). Short-term CR also improves flow-mediated vasodilatation and increases eNOS activity and NO bioavailability in spontaneously hypertensive rats (Dolinsky et al., 2010). These effects are associated with elevated levels of circulating adiponectin and enhanced AMPK activity in mesenteric arteries, suggesting an involvement of the adiponectin/AMPK/eNOS signalling axis in the vasoprotective effects of CR (Dolinsky et al., 2010). Accordingly, administration of a NOS inhibitor abolishes the protective effects of adiponectin on infarct size and LV function following I/R (Gonon et al., 2008). Mice with cardiomyocyte-specific overexpression of a dominant negative AMPKα2 demonstrate larger infarcts, reduced LV function associated with a higher superoxide formation and increased apoptosis compared with wild-type mice after ischaemia/reperfusion (Wang et al., 2009). Exogenous adiponectin reduces infarct size, apoptotic activation and ROS production in both groups of mice similarly, suggesting AMPK-independent protective effects of adiponectin (Wang et al., 2009).
Recently, a family of structural and functional adiponectin paralogues, comprising 15 members so far, was discovered and designated as C1q/TNF-α-related proteins (CTRPs; Wong et al., 2004; 2008; 2009; Peterson et al., 2009; 2010; Wei et al., 2011; 2012a,b; Seldin et al., 2012). CTRPs are widely expressed and may exert their biological effects in a paracrine or autocrine fashion (Wong et al., 2004). Although adiponectin was described to be secreted by cardiomyocytes and to protect against I/R injury (Pineiro et al., 2005; Wang et al., 2010), these locally produced and secreted CTRPs may emerge as major mediators of AMPK-mediated cardioprotection in the heart in addition to the fat cell-derived high amounts of adiponectin. The CTRPs are predicted to be secreted proteins and may form heteromultimers with adiponectin in vivo, but they do not circulate in as high concentrations in blood as adiponectin (Wong et al., 2004). Ageing is associated with a reduction in plasma adiponectin and this reduction can only partially be overcome by a higher degree and duration of CR (Niemann et al., 2008). The adiponectin paralogues CTRP2 and CTRP7, which were among the first described CTRPs, cannot sufficiently compensate the loss of adiponectin in aged animals (Rohrbach et al., 2007). Putatively, other CTRPs could locally compensate in situations of adiponectin deficiency such as obesity or ageing. One of the most relevant CTRPs in terms of cardioprotective effects appears to be CTRP9. Cardiac expression of CTRP9 exceeds adiponectin by more than 100-fold (Su et al., 2013). CTRP9, which is most probably released by the cardiomyocyte itself, reduces myocardial infarct size and cardiomyocyte apoptosis following I/R. These effects appear to be mediated via CTRP9-induced activation of the adiponectin receptor-1 (Adipo1 receptor)-AMPK-signalling pathway (Kambara et al., 2012). Furthermore, CTRP9 administration significantly attenuates NOX expression and superoxide generation, reduces infarct size, and improves cardiac function in diabetic mice (Su et al., 2013). Both conditions, high-fat diet-induced diabetes and I/R injury, result in a strongly reduced CTRP9 expression in vivo (Kambara et al., 2012; Su et al., 2013). Recently, the adipose-derived CTRP3 was also demonstrated to act as an anti-apoptotic, pro-angiogenic and cardioprotective adipokine in a mouse model of myocardial infarction (Yi et al., 2012). CTRP3 improves survival and restores cardiac function after myocardial infarction and attenuates post-ischaemic pathological remodelling, which involves Akt-HIF-1α-VEGF signalling (Yi et al., 2012). The influence of CR on CTRP9 expression in the heart or CTRP3 expression in adipose tissue has not been studied so far.
Drug targets to mimic CR
It is unlikely that CR will be widely adopted because of the difficulty in maintaining long-term CR in modern society and because of potential side effects such as hypotension, infertility, bone thinning and osteoporosis, cold sensitivity, loss of strength and sarcopoenia, slower wound healing, depression, and emotional deadening. Therefore, efforts have been made to develop pharmacological agents to mimic CR as summarized in Figure 2. Such agents, which have been termed CRM, could provide the beneficial metabolic, hormonal and physiological effects of CR without altering dietary intake (Ingram et al., 2006).
Figure 2.
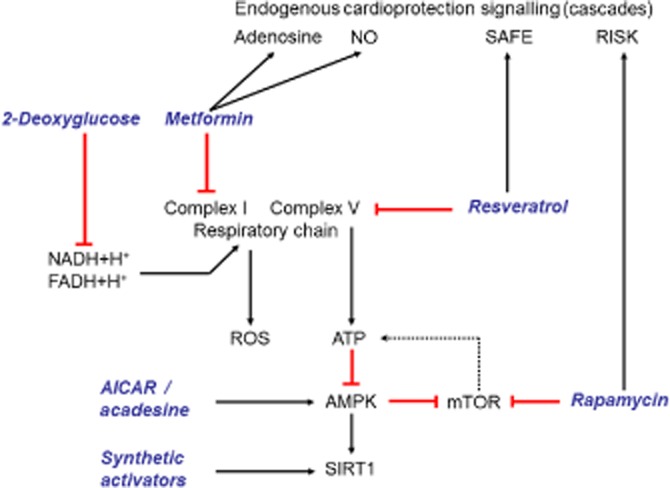
Possible drug targets to mimic the cardioprotective effects of CR and their mechanisms of action.
2-Deoxyglucose (2-DG)
2-DG was the very first agent that was formally investigated as a putative CRM (Lane et al., 1998) via inhibition of the glycolytic pathway. 2-DG is a synthetic analogue of glucose in which a hydroxyl group at position C2 is replaced with a hydrogen atom. It enters the normal glycolysis pathway, however, inhibits the activity of phosphoglucose isomerase, thereby, blocks glycolysis at the second step. With this property, 2-DG could induce the CR-like physiological phenotype in animal models. Feeding rats with low to moderate doses (0.2–0.4%) of 2-DG for up to 7 days lowers the serum insulin and glucose levels, lowers heart rate, basal BP and circulating free fatty acids without affecting the daily food intake. Long-term administration of 2-DG every second day decreases resting BP and heart rate and markedly enhances cardiovascular adaptation to stress (Wan et al., 2004). Likewise, 24 h incubation of neonatal rat cardiomyocytes with 2-DG protects the myocytes against doxorubicin-induced toxicity (Chen et al., 2011). However, feeding of rats with 0.4% 2-DG every second day for 6 months results in premature death (Wan et al., 2003), most likely by vacuolization of the heart, impaired cardiac function, atrial thrombosis and lung and liver congestion (Minor et al., 2010). The toxicity of 2-DG has been related to increased ER stress and is independent of metabolic inhibition. A striking difference between CR and 2-DG ingestion is that CR reduces the expression of the ER stress marker glucose regulated protein 78 while 2-DG ingestion induces it, thereby leading to autophagy – a main cause of its cardiac toxicity (Minor et al., 2010). Although increasing data show that long-term use of 2-DG as a CRM results in cardiac toxicity, a short-term use is protective against apoptosis in both endothelial cells and cardiomyocytes. Clearly, more studies are required to determine whether or not short-term use of 2-DG can be used to pharmacologically precondition the heart.
Metformin
Metformin is a member of biguanides and is the drug of choice for the treatment of type-2 diabetes. Metformin reduces blood glucose levels by decreasing hepatic glucose production and increasing insulin sensitivity in muscular tissue at least in part via activation of LKB1/AMPK signalling (Zhou et al., 2001; Shaw et al., 2005). In addition to its use in diabetes, recent animal studies and clinical trials suggest that the drug has therapeutic potential in other clinical disorders including cardiovascular diseases. Acute administration of metformin before ischaemia or at the onset of reperfusion reduced infarct size in isolated rat hearts (Legtenberg et al., 2002; Bhamra et al., 2008; Calvert et al., 2008). Although administration of a single dose of metformin reduces infarct size after 24 h, this dose does not prevent development of severe cardiomyopathies after 4 weeks. However, cardiomyopathy is prevented when metformin is administered daily for 4 weeks, showing its efficacy in post-infarction cardiac remodelling (Gundewar et al., 2009). Similarly, administration of metformin in murine models of permanent left coronary artery ligation and acute or chronic I/R improves cardiac and mitochondrial function, effects ablated in mice lacking functional AMPK (Calvert et al., 2008; Gundewar et al., 2009). Chronic intake of metformin for 30 days reduces infarct size and elicits cardioprotection both in diabetic and non-diabetic rats (Whittington et al., 2013) and preserves LV ejection fraction. Similarly, metformin improves cardiac function in a ventricular pacing model of heart failure in dogs (Sasaki et al., 2009), and it reduces acute graft rejection and apoptosis in cardiac allografts in a murine model of heart transplantation, when given daily for 4 weeks post-surgery (Chin et al., 2011). It must be noted, however, that metformin does not provide cardioprotection in all models of cardiomyopathy, that is, metformin does not preserve cardiac structure and function in a volume overload-induced model of heart failure (Benes et al., 2011). Beyond its specific cardioprotective effect, metformin improves endothelial function via activation of eNOS (Davis et al., 2006), inhibits platelet aggregation (Gin et al., 1989) and ROS production in endothelial cells (Ouslimani et al., 2005).
Metformin directly interacts with complex I of the mitochondrial respiratory chain and inhibits its activity, thus shutting off the ATP generation along with reduction in mitochondrial ROS production (El-Mir et al., 2000; Owen et al., 2000). This lowers the ATP/AMP ratio, thus activating the LKB1/AMPK pathway. Additionally, metformin inhibits the opening of MPTP and protects mitochondria against swelling, effects involved in cardioprotection against reperfusion injury (Bhamra et al., 2008). This effect was suggested to be mediated via PI3K activation (Bhamra et al., 2008). The metformin-induced cardioprotective effect is dependent at least in part on AMPK activation (Calvert et al., 2008; Gundewar et al., 2009), and part of its protective effect is lost in AMPKα2-dominant negative transgenic mice. Similarly, low-dose metformin was able to activate AMPK in isolated rat hearts and co-perfusion with the AMPK inhibitor compound C attenuates the protective effects of metformin (Paiva et al., 2010). Recently, long-term treatment with low-dose metformin (0.1% w w−1 in diet) was shown to extend lifespan in male mice, while a higher dose (1% w w−1 in diet) was toxic (Martin-Montalvo et al., 2013). Chronic metformin treatment increases AMPK activity and antioxidant protection (Martin-Montalvo et al., 2013). Metformin also induces eNOS phosphorylation and its cardioprotective effect is lost in eNOS KO mice. In contrast to the studies mentioned earlier, Bhamra et al. (2008) showed that acute administration of metformin protects rat hearts against reperfusion injury without activating AMPK, but instead Akt phosphorylation is increased following metformin administration; protection of rat hearts is lost following PI3K inhibition (Solskov et al., 2008). Finally, metformin induces the release of adenosine from cardiac tissue and a non-specific adenosine receptor antagonist attenuated metformin-mediated cardioprotection (Paiva et al., 2009). Thus, metformin exerts its beneficial effects via multiple molecular targets, potentially depending on the animal model used. Other drugs or xenobiotics that have been described to activate AMPK, and may thus also mimic some of the CR effects are the nucleoside aminoimidazole carboxamide ribonucleotide (AICAR), thiazolidindiones, resveratrol, capsaicin, berberine and quercetin (Hardie, 2011). Some of these pharmacological AMPK activators have been tested with regard to their efficiency in limiting the I/R injury. Ex vivo models have shown that AMPK activation by AICAR results in a reduction in infarct size in young hearts, an effect attenuated by AMPK inhibition (Paiva et al., 2010). However, AMPK activation by AICAR failed to reduce infarct size in rabbits (Dorion et al., 1992). Ischaemia/reperfusion injury is an important cause of morbidity and mortality after coronary artery bypass graft (CABG) surgery. In a meta-analysis of five studies, using the adenosine-regulating agent and AMPK activator acadesine, the use of acadesine correlated with significant reductions in the occurrence of perioperative myocardial infarction, cardiac death and reduction in a composite of cardiac death, stroke and myocardial infarction (Mangano, 1997). However, in a randomized, double-blind, placebo-controlled trial (RED-CABG study: Reduction in Cardiovascular Events by Acadesine in Patients Undergoing CABG) acadesine did not reduce all-cause mortality, non-fatal stroke, or need for mechanical support in intermediate to high-risk patients undergoing CABG surgery (Newman et al., 2012).
Resveratrol
The polyphenol resveratrol is a potent activator of SIRT1, possibly through increasing its binding affinity to NAD+ and the acetylated substrate (Howitz et al., 2003). Resveratrol is a plant phenolic compound found in the skin of red grapes and other fruits, which has been shown to expand lifespan in yeast, worms and flies (Howitz et al., 2003; Wood et al., 2004). Notably, the life-expanding benefits of resveratrol have been reproduced (Bauer et al., 2004; Jarolim et al., 2004; Viswanathan et al., 2005; Yang et al., 2007a; Greer and Brunet, 2009) in all three organisms, although some negative studies exist as well (Kaeberlein et al., 2005; Bass et al., 2007). Supporting the latter finding, resveratrol does not extend lifespan in mice (Barger et al., 2008; Pearson et al., 2008), but was shown to improve insulin sensitivity and reduce mortality in obese mice (Baur et al., 2006). The ability of resveratrol to directly activate SIRT1 in vitro has recently been questioned (Borra et al., 2005; Kaeberlein et al., 2005; Beher et al., 2009; Pacholec et al., 2010), suggesting that resveratrol modulates SIRT1 activity by regulating other signalling cascades. Furthermore, although many effects of resveratrol are mediated via SIRT1-dependent pathways, there are also a number of SIRT1-independent mechanisms. Many pharmacological compounds that interfere with the mitochondrial respiratory chain are known activators of AMPK and have been used to mimic some of the CR effects. Resveratrol for example has an impact on mitochondrial respiratory chain by inhibiting ATP synthase (Zheng and Ramirez, 2000). Many metabolic effects of resveratrol, including increases in insulin sensitivity, mitochondrial biogenesis, physical endurance and elevation of the NAD/NADH ratio appear to be AMPK-dependent (Um et al., 2010). AMPK may thus serve as a convergent point for many CRM compounds including resveratrol and metformin.
Resveratrol also prevents cardiac hypertrophy in SHR both by reducing haemodynamic load (Dolinsky and Dyck, 2011) and by direct action on cardiomyocytes (Chan et al., 2008) and has been proven beneficial by reducing myocardial infarct size and improving post-ischaemic functional recovery (Ray et al., 1999; Kaga et al., 2005; Shen et al., 2006; Goh et al., 2007). The cardioprotective effects of resveratrol have been attributed to its capacity to induce adenosine release, enhance NO production (Bradamante et al., 2003), increase neovascularization via VEGF signalling (Kaga et al., 2005), its antioxidant activity (Hung et al., 2002; Shen et al., 2006) and anti-inflammatory effects (Shigematsu et al., 2003). Furthermore, resveratrol activates SIRT1 thereby inducing mitochondrial biogenesis (Price et al., 2012), it inhibits hypoxia-induced apoptosis via the SIRT1-FOXO1 pathway in H9C2 cardiomyoblasts (Chen et al., 2009a) and protects the heart from I/R injury by inducing autophagy (Gurusamy et al., 2010). Resveratrol provides significant cardioprotection during ex vivo I/R as evidenced by better post-ischaemic ventricular recovery, reduced infarct size, concentration-dependent protective actions on the vasculature and is associated with enhanced NO-signalling, Akt and p38 MAPK activation (Das et al., 2006; Goh et al., 2007). The survivor activating factor enhancement (SAFE) pathway, which requires the activation of STAT-3 and significantly reduces cardiomyocyte death at the time of reperfusion, is activated by resveratrol in isolated cardiomyocytes (Palomer et al., 2013) and mouse hearts during I/R (Lamont et al., 2011). The cardioprotective effects of resveratrol, at concentrations found in red wine, seem to depend on TNF-α and STAT-3, as resveratrol significantly reduces infarct size in wild-type mice, but fails to protect in TNFRSF1B (also know as TNF receptor 2; see Alexander et al., 2013b)-KO or STAT-3-KO mice after I/R (Lamont et al., 2011).
The expanding body of preclinical evidence for beneficial effects has encouraged the translational researchers to analyse resveratrol for potential clinical use. Several clinical trials are being carried out for potential use of resveratrol in different diseases (http://clinicaltrials.gov). Most of the available data are about the pharmacokinetic and safety profile of resveratrol (review in Patel et al., 2011). The major challenge is the bioavailability of resveratrol after oral administration. A phase I clinical study showed that after a single oral dose of 5 g, the peak plasma concentration reached approximately 0.53 μg·mL−1 within 1.5 h (Boocock et al., 2007). Upon an oral dose, resveratrol is almost completely conjugated to its glucuronide and sulfate metabolites that show several fold higher plasma concentration compared with free resveratrol (Boocock et al., 2007; Brown et al., 2010). Whether these metabolites are therapeutically active, however, remains unknown. The bioavailability of resveratrol could be improved using micronized forms (SRT501, GlaxoSmithKline, Middlesex, UK), reaching plasma concentrations up to 2.2 μg·mL−1 after a single 5 g daily dose (Howells et al., 2011). These high doses of resveratrol are generally well tolerated and show only mild adverse reaction such as diarrhoea, nausea and vomiting (Patel et al., 2011). The most severe adverse effects are observed with the micronized form of resveratrol (SRT501) in a phase II clinical study where the drug was administered to multiple myeloma patients (Popat et al., 2013). The study reported that 38 and 21% of the patients developed anaaemia and acute renal failure, and finally the study was halted following two deaths because of nephrotoxicity. In contrast to the high-dose strategy, recently, two studies reported cardiovascular protective effects with long-term use of very low dosages of resveratrol, although they did not analyse the plasma concentration of the drug. Administration of 10 mg resveratrol day−1 to post-myocardial infarction, patients for 3 months reduces plasma low-density lipoprotein levels and platelet aggregation, and improves endothelial function (Magyar et al., 2012). In another 1 year follow-up of a study in patients undergoing primary prevention of cardiovascular disease, administration of 8 mg daily dose of resveratrol was reported to reduce plasma CRP and TNF-α concentrations and increase the adiponectin levels (Tome-Carneiro et al., 2012). Similarly, administration of 150 mg day−1 for 30 days to healthy volunteers reduces metabolic rate, activates AMPK, induces the expression of SIRT1 and PGC-1α, enhances intramyocellular and reduces intrahepatic lipid levels (Timmers et al., 2011). Finally, resveratrol improves flow-mediated vasodilatation in patients with coronary heart disease (Timmers et al., 2011).
Other SIRT activators
A number of synthetic SIRT1-activating compounds (STACs), that are 1000 times more effective than resveratrol in vitro (Milne et al., 2007), such as SRT1720, SRT2104, SRT2183 and SRT2379, have been developed (Sirtis). Currently, several STACs have already entered clinical trials (see the ClinicalTrials.gov website; http://clinicaltrials.gov). SRT1720, one of the first synthetic SIRT1 activators developed, induces mitochondrial biogenesis via SIRT1-dependent activation of PGC-1α and increases the health span of obese mice on high-fat diet (Feige et al., 2008; Minor et al., 2011). However, its efficacy in metabolic disorders is still controversial (Huber et al., 2010; Pacholec et al., 2010). Although acute perfusion of SRT1720 reduces infarct size in aged as well as SIRT1+/− mice following I/R (Tong et al., 2013), more work is needed to evaluate and confirm the beneficial effects of SRT1720 in vitro as well as in vivo. Although pharmacological strategies to influence SIRT3 activity are not yet on the market, there are good reasons for considering SIRT3 and its biological effects, as described earlier, as a promising new target for future therapeutic strategies. SIRT3 activation seems to provide beneficial effects in ageing, metabolic and cardiac diseases as well as neurological disorders. However, increasing SIRT3 activity might turn out to be a double-edged sword. Fasting-induced, SIRT3-mediated deacetylation of mitochondrial aldehyde dehydrogenase 2 was reported to be involved in exacerbated hepatotoxicity observed after paracetamol (Lu et al., 2011). However, SIRT3 KO mice are protected from paracetamol-induced hepatotoxicity, recommending at least caution regarding therapeutic strategies aimed at increasing SIRT3 activity (Lu et al., 2011).
Rapamycin and mTOR
Rapamycin (sirolimus) is a natural macrolide antibiotic obtained from Streptomyces hygroscopicus and commonly used as an immunosuppressant. The kinase mTOR is an important mediator of insulin- and growth factor-mediated signalling in multiple organs including the heart. Structurally, mTOR exists with other molecular components in two enzyme complexes, that is rapamycin-sensitive complex I (mTORC1) and rapamycin-insensitive complex 2 (mTORC2). Rapamycin is the best-characterized pharmacological autophagy inducer and inhibits mTOR by directly binding mTORC1.
Pharmacological or genetic inhibition of mTOR extends the lifespan in yeast, nematodes, flies and different mouse strains (Vellai et al., 2003; Jia et al., 2004; Kapahi et al., 2004; Harrison et al., 2009). Moreover, deletion of mTORC1 substrate S6K1 (ribosomal S6 kinase) is sufficient to confer increased lifespan in mice (Selman et al., 2009) and mice lacking S6K1 are protected from obesity and metabolic disorders (Um et al., 2004). Although the role of mTORC1-S6K1 inhibition in longevity is consistently demonstrated in animal models, little information is available about the role of mTORC1-S6K1 in age-associated cardiovascular diseases (Ming et al., 2012).
The most data in relation to cardiovascular disorders are available on pathological cardiac hypertrophy. In cultured cardiomyocytes, treatment with rapamycin attenuates angiotensin- and phenylephrine-induced hypertrophic response (Sadoshima and Izumo, 1995; Boluyt et al., 1997). A decrease in protein degradation because of inhibition of autophagy and an increase in protein synthesis upon stress stimulation by mTORC1-S6K1 signalling have been proposed as underlying mechanisms (Hands et al., 2009). In mice with pressure overload-induced cardiac hypertrophy, acute administration of rapamycin reduces cardiac growth without compromising cardiac function (Shioi et al., 2003), and it even reverses established hypertrophy (McMullen et al., 2004). Likewise, rapamycin treatment also attenuates thyroid hormone-induced cardiomyopathy (Kuzman et al., 2007). Similarly, partial genetic deletion of TOR in a heterozygous zebrafish model protects the animals against doxorubicin-induced cardiac toxicity and remodelling (Ding et al., 2011). In isolated mice hearts and in cultured cardiomyocytes, rapamycin protects against I/R-induced irreversible injury (Khan et al., 2006) and attenuates LV remodelling following myocardial infarction in rats (Buss et al., 2009). Rapamycin was suggested to confer cardioprotection against myocardial infarction through potent preconditioning-like effects involving the PI3K pathway, and opening of mitochondrial KATP channels (Khan et al., 2006), but appears to be independent of rapamycin-induced autophagy (Yang et al., 2010).
In contrast to rapamycin, which directly inhibits mTOR, AMPK activates the mTOR inhibitors TSC1/TSC2, thus indirectly inhibiting mTOR-S6K1 signalling (Shaw, 2009). In mammals, CR, which is known to activate AMPK, also suppresses mTOR signalling (Stanfel et al., 2009; Dogan et al., 2011; Shinmura et al., 2011a). The AMPK activators AICAR and metformin also attenuate pressure overload-induced cardiac hypertrophy (Li et al., 2007a; Fu et al., 2011). Conversely, reduced AMPK activity either by genetic deletion of AMPKα2 (Zhang et al., 2008) or lack of adiponectin (Shibata et al., 2004a) increases mTOR-S6K1 signalling and exacerbates cardiac hypertrophy in response to pressure overload. In contrast to the studies mentioned earlier demonstrating a consistent cardioprotection of mTOR inhibition, genetic ablation of mTORC1 signalling either in cardiac-specific KD mTOR transgenic mice or by deletion of raptor, a component of mTORC1 complex, induces dilated cardiomyopathy with enhanced autophagy, apoptosis and mitochondrial structural changes (Zhang et al., 2010; Shende et al., 2011). Interestingly, another group using the same transgenic mouse model (Shen et al., 2008) was unable to observe any protective or destructive effect of mTOR ablation on cardiac morphology or structure in response to exercise-induced hypertrophy and rapamycin again protected against hypertrophic growth (Shioi et al., 2002). Finally, cardiac-specific overexpression of mTOR in mice attenuates pressure overload-induced cardiac hypertrophy (Song et al., 2010) and protects the heart against irreversible injury following I/R and reduces mortality (Aoyagi et al., 2012). Thus, genetic and pharmacological studies differ in their results, and more basic research is needed to understand the underlying causes.
In the clinical arena, pharmacological inhibition of mTOR was proven beneficial. In a retrospective study in cardiac transplant patients receiving rapamycin, cardiac function was shown to be improved and LV mass was reduced compared with patients who were treated with a calcineurin inhibitor (CNI) over 1 year (Kushwaha et al., 2008). In a similar study in kidney transplant patients when CNI was replaced by rapamycin, a marked regression in LV hypertrophy was observed (Paoletti et al., 2008). A similar beneficial effect of CNI to rapamycin switching was also observed in patients who underwent cardiac transplantation (Raichlin et al., 2007). Overall, the current animal and clinical data are consistent in demonstrating some cardioprotective effects by pharmacological mTOR signalling inhibition. However, several challenges, particularly related to the adverse effects need to be addressed in clinical studies. A major adverse effect of chronic rapamycin therapy is glucose intolerance and insulin resistance (Houde et al., 2010; Deblon et al., 2012), causing a condition known as ‘starvation-like diabetes’ (Lamming et al., 2012). Thus, further analyses of the mTOR signalling pathway are required to potentially develop novel therapeutic approaches for the treatment of cardiomyopathies of different aetiologies.
Conclusions
CR has proven to be a valuable experimental tool for studying the process of ageing and its interaction with chronic diseases. Furthermore, results obtained from CR animals have also stimulated the search for drugs that mimic the beneficial metabolic, hormonal and physiological effects of CR without the potential side effects and without altering dietary intake. Evidence from animal studies and a limited number of human trials indicates that CR has the potential to both delay cardiac ageing and help prevent cardiovascular diseases. The mechanisms involved in this cardioprotection are diverse, but many of them involve mitochondrial adaptations. Data obtained from experimental models also suggest that pre-interventional, short-term CR or the pre-interventional application of CRMs may be a promising strategy in cardiac surgery and cardiology to improve tolerance to ischaemia and post-ischaemic cardiac recovery in their patient population. However, it is unlikely that CR will be widely adopted because of the difficulty in maintaining long-term CR in modern society and because of potentially serious side effects. Therefore, several challenges related to the adverse effects of CR and CRMs, and their long-term applicability for older adults and for those with significant comorbidities including cardiovascular diseases need to be addressed in clinical studies. Therefore, currently no general recommendation to implement CR in the general population for extending maximal lifespan in humans and improving cardiovascular diseases can be made.
Acknowledgments
We apologize to all colleagues whose work we could not cite owing to space limitations.
Glossary
- 2-DG
2-deoxyglucose
- Adipo1 receptor
adiponectin receptor 1
- AICAR
aminoimidazole carboxamide ribonucleotide
- AMPK
AMP-activated PK
- ANT
adenine nucleotide translocator
- Bax
BCL2-associated X protein
- Bnip3
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- CABG
coronary artery bypass graft
- CALERIE
Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy
- CAMKK
calcium/calmodulin-dependent PK kinase
- CNI
calcineurin inhibitor
- CR
caloric restriction
- CREB
cAMP response element-binding protein
- CRM
CR mimetic
- CTRP
C1Q/TNF-related protein
- CypD
cyclophilin D
- Drp1
dynamin related protein 1
- eNOS
endothelial NOS
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FAO
fatty acid oxidation
- Fis1
mitochondrial fission protein 1
- FOXO3a
forkhead box O3
- HIF-1α
hypoxia-inducible factor 1α
- I/R
ischaemia and reperfusion
- IDH2
isocitrate dehydrogenase 2
- IFM
interfibrillar mitochondria
- IGF
insulin-like growth factor
- iNOS
inducible NOS
- IPC
ischaemic preconditioning
- KD
kinase dead
- KO
knockout
- LKB1
liver kinase B1
- LV
left ventricle/left ventricular
- Mfn
mitofusin
- miR
micro RNA
- MnSOD
manganese superoxide dismutase
- MPTP
mitochondrial permeability transition pore
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin kinase
- mTORC1
mTOR complex 1
- Nampt
nicotinamide phosphoribosyltransferase
- NOX
NADPH oxidase
- NRF
nuclear respiratory factor
- Opa1
optic atrophy protein 1
- PGC-1α
PPARγ coactivator-1α
- PI3K
phosphatidylinositide 3-kinase
- PINK1
PTEN-induced novel kinase 1
- PRC
PGC-1-related coactivator
- ROS
reactive oxygen species
- S6K1
ribosomal S6 kinase
- Sdh
succinate dehydrogenase
- SHR
spontaneously hypertensive rat
- SIRT
sirtuin
- SR
sarcoplasmic reticulum
- SSM
subsarcolemmal mitochondria
- STACs
sirtuin-activating compounds
- TAK1
transforming growth factor-β-activating kinase 1
- TCA
tricarboxylic acid
- Tfam
mitochondrial transcription factor A
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- TORC1
transducer of regulated cAMP-regulated element-binding protein 1
- TSC1 and 2
mTOR inhibiting proteins
Conflict of interest
No conflict of interests.
References
- Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, et al. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, et al. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol. 2002;282:H1978–H1987. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- Abete P, Testa G, Galizia G, Mazzella F, Della Morte D, de Santis D, et al. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase SIRT3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, et al. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. SIRT1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013b;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, et al. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2012;303:H75–H85. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992;86(5 Suppl):II371–II376. [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Chandrashekhar Y, McFalls E, Anand I, Liu D, Jaimes D, et al. Ischemic preconditioning prior to aortic cross-clamping protects high-energy phosphate levels, glucose uptake, and myocyte contractility. J Surg Res. 2002;105:153–159. doi: 10.1006/jsre.2002.6394. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1:397–411. doi: 10.1016/s1568-1637(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277((6 Pt 2)):H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Benes J, Kazdova L, Drahota Z, Houstek J, Medrikova D, Kopecky J, et al. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin Sci (Lond) 2011;121:29–41. doi: 10.1042/CS20100527. [DOI] [PubMed] [Google Scholar]
- Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- Boengler K, Gres P, Dodoni G, Konietzka I, Di Lisa F, Heusch G, et al. Mitochondrial respiration and membrane potential after low-flow ischemia are not affected by ischemic preconditioning. J Mol Cell Cardiol. 2007;43:610–615. doi: 10.1016/j.yjmcc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009a;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, et al. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009b;104:141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- Boengler K, Heusch G, Schulz R. Nuclear-encoded mitochondrial proteins and their role in cardioprotection. Biochim Biophys Acta. 2011;1813:1286–1294. doi: 10.1016/j.bbamcr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, et al. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med. 2012;16:1649–1655. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Ungefug E, Heusch G, Schulz R. The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr Pharm Des. 2013;19:6890–6895. doi: 10.2174/138161281939131127115940. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluyt MO, Zheng JS, Younes A, Long X, O'Neill L, Silverman H, et al. Rapamycin inhibits alpha 1-adrenergic receptor-stimulated cardiac myocyte hypertrophy but not activation of hypertrophy-associated genes. Evidence for involvement of p70 S6 kinase. Circ Res. 1997;81:176–186. doi: 10.1161/01.res.81.2.176. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, et al. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465:115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- Bugger H, Chemnitius JM, Doenst T. Differential changes in respiratory capacity and ischemia tolerance of isolated mitochondria from atrophied and hypertrophied hearts. Metabolism. 2006;55:1097–1106. doi: 10.1016/j.metabol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, et al. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Cai Y, Yu SS, Chen SR, Pi RB, Gao S, Li H, et al. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett. 2012;586:866–874. doi: 10.1016/j.febslet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13:215–235. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus A, Uryga AK, Walters G, Erusalimsky JD. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. 2013;97:571–579. doi: 10.1093/cvr/cvs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–472. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, et al. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2007;292:H3136–H3147. doi: 10.1152/ajpheart.00683.2006. [DOI] [PubMed] [Google Scholar]
- Cerqueira FM, Laurindo FR, Kowaltowski AJ. Mild mitochondrial uncoupling and calorie restriction increase fasting eNOS, akt and mitochondrial biogenesis. PLoS ONE. 2011;6:e18433. doi: 10.1371/journal.pone.0018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Brandizzi LI, Cunha FM, Laurindo FR, Kowaltowski AJ. Serum from calorie-restricted rats activates vascular cell eNOS through enhanced insulin signaling mediated by adiponectin. PLoS ONE. 2012a;7:e31155. doi: 10.1371/journal.pone.0031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Cunha FM, Laurindo FR, Kowaltowski AJ. Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO*-mediated mechanism: impact on neuronal survival. Free Radic Biol Med. 2012b;52:1236–1241. doi: 10.1016/j.freeradbiomed.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, et al. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FOXO1 pathway. Biochem Biophys Res Commun. 2009a;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires SIRT1. Science. 2005;310(5754):1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010a;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu X, Kobayashi S, Timm D, Jepperson T, Liang Q. Caloric restriction mimetic 2-deoxyglucose antagonizes doxorubicin-induced cardiomyocyte death by multiple mechanisms. J Biol Chem. 2011;286:21993–22006. doi: 10.1074/jbc.M111.225805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Knowlton AA. Mitochondrial dynamics in heart failure. Congest Heart Fail. 2011;17:257–261. doi: 10.1111/j.1751-7133.2011.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009b;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yin G, Stewart S, Hu Y, Lesnefsky EJ. Isolating the segment of the mitochondrial electron transport chain responsible for mitochondrial damage during cardiac ischemia. Biochem Biophys Res Commun. 2010b;397:656–660. doi: 10.1016/j.bbrc.2010.05.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JT, Troke JJ, Kimura N, Itoh S, Wang X, Palmer OP, et al. A novel cardioprotective agent in cardiac transplantation: metformin activation of AMP-activated protein kinase decreases acute ischemia-reperfusion injury and chronic rejection. Yale J Biol Med. 2011;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J Biol Chem. 2010;285:142–152. doi: 10.1074/jbc.M109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFkappaB. Free Radic Res. 2006;40:1066–1075. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Guerrero N, Boffelli D, et al. Deep sequencing identifies circulating mouse miRNAs that are functionally implicated in manifestations of aging and responsive to calorie restriction. Aging (Albany NY) 2013;5:130–141. doi: 10.18632/aging.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Carpi A, Giorgio V, Bernardi P. The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim Biophys Acta. 2011;1813:1316–1322. doi: 10.1016/j.bbamcr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Ding Q, Ash C, Mracek T, Merry B, Bing C. Caloric restriction increases adiponectin expression by adipose tissue and prevents the inhibitory effect of insulin on circulating adiponectin in rats. J Nutr Biochem. 2012;23:867–874. doi: 10.1016/j.jnutbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, et al. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res. 2011;109:658–669. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63:389–401. doi: 10.1080/01635581.2011.535968. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, et al. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1alpha's acetylation state and metabolic function through the enzymatic activities of SIRT1/GCN5. Biochim Biophys Acta. 2010;1804:1676–1683. doi: 10.1016/j.bbapap.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion M, Rouleau J, Kingma JG., Jr Failure of AICA riboside to limit infarct size during acute myocardial infarction in rabbits. J Cardiovasc Pharmacol. 1992;19:69–77. doi: 10.1097/00005344-199201000-00010. [DOI] [PubMed] [Google Scholar]
- Dorn GW., 2nd Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013;1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, et al. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18:278–289. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5′-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol. 2009;297:H313–H321. doi: 10.1152/ajpheart.01298.2008. [DOI] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Frolkis VV, Frolkis RA, Mkhitarian LS, Fraifeld VE. Age-dependent effects of ischemia and reperfusion on cardiac function and Ca2+ transport in myocardium. Gerontology. 1991;37:233–239. doi: 10.1159/000213266. [DOI] [PubMed] [Google Scholar]
- Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol Sin. 2011;32:879–887. doi: 10.1038/aps.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco S, Ripoli C, Podda MV, Ranieri SC, Leone L, Toietta G, et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci U S A. 2012;109:621–626. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin H, Freyburger G, Boisseau M, Aubertin J. Study of the effect of metformin on platelet aggregation in insulin-dependent diabetics. Diabetes Res Clin Pract. 1989;6:61–67. doi: 10.1016/0168-8227(89)90058-2. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart S, Wiesner RJ. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp Physiol. 2003;88:33–40. doi: 10.1113/eph8802500. [DOI] [PubMed] [Google Scholar]
- Goh SS, Woodman OL, Pepe S, Cao AH, Qin C, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, et al. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Finley KD, Mentzer RM., Jr Cardioprotection requires taking out the trash. Basic Res Cardiol. 2009;104:169–180. doi: 10.1007/s00395-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007a;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007b;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ. Mitochondria and heart disease. Adv Exp Med Biol. 2012;942:249–267. doi: 10.1007/978-94-007-2869-1_11. [DOI] [PubMed] [Google Scholar]
- Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006a;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006b;2:307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- Han X, Turdi S, Hu N, Guo R, Zhang Y, Ren J. Influence of long-term caloric restriction on myocardial and cardiomyocyte contractile function and autophagy in mice. J Nutr Biochem. 2012;23:1592–1599. doi: 10.1016/j.jnutbio.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hands SL, Proud CG, Wyttenbach A. mTOR's role in ageing: protein synthesis or autophagy? Aging (Albany NY) 2009;1:586–597. doi: 10.18632/aging.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway – new players upstream and downstream. J Cell Sci. 2004;117((Pt 23)):5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FOXO by SIRT1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R, Marina Prendes MG, Torresin ME, Velez D, Savino EA, Varela A. Effects of the AMP-activated protein kinase inhibitor compound C on the postconditioned rat heart. J Physiol Sci. 2012;62:333–341. doi: 10.1007/s12576-012-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, et al. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Ning J, Yang X, Liu HY, Han J, Liu Z, et al. Fine-tuned regulation of the PGC-1alpha gene transcription by different intracellular signaling pathways. Am J Physiol Endocrinol Metab. 2011;300:E500–E507. doi: 10.1152/ajpendo.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Matoba S, Iwai-Kanai E, Nakamura H, Kimata M, Nakaoka M, et al. p53-TIGAR axis attenuates mitophagy to exacerbate cardiac damage after ischemia. J Mol Cell Cardiol. 2012;52:175–184. doi: 10.1016/j.yjmcc.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases – safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS ONE. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, McBurney MW, Distefano PS, McDonagh T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med Chem. 2010;2:1751–1759. doi: 10.4155/fmc.10.257. [DOI] [PubMed] [Google Scholar]
- Hung LM, Su MJ, Chu WK, Chiao CW, Chan WF, Chen JK. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br J Pharmacol. 2002;135:1627–1633. doi: 10.1038/sj.bjp.0704637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. doi: 10.1161/CIRCULATIONAHA.109.871137. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim S, Millen J, Heeren G, Laun P, Goldfarb DS, Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, et al. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal. 2014;20:267–280. doi: 10.1089/ars.2012.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, et al. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kanki T, Klionsky DJ, Okamoto K. Mitochondria autophagy in yeast. Antioxid Redox Signal. 2011;14:1989–2001. doi: 10.1089/ars.2010.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Inuzuka Y, Okuda J, Kato T, Niizuma S, Tamaki Y, et al. Constitutive SIRT1 overexpression impairs mitochondria and reduces cardiac function in mice. J Mol Cell Cardiol. 2011;51:1026–1036. doi: 10.1016/j.yjmcc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Pilvi T, Mervaala E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels. 2010;25:254–262. doi: 10.1007/s00380-009-1182-x. [DOI] [PubMed] [Google Scholar]
- Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 2011;3:223–236. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge P, Yang L, Yang JH, Wang Y, Qu Z, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. J Biol Chem. 2011;286:34851–34857. doi: 10.1074/jbc.M111.239921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen SB, Solskov L, Jessen N, Lofgren B, Schmitz O, Nielsen-Kudsk JE, et al. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases myocardial glucose uptake during reperfusion and induces late pre-conditioning: potential role of AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2009;105:10–16. doi: 10.1111/j.1742-7843.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha SS, Raichlin E, Sheinin Y, Kremers WK, Chandrasekaran K, Brunn GJ, et al. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–2750. doi: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzman JA, O'Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148:3477–3484. doi: 10.1210/en.2007-0099. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J Pineal Res. 2011;50:374–380. doi: 10.1111/j.1600-079X.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of calorie restriction. J Anti Aging Med. 1998;1:327–337. [Google Scholar]
- Leary SC, Shoubridge EA. Mitochondrial biogenesis: which part of ‘NO’ do we understand? Bioessays. 2003;25:538–541. doi: 10.1002/bies.10298. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase SIRT1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY) 2010;2:527–534. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res. 2002;34:182–185. doi: 10.1055/s-2002-26705. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire BD, Behrendt M, DeCorby A, Gaskova D. C. elegans longevity pathways converge to decrease mitochondrial membrane potential. Mech Ageing Dev. 2009;130:461–465. doi: 10.1016/j.mad.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescai F, Blanche H, Nebel A, Beekman M, Sahbatou M, Flachsbart F, et al. Human longevity and 11p15.5: a study in 1321 centenarians. Eur J Hum Genet. 2009;17:1515–1519. doi: 10.1038/ejhg.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273((3 Pt 2)):H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, et al. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem. 2007a;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Pan R, Li R, Niemann B, Aurich AC, Chen Y, et al. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes. 2011;60:157–167. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007b;447(7147):1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Beyer RP, Gollahon K, Krajcik RA, Malloy VL, Demas V, et al. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Liu HY, Yehuda-Shnaidman E, Hong T, Han J, Pi J, Liu Z, et al. Prolonged exposure to insulin suppresses mitochondrial production in primary hepatocytes. J Biol Chem. 2009;284:14087–14095. doi: 10.1074/jbc.M807992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002;39:718–725. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. 1993;264:135–144. [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Bourdi M, Li JH, Aponte AM, Chen Y, Lombard DB, et al. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity. EMBO Rep. 2011;12:840–846. doi: 10.1038/embor.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo LL, Chen XC, Fu YC, Xu JJ, Li L, Lin XH, et al. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin Exp Res. 2012;24:125–133. doi: 10.3275/7660. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–292. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- Marina Prendes MG, Hermann R, Torresin ME, Souto P, Tallis S, Savino EA, et al. Involvement of energetic metabolism in the effects of ischemic postconditioning on the ischemic-reperfused heart of fed and fasted rats. J Physiol Sci. 2011;61:303–312. doi: 10.1007/s12576-011-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Food restriction in rodents: an evaluation of its role in the study of aging. J Gerontol. 1988;43:B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Gomi F, Kuramoto K, Sagai M. Food restriction suppresses an age-dependent increase in the exhalation rate of pentane from rats: a longitudinal study. J Gerontol. 1993;48:B133–B136. doi: 10.1093/geronj/48.4.b133. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, et al. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming XF, Montani JP, Yang Z. Perspectives of targeting mTORC1-S6K1 in cardiovascular aging. Front Physiol. 2012;3:5. doi: 10.3389/fphys.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Smith DL, Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, et al. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–339. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010;13:40–45. doi: 10.1097/MCO.0b013e3283331384. [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011a;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, et al. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol. 2011b;301:H1506–H1512. doi: 10.1152/ajpheart.00587.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, et al. Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med. 2001;7:934–940. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, et al. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MF, Ferguson TB, White JA, Ambrosio G, Koglin J, Nussmeier NA, et al. Effect of adenosine-regulating agent acadesine on morbidity and mortality associated with coronary artery bypass grafting: the RED-CABG randomized controlled trial. JAMA. 2012;308:157–164. doi: 10.1001/jama.2012.7633. [DOI] [PubMed] [Google Scholar]
- Niemann B, Silber RE, Rohrbach S. Age-specific effects of short- and long-term caloric restriction on the expression of adiponectin and adiponectin receptors: influence of intensity of food restriction. Exp Gerontol. 2008;43:706–713. doi: 10.1016/j.exger.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res. 2010;88:267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol. 2011;57:577–585. doi: 10.1016/j.jacc.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Niemann B, Pan R, Teschner M, Boening A, Silber RE, Rohrbach S. Age and obesity-associated changes in the expression and activation of components of the AMPK signaling pathway in human right atrial tissue. Exp Gerontol. 2013;48:55–63. doi: 10.1016/j.exger.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119((Pt 14)):2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–275. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- Oosterlinck W, Dresselaers T, Geldhof V, Nevelsteen I, Janssens S, Himmelreich U, et al. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg. 2013;145:1595–1602. doi: 10.1016/j.jtcvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348((Pt 3)):607–614. [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalko VI. Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry (Mosc) 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, et al. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009;53:373–378. doi: 10.1097/FJC.0b013e31819fd4e7. [DOI] [PubMed] [Google Scholar]
- Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther. 2010;24:25–32. doi: 10.1007/s10557-010-6222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomer X, Capdevila-Busquets E, Alvarez-Guardia D, Barroso E, Pallas M, Camins A, et al. Resveratrol induces nuclear factor-kappaB activity in human cardiac cells. Int J Cardiol. 2013;167:2507–2516. doi: 10.1016/j.ijcard.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Paoletti E, Amidone M, Cassottana P, Gherzi M, Marsano L, Cannella G. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: a 1-year nonrandomized controlled trial. Am J Kidney Dis. 2008;52:324–330. doi: 10.1053/j.ajkd.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- Passariello CL, Zini M, Nassi PA, Pignatti C, Stefanelli C. Upregulation of SIRT1 deacetylase in phenylephrine-treated cardiomyoblasts. Biochem Biophys Res Commun. 2011;407:512–516. doi: 10.1016/j.bbrc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun. 2009;388:360–365. doi: 10.1016/j.bbrc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–39701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Kim G, Samant S, Moreno-Vinasco L, Garcia JG, et al. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304:H415–H426. doi: 10.1152/ajpheart.00468.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Pinotti MF, Leopoldo AS, Silva MD, Sugizaki MM, do Nascimento AF, Lima-Leopoldo AP, et al. A comparative study of myocardial function and morphology during fasting/refeeding and food restriction in rats. Cardiovasc Pathol. 2010;19:e175–e182. doi: 10.1016/j.carpath.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Piper HM, Kasseckert S, Abdallah Y. The sarcoplasmic reticulum as the primary target of reperfusion protection. Cardiovasc Res. 2006;70:170–173. doi: 10.1016/j.cardiores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Planavila A, Dominguez E, Navarro M, Vinciguerra M, Iglesias R, Giralt M, et al. Dilated cardiomyopathy and mitochondrial dysfunction in SIRT1-deficient mice: a role for SIRT1-Mef2 in adult heart. J Mol Cell Cardiol. 2012;53:521–531. doi: 10.1016/j.yjmcc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28:331–343. doi: 10.2165/11587190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Quinsay MN, Lee Y, Rikka S, Sayen MR, Molkentin JD, Gottlieb RA, et al. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J Mol Cell Cardiol. 2010;48:1146–1156. doi: 10.1016/j.yjmcc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae M. It's never too late: calorie restriction is effective in older mammals. Rejuvenation Res. 2004;7:3–8. doi: 10.1089/154916804323105026. [DOI] [PubMed] [Google Scholar]
- Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM) Biochim Biophys Acta. 2013;1833:213–224. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Aurich AC, Li L, Niemann B. Age-associated loss in adiponectin-activation by caloric restriction: lack of compensation by enhanced inducibility of adiponectin paralogs CTRP2 and CTRP7. Mol Cell Endocrinol. 2007;277:26–34. doi: 10.1016/j.mce.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, et al. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2009;296:H1125–H1132. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15((4 Pt 1)):302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins SIRT3 and SIRT5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101:6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Crowley VC, Sewter CP, Laudes M, Christodoulides C, Considine RV, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord. 2004;28:176–179. doi: 10.1038/sj.ijo.0802482. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Yutzey KE. FOXO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TB. Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology. 2006;7:165–168. doi: 10.1007/s10522-006-9006-1. [DOI] [PubMed] [Google Scholar]
- Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Jia GL, Wang YM, Ma H. Cardioprotective effect of resvaratrol pretreatment on myocardial ischemia-reperfusion induced injury in rats. Vascul Pharmacol. 2006;45:122–126. doi: 10.1016/j.vph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, et al. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- Sheng R, Zhang LS, Han R, Liu XQ, Gao B, Qin ZH. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy. 2010;6:482–494. doi: 10.4161/auto.6.4.11737. [DOI] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004a;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004b;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34:810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear SIRT1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011a;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, et al. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011b;109:396–406. doi: 10.1161/CIRCRESAHA.111.243097. [DOI] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123((Pt 6)):894–902. doi: 10.1242/jcs.061846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Solskov L, Lofgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, et al. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 hours after administration. Basic Clin Pharmacol Toxicol. 2008;103:82–87. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. SIRT3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–C1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, et al. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol. 2013;108:315. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. SIRT3 blocks the cardiac hypertrophic response by augmenting FOXO3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MM, Soltys CL, Masson G, Boisvenue JJ, Dyck JR. Improved cardiac metabolism and activation of the RISK pathway contributes to improved post-ischemic recovery in calorie restricted mice. J Mol Med (Berl) 2011;89:291–302. doi: 10.1007/s00109-010-0703-5. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev. 2012;11:254–270. doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y, et al. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29:3081–3089. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, et al. SIRT3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennen RI, Bua DJ, Wright WE, Chua KF. SIRT6 is required for maintenance of telomere position effect in human cells. Nat Commun. 2011;2:433. doi: 10.1038/ncomms1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than TA, Lou H, Ji C, Win S, Kaplowitz N. Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J Biol Chem. 2011;286:22047–22054. doi: 10.1074/jbc.M111.240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, et al. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, Del Campo A, et al. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res. 2012;93:320–329. doi: 10.1093/cvr/cvr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552((Pt 2)):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, et al. SIRT7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Kupai K, Szucs G, Gaspar R, Paloczi J, Farago N, et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Vora M, Shah M, Ostafi S, Onken B, Xue J, Ni JZ, et al. Deletion of microRNA-80 activates dietary restriction to extend C. elegans healthspan and lifespan. PLoS Genet. 2013;9:e1003737. doi: 10.1371/journal.pgen.1003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–1134. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Dietary supplementation with 2-deoxy-D-glucose improves cardiovascular and neuroendocrine stress adaptation in rats. Am J Physiol Heart Circ Physiol. 2004;287:H1186–H1193. doi: 10.1152/ajpheart.00932.2003. [DOI] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- Wang K, Long B, Jiao JQ, Wang JX, Liu JP, Li Q, et al. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun. 2012;3:781. doi: 10.1038/ncomms1770. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–E670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem. 2011;286:15652–15665. doi: 10.1074/jbc.M110.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem. 2012a;287:35804–35814. doi: 10.1074/jbc.M112.365965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012b;287:10301–10315. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215(4538):1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther. 2013;27:5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Steenbergen C, Murphy E. Mitochondrial permeability transition pore and calcium handling. Methods Mol Biol. 2012;810:235–242. doi: 10.1007/978-1-61779-382-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Gullicksen PS, Bare O, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Bessho M, Yanagida S, Nishizawa K, Kusuhara M, Ohsuzu F, et al. Severe, short-term food restriction improves cardiac function following ischemia/reperfusion in perfused rat hearts. Heart Vessels. 2010;25:417–425. doi: 10.1007/s00380-009-1222-6. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yang H, Baur JA, Chen A, Miller C, Adams JK, Kisielewski A, et al. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007a;6:35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007b;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Liu YB, Yu JB, Fan Y, Tang SY, Duan WT, et al. Rapamycin protects heart from ischemia/reperfusion injury independent of autophagy by activating PI3 kinase-Akt pathway and mitochondria K(ATP) channel. Pharmazie. 2010;65:760–765. [PubMed] [Google Scholar]
- Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, et al. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125:3159–3169. doi: 10.1161/CIRCULATIONAHA.112.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Cai Y, Ye JT, Pi RB, Chen SR, Liu PQ, et al. Sirtuin 6 protects cardiomyocytes from hypertrophy in vitro via inhibition of NF-kappaB-dependent transcriptional activity. Br J Pharmacol. 2013;168:117–128. doi: 10.1111/j.1476-5381.2012.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2:e115. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M, Gortan Cappellari G, Burekovic I, Barazzoni R, Stebel M, Guarnieri G. Caloric restriction improves endothelial dysfunction during vascular aging: effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol. 2010;45:848–855. doi: 10.1016/j.exger.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Liu Y, Cui AF, Shao D, Liang JC, Liu XJ, et al. PGC-1alpha coactivates estrogen-related receptor-alpha to induce the expression of glucokinase. Am J Physiol Endocrinol Metab. 2010;298:E1210–E1218. doi: 10.1152/ajpendo.00633.2009. [DOI] [PubMed] [Google Scholar]
- Zhu M, Lee GD, Ding L, Hu J, Qiu G, de Cabo R, et al. Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp Gerontol. 2007;42:733–744. doi: 10.1016/j.exger.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]


