Abstract
Background
Despite a wide body of literature supporting the use of antenatal antiretrovirals (ARV) for the prevention of mother to child transmission, there remains a need for continued monitoring as the intrauterine interval is a critical period during which fetal programming influences the future health and development of the child.
Methods
We conducted a systematic review of the current literature addressing potential metabolic complications of in utero HIV and ARV exposure. We describe studies evaluating metabolic outcomes such as intrauterine and early postnatal growth, bone health, and mitochondrial toxicity.
Results
Overall, infants exposed to HIV/ARV do not appear to exhibit vastly compromised intrauterine or early postnatal growth. However, some studies on the effect of combination antiretroviral therapy (cART) on small for gestational age (SGA) and low birth weight (LBW) outcomes in low-middle income countries show a risk for SGA/LBW while those in the U.S. do not. Postnatal growth to 1 year does not appear to be affected by intrauterine tenofovir exposure in African studies, but a U.S. study found statistically significant differences in length for age z scores (LAZ) at 1 year. Little data exists on long term bone health. Mitochondrial toxicity including abnormal mitochondrial morphology and DNA content, as well as neurologic deficits and death have been demonstrated in HIV/ARV–exposed infants.
Conclusion
Though gross measures of metabolic well-being appear to be reassuring, careful vigilance of even small risks for potential serious adverse effects to infants exposed to intrauterine HIV/ARVs is warranted as intrauterine fetal metabolic programming may substantially impact the future health of the child.
Keywords: Metabolic, Complications, Exposure, HIV, ARV
Since the introduction of zidovudine (AZT) for the prevention of mother-to-child transmission (PMTCT) in 1994(1), a wide body of literature has emerged evaluating the overall safety of in utero antiretroviral (ARV) exposure.(2–4) While some uncertainty still shrouds this issue, research largely suggests that the benefits of perinatal ARVs far outweigh the potential risks, supporting the use of antenatal antiretroviral therapy (ART) for PMTCT. Nonetheless, continued monitoring is necessary as the intrauterine interval is a critical period in which fetal programming influences the future health of a child. Fetal programming has been implicated as an important epigenetic mechanism whereby changes in the in utero environment can affect later disease development.(5, 6) In essence, the prenatal environment may have profound effects on intracellular signaling and metabolic pathways which, in turn, affect future development of chronic diseases.
Fetal metabolism, and consequently growth, depends on placental sufficiency and nutrients crossing the placenta. Maternal HIV may restrict placental size (7, 8) and cause morphologic changes,(8, 9) leading to deficient nutrient transfer and aberrant fetal metabolism. Metabolic complications from these changes may include compromised intrauterine and postnatal growth from placental insufficiency, poor bone health from decreased vitamin D and calcium transfer, as well as mitochondrial toxicities attributed to ARV exposure. In this review, we summarize the current literature addressing potential metabolic complications of in utero HIV/ARV exposure.
METHODS
We reviewed all English, French, and Spanish articles identified using a PubMed/Medline database search up to October 2013 using combinations of keywords including fetal, intrauterine, growth, birth weight (BW), low birth weight (LBW), small for gestational age (SGA), bone, mitochondrial toxicity, mitochondrial dysfunction, pregnancy, outcomes, infant, neonatal, perinatal, HIV exposure, maternal HIV, antiretrovirals, zidovudine, tenofovir, nucleoside analogues, combination antiretroviral therapy (cART), and highly active antiretroviral therapy (HAART) as well as a MeSH term search. Reference lists of all papers identified were reviewed for additional papers. We considered the article relevant if it contained information on HIV-infected pregnant women and any infant metabolic outcome including but not limited to fetal growth, postnatal growth, bone health, and mitochondrial toxicity (MT).
INTRAUTERINE GROWTH
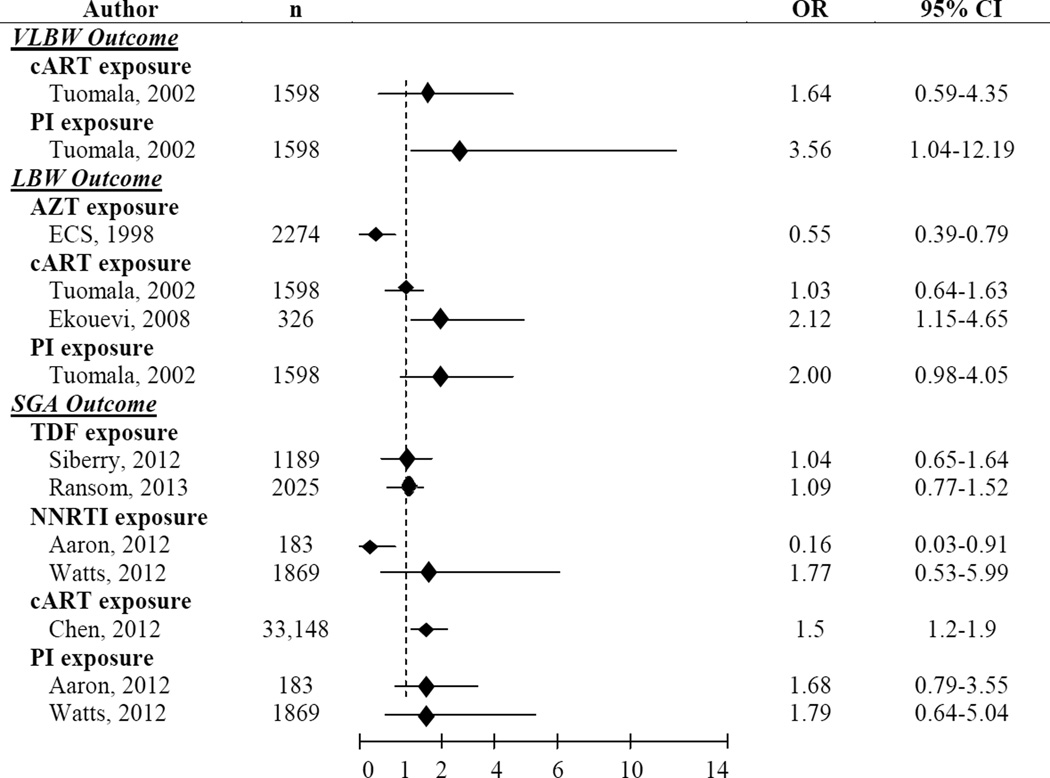
Several studies have evaluated the association of maternal HIV and in utero ARVs with birth outcomes such as BW, LBW (<2500 g), SGA, and preterm birth. (Figure 1) We focus on BW, LBW, and SGA in our discussion, as the causes of preterm birth, while often overlapping with those of LBW/SGA, are generally more multifactorial in nature and may be less directly associated with metabolic complications related to placental insufficiency. Unless otherwise noted, outcomes of SGA for BW were defined in the highlighted studies as <10th percentile according to standards specific to each study.
Figure 1.
Studies Evaluating Multivariate Associations with VLBW, LBW and SGA in HIV/ARV-exposed Infants
OR=Odds Ratio, CI=Confidence Interval, VLBW=Very Low Birth Weight (<1500 g), cART=Combination Antiretroviral Therapy, PI=Protease Inhibitor, LBW=Low Birth Weight (<2500 g), AZT=Zidovudine, ECS=European Collaborative Study, TDF=Tenofovir, SGA=Small for Gestational Age, NNRTI=Non-Nucleoside Reverse Transcriptase Inhibitor
Maternal HIV Exposure
Since most HIV-infected pregnant women now receive antenatal ARVs, it has been increasingly difficult to disentangle the effects of in utero HIV and ARV exposure. One study in Tanzania demonstrated that untreated HIV-infected women were more likely to have SGA infants than HIV-uninfected women [Odds Ratio (OR):1.64, Confidence Interval (CI):1.1–2.44]. (10) (see Table, SDC 1) Another South African study reported comparable associations [Relative Risk (RR): 1.28, CI: 1.06–1.53) (11), suggesting an association between maternal HIV infection and poor BW outcomes. The latter study also found evidence for an association between maternal CD4 cell count <200 cells/mm3 and SGA in adjusted subgroup analyses (RR: 1.43, CI: 1.0–2.07, p=0.05) as well as a lack of difference in SGA by infant HIV infection status. Two African studies conducted prior to the availability of ARVs for PMTCT report similar associations with LBW,(12, 13) and another reported lower mean BWs and birth lengths (p=0.01 for both) in infants born to HIV-infected women.(14) Several of these studies did not report results stratified by maternal immunosuppression or disease severity, limiting interpretation of these findings.(10, 12, 13)
ARV Exposure
As PMTCT prophylaxis has advanced from AZT monotherapy to combination antiretroviral therapy (cART), we highlight studies assessing effects of specific ARVs as well as cART. Unless otherwise noted, cART refers to the use of three drugs, generally two NRTI with a PI or NNRTI.
Zidovudine
Several studies have confirmed the overall safety of AZT on fetal growth.(15–19) Secondary data analysis on the Pediatric AIDS Clinical Trials Groups (PACTG) 076 revealed no difference in mean weight, length or head circumference through 18 months amongst uninfected infants between AZT and placebo groups. Rates of SGA in this study were also no different, though this latter finding should be interpreted with caution as both infected and uninfected infants were included in the birth cohort. (15) In addition, randomized clinical trials (RCTs) from Africa and Thailand revealed no increased risk of SGA births in women receiving AZT vs. placebo.(16–18) The West African DITRAME study reported no differences in LBW between infants exposed to in utero AZT vs. placebo, but this analysis included infected infants.(16) One RCT in Thailand also did not demonstrate any differences in mean BW (17) between AZT-exposed and unexposed infants. Another reported decreased birth weight-for-age (WAZ) and weight-for-length (WLZ) z scores in those with ≥7.5 vs. <7.5 weeks of in utero AZT exposure, though the actual difference, 50g, was small. (18) Lastly, the European Collaborative Study (ECS) reported a protective effect of antenatal AZT against LBW (OR: 0.55, CI: 0.39–0.79). (19) The varied nature of these cohorts, particularly around the inclusion of HIV-infected infants, has made it challenging to properly compare studies and reach consistent conclusions.
Tenofovir disoproxil fumarate
The introduction of the TDF/emcitritabine once-daily combination pill has shifted the landscape of cART administered in pregnancy. Its benefits in ease of dosing, decreased risk of MT,(20, 21) and 2010 designation by the World Health Organization (22) as first-line ART in HIV-infected adults have played a role in the doubling of its use in developing countries.(23, 24)
The Pediatric HIV/AIDS Cohort Study (PHACS) evaluated 2029 HIV/ARV-exposed infants and found no increased risk of LBW (OR:0.87, CI:0.63–1.2), SGA (OR:1.01, CI:0.65–1.64), or birth LAZ < −1.5 (OR:1.28, CI:0.73–2.25) or differences in birth WAZ between TDF-exposed (21%) and TDF-unexposed infants.(25) In an analysis of 2025 infants exposed to maternal HIVARV, the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) protocol P1025 found no differences in rates of SGA (as defined by BW z score <10th percentile) (OR: 1.09, CI: 0.77–1.52) or mean BW z scores between TDF-exposed and -unexposed neonates (p=0.9).(26) The DART study in Uganda and Zimbabwe assessed infants born to women enrolled in this RCT from 2004–2009 and also found no difference in rates of LBW by TDF exposure [19% (19/130) in TDF-exposed vs. 15% (13/69) in TDF-unexposed, p=0.44]. (27)
Combination Antiretrovirals
Combination ART has been shown to have inconsistent associations with LBW and SGA. Studies in the U.S./Europe have largely reported no associations between cART and LBW/SGA, whilst some in low-middle income countries have reported otherwise. This may be explained by differences in sample size, population characteristics, and access to obstetrical care. A combined analysis of 3266 participants including three single site studies, PACTG 076 & 185, the Perinatal AIDS Collaborative Transmission Study (PACTS), and the Women and Infants Transmission Study (WITS) reported no increased risk for LBW when cART exposure was compared to both AZT monotherapy and no cART in multivariate analysis.(28) The ECS reported no evidence of increased LBW rates in children exposed to any vs. no in utero ART.(29) An analysis of 9504 infants born to Botswanan women, however, identified cART in pregnancy as an independent predictor of SGA. Other factors associated with SGA included a prior adverse pregnancy outcome, alcohol use, gestational hypertension, and a maternal CD4 cell count ≤200 cells/mm3. (30) Another study in Côte d’Ivoire evaluated LBW outcomes and reported similar results.(31) Both reported an additional risk with cART initiated pre-pregnancy compared to that initiated during pregnancy, a finding which was not seen in the U.S. PHACS.(32) When evaluating the effect of maternal CD4, it is interesting that both the Botswanan and U.S. PHACS studies found maternal CD4 cell count ≤200 cell/mm3 to be associated with SGA while the study in Côte d’Ivoire did not find a similar association with LBW. Unlike these studies, a combined analysis of ACTG 5190/IMPAACT 1054 including participants in Africa, Thailand, India, and Brazil found no risk of SGA in infants exposed to in utero cART vs. AZT in univariate analysis, though this study may not have been adequately powered to detect true differences in SGA as only 10% (n=24/236) were born to women on cART. Maternal ARV exposure groups were also reasonably varied in levels of viremic control: 92% of women on cART reached HIV RNA levels <1000 copies/mL but only 36% of those on AZT plus intrapartum ARV reached this level of viremic control. The study did not report multivariate analyses adjusting for these or other factors such as maternal regimen or immunosuppression. (33)
Because of a wider range of ART choices available in North America, studies in the U.S. have attempted to distinguish effects between ART classes on intrauterine growth. Though the earlier PACTG 076 & 185/ PACTS/WITS combined analysis demonstrated a lack of association between cART and LBW, it did find a slightly increased risk of very (V)LBW with PI-based cART (OR=3.03, 95% CI: 1.04–12.19).(28) Neither the PHACS study (32) nor a U.S. study of 183 HIV-infected pregnant women (34) revealed an association of PI-based cART with SGA. However the latter did report a small protective effect of non-nucleoside reverse transcriptase inhibitors (NNRTIs) on SGA (OR=0.28, 95% CI: 0.1–0.75).(34)
POSTNATAL GROWTH
While some infants may be born SGA, whether in utero maternal HIV/ARV exposure has lasting impact on infant growth remains unknown. The worldwide heterogeneity of HIV-infected maternal/child dyad cohorts, chiefly around risk behaviors and nutritional/socioeconomic status, has complicated our ability to properly assess postnatal growth in HIV-exposed uninfected (HEU) children.
Maternal HIV Exposure
Unlike studies assessing intrauterine growth, studies assessing postnatal growth have not reported a direct association with maternal HIV.(35, 36) This may be because a number of studies assessing intrauterine growth have included both HIV-infected and uninfected infants, while those assessing postnatal growth have excluded HIV-infected infants. A British study conducted prior to the widespread use of ARVs in pregnancy (1984–1992) showed no differences in growth to 3 years between HEU and HIV-unexposed children.(35) In a study of 1403 HEU children, the ECS reported no differences in growth to 10 years when comparing HEU children to British 1990 standards.(36)
ARV Exposure
Zidovudine
Consistent with results evaluating intrauterine growth, long term follow-up of infants exposed to in utero AZT has not revealed detrimental effects on postnatal growth. PACTG 076 & 219 reported no differences in growth to 4 years between those exposed to in utero AZT and placebo.(37) RCTs in Thailand have reported the same after an 18 month follow up.(17, 18)
Tenofovir disoproxil fumarate
The only studies which have evaluated the effect of in utero TDF exposure on postnatal growth in HEU infants are those mentioned above. (25–27) DART reported no differences in mean WAZ between groups when growth was followed to 3 years.(27) Increased LAZ in TDF-exposed infants were noted during year 1 to year 2, but this difference did not persist thereafter. PHACS evaluated WAZ, LAZ, and HCAZ at 1 year of age and found that TDF-containing regimens were associated with lower mean LAZ and HCAZ (p=0.04 & 0.02).(25) IMPAACT 1025 reported no differences in WAZ at 6 months between groups (p=0.61).(26) Further studies will be required to assess in utero TDF exposure and long term growth.
Combination Antiretrovirals
Two large European cohorts have evaluated the impact of cART on growth through 18 months in HEU but reported somewhat contradictory findings. The ECS reported decreased WAZ (−0.1, p=0.019), LAZ (−0.12, p=0.008), and HCAZ (−0.14, p=0.001) associated with cART vs. no ART or AZT monotherapy.(38) A study in Spain found no evidence for abnormal WAZ, LAZ, or HCAZ when comparing infants exposed to any vs. no in utero ARVs and those exposed to PI vs. non-PI based cART. (39) A small U.S. study comparing growth to 2 years in HEU and HIV-unexposed children found no differences in growth.(40) A combined analysis of ACTG 5190/IMPAACT 1054 participants did not find evidence of decreased WAZ, LAZ, or HCAZ in infants exposed to cART vs. AZT.(33) Lastly, a secondary analysis of two RCTs in Botswana reported no differences in WAZ or WLZ by 6 months of age in infants exposed to in utero cART vs. AZT prophylaxis.(41)
BONE HEALTH
Bone mineral content has been shown to be decreased in HIV-infected adults(42) and children(43–45) on ART. TDF-containing regimens have been associated with decreased bone density in adults (46, 47), though reports in children have been conflicting.(48–50) Studies in rhesus macaques have demonstrated compromised intrauterine growth, diminished insulin-like growth factor-1 (IGF-1), and slightly decreased fetal bone porosity in infants born to high dose TDF-treated SIV-infected and –uninfected monkeys,(51, 52) raising concern regarding possible detrimental effects of in utero TDF exposure on infant bone health.
Few studies have directly evaluated early markers of bone health in ART-exposed infants. One study assessed neonatal bone status by quantitative ultrasonography as well as bone formation and resorption via bone alkaline phosphatase and C-terminal telopeptide of type I collagen in cord blood.(53) No differences were found between HIV/ARV-exposed and unexposed infants. Another study specifically evaluated the effect of in utero TDF exposure on bone health using these measurements in addition to serum calcium, phosphate, albumin, parathyroid hormone, 25-hydroxyvitamin D, 1,25-hydroxyvitamin D, IGF-1, and urinary calcium and creatinine.(54) Neither growth, measured via quantitative ultrasound, nor parameters of bone metabolism were statistically different between groups. As these studies were small and conducted in the same cohort their generalizability should be interpreted with caution. A current TDF safety substudy of the IMPAACT 1077 Promoting Maternal and Infant Survival Everywhere (PROMISE) study evaluating bone health via serum markers and dual energy x-ray absorptiometry (DEXA) in mothers/infants exposed to TDF-containing antepartum regimens is underway and will bridge gaps in knowledge in this critical area.(55)
MITOCHONDRIAL TOXICITY
Since the first report of mitochondrial dysfunction in HIV/NRTI-exposed infants exhibiting neurologic impairment, concern has evolved regarding the effects of in utero ART on the fetus.(56) Proposed mechanisms of MT include: 1) inhibition of mitochondrial DNA (mtDNA) polymerase-γ, 2) production of defective mtDNA, and 3) inefficient repair of errors in mtDNA replication. NRTIs inhibit mtDNA polymerase-γ, required for mtDNA replication.(57) This results in decreased levels of mtDNA/RNA and disruption of proper oxidative phosphorylation (OXPHOS), thereby leading to mitochondrial dysfunction.(58, 59) Additionally, because NRTIs can be incorporated into mtDNA by mtDNA polymerase, early mtDNA chain termination and inefficient NRTI excision may occur resulting in defective mtDNA.(60–62)
Animal Studies of MT from in utero ART
Studies in HIV-uninfected pregnant Erythrocebus patas mother/infant monkey dyads exposed to AZT have shown dose-dependent decreases in mtDNA levels, decreases in OXPHOS Complex I activity and abnormal mitochondrial morphology in cardiac and skeletal muscle cells at birth.(63) Similar findings were reported in studies evaluating brain cells of patas monkeys at birth, though no abnormal mitochondrial morphology was noted.(64) (see Table, SDC 2) Another study found evidence of abnormal mitochondrial morphology, and specific NRTIs (3TC, d4T) were associated with abnormal respiratory chain activity.(65) Lastly, a recent study evaluating the effects of NRTI incorporation into nuclear DNA (nDNA) and mtDNA in patas monkeys from birth to 3 years reported an 8-fold increase in cells with centrosomal amplification and an increase in cells with genetic material separated from nuclei in AZT/3TC-exposed patas at birth.(66)
Abnormal mtDNA
Both mtDNA mutations as well as overall levels of mtDNA have been studied in humans. When compared to HIV-unexposed infants, infants exposed to HIV/AZT-containing cART have demonstrated increased mtDNA(67) and nDNA abnormalities(68) in umbilical cord cells. A recent study on the latter even found evidence of increased aneuploidy and consistent alterations in gene expression affecting pathways of cell signaling, transcription, DNA recombination, replication, and repair in cord cells of infants exposed to AZT-containing cART.(68) Reports of mtDNA levels in infants exposed to in utero HIV/ART are conflicting. Earlier studies in smaller U.S. cohorts reported decreased mtDNA levels in cord blood mononuclear cells (CBMCs), peripheral blood mononuclear cells (PBMCs), and placental cells(69–71) in those exposed to HIV/AZT in utero compared to those unexposed to HIV as well as in those exposed to AZT vs. no AZT. A subsequent larger U.S. study confirmed this former finding when evaluating PBMCs but did not report the latter association with AZT. In fact, infants exposed to in utero AZT-3TC or AZT were found to have higher mtDNA levels than those unexposed to either.(72) Other studies in North America(73, 74) and Africa(75) have shown similar findings of increased mtDNA levels or no differences in mtDNA levels(76) in those exposed to cART. Several explanations may account for these conflicting results. Maternal HIV infection itself causes mitochondrial injury which may be mitigated by initial ART use, causing a rise in mtDNA levels. As women in the smaller studies were significantly more immune-compromised than those in subsequent studies, the effect of HIV on MT may have outweighed a possible beneficial effect of ART. In addition, the heterogeneity of results may reflect the wide range of cell line/tissue types used to detect mtDNA toxicity in these studies. Lymphocytes may produce more mtDNA, and mtDNA changes in PMBCs have not been shown to correlate well with mtDNA in tissue.(77) Another possibility is that fetal tissues may respond to maternal HIV/AZT-induced MT through a compensatory rise in mtDNA genesis.
Aberrant Mitochondrial Morphology
In the first case report of mitochondrial dysfunction from in utero ART, two of eight infants were found to have ultra-structural mitochondrial abnormalities by electron microscopy, one of whom died and both of whom exhibited seizures and neuromuscular abnormalities.(56) Aberrant mitochondrial morphology has also been demonstrated in endothelial cells of umbilical cord arteries (70) but not placental tissue (78) of infants exposed to in utero HIV/cART vs. those unexposed. Abnormal histology included excessive and swollen mitochondria with multiple membrane disruptions, extensive loss of cristae and matrix material, and in some, complete effacement of the central architecture.
Respiratory Chain Compromise
Mitochondrial DNA specifically encodes the subunit II of cytochrome c-oxidase (Complex II), while nDNA encodes subunit IV (Complex IV). Several studies have evaluated Complex II:IV ratios to further assess mitochondrial function in infants exposed to ART. Decreased Complex II:IV ratios have been reported in placental tissue (78) and CBMCs(76) of HIV/ART-exposed infants vs. -unexposed infants.
Pathways of intermediary metabolism such as fatty acid oxidation, organic acid and amino acid metabolism also require normal respiratory chain function. One study examined acylcarnitine and amino acid profiles, products of intermediary metabolism, from newborn metabolic screens in the U.S. and found a higher rate of abnormal screens in infants who also screened positive for HIV infection (2.2% vs. 1.2%, p=0.00025).(79) In addition, the rate of abnormal acylcarnitine levels was increased in ARV-exposed infants (43% vs. 0%, p=0.02).
Clinical Mitochondrial Dysfunction
Clinical manifestations of mitochondrial dysfunction may be varied as they depend on the tissue type affected. Lactate build-up is a result of mitochondrial dysfunction and known adverse effect of NRTIs. (80) Several studies have evaluated hyperlactatemia in infants exposed to in utero ART/HIV.(81–84) A Spanish study reported a 49.6% rate of hyperlactatemia (defined as >2.5 mmol/L) and reported an association with in utero didanosine exposure (OR: 1.06 per 1 week of fetal exposure, CI: 1.01–1.11).(82) An Ivorian study required ≥ 2 increased values and reported 13.4% with hyperlactatemia. No association was found between AZT or AZT-3TC in utero and hyperlactatemia, but in utero AZT-3TC was associated with increased mean lactate levels when compared with AZT monotherapy (p=0.009).(83) In a large U.S. cohort of HEU infants, only 3.4% were found to have hyperlactatemia when using a lactate threshold of >3 mmol/L.(84) Use of emcitritabine (OR: 2.23, CI: 1.12–4.42) and efavirenz (OR: 4.05, CI: 1.62–10.1) were found to be associated with hyperlactatemia. Variations in methods of lactate measurements as well as definitions of hyperlactatemia may account for the discrepancies in study findings.
Studies in France have shown an association of in utero HIV/ART with significant mitochondriopathy presenting as seizures, cognitive delays, motor and cardiac dysfunction, and even death.(56, 85) The French Pediatric Cohort (EPF) reported a significantly increased incidence of established/possible mitochondrial dysfunction in ARV-exposed infants (21/2644 vs. 0/1748, p=0.002). The relative risk (RR) of mitochondrial dysfunction was increased in those with in utero combination NRTI vs. AZT monotherapy exposure (RR: 2.5, CI: 1–6.5, p=0.046). Defects in respiratory chain complex enzyme units and abnormal mitochondrial morphology were also identified in available specimens.
In constrast, the Perinatal Safety Review Working Group reviewed 223 deaths amongst five cohorts of HIV-exposed children <60 months of age (n=23,265) and found no deaths associated with signs/ symptoms either suggestive of or proven to result from MT. (86) In addition, based on a population-based surveillance study of 9,067 HEU/ indeterminate infants, no deaths thus far have been directly linked to in utero HIV/AZT-induced MT in the U.S.(87) A secondary analysis of 984 HEU infants in the U.S. found only three cases of possible/established cases of MT. (88) No association between perinatal AZT and cardiac toxicity has been demonstrated in the U.S.(89) The PACTG 219 study did find an association between first exposure of 3TC or AZT-3TC in 3rd trimester with increased risk of mitochondrial dysfunction (RR: 10.57, CI: 1.93–75.61 and RR: 9.84, CI: 1.77–71.68).(90) However, the authors were unable to control for important factors such as maternal viremia or antenatal drug use.
CONCLUSION
The use of ARVs for HIV treatment and PMTCT has expanded tremendously since 1994. Possible adverse metabolic effects must be tempered with the overwhelming benefits of ARVs administered to HIV-infected pregnant women. Though gross measures of metabolic well-being such as intrauterine and early postnatal growth appear to be reassuring, more complex appraisals of metabolic health will require more sophisticated measures of bone health, mitochondrial function, and even pathways of intermediary metabolism. Careful vigilance of even small risks for potential serious adverse effects to infants exposed to in utero HIV/ARVs is warranted as intrauterine fetal metabolic programming poses a substantial impact on the future health of the child. Close monitoring of HEU children is essential in order to firmly establish the perinatal ART regimen with the most optimal long term outcomes.
Supplementary Material
Acknowledgments
Funding sources: JJ received salary support from the National Institute of Child Health and Human Development 1K23HD070760-01A1 during the preparation of this manuscript.
REFERENCES
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Stek AM. Antiretroviral treatment in pregnancy. Curr Opin HIV AIDS. 2008;3:155–160. doi: 10.1097/COH.0b013e3282f50bfe. [DOI] [PubMed] [Google Scholar]
- 3.Thorne C, Newell ML. The safety of antiretroviral drugs in pregnancy. Expert Opin Drug Saf. 2005;4:323–335. doi: 10.1517/14740338.4.2.323. [DOI] [PubMed] [Google Scholar]
- 4.Watts DH, Covington DL, Beckerman K, et al. Assessing the risk of birth defects associated with antiretroviral exposure during pregnancy. Am J Obstet Gynecol. 2004;191:985–992. doi: 10.1016/j.ajog.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. Rise and fall of Western diseases. Nature. 1989;338:371–372. doi: 10.1038/338371a0. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 7.Jauniaux E, Nessmann C, Imbert MC, Meuris S, Puissant F, Hustin J. Morphological aspects of the placenta in HIV pregnancies. Placenta. 1988;9:633–642. doi: 10.1016/0143-4004(88)90007-0. [DOI] [PubMed] [Google Scholar]
- 8.Vermaak A, Theron GB, Schubert PT, et al. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int J Gynaecol Obstet. 2012;119:239–243. doi: 10.1016/j.ijgo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz DA, Sungkarat S, Shaffer N, et al. Placental abnormalities associated with human immunodeficiency virus type 1 infection and perinatal transmission in Bangkok, Thailand. J Infect Dis. 2000;182:1652–1657. doi: 10.1086/317634. [DOI] [PubMed] [Google Scholar]
- 10.Habib NA, Daltveit AK, Bergsjo P, Shao J, Oneko O, Lie RT. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. BJOG. 2008;115:616–624. doi: 10.1111/j.1471-0528.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 11.Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Hum Reprod. 2012;27:1846–1856. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taha TE, Dallabetta GA, Canner JK, et al. The effect of human immunodeficiency virus infection on birthweight, and infant and child mortality in urban Malawi. Int J Epidemiol. 1995;24:1022–1029. doi: 10.1093/ije/24.5.1022. [DOI] [PubMed] [Google Scholar]
- 13.Ezeaka VC, Iroha EO, Akinsulie AO, Temiye EO, Adetifa IM. Anthropometric indices of infants born to HIV-1-infected mothers: a prospective cohort study in Lagos, Nigeria. Int J STD AIDS. 2009;20:545–548. doi: 10.1258/ijsa.2008.008446. [DOI] [PubMed] [Google Scholar]
- 14.Lepage P, Dabis F, Hitimana DG, et al. Perinatal transmission of HIV-1: lack of impact of maternal HIV infection on characteristics of livebirths and on neonatal mortality in Kigali, Rwanda. AIDS. 1991;5:295–300. [PubMed] [Google Scholar]
- 15.Sperling RS, Shapiro DE, McSherry GD, et al. Safety of the maternal-infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS. 1998;12:1805–1813. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Dabis F, Msellati P, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 17.Chotpitayasunondh T, Vanprapar N, Simonds RJ, et al. Safety of late in utero exposure to zidovudine in infants born to human immunodeficiency virus-infected mothers: Bangkok. Bangkok Collaborative Perinatal HIV Transmission Study Group. Pediatrics. 2001;107:E5. doi: 10.1542/peds.107.1.e5. [DOI] [PubMed] [Google Scholar]
- 18.Briand N, Le Coeur S, Traisathit P, et al. Growth of human immunodeficiency virus-uninfected children exposed to perinatal zidovudine for the prevention of mother-to-child human immunodeficiency virus transmission. Pediatr Infect Dis J. 2006;25:325–332. doi: 10.1097/01.inf.0000207398.10466.0d. [DOI] [PubMed] [Google Scholar]
- 19.Is zidovudine therapy in pregnant HIV-infected women associated with gestational age and birthweight? The European Collaborative Study. AIDS. 1999;13:119–124. doi: 10.1097/00002030-199901140-00016. [DOI] [PubMed] [Google Scholar]
- 20.Birkus G, Hitchcock MJ, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng JY, Murakami E, Zorca SM, et al. Relationship between antiviral activity and host toxicity: comparison of the incorporation efficiencies of 2',3'-dideoxy-5-fluoro-3'-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrob Agents Chemother. 2004;48:1300–1306. doi: 10.1128/AAC.48.4.1300-1306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Antiretroviral Therapy for HIV Infections in Adults and Adolescents. Recommendations for a Public Health Approach. Geneva: WHO; 2010. 2010 Revision. [PubMed] [Google Scholar]
- 23. [Accessed May 10, 2013];Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report 2009. Available at http://www.unaidsrstesa.org/resources/reports/towards-universal-access-scaling-priority-hivaids-interventions-health-sector.
- 24.Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. Progress Report 2011. WHO; [Accessed May 10, 2012]. Available at http://www.who.int/hiv/pub/progress_report2011/en/. [Google Scholar]
- 25.Siberry GK, Williams PL, Mendez H, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–1159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ransom CE, Huo Y, Patel K, et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64:374–381. doi: 10.1097/QAI.0b013e3182a7adb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9:e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–1870. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 29.Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 30.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS. 2008;22:1815–1820. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 32.Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207:612–621. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen-Saines K, Komarow L, Cu-Uvin S, et al. Infant outcomes after maternal antiretroviral exposure in resource-limited settings. Pediatrics. 2012;129:e1525–e1532. doi: 10.1542/peds.2011-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaron E, Bonacquisti A, Mathew L, Alleyne G, Bamford LP, Culhane JF. Small-for-gestational-age births in pregnant women with HIV, due to severity of HIV disease, not antiretroviral therapy. Infectious diseases in obstetrics and gynecology. 2012;2012 doi: 10.1155/2012/135030. 135030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross A, Raab GM, Mok J, Gilkison S, Hamilton B, Johnstone FD. Maternal HIV infection, drug use, growth of uninfected children in their first 3 years. Arch Dis Child. 1995;73:490–495. doi: 10.1136/adc.73.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newell ML, Borja MC, Peckham C. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–e60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- 37.Culnane M, Fowler M, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 38.Hankin C, Thorne C, Newell ML. Does exposure to antiretroviral therapy affect growth in the first 18 months of life in uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2005;40:364–370. doi: 10.1097/01.qai.0000162417.62748.cd. [DOI] [PubMed] [Google Scholar]
- 39.Ibieta MF, Cano JM, Amador JT, et al. [Growth of uninfected infants exposed to antiretrovirals born to HIV-infected woman] An Pediatr (Barc) 2009;71:299–309. doi: 10.1016/j.anpedi.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Neri D, Somarriba GA, Schaefer NN, et al. Growth and Body Composition of Uninfected Children Exposed to Human Immunodeficiency Virus: Comparison with a Contemporary Cohort and United States National Standards. J Pediatr. 2013 doi: 10.1016/j.jpeds.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powis KM, Smeaton L, Ogwu A, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56:131–138. doi: 10.1097/QAI.0b013e3181ffa4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone B, Dockrell D, Bowman C, McCloskey E. HIV and bone disease. Arch Biochem Biophys. 2010;503:66–77. doi: 10.1016/j.abb.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr. 2002;29:450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Mora S, Sala N, Bricalli D, Zuin G, Chiumello G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001;15:1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 45.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 46.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 47.Jones S, Restrepo D, Kasowitz A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int. 2008;19:913–918. doi: 10.1007/s00198-007-0524-8. [DOI] [PubMed] [Google Scholar]
- 48.Gafni RI, Hazra R, Reynolds JC, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–e718. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 49.Purdy JB, Gafni RI, Reynolds JC, Zeichner S, Hazra R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr. 2008;152:582–584. doi: 10.1016/j.jpeds.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giacomet V, Mora S, Martelli L, Merlo M, Sciannamblo M, Vigano A. A 12-month treatment with tenofovir does not impair bone mineral accrual in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:448–450. doi: 10.1097/01.qai.0000184860.62189.c8. [DOI] [PubMed] [Google Scholar]
- 51.Tarantal AF, Marthas ML, Shaw JP, Cundy K, Bischofberger N. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1999;20:323–333. doi: 10.1097/00042560-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 52.Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta) J Acquir Immune Defic Syndr. 2002;29:207–220. doi: 10.1097/00042560-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 53.Mora S, Giacomet V, Vigano A, et al. Exposure to antiretroviral agents during pregnancy does not alter bone status in infants. Bone. 2012;50:255–258. doi: 10.1016/j.bone.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Vigano A, Mora S, Giacomet V, et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther. 2011;16:1259–1266. doi: 10.3851/IMP1909. [DOI] [PubMed] [Google Scholar]
- 55. [Accessed June 5];Study of Effects of Tenofovir on Bone Health and Kidneys During Pregnancy and Breastfeeding. http://clinicaltrials.gov/ct2/show/NCT01066858?term=impaact+1077&rank=1.
- 56.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 57.Konig H, Behr E, Lower J, Kurth R. Azidothymidine triphosphate is an inhibitor of both human immunodeficiency virus type 1 reverse transcriptase and DNA polymerase gamma. Antimicrob Agents Chemother. 1989;33:2109–2114. doi: 10.1128/aac.33.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322:1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- 59.Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circulation research. 1994;74:344–348. doi: 10.1161/01.res.74.2.344. [DOI] [PubMed] [Google Scholar]
- 60.Copeland WC, Chen MS, Wang TS. Human DNA polymerases alpha and beta are able to incorporate anti-HIV deoxynucleotides into DNA. J Biol Chem. 1992;267:21459–21464. [PubMed] [Google Scholar]
- 61.Toji L, Cohen SS. The enzymatic termination of polydeoxynucleotides by 2',3'-dideoxyadenosine triphosphate. Proc Natl Acad Sci U S A. 1969;63:871–877. doi: 10.1073/pnas.63.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eriksson S, Xu B, Clayton DA. Efficient incorporation of anti-HIV deoxynucleotides by recombinant yeast mitochondrial DNA polymerase. J Biol Chem. 1995;270:18929–18934. doi: 10.1074/jbc.270.32.18929. [DOI] [PubMed] [Google Scholar]
- 63.Gerschenson M, Erhart SW, Paik CY, et al. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3'-azido-3'-deoxythymidine. AIDS Res Hum Retroviruses. 2000;16:635–644. doi: 10.1089/088922200308864. [DOI] [PubMed] [Google Scholar]
- 64.Ewings EL, Gerschenson M, St Claire MC, et al. Genotoxic and functional consequences of transplacental zidovudine exposure in fetal monkey brain mitochondria. J Acquir Immune Defic Syndr. 2000;24:100–105. doi: 10.1097/00126334-200006010-00003. [DOI] [PubMed] [Google Scholar]
- 65.Divi RL, Leonard SL, Kuo MM, et al. Cardiac mitochondrial compromise in 1-yr-old Erythrocebus patas monkeys perinatally-exposed to nucleoside reverse transcriptase inhibitors. Cardiovasc Toxicol. 2005;5:333–346. doi: 10.1385/ct:5:3:333. [DOI] [PubMed] [Google Scholar]
- 66.Olivero OA, Torres LR, Gorjifard S, et al. Perinatal Exposure of Patas Monkeys to Antiretroviral Nucleoside Reverse-Transcriptase Inhibitors Induces Genotoxicity Persistent for up to 3 Years of Age. J Infect Dis. 2013 doi: 10.1093/infdis/jit146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres SM, Walker DM, McCash CL, et al. Mutational analysis of the mitochondrial tRNA genes and flanking regions in umbilical cord tissue from uninfected infants receiving AZT-based therapies for prophylaxis of HIV-1. Environmental and molecular mutagenesis. 2009;50:10–26. doi: 10.1002/em.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andre-Schmutz I, Dal-Cortivo L, Six E, et al. Genotoxic Signature in Cord Blood Cells of Newborns Exposed In Utero to a Zidovudine-Based Antiretroviral Combination. J Infect Dis. 2013 doi: 10.1093/infdis/jit149. [DOI] [PubMed] [Google Scholar]
- 69.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 70.Divi RL, Walker VE, Wade NA, et al. Mitochondrial damage and DNA depletion in cord blood and umbilical cord from infants exposed in utero to Combivir. AIDS. 2004;18:1013–1021. doi: 10.1097/00002030-200404300-00009. [DOI] [PubMed] [Google Scholar]
- 71.Shiramizu B, Shikuma KM, Kamemoto L, et al. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleoside reverse transcriptase inhibitors during pregnancy. J Acquir Immune Defic Syndr. 2003;32:370–374. doi: 10.1097/00126334-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 72.Aldrovandi GM, Chu C, Shearer WT, et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics. 2009;124:e1189–e1197. doi: 10.1542/peds.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cote HC, Raboud J, Bitnun A, et al. Perinatal exposure to antiretroviral therapy is associated with increased blood mitochondrial DNA levels and decreased mitochondrial gene expression in infants. J Infect Dis. 2008;198:851–859. doi: 10.1086/591253. [DOI] [PubMed] [Google Scholar]
- 74.McComsey GA, Kang M, Ross AC, et al. Increased mtDNA levels without change in mitochondrial enzymes in peripheral blood mononuclear cells of infants born to HIV-infected mothers on antiretroviral therapy. HIV Clin Trials. 2008;9:126–136. doi: 10.1310/hct0902-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunz A, von Wurmb-Schwark N, Sewangi J, et al. Zidovudine exposure in HIV-1 infected Tanzanian women increases mitochondrial DNA levels in placenta and umbilical cords. PLoS One. 2012;7:e41637. doi: 10.1371/journal.pone.0041637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross AC, Leong T, Avery A, et al. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV Med. 2012;13:98–106. doi: 10.1111/j.1468-1293.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maagaard A, Holberg-Petersen M, Kollberg G, Oldfors A, Sandvik L, Bruun JN. Mitochondrial (mt)DNA changes in tissue may not be reflected by depletion of mtDNA in peripheral blood mononuclear cells in HIV-infected patients. Antivir Ther. 2006;11:601–608. [PubMed] [Google Scholar]
- 78.Gingelmaier A, Grubert TA, Kost BP, et al. Mitochondrial toxicity in HIV type-1-exposed pregnancies in the era of highly active antiretroviral therapy. Antivir Ther. 2009;14:331–338. [PubMed] [Google Scholar]
- 79.Kirmse B, Hobbs CV, Peter I, et al. Abnormal newborn screens and acylcarnitines in HIV-exposed and ARV-exposed infants. Pediatr Infect Dis J. 2013;32:146–150. doi: 10.1097/INF.0b013e31827030a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 81.Shah I. Lactic acidosis in HIV-exposed infants with perinatal exposure to antiretroviral therapy. Ann Trop Paediatr. 2009;29:257–261. doi: 10.1179/027249309X12547917868880. [DOI] [PubMed] [Google Scholar]
- 82.Noguera A, Fortuny C, Munoz-Almagro C, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–e603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 83.Ekouevi DK, Toure R, Becquet R, et al. Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV: Agence Nationale de Recherches Sur le SIDA et les Hepatites Virales 1209 study, Abidjan, Ivory Coast. Pediatrics. 2006;118:e1071–e1077. doi: 10.1542/peds.2006-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crain MJ, Williams PL, Griner R, et al. Point-of-care capillary blood lactate measurements in human immunodeficiency virus-uninfected children with in utero exposure to human immunodeficiency virus and antiretroviral medications. Pediatr Infect Dis J. 2011;30:1069–1074. doi: 10.1097/INF.0b013e318234c886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 86.Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25:261–268. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 87.Lindegren ML, Rhodes P, Gordon L, Fleming P. Drug safety during pregnancy and in infants. Lack of mortality related to mitochondrial dysfunction among perinatally HIV-exposed children in pediatric HIV surveillance. Ann N Y Acad Sci. 2000;918:222–235. [PubMed] [Google Scholar]
- 88.Brogly SB, Foca M, Deville JG, et al. Potential confounding of the association between exposure to nucleoside analogues and mitochondrial dysfunction in HIV-uninfected and indeterminate infants. J Acquir Immune Defic Syndr. 2010;53:154–157. doi: 10.1097/QAI.0b013e3181b3adc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lipshultz SE, Easley KA, Orav EJ, et al. Absence of cardiac toxicity of zidovudine in infants. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 2000;343:759–766. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- 90.Brogly SB, Ylitalo N, Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.