Abstract
Objective
To determine the effects of cognitive training on cognitive abilities and everyday function over 10 years.
Design, Setting, and Participants
Ten-year follow-up of a randomized, controlled single-blind trial with 3 intervention groups and a no-contact control group. A volunteer sample of 2832 persons (mean baseline age, 73.6 years; 26% African American) living independently in 6 US cities.
Interventions
Ten-session training for memory, reasoning, or speed-of-processing.; 4-session booster training at 11 and at 35 months after training.
Measurements
Objectively measured cognitive abilities and self-reported and performance-based measures of everyday function.
Results
Participants in each intervention group reported less difficulty with instrumental activities of daily living (IADL) (memory: effect size, 0.48 [99% CI, 0.12-0.84]; reasoning: effect size, 0.38 [99% CI, 0.02-0.74]; speed-of-processing: effect size, 0.36 [99% CI, 0.01-0.72]). At mean age of 82 years, about 60% of trained participants compared to 50% of controls (p<.05) were at or above their baseline level of self-reported IADL function at 10 years. The reasoning and speed-of-processing interventions maintained their effects on their targeted cognitive abilities at 10 years (reasoning: effect size, 0.23 [99% CI, 0.09-0.38]; speed-of-processing: effect size, 0.66 [99% CI, 0.43-0.88]). Memory training effects were no longer maintained for memory performance. Booster training produced additional and durable improvement for the reasoning intervention for reasoning performance (effect size, 0.21 [99% CI, 0.01-0.41]) and the speed-of-processing intervention for speed-of-processing performance (effect size, 0.62 [99% CI, 0.31-0.93]).
Conclusions
Each ACTIVE cognitive intervention resulted in less decline in self-reported IADL compared with the control group. Reasoning and speed, but not memory, training resulted in improved targeted cognitive abilities for 10 years.
Keywords: cognitive training, elderly, cognitive abilities, everyday function, training maintenance
INTRODUCTION
Cognitive decline is prevalent in older adults and is associated with decline in performance of instrumental activities of daily living (IADLs). Cognitive training has demonstrated utility for reducing cognitive declines in normal aging (1, 2), but evidence of its effectiveness in delaying difficulties in daily function has been limited (3).
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study is the first large-scale, randomized trial to show that cognitive training improves cognitive function in community-dwelling older adults up to 5 years and to show evidence of transfer of that training to daily function (4, 5). Given the time lag in the relationship between cognitive change and appearance of functional deficits, the full extent of the intervention effects on daily function was expected to take longer than 5 years to observe in this well-functioning study population (5).
Two hypotheses are derived from the trial’s conceptual model (4, 6) and prior findings: 1) the effects of cognitive training are specific to the trained cognitive ability and durable to 10 years; and 2) the effects of cognitive training will show positive transfer from cognitive function to delays in difficulties in daily function (7, 8) at 10 years.
METHODS
Design and Participants
ACTIVE is a multi-site, randomized, controlled clinical trial (see Ball et al (4) and Jobe et al (6) for details), with recruitment from March 1998 through October 1999 in six metropolitan areas. Community-dwelling adults aged 65 years and older were eligible. Exclusion criteria included: significant cognitive dysfunction (Mini-Mental State Examination [MMSE] score < 23) (9); functional impairment (dependency or regular assistance in activities of daily living (ADL) on Minimum Dataset (MDS) Home Care (10)); self-reported diagnoses of Alzheimer disease, stroke within the last 12 months, or certain cancers; current chemotherapy or radiation therapy; or poor vision, hearing, or communicative ability that would have interfered with the interventions or outcome assessments. A sample of 2,832 individuals (average age 73.6 years, average education 13 years, 74% white and 26% African American, and 76% women) were randomly assigned to one of three intervention groups (memory, reasoning, or speed-of-processing training) or a no-contact control group. Outcome assessments were conducted immediately following and at 1, 2, 3, 5, and 10 years after intervention. Study procedures were approved by institutional review boards at participating institutions, and all participants provided written informed consent.
Interventions
ACTIVE training focused on memory, reasoning, and speed-of-processing because prior research indicated that these abilities show early age-related decline and are related to activities of daily living. Training was conducted in small groups in ten 60-75 minute sessions over 5-6 weeks. Memory training focused on improving verbal episodic memory through instruction and practice in strategy use. Reasoning training focused on improving the ability to solve problems that contained a serial pattern. Speed-of-processing training focused on visual search and ability to process increasingly more complex information presented in successively shorter inspection times. Booster training (four 75-minute sessions) was provided at 11 and 35 months after training to a random subset (39%) of participants in each training group who completed at least 8 of 10 training sessions. Sixty percent of selected participants completed booster training at year 1 and year 3; 19% completed year 1 booster only; 6% completed year 3 booster only; and 15% did not complete any booster training. Sixty-one percent of the total sample (n=1694) was not selected to receive booster training.
Outcome Measures
Cognitive outcome measures assessed the effect of each cognitive training intervention on its targeted cognitive ability. Memory outcomes involved measures of episodic verbal memory: Rey Auditory-Verbal Learning Test (AVLT) total of five learning trials, the Hopkins Verbal Learning Test (HVLT) total of three learning trials, and the Rivermead Behavioural Paragraph Recall test immediate recall (11-13). Reasoning outcomes involved measures requiring identification of patterns including total correct for Letter Series (14), Letter Sets (15), and Word Series (16). Speed-of-processing outcomes involved three Useful Field of View (UFOV) tasks requiring identification and localization of information, with 75% accuracy, under varying levels of cognitive demand (17-19).
Functional outcomes assessed whether training-related cognitive improvements improved everyday function. There were three measure of daily function. The self-reported measure of Everyday IADL function was the IADL difficulty sub-score from the Minimum Dataset - Home Care (MDS-HC) which assesses performance in the past 7 days on 19 daily tasks spanning meal preparation, housework, finances, health care, telephone, shopping, travel, and need for assistance in dressing, personal hygiene, and bathing (20). Validity and clinical utility of the MDS scores have been established (21, 22). The two performance-based measures of daily function included Everyday Problem Solving, comprised of the Everyday Problems Test (EPT) (23) and Observed Tasks of Daily Living (OTDL) (24), and Everyday Speed, comprised of Complex Reaction Time (CRT) (25) and Timed IADL (TIADL) (26).
There were multiple measures of the cognitive and daily function outcomes. Because we were interested in training effects on an outcome such as memory function, rather than the effects on each single test of memory function, we created composite scores for each area of cognitive and daily function using the average of the standardized scores for each test in that composite measure (4,5,6).
Analysis
To evaluate the effects of ACTIVE training, an intention-to-treat analysis was conducted using a repeated-measures mixed-effects model (27) for each cognitive and daily function composite outcome. In these models, we included several design features and three interaction terms to measure the net effect of training and both the net effect and added effect of booster training. Time was treated as a categorical variable (baseline, 1, 2, 3, 5, 10 years). The following baseline measures also were included: age, sex, cognitive status (MMSE score), years of education, and visual acuity.
Training effects were assessed by comparing mean improvement from baseline to year 10 in each of the three training groups to mean improvement from baseline to year 10 in the non-trained control group. Effects of booster training were assessed similarly by comparing mean improvement from baseline to year 10 in subjects receiving booster training to mean improvement from baseline to year 10 in subjects who did not receive booster training. This comparison was made for each of the three cognitive interventions. The analyses were first performed using available data. Then we assessed the impact of missing data by repeating the analysis with multiple imputation (28, 29) and by conducting a sensitivity analysis that forced missing cognitive and daily function scores to be low. All statistical tests were two-sided. Analyses were conducted at the data coordinating center using R version 2.12.0 (30).
Results are presented as effect sizes which quantify the size of the difference between a training group and the control group and provide a way to compare this difference across the training groups (e.g., does reasoning training have a better effect than memory training on each cognitive and daily function outcome). Cohen describes an effect size of 0.2 as small, 0.5 as medium, and 0.8 as large (27). Because the analyses included 6 comparisons, we use a corrected significance level (31) of p< 0.008.
In addition, we investigated the percent of participants who were at or above their baseline performance level at 10 years after training (reliable change) using standard error of measurement (SEM) (32). A participant was classified as reliably at or above baseline level if their score at 10 years was within a 0.66 SEM confidence interval or more of the baseline score (33). For our purposes, this was considered maintenance of performance. For each training group, we compared the percent with reliable change on each cognitive and daily function outcome to that of the control group.
RESULTS
Sample Characteristics
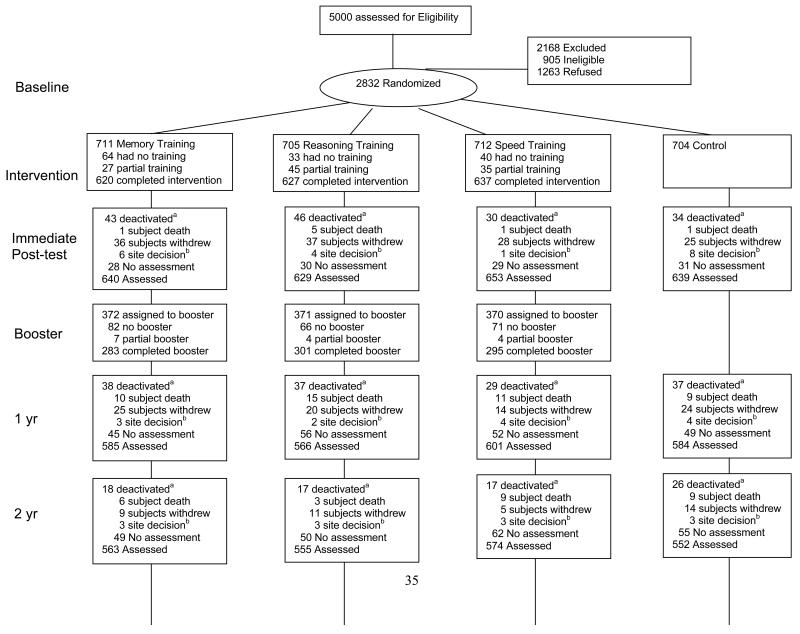
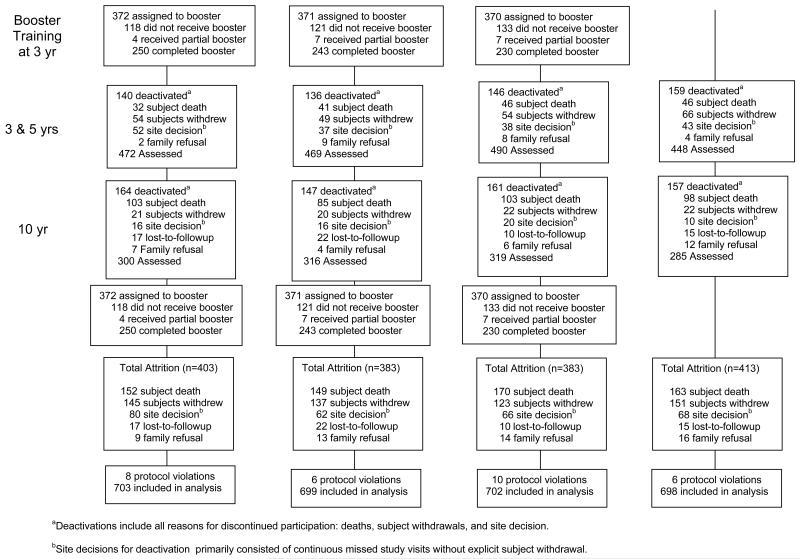
Of 5000 individuals contacted for participation, 2802 were randomized in accord with the protocol and comprise the analytical sample. Of those not randomized, about 41% were ineligible, 57% refused, and 1% were improperly randomized (FIGURE 1). Compared to refusers, participants were less likely to be women (76% vs. 79%), were younger (mean age 74 vs. 75 years), more likely to be white (73% vs. 60%), married (36% vs. 27%), and better educated (mean of 13.5 vs. 12.3 years). Participants had higher MMSE scores (mean 27.3 vs. 26.8) and were less likely to have heart disease (11% vs. 14%) and diabetes (13% vs. 17%) than were refusers.
Figure 1.
Profile of the ACTIVE trial
Baseline characteristics by intervention group appear in TABLE 1. Eighty-nine percent of participants completed the training intervention. Completers were younger, had more education, and had higher baseline MMSE and cognitive function scores.
Table 1.
Baseline Characteristics.
| Memory | Reasoning | Speed of Processing | Control | |
|---|---|---|---|---|
| (n=703) | (n=699) | (n=702) | (n=698) | |
| Age, mean (±SD) [range] | 73.5 (±6.0) [65-93] | 73.5 (±5.8) [65-91] | 73.4 (±5.8) [65-91] | 74.1 (±6.1)[65-94] |
| Female sex | 537 (±76.4) | 537 (±76.8) | 538 (±76.6) | 514 (±73.6) |
| Race | ||||
| White | 524 (±74.5) | 504 (±72.1) | 523 (±74.5) | 503 (±72.1) |
| Black | 176 (±25.0) | 190 (±27.2) | 175 (±24.9) | 187 (±26.8) |
| Other or unknown | 3 (±0.4) | 5 (±0.7) | 4 (±0.6) | 8 (±1.2) |
| Years of education, mean (±SD) [range] | 13.6 (±2.7) [5-20] | 13.5 (±2.7) [4-20] | 13.7 (±2.7) [5-20] | 13.4 (±2.7) [6-20] |
| Married | 257 (±36.6) | 249 (±35.6) | 242 (±34.5) | 259 (±37.1) |
| Mini-Mental State Examination score, mean (±SD) [range] |
27.3 (±2.1) [23-30] | 27.3 (±2.0) [23-30] | 27.4 (±2.0) [23-30] | 27.3 (±2.0) [23-30] |
| Short-Form 36 physical function score, mean (±SD) [range] |
69.1 (±23.5) [5-100] | 67.4 (±24.1) [5-100] | 69.7 (±24.1) [0-100] | 68.9 (±24.6) [5-100] |
| Alcohol consumption † | ||||
| Nondrinker | 298 (±43) | 302 (±43) | 295 (±42) | 350 (±51) |
| Light drinker | 341 (±49) | 347 (±50) | 362 (±52) | 313 (±45) |
| Heavy drinker | 60 (±8) | 46 (±7) | 42 (±6) | 30 (±4) |
| Center for Epidemiologic Studies Depression | ||||
| Scale score, mean (±SD) [range] | 5.1 (±5.3) [0-36] | 5.5 (±5.3) [0-36] | 5.2 (±5.0) [0-36] | 5.1 (±4.9) [0-36] |
| Disease history | ||||
| Hypertension | 372 (±53.1) | 369 (±53.2) | 350 (±50.1) | 337 (±48.8) |
| Diabetes | 95 (±13.5) | 99 (±14.2) | 87 (±12.4) | 77 (±11) |
| Transient ischemic attack or stroke | 46 (±6.6) | 54 (±7.8) | 51 (±7.3) | 44 (±6.3) |
| Ischemic heart disease | 108 (±15.5) | 117 (±17) | 94 (±13.5) | 102 (±14.7) |
| Congestive heart failure | 30 (±4.3) | 44 (±6.4) | 27 (±3.9) | 37 (±5.4) |
| High cholesterol | 309 (±44.6) | 316 (±46.4) | 305 (±44.3) | 296 (±43.1) |
| Myocardial infarction | 79 (±11.3) | 78 (±11.2) | 76 (±10.9) | 76 (±10.9) |
Data presented as N(%) unless otherwise indicated.
Based on frequency of drinking alcohol and number of drinks on a typical day when drinking.
Sixty-seven percent of the sample was retained 5 years after training, and 44% were retained at 10 years. Death (40%) was the primary reason for non-participation at 10 years, followed by the participant’s decision to withdraw (35%) and site’s decision to withdraw the participant due to continued missed visits in the absence of explicit refusal (17%). Predictors of attrition at 10 years include older age, male gender, non-married, higher alcohol consumption, more physical and mental health problems, and worse performance on cognitive outcomes. Attrition rates and predictors of attrition were similar across intervention groups.
Training Effects on Cognitive Abilities
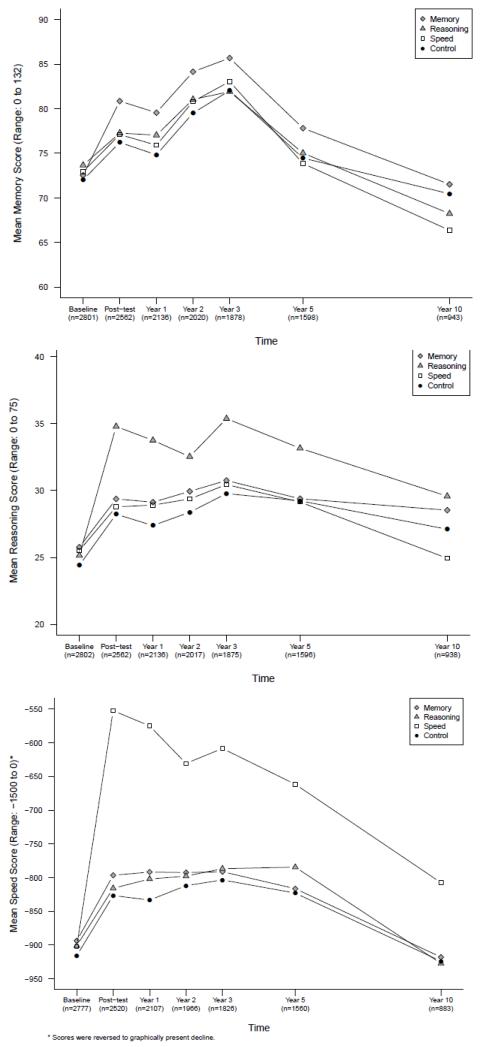
Data in TABLE 2 report the mean scores at baseline and change from baseline to year 10 as well as the effect size of the intervention on each cognitive outcome. All interventions produced immediate improvement in the trained cognitive ability (6) (FIGURE 2). This improvement was retained for 10 years in the reasoning and speed trained groups (TABLE 2). The effect sizes (shaded in TABLE 2) indicate a small effect of the reasoning intervention (0.23) on the reasoning outcome and a medium-to-large effect of the speed intervention (0.66) on the speed outcome at 10 years. The effect of the memory intervention (0.06) on the memory outcome at 10 years was not significant. Similarly, there were significant effects of booster training for the reasoning (effect size=0.21, CI: 0.01, 0.41) and speed (effect size = 0.62, 99% CI: 0.31, 0.93) interventions but not for the memory intervention.
Table 2. Effect of Training on Cognitive and Functional Outcomes From Baseline to Year 10.
| Intervention Groups | ||||
|---|---|---|---|---|
| Memory | Reasoning | Speed | Control Group | |
| Memory (possible range: 0 to 132, N=943) | ||||
|
| ||||
| Score at baseline, mean (±SD) | 82.1 (±25.7) | 79.5 (±26.3) | 79.1 (±25.5) | 79.8 (±27.3) |
|
| ||||
| Mean change from baseline to year 10 | −10.6 | −11.2 | −12.7 | −9.4 |
|
| ||||
| Effect size (99% CI)* | 0.06 (−0.14,0.27) | −0.11 (−0.31,0.10) | −0.05 (−0.25,0.15) | |
|
| ||||
| % at or above baseline level § | 35.9% | 28.6% | 31.0% | 31.0% |
|
| ||||
| Reasoning (possible range: 0 to 75, N=938) | ||||
|
| ||||
| Score at baseline, mean (±SD) | 31.8 (±11.7) | 29.6 (±12.3) | 28.9 (±12.0) | 30.2 (±12.8) |
|
| ||||
| Mean change from baseline to year 10 | −3.2 | −0.05 | −3.9 | −3.0 |
|
| ||||
| Effect size (99% CI)* | −0.02 (−0.17,0.12) | 0.23 (0.09,0.38) | −0.06 (−0.20,0.08) | |
|
| ||||
| % at or above baseline level § | 60.0% | 73.6% (p<.01) | 59.3% | 61.7% |
|
| ||||
| Speed of Processing (possible range: 0 to 1500, N=883) | ||||
| Score at baseline, mean (±SD) | 774.1 (±216.9) | 800.9 (±231.0) | 830.0 (±231.9) | 800.6 (±231.8) |
|
| ||||
| Mean change from baseline to year 10 | −144.4 | −126.2 | 24.3 | −123.3 |
|
| ||||
| Effect size (99% CI)* | −0.07 (−0.29,0.16) | 0.005 (−0.22,0.23) | 0.66 (0.43,0.88) | |
|
| ||||
| % at or above baseline level § | 47.2% | 48.5% | 70.7% (p<.01) | 47.8% |
|
| ||||
| IADL difficulty (possible range: 0 to 38**, N=1211) | ||||
|
| ||||
| Score at baseline, mean (±SD) | 1.0 (±1.8) | 1.2 (±2.0) | 1.1 (±2.0) | 0.9 (±2.1) |
|
| ||||
| Mean change from baseline to year 10 | −3.1 | −2.7 | −2.3 | −3.6 |
|
| ||||
| Effect size (99% CI)* | 0.48 (0.12,0.84) | 0.38 (0.02,0.74) | 0.36 (0.01,0.72) | |
|
| ||||
| % at or above baseline level § | 61.6% (p<.01) | 60.2% (p<.01) | 58.5% (p<.05) | 49.3% |
|
| ||||
| Everyday problem solving (possible range: 0 to 56, N=1104) | ||||
|
| ||||
| Score at baseline, mean (±SD) | 40.7 (±7.7) | 39.2 (±8.1) | 38.7 (±7.7) | 39.4 (±9.1) |
|
| ||||
| Mean change from baseline to year 10 | −6.1 | −5.6 | −6.0 | −5.7 |
|
| ||||
| Effect size (99% CI)* | 0.004 (−0.23,0.24) | −0.02 (−0.25,0.22) | 0.008 (−0.23,0.24) | |
|
| ||||
| % at or above baseline level § | 59.6% | 63.1% | 61.0% | 61.4% |
|
| ||||
| Everyday speed of processing (possible range: −3 to 100, N=938)+ | ||||
|
| ||||
| Score at baseline, mean (±SD) | 3.2 (±1.0) | 3.3 (±1.2) | 3.4 (±1.3) | 3.4 (±1.1) |
|
| ||||
| Mean change from baseline to year 10 | −1.5 | −1.4 | −1.5 | −1.4 |
|
| ||||
| Effect size (99% CI)* | 0.02 (−0.19,0.23) | −0.004 (−0.21,0.21) | −0.05 (−0.26,0.16) | |
|
| ||||
| % at or above baseline level § | 34.9% | 30.5% | 29.0% | 30.2% |
Abbreviations: CI, confidence interval; SD, standard deviation; IADL, instrumental activities of daily living.
Effect size defined as training improvement from baseline to year 10 minus control improvement from baseline to year 10 divided by the intrasubject SD of the composite score. Positive effect sizes indicate improvement.
Coded as 0=no difficulty; 1=some help needed or participant is slow or becomes tired’; 2=great difficulty
One component of this composite score is a standardized z score with a potential range of −∞to ∞.
Calculated as the percentage of participants in each group who were ≥0.66 SEM above baseline.
Figure 2. Cognitive Outcomes by Time and Training Group.
The figures displays mean scores for the three cognitive outcomes - memory (panel A), reasoning (panel B), speed-of-processing (panel C) - for each training group at each time point. Higher scores indicate better performance. The sample sizes show the number of participants with complete data for each cognitive outcome at each time point.
Results of the analyses of reliable maintenance of cognitive function at 10 years (TABLE 2) show that 73.6% of reasoning-trained participants and 70.7% of speed-trained participants were performing at or above their respective cognitive ability compared to 61.7% and 48.8% respectively of control participants (p<.01). The results for memory-trained participants were not significant.
Training Effects on Daily Function
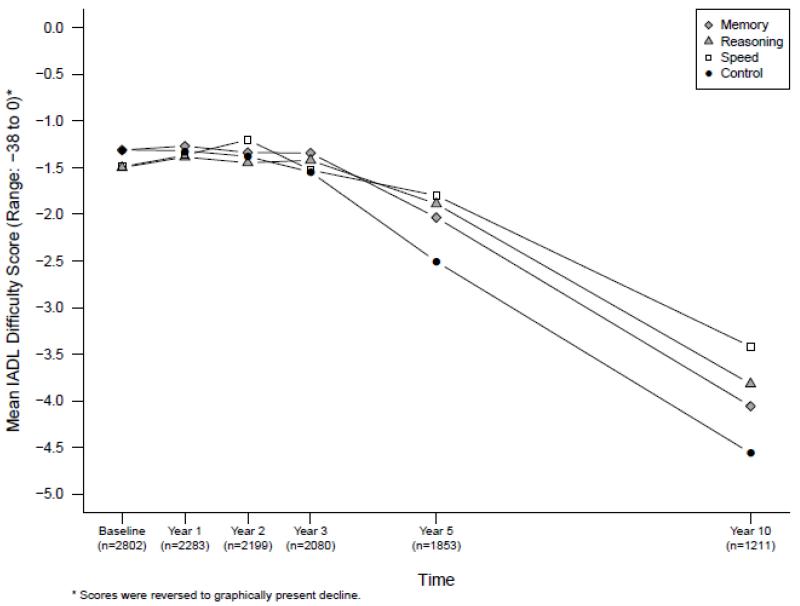
At year 10, participants in all three intervention groups reported less difficulty in performing IADL activities than did participants in the control group (TABLE 2, FIGURE 3). The effects of the interventions (shaded in Table 2) were small to medium (i.e., 0.48 for memory, 0.38 for reasoning and 0.36 for speed). As displayed in FIGURE 3, self-reported IADL function improved through 2 years. Then functional decline is first evident between years 2 and 3 for all groups. From years 3 to 5, the decline is less in the three intervention groups than in the control group. This difference in self-reported IADL function between trained participants and the non-trained control participants is then maintained as all participants continue to decline (i.e. report more IADL difficulties) from years 5 to 10.
Figure 3.
Training effects on self-reported Instrumental Activities of Daily Living (IADL) difficulty scores.
The figure displays mean IADL difficulty scores for each training group at each time point. Higher scores indicate better functioning. The sample sizes show the number of participants with complete data for the IADL difficulty score at each time point.
Results of the reliable maintenance analysis (TABLE 2) are consistent with this pattern of temporal decline. Whereas at 10 years half (49.3%) of control participants reported the same or improved level of IADL difficulty as at baseline, the proportions of trained participants reporting the same or improved level of IADL difficulty were significantly higher (Memory: 61.6%, p<.01; Reasoning: 60.2%, p<.01; Speed: 59.5%, p<.05). There was no effect of training (TABLE 2) or added booster training (not shown) on the performance-based measures of everyday function. Finally, the results of models using multiple imputation for missing data as well as results of the sensitivity analysis (data not shown) were the same as the main results reported above.
DISCUSSION
In the ACTIVE trial, 10-14 weeks of organized cognitive training delivered to community-dwelling older adults resulted in significant improvements in cognitive abilities and better preserved functional status compared to non-trained persons 10 years later. Each training intervention produced large and significant improvements in the trained cognitive ability. These improvements dissipated slowly but persisted to at least 5 years for memory training and to 10 years for reasoning and speed-of-processing training. This is the first demonstration of long-term transfer of the training effects on cognitive abilities to daily function.
Compared to non-trained participants, cognitive function for the majority of the reasoning and speed-trained participants was at or above their baseline level for the trained cognitive ability, 10 years later. A significant percent of participants in all trained groups (at least 60%) continued to report less difficulty performing IADLs compared to non-trained participants (49%). After 10 years, 60-70% of participants were as well or better off than when they started.
The absence of long-term memory training effects has been reported by others (34). It is possible that the memory training used in ACTIVE requires more extensive practice or dosing to reach durability levels comparable to reasoning and speed training. It is also possible that the durability of memory training is limited in older adults due to age-related structural changes in the medial temporal lobe, including age-related neuropathology and even incipient Alzheimer disease in some participants (35, 36).
There are a number of possible reasons for the finding that training effects on self-reported daily function are maintained over time while the training effects on cognitive abilities dissipate over time. First, this could reflect a cascade relationship between cognitive ability and daily function. Prospective observational studies indicate that changes in cognition precede changes in daily function by several years (37). Second, improved cognitive processing may alter patterns of neural activation over the long-term (38, 39). Third, training-based improvements in cognitive abilities may produce changes in behavior and social interaction that promote broad-based engagement in functional activities and maintenance over many years.
The effects of cognitive training on daily function in this study were modest. This is likely due to the fact that many factors beyond cognition affect daily function and functional independence, including gender, social class, mood, sarcopenia, obesity, chronic diseases, and social isolation to name a few (40, 41). Even within the cognitive realm, some domains like general cognitive status and executive cognitive ability may be more closely related to daily function than other domains (e.g., spatial skills) (42, 43).
Our study showed weak to absent effects of cognitive training on performance-based measures of daily function. It is probably a mistake to conceive of these performance-based functional measures as something other than cognitive tests. The administration formats, task demands, and scoring all have more in common with standard cognitive tests than with actual acts of daily living. In addition, these performance-based measures call on multiple cognitive skills. A main lesson of the ACTIVE study and other cognitive intervention trials is that the benefits of cognitive training are specific to the cognitive ability trained. Viewed in this way, it is not surprising that the specific forms of cognitive training used in ACTIVE did not result in improvements on performance-based measures of daily function that are really multi-ability cognitive tests.
The ACTIVE 10-year retention rate was 44%. Death was the primary reason for non-participation (40%), followed by the subject’s decision to stop participation (35%) and the site’s decision to withdraw the subject (17%). In comparison, the Diabetes Prevention Program (DPPP) reported a 10-year retention rate of 59% (44). However, DPPP participants were more than 20 years younger (50.6 yrs) at enrollment than were ACTIVE participants at enrollment (73.0 yrs). Our 10-year retention rate compares favorably with rates in observational studies of similar duration and samples of similar ages and ethnic diversity (45, 46). While retained subjects were younger and had fewer physical and mental health problems at baseline, there was no difference across groups in attrition. This means that the training effects we observed are not an artifact of differential attrition. Further, in recognition of this attrition, we used appropriate methods to test our assumptions about the missing data and the validity of our inferences. First, the linear mixed-effects models are appropriate for situations with informative missingness and informative censoring (47). In addition, we analyzed the effect of missing data on the outcomes with both multiple imputation and a sensitivity analysis that assumed missing outcome scores to be low. Results of the analysis using multiple imputation and the sensitivity analysis were similar to the results of the mixed effects models. Therefore, our results regarding the effects of cognitive training interventions are likely robust.
We note that the evaluation of the effect of booster training is limited because the two groups of interest (booster trained and non-booster trained) are not comparable. In order to be eligible for selection for booster training, participants had to have completed at least 80% of baseline training. In contrast, only 20% of non-booster trained participants completed baseline training. Therefore, the non-booster trained group is overrepresented by persons who did not complete baseline training, and reflects neither participants who completed baseline training nor non-trained participants (i.e., the control group) but something in between.
In summary, ACTIVE was the first multi-site clinical trial to test the effects of cognitive training interventions on cognitive abilities and daily function. Results at 10 years demonstrate that cognitive training has beneficial effects on cognitive abilities and on self-reported IADL function. These results provide support for the development of other interventions, particularly those that target multiple cognitive abilities and are more likely to have an effect on IADL performance. Such interventions hold the potential to delay onset of functional decline and possibly dementia and are consistent with comprehensive geriatric care that strives to maintain and support functional independence. If interventions that could delay onset of functional impairment by even 6 years were introduced, the number of people affected by 2050 would be reduced by 38 percent (48) which would be of great public health significance.
ACKNOWLEDGEMENTS
The principal investigators thank the following NIH project officers who were at their respective Institutes during some or all of the project period: Jared Jobe, Daniel Berch, Jeffrey Elias, Sidney Stahl, and Jonathan King of the National Institute on Aging, and Taylor Harden, Karin Helmers, Mary Leveck, Nell Armstrong, Kathy Koepke, and Susan Marden of the National Institute of Nursing Research. We also thank the ACTIVE participants and the research staff at each field site and the data coordinating center.
Funding/Support: ACTIVE is supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew SeniorLife (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), the University of Alabama at Birmingham (U01 AG14289), and the University of Florida (U01AG14276). Drs. Jones and Morris were also supported in part by the Edward Fein Foundation (Nevada) and through the generosity of Vicki and Arthur Loring (Massachusetts). Representatives of NIA and NINR were involved in the design of the study, interpretation of the data, and preparation, review, and approval of the manuscript.
Role of the Sponsor: Representatives of the National Institute on Aging and the National Institute of Nursing Research were directly involved in the design of the study, interpretation of the data, and preparation, review, and approval of the manuscript. These representatives also monitored the conduct of the study, collection, management, and analysis of the data.
APPENDIX
Conflict of Interest
| Elements of Financial/Personal Conflicts |
*Author 1 (GWR) |
Author 2 (KB) |
Author 3 (LTG) |
Author 4 (RNJ) |
Author 5 (HYK) |
Author 6 (JK) |
Author 7 (MM) |
Author 8 (JNM) |
Author 9 (SLT) |
Author 10 (FWU) |
Author 11 (SLW) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Honoraria | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Consultant | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Stocks | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Royalties | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Board Member | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Patents | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Other | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
Footnotes
Financial Disclosures:
Dr. Unverzagt has received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for investigator-initiated research projects.
Dr. Marsiske has received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for investigator-initiated research projects. Dr. Marsiske has received research support from Robert Wood Johnson Foundation and McKnight Brain Research Foundation. Dr. Marsiske has received payment for development of education presentations from the National Academy of Neuropsychology and the International Neuropsychological Society for workshops on cognitive interventions. Dr. Marsiske has received payment for development of education presentations from the National Institute on Aging and American Society on Aging for overview presentation on cognitive interventions.
Dr. Ball is a consultant and owns stock in the Visual Awareness Research Group and Posit Science, Inc., the companies that market the Useful Field of View Test (UFOV®) and speed of processing training software now called Insight (the Visual Awareness Research Group invented Insight and the UFOV®). Dr. Ball serves as a member of the Posit Science Scientific Advisory Board. Posit Science paid royalties to the Visual Awareness Research Group (unrelated to the study described). The Visual Awareness Research Group is an S Corp; all profits and losses flow to stockholders.
Dr. Rebok is an investigator with Compact Disc Incorporated for the development of an electronic version of the ACTIVE memory intervention.
Drs. Morris and Jones received support from the Edward Fein Foundation and Vicki and Arthur Loring for research activities.
The views expressed in this article are those of the authors and not to be ascribed to the National Institute on Aging, National Institute of Nursing Research or the Department of Health and Human Services.
Author Contributions: Drs. Guey and Kim had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rebok, Ball, Jones, Marsiske, Morris, Tennstedt, Unverzagt, Willis.
Acquisition of data: Rebok, Ball, Marsiske, Morris, Unverzagt, Willis
Analysis and interpretation of data: Rebok, Ball, Jones, King, Marsiske, Morris, Tennstedt, Unverzagt, Willis, Guey, Kim.
Drafting of the manuscript: Rebok, Ball, Jones, King, Marsiske, Tennstedt, Unverzagt, Willis, Guey.
Critical revision of the manuscript for important intellectual content: Rebok, Ball, Jones, King, Marsiske, Tennstedt, Unverzagt, Willis, Guey, Kim.
Statistical analysis: Jones, Marsiske, Guey, Kim
Obtained funding: Rebok, Ball, Marsiske, Morris, Tennstedt, Unverzagt, Willis.
Administrative, technical, or material support: Rebok, Ball, Jones, King, Marsiske, Morris, Tennstedt, Unverzagt, Willis, Guey, Kim.
Study supervision: Rebok, Ball, Jones, Marsiske, Morris, Tennstedt, Unverzagt, Willis.
Trial Registration: clinicaltrials.gov Identifier: NCT00298558
ACTIVE Study Investigators: In addition to the principal investigators and program officers, the following persons participated in the ACTIVE study: Hebrew SeniorLife - Adrienne L. Rosenberg MS; Indiana University School of Medicine - Daniel F. Rexroth PsyD., David M. Smith MD, Lyndsi Moser CCRP, Fredric D. Wolinsky PhD; Johns Hopkins University - Jason Brandt, PhD, Kay Cresci PhD, RN, Joseph Gallo MD, MPH, Laura Talbot PhD, EdD, RN, CS; New England Research Institutes (Data Coordinating Center) - Kathleen Cannon BS, Michael Doherty MS, Henry Feldman PhD, Patricia Forde BS, Nancy Gee MPH, Eric Hartung EdD, Linda Kasten MS, Ken Kleinman ScD, Herman Mitchell PhD, George Reed PhD, Anne Stoddard ScD, Yan Xu MS, Elizabeth Wright PhD; Pennsylvania State University – Pamela Davis MS, Scott Hofer PhD, K. Warner Schaie PhD; University of Alabama at Birmingham - Jerri Edwards PhD, Martha Frankel, Cynthia Owsley PhD, Dan Roenker PhD, David Vance PhD, Virginia Wadley PhD; University of Florida / Wayne State University - Manfred K. Diehl, PhD, Ann L. Horgas, RN, PhD, FAAN, Peter A. Lichtenberg, PhD, ABPP
Contributor Information
Dr. George W. Rebok, Department of Mental Health and Johns Hopkins Center on Aging and Health, Johns Hopkins University, Hampton House 891, 624 North Broadway, Baltimore, MD 21205.
Dr. Karlene Ball, Department of Psychology, University of Alabama at Birmingham, 1300 University Blvd, Birmingham, AL 35294.
Dr. Lin T. Guey, Shire HGT, Lexington, MA 02420.
Dr. Richard N. Jones, Social and Health Policy Research, Hebrew SeniorLife, 1200 Centre Street, Boston MA 02131.
Dr. Hae-Young Kim, New England Research Institutes, 9 Galen Street, Watertown, MA 02472.
Dr. Jonathan W. King, Division of Behavioral and Social Research, National Institute on Aging, Bethesda, MD 20892.
Dr. Michael Marsiske, Institute on Aging and Department of Clinical and Health Psychology, University of Florida, PO Box 100165 HSC, Gainesville, FL 32610.
Dr. John N. Morris, Hebrew SeniorLife, 1200 Centre Street, Boston MA 02131.
Dr. Sharon L. Tennstedt, New England Research Institutes, 9 Galen Street, Watertown, MA 02472.
Dr. Frederick W. Unverzagt, Department of Psychiatry, Indiana University School of Medicine, 1111 W. 10th Street, RM Suite PB 218A, Indianapolis, IN 46202.
Dr. Sherry L. Willis, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195.
REFERENCES
- 1.Hertzog C, Kramer A, Wilson R, et al. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol Sci. 2008;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 2.Rebok G. State-of-Science Review: SR:E22 UK. Government Foresight Mental Capital and Mental Wellbeing Project. Government Office for Science; 2008. Cognitive training: influence on neuropsychological and brain function in later life. [Google Scholar]
- 3.Pappa K, Walsh S, Snyder P. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement. 2009;5(1):50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Controlled clinical trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazaridis EN, Rudberg MA, Furner SE, et al. Do activities of daily living have a hierarchical structure? An analysis using the longitudinal study of aging. Journal of gerontology. 1994;49(2):M47–51. doi: 10.1093/geronj/49.2.m47. [DOI] [PubMed] [Google Scholar]
- 8.Wolinsky F, Miller D. Disability concepts and measurement: contributions of the epidemiology of disability to gerontological inquiry. In: Wilmoth J, Ferraro K, editors. Gerontology: Perspectives and issues. Springer Publishing; New York, New York: 2006. [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Morris J, Morris S. ADL Assessment Measures for Use with Frail Elders. In: Teresi J, Lawton M, Holmes D, et al., editors. Measurement in elderly chronic care populations. Springer Publishing Co; New York, NY: 1997. [Google Scholar]
- 11.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- 12.Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. (Les problems.). / The psychological examination in cases of traumatic encepholopathy. Problems. Archives de Psychologie. 1941;28:215–285. [Google Scholar]
- 13.Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Thames Valley Test Company; 1985. 34 The Square, Titchfield, Fareham, Hampshire PO14 4AF. [Google Scholar]
- 14.Thurstone L, Thurstone T. Examiner Manual for the SRA Primary Mental Abilities Test (Form 10-14) Science Research Associates; Chicago: 1949. [Google Scholar]
- 15.Ekstrom R, French J, Harman H, et al. Kit of Factor-Referenced Cognitive Tests (Rev. ed.) Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- 16.Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Consulting Psychologists Press; Palo Alto, CA: 1985. [Google Scholar]
- 17.Owsley C, Ball K, Sloane ME, et al. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and aging. 1991;6(3):403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- 18.Owsley C, Ball K, McGwin G, Jr., et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 19.Ball KK, Beard BL, Roenker DL, et al. Age and visual search: expanding the useful field of view. Journal of the Optical Society of America. 1988;5(12):2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 20.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. Journal of the American Geriatrics Society. 1997;45(8):1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 21.Landi F, Tua E, Onder G, et al. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Medical care. 2000;38(12):1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hirdes JP, Fries BE, Morris JN, et al. Home care quality indicators (HCQIs) based on the MDS-HC. The Gerontologist. 2004;44(5):665–679. doi: 10.1093/geront/44.5.665. [DOI] [PubMed] [Google Scholar]
- 23.Willis S, Marsiske M. Manual for the Everyday Problems Test. Pennsylvania State University; University Park, PA: 1993. [Google Scholar]
- 24.Diehl M, Marsiske M, Horgas AL, et al. The Revised Observed Tasks of Daily Living: A performance-based assessment of everyday problem solving in older adults. J Appl Gerontol. 2005;24(3):211–230. doi: 10.1177/0733464804273772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball K. Increased mobility and reducing accidents of older drivers. In: Schaie K, Pietrucha M, editors. Mobility and Transportation in the Elderly. Springer; New York, NY: 2000. [Google Scholar]
- 26.Owsley C, Sloane M, McGwin G, Jr., et al. Timed instrumental activities of daily living tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48(4):254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- 27.Brown H, Prescott R. Applied Mixed Models in medicine. 2nd Ed. John Wiley; Chichester England; Hoboken NJ: 2006. [Google Scholar]
- 28.Schafer J. Analysis of Incomplete Multivariate Data. Chapman & Hall; London: 1997. [Google Scholar]
- 29.van-Buuren S, Oudshoorn C. The mice Package: Multivariate Imputation by Chained Equations. 2007. [Google Scholar]
- 30.Team RDC . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- 31.Abdi H, editor. Bonferroni and Sidák corrections for multiple comparisons. Sage; Thousand Oaks, CA: 2007. [Google Scholar]
- 32.Dudek F. The continuing misinterpretation of the standard error of measurement. Psychological Bulletin. 1979;86(2):335–337. [Google Scholar]
- 33.Garrett H. Statistics in psychology and education. Longsman; New York: 1937. [Google Scholar]
- 34.Scogin F, Bienias JL. A three-year follow-up of older adult participants in a memory-skills training program. Psychology and aging. 1988;3(4):334–337. doi: 10.1037//0882-7974.3.4.334. [DOI] [PubMed] [Google Scholar]
- 35.Singer T, Lindenberger U, Baltes PB. Plasticity of memory for new learning in very old age: a story of major loss? Psychology and aging. 2003;18(2):306–317. doi: 10.1037/0882-7974.18.2.306. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaie K. Developmental influences on adult intellectual development: The Seattle Longitudinal Study. Oxford University Press; New York: 2005. [Google Scholar]
- 38.Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15(8):1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- 39.May A, Hajak G, Ganssbauer S, et al. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17(1):205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- 40.Beland F, Zunzunegui MV. Predictors of functional status in older people living at home. Age and ageing. 1999 Mar;28(2):153–159. doi: 10.1093/ageing/28.2.153. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obesity research. 2004 Dec;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 42.Royall D, Lauterbach E, Kaufer D, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 43.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007 Oct;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson MC, Xue QL, Zhou J, et al. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. The journals of gerontology. 2009;64(1):110–117. doi: 10.1093/gerona/gln008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S, Thiébaut R. Mixed-effect models for truncated longitudinal outcomes with nonignorable missing data. J Data Sci. 2009;7(1):27–42. [Google Scholar]
- 47.Park S, Palta M, Shao J, et al. Bias adjustment in analysing longitudinal data with informative missingness. Statistics in medicine. 2002 Jan 30;21(2):277–291. doi: 10.1002/sim.992. [DOI] [PubMed] [Google Scholar]
- 48.Sloane PD, Zimmerman S, Suchindran C, et al. The public health impact of Alzheimer’s disease, 2000-2050: potential implication of treatment advances. Annual review of public health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]