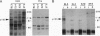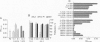Abstract
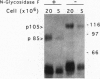
Hemopoietic stem cells are a distinct population of cells that can differentiate into multilineages of hemopoietic cells and have long-term repopulation capability. A few membrane-bound molecules have been found to be preferentially, but not uniquely, present on the surface of these primitive cells. We report here the identification of a unique 105-kDa glycoprotein on the surface of hemopoietic stem cell line BL3. This molecule, recognized by the absorbed antiserum, is not present on the surface of myeloid progenitors 32D and FDC-P1 cells, EL4 T cells, and NIH 3T3 fibroblasts. This antiserum can also be used to block the proliferation of BL3 cells even in the presence of mitogen-stimulated spleen cell conditioned medium, which is known to have a stimulating activity on BL3 cells. It can also inhibit development of in vitro, fetal liver cell-derived multilineage colonies, but not other types of colonies, and of in vivo bone marrow cell-derived colony-forming unit spleen foci. These data suggest that gp105 plays an important role in hemopoietic stem cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brecher G., Bookstein N., Redfearn W., Necas E., Pallavicini M. G., Cronkite E. P. Self-renewal of the long-term repopulating stem cell. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6028–6031. doi: 10.1073/pnas.90.13.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briddell R. A., Broudy V. C., Bruno E., Brandt J. E., Srour E. F., Hoffman R. Further phenotypic characterization and isolation of human hematopoietic progenitor cells using a monoclonal antibody to the c-kit receptor. Blood. 1992 Jun 15;79(12):3159–3167. [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. W., Wong P. M., Durkin H., Wu Y. S., Petersen J. Leukemia initiated by hemopoietic stem cells expressing the v-abl oncogene. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1585–1589. doi: 10.1073/pnas.88.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Scott D., Teich N. M. Isolation and characterisation of a bipotential haematopoietic cell line. Nature. 1979 Feb 8;277(5696):471–474. doi: 10.1038/277471a0. [DOI] [PubMed] [Google Scholar]
- Fackler M. J., Civin C. I., May W. S. Up-regulation of surface CD34 is associated with protein kinase C-mediated hyperphosphorylation of CD34. J Biol Chem. 1992 Sep 5;267(25):17540–17546. [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Weissman I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Krause D. S., Ito T., Fackler M. J., Smith O. M., Collector M. I., Sharkis S. J., May W. S. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994 Aug 1;84(3):691–701. [PubMed] [Google Scholar]
- Lyman S. D., James L., Vanden Bos T., de Vries P., Brasel K., Gliniak B., Hollingsworth L. T., Picha K. S., McKenna H. J., Splett R. R. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993 Dec 17;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- Lyman S. D., James L., Zappone J., Sleath P. R., Beckmann M. P., Bird T. Characterization of the protein encoded by the flt3 (flk2) receptor-like tyrosine kinase gene. Oncogene. 1993 Apr;8(4):815–822. [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991 Jun 28;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Baum C., Davie J. M. Cell surface antigens expressed by subsets of pre-B cells and B cells. J Immunol. 1984 Jan;132(1):332–339. [PubMed] [Google Scholar]
- McKearn J. P., McCubrey J., Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Anthony K., Litwin S. Monoclonal derivation of mouse myeloid and lymphoid lineages from totipotent hematopoietic stem cells experimentally engrafted in fetal hosts. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7835–7839. doi: 10.1073/pnas.81.24.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Matsuzaki Y., Nishikawa S., Hayashi S., Kunisada T., Sudo T., Kina T., Nakauchi H., Nishikawa S. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991 Jul 1;174(1):63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Nakauchi H., Nagayoshi K., Nishikawa S., Miura Y., Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992 Dec 15;80(12):3044–3050. [PubMed] [Google Scholar]
- Orlic D., Fischer R., Nishikawa S., Nienhuis A. W., Bodine D. M. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993 Aug 1;82(3):762–770. [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Broudy V. C., Zsebo K. M. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood. 1991 Sep 15;78(6):1403–1412. [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Ray P., Higgins K. M., Tan J. C., Chu T. Y., Yee N. S., Nguyen H., Lacy E., Besmer P. Ectopic expression of a c-kitW42 minigene in transgenic mice: recapitulation of W phenotypes and evidence for c-kit function in melanoblast progenitors. Genes Dev. 1991 Dec;5(12A):2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- Reith A. D., Rottapel R., Giddens E., Brady C., Forrester L., Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990 Mar;4(3):390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Rosnet O., Marchetto S., deLapeyriere O., Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991 Sep;6(9):1641–1650. [PubMed] [Google Scholar]
- Shigeno N., Hämmerling U., Arpels C., Boyse E. A., Old L. J. Preparation of lymphocyte-specific antibody from anti-lymphocyte serum. Lancet. 1968 Aug 10;2(7563):320–323. doi: 10.1016/s0140-6736(68)90530-8. [DOI] [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Uchida N., Aguila H. L., Fleming W. H., Jerabek L., Weissman I. L. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1lo Lin-Sca-1+ hematopoietic stem cells. Blood. 1994 Jun 15;83(12):3758–3779. [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Chui D. H., Eaves C. J. Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3851–3854. doi: 10.1073/pnas.83.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Han X. D., Ruscetti F. W., Chung S. W. Immortalized hemopoietic cells with stem cell properties. Immunity. 1994 Oct;1(7):571–583. doi: 10.1016/1074-7613(94)90047-7. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Heimfeld S., Spangrude G. J., Weissman I. L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]