Abstract
Fundamental knowledge gaps in relation to the 3 epimer of 25-hydroxycholecalciferol [3-epi-25(OH)D3] limit our understanding of its relevance for vitamin D nutrition and health. The aims of this study were to characterize the 3-epi-25(OH)D3 concentrations in a nationally representative sample of adults and explore its determinants. We also used data from a recent randomized controlled trial (RCT) of supplemental cholecalciferol (vitamin D3) conducted in winter in older adults to directly test the impact of changes in vitamin D status on serum 3-epi-25(OH)D3 concentrations. Serum 25-hydroxycholecalciferol [25(OH)D3] and 3-epi-25(OH)D3 concentrations (via LC-tandem mass spectrometry) from our vitamin D3 RCT in adults (aged ≥50 y) and data on dietary, lifestyle, and biochemical characteristics of participants of the recent National Adult Nutrition Survey in Ireland (aged 18–84 y; n = 1122) were used in the present work. In the subsample of participants who had serum 3-epi-25(OH)D3 concentrations greater than the limit of quantification (n = 1082; 96.4%), the mean, 10th, 50th (median), and 90th percentile concentrations were 2.50, 1.05, 2.18, and 4.30 nmol/L, respectively, whereas the maximum 3-epi-25(OH)D3 concentration was 15.0 nmol/L. A regression model [explaining 29.9% of the variability in serum 3-epi-25(OH)D3] showed that age >50 y, vitamin D supplement use, dietary vitamin D, meat intake, season of blood sampling, and sun exposure habits were significant positive determinants, whereas increasing waist circumference and serum 25-hydroxyergocalciferol concentration were significant negative determinants. The RCT data showed that mean serum 25(OH)D3 and 3-epi-25(OH)D3 concentrations increased (49.3% and 42.1%, respectively) and decreased (−28.0% and −29.1%, respectively) significantly (P < 0.0001) with vitamin D3 (20 μg/d) and placebo supplementation, respectively, over 15 wk of winter. In conclusion, we provide data on serum 3-epi-25(OH)D3 in a nationally representative sample of adults. Our combined observational and RCT data might suggest that both dietary supply and dermal synthesis of vitamin D3 contribute to serum 3-epi-25(OH)D3 concentration. This trial was registered at clinicaltrials.gov as NCT01990872.
Introduction
Synthesis in the skin on exposure to UVB sunshine and dietary sources (food and supplements) are the main sources of vitamin D for humans (1). The metabolic pathway of this endogenously and exogenously derived vitamin D through its various hydroxylation and oxidation events was described comprehensively previously (1–3). Recently, it was discovered that vitamin D can alternatively be metabolized through a 3 epimerization pathway that parallels the standard metabolic pathway (4, 5) and thus accounts for the presence of epimerized vitamin D metabolites in human sera.
Although it was suggested that the 3 epimer of 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D3) possesses some but not all of the physiologic functionality of 1,25-dihydroxyvitamin D3 per se (6–9), there is a general consensus that additional priority research is necessary to establish the physiologic function of 3 epimers of vitamin D (5, 10, 11). Furthermore, whether the 3 epimer of 25-hydroxycholecalciferol [3-epi-25(OH)D3]6, which will influence the concentration of the 3 epimer of 1,25-dihydroxyvitamin D3, should or should not be included in the serum total 25-hydroxycholecalciferol [25(OH)D3] estimate and the consequences for associated analytics was also discussed (5, 10–12). In addition to these key considerations, other fundamental knowledge gaps also pertain to other aspects of 3-epi-25(OH)D3, all of which are in need of being addressed to have the fullest understanding of the relevance of this metabolite for vitamin D nutrition and indeed health in early and later life. For example, the percentage detectable 3-epi-25(OH)D3 in sera from adults was reported variably from 0% to 100%, as recently comprehensively reviewed by Bailey et al. (5). However, the 9 studies included in that systematic review were of relatively small sample sizes (n = 5–212 and 1 with n = 501), some were in not very well-characterized individuals, and some used a range of different LC-MS/MS methods (5).
The putative enzyme responsible for the 3 epimerization has yet to be identified (5), leading to calls for clarification on whether the 3-epi-25(OH)D3 is obtained exogenously (e.g., from food or supplemental sources) or made endogenously (5, 10, 11, 13). Engelman et al. (14), in their recent multivariable regression analysis of serum 3-epi-25(OH)D3 in a population-based sample of 303 adults, showed that being sampled in summer, greater sun exposure, vitamin D supplement use, and the number of alcoholic drinks were all positive determinants.
Thus, the aims of the present work were 1) to characterize the 3-epi-25(OH)D3 concentrations in participants (aged 18–84 y) of the recent National Adult Nutrition Survey (NANS) in Ireland, 2) to explore its determinants, and 3) to evaluate the impact of its inclusion in serum total 25-hydroxyvitamin D [25(OH)D; i.e., 25-hydroxyergocalciferol [25(OH)D2] plus 25(OH)D3] on the prevalence of vitamin D deficiency/inadequacy. To complement these observational data, we used data from a recent randomized controlled trial (RCT) of supplemental cholecalciferol (vitamin D3) conducted in winter in older adults (≥50 y) to test the impact of increases and decreases in vitamin D status on serum 3-epi-25(OH)D3 concentrations.
Participants and Methods
The NANS sample
Participant sampling and recruitment procedures and methods of data collection.
A detailed description of the methodology used in NANS, including the sampling procedure and sample recruitment, was reported previously (15, 16). Briefly, the fieldwork phase of NANS was performed between October 2008 and April 2010, providing a seasonal balance to the data and biologic sample collection. To achieve a nationally representative sample of community-dwelling adults aged ≥18 y, a quota sampling approach was adopted using data from the 2006 Census (17). A sample of 1500 free-living adults to represent a population of ∼4.2 million participated in the dietary survey. There were few exclusion criteria, other than pregnancy/lactation and inability to complete the survey due to disability. The study was approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, University College Cork and the Human Ethics Research Committee of University College Dublin. All eligible and willing participants gave their written consent according to the Helsinki Declaration.
Analysis of the demographic features in this sample showed it to be a representative sample of Irish adults with respect to age, sex, social class, and geographical location when compared with census data (15–17). Although participation in the survey did not require provision of a blood sample as an eligibility criterion, all participants were asked whether they were willing to provide a blood sample. Of the total group of respondents, 75.5% (n = 1132) provided a blood sample. The demographic features of the group of participants who provided a blood sample and those in the entire sample were described previously (15, 16). Seasonality was based on the date the respondents provided the blood sample—November to March (representing the “winter” period) or April to October (representing the “summer” period)—consistent with studies based on NHANES (18) and the recent analysis of Canadian Health Measures Survey for vitamin D status (19).
The approach to food intake data collection, food quantification, and estimation of vitamin D intake in NANS was provided in detail previously (15, 16). Of note, food intake data were analyzed using WISP version 3.0 (Tinuviel Software), which uses data from McCance and Widdowson’s The Composition of Foods, 5th and 6th editions plus all 9 supplemental volumes, to generate nutrient intake data, as described previously (15, 20). Information on social class and education level, smoking status, alcohol intake, and medication usage (including those that contained nutrients) was also collected (15, 16). Anthropometric measures, including height, weight, waist and hip circumference, and measures of body composition, were taken in the respondent’s homes, as described previously (15). The approach toward assessment of vitamin D–containing supplement consumption was described previously (15, 16).
Blood collection.
Blood samples were collected by venipuncture into a vacutainer tube by a qualified nurse at designated centers within the survey area or in the respondent’s home if the respondent could not travel. Bloods were transported to the laboratory for additional processing, and serum was stored at −80°C until required for analysis.
The vitamin D RCT in older adults
A 15-wk randomized, placebo-controlled, double-blind vitamin D3 intervention (20 μg/d) study conducted in the winter in free-living women and men (ratio of ∼2.5:1) aged ≥50 y (n = 125) stratified according to calcium intake (moderate-low: <700 mg/d; or high: >1000 mg/d) was described in detail previously (21). The baseline characteristics of the older adults who completed the intervention are shown in Supplemental Table 1. The study was registered at clinicaltrials.gov as NCT01990872. The vitamin D3 capsules contained 20 μg of vitamin D3 (as Quali-D, a pure crystalline powder form of vitamin D3), and the placebo and vitamin D3 capsules contained filling additives (potato starch and talcum). The vitamin D3 content of the capsules was confirmed by in-house laboratory HPLC analysis within the Vitamin D Research Group at University College Cork. The capsules were also analyzed for 3-epi-25(OH)D3, which was shown to be absent. Repeated-measures ANOVA showed that there was no significant time × vitamin D treatment × calcium intake grouping interaction effect (P = 0.20 for interaction in both cases) or calcium intake grouping × time interaction effects (P > 0.72 for interaction in both cases) on mean serum total 25(OH)D or 3-epi-25(OH)D3 concentrations over the 15-wk intervention period. Serum total 25(OH)D increased and decreased in the vitamin D3 and placebo groups, respectively, and of similar magnitudes in those with calcium intakes <700 and >1000 mg/d (21). Therefore, because of the lack of calcium intake grouping effect, we were able to combine groups and examine the effect of vitamin D3 supplementation (n = 64) or placebo (n = 61) on serum 3-epi-25(OH)D3 and 25(OH)D3 concentrations and their ratio over winter. There was no difference (P > 0.32 in all cases) in the mean age, weight, height, BMI, and the proportion of men-to-women mean habitual dietary calcium or vitamin D at baseline among the placebo and vitamin D3–supplemented groups.
Analysis of serum 3 epimer of 25-hydroxyvitamin D3, 25-hydroxyvitamin D3, and 25-hydroxyvitamin D2.
The concentrations of total 25(OH)D and 3-epi-25(OH)D3 in serum samples were measured by the Vitamin D Research Group at University College Cork using an LC-MS/MS method. In brief, the LC-MS/MS method resolves and measures 3-epi-25(OH)D3 (22), which is not chromatographically resolved from 25(OH)D3 by most routine LC-MS/MS methods, in addition to 25(OH)D2 and 25(OH)D3 in serum. The presence of 3-epi-25(OH)D3 can pose problems for LC-MS/MS methods because the mass and fragmentation patterns are the same as 25(OH)D. The present method does not measure the 3 epimer of 25(OH)D2, but, with the mean and 90th percentile of serum 25(OH)D2 in NANS being 3.7 and 6.3 nmol/L, respectively (22), the concentration of the 3 epimer of 25(OH)D2 would be expected to be extremely low for most. A detailed description of the LC-MS/MS method was described previously (22). The interassay CV of the method was <5% for all 25(OH)D metabolites, whereas the intra-assay CV was <6%. The limit of detection (LOD) for 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3 in serum was 0.44, 0.31, and 0.20 nmol/L, respectively, using the LC-MS/MS method by the Vitamin D Research Group at University College Cork. The limit of quantification (LOQ) for 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3 in serum was 1.43, 1.03, and 0.66 nmol/L, respectively. The quality and accuracy of serum 25(OH)D analysis using the LC-MS/MS method in our laboratory are monitored on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (Charing Cross Hospital, London, UK), but this scheme is for total serum 25(OH)D and does not delineate 25(OH)D2 and 25(OH)D3 or their respective 3 epimers. In addition, the Vitamin D Research Group is a participant in the CDC Vitamin D Standardization Certification Program (23), which reports total 25(OH)D, 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3. The Vitamin D Research Group was also a participant in the Vitamin D Standardization Program Interlaboratory Comparison Study in which 50 especially commissioned patient sera [covering a wide range of serum 25(OH)D concentrations] were analyzed by each national survey laboratory and 2 higher-order reference laboratories who assigned values for total 25(OH)D, 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3 using a reference measurement LC-MS procedure (24). Of the 50 patient sera, 45 had serum 3-epi-25(OH)D3 concentrations greater than the LOQ of the method (and ranged from 1.60 to 15.9 nmol/L). The relation between serum 3-epi-25(OH)D3 concentrations by the reference measurement LC-MS procedure at the University of Ghent (25) and the Vitamin D Research Group LC-MS/MS was 0.99 × X + 0.02 (r2 = 0.99). In addition to this very close relation between the 2 methods, the mean bias for the Vitamin D Research Group LC-MS/MS was only −0.3%.
Although the data on concentration of serum total 25(OH)D and 25(OH)D2 in NANS (22, 26) and serum total 25(OH)D in the vitamin D RCT (21) was reported previously, the serum 3-epi-25(OH)D3 or 25(OH)D3 was not, and this forms the basis of the present analysis.
Data interpretation and statistical analysis.
Data and statistical analyses were conducted using SPSS version 20.0 for Windows (SPSS). Descriptive statistics (frequencies, means, medians, 95% CIs, and percentiles, when relevant) were generated for serum 3-epi-25(OH)D3, 25(OH)D3, and the 3 epimer of 25-hydroxycholecalciferol as a percentage of 25-hydroxycholecalciferol [%3-epi-25(OH)D3] data. ANOVA was used to compare means across quartiles of these variables, and an unpaired t test was used to compare the ratio of 3-epi-25(OH)D3 to 25(OH)D3 between extended summer and winter. Pearson’s correlations were performed between serum 25(OH)D3 and 3-epi-25(OH)D3 and between serum 3-epi-25(OH)D3 and age. Linear regression analysis was performed to identify independent predictors of serum 3-epi-25(OH)D3, 25(OH)D3, and %3-epi-25(OH)D3. The following categorical variables were included: 1) vitamin D–containing supplement use (0, no supplements; 1, taking supplements); 2) calcium-containing supplement use (0, no supplements; 1, taking supplements); 3) season (0, winter; 1, spring; 2, autumn; 3, summer); 4) sex (0, female; 1, male); 5) sun habits (0, avoids sunshine; 1, sometimes in the sun; 2, prefers sunshine exposure); 6) frequency of sunscreen use (0, never; 1, rarely; 2, sometimes; 3, always); and 7) smoking status (0, smoker; 1, ex-smoker; 2, non-smoker). The following continuous numerical variables were included: 1) age (years); 2) waist circumference (centimeters); 3) serum creatinine (millimoles per liter); 4) serum 25(OH)D2 (nanomoles per liter); 5) mean daily intake (MDI) of magnesium (milligrams per day); 6) MDI of calcium (excluding that from supplements; milligrams per day); 7) MDI of vitamin D (excluding that from supplements; micrograms per day); 8) MDI of total ethanol intake (grams per day); and 9) total meat intake (which included fresh, processed, and meat dishes for beef, pork, lamb, and poultry; grams per day). Linear models of the response in a repeated-measures analysis for the differences in serum 25(OH)D3, 3-epi-25(OH)D3, and the ratio of 3-epi-25(OH)D3 to 25(OH)D3 were constructed for vitamin D RCT data. The linear models explored the effect of the main factor (vitamin D treatment) and the vitamin D treatment × time interactions. Bonferroni’s-adjusted t tests were used for post hoc analysis to compare within-treatment group changes from baseline to endpoint. Statistical significance was defined as a P value ≤0.05. In the case of the regression models, a P value >0.05 but <0.09 was considered suggestive of a trend toward significance.
Results
Distribution of serum 3-epi-25(OH)D3 concentrations in NANS.
Of the entire NANS population for which serum 3-epi-25(OH)D3 concentrations existed (n = 1122), only 2 participants had serum 3-epi-25(OH)D3 concentrations less than the LOD [both had serum 25(OH)D3 concentrations < 10.4 nmol/L], 38 participants had concentrations between the LOD and LOQ, and 96.4% (n = 1082) had serum 3-epi-25(OH)D3 concentrations greater than the LOQ. In the subsample of NANS participants who had serum 3-epi-25(OH)D3 concentrations greater than the LOQ, the mean, 10th, 50th (median), and 90th percentiles were 2.50, 1.05, 2.18, and 4.30 nmol/L, respectively, whereas the maximum serum 3-epi-25(OH)D3 concentration was 15.0 nmol/L [participant had a serum 25(OH)D3 of 105 nmol/L and was a vitamin D supplement user and sampled in the summer]. The mean, 10th, 50th (median), and 90th percentiles of serum 25(OH)D3 concentrations in the same subsample were 53.2, 27.2, 50.9, and 84.6 nmol/L, respectively, whereas the maximum serum 25(OH)D3 concentration was 168 nmol/L.
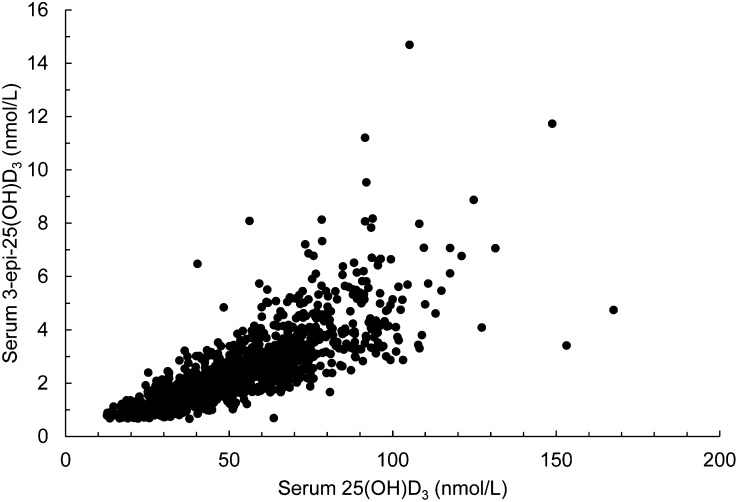
The relation between serum 3-epi-25(OH)D3 to 25(OH)D3 is shown in Figure 1. There was a highly significant correlation between these 2 metabolites (Pearson’s correlation coefficient, r = 0.78; P < 0.0001; n = 1082), and the linear relation was serum 3-epi-25(OH)D3 = 0.05 × [serum 25(OH)D3] − 0.28 (r2 = 0.64; P < 0.0001; n = 1082). There was a large variability in serum 3-epi-25(OH)D3 concentration at any 1 concentration of serum 25(OH)D3, particularly at the higher end of the concentration range (Fig. 1).
FIGURE 1.
Scatter plot of serum 3-epi-25(OH)D3 and 25(OH)D3 in adults aged 18–84 y (National Adult Nutrition Survey participants, n = 1082). 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol.
There was no significant correlation between serum 3-epi-25(OH)D3 and age (r = 0.005; P > 0.42, n = 1082).
The mean 3-epi-25(OH)D3 concentration and %3-epi-25(OH)D3 by quartile of serum 25(OH)D3 concentration are shown in Table 1. Although the mean 3-epi-25(OH)D3 concentration increased linearly by quartile of serum 25(OH)D3 concentration, the %3-epi-25(OH)D3 was similar in the lowest 3 quartiles and only significantly (P < 0.05) increased in the highest quartile. Overall, the mean, 10th, 50th (median), and 90th percentiles of %3-epi-25(OH)D3 were 4.6%, 3.1%, 4.4%, and 6.5%, respectively, whereas the maximum %3-epi-25(OH)D3 in serum was 16.0%.
TABLE 1.
Serum 3-epi-25(OH)D3 and %3-epi-25(OH)D3 by quartile of serum 25(OH)D3 in adults aged 18–84 y (National Adult Nutrition Survey participants, n = 1082)1
| Quartiles of 25(OH)D3 concentration (nmol/L) |
||||
| <35.3 | ≥35.3–50.8 | ≥50.9–67.7 | ≥67.8 | |
| Participants, n | 269 | 272 | 270 | 271 |
| 3-epi-25(OH)D3, nmol/L | 1.22 ± 0.38a | 1.91 ± 0.64b | 2.69 ± 0.85c | 4.16 ± 1.66d |
| %3-epi-25(OH)D3 | 4.51 ± 1.26a | 4.46 ± 1.43a | 4.58 ± 1.40a | 4.97 ± 1.66b |
Values are means ± SDs. Means in a row without a common letter differ, P < 0.05 (Bonferroni’s test for post hoc comparison) and P < 0.0001 [1-factor ANOVA for both 3-epi-25(OH)D3 and %3-epi-25(OH)D3]. 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol; %3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol as a percentage of 25-hydroxycholecalciferol.
Impact of inclusion of 3-epi-25(OH)D3 in serum total 25(OH)D concentration on the prevalence of vitamin D deficiency and inadequacy.
The percentage of participants with serum total 25(OH)D below variously proposed thresholds of serum 25(OH)D and the impact of inclusion of the serum 3-epi-25(OH)D3 concentration on same are shown in Table 2. Accounting for the 3-epi-25(OH)D3 concentration in the estimate of serum total 25(OH)D reduced the prevalence of serum 25(OH)D <30, <50, and <75 nmol/L [representing vitamin D deficiency, inadequacy, and suboptimal, respectively (1,27)] by 1.7, 2.8, and 3.6 percentage points, respectively. The prevalence of serum total 25(OH)D > 125 nmol/L, a concentration that might be of concern if chronically sustained, according to the Institute of Medicine (1), increased from 3.4% to 5.5%, with the inclusion of the 3-epi-25(OH)D3 concentration in the total estimate.
TABLE 2.
Adults aged 18–84 y with serum total 25(OH)D below various thresholds, with and without inclusion of serum 3-epi-25(OH)D3 (National Adult Nutrition Survey participants, n = 1082)1
| Threshold (nmol/L) | Without 3-epi-25(OH)D3 | With 3-epi-25(OH)D3 |
| % | % | |
| <20 | 2.9 | 2.1 |
| <30 | 12.5 | 10.8 |
| <40 | 29.1 | 26.2 |
| <50 | 45.8 | 43.0 |
| <60 | 61.9 | 57.7 |
| <70 | 75.2 | 71.6 |
| <75 | 81.0 | 77.4 |
| >125 | 3.4 | 5.5 |
25(OH)D, 25-hydroxyvitamin D; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol.
Exploration of the determinants of serum 3-epi-25(OH)D3 concentrations: possible clues to its exogenous and endogenous sources.
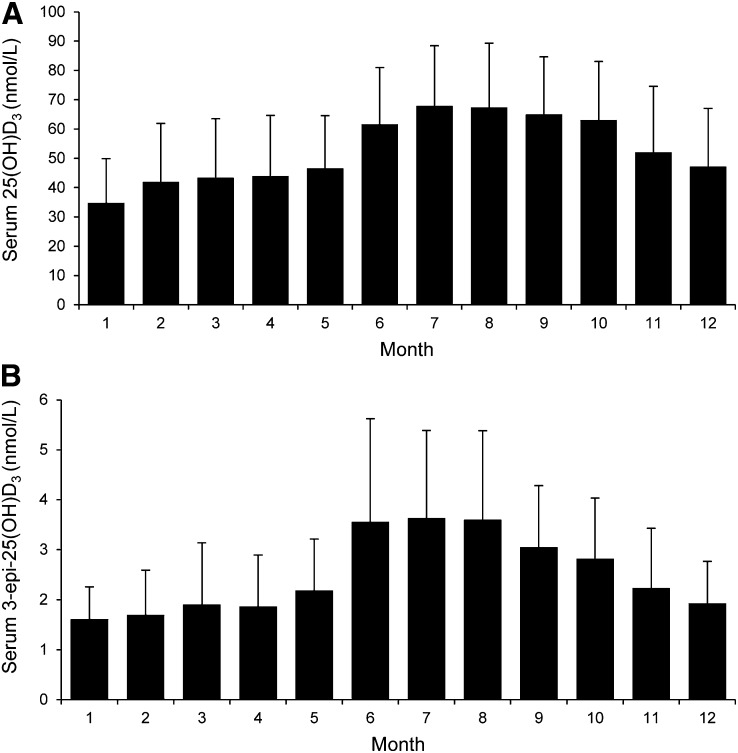
A regression model that included age, sex, waist circumference, serum creatinine, serum 25(OH)D2, vitamin D supplement use, dietary vitamin D intake, calcium supplement use, food calcium intake, dietary magnesium intake, total ethanol intake, total meat intake, season of blood sampling, sun exposure habits, frequency of sunscreen use, and smoking status explained 30.7% of the variability in serum 25(OH)D3, with sunnier seasons being the strongest positive determinants (Table 3). The mean concentration of serum 25(OH)D3 and 3-epi-25(OH)D3 by month is shown in Figure 2. The mean monthly ratio of 3-epi-25(OH)D3 to 25(OH)D3 increased in the sunnier periods of the year, with the mean (± SD) ratio in an extended summer (April to October) being significantly (P < 0.0001) higher than that in an extended winter (November to March) (0.049 ± 0.015 vs. 0.043 ± 0.014, respectively).
TABLE 3.
Multiple regression model for serum 25(OH)D3 and 3-epi-25(OH)D3 in adults aged 18–84 y (National Adult Nutrition Survey participants, n = 1082)1
| Serum 25(OH)D3 |
Serum 3-epi-25(OH)D3 |
|||
| B (95% CI) | P | B (95% CI) | P | |
| Age group (vs. aged 18–35 y) (y) | ||||
| 36–50 | 4.32 (0.86, 7.78) | 0.014 | 0.13 (−0.08, 0.36) | 0.22 |
| 51–64 | 6.62 (2.38, 10.8) | 0.002 | 0.46 (0.19, 0.73) | 0.001 |
| ≥65 | 5.41 (0.37, 10.4) | 0.036 | 0.54 (0.22, 0.86) | 0.001 |
| Sex (F vs. M) | 2.18 (−1.21, 5.58) | 0.21 | −0.15 (−0.37, 0.06) | 0.16 |
| Waist circumference (cm) | −0.22 (−0.33, −0.10) | <0.0001 | −0.009 (−0.016, −0.001) | 0.019 |
| Serum creatinine (mmol/L) | −0.03 (−0.12, 0.06) | 0.47 | −0.002 (−0.007, 0.004) | 0.60 |
| Serum 25(OH)D2 (nmol/L) | −1.01 (−1.53, −0.49) | <0.0001 | −0.05 (−0.08, −0.02) | 0.002 |
| Using a vitamin D supplement | 8.89 (4.61, 13.2) | <0.0001 | 0.36 (0.09, 0.64) | 0.009 |
| Dietary vitamin D intake (μg/d) | 1.49 (0.93, 2.05) | <0.0001 | 0.04 (0.004, 0.076) | 0.027 |
| Using a calcium supplement | 1.46 (−3.58, 6.50) | 0.57 | 0.23 (−0.09, 0.56) | 0.115 |
| Dietary calcium intake (mg/d) | −0.001 (−0.006, 0.004) | 0.58 | 0.00 (0.000, 0.000) | 0.84 |
| Dietary magnesium intake (mg/d) | 0.009 (−0.008, 0.027) | 0.28 | 0.000 (−0.001, 0.001) | 0.57 |
| Total ethanol intake (g/d) | 0.020 (−0.002, 0.041) | 0.07 | 0.001 (0.000, 0.003) | 0.10 |
| Meat intake (g/d) | 0.009 (−0.006, 0.024) | 0.24 | 0.001 (0.000, 0.002) | 0.032 |
| Season of blood sampling (vs. winter) | ||||
| Spring | 0.91 (−3.05, 4.86) | 0.65 | 0.26 (0.003, 0.509) | 0.047 |
| Summer | 20.8 (16.6, 25.1) | <0.0001 | 1.75 (1.48, 2.02) | <0.0001 |
| Autumn | 14.1 (10.3, 18.0) | <0.0001 | 0.77 (0.53, 1.02) | <0.0001 |
| Sun habits (vs. avoids sunshine exposure) | ||||
| Sometimes in the sun | 1.53 (−2.11, 5.17) | 0.41 | 0.24 (0.01, 0.48) | 0.040 |
| Prefers sunshine exposure | 8.53 (4.47, 12.6) | <0.0001 | 0.73 (0.47, 0.99) | <0.0001 |
| Frequency of sunscreen use (vs. never) | ||||
| Rarely | −0.68 (−6.24, 6.11) | 0.98 | 0.08 (−0.32, 0.47) | 0.69 |
| Sometimes | 3.36 (−2.27, 8.99) | 0.24 | 0.24 (−0.12, 0.60) | 0.20 |
| Always | 2.32 (−3.66, 8.30) | 0.45 | 0.29 (−0.09, 0.67) | 0.13 |
| Smoking status (vs. smoker) | ||||
| Ex-smoker | 5.65 (1.46, 9.84) | 0.008 | 0.26 (−0.003, 0.532) | 0.053 |
| Non-smoker | 7.05 (3.43, 10.7) | <0.0001 | 0.28 (0.05, 0.51) | 0.017 |
| R2 for model, % | 30.7 | 29.9 | ||
Values are unstandardized coefficients (B) (95% CIs) unless otherwise indicated. 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol.
FIGURE 2.
Mean serum 25(OH)D3 (A) and 3-epi-25(OH)D3 (B) of adults aged 18–84 y (National Adult Nutrition Survey, n = 1082) by month of the year (1, January; 12, December). Bars and error bars represent means ± SDs for n = 24–121. 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol.
The same regression model as used for serum 25(OH)D3 explained 29.9% and 15.0% of the variability in serum 3-epi-25(OH)D3 and %3-epi-25(OH)D3, respectively. Increasing age, use of vitamin D–containing supplements, increasing dietary vitamin D, total meat intake, being sampled in sunnier periods of the year, sun exposure, and being a non-smoker or ex-smoker were significant positive determinants of serum 3-epi-25(OH)D3, whereas increasing waist circumference and serum 25(OH)D2 concentration were significant negative determinants (Table 3). Inclusion of serum 25(OH)D3 in the model increased the percentage explained variability in serum 3-epi-25(OH)D3 to 67.0%, but there was significant multicollinearity in the model (data not shown).
Being aged 50–64 or ≥65 y [unstandardized coefficient B = 0.004 (95% CI: 0.001, 0.007) and B = 0.005 (95% CI: 0.001, 0.009), P = 0.014 and 0.008, respectively], being sampled in spring or summer [B = 0.004 (95% CI: 0.001, 0.007) and B = 0.012 (95% CI: 0.009, 0.015), P = 0.007 and ≤0.0001, respectively], and preference for sun exposure [B = 0.005 (95% CI: 0.002, 0.008), P = 0.001] were significant positive determinants of serum %3-epi-25(OH)D3, whereas being female was a significant negative determinant of serum %3-epi-25(OH)D3 [B = −0.005 (95% CI: −0.008, −0.003), P ≤ 0.0001].
The effect of daily supplementation with vitamin D3 or placebo on serum 3-epi-25(OH)D3, 25(OH)D3, or %3-epi-25(OH)D3 in older adults over the winter.
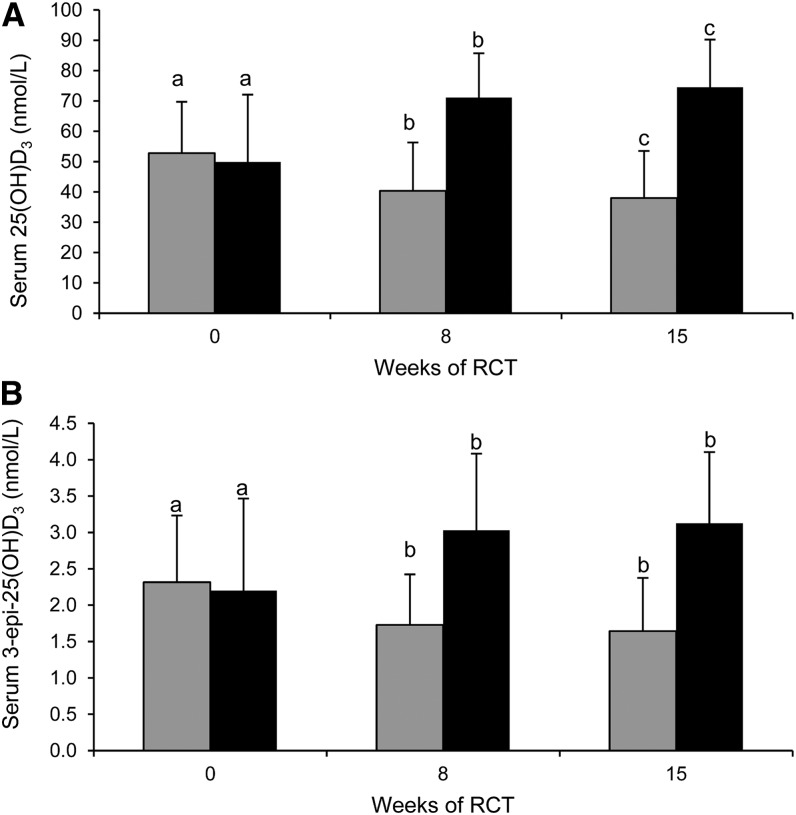
The vitamin D3 treatment group × time interactions on serum 3-epi-25(OH)D3 and 25(OH)D3 in older adults over 15 wk of winter were highly significant (P < 0.0001 for interaction in both cases). Post hoc analysis showed that vitamin D3 treatment (20 μg/d) significantly (P < 0.001) increased mean serum 25(OH)D3 and 3-epi-25(OH)D3 concentrations from baseline to midpoint (8 wk), and, although serum 25(OH)D3 continued to increase modestly but significantly (P < 0.003) by endpoint (7 wk later), serum 3-epi-25(OH)D3 concentrations remained similar (P = 0.19) (Fig. 3). Mean serum 25(OH)D3 and 3-epi-25(OH)D3 concentrations decreased significantly (P < 0.0001) in the placebo group from baseline to midpoint, and, although serum 25(OH)D3 continued to decline modestly to endpoint (P < 0.001), serum 3-epi-25(OH)D3 concentrations remained similar (P = 0.16) (Fig. 3).
FIGURE 3.
Serum 25(OH)D3 (A) and 3-epi-25(OH)D3 (B) in placebo- (gray bars) and vitamin D3 (20 μg/d; black bars)–supplemented older adults (aged ≥50 y) at baseline and 8 and 15 wk of winter. Bars and error bars represent means ± SDs for n = 61–64. Means within a treatment group without a common letter differ, P < 0.0001 [repeated-measures ANOVA for both 25(OH)D3 and 3-epi-25(OH)D3] and P < 0.05 (Bonferroni’s-adjusted t tests for post hoc comparison of means). RCT, randomized controlled trial; 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol.
There was no significant vitamin D3 treatment group × time interaction effects (P = 0.54) on serum %3-epi-25(OH)D3 in older adults over 15 wk of winter (data not shown).
Discussion
To our knowledge, the present study is the first description of the distribution of serum 3-epi-25(OH)D3 in a nationally representative sample of adults. The vast majority of participants (96.4%) had quantifiable (i.e., greater than the LOQ) serum 3-epi-25(OH)D3 concentrations, which is in line with the findings from some (range, 90–99% detectable) (10,28–30) but not all studies in adults (range, 0–80% detectable) (31–35), which were of relatively smaller sample size (n = 5–212 and 1 with n = 501). The mean serum 3-epi-25(OH)D3 concentration and %3-epi-25(OH)D3 in our sample of adults, aged 18–84 y, were 2.5 nmol/L and 4.6%, respectively. Bailey et al. (5) in their recent systematic review reported a weighted mean (range) serum 3-epi-25(OH)D3 concentration and %3-epi-25(OH)D3 of 4.3 (0–22.5) nmol/L and 6.1 (0–47.0)%, respectively, from the 9 studies that reported adult data. Subsequent to the Bailey et al. (5) systematic review, Zhang et al. (38) reported a mean 3-epi-25(OH)D3 concentration and %3-epi-25(OH)D3 in their Irish adult pregnancy cohort (n = 1768) of 2.6 nmol/L and 4.9%, respectively, whereas Engelman et al. (14) reported in their epidemiologic study of 3-epi-25(OH)D3 in a population-based sample of non-Hispanic white adults (n = 303; mean age, 48.1 y) that the mean and percentage 3-epi-25(OH)D3 were 3.2 nmol/L and 3.9%, respectively.
There was a strong relation between serum 3-epi-25(OH)D3 and 25(OH)D3. Furthermore, the ratio of 3-epi-25(OH)D3 to 25(OH)D3 appeared to be greater (∼5.0) at relatively high concentrations of serum 25(OH)D3 (highest quartile, >67.8 nmol/L) but relatively stable (∼4.5) at concentrations below that (quartiles 1–3). In contrast, Engelman et al. (14) reported that the percentage of the epimer increases approximately linearly to a mean of 5.1% in individuals in the highest quartile. However, the quartile distribution of serum 25(OH)D3 in that study was much higher than that in the present study, with a mean serum 25(OH)D3 of 75.5 and 53.2 nmol/L, respectively. The higher ratio of 3-epi-25(OH)D3 to 25(OH)D3 at higher concentrations of serum 25(OH)D3 attenuates (by 3.6 percentage points) the prevalence of suboptimal vitamin D status [serum 25(OH)D < 75 nmol/L] (27) when the contribution of 3-epi-25(OH)D3 is included in the total serum 25(OH)D concentration. This attenuation by inclusion of 3-epi-25(OH)D3 in total serum 25(OH)D concentration was lessened at the <50 and <30 nmol/L cutoffs (by 2.8 and 1.7 percentage points, respectively). Engelman et al. (14) reported that only 1% of their population-based sample of adults, aged 21–74 y, would have been classified as sufficient if the 3 epimer was included in the total 25(OH)D but deficient [serum 25(OH)D < 50 nmol/L] if it was excluded.
The lack of understanding in relation to the source, not to mention the reason for the presence, of the 3-epi-25(OH)D3 in serum was reported widely (5, 10, 11, 14). Although a number of reports investigated associations between 3-epi-25(OH)D3 and individual factors, such as age (10, 30) and season (36), Engelman et al. (14) in their multivariable regression analysis of serum 25(OH)D3, 3-epi-25(OH)D3, and %3-epi-25(OH)D3 in a population-based sample of 303 adults showed that being sampled in summer vs. winter season, more sun exposure, vitamin D intake from supplements, and the number of alcoholic drinks were positive determinants of serum 3-epi-25(OH)D3, whereas waist circumference was a significant negative determinant, and age, sex, and vitamin D intake from foods were not associated with the 3 epimer. Their model explained 37% and 26% of variability in serum 25(OH)D3 and 3-epi-25(OH)D3 concentrations, respectively (14). In the present study, in a much larger and nationally representative sample of adults, an expanded list of potential determinants explained a similar extent of the variability in serum 25(OH)D3 and 3-epi-25(OH)D3 concentration (30.7% and 29.9%, respectively). In general, factors that are known to increase and decrease serum 25(OH)D3 were positively and negatively associated with serum 3-epi-25(OH)D3 concentration, respectively. The increased supply of vitamin D, either orally by greater dietary intake and/or supplement use or enhanced dermal synthesis by more UVB sunlight exposure, was associated with increased serum 3-epi-25(OH)D3 concentration, whereas increasing adiposity, smoking, and high serum 25(OH)D2 concentration, all known or suggested to reduce circulating vitamin D and/or 25(OH)D (37–39), were associated with reduced serum 3-epi-25(OH)D3 concentrations in the present study.
Although serum 3-epi-25(OH)D3 was not significantly correlated with age in the present study in agreement with the lack of association with age in studies by Engelman et al. (14) and Lensmeyer et al. (10), being aged 50–64 and ≥65 y was a significant positive predictor of serum 3-epi-25(OH)D3 concentration. This may relate to the higher intake of vitamin D from supplements in older participants in our survey (16), which will increase the serum 25(OH)D3 concentration. Interestingly, the factors included in our regression model only explained 15% of the variability in %3-epi-25(OH)D3, in close agreement with the findings by Engelman et al. (14). Availability of UVB sunshine, in the context of sampling season and sun preference, was the strongest positive determinant, whereas dietary vitamin D intake (but not supplement use) was only a borderline significant positive determinant of %3-epi-25(OH)D3.
Although the associations reported in the present study and those by Engelman et al. (10) provide some biologic plausibility and insight into the possible role of factors that increase and decrease serum 25(OH)D3 in determining serum 3-epi-25(OH)D3 concentrations, they do not provide evidence of causal relations. In this regard, the present data on the response of serum 3-epi-25(OH)D3 concentrations to vitamin D3 supplementation over winter in our RCT in older adults is the first, to our knowledge, to show that, during winter, the concentration of the epimer decreases (as shown in the placebo group) unless additional vitamin D3 is supplied (as shown in the vitamin D3–supplemented group), in which case it increases significantly. These changes closely mirrored the changes in serum 25(OH)D3 concentrations, and the ratio of 3-epi-25(OH)D3 to 25(OH)D3 was constant.
The quality control measures used in the present study demonstrate that the epimer was not an artifact of the extraction or LC-MS/MS procedures. We acknowledge the possibility that what we are identifying as 3-epi-25(OH)D3 could plausibly be 25-hydroxyprevitamin D3. However, as suggested recently by Lensmeyer et al. (10), we also believe this unlikely given that the peak we label as 3-epi-25(OH)D3 is identical to what the National Institute of Science and Technology has identified as 3-epi-25(OH)D3 based on the ability of our LC-MS/MS method to recover the values of 3-epi-25(OH)D3 in the human serum reference material. This is also the case for our analysis of the sera used in the CDC Vitamin D Standardization Certification Program (23).
In conclusion, to our knowledge, we provide for the first time data on serum 3-epi-25(OH)D3 in a nationally representative sample of adults. Furthermore, combining the observational and RCT data from the present study seems to suggest that both dietary supply and dermal synthesis of vitamin D3 contribute to the concentration of 3-epi-25(OH)D3 in serum. This in turn suggests, at least in part, the involvement of a 3-epimerase enzyme, although this has not yet been isolated. However, the data do not discount the possibility of an additional direct dietary route of exposure to 3-epi-25(OH)D3 per se, as might occur with consumption of foods of animal origin, in particular meat and eggs that contain vitamin D3 but also 25(OH)D3 (40), and therefore the possibility of 3-epi-25(OH)D3, although this has yet to be reported. Meat intake was a significant determinant of serum 3-epi-25(OH)D3 but not 25(OH)D3 in the present regression analysis. Clearly, more research in terms of the functionality and sources of 3-epi-25(OH)D3 is warranted.
Supplementary Material
Acknowledgments
A.F., M. Kiely, and K.D.C. were involved in conception of work and are grant holders; J.W., A.F., K.M.S., A.H., M. Kiely, and K.D.C. contributed to the study design and execution of the study; M. Kinsella, A.J.L., A.H., and K.D.C. contributed to the sample and data analysis; and all authors contributed to the data analysis and writing of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: LOD, limit of detection; LOQ, limit of quantification; MDI, mean daily intake; NANS, National Adult Nutrition Survey; RCT, randomized controlled trial; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol; 3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol; %3-epi-25(OH)D3, 3 epimer of 25-hydroxycholecalciferol as a percentage of 25-hydroxycholecalciferol.
References
- 1.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington: National Academy Press; 2011. [Google Scholar]
- 2.Jones G. Extrarenal vitamin D activation and interactions between vitamin D2, vitamin D3, and vitamin D analogs. Annu Rev Nutr 2013;33:23–44. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6S. [DOI] [PubMed] [Google Scholar]
- 4.Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA. Metabolism of 1alpha,25-dihydroxyvitamin D(3) and its C-3 epimer 1alpha,25-dihydroxy-3-epivitamin D(3) in neonatal human keratinocytes. Steroids 2001;66:441–50. [DOI] [PubMed] [Google Scholar]
- 5.Bailey D, Veljkovic K, Yazdanpanah M, Adeli K. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin Biochem 2013;46:190–6. [DOI] [PubMed] [Google Scholar]
- 6.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem 2004;279:15897–907. [DOI] [PubMed] [Google Scholar]
- 7.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25 dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem 1999;73:106–13. [PubMed] [Google Scholar]
- 8.Brown AJ, Ritter CS, Weiskopf AS, Vouros P, Sasso GJ, Uskokovic MR, Wang G, Reddy GS. Isolation and identification of 1alpha-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion. J Cell Biochem 2005;96:569–78. [DOI] [PubMed] [Google Scholar]
- 9.Morrison NA, Eisman JA. Nonhypercalcemic 1,25-(OH)2D3 analogs potently induce the human osteocalcin gene promoter stably transfected into rat osteosarcoma cells (ROSCO-2). J Bone Miner Res 1991;6:893–9. [DOI] [PubMed] [Google Scholar]
- 10.Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J Clin Endocrinol Metab 2012;97:163–8. [DOI] [PubMed] [Google Scholar]
- 11.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, et al. ; Vitamin D Roundtable on the NHANES Monitoring of Serum 25(OH)D: Assay Challenges and Options for Resolving Them. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr 2010;140:2030S–45S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binkley N, Wiebe D. Clinical controversies in vitamin D: 25(OH)D measurement, target concentration, and supplementation. J Clin Densitom 2013;16:402–8. [DOI] [PubMed] [Google Scholar]
- 13.Jones G. Metabolism and biomarkers of vitamin D. Scand J Clin Lab Invest Suppl 2012;243:7–13. [DOI] [PubMed] [Google Scholar]
- 14.Engelman CD, Bo R, Zuelsdorff M, Steltenpohl H, Kirby T, Nieto FJ. Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D3 in a population-based sample. Clin Nutr 2013;pii:S0261-5614(13)00177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irish Universities Nutrition Alliance. National Adult Nutrition Survey: summary report. 2011 (cited 2011 June 1). Available from: http://www.iuna.net/wp-content/uploads/2010/12/National-Adult-Nutrition-Survey-Summary-Report-March-2011.pdf.
- 16.Cashman KD, Muldowney S, McNulty B, Nugent A, FitzGerald AP, Kiely M, Walton J, Gibney MJ, Flynn A. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr 2013;109:1248–56. [DOI] [PubMed] [Google Scholar]
- 17.Central Statistics Office (CSO). Census 2006 principal demographic results. Dublin, Ireland: The Stationery Office. 2007. [Google Scholar]
- 18.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011;(59):1–8. [PubMed] [Google Scholar]
- 19.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 2011;94:128–35. [DOI] [PubMed] [Google Scholar]
- 20.Hill TR, O’Brien MM, Cashman KD, Flynn A, Kiely M. Vitamin D intakes in 18–64-y-old Irish adults. Eur J Clin Nutr 2004;58:1509–17. [DOI] [PubMed] [Google Scholar]
- 21.Cashman KD, Hayes A, O’Donovan SM, Zhang JY, Kinsella M, Galvin K, Kiely M, Seamans KM. Dietary calcium does not interact with vitamin D3 in terms of determining the response and catabolism of serum 25-hydroxyvitamin D during winter in older adults. Am J Clin Nutr 2014;Apr 2 (Epub ahead of print; DOI:10.3945/ajcn.113.080358). [DOI] [PubMed] [Google Scholar]
- 22.Cashman KD, Kinsella M, Walton J, Gibney MJ, Flynn A, Mairead KM. Dietary vitamin D2 – a potentially under-estimated contributor to vitamin D nutritional status of adults? Br J Nutr 2014:Apr 29 (Epub ahead of print; DOI:10.1017/S0007114514000725). [DOI] [PubMed] [Google Scholar]
- 23.Rahmani YE, Botelho JC, Vesper HW. CDC vitamin D standardization certification program. Endocr Rev. 2013;34(03_MeetingAbstracts):SUN-277. [Google Scholar]
- 24.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 25.Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography–tandem mass spectrometry. Clin Chem 2011;57:441–8. [DOI] [PubMed] [Google Scholar]
- 26.Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 2013;97:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab 2012;97:1153–8. [DOI] [PubMed] [Google Scholar]
- 28.Stepman HC, Vanderroost A, Stockl D, Thienpont LM. Full-scan mass spectral evidence for 3-epi-25-hydroxyvitamin D(3) in serum of infants and adults. Clin Chem Lab Med 2011;49:253–6. [DOI] [PubMed] [Google Scholar]
- 29.Keevil B. Does the presence of 3-epi-25OHD3 affect the routine measurement of vitamin D using liquid chromatography tandem mass spectrometry? Clin Chem Lab Med 2012;50:181–3. [DOI] [PubMed] [Google Scholar]
- 30.Strathmann FG, Sadilkova K, Laha TJ, LeSourd SE, Bornhorst JA, Hoofnagle AN, Jack R. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta 2012;413:203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 2006;91:3055–61. [DOI] [PubMed] [Google Scholar]
- 32.van den Ouweland JM, Beijers AM, van Daal H. Fast separation of 25-hydroxyvitamin D3 from 3-epi-25-hydroxyvitamin D3 in human serum by liquid chromatography-tandem mass spectrometry: variable prevalence of 3-epi-25 hydroxyvitamin D3 in infants, children, and adults. Clin Chem 2011;57:1618–9. [DOI] [PubMed] [Google Scholar]
- 33.Baecher S, Leinenbach A, Wright JA, Pongratz S, Kobold U, Thiele R. Simultaneous quantification of four vitamin D metabolites in human serum using high performance liquid chromatography tandem mass spectrometry for vitamin D profiling. Clin Biochem 2012;45:1491–6. [DOI] [PubMed] [Google Scholar]
- 34.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 2011;412:1594–9. [DOI] [PubMed] [Google Scholar]
- 35.Shah I, James R, Barker J, Petroczi A, Naughton DP. Misleading measures in Vitamin D analysis: a novel LC-MS/MS assay to account for epimers and isobars. Nutr J 2011;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JY, Kinsella M, Khashan AS, Kenny LC, Kiely ME. The C3-epimer of 25-hydroxyvitamin D3 (3-epi-25(OH)D3) is quantifiable in almost all pregnant women and mirrors variation in serum 25(OH)D3 concentrations. Am J Clin Nutr. In press. 2014. [Google Scholar]
- 37.Hill TR, O’Brien MM, Lamberg-Allardt C, Jakobsen J, Kiely M, Flynn A, Cashman KD. Vitamin D status of 51–75-year-old Irish women: its determinants and impact on biochemical indices of bone turnover. Public Health Nutr 2006;9:225–33. [DOI] [PubMed] [Google Scholar]
- 38.Andersen R, Mølgaard C, Skovgaard LT, Brot C, Cashman KD, Chabros E, Charzewska J, Flynn A, Jakobsen J, Kärkkäinen M, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr 2005;59:533–41. [DOI] [PubMed] [Google Scholar]
- 39.Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr 2013;109:1082–8. [DOI] [PubMed] [Google Scholar]
- 40.Food Standards Agency. McCance and Widdowson’s The Composition of Foods. 6th summary ed. Cambridge, UK: Royal Society of Chemistry. 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.