Abstract
Asthma is characterized by airway inflammation rich in eosinophils. Airway eosinophilia is associated with exacerbations and has been suggested to play a role in airway remodeling. Recruitment of eosinophils from the circulation requires that blood eosinophils become activated, leading to their arrest on the endothelium and extravasation. Circulating eosinophils can be envisioned as potentially being in different activation states, including non-activated, pre-activated or “primed”, or fully activated. In addition, the circulation can potentially be deficient of pre-activated or activated eosinophils, because such cells have marginated on activated endothelium or extravasated into the tissue. A number of eosinophil-surface proteins, including CD69, L-selectin, intercellular adhesion molecule-1 (ICAM-1, CD54), CD44, P-selectin glycoprotein ligand-1 (PSGL-1, CD162), cytokine receptors, Fc receptors, integrins including αM integrin (CD11b), and activated conformations of Fc receptors and integrins have been proposed to report cell activation. Variation in eosinophil activation states may be associated with asthma activity. Eosinophil-surface proteins proposed to be activation markers, with a particular focus on integrins, and evidence for associations between activation states of blood eosinophils and features of asthma are reviewed here. Partial activation of β1 and β2 integrins on blood eosinophils, reported by monoclonal antibodies (mAb) N29 and KIM-127, is associated with impaired pulmonary function and airway eosinophilia, respectively, in non-severe asthma. The association with lung function does not occur in severe asthma, presumably due to greater eosinophil extravasation, specifically of activated or pre-activated cells, in severe disease.
Introduction
Asthma is frequently characterized by airway inflammation rich in eosinophils [1–32]. Airway eosinophilia is associated with exacerbations [1, 8, 9, 14, 33–37] and likely plays a role in airway remodeling [1, 8, 36–39]. Recruitment of eosinophils from the circulation requires that blood eosinophils become activated, leading to their arrest on activated endothelium and extravasation [40–44]. This review will discuss cell-surface proteins proposed to report or potentially reporting eosinophil activation. It will particularly focus on the integrin family of cell adhesion receptors [45–50], the activation states or conformations of integrins [47, 48, 50–52], and evidence for associations between activation states of integrins on blood eosinophils and features of asthma, such as pulmonary function, and airway inflammation and eosinophilia. Arrest of eosinophils in vessels and their extravasation into the airway wall and through the bronchial tissue and epithelium to the airway lumen are mediated by integrins [12, 41–43, 53, 54]. Thus, there is a biological rationale for integrin conformation states as markers of eosinophil activation in asthma and as potential correlates with disease activity.
Eosinophil-surface proteins proposed to report cell activation
General remarks
The eosinophil surface phenotype, consisting of numerous cell-surface proteins, including adhesion molecules and cytokine, chemoattractant, complement, Fc, and innate immune receptors, has been reviewed extensively [5, 12, 13, 55–57]. Induction or upregulation, in some cases, downregulation, of a number of eosinophil-surface proteins, e.g., CD69 and αM integrin (CD11b), as well as activated conformations of Fc receptors (FcγRII = CD32) and integrins (β1 and β2) potentially report cell activation or have been proposed to be biomarkers in asthma (Table 1) [4, 40, 58–62]. Such suggestions have often been based on the response of blood eosinophils to various cytokines or other factors in vitro. Table 1 lists such suggested cell-surface proteins and alterations in the cell-surface protein expression, usually detected by flow cytometry. Unless indicated otherwise, alterations in Table 1 are on blood eosinophils.
Table 1.
Eosinophil-surface proteins potentially reporting cell activation.
| Protein | Obervation | Reference |
|---|---|---|
| CD4 | Upregulated by GM-CSF or IL-3 | [135] |
| CD9 | Upregulated during allergy season | [170] |
| CD35 (CR1) | Upregulated by fMLF, downregulated in BAL | [171, 210] |
| CD44 | Upregulated by IL-5, after segmental antigen challenge, in BAL, or in sputum, higher in well-controlled than poorly controlled asthma | [103, 106, 110, 124] |
| CD45RO | Upregulated in mild-moderate asthma | [125] |
| CD48 | Upregulated by IL-3 or in asthma | [199] |
| CD58 | Upregulated in BAL | [118] |
| CD63 (LAMP-3) | Upregulated by IFN-γ or in BAL | [118, 211] |
| CD66e (CEACAM5) | Upregulated after segmental antigen challenge or in BAL | [110] |
| CD67 | Upregulated by PAF+fMLF or in BAL | [116, 118] |
| CD69 | Upregulated by IL-5, GM-CSF, IL-3, IFN-γ, IL-4, IL-13, TNF-α, or IL-17; after whole-lung antigen challenge; or in BAL | [65, 101–111] |
| CD81 | Upregulated by IL-5, GM-CSF or IL-3 | [65] |
| αL integrin (CD11a) | Upregulated in asthma or after segmental antigen challenge, decreased by anti-IL-5 after segmental antigen challenge | [100, 161] |
| αM integrin (CD11b) | Upregulated by IL-3, GM-CSF, IL-5, eotaxin-1, fMLF, PAF, RANTES, C5a, TNF-α, IL-33, or ATP; during allergy season; after segmental antigen challenge; on cells with platelet satellitism after whole-lung antigen challenge; in BAL; or in sputum; decreased by anti-IL-5 after segmental antigen challenge | [63, 68, 100, 101, 112, 113, 117–120, 123, 151, 160, 162–171] |
| αX integrin (CD11c) | Upregulated in BAL or in sputum | [118, 120] |
| αD integrin | Upregulated by IL-5 or in BAL | [63, 66, 100, 172] |
| β2 integrin (CD18) | Upregulated by IL-5, GM-CSF, IL-3, eotaxin-1 or TSLP; after segmental antigen challenge; on cells with platelet satellitism in AERD; in BAL; decreased by anti-IL-5 after segmental antigen challenge | [63, 68, 100, 114, 123, 149, 163] |
| Aminopeptidase N (CD13) | Upregulated by IL-3, IL-5, or GM-CSF, or in BAL | [212] |
| FcαRI (CD89) | Upregulated in asthma or allergy | [141] |
| FcεRII (CD23) | Upregulated by IL-5, GM-CSF or IL-3 | [65] |
| FcγRI (CD64) | Upregulated by IFN-γ | [142] |
| FcγRII (CD32) | Upregulated by IFN-γ or IL-3 | [142, 143] |
| FcγRIII (CD16) | Upregulated by C5a, fMLF, PAF, or IFN-γ; in allergy or allergic asthma; or after whole-lung antigen challenge | [141, 142, 144, 145] |
| Galectin-3 | Upregulated in allergy | [200] |
| HLA-DR | Upregulated by IFN-γ, GM-CSF, or IL-4; in BAL; or in sputum | [118, 120, 122, 171, 213] |
| ICAM-1 (CD54) | Upregulated by IL-3, GM-CSF, IL-5, IFN-γ, or TSLP; in BAL; or in sputum | [114, 118, 120–123] |
| IL-2Rα (CD25) | Upregulated by GM-CSF or IL-3, or in BAL, downregulated by IFN-γ | [110, 134, 135] |
| IL-3Rα (CD123) | Upregulated by IL-3, IL-5, or GM-CSF, after segmental antigen challenge, or in BAL | [108, 110, 136] |
| IL-5Rα (CD125) | Downregulated by IL-5, GM-CSF, or IL-3, or in BAL; increased by anti-IL-5 | [107, 108, 136–138] |
| IL-13Rα1 (CD213a1) | Upregulated by TGF-β, IFN-γ, TNF-α, IL-5, or GM-CSF; downregulated by IL-13 or IL-4 | [139] |
| IL-17RA (subunit of IL-25R) | Upregulated in mild allergic asthma | [140] |
| IL-17RB (subunit of IL-25R) | Upregulated in mild allergic asthma | [140] |
| L-selectin (CD62L) | Downregulated by IL-5, PAF, fMLF, or TSLP; in BAL; or in sputum | [112–119] |
| Neuropeptide S receptor | Upregulated in severe asthma | [203] |
| PSGL-1 (CD162) | Downregulated by PAF, upregulated after segmental antigen challenge, decreased transiently after whole-lung antigen challenge, decreased by anti-IL-5 after segmental antigen challenge | [100, 130, 131] |
| Semaphorin 7A (CD108) | Upregulated by IL-3, GM-SCF, or IL-5; or in BAL | [136] |
| TSLP receptor | Upregulated by TNF-α and IL-3 | [214] |
| Activated αM integrin (CD11b) | High-activity state induced by IL-5, RANTES, MCP-3, or C5a; or in BAL | [66, 68, 100, 160, 197, 198] |
| Activated β1 integrin (CD29) | Intermediate-activity state induced by P-selectin, in non-severe but not severe asthma, or after segmental antigen challenge in dual responders; correlates with eosinophil-bound or platelet-surface P-selectin; increased on cells with high level of surface-associated P-selectin; decreased on cells non-adherent to VCAM-1 or transiently after whole-lung antigen challenge | [63, 64, 130] |
| High-activity state in BAL | [100] | |
| Activated β2 integrin (CD18) | Intermediate-activity state decreased by anti-IL-5 | [100] |
| High-activity state induced by IL-5 or in BAL | [63, 64, 68, 100] | |
| Activated FcγRII (CD32) | Activated state induced by IL-5, GM-CSF, or fMLF; in mild asthma; after whole-lung antigen challenge in dual responders; or in BAL | [112, 146] |
Observations refer to cell-surface expression level, usually determined by flow cytometry, and are, if not indicated otherwise, on blood eosinophils.
AERD, aspirin-exacerbated respiratory disease; ATP, adenosine triphosphate; BAL, bronchoalveolar lavage; C, complement (factor); CEACAM, carcinoembryonic antigen-related cell adhesion molecule; Fc, fragment, crystallizable (of immunoglobulin); fMLF, formyl-methionyl-leucyl-phenylalanine; GM-CSF, granulocyte macrophage-colony stimulating factor; HLA, human leukocyte antigen; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; LAMP, lysosomal-associated membrane protein; MCP, monocyte chemotactic protein; PAF, platelet activating factor; PSGL, P-selectin glycoprotein ligand; R, receptor; RANTES, regulated on activation, normal T cell expressed and secreted; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin; VCAM, vascular cell adhesion molecule.
“Upregulation” and “downregulation”, etc., in Table 1 and throughout the text of this review refer to increased cell-surface protein expression, regardless of the mechanism in the individual case, which may be mobilization from intracellular stores or the result of increased transcription or translation. Further, upregulation may mean that an increase in average level on all eosinophils has been reported and/or an increase in the percentage of eosinophils positive for a particular protein. Although the percentage positive cells and expression level often appear to correlate, percentage positivity plateaus and is no longer informative beyond a certain level; when positivity reaches close to or 100%, expression level continues to increase and thus has a greater dynamic range [63]. In addition, expression level has been reported in various ways, including by arithmetic or geometric mean or median fluorescent intensity or channel fluorescence (CF). Whenever possible, the dynamic range in percentage positive cells or expression level among subjects, upon stimulation in vitro, or, e.g., between airway and blood eosinophis, is given in the text. However, considering the various different ways that data have been reported, the dynamic ranges of different proteins or in different publications are not always directly comparable. Further, Table 1 does not distinguish between different incubation times for the in vitro experiments, which range from minutes to days. In some cases, in vitro studies of blood eosinophils have been complemented by comparisons of blood eosinophils among subjects with allergic or non-allergic asthma, allergy without asthma, or normal healthy control subjects, or observations of blood eosinophils after whole or segmental lung antigen challenge, BAL eosinophils, or sputum eosinophils.
Many of the studies are on purified eosinophils, whereas some are on whole blood or BAL cells. Use of unfractionated cells has several advantages, including the requirement for only a small volume of blood making repeated sampling in the same subject possible, and the fact that purified eosinophils are not a completely accurate reflection of eosinophils in vivo. For instance, the signal for mAb N29, reporting the intermediate-activity conformation of β1 integrins [41], increases upon purification of blood eosinophils [64]; thus, β1 becomes more activated during the purification process. Similarly, CD35 and CD81 expression on purified eosinophils is higher and less variable among subjects than that on eosinophils in whole blood [65].
As will become evident below, high expression levels or activation states of cell-surface proteins achieved on BAL eosinophils are often not observed on circulating eosinophils. In addition, concentrations of cytokines or other factors required to produce a fully activated state in vitro, e.g., with highly activated αMβ2 integrin or high degree of adhesion, are often relatively high, ≥ 10 ng/ml interleukin (IL)-5, granulocyte macrophage-colony stimulating factor (GM-CSF), or IL-3 [66–68]. In asthmatic lung, extrapolated from levels found in BAL [63, 69], IL-5 family cytokines range from 0.1 to 100 ng/ml [63, 70–74], whereas levels in peripheral blood in asthma are lower, e.g., 1–10 pg/ml [75–80]. Also for other stimuli, such as eotaxins, IL-4, IL-13, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, the same discrepancy between lower concentrations in blood [77–85] and higher concentrations in inflamed airway [63, 73, 86–90] exists, supporting a scenario in asthma where eosinophils likely are exposed to high mediator concentrations only after entering vasculature of the lung or airway tissue.
Finally, it is worth mentioning in this context, although it is not the focus of this review, that in addition to expression of potential activation markers, pre-activation or “priming” of blood eosinophils as a result of systemic inflammation have been evaluated using functional assays [40, 91, 92]. Such experiments have shown that blood eosinophils from subjects with allergy or asthma, particularly after antigen challenge, have a greater degree of adhesion or transendothelial migration or greater responsiveness to chemoattractants for chemotaxis or activation of the respiratory burst, whereas blood eosinophils from normal donors can be “primed” for greater responses by IL-5 family cytokines in vitro [40, 93–99]. A disadvantage with the functional assays is the need for isolated eosinophils; cell purification requires a larger blood volume and may in itself promote more activation, as discussed above. It is possible that the “priming” seen with the functional assays and believed to be a result of exposure to IL-5 or similar cytokines in vivo [40, 91, 92] is associated with changes in activation markers, such as seen in the IL-5-dependent (decreased by anti-IL-5 in vivo) presence of the intermediate-activity state of β2 integrins or the upregulation of αL, αL, and β2 after segmental antigen challenge (see below and Table 1) [100].
CD69
CD69, an early activation antigen of T cells, was suggested in the beginning of the 1990s as a marker of eosinophil activation [4, 59, 60, 101, 102]. It is absent or expressed at a low level on unstimulated blood eosinophils, with from 0% to about 30% positive cells [65, 101–109]. The expression level, although low, varies up to 50-fold among subjects (by median CF, defined as described [66]) [110]. It is induced in vitro by IL-5 or the other IL-5 family cytokines IL-3, GM-CSF, or by cytokines of other classes (Table 1) [65, 101–110]. In different studies percentage positive cells were increased to 50–90% and level about 3–50-fold by IL-5 family cytokines (by fluorescence intensity or CF) [65, 101, 104–110]. Further, CD69 is induced transiently on blood eosinophils after whole-lung antigen challenge (from 1–4% to 10–20% positive cells) [111] and on BAL eosinophils (up to four-fold level compared to blood eosinophils) [101, 103, 105]. In one study of approximately 350 mAbs, only those against CD69 reacted with cytokine-stimulated blood eosinophils and BAL eosinophils but not with unstimulated blood eosinophils [103], supporting the suggestion of CD69 as an eosinophil activation marker [59, 60]. However, a comparison by Johnsson and others among patients with asthma or airway allergy, other eosinophilic diseases, and normal healthy control subjects of expression levels of multiple surface proteins on eosinophils in blood did not find any differences in CD69 expression among the groups [77].
L-selectin
L-selectin (CD62L) is another proposed eosinophil activation marker [40, 59, 60]. L-selectin is constitutively expressed by blood eosinophils, with level varying about six-fold among subjects (by fluorescence intensity) [112]. It is downregulated in response various mediators (Table 1) (to an 0.2–0.6-fold level, by intensity or CF), through a mechanism involving metalloproteinase-mediated shedding [112–116]. It is also downregulated on BAL eosinophils (to an 0.2-fold level of that on blood eosinophils or from about 70% to 20% positive cells) [116–118] and sputum eosinophils (to an 0.1–0.3-fold level) [119]. However, comparing subjects with severe or mild asthma, or normal subjects, no difference in blood eosinophil L-selectin expression was found [112, 119].
ICAM-1
ICAM-1 (CD54) is not expressed or only expressed at a low level on blood eosinophils [60, 118, 120, 121]. ICAM-1 is induced by IL-5 family cytokines and other cytokines, about 3–20-fold (by fluorescence intensity) (Table 1) [114, 121–123]. It is expressed on BAL (1.8-fold of the level on blood eosinophils) [118] and sputum [120] eosinophils. Patients with asthma or airway allergy as a group do not have higher expression than normal subjects [77].
CD44
CD44, a hyaluronan receptor and another suggested activation marker [59, 60, 110], is normally expressed on blood eosinophils [59, 60, 103, 124] (with 40–60% positive cells [106] and level varying about at least four-fold among subjects, by fluorescence intensity [124], more by CF [110]). CD44 is upregulated by IL-5 (1.6-fold and to 60–70% positivity) (Table 1) [103, 106]. Further, it is modestly upregulated on blood eosinophils after segmental lung antigen challenge (less than two-fold by CF) [110] and upregulated on BAL (five-to-six-fold) [110] and sputum (about 1.4-fold by intensity) [124] eosinophils. No differences have been found between CD44 expression on blood eosinophils in patients with asthma as a group and normal subjects [77, 125]. However, interestingly, Sano and colleagues found that the level of blood eosinophil CD44 expression was higher in patients with well-controlled than poorly controlled asthma (about 1.7-fold) and suggested that this implies that the transmigration of activated, CD44-high eosinophils from the circulation is facilitated [124]. Extravasation of eosinophils with the highest levels of CD44 is compatible with a contribution for CD44 to eosinophil recruitment to the airway in a mouse asthma model [126–128]. Further, CD44 becomes redistributed on blood eosinophils within minutes after addition of IL-5, GM-CSF, IL-3, or eotaxin-1, when the cell undergoes shape change and polarization, concentrating at and covering one pole of the cell, the nucleopod, which constitutes a specialized uropod occupied by the nucleus; such reorganization of CD44 and other receptors may promote eosinophil arrest, extravasation, and migration [68].
PSGL-1
P-selectin glycoprotein ligand-1 (PSGL-1, CD162), the eosinophil counter-receptor for P-selectin [129], is constitutively expressed at a high level on blood eosinophils [64, 100, 130, 131] (varying about two-fold among subjects, by geometric mean CF) [64, 130]. It is downregulated in vitro in response to platelet-activating factor (PAF), presumably by shedding [131]. However, whether such shedding occurs in vivo is uncertain. Unlike L-selectin, PSGL-1 is not downregulated on BAL eosinophils [100]. It is modestly upregulated on blood eosinophils 48 h after segmental antigen challenge (1.1-fold by CF); this increase is ablated after anti-IL-5 administration, indicating that IL-5 can be responsible for PSGL-1 upregulation in vivo [100]. After whole-lung antigen challenge, which is a more major insult and a model of asthma exacerbation [132, 133], blood eosinophil PSGL-1 is first modestly decreased at 8 h (to about 0.8-fold of the baseline level) followed by a recovery and increase at 48 h to about 1.1-fold above baseline, supporting a scenario in which the cells with the highest PSGL-1 expression extravasate [130]. Like CD44, PSGL-1 on blood eosinophils becomes localized at the nucleopod during IL-5-stimulated cell polarization [68].
Cytokine receptors
Several cytokine receptors, upregulated or downregulated, have been proposed to report eosinophil activation (Table 1) [4, 59, 60, 110]. IL-2 receptor (IL-2R, CD25) expression on blood eosinophils (varying from 3% to about 60% positive cells [134, 135]) is upregulated by GM-CSF [134, 135] but downregulated by IFN-γ [134]. IL-2R is upregulated on BAL eosinophils (about 1.4-fold by median channel fluorescence) [110], but not increased on blood eosinophils after segmental antigen challenge [110]. It was found not be different between asthma and allergy, and normal subjects [77].
IL-5Rα (CD125) on blood eosinophils is downregulated in vitro by its own ligand IL-5 as well as by the related cytokines GM-CSF and IL-3 (to about 0.1–0.3-fold level, by intensity or CF, or from about 80–90% to 10% positive cells) [107, 108, 136, 137], through the involvement of metalloproteinase-mediated shedding [107]. Further, it is downregulated on BAL eosinophils (to 0.4-fold of the blood eosinophil level and to 10% positivity) [137]. Blood eosinophil IL-5Rα expression has been reported to be increased after administration of anti-IL-5 mepolizumab [138], supporting the scenario that IL-5 regulates the expression of its own receptor in vivo.
In contrast to IL-5Rα, IL-3Rα (CD123), expressed on blood eosinophils at a level varying about four-fold among subjects (by CF) [110], is upregulated by its own ligand and the other IL-5 family cytokines (about three- to ten-fold by fluorescence intensity) [108, 136]. It is modestly upregulated on blood eosinophils after segmental antigen challenge (about 1.2-fold) and more highly upregulated on BAL eosinophils (about 2.3-fold) [110].
IL-13Rα1 (CD213a1) on blood eosinophils is upregulated by various cytokines (up to about 2.5-fold) (Table 1) but downregulated by its own ligand IL-13 and the related cytokine IL-4 (to about a 0.5-fold level) [139].
Finally, expression of the subunits of the IL-25 receptor, IL-17RA and IL-17RB (varying among subjects from about 0% to 100% positive blood eosinophils) was recently found to be elevated in patients with mild allergic asthma compared to atopic non-asthmatic patients and normal subjects (median about 100% and 50% positive for IL-17RA and IL7RB, respectively, in asthma versus about 70% and 20% in the other groups) [140].
To summarize, various cytokine receptors are up- or down-regulated on blood eosinophils in response to their own ligands or other cytokines in vitro, have altered expression levels on BAL eosinophils, and may have moderately altered expression on blood eosinophils after antigen challenge or when comparing subjects with asthma to normal subjects.
Fc receptors
Various Fc receptors (i.e., receptors for immunoglobulins), their induction, upregulation, or activation, have been suggested to report eosinophil activation (Table 1) [4, 40, 58, 60, 61]. Expression of FcαRI (CD89), an immunoglobulin (Ig) A receptor, on blood eosinophils is higher in subjects with asthma and/or allergic rhinitis than in normal subjects (about two-fold, by fluorescence intensity) [141]. FcεRII (CD23), an IgE receptor, on blood eosinophils (varying from 0% to about 50% among subjects and three-fold, by intensity) is upregulated by IL-5 family cytokines (1.1–1.3-fold) [65], but was found not to be different between subjects with asthma or airway allergy compared to normal subjects [77]. The IgG receptor FcγRI (CD64) is induced on blood eosinophils by IFN-γ (about eight-fold) [142]. FcγRII (CD32), another IgG receptor, on blood eosinophils is upregulated by IFN-γ or IL-3 (1.7-fold by intensity or from 15–59% to 32–72% positive cells) [142, 143], but is not different in subjects with asthma and/or allergic rhinitis from normals [141].
FcγRIII (CD16), a third IgG receptor, is not expressed or expressed at a low level on unstimulated blood eosinophils [141, 142, 144, 145] but is induced by various chemoattractants and other mediators (from 0% up to about 30% positive cells) (Table 1) [142, 144]. CD16 expression of blood eosinophils is increased after whole-lung antigen challenge [145]. Monteiro and colleagues as well as Davoine and others found it to be higher in allergic asthma and/or allergic rhinitis than in normal subjects (ranging about 0–30% in allergic asthma and 0–10% in normals) [141, 145], whereas Johnsson et al. found no difference between asthma or airway allergy as a group and normals [77].
Interestingly, two activation-sensitive mAbs, A17 and A27, have been developed [146] that recognize an activated form of FcγRII (CD32) [40, 112]. Using these mAbs, the signal of unstimulated blood eosinophils varied at least 20-fold among subjects [112, 146], and CD32 was found to become more activated in response to IL-5, GM-CSF, or fMLF in vitro (up to about tenfold, by intensity) [112, 146]. CD32 on eosinophils in blood from patients with mild asthma is more activated than on cells from normal subjects (two-four-fold) [112, 146]. Blood eosinophil CD32 becomes more activated after whole-lung challenge in patients with a dual-response asthma phenotype (up to 1.8-fold) [112, 146]. Finally, CD32 is more activated on BAL eosinophils than on blood eosinophils (about three-fold) [146].
Taken together, Fc receptors are induced, upregulated, or, at least in the case of CD32, activated by cytokines or chemoattractants and may be upregulated or activated by antigen challenge, in subjects with asthma, or on BAL eosinophils.
Integrins
Integrins are heterodimers of α and β subunits [45, 46]. Eosinophils express seven integrins, α4β1 (CD49d/CD29), α6β1 (CD49f/CD29), αLβ2 (CD11a/CD18), αMβ2 (CD11b/CD18), αXβ2 (CD11c/CD18), αDβ2, and α4β7 [5, 12, 13, 41, 42, 55, 128], which potentially interact with ligands including vascular cell adhesion molecule (VCAM)-1, ICAM-1, laminin, fibrinogen/fibrin, vitronectin, and periostin, on other cells or in the extracellular matrix (ECM) [41, 42, 67]. The platelet integrin αIIbβ3 (CD41/CD61) [147] is not synthesized by eosinophils [110] but can be detected by flow cytometry or immunofluorescence microscopy staining on a variable proportion of eosinophils (in humans and mice), due to association of platelets or platelet fragments with some of the cells [64, 130, 148–152]; activated platelets are known to bind leukocytes via P-selectin [153]. Some workers have detected low levels of additional integrins, including α2β1 (CD49b/CD29), which is also not synthesized by eosinophils [110], on the eosinophil surface [154–156]; this is similarly likely due to platelet “satellitism”. It has been reported that (human and mouse) eosinophils with platelet satellitism also can have increased levels of endogenous eosinophil integrins, including α4, αM, and β2 (Table 1) [149–151]. Similarly, eosinophils with high level of surface-associated P-selectin (presumably primarily derived from activated platelets, most of these cells also have platelet satellitism [64]) have increased reactivity with mAb N29, demonstrating that they have a higher number of their β1 integrins in the intermediate-activity conformation [64] (see Table 1 and more below).
Studies with β2 integrin-deficient and conditionally α4 integrin-deficient mice, or with mAbs in wild-type mice, indicate that both α4 and β2 integrins mediate eosinophil recruitment to the airway [53, 54, 157, 158]. Such data together with results from in vitro adhesion experiments on human cells [41, 42, 66, 67, 159, 160] indicate that α4β1 and αMβ2 are the principal integrins mediating eosinophil adhesion, with α4β1 largely responsible for arrest of blood eosinophils on VCAM-1 on activated endothelium in vessels of the asthmatic lung, with a more minor contribution by αMβ; whereas activated αMβ2, by interacting with periostin and possibly other ligands, is involved in subsequent eosinophil movement to and persistence in the ECM of the bronchi in asthma [41] (see Fig. 1 for model).
Fig. 1. Model of activation states of eosinophils in circulation and during arrest and extravasation in asthma.
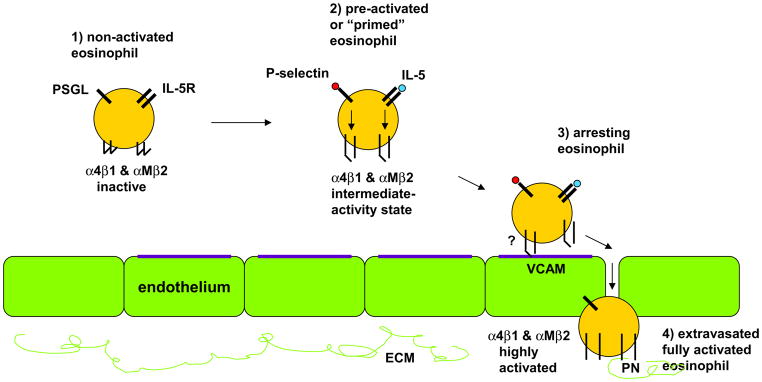
- Circulating non-activated eosinophil with α4β1 and αMβ2 in the inactive integrin conformation, as found in normal subjects, some subjects with non-severe asthma, or in severe asthma; sampled in severe asthma due to great extravasation of activated cells.
- Pre-activated or “primed” circulating eosinophil with (variable numbers of) α4β1 and αMβ2 in the intermediate-activity integrin conformation, as a result of P-selectin- and IL-5-triggered signaling, respectively, as found to varying degree primarily in some subjects with non-severe asthma (in vivo it is most likely P-selectin on the surface of activated platelets that is responsible for this activation of α4β1).
- Eosinophil arresting on activated endothelium in asthma with α4β1 and αMβ2 in unknown state, likely in the intermediate-activity integrin conformation, with α4β1 primarily mediating arrest on VCAM-1 with a possible minor contribution of αMβ2.
- Extravasated or tissue eosinophil in asthma with α4β1 and αMβ2 in the high-activity integrin conformation (and with down-regulated IL-5 receptor), and αMβ2 interacting with periostin in the extracellular matrix.
Please see text for references.
ECM, extracellular matrix; IL-5, interleukin-5; IL-5R, interleukin-5 receptor; PN, periostin; PSGL, P-selectin glycoprotein-1; VCAM, vascular cell adhesion molecule-1.
The expression level of several integrins has been proposed to report eosinophil activation (Table 1) [4, 40, 59, 60]. αL expression on blood eosinophils (varying about 20-fold, by CF), was found to be higher (about five-fold) in patients with asthma than in normal subjects [161]. It was modestly upregulated on blood eosinophils after segmental antigen challenge (1.2-fold by CF); this increase was abolished by anti-IL-5, indicating that IL-5 upregulates αLβ2 in vivo [100]. αM level (varying up to about 20-fold among subjects [112]) is upregulated by the IL-5 family cytokines or various other cytokines, chemoattractants and mediators (up to about fourfold by fluorescence intensity) (Table 1) [68, 101, 112, 113, 123, 160, 162–169]. It was reported to be higher (about 1.5-fold) during the allergy season [170]. Like αL, αM is modestly increased (1.2-fold by CF) on blood eosinophils after segmental antigen challenge in a manner prevented by anti-IL-5 [100]. It is upregulated to a greater degree (1.5–1.8-fold by CF or about three-fold by intensity) on BAL [63, 100, 117, 118, 164, 171] and sputum (up to 18-fold) [119, 120] eosinophils. However, blood eosinophil αM level is not significantly different among patients with mild and severe asthma, and normal subjects [77, 112, 119, 125, 161]. αX is increased (1.8-fold by intensity) on BAL [118] and (about three-fold) on sputum [120] eosinophils but was found not to be different between subjects with asthma or airway allergy and normal subjects [77]. αD is expressed at a low level on eosinophils in blood [100] and is induced (about four-fold by intensity) on blood eosinophils in response to IL-5 [172], as well as is induced (12-fold by CF, two-fold by intensity) on BAL eosinophils [63, 66, 100, 172]. β2 is upregulated by the IL-5 family cytokines and other factors (about 1.5-fold by intensity) (Table 1) [68, 114, 123, 163]. Like αL and αM, it is modestly upregulated after segmental antigen challenge and upregulated to a somewhat greater degree on BAL eosinophils (1.5-fold) [63, 100]. Blood eosinophil β2 expression is not different between subjects with asthma or allergy and normals [77].
Whether an integrin mediates adhesion to and migration on a particular ligand depends on the activation state of the integrin [50, 173–175]; this is at least as important as the expression level. Integrins exist in three major conformations, one inactive, one intermediate-activity, and one high-activity conformation [47, 48, 50–52]. The activation states of integrins can be monitored by conformation-specific monoclonal antibodies (mAbs) [41, 176, 177]. Integrins are activated by so-called “inside-out” signaling triggered through other receptors, including G-protein coupled receptors (GPCRs), and mediated by proteins including talin and kindlins that bind the cytoplasmic tail of integrin β subunits [47, 48, 50, 178, 179].
Eosinophils in blood, on the average, express the epitopes for mAbs N29 and 8E3 [63, 64, 130, 180], which recognize intermediate- and high-activity β1 [41, 177, 181–184], but have no or very low expression of epitopes for mAbs HUTS-21 and 9EG7, which recognize only high-activity β1 [41, 177, 185–187], indicating that their β1 integrins, including α4β1, are in the intermediate conformation. However, N29 and 8E3 reactivities are variable among subjects, ranging from subjects with no signal and thus inactive β1 integrins over some with low but detectable N29 signal (i.e, a number or a fraction of β1 integrin molecules on each cell in the intermediate-activity state) to some with high N29 signal (i.e., presumably most molecules on each cell having the intermediate conformation), with N29 varying from a geometric mean CF of 0 to about 700 and positivity up to about 80%) [41, 63, 64, 130], presumably conferring greatly variable capacity to arrest on VCAM-1 on activated endothelium [41] (Fig. 1). N29 reactivity of eosinophils in whole blood can be increased by P-selectin, but not by IL-5, in vitro (up to about 1.5-fold by CF, to a greater degree in normal than in subjects with asthma and/or allergy) [64]. The N29 signal correlates with eosinophil-bound [64, 130] or platelet-surface P-selectin [130] in vivo. Thus, in vivo it is most likely the P-selectin on the surface of activated platelets that is responsible for inducing the intermediate-activity conformation of β1 integrins on blood eosinophils [41, 130], even though a proportion of soluble plasma P-selectin in asthma appears to be derived from activated endothelial cells [188]. The results on platelet P-selectin are compatible with data showing that activated platelets promote eosinophil recruitment to the airway in mice in a P-selectin-dependent manner [150, 152] and an in vitro study that observed increased complex formation between activated P-selectin-bearing platelets and human blood eosinophils from subjects with allergic asthma and indicated that platelet association contributes to the enhanced tethering of such eosinophils to activated endothelium in a P-selectin-dependent manner [189]. As a group, patients with asthma or non-severe asthma, but not severe asthma, have a higher N29 reactivity than normal subjects (2.2-fold for non-severe asthma) [130]. In dual responders, the N29 signal is increased 48 h after segmental antigen challenge (about 1.6-fold) [63]. After whole-lung antigen challenge, N29 reactivity decreases at 8 h (to about an 0.5-fold level) and recovers at 48 h [130], indicating that eosinophils with the highest proportion of activated β1 integrins are the ones that extravasate. We have suggested that a similar phenomenon, i.e., that the eosinophils with the most activated α4β1 are efficiently removed from the circulation, occurs continuously in severe asthma [130] (Fig. 1). One possible reason for such efficient extravasation may be the greater lung endothelial VCAM-1 expression in severe asthma, as observed in bronchial biopsies [190]. In vitro, the proportion of eosinophils that do not attach to VCAM-1 have lower N29 signal [64], also supporting the idea that the cells with the most activated α4β1 are the ones that preferentially adhere. BAL eosinophils have β1 integrins in the high-activity conformation, judged by their reactivity with mAbs HUTS-21 and 9EG7 [100].
Eosinophils in blood have a low but detectable reactivity with mAb KIM-127 [100], which recognizes intermediate- and high-activity β2 integrins [41, 47, 177, 191, 192], but very low reactivity with mAb24 [63], which recognizes only high-activity β2 integrins [41, 47, 177, 193–195]. The KIM-127 signal is decreased after anti-IL-5 administration [100]. In addition, blood eosinophils have no or very low reactivity with activation-sensitive anti-αM mAb CBRM1/5 [66, 100], which reports the high-activity state of αMβ2 integrin [41, 177, 196]. Together, these data indicate that blood eosinophils have a fraction of their β2 integrins, including αMβ2, in the intermediate-activity conformation [41] (Fig. 1), as a result of in vivo exposure to either the low concentrations of IL-5 present in blood [75–79] or higher concentrations in, e.g., the bone marrow [7, 84]. In vitro, high (ng/ml) doses of IL-5, but not P-selectin, induces mAb24 (1.6-fold by CF, four-fold by intensity) [64, 68] or CBRM1/5 (up to about three-fold by intensity) [66, 68, 160] reactivity of blood eosinophils. In addition, CBRM1/5 reactivity is induced by various, but not all, chemoattractants, e.g., C5a and others but not eotaxin-1 or IL-8 [197, 198]. BAL eosinophils recognize mAb24 [63, 100] and CBRM1/5 [66, 100] and thus display high-activity αMβ2. As with CD44 and PSGL-1, activated αMβ2 becomes localized to the nucleopod in IL-5-polarized blood eosinophils [68].
In summary, αM, αD, and β2, are upregulated by IL-5 in vitro and αM and β2 also by other mediators; αL, αM, and β2 are modestly upregulated after segmental antigen challenge in an apparently IL-5-dependent manner; and αM, αX, αD, and β2 are upregulated on BAL eosinophils. β1 and β2 integrins are in the intermediate-activity state on blood eosinophils to varying degree among subjects, β1 likely as a result of interaction with P-selectin, primarily on activated platelets, and β2 as a result of low levels of IL-5 (Fig. 1). The high-activity state of αMβ2 can be induced in vitro by IL-5 or some chemoattractants. β1 and αMβ2 are in high-activity states on BAL eosinophils.
Others
Various other cell-surface proteins are potential eosinophil activation markers (Table 1) [4, 40, 60, 110]. These include CD9, whose expression on blood eosinophils from subjects with allergic rhinitis and occasional asthma was found to be higher during a high-pollen load season (1.2-fold by fluorescence intensity) [170] but was in another study not different between subjects with asthma or airway allergy and normal subjects [77]. CD45RO expression on blood eosinophils is higher in patients with mild-moderate asthma than in normals (about 65% positive cells versus 5%) [125]. CD48 on blood eosinophils is upregulated by IL-3 (about two-fold by intensity), but not by IL-5 or GM-CSF, and is higher in patients with asthma (two-fold) than in normal subjects [199]. CD66e is modestly upregulated (about 1.3-fold by CF) on blood eosinophils after segmental antigen challenge and more highly upregulated (three-fold) on BAL eosinophils [110]. Galectin-3 expression on blood eosinophils is higher in allergic than in normal subjects (about 12% versus 4% positive cells) [200], compatible with the ability of galectin-3 to interact with α4β1 integrin and contribute to eosinophil rolling and arrest on VCAM-1 or activated endothelium in vitro [200] and to eosinophil recruitment to the airway in vivo [158, 201, 202]. Neuropeptide S receptor expression is higher in patients with severe asthma than in patients with mild asthma or normal subjects (two-to-three-fold by intensity) [203].
Some final remarks on activation markers
As described above, multiple eosinophil-surface proteins are potential markers of or have been proposed to report cell activation, some of which are altered in asthma, upon antigen challenge, or on BAL or sputum eosinophils (Table 1). There are various aspects or patterns of eosinophil activation in vivo and in vitro. Many proteins are upregulated on airway eosinophils, while a few, like L-selectin or IL-5Rα, are downregulated. One group of proteins, including αL, αM, and β2, integrins, PSGL-1, CD44, CD66e, and IL-3Rα, are modestly upregulated on blood eosinophils after segmental antigen challenge [100, 110], at least the first four of these in an apparently IL-5-dependent fashion [100], and more highly upregulated on BAL eosinophils [100, 110]. Many but not all proteins mentioned are upregulated or activated in vitro by IL-5 family cytokines or chemoattractants. For instance, αMβ2 integrin is upregulated or activated by IL-5 but not P-selectin; whereas β1 integrins are activated by P-selectin and not IL-5 [41, 64]. IL-13Rα1 is upregulated by various cytokines including IL-5 or GM-CSF (Table 1) but downregulated by IL-4 or IL-13 [139]. IL-2Rα is upregulated by GM-CSF but downregulated by IFN-γ [134]. Further, there are different time frames of activation in vitro, ranging from minutes over hours to days. Activation of integrins or FcγRII occurs within minutes to an hour [64, 66, 68, 112, 146, 160, 197, 198]. One group, including αM integrin, CD16, and CD63, are rapidly mobilized to the cell surface [40, 144], presumably due to transport of preformed proteins from granules [204]. Another group, including CD48, αM integrin, aminopeptidase N (CD13), ICAM-1, and semaphorin 7A, appear to be upregulated to a greater degree in response to IL-3 than to other cytokines over an approximately 16–24 h period [101, 123, 136, 199]. In summary, the potential activation markers reflect exposure to partly similar and partly different stimuli and time frames.
Associations with features of asthma
Although many surface proteins have been proposed as eosinophil activation markers or biomarkers in asthma, for only a few of those described above have their expression level or activation state been shown to correlate with features of asthma (Table 2). For these studies, unfractionated, whole blood has been used for flow cytometry as described above [63, 100, 112, 119, 130, 146, 180].
Table 2.
Correlations between activation states of blood eosinophils and features of asthma.
| Protein/Activation state | Correlation | Reference |
|---|---|---|
| Intermediate-activity β1 integrin | Inverse with FEV1 after or during ICS withdrawal in mild asthma, predicts decreased FEV1 in ROC curve analysis | [180] |
| FENO after ICS withdrawal in mild asthma | [180] | |
| Inverse with FEV1/FVC in younger subjects with non-severe asthma | [130] | |
| Inverse with FEV1/FVC in phenotype cluster 1 (mild atopic asthma) | [207] | |
| 48 h after segmental antigen challenge, correlates with late-phase FEV1 fall after whole-lung antigen challenge in mild allergic asthma | [63] | |
| Intermediate-activity β2 integrin | Percentage BAL eosinophils in mild allergic asthma | [100] |
| αM integrin level | Inverse with PC20 | [112, 119] |
| Activated FcγRII | FENO | [40] |
BAL, bronchoalveolar lavage; FENO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 s: FVC, forced vital capacity; ICS, inhaled corticosteroid; PC20, provocative concentration of methacholine or histamine producing a 20% fall in FEV1; R, receptor; ROC, receiver-operator characteristic.
Activated FcγRII (CD32) on blood eosinophils has been reported to correlate with fraction of exhaled nitric oxide (FENO), an indicator of airway inflammation, in asthma (Table 2) [40].
The expression level of αM integrin has been shown to correlate with airway hyperresponsiveness in moderate to severe asthma [119] or in patients with a dual-response asthma phenotype [112] (Table 2).
Variation in integrin activation states is likely more important than variation in expression levels. N29 reactivity of blood eosinophils, indicative of the intermediate-activity state of β1 integrins, correlated inversely with forced expiratory volume in 1 s (FEV1, as percentage of baseline) after inhaled corticosteroid (ICS) withdrawal or across all visits during a double-blind placebo-controlled, two-period crossover study in patients with mild asthma (Table 2) [180]. Receiver-operator characteristic (ROC) curve analysis demonstrated that the N29 signal predicted decreased FEV1 and performed better than did the established asthma markers sputum eosinophil percentage or FENO [180]. Further, N29 correlated with FENO after ICS withdrawal [180]. In another study, blood eosinophil N29 reactivity 48 h after segmental antigen challenge in subjects with mild allergic asthma correlated with the late-phase fall in FEV1 3–8 h after the whole-lung antigen challenge performed during screening [63]. The ICS withdrawal and antigen challenge studies were on subjects with non-severe asthma who were young (mean 21 years) [63, 180]. In an observational study that was part of the Severe Asthma Research Program (SARP) [205] on a population with asthma of varying severity and a higher mean age, N29 reactivity correlated with FEV1/FVC (FVC = forced vital capacity) in young subjects (under 30 years old) with non-severe asthma but did not correlate in severe asthma [130]. The subjects in that study belonged to a population that had been classified using cluster analysis [22, 206]. N29 correlated best and significantly with FEV1/FVC in cluster 1 [207], which consists of subjects with mild allergic asthma [22, 205, 206]. Blood eosinophil reactivity with mAb KIM-127, indicative of the intermediate-activity state of β2 integrins, at baseline (the time of segmental antigen challenge in subjects with mild allergic asthma) correlated with BAL eosinophil percentage 48 h later (Table 2) [100]. Unfortunately, KIM-127 was not assayed in the earlier studies.
A possible explanation for the lack of correlation between the N29 signal and lung function in severe asthma and older patients is high degree of ongoing extravasation of eosinophils with the most activated integrins, as discussed above. An additional possible explanation is that a subpopulation of subjects with asthma, likely particularly patients with severe asthma or older patients, do not have a persistent predominantly eosinophilic airway inflammatory phenotype but rather an intermittent or persistent mixed eosinophilic-neutrophilic, neutrophilic, or non-eosinophilic paucigranulocytic phenotype [18, 21, 23–27, 29–32], which may contribute to the weakening of the association between eosinophil activation and lung function. Future studies would be needed to address the question whether activation of, e.g, neutrophil integrins is associated with such less eosinophilic phenotypes.
Conclusions
This review has described multiple eosinophil-surface proteins that have been proposed to report or potentially report cell activation. Although many of these are upregulated or otherwise altered in response to cytokines, chemoattractants, or other stimuli in vitro, or after antigen challenge, in asthma, or on airway eosinophils in vivo, for only a few of those suggested have associations been found between their level or activation state and features of asthma.
These include greater degree of the intermediate-activity state of β1 integrins on blood eosinophils, reported by mAb N29, which is associated with decreased pulmonary function, late-phase response, and airway inflammation in subjects with non-severe asthma but not in severe asthma. In addition, intermediate β2 integrin activation, assessed by mAb KIM-127, is associated with airway eosinophilia in non-severe asthma.
The results from these studies indicate that the activation state of blood eosinophils varies among subjects, with normal subjects and some subjects with asthma having non-activated β1 integrins or only a small number of their β1 integrins in the intermediate-activity state, over subjects with non-severe asthma with a variable number of their β1 and β2 integrins in the intermediate-activity or partly activated state, to subjects with severe asthma that have a lower degree of intermediate-activity β1 integrins, presumably because the most activated cells have marginated on activated endothelium or extravasated [41, 130] (Fig. 1). Together with other data mentioned above, this indicates that in severe or uncontrolled asthma, the circulation becomes depleted of eosinophils that have the greatest degree of integrin activation, and possibly the highest levels of PSGL-1 and CD44. A similar model of FcγRII activation on circulating eosinophils was recently presented, in which activation first increases with increasing degree of systemic inflammation and then decreases at the highest level of systemic inflammation [40]. Fully activated eosinophils, characterized by integrins in the high-activity state and highly upregulated αMβ2 integrin and other proteins, as is the case on airway eosinophils (Table 1), are not or seldom seen in a blood sample, possibly because such cells may transiently be present immediately before arrest, or may only occur on arrested eosinophils before extravasation, or may occur only in the tissue and not in the circulation (Fig. 1).
The classical paradigm for leukocyte extravasation [208], which has been applied to eosinophils [43], depict circulating cells as having inactive integrins that become activated when rolling cells are exposed to chemokines associated with the surface of activated endothelium. This paradigm now needs to be modified to include in vivo pre-activation or “priming” [40, 91, 92], mediated by P-selectin (primarily on activated platelets) and IL-5, causing eosinophils to display integrins in partially activated conformations [41] (Fig. 1). The modified paradigm is in accord with other recent evidence that subsets of leukocytes have a fraction of their integrins in an intermediate-activity state [209].
Acknowledgments
I thank Deane Mosher and Stephane Esnault for excellent suggestions and comments on this manuscript and helpful discussions. I am grateful to many others, including William Busse, Nizar Jarjour, Loren Denlinger, Sameer Mathur, and Ronald Sorkness, without whom our studies on human subjects would not have been possible, and to them, Elizabeth Kelly, Michael Evans, and Gina Crisafi for discussions and steadfast research, administrative, and statistical support and advice. I thank Martin Humphries and Nancy Hogg for providing some of the activation-sensitive anti-integrin mAbs used in our studies and for discussions on integrin conformations. Our research and this review were mainly supported by Program Project grant P01 HL088594 and the Severe Asthma Research Program (SARP) grants R01 HL69116 and 1U10 HL109168 from the National Institutes of Health.
Footnotes
Conflict of interest: M.W. Johansson received a fee for consulting from Guidepoint Global.
References
- 1.Thomas A, Busse WW. The evolving role of eosinophils in asthma. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 448–62. [Google Scholar]
- 2.Scott KA, Wardlaw AJ. Eosinophilic airway disorders. Semin Respir Crit Care Med. 2006;27:128–33. doi: 10.1055/s-2006-939515. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–77. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochner BS, Book W, Busse WW, Butterfield J, Furuta GT, Gleich GJ, Klion AD, Lee JJ, Leiferman KM, Minnicozzi M, Moqbel R, Rothenberg ME, Schwartz LB, Simon HU, Wechsler ME, Weller PF. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) J Allergy Clin Immunol. 2012;130:587–96. doi: 10.1016/j.jaci.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauvreau GM, Denburg JA. Eosinophils and hemopoietic processes in allergic asthma. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 466–76. [Google Scholar]
- 8.Haldar P, Pavord ID, Wardlaw AJ. Insights into the pathogenesis of asthma and other eosinophil-mediated diseases from antagonists of interleukin-5 and its receptor. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 579–87. [Google Scholar]
- 9.Nair P. Eosinophil-targeted treatment of asthma. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 462–65. [Google Scholar]
- 10.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nature Rev Drug Discovery. 2013;12:117–29. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadif R, Zerimech F, Bouzigon E, Matran R. The role of eosinophils and basophils in allergic diseases considering genetic findings. Curr Opin Allergy Clin Immunol. 2013;13:507–13. doi: 10.1097/ACI.0b013e328364e9c0. [DOI] [PubMed] [Google Scholar]
- 12.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 14.Pavord ID. Eosinophilic phenotypes of airway disease. Ann Am Thoracic Soc. 2013;10(supplement):S143–S49. doi: 10.1513/AnnalsATS.201306-168AW. [DOI] [PubMed] [Google Scholar]
- 15.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegmann M. Targeting eosinophil biology in asthma therapy. Am J Respir Cell Mol Biol. 2011;45:667–74. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- 17.Arron JR, Scheerens H, Matthews JG. Redefining approaches to asthma: developing targeted biologic therapies. Adv Pharmacol. 2013;66:1–49. doi: 10.1016/B978-0-12-404717-4.00001-9. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 19.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–36. e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F, Gibson PG. Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J. 2011;38:567–74. doi: 10.1183/09031936.00170110. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 25.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–9. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavord ID, Gibson PG. Inflammometry: the current state of play. Thorax. 2012;67:191–2. doi: 10.1136/thoraxjnl-2012-201712. [DOI] [PubMed] [Google Scholar]
- 27.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.10.011. doi:10, 1016/j.jaci.2013.10.011 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin TY, Poon AH, Hamid Q. Asthma phenotypes and endotypes. Curr Opin Pulm Med. 2013;19:18–23. doi: 10.1097/MCP.0b013e32835b10ec. [DOI] [PubMed] [Google Scholar]
- 29.Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration. 2010;79:147–51. doi: 10.1159/000245899. [DOI] [PubMed] [Google Scholar]
- 30.Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67:835–46. doi: 10.1111/j.1398-9995.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 31.Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11. doi: 10.1186/1471-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67:675–81. doi: 10.1136/thoraxjnl-2011-201064. [DOI] [PubMed] [Google Scholar]
- 33.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 35.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 36.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol. 2010;125:803–13. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 37.Robinson DS. Mepolizumab treatment for asthma. Expert Opin Biol Ther. 2013;13:295–302. doi: 10.1517/14712598.2012.725717. [DOI] [PubMed] [Google Scholar]
- 38.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–82. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Koenderman L. Priming: a critical step in the control of eosinophil activation. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 170–79. [Google Scholar]
- 41.Johansson MW, Mosher DF. Integrin activation states and eosinophil recruitment in asthma. Front Pharmacol. 2013;4:33. doi: 10.3389/fphar.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 44.Wardlaw AJ. Eosinophil trafficking in asthma. Clin Med. 2001;1:214–8. doi: 10.7861/clinmedicine.1-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hynes RO. Integrins: A family of cell surface receptors. Cell. 1987;48:549–54. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 46.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–25. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 48.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–26. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 49.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–14. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–86. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee ER, Jiang Y, Henderson WR, Jr, Latchman Y, Papayannopoulou T. Absence of alpha 4 but not beta 2 integrins restrains development of chronic allergic asthma using mouse genetic models. Exp Hematol. 2009;37:715–27. e3. doi: 10.1016/j.exphem.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banerjee ER, Jiang Y, Henderson WR, Jr, Scott LM, Papayannopoulou T. Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol. 2007;35:605–17. doi: 10.1016/j.exphem.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 55.Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol. 2000;76:45–62. doi: 10.1159/000058780. [DOI] [PubMed] [Google Scholar]
- 56.Ebisawa M, Schleimer RP, Bickel C, Bochner BS. Phenotyping of purified human peripheral blood eosinophils using Blind Panel mAb. In: Schlossman S, Boumsell L, Gilks W, Harlan J, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T, Todd R, editors. Leukocyte Typing V: white cell differentiation antigens. New York: Oxford University Press; 1995. pp. 1036–38. [Google Scholar]
- 57.Driss V, Legrand F, Capron M. Eosinophil receptor profile. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 30–38. [Google Scholar]
- 58.Vijverberg SJ, Koenderman L, Koster ES, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. Biomarkers of therapy responsiveness in asthma: pitfalls and promises. Clin Exp Allergy. 2011;41:615–29. doi: 10.1111/j.1365-2222.2011.03694.x. [DOI] [PubMed] [Google Scholar]
- 59.Na HJ, Hamilton RG, Klion AD, Bochner BS. Biomarkers of eosinophil involvement in allergic and eosinophilic diseases: review of phenotypic and serum markers including a novel assay to quantify levels of soluble Siglec-8. J Immunol Methods. 2012;383:39–46. doi: 10.1016/j.jim.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bochner BS. Systemic activation of basophils and eosinophils: markers and consequences. J Allergy Clin Immunol. 2000;106:S292–302. doi: 10.1067/mai.2000.110164. [DOI] [PubMed] [Google Scholar]
- 61.Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland-van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench to bedside. Biologics Targets Therapy. 2013;7:199–210. doi: 10.2147/BTT.S29976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kita H. Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol. 2013;161 (Suppl 2):3–9. doi: 10.1159/000350662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–35. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson MW, Mosher DF. Activation of beta1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol. 2011;45:889–97. doi: 10.1165/rcmb.2010-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mawhorter SD, Stephany DA, Ottesen EA, Nutman TB. Identification of surface molecules associated with physiologic activation of eosinophils. Application of whole-blood flow cytometry to eosinophils. J Immunol. 1996;156:4851–8. [PubMed] [Google Scholar]
- 66.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35:378–86. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson MW, Annis DS, Mosher DF. Alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–10. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han S-T, Mosher DF. IL-5 induces suspended eosinophils to undergo unique global reorganization associated with priming. Am J Respir Cell Mol Biol. 2014;50:654–64. doi: 10.1165/rcmb.2013-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–8. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 70.Teran LM, Carroll MP, Shute JK, Holgate ST. Interleukin 5 release into asthmatic airways 4 and 24 hours after endobronchial allergen challenge: its relationship with eosinophil recruitment. Cytokine. 1999;11:518–22. doi: 10.1006/cyto.1998.0457. [DOI] [PubMed] [Google Scholar]
- 71.Kelly EAB, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111:79–86. doi: 10.1067/mai.2003.28. [DOI] [PubMed] [Google Scholar]
- 72.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O’Byrne PM. Effects of allergen challenge on eosinophils, eosinophil cationic protein, and granulocyte-macrophage colony-stimulating factor in mild asthma. Am J Respir Crit Care Med. 1995;151:1915–24. doi: 10.1164/ajrccm.151.6.7767540. [DOI] [PubMed] [Google Scholar]
- 73.Evans DJ, Barnes PJ, Spaethe SM, van Alstyne EL, Mitchell MI, O’Connor BJ. Effect of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax. 1996;51:1178–84. doi: 10.1136/thx.51.12.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarjour NN, Calhoun WJ, Kelly EA, Gleich GJ, Schwartz LB, Busse WW. The immediate and late allergic response to segmental bronchopulmonary provocation in asthma. Am J Respir Crit Care Med. 1997;155:1515–21. doi: 10.1164/ajrccm.155.5.9154851. [DOI] [PubMed] [Google Scholar]
- 75.Mastalerz L, Sanak M, Szczeklik A. Serum interleukin-5 in aspirin-induced asthma. Clin Exp Allergy. 2001;31:1036–40. doi: 10.1046/j.1365-2222.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- 76.Joseph J, Benedict S, Safa W, Joseph M. Serum interleukin-5 levels are elevated in mild and moderate persistent asthma irrespective of regular inhaled glucocorticoid therapy. BMC Pulm Med. 2004;4:2. doi: 10.1186/1471-2466-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, Wold AE, Wenneras C. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J Innate Immun. 2011;3:594–604. doi: 10.1159/000331326. [DOI] [PubMed] [Google Scholar]
- 78.Pelikan Z. Delayed type of asthmatic response to allergen challenge and cytokines in the peripheral blood. Respiration. 2012;84:385–95. doi: 10.1159/000335258. [DOI] [PubMed] [Google Scholar]
- 79.Pelikan Z. Delayed asthmatic response to allergen challenge and cytokines released by nonspecifically stimulated blood cells. ISRN Inflamma. 2013;2013:496208. doi: 10.1155/2013/496208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patil SP, Wisnivesky JP, Busse PJ, Halm EA, Li XM. Detection of immunological biomarkers correlated with asthma control and quality of life measurements in sera from chronic asthmatic patients. Ann Allergy Asthma Immunol. 2011;106:205–13. doi: 10.1016/j.anai.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang TN, Chiang W, Tseng HI, Chu YT, Chen WY, Shih NH, Ko YC. The polymorphisms of eotaxin 1 and CCR3 genes influence on serum IgE, eotaxin levels and mild asthmatic children in Taiwan. Allergy. 2007;62:1125–30. doi: 10.1111/j.1398-9995.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 82.Pelikan Z. Chemokine profiles in blood associated with delayed asthmatic response to allergen challenge. Respir Med. 2013;107:47–59. doi: 10.1016/j.rmed.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Arima K, Umeshita-Suyama R, Sakata Y, Akaiwa M, Mao XQ, Enomoto T, Dake Y, Shimazu S, Yamashita T, Sugawara N, Brodeur S, Geha R, Puri RK, Sayegh MH, Adra CN, Hamasaki N, Hopkin JM, Shirakawa T, Izuhara K. Upregulation of IL-13 concentration in vivo by the IL13 variant associated with bronchial asthma. J Allergy Clin Immunol. 2002;109:980–7. doi: 10.1067/mai.2002.124656. [DOI] [PubMed] [Google Scholar]
- 84.Dorman SC, Sehmi R, Gauvreau GM, Watson RM, Foley R, Jones GL, Denburg JA, Inman MD, O’Byrne PM. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;169:565–72. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- 85.Sumimoto S, Kawai M, Kasajima Y, Hamamoto T. Increased plasma tumour necrosis factor-alpha concentration in atopic dermatitis. Arch Dis Child. 1992;67:277–9. doi: 10.1136/adc.67.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–75. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- 87.Krisiukeniene A, Babusyte A, Stravinskaite K, Lotvall J, Sakalauskas R, Sitkauskiene B. Smoking affects eotaxin levels in asthma patients. J Asthma. 2009;46:470–6. doi: 10.1080/02770900902846349. [DOI] [PubMed] [Google Scholar]
- 88.Min JW, Lee JH, Park CS, Chang HS, Rhim TY, Park SW, Jang AS, Shin HD. Association of eotaxin-2 gene polymorphisms with plasma eotaxin-2 concentration. J Hum Genet. 2005;50:118–23. doi: 10.1007/s10038-005-0230-3. [DOI] [PubMed] [Google Scholar]
- 89.Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004;34:437–44. doi: 10.1111/j.1365-2222.2004.01885.x. [DOI] [PubMed] [Google Scholar]
- 90.Kroegel C, Julius P, Matthys H, Virchow JC, Jr, Luttmann W. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur Respir J. 1996;9:899–904. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- 91.Koenderman L, van der Bruggen T, Schweizer RC, Warringa RA, Coffer P, Caldenhoven E, Lammers JW, Raaijmakers JA. Eosinophil priming by cytokines: from cellular signal to in vivo modulation. Eur Respir J Suppl. 1996;22:119s–25s. [PubMed] [Google Scholar]
- 92.Coffer PJ, Koenderman L. Granulocyte signal transduction and priming: cause without effect? Immunol Lett. 1997;57:27–31. doi: 10.1016/s0165-2478(97)00067-9. [DOI] [PubMed] [Google Scholar]
- 93.Hakansson L, Heinrich C, Rak S. Priming of eosinophil adhesion in patients with birch pollen allergy during pollen season: effect of immunotherapy. J Allergy Clin Immunol. 1997;99:551–62. doi: 10.1016/s0091-6749(97)70084-8. [DOI] [PubMed] [Google Scholar]
- 94.Moser R, Fehr J, Bruijnzeel PLB. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992;149:1432–38. [PubMed] [Google Scholar]
- 95.Sehmi R, Wardlaw AJ, Cromwell O, Kurihara K, Waltmann P, Kay AB. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992;79:2952–9. [PubMed] [Google Scholar]
- 96.Warringa RA, Mengelers HJ, Kuijper PH, Raaijmakers JA, Bruijnzeel PL, Koenderman L. In vivo priming of platelet-activating factor-induced eosinophil chemotaxis in allergic asthmatic individuals. Blood. 1992;79:1836–41. [PubMed] [Google Scholar]
- 97.Sannohe S, Adachi T, Hamada K, Honda K, Yamada Y, Saito N, Cui CH, Kayaba H, Ishikawa K, Chihara J. Upregulated response to chemokines in oxidative metabolism of eosinophils in asthma and allergic rhinitis. Eur Respir J. 2003;21:925–31. doi: 10.1183/09031936.03.00028103a. [DOI] [PubMed] [Google Scholar]
- 98.Moser R, Fehr J, Olgiati L, Bruijnzeel PL. Migration of primed human eosinophils across cytokine-activated endothelial cell monolayers. Blood. 1992;79:2937–45. [PubMed] [Google Scholar]
- 99.Warringa RA, Mengelers HJ, Raaijmakers JA, Bruijnzeel PL, Koenderman L. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J Allergy Clin Immunol. 1993;91:1198–205. doi: 10.1016/0091-6749(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 100.Johansson MW, Gunderson KA, Kelly EA, Denlinger LC, Jarjour NN, Mosher DF. Anti-IL-5 attenuates activation and surface density of beta(2) -integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy. 2013;43:292–303. doi: 10.1111/j.1365-2222.2012.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–6. [PMC free article] [PubMed] [Google Scholar]
- 102.Nishikawa K, Morii T, Ako H, Hamada K, Saito S, Narita N. In vivo expression of CD69 on lung eosinophils in eosinophilic pneumonia: CD69 as a possible activation marker for eosinophils. J Allergy Clin Immunol. 1992;90:169–74. doi: 10.1016/0091-6749(92)90068-d. [DOI] [PubMed] [Google Scholar]
- 103.Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell-surface activation markers for human eosinophils. Am J Respir Cell Mol Biol. 1998;18:860–6. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- 104.Luttmann W, Matthiesen T, Matthys H, Virchow JC., Jr Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. Am J Respir Cell Mol Biol. 1999;20:474–80. doi: 10.1165/ajrcmb.20.3.3326. [DOI] [PubMed] [Google Scholar]
- 105.Julius P, Luttmann W, Knoechel B, Kroegel C, Matthys H, Virchow JC., Jr CD69 surface expression on human lung eosinophils after segmental allergen provocation. Eur Respir J. 1999;13:1253–9. doi: 10.1183/09031936.99.13612609. [DOI] [PubMed] [Google Scholar]
- 106.Dallaire MJ, Ferland C, Lavigne S, Chakir J, Laviolette M. Migration through basement membrane modulates eosinophil expression of CD44. Clin Exp Allergy. 2002;32:898–905. doi: 10.1046/j.1365-2222.2002.01377.x. [DOI] [PubMed] [Google Scholar]
- 107.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–66. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 108.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170:5359–66. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 109.Kelly EA, Koziol-White CJ, Clay KJ, Liu LY, Bates ME, Bertics PJ, Jarjour NN. Potential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthma. J Immunol. 2009;182:1404–10. doi: 10.4049/jimmunol.182.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EA, Bates DM, Malter JS, Busse WW, Bertics PJ. Expression of interleukin-5- and granulocyte macrophage-colony-stimulating factor-responsive genes in blood and airway eosinophils. Am J Respir Cell Mol Biol. 2004;30:736–43. doi: 10.1165/rcmb.2003-0234OC. [DOI] [PubMed] [Google Scholar]
- 111.Pignatti P, Perfetti L, Galdi E, Pozzi V, Bossi A, Biale C, Moscato G. Increased CD69 expression on peripheral blood eosinophils after specific inhalation challenge. Allergy. 2002;57:411–6. doi: 10.1034/j.1398-9995.2002.23454.x. [DOI] [PubMed] [Google Scholar]
- 112.Kanters D, ten Hove W, Luijk B, van Aalst C, Schweizer RC, Lammers JW, Leufkens HG, Raaijmakers JA, Bracke M, Koenderman L. Expression of activated Fc gamma RII discriminates between multiple granulocyte-priming phenotypes in peripheral blood of allergic asthmatic subjects. J Allergy Clin Immunol. 2007;120:1073–81. doi: 10.1016/j.jaci.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Neeley SP, Hamann KJ, White SR, Baranowski SL, Burch RA, Leff AR. Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. Am J Respir Cell Mol Biol. 1993;8:633–9. doi: 10.1165/ajrcmb/8.6.633. [DOI] [PubMed] [Google Scholar]
- 114.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–15. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 115.Bennett TA, Lynam EB, Sklar LA, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol. 1996;156:3093–7. [PubMed] [Google Scholar]
- 116.Mengelers HJ, Maikoe T, Hooibrink B, Kuypers TW, Kreukniet J, Lammers JW, Koenderman L. Down modulation of L-selectin expression on eosinophils recovered from bronchoalveolar lavage fluid after allergen provocation. Clin Exp Allergy. 1993;23:196–204. doi: 10.1111/j.1365-2222.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 117.Georas SN, Liu MC, Newman W, Dawson Beall L, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992;7:261–69. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- 118.Mengelers HJ, Maikoe T, Brinkman L, Hooibrink B, Lammers JW, Koenderman L. Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994;149:345–51. doi: 10.1164/ajrccm.149.2.8306028. [DOI] [PubMed] [Google Scholar]
- 119.in ‘t Veen JC, Grootendorst DC, Bel EH, Smits HH, Van Der Keur M, Sterk PJ, Hiemstra PS. CD11b and L-selectin expression on eosinophils and neutrophils in blood and induced sputum of patients with asthma compared with normal subjects. Clin Exp Allergy. 1998;28:606–15. doi: 10.1046/j.1365-2222.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 120.Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PL, Virchow JC, Jr, Virchow C., Sr Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–7. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czech W, Krutmann J, Budnik A, Schopf E, Kapp A. Induction of intercellular adhesion molecule 1 (ICAM-1) expression in normal human eosinophils by inflammatory cytokines. J Invest Dermatol. 1993;100:417–23. doi: 10.1111/1523-1747.ep12472082. [DOI] [PubMed] [Google Scholar]
- 122.Hansel TT, De Vries IJ, Carballido JM, Braun RK, Carballido-Perrig N, Rihs S, Blaser K, Walker C. Induction and function of eosinophil intercellular adhesion molecule-1 and HLA-DR. J Immunol. 1992;149:2130–6. [PubMed] [Google Scholar]
- 123.Wong CK, Ip WK, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor-induced adhesion molecule expression on eosinophils by p38 mitogen-activated protein kinase and nuclear factor-[kappa] B. Am J Respir Cell Mol Biol. 2003;29:133–47. doi: 10.1165/rcmb.2002-0289OC. [DOI] [PubMed] [Google Scholar]
- 124.Sano K, Yamauchi K, Hoshi H, Honma M, Tamura G, Shirato K. CD44 expression on blood eosinophils is a novel marker of bronchial asthma. Int Arch Allergy Immunol. 1997;114 (Suppl 1):67–71. doi: 10.1159/000237722. [DOI] [PubMed] [Google Scholar]
- 125.Sakamoto S, Oki K, Takahashi H, Arakawa Y, Sugita H, Hirano H, Takeuchi K, Tomichi N, Sakuma T, Futai K, Kawabata Y. Comparison of surface antigens on eosinophils from patients with eosinophilia. Int Arch Allergy Immunol. 1996;111 (Suppl 1):26–8. doi: 10.1159/000237410. [DOI] [PubMed] [Google Scholar]
- 126.Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest. 2003;111:1563–70. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rothenberg ME. CD44--a sticky target for asthma. J Clin Invest. 2003;111:1460–2. doi: 10.1172/JCI18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsumoto K, Bochner BS. Adhesion molecules. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 131–39. [Google Scholar]
- 129.Symon FA, Lawrence MB, Williamson ML, Walsh GM, Watson SR, Wardlaw AJ. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol. 1996;157:1711–9. [PubMed] [Google Scholar]
- 130.Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation. P-selectin, and eosinophil beta1-integrin activation in asthma. Am J Respir Crit Care Med. 2012;185:498–507. doi: 10.1164/rccm.201109-1712OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davenpeck KL, Brummet ME, Hudson SA, Mayer RJ, Bochner BS. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGL-1, CD162) and adhesion to P-selectin in vitro. J Immunol. 2000;165:2764–72. doi: 10.4049/jimmunol.165.5.2764. [DOI] [PubMed] [Google Scholar]
- 132.Gauvreau GM, Evans MY. Allergen inhalation challenge: a human model of asthma exacerbation. Contrib Microbiol. 2007;14:21–32. doi: 10.1159/000107052. [DOI] [PubMed] [Google Scholar]
- 133.Diamant Z, Gauvreau GM, Cockcroft DW, Boulet LP, Sterk PJ, de Jongh FH, Dahlen B, O’Byrne PM. Inhaled allergen bronchoprovocation tests. J Allergy Clin Immunol. 2013;132:1045–55. e6. doi: 10.1016/j.jaci.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 134.Rand TH, Silberstein DS, Kornfeld H, Weller PF. Human eosinophils express functional interleukin 2 receptors. J Clin Invest. 1991;88:825–32. doi: 10.1172/JCI115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Riedel D, Lindemann A, Brach M, Mertelsmann R, Herrmann F. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce surface expression of interleukin-2 receptor p55-chain and CD4 by human eosinophils. Immunology. 1990;70:258–61. [PMC free article] [PubMed] [Google Scholar]
- 136.Esnault S, Kelly EA, Johansson MW, Liu LY, Han S-T, Akhtar M, Sandbo N, Mosher DF, Denlinger LC, Mathur SK, Malter JS, Jarjour NN. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clin Immunol. 2013;150:90–100. doi: 10.1016/j.clim.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–8. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 138.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–83. 83 e1–4. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Myrtek D, Knoll M, Matthiesen T, Krause S, Lohrmann J, Schillinger D, Idzko M, Virchow JC, Friedrich K, Luttmann W. Expression of interleukin-13 receptor alpha 1-subunit on peripheral blood eosinophils is regulated by cytokines. Immunology. 2004;112:597–604. doi: 10.1046/j.1365-2567.2004.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang W, Smith SG, Beaudin S, Dua B, Howie K, Gauvreau G, O’Byrne PM. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int Arch Allergy Immunol. 2013;163:5–10. doi: 10.1159/000355331. [DOI] [PubMed] [Google Scholar]
- 141.Monteiro RC, Hostoffer RW, Cooper MD, Bonner JR, Gartland GL, Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–5. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hartnell A, Kay AB, Wardlaw AJ. IFN-gamma induces expression of Fc gamma RIII (CD16) on human eosinophils. J Immunol. 1992;148:1471–8. [PubMed] [Google Scholar]
- 143.Valerius T, Repp R, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines. A novel class of eosinophil activators with delayed onset of action. J Immunol. 1990;145:2950–8. [PubMed] [Google Scholar]
- 144.Zhu X, Hamann KJ, Munoz NM, Rubio N, Mayer D, Hernrreiter A, Leff AR. Intracellular expression of Fc gamma RIII (CD16) and its mobilization by chemoattractants in human eosinophils. J Immunol. 1998;161:2574–9. [PubMed] [Google Scholar]
- 145.Davoine F, Lavigne S, Chakir J, Ferland C, Boulay ME, Laviolette M. Expression of FcgammaRIII (CD16) on human peripheral blood eosinophils increases in allergic conditions. J Allergy Clin Immunol. 2002;109:463–9. doi: 10.1067/mai.2002.121952. [DOI] [PubMed] [Google Scholar]
- 146.Luijk B, Lindemans CA, Kanters D, van der Heijde R, Bertics P, Lammers JW, Bates ME, Koenderman L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol. 2005;115:997–1003. doi: 10.1016/j.jaci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 147.Kishimoto T, Kikutani H, von dem Borne AEGK, Goyert SM, Mason DY, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer TA, Sugamura K, Zola H. Leucocyte typing VI: white cell differentiation antigens. New York: Garland Publishing, Inc; 1997. [Google Scholar]
- 148.Wardlaw AJ, Jeffrey PK, Majumdar S, Dewar A, Anwar ARE, Walsh G, Kay AB. Platelet adhesion to eosinophils. Am Rev Respir Disease. 1992;145:A664. [Google Scholar]
- 149.Laidlaw TM, Boyce JA. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy. 2012;42:1313–20. doi: 10.1111/j.1365-2222.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–81. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- 151.Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Rose MJ, Giannini S, Momi S, Spina D, O’Connor B, Gresele P, Page CP. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol. 2003;112:109–18. doi: 10.1067/mai.2003.1514. [DOI] [PubMed] [Google Scholar]
- 152.Page C, Pitchford S. Platelets coming of age: implications for our understanding of allergic inflammation. Am J Respir Crit Care Med. 2013;187:459–60. doi: 10.1164/rccm.201301-0085ED. [DOI] [PubMed] [Google Scholar]
- 153.de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 1992;80:134–42. [PubMed] [Google Scholar]
- 154.Bazan-Socha S, Bukiej A, Pulka G, Marcinkiewicz C, Musial J. Increased expression of collagen receptors: alpha1beta1 and alpha2beta1 integrins on blood eosinophils in bronchial asthma. Clin Exp Allergy. 2006;36:1184–91. doi: 10.1111/j.1365-2222.2006.02540.x. [DOI] [PubMed] [Google Scholar]
- 155.Bazan-Socha S, Zuk J, Plutecka H, Marcinkiewicz C, Zareba L, Musial J. Collagen receptors alpha(1)beta(1) and alpha(2)beta(1) integrins are involved in transmigration of peripheral blood eosinophils, but not mononuclear cells through human microvascular endothelial cells monolayer. J Physiol Pharmacol. 2012;63:373–9. [PubMed] [Google Scholar]
- 156.Kuijpers TW, Mul EPJ, Blom M, Kovach NL, Gaeta FCA, Tollefson V, Elices MJ, Harlan JM. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–84. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, Staite ND, Richards IM. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–29. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 158.Cook-Mills JM. Eosinophil-endothelial cell interactions during inflammation. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Amsterdam: Elsevier; 2013. pp. 139–53. [Google Scholar]
- 159.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem. 2006;281:32175–87. doi: 10.1074/jbc.M600943200. [DOI] [PubMed] [Google Scholar]
- 160.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin-dependent adhesion of human eosinophils. J Immunol. 1999;163:3423–9. [PubMed] [Google Scholar]
- 161.Lantero S, Alessandri G, Spallarossa D, Scarso L, Rossi GA. LFA-1 expression by blood eosinophils is increased in atopic asthmatic children and is involved in eosinophil locomotion. Eur Respir J. 1998;12:1094–8. doi: 10.1183/09031936.98.12051094. [DOI] [PubMed] [Google Scholar]
- 162.Tenscher K, Metzner B, Schopf E, Norgauer J, Czech W. Recombinant human eotaxin induces oxygen radical production, Ca(2+)-mobilization, actin reorganization, and CD11b upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood. 1996;88:3195–9. [PubMed] [Google Scholar]
- 163.Liu L, Hakansson L, Ridefelt P, Garcia RC, Venge P. Priming of eosinophil migration across lung epithelial cell monolayers and upregulation of CD11b/CD18 are elicited by extracellular Ca2+ Am J Respir Cell Mol Biol. 2003;28:713–21. doi: 10.1165/rcmb.4771. [DOI] [PubMed] [Google Scholar]
- 164.Kroegel C, Liu MC, Hubbard WC, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J Allergy Clin Immunol. 1994;93:725–34. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 165.Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttmann W, Virchow C, Jr, Di Virgilio F, Norgauer J. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–8. [PubMed] [Google Scholar]
- 166.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–53. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 167.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology. 1990;71:258–65. [PMC free article] [PubMed] [Google Scholar]
- 168.Thorne KJ, Richardson BA, Mazza G, Butterworth AE. A new method for measuring eosinophil activating factors, based on the increased expression of CR3 alpha chain (CD11b) on the surface of activated eosinophils. J Immunol Methods. 1990;133:47–54. doi: 10.1016/0022-1759(90)90317-o. [DOI] [PubMed] [Google Scholar]
- 169.Lundahl J, Hallden G, Hed J. Differences in intracellular pool and receptor-dependent mobilization of the adhesion-promoting glycoprotein Mac-1 between eosinophils and neutrophils. J Leukoc Biol. 1993;53:336–41. doi: 10.1002/jlb.53.3.336. [DOI] [PubMed] [Google Scholar]
- 170.Fernvik E, Gronneberg R, Lundahl J, Hed J, Andersson O, Johansson SG, Hallden G. The degree of natural allergen exposure modifies eosinophil activity markers in the circulation of patients with mild asthma. Allergy. 1996;51:697–705. [PubMed] [Google Scholar]
- 171.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–8. [PubMed] [Google Scholar]
- 172.Grayson MH, Van der Vieren M, Sterbinsky SA, Gallatin WM, Hoffman PA, Staunton DE, Bochner BS. AlphaDbeta2 Integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1998;188:2187–91. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–62. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 175.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–70. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans. 2004;32:407–11. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- 177.Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–11. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta 1 integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol. 2006;117:1502–4. doi: 10.1016/j.jaci.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 181.Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–51. [PubMed] [Google Scholar]
- 182.Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- 183.Coe AP, Askari JA, Kline AD, Robinson MK, Kirby H, Stephens PE, Humphries MJ. Generation of a minimal alpha5beta1 integrin-Fc fragment. J Biol Chem. 2001;276:35854–66. doi: 10.1074/jbc.M103639200. [DOI] [PubMed] [Google Scholar]
- 184.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem. 2005;280:4238–46. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A. 1993;90:9051–5. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–7. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 187.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–75. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 188.Johansson MW, Kruger SJ, Schiebler ML, Evans MD, Sorkness RL, Denlinger LC, Busse WW, Jarjour NN, Montgomery RR, Mosher DF, Fain SB. Markers of vascular perturbation correlate with airway structural change in asthma. Am J Respir Crit Care Med. 2013;188:167–78. doi: 10.1164/rccm.201301-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ulfman LH, Joosten DP, van Aalst CW, Lammers JW, van de Graaf EA, Koenderman L, Zwaginga JJ. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol. 2003;28:512–9. doi: 10.1165/rcmb.4806. [DOI] [PubMed] [Google Scholar]
- 190.Ramos-Barbon D, Fraga-Iriso R, Brienza NS, Montero-Martinez C, Verea-Hernando H, Olivenstein R, Lemiere C, Ernst P, Hamid QA, Martin JG. T Cells localize with proliferating smooth muscle alpha-actin+ cell compartments in asthma. Am J Respir Crit Care Med. 2010;182:317–24. doi: 10.1164/rccm.200905-0745OC. [DOI] [PubMed] [Google Scholar]
- 191.Robinson MK, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–5. [PubMed] [Google Scholar]
- 192.Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9:282–7. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- 193.Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989;8:3759–65. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Leitinger B, Hogg N. Effects of I domain deletion on the function of the beta2 integrin lymphocyte function-associated antigen-1. Mol Biol Cell. 2000;11:677–90. doi: 10.1091/mbc.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci U S A. 2001;98:2393–8. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proc Natl Acad Sci U S A. 1999;96:2215–20. doi: 10.1073/pnas.96.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weber C, Kitayama J, Springer TA. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci U S A. 1996;93:10939–44. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ulfman LH, Alblas J, van Aalst CW, Zwaginga JJ, Koenderman L. Differences in potency of CXC chemokine ligand 8-, CC chemokine ligand 11-, and C5a-induced modulation of integrin function on human eosinophils. J Immunol. 2005;175:6092–9. doi: 10.4049/jimmunol.175.9.6092. [DOI] [PubMed] [Google Scholar]
- 199.Munitz A, Bachelet I, Eliashar R, Khodoun M, Finkelman FD, Rothenberg ME, Levi-Schaffer F. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J Immunol. 2006;177:77–83. doi: 10.4049/jimmunol.177.1.77. [DOI] [PubMed] [Google Scholar]
- 200.Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, Liu FT, Sriramarao P. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol. 2007;179:7800–7. doi: 10.4049/jimmunol.179.11.7800. [DOI] [PubMed] [Google Scholar]
- 201.Ge XN, Bahaie NS, Kang BN, Hosseinkhani MR, Ha SG, Frenzel EM, Liu FT, Rao SP, Sriramarao P. Allergen-induced airway remodeling is impaired in galectin-3-deficient mice. J Immunol. 2010;185:1205–14. doi: 10.4049/jimmunol.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, Apgar JR, Kawakami T, Lilly CM, Liu FT. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–53. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ilmarinen P, James A, Moilanen E, Pulkkinen V, Daham K, Saarelainen S, Laitinen T, Dahlen SE, Kere J, Dahlen B, Kankaanranta H. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100IU/ml. Peptides. 2014;51:100–9. doi: 10.1016/j.peptides.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 204.Calafat J, Kuijpers TW, Janssen H, Borregaard N, Verhoeven AJ, Roos D. Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood. 1993;81:3122–9. [PubMed] [Google Scholar]
- 205.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW. Severe Asthma: Lessons Learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moore WC, Fitzpatrick AM, Li X, Hastie AT, Li H, Meyers DA, Bleecker ER. Clinical heterogeneity in the Severe Asthma Research Program. Ann Am Thoracic Soc. 2013;10(supplement):S118–S24. doi: 10.1513/AnnalsATS.201309-307AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Johansson MW, Han S-T, Gunderson KA, Montgomery RR, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin mobilization, and eosinophil beta1 integrin activation occur in asthma and are associated with clinical phenotypes [abstract] Am J Respir Crit Care Med. 2011;183:A4335. [Google Scholar]
- 208.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 209.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 210.Berends C, Hoekstra MO, Dijkhuizen B, DeMonchy JGR, Gerritsen J, Kauffman HF. Expression of CD35 (CR1) and CD11b (CR3) on circulating neutrophils and eosinophils from allergic asthmatic children. Clin Exp Allergy. 1993;23:926–33. doi: 10.1111/j.1365-2222.1993.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 211.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–47. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 212.Braun RK, Foerster M, Workalemahu G, Haefner D, Kroegel C, Walker C. Differential regulation of aminopeptidase N (CD13) by transendothelial migration and cytokines on human eosinophils. Exp Lung Res. 2003;29:59–77. doi: 10.1080/01902140303766. [DOI] [PubMed] [Google Scholar]
- 213.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993;150:2554–62. [PubMed] [Google Scholar]
- 214.Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFalpha increase Thymic Stromal Lymphopoietin Receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin Mol Allergy. 2012;10:8. doi: 10.1186/1476-7961-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


