Abstract
Renovascular hypertension alters cardiac structure and function. Autophagy is activated during left ventricular hypertrophy and linked to adverse cardiac function. The Angiotensin II receptor blocker Valsartan lowers blood pressure and is cardioprotective, but whether it modulates autophagy in the myocardium is unclear. We hypothesized that Valsartan would alleviate autophagy and improve left ventricular myocardial mitochondrial turnover in swine renovascular hypertension. Domestic pigs were randomized to control, unilateral renovascular hypertension, and renovascular hypertension treated with Valsartan (320 mg/day) or conventional triple therapy (Reserpine+hydralazine+hydrochlorothiazide) for 4 weeks post 6-weeks of renovascular hypertension (n=7 each group). Left ventricular remodeling, function and myocardial oxygenation and microcirculation were assessed by multi-detector computer tomography, blood-oxygen-level-dependent magnetic resonance imaging and microcomputer tomography. Myocardial autophagy, markers for mitochondrial degradation and biogenesis, and mitochondrial respiratory-chain proteins were examined ex vivo. Renovascular hypertension induced left ventricular hypertrophy and myocardial hypoxia, enhanced cellular autophagy and mitochondrial degradation, and suppressed mitochondrial biogenesis. Valsartan and triple therapy similarly decreased blood pressure, but Valsartan solely alleviated left ventricular hypertrophy, ameliorated myocardial autophagy and mitophagy, and increased mitochondrial biogenesis. In contrast, triple therapy only slightly attenuated autophagy and preserved mitochondrial proteins, but elicited no improvement in mitophagy. These data suggest a novel potential role of Valsartan in modulating myocardial autophagy and mitochondrial turnover in renovascular hypertension-induced hypertensive heart disease, which may possibly bolster cardiac repair via a blood pressure-independent manner.
Keywords: hypertension, left ventricular hypertrophy, mitophagy, mitochondrial biogenesis, angiotensin-receptor blocker, autophagy
Introduction
Hypertension is a leading risk factor for mortality worldwide. In the United States, its prevalence in 2009–2010 among adults aged 18 and over was up to 28.6% (to about 70 million cases),1 and about 74% of chronic heart failure cases are associated with hypertension.2 Renal artery stenosis (RAS), leading to renovascular hypertension (RVH), constitutes under 5% of hypertensive cases, yet is more closely linked to hypertensive heart diseases. Indeed, left ventricular (LV) hypertrophy (LVH) is 3 times more prevalent in patients with RVH compared to essential hypertension,3 possibly attributed to greater activation of angiotensin (Ang)-II that contributes to LVH.4
Recent investigations have shed light on the link between autophagy and pathophysiologic LV remodeling in response to pressure overload.5 During adaptive remodeling toward LVH, a compensatory increase in protein synthesis causes accumulation of toxic misfolded molecules and protein aggregates. To maintain cardiac integrity,6, 7 autophagy serves as a major cellular mechanism for clearing these toxic protein aggregates and dysfunctional organelles. Moreover, energy deprivation during hypertension secondary to myocardial hypoxia8 or ischemia9 might also enhance autophagy in order to promote cell survival by releasing energy substrates, via degradation of cellular constituents.10 However, excessive autophagic activity may result in elimination of essential molecules and organelles, and contribute to LV dysfunction and adverse events.11, 12 Therefore, modulation of autophagy is important to functional homeostasis in the hypertensive heart.
Anti-hypertensive drugs greatly improve cardiovascular outcomes in hypertensive patients. In particular, angiotensin-converting-enzyme inhibitors (ACEI) and AngII receptor blockers (ARB) efficiently decrease blood pressure in RVH. Further, some of their cardio-protective effects, including reversal of LVH and prevention of heart failure, exceed blood pressure control.13 By blocking the AT1 receptor (AT1R), ARBs allow AngII to bind to AT2R, thereby ameliorating AT1R-mediated deleterious cardiac effects of AngII like oxidative stress, apoptosis,14 and inflammation. However, whether the benefit of ARB in hypertensive heart disease involves modulation of autophagy is poorly characterized. We hypothesized that ARB Valsartan would alleviate myocardial autophagy and improve bioenergetic metabolism in RVH-induced LVH.
Methods
Domestic pigs were randomized to control, RVH, and RVH treated with Valsartan (320 mg/day; RVH+Valsartan) or triple-therapy (Reserpine+hydralazine+hydrochlorothiazide, RVH+TT) for 4 wks post 6-wks of RVH (n=7 each). Cardiac function and myocardial oxygenation were then studied in-vivo using multi-detector computer tomography (CT) and blood oxygen level dependent (BOLD)-magnetic resonance imaging (MRI), respectively, and microvascular architecture ex-vivo with micro-CT. Myocardial protein expression and staining were measured ex-vivo. (Detailed descriptions of all experimental methods are included in the Online-only Data Supplement http://hyper.ahajournals.org).
Results
1. Animal characteristics
There were no differences in body weight or degree of RAS among the groups (Table 1). Mean arterial pressure (MAP) increased in RVH groups by 6 weeks. Subsequent 4-week treatment by TT and Valsartan similarly decreased MAP compared to RVH, but neither normalized it (Table 1). Nocturnal MAP was lower than daytime in Normal pigs; this pattern was lost in RVH (Figure S1A–B), and restored only by Valsartan. Plasma creatinine was increased in all RVH groups, and unaltered by either treatment. RAS also elevated plasma aldosterone, which only Valsartan normalized.
Table 1.
Systemic characteristics in normal, renovascular hypertension (RVH), and triple-therapy (TT) or Valsartan-treated RVH pigs (n=7 each).
| Characteristics | Normal | RVH | RVH+TT | RVH+Valsartan |
|---|---|---|---|---|
| Body Weight (kg) | 48.6±0.6 | 49.1±2.4 | 50.3±2.6 | 48.4±0.7 |
| Degree of stenosis (%) | - | 76 ± 8 | 78±5 | 77±6 |
| Mean arterial pressure (mmHg) | ||||
| Baseline | 108.9±4.5 | 111.1±1.4 | 109.6±1.1 | 107.9±2.9 |
| 3 wks | 112.2±4.8 | 144.7±8.4* | 134.5±7.6* | 137.5±8.6* |
| 6 wks | 111.6±4.7 | 163.1±11.8* | 146.9±8.8* | 151.1±10.4* |
| 10 wks | 107.6±4.9 | 169.8±7.3* | 132.2±10.7*†‡ | 133.1±5.8*†‡ |
| Serum Creatinine (mg/dl) | 1.2±0.0 | 1.6±0.1* | 1.7±0.1* | 1.6±0.0* |
| Plasma aldosterone (pg/ml) | 586.9±30.0 | 897.2±62.3* | 743.5±86.2* | 697.3±61.5 |
Data are mean±SEM.
p<0.05 vs. Normal;
p<0.05 vs. 6 weeks;
p<0.05 vs. RVH.
2. Left Ventricle (LV) remodeling, function and oxygenation
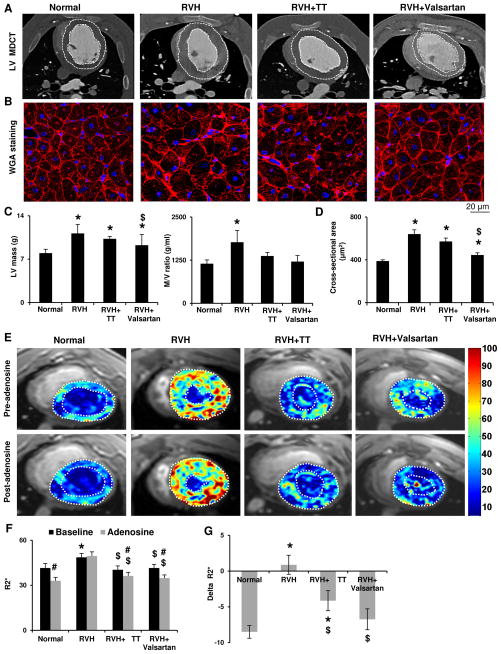
RVH increased LV mass and its ratio to chamber volume (M/V ratio), suggesting LVH and concentric remodeling (Figure 1A, C), and induced cardiomyocyte hypertrophy (Figure 1B, D). Both drugs improved M/V ratio, but only Valsartan alleviated LVH and cardiomyocyte hypertrophy compared to RVH. Left ventricle end diastolic volume (LVEDV) and early (E)/late (A) relaxations ratio were comparable among the groups, as were stroke volume and ejection fraction (EF, Figure S1C–F), indicating relatively preserved diastolic and contractile function at early RVH.
Figure 1.
A,C: Representative computed tomography images of left ventricular (LV) structure in Normal, renovascular hypertension (RVH), RVH+triple therapy (TT) and RVH+Valsartan pigs, and quantification of LV mass and its ratio to chamber volume (M/V ratio). White dotted lines outline the inner and outer LV wall boundaries. B and D: Wheat-germ-agglutinin staining and quantification of myocyte cross-sectional area. Valsartan alleviated RVH-induced concentric LV and myocyte hypertrophy. E: Representative blood-oxygen-level-dependent MRI LV images. Hypoxic myocardial regions are indicated as yellow and red colors, in contrast to blue LV chambers filled with oxygenated blood (scale bar). RVH induced myocardial hypoxia, reflected by increased R2* (F), and inhibited its response to adenosine (Delta-R2*, G). Valsartan and TT both improved basal oxygenation, while Valsartan also normalized response to adenosine. *p<0.05 vs. Normal; $p<0.05 vs. RVH; #p<0.05 vs. Baseline.
Basal myocardial perfusion in RVH did not differ from Normal, but the adenosine-induced increase observed in Normal pigs was abrogated in RVH (Figure S1G), suggesting impaired microvascular function, which was restored by Valsartan but not TT. RVH also increased LV oxygen consumption reflected by rate-pressure product (RPP), which was restored by both drugs (Figure S1H). Congruently, BOLD MRI indicated myocardial hypoxia in RVH (R2*, p<0.001 vs. Normal) and impaired response to adenosine (p=0.26 vs. baseline; Delta-R2* p<0.001 vs. Normal, Figure 1E–G). Although both drugs improved basal myocardial oxygenation, only Valsartan bolstered its response to adenosine to normal levels (Figure 1G).
3. Myocardial microcirculation
RVH suppressed the numbers of both small (20–200 μm) and large (200–500 μm) microvessels in the subendocardium, and large vessels in the subepicardium (Figure S2A,B), associated with decreased expression of vascular endothelial growth factor (VEGF) and angiopoietin-1, and increased hypoxia-inducible factor (HIF)-1α (Figure S2C–E). Hence, insufficient microvascular spawning accompanied by increased oxygen consumption might promote myocardial hypoxia in RVH. TT normalized the density of both subendocardial and subepicardial large vessels, and restored the expression of VEGF, angiopoietin-1 and HIF-1α. Moreover, Valsartan additionally increased density of subepicardial small vessels, and achieved greater levels of VEGF and Angiopoietin-1 than TT (Figure S2).
4. Myocardial autophagy and mitochondrial activity
Myocardial autophagy
RVH increased the expression of the autophagy initiator Beclin, of the autophagosome formation hallmarks autophagy-related gene (Atg)12-Atg5 and microtubule-associated protein1 light chain (LC3)-II, and the LC3-II/LC3-I ratio, indicating augmented autophagic activity (Figure S3). Valsartan normalized Beclin, Atg12-Atg5, LC3II and the conversion of LC3 II to LC3 I, whereas TT did not reverse enhanced autophagy. Interestingly, increased mTOR expression in RVH was similarly restored by both drugs (Figure S3).
Apoptosis
RVH activated myocardial apoptosis, indicated by increased number of caspase-3+ and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ cells (Figure S4A), accompanied by upregulation of the pro-apoptotic Bax and its ratio to Bcl-xL (Figure S4B). Valsartan normalized Caspse-3 and TUNEL staining, and restored Bax and Bax/Bcl-xL. TT showed anti-apoptotic effects, but lesser than Valsartan (Figure S4A–B).
Mitochondrial activity
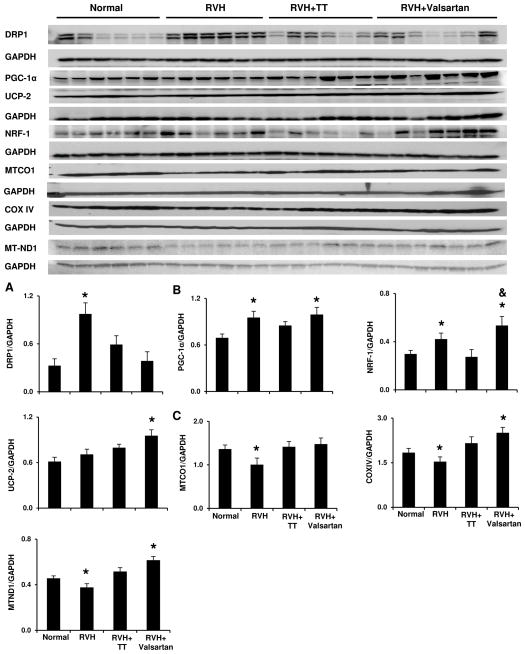
RVH enhanced myocardial mitophagy, indicated by elevated dynamin-related protein (DRP)1 (Figure 2A), and increased translocation of Parkin from the cytosol to mitochondrial outer membrane (Figure S5A–C). This was accompanied by elevated expression of mitochondria biogenesis regulator peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α and nuclear respiratory factor (NRF)-1 (Figure 2B), possibly in compensation for hypoxia and mitochondrial degradation. However, the mitochondrial respiratory-chain proteins mitochondrial cytochrome c oxidase (MTCO)1, COXIV, and mitochondrial NADH dehydrogenase (MTND)1 were uniformly lower than Normal, suggesting suppressed mitochondrial production (Figure 2C). TT did not reverse mitophagy, but restored mitochondrial proteins, possibly as a compensatory response to mitochondrial degradation. Contrarily, Valsartan alleviated mitophagy (Figure 2A, S5), increased mitochondrial biogenesis signals compared to Normal (Figure 2B), and further stimulated the production of mitochondrial proteins (Figure 2C). Collectively, these data link Valsartan to myocardial energy metabolism by regulating the balance between mitochondria degradation and biogenesis.
Figure 2.
Valsartan alleviated mitophagy and stimulated mitochondrial biogenesis in RVH myocardium. DRP: dynamin-related protein; PGC: proliferator-activated receptor-gamma coactivator; UCP: uncoupling protein; NRF: nuclear respiratory factor; MTCO1: mitochondrial cytochrome-c-1; COXIV: cytochrome c IV; MTND-1: mitochondrial NADH dehydrogenase 1. *p<0.05 vs. Normal; &p<0.05 vs. RVH+TT.
5. Myocardial oxidative stress and fibrosis
RVH enhanced oxidative stress as demonstrated by increased myocardial dihydroethidium (DHE) staining and expression of NAD(P)H oxidase gp91 (Figure S6A,C,E), alleviated by both drugs.
RVH induced myocardial fibrosis (Figure S6B,D), increased expression of TGF-β but decreased MMP-2 (Figure S6F). Both drugs similarly alleviated fibrosis, although neither downregulated TGF-β, and only Valsartan normalized MMP-2.
Discussion
The present study shows that in addition to inducing LV hypertrophy, RVH reduced microvascular growth and function, decreased oxygen supply, and increased fibrosis. Although overall cardiac function was relatively preserved at this early stage of RVH, its biological effects were highlighted by altered bioenergetic metabolism, including enhanced cellular autophagy, apoptosis, and signals for mitochondrial degradation, but impeded production of mitochondrial respiratory-chain subunits proteins. Valsartan showed effects superior to conventional therapy in alleviating LVH, improving myocardial microcirculation and oxygenation, associated with restored cellular survival and instigation of mitochondrial biogenesis. Accordingly, this study suggests novel, blood-pressure-independent cardioprotective effects of Valsartan in RVH-induced heart disease.
The role of myocardial autophagy in advanced hypertensive cardiomyopathy has been described,15 while its involvement in early RVH is unclear. Interestingly, despite interactive crosstalk between autophagy and apoptosis, two cell death processes that regulate and balance each other,16–18 we found enhanced activities of both, suggesting maladaptive cell survival in RVH-induced cardiomyopathy. While the underlying mechanism is unclear, excessive AngII, via its AT1R, may be partly responsible.19, 20 Furthermore, prominent mitophagic activity was observed in RVH. DRP-1, richly expressed in heart,21 might have been triggered by upregulated HIF-1α, secondary to compromised microcirculation and oxygenation in RVH. Activated DRP-1 promotes mitochondrial fragmentation, an early step in mitophagy22 and its interaction with E3 ubiquitin ligase Parkin.23 Parkin translocates to damaged mitochondrial outer-membrane,24 ubiquitinates mitochondrial proteins, and signals mitochondrial degradation.25 Despite increased expression of mitochondrial biogenesis signals (PGC-1α, NRF-1), which may reflect an attempt to replenish energy production during exposure to AngII,26 the expression of mitochondrial proteins in RVH was suppressed. As a result, inadequate mitochondrial production coexisting with increased oxygen demand in RVH may deplete bioenergetic reserve in remaining mitochondria, thereby accounting for overactivated cell death processes. Whether the imbalanced cell death processes and mitochondrial turnover underpin development of myocardial hypertrophy in RVH and progression of cardiac dysfunction needs further investigation.
Although LVH is a major factor triggering myocardial autophagy, its partial reversal by Valsartan may not suffice to achieve normalization of autophagic activity. The present study hence discloses a novel role of Valsartan in regulating myocardial autophagy in RVH, which involves several potential mechanisms. AT1R and AT2R in essence play opposite roles in autophagy, where the stimulatory effect of AT1R is antagonized by AT2R.19 AT1R-mediated aldosterone production also elevates LC3II expression in cardiomyocytes.27 Valsartan has moderate binding affinity, but is highly specific and selective for AT1R, with low (10%) non-specific binding and virtually no affinity for AT2R.28, 29 Hence, by selectively blocking the binding of AngII to AT1R in myocardium, Valsartan impedes the pro-autophagic effect of AT1R. Conceivably, Valsartan may also anneal AngII-driven mitochondrial degradation in response to hypoxia21 and oxidative stress30 via conferring AngII binding to AT2R. Despite minor regulation of autophagy, TT also restored the anti-autophagic mTOR similarly to Valsartan. Given its important role in regulating cardiac hypertrophy in pressure overload,31 the activity of mTOR is likely regulated by blood pressure level. Moreover, Valsartan distinctly upregulated mitochondrial biogenesis signals and subsequent synthesis of core proteins that carry out mitochondrial function, possibly via release of nitric oxide, which, through activation of AT2R,32, 33 modulates mitochondrial electron-transport-chain subunits, biogenesis, and degradation.34 Collectively, our findings imply potential biological benefits of AT2R in regulation of mitochondrial turnover. Further studies need to identify the causal link of AngII receptors to mitochondrial dynamics and its therapeutic effect by ARB in myocardial repair.
Microvascular remodeling characterizes tissue adaptation in hypertension.8, 35 Early RVH is associated with myocardial neovascularization to match blood supply to the evolving LVH, but subsequent vascular loss ensues,8, 36 which is tightly linked to oxidative stress.9 In the current study, basal myocardial perfusion was relatively preserved in RVH, yet reduced VEGF expression suggested a transition from “functional” to “structural” rarefaction that characterizes disease progression.9 Importantly, autophagy which was magnified by LVH and hypoxia in RVH, inhibits angiogenesis37 and mediates the effect of angiogenesis inhibitors,38, 39 thereby constituting a vicious circle interfering with maintenance of the microcirculation. Indeed, despite similar alleviation of oxidative stress to TT, Valsartan alone preserved microvascular density and function (myocardial perfusion and oxygenation), which paralleled amelioration of autophagy. In addition, enhanced mitochondrial biogenesis by Valsartan may also boost energy provision for restoration and maintenance of microvascular function. The role of autophagy in modulating myocardial microcirculation merits further examination.
Our study is limited by short duration of the disease, yet cardiac structure and function in our swine model are similar to human. We were also unable to explore autophagosome formation or mitophagic activity using electron microscopy or isolated mitochondria, and relied on changes in protein expression in tissue. The effects of Valsartan on myocardial autophagy and mitochondrial turnover under more aggressive blood pressure control warrant further studies. Further studies also need to clarify the causal link between myocardial bioenergetic metabolism and LVH reversal, and explore strategies for alleviating tissue fibrosis and slowing the progression of cardiac dysfunction.
Perspectives
Our study demonstrated that improvement of LVH and myocardial microcirculation by Valsartan is associated with restoration of autophagy activity and regulation of mitochondria-related bioenergetic metabolism. Conventional therapy also confers some cardio-protective benefits, possibly linked to blood pressure control, vasodilation, and direct anti-oxidant effects. However, the distinct effects of Valsartan in regulating myocardial autophagy and mitochondrial turnover may contribute to its superior efficacy in relieving cardiovascular complication, and therefore constitutes a unique and important therapeutic component in the management of cardiovascular disease in RVH.
Supplementary Material
Novelty and Significance.
-
What Is New:
Our study implicates restoration of autophagic activity and regulation of mitochondrial turnover as novel protective targets in management of hypertensive heart secondary to renovascular hypertension.
What Is Relevant: Renovascular hypertension is linked to adverse cardiac outcomes, and the mechanism by which ARB protect the hypertensive heart is incompletely understood. Out study proposes that unique modulation of autophagy and mitochondria turnover by Valsartan may mediate its therapeutic benefit in hypertensive heart disease.
Summary: Angiotensin receptor blocker, Valsartan, may convey blood-pressure-independent cardiac repair in RVH by directly improving cell survival and mitochondrial turnover.
Acknowledgments
Novartis Pharmaceuticals Corporation generously providing Valsartan for this study.
Source(s) of Funding
This work was partly supported by the National Institutes of Health (HL085307, DK73608, HL121561, and HL77131), and the American Heart Association.
References
- 1.Yoon SS, Burt V, Louis T, Carroll MD. Hypertension among adults in the united states, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hypertens. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin ii cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 7.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of amp-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol. 2007;18:1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 9.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 12.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 13.Muramatsu T, Matsushita K, Yamashita K, Kondo T, Maeda K, Shintani S, Ichimiya S, Ohno M, Sone T, Ikeda N, Watarai M, Murohara T. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: Nagoya heart study. Hypertension. 2012;59:580–586. doi: 10.1161/HYPERTENSIONAHA.111.184226. [DOI] [PubMed] [Google Scholar]
- 14.Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, Roden DM, Wagner T, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Cardiac-specific overexpression of at1 receptor mutant lacking g alpha q/g alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. J Biol Chem. 2010;285:8509–8514. doi: 10.1074/jbc.R109.025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorburn A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porrello ER, D’Amore A, Curl CL, Allen AM, Harrap SB, Thomas WG, Delbridge LM. Angiotensin ii type 2 receptor antagonizes angiotensin ii type 1 receptor-mediated cardiomyocyte autophagy. Hypertension. 2009;53:1032–1040. doi: 10.1161/HYPERTENSIONAHA.108.128488. [DOI] [PubMed] [Google Scholar]
- 20.Diep QN, El Mabrouk M, Yue P, Schiffrin EL. Effect of at (1) receptor blockade on cardiac apoptosis in angiotensin ii-induced hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H1635–1641. doi: 10.1152/ajpheart.00984.2001. [DOI] [PubMed] [Google Scholar]
- 21.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The pink1/parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. Pink1/parkin-mediated mitophagy is dependent on vdac1 and p62/sqstm1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 26.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F. Interferon-gamma ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H587–596. doi: 10.1152/ajpheart.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Gasparo M, Whitebread S. Binding of valsartan to mammalian angiotensin at1 receptors. Regul Pept. 1995;59:303–311. doi: 10.1016/0167-0115(95)00085-p. [DOI] [PubMed] [Google Scholar]
- 29.Criscione L, de Gasparo M, Buhlmayer P, Whitebread S, Ramjoue HP, Wood J. Pharmacological profile of valsartan: A potent, orally active, nonpeptide antagonist of the angiotensin ii at1-receptor subtype. Br J Pharmacol. 1993;110:761–771. doi: 10.1111/j.1476-5381.1993.tb13877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe T, Barker TA, Berk BC. Angiotensin ii and the endothelium: Diverse signals and effects. Hypertension. 2005;45:163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]
- 33.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 34.Miller MW, Knaub LA, Olivera-Fragoso LF, Keller AC, Balasubramaniam V, Watson PA, Reusch JE. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am J Physiol Heart Circ Physiol. 2013;304:H1624–1633. doi: 10.1152/ajpheart.00987.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibirica E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens. 2006;19:477–483. doi: 10.1016/j.amjhyper.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Zhu X, Chade AR, Jordan KL, Lavi R, Daghini E, Gibson ME, Guglielmotti A, Lerman A, Lerman LO. Monocyte chemoattractant proteins mediate myocardial microvascular dysfunction in swine renovascular hypertension. Arterioscler Thromb Vasc Biol. 2009;29:1810–1816. doi: 10.1161/ATVBAHA.109.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Guru SK, Pathania AS, Kumar A, Bhushan S, Malik F. Autophagy triggered by magnolol derivative negatively regulates angiogenesis. Cell Death Dis. 2013;4:e889. doi: 10.1038/cddis.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TM, Subramanian IV, Kelekar A, Ramakrishnan S. Kringle 5 of human plasminogen, an angiogenesis inhibitor, induces both autophagy and apoptotic death in endothelial cells. Blood. 2007;109:4793–4802. doi: 10.1182/blood-2006-11-059352. [DOI] [PubMed] [Google Scholar]
- 39.Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O’Dwyer PJ. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19:2995–3007. doi: 10.1158/1078-0432.CCR-12-1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.