Abstract
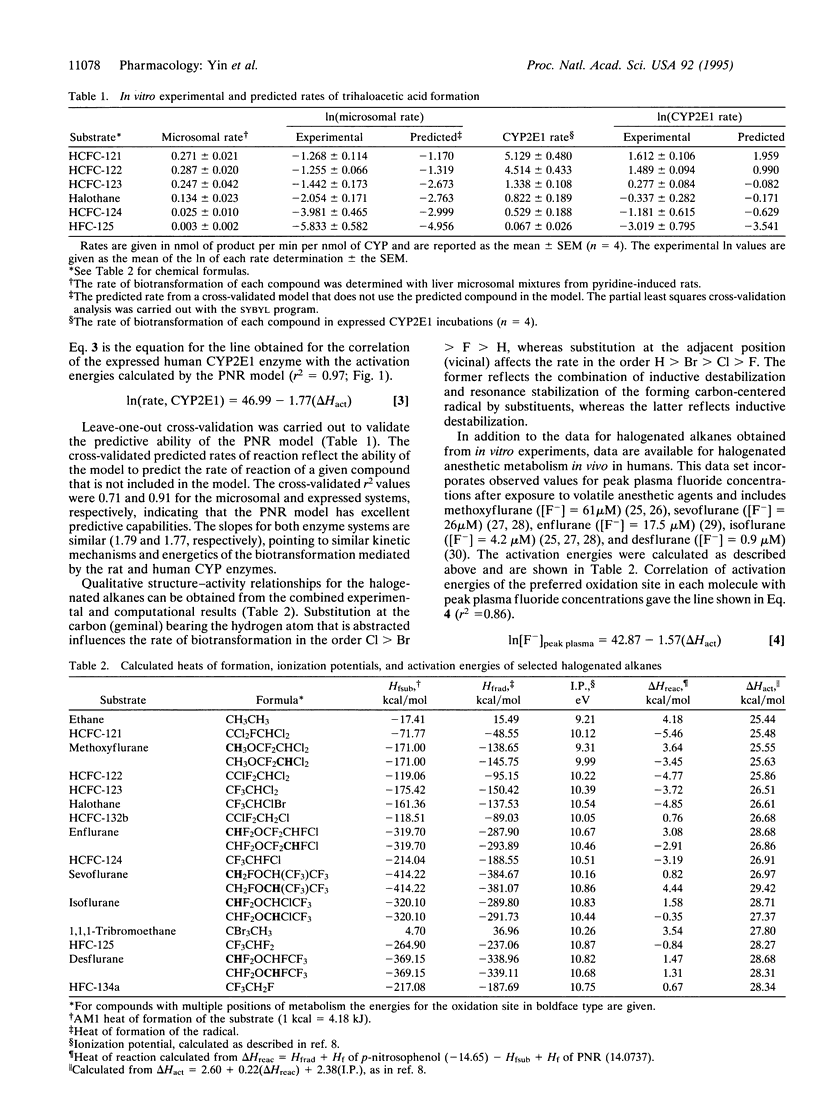
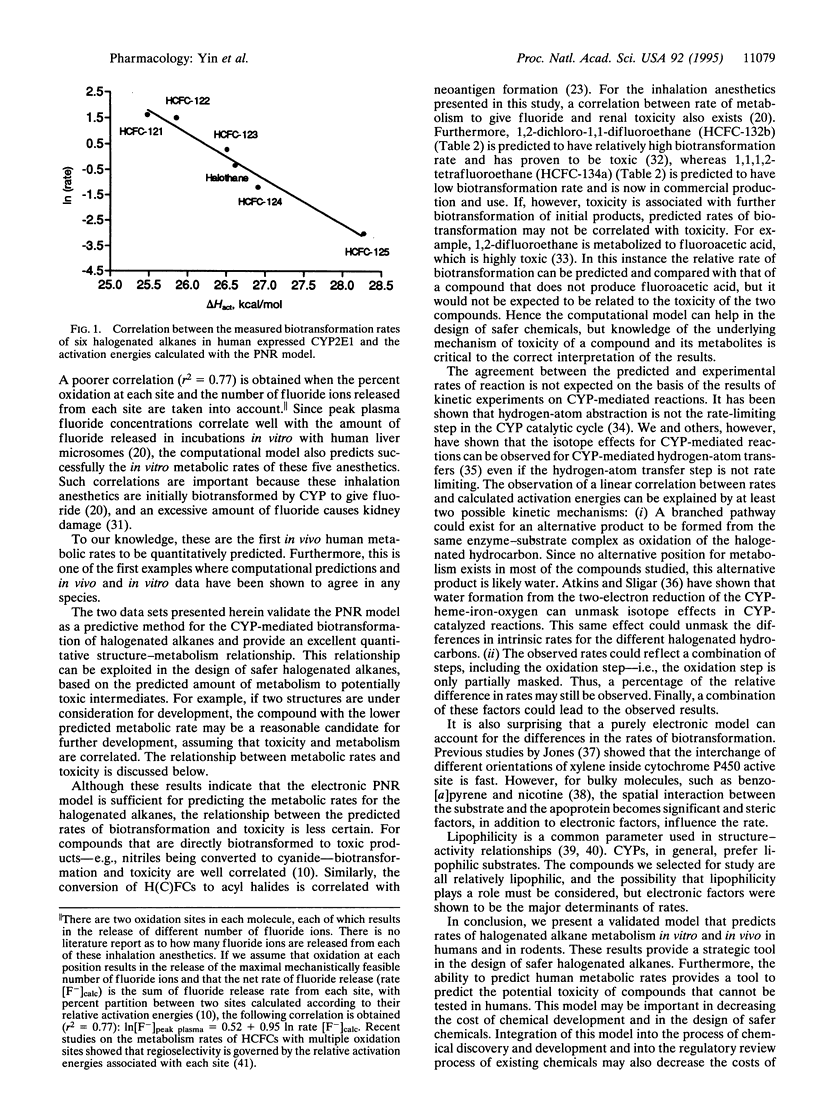
A computational model is presented that can be used as a tool in the design of safer chemicals. This model predicts the rate of hydrogen-atom abstraction by cytochrome P450 enzymes. Excellent correlations between biotransformation rates and the calculated activation energies (delta Hact) of the cytochrome P450-mediated hydrogen-atom abstractions were obtained for the in vitro biotransformation of six halogenated alkanes (1-fluoro-1,1,2,2-tetrachloroethane, 1,1-difluoro-1,2,2-trichloroethane, 1,1,1-trifluro-2,2-dichloroethane, 1,1,1,2-tetrafluoro-2-chloroethane, 1,1,1,2,2,-pentafluoroethane, and 2-bromo-2-chloro-1,1,1-trifluoroethane) with both rat and human enzyme preparations: In(rate, rat liver microsomes) = 44.99 - 1.79(delta Hact), r2 = 0.86; In(rate, human CYP2E1) = 46.99 - 1.77(delta Hact), r2 = 0.97 (rates are in nmol of product per min per nmol of cytochrome P450 and energies are in kcal/mol). Correlations were also obtained for five inhalation anesthetics (enflurane, sevoflurane, desflurane, methoxyflurane, and isoflurane) for both in vivo and in vitro metabolism by humans: In[F(-)]peak plasma = 42.87 - 1.57(delta Hact), r2 = 0.86. To our knowledge, these are the first in vivo human metabolic rates to be quantitatively predicted. Furthermore, this is one of the first examples where computational predictions and in vivo and in vitro data have been shown to agree in any species. The model presented herein provides an archetype for the methodology that may be used in the future design of safer chemicals, particularly hydrochlorofluorocarbons and inhalation anesthetics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders M. W. Metabolism and toxicity of hydrochlorofluorocarbons: current knowledge and needs for the future. Environ Health Perspect. 1991 Dec;96:185–191. doi: 10.1289/ehp.9196185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomont H. L., Tarbit M. H., Humphrey M. J., Houston J. B. Disposition of azole antifungal agents. II. Hepatic binding and clearance of dichlorophenyl-bis-triazolylpropanol (DTP) in the rat. Pharm Res. 1994 Jul;11(7):951–960. doi: 10.1023/a:1018966800208. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson G. P., Day B. J. Induction by pyridine of cytochrome P450IIE1 and xenobiotic metabolism in rat lung and liver. Pharmacology. 1992;44(3):117–123. doi: 10.1159/000138904. [DOI] [PubMed] [Google Scholar]
- Cousins M. J., Mazze R. I. Methoxyflurane nephrotoxicity. A study of dose response in man. JAMA. 1973 Sep 24;225(13):1611–1616. doi: 10.1001/jama.225.13.1611. [DOI] [PubMed] [Google Scholar]
- Flam F. EPA campaigns for safer chemicals. Science. 1994 Sep 9;265(5178):1519–1519. doi: 10.1126/science.8079163. [DOI] [PubMed] [Google Scholar]
- Frink E. J., Jr, Ghantous H., Malan T. P., Morgan S., Fernando J., Gandolfi A. J., Brown B. R., Jr Plasma inorganic fluoride with sevoflurane anesthesia: correlation with indices of hepatic and renal function. Anesth Analg. 1992 Feb;74(2):231–235. doi: 10.1213/00000539-199202000-00010. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Aoyama T., Gelboin H. V. Expression of mammalian cytochrome P450 using vaccinia virus. Methods Enzymol. 1991;206:85–92. doi: 10.1016/0076-6879(91)06079-i. [DOI] [PubMed] [Google Scholar]
- Grogan J., DeVito S. C., Pearlman R. S., Korzekwa K. R. Modeling cyanide release from nitriles: prediction of cytochrome P450 mediated acute nitrile toxicity. Chem Res Toxicol. 1992 Jul-Aug;5(4):548–552. doi: 10.1021/tx00028a014. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., MacDonald T. L. Mechanisms of cytochrome P-450 catalysis. FASEB J. 1990 May;4(8):2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Gyllenhaal O., Ehrsson H. Determination of sulphonamides by electron-capture gas chromatography. Preparation and properties of perfluoroacyl and pentafluorobenzyl derivatives. J Chromatogr. 1975 Apr 16;107(2):327–333. doi: 10.1016/0021-9673(75)80008-2. [DOI] [PubMed] [Google Scholar]
- Harris J. W., Anders M. W. Metabolism of the hydrochlorofluorocarbon 1,2-dichloro-1,1-difluoroethane. Chem Res Toxicol. 1991 Mar-Apr;4(2):180–186. doi: 10.1021/tx00020a009. [DOI] [PubMed] [Google Scholar]
- Harris J. W., Jones J. P., Martin J. L., LaRosa A. C., Olson M. J., Pohl L. R., Anders M. W. Pentahaloethane-based chlorofluorocarbon substitutes and halothane: correlation of in vivo hepatic protein trifluoroacetylation and urinary trifluoroacetic acid excretion with calculated enthalpies of activation. Chem Res Toxicol. 1992 Sep-Oct;5(5):720–725. doi: 10.1021/tx00029a020. [DOI] [PubMed] [Google Scholar]
- Harris J. W., Pohl L. R., Martin J. L., Anders M. W. Tissue acylation by the chlorofluorocarbon substitute 2,2-dichloro-1,1,1-trifluoroethane. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1407–1410. doi: 10.1073/pnas.88.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. P., Rettie A. E., Trager W. F. Intrinsic isotope effects suggest that the reaction coordinate symmetry for the cytochrome P-450 catalyzed hydroxylation of octane is isozyme independent. J Med Chem. 1990 Apr;33(4):1242–1246. doi: 10.1021/jm00166a024. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Hankins D. C., Thummel K. E. Human kidney methoxyflurane and sevoflurane metabolism. Intrarenal fluoride production as a possible mechanism of methoxyflurane nephrotoxicity. Anesthesiology. 1995 Mar;82(3):689–699. doi: 10.1097/00000542-199503000-00011. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Thummel K. E. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993 Oct;79(4):795–807. doi: 10.1097/00000542-199310000-00023. [DOI] [PubMed] [Google Scholar]
- Korzekwa K. R., Jones J. P. Predicting the cytochrome P450 mediated metabolism of xenobiotics. Pharmacogenetics. 1993 Feb;3(1):1–18. doi: 10.1097/00008571-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Loizou G. D., Urban G., Dekant W., Anders M. W. Gas-uptake pharmacokinetics of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Drug Metab Dispos. 1994 Jul-Aug;22(4):511–517. [PubMed] [Google Scholar]
- Manzer L. E. The CFC-Ozone Issue: Progress on the Development of Alternatives to CFCs. Science. 1990 Jul 6;249(4964):31–35. doi: 10.1126/science.249.4964.31. [DOI] [PubMed] [Google Scholar]
- Mazze R. I., Cousins M. J., Barr G. A. Renal effects and metabolism of isoflurane in man. Anesthesiology. 1974 Jun;40(6):536–542. doi: 10.1097/00000542-197406000-00006. [DOI] [PubMed] [Google Scholar]
- Mazze R. I., Woodruff R. E., Heerdt M. E. Isoniazid-induced enflurane defluorination in humans. Anesthesiology. 1982 Jul;57(1):5–8. doi: 10.1097/00000542-198207000-00002. [DOI] [PubMed] [Google Scholar]
- Olson M. J., Johnson J. T., O'Gara J. F., Surbrook S. E., Jr Metabolism in vivo and in vitro of the refrigerant substitute 1,1,1,2-tetrafluoro-2-chloroethane. Drug Metab Dispos. 1991 Sep-Oct;19(5):1004–1011. [PubMed] [Google Scholar]
- Olson M. J., Kim S. G., Reidy C. A., Johnson J. T., Novak R. F. Oxidation of 1,1,1,2-tetrafluoroethane in rat liver microsomes is catalyzed primarily by cytochrome P-450IIE1. Drug Metab Dispos. 1991 Mar-Apr;19(2):298–303. [PubMed] [Google Scholar]
- Olson M. J., Reidy C. A., Johnson J. T., Pederson T. C. Oxidative defluorination of 1,1,1,2-tetrafluoroethane by rat liver microsomes. Drug Metab Dispos. 1990 Nov-Dec;18(6):992–998. [PubMed] [Google Scholar]
- Smith I., Ding Y., White P. F. Comparison of induction, maintenance, and recovery characteristics of sevoflurane-N2O and propofol-sevoflurane-N2O with propofol-isoflurane-N2O anesthesia. Anesth Analg. 1992 Feb;74(2):253–259. doi: 10.1213/00000539-199202000-00015. [DOI] [PubMed] [Google Scholar]
- Sutton T. S., Koblin D. D., Gruenke L. D., Weiskopf R. B., Rampil I. J., Waskell L., Eger E. I., 2nd Fluoride metabolites after prolonged exposure of volunteers and patients to desflurane. Anesth Analg. 1991 Aug;73(2):180–185. doi: 10.1213/00000539-199108000-00011. [DOI] [PubMed] [Google Scholar]
- Tanii H., Hashimoto K. Studies on the mechanism of acute toxicity of nitriles in mice. Arch Toxicol. 1984 Mar;55(1):47–54. doi: 10.1007/BF00316585. [DOI] [PubMed] [Google Scholar]
- Yin H., Bennett G., Jones J. P. Mechanistic studies of uridine diphosphate glucuronosyltransferase. Chem Biol Interact. 1994 Jan;90(1):47–58. doi: 10.1016/0009-2797(94)90110-4. [DOI] [PubMed] [Google Scholar]


