Abstract
Background
Thoracic aortic aneurysms (TAAs) develop through an asymptomatic process resulting in gross dilatation that progresses to rupture if left undetected and untreated. If detected, TAA patients are followed over time until the risk of rupture outweighs the risk of surgical repair. Current methodologies for tracking TAA size are limited to expensive computed tomography or magnetic resonance imaging, as no acceptable population screening tools are currently available. Previous studies from this laboratory and others have identified differential protein profiles for the matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs), in ascending TAA tissue from patients with bicuspid aortic valves (BAV), versus patients with idiopathic degenerative disease and a tricuspid aortic valve (TAV). Additionally, altered microRNA (miR) expression levels have also been reported in TAAs as compared to normal aortic tissue. The objective of the present study was to identify circulating factors within the plasma that could serve as potential biomarkers for distinguishing etiological subtypes of aneurysm disease.
Methods
Ascending TAA tissue and plasma specimens were obtained from BAV (n=21) and TAV (n=21) patients at the time of surgical resection. The protein abundance of key MMPs (-1, -2, -3, -8, -9) and TIMPs (-1, -2, -3, -4), and microRNAs (-1, -21, -29a, -133a, -143, -145) was examined using a multi-analyte protein profiling system or by quantitative PCR, respectively. Results were compared to normal aortic tissue and plasma obtained from patients without aortic disease (n=10).
Results
Significant (p < 0.05) differences in standardized miR-1 and miR-21 abundance between BAV and TAV aortic tissue samples and different tissue and plasma profiles of analyte differences from normal aorta where observed between the BAV and TAV groups. Linear regression analysis significant linear relationships in plasma and tissue measurements only for MMP-8 and TIMPs -1, -3 and -4 (p < 0.05). Receiver operator curve analysis revealed specific cassettes of analytes predictive of TAA disease. Relative to normal aorta, BAV proteolytic balance was significantly increased for MMP-1, -2 and -7, and for decreased MMP-8 and -9. In contrast, TAV proteolytic balance relative to normal aorta was significantly increased only for MMP-1 and decreased for MMP-8 and -9.
Conclusions
Taken together these unique data demonstrate differential plasma profiles of MMPs, TIMPs, and miRs in ascending TAA specimens from patients with BAV and TAV. These results suggest that circulating biomarkers may form the foundation for a broader platform of biomarkers capable of detecting the presence of TAA using a simple blood test and may also be useful in personalized medicine strategies to distinguish between etiological subtypes of TAAs in patients with aneurysm disease.
Introduction
Thoracic aortic aneurysm (TAA) is an insidious and potentially devastating disease process. Despite advancements in our understanding of the pathobiology of thoracic aortic aneurysms, these advancements have yet to be translated into significant advancements in screening, diagnosis, tracking and treatment of TAAs.
From a biological standpoint, numerous studies have confirmed that specific proteinases such as the matrix metalloproteinases (MMPs) and their endogenous inhibitors (TIMPs) are implicated in the pathogenesis of ascending thoracic aortic aneurysms.1–4 In addition, specific and different cassettes of MMPs and TIMPs have been demonstrated in ascending TAAs with different etiologies, such as those associated with congenitally bicuspid aortic valves (BAVs) or tricuspid aortic valves (TAVs).1–4 Similar findings have been demonstrated with microRNAs (miRs) in which different types are also seen to be expressed within these aneurysms5.
Many of these agents can be reliably measured in plasma, providing a potentially novel strategy to identify and follow the progression of TAAs. Accordingly, the present study sought to identify circulating plasma factors that could distinguish and predict the etiological subtypes of aneurysm disease.
Methods
Patient Demographics
Matched tissue and plasma specimens from 42 patients with ascending aortic aneurysms (n=21 BAV patients, n=21 TAV patients) were taken from the widest region of the ascending aorta at the time of surgical resection or aortic valve replacement. No patients had aortic dissection, inflammatory aortic disease, or known connective tissue disorder. Normal aortic specimens were similarly harvested from the ascending aorta of heart transplant donors or recipients (n=10). Group mean ages were 58 ± 6 years Normal, 59 ± 2 years BAV and 70 ± 2 years TAV (TAV p < 0.05 from BAV and Normal). Seventy percent of Normal, 71% of BAV and 52% of TAV patients were male. Aortic diameters were 3.8 ± 0.2 cm Normal, 5.2 ± 0.2 cm BAV and 5.7 ± 0.2 cm TAV (TAV, BAV p < 0.05 from Normal). Normal aortic tissue and plasma specimens were snap frozen and stored at −80°C until analyzed. This study was approved by the institutional review boards of the Medical University of South Carolina, Duke University, and the University of Pennsylvania. Informed consent was obtained from all patients.
miR Isolation
Tissue Samples
For each tissue sample, 5 mg of frozen tissue was weighed and homogenized using a bead-mill homogenizer (Qiagen, Valencia, CA). Total RNA was extracted from tissue homogenates (mirVana PARIS miRNA Isolation kit; Applied Biosystems/Ambion Austin, TX) and analyzed for RNA quality and quantity using an Experion Automated Electrophoresis System (RNA StdSens Analysis Kit, Bio-Rad Laboratories, Hercules, CA). Ten ng of total RNA was reversed transcribed (TaqMan MicroRNA Reverse Transcription Kit; Applied Biosystems) for each miR of interest, and quantitative PCR was performed. Each tissue sample was analyzed for the following miRs: hsa-miR-1, hsa-miR-21, hsa-miR-29a, has-miR-133a, hsa-miR-143, and hsa-miR-145. These were selected because of the results from a previous manuscript published by our group,5 their reported involvement6 in the regulation of specific protein targets within the cardiovascular system, including several MMPs and ECM components (collagens, elastin, and microfibrillar proteins), and because of a previous report of involvement (miR-143/-145) in ascending TAA formation.7
Plasma Samples
RNA was isolated from 50 μl of plasma (mirVana PARIS Protein and RNA isolation System for Small RNAs; Ambion, AM1556) following the manufacturer’s instructions. The isolated RNA (40 μl) was then incubated for one hour at room temperature with 1.3 units of Heparinase-I (IBEX Pharmaceuticals Inc., PN 50-010-001) in a buffer containing 20mM Tris, pH 7.5, 50mM NaCl, 4mM CaCl2 and 0.01% BSA. Five μl of treated RNA was reverse transcribed for each miR of interest and quantitative PCR was performed. Each plasma sample was analyzed for the following miRs: hsa-miR-1, hsa-miR-21, hsa-miR-29a, has-miR-133a, hsa-miR-143, and hsa-miR-145.
Quantitative Polymerase Chain Reaction (QPCR)
QPCR
For both tissue and plasma samples, the reverse transcription product was amplified with gene specific TaqMan primer/probe sets using the TaqMan Universal PCR Master Mix with no AmperErase UNG (Cat# 4324020, Applied Biosystems, Carlsbad, CA) in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The thermal cycling protocol was conducted as follows: 10 minutes at 95°C, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Negative PCR controls were run to verify the absence of genomic DNA contamination (no reverse transcription control). Fluorescence was recorded at regular intervals following the 60°C annealing/extension segment of the PCR reaction and real-time data showing relative fluorescence versus cycle number was analyzed. Because of the paucity of good internal PCR controls for plasma specimens, miR expression in both tissue and plasma (for consistency of measurement) was determined from a ΔCt value (expression = 2(−ΔCt)) where ΔCt was derived for each individual specimen, and calculated by subtracting the mean Ct value for all targets measured from the individual Ct value of a given PCR target, as previously described.8–9 Results were then reported as a mean ± SEM for each miR measured in either tissue or plasma.
MMP/TIMP Multiplex Suspension Array (MSA)
MMP/TIMP MSA
utilizing an MSA approach. For the tissue specimens, thawed tissue was transferred to a cold buffer (volume 1:6 w/v) containing 10 mM cacodylic acid pH 5.0, 0.15 M NaCl, 10 mM ZnCl2, 1.5 mM NaN3, and 0.01% Triton X-100 (v/v), and homogenized using a bead-mill homogenizer (Qiagen, Valencia, CA). The homogenates were then centrifuged (800 x g, 10 min, 4°C), and 20 μg was analyzed using a an MSA approach. The following MMPs (-1, -2, -3, -7, -8, -9, -12, and -13) and TIMPs (-1, -2, -3, and -4) were examined as previously described. 10 The plasma specimens were analyzed in a similar fashion following dilution (1:100 for MMPs -2, and -9; 1:10 for the MMPs -1, -3, -7 -8, -12, and -13; and 1:20 for the TIMPs), as previously described.11 In both cases, samples were incubated on a microplate shaker (room temperature, 2 hours), filtered, and washed 3 times with 100 μl of wash buffer. Diluted goat anti-human polyclonal biotinylated antibodies (50 μl, analyte specific [included with antibody-conjugated bead kits], R&D Systems) were then added to each well and the specimens were again incubated on a microplate shaker (room temperature, 1 hour). The beads were filtered and washed as before, and streptavidin-phycoerythrin (50 μl, R&D Systems) was added to each well for 30 minutes at room temperature. After a final filtration and wash, the beads were analyzed using the Bio-Plex System; fluorescence was measured and then compared with standard curves for each analyte also run on the same plate. Protein quantities were calculated using Bio-Plex Manager Software 4.1 and expressed as absolute concentration in pg/ml.
Data Analysis
Expression levels of miRs and protein abundance of the MMPs/TIMPs were analyzed in two ways. First, all QPCR and MSA results were subjected to a Shapiro-Wilk test for normality. For the unequally distributed analytes, the absolute values were log transformed. Then all values were subjected to a one-way analysis of variance (prcomp module, Stata) with Tukey’s wholly significant difference post-hoc analysis for separation of means to determine differences between the referent controls, BAV, and TAV groups. Second, the percent change of miR and MMP/TIMP levels in the BAV and TAV groups were computed and compared to the referent controls using a one-sample mean comparison test with the hypothesized mean set at 100%. Analysis of variance with Tukey’s wholly significant difference post-hoc analysis (prcomp module) was used to determined differences between BAV and TAV groups. Linear regression analysis was performed to identify significant relationships between tissue and plasma levels of each analyte. Additionally, plasma biomarkers were assessed for univariate association with the presence of aortic aneurysm using logistic regression models. Receiver operating characteristic curves was then generated to compute an area under the curve (AUC) for each individual biomarker. Those biomarkers with p values of less than 0.25 were considered for inclusion in a multiple logistic regression model. Using forward stepwise variable selection, biomarkers were added to the model with the variable most strongly associated with outcome (presence of a TAA) being added to the model until no more variables met the entry criterion of α<=0.20. The α-level was set at 0.20 to ensure that even marginally predictive biomarkers were captured. Logistic regression analysis was performed to determine the coefficients and intercepts for biomarkers that were found to be significant predictors of aneurysm development in multivariable analysis. Discrimination and classification of the fitted multivariable models were assessed by using the generated equation and computing the corresponding sensitivity, specificity, positive predictive, and negative predictive values.12 Finally, relative proteolytic balance was expressed as the ratio of MMP abundance to a composite TIMP score composed of the sum of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 abundance in each sample. Changes in the ratio of MMP abundance to a composite TIMP score were determined by using a one-sample mean comparison test with the hypothesized mean for the referent controls set at 100%. All statistical calculations were made using the Stata software package (v8.2; StataCorp LP, College Station, TX). In all cases, p<0.05 was considered significant.
Results
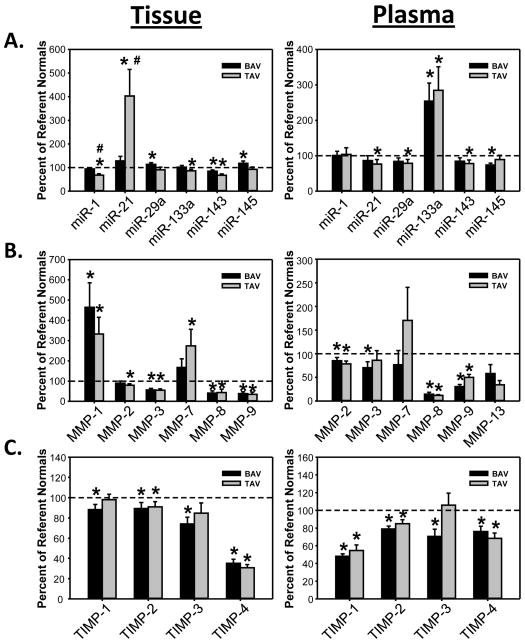
Tissue and plasma measurements of MMPs, TIMPs and miRs standardized to normal aorta or normal plasma are shown in Figure 1. Absolute value measurements are summarized in Table 1 (Tissue) and Table 2 (Plasma). There were significant differences in the tissue and/or plasma levels of several analytes with respect to control values. Moreover, a differential expression of certain analytes was observed in TAAs from the BAV and TAV groups. For example, tissue levels of miR-1 and miR-21 were differentially altered the TAV group compared to the BAV group (Table 1) Lastly, relative proteolytic balance in the tissue specimens was expressed as the ratio of MMP abundance to a composite TIMP score composed of the sum of TIMP-1, TIMP-2, TIMP-3, and TIMP-4 abundance in each sample, shown in the Supplemental Figure. Relative to normal aorta, BAV proteolytic balance was significantly increased for MMP-1, -2 and -7, and for decreased MMP-8 and -9. In contrast, TAV proteolytic balance relative to normal aorta was significantly increased only for MMP-1 and decreased for MMP-8 and -9.
Figure 1.
Aortic tissue and plasma analysis for miRs, MMPs and TIMPs, comparing ascending TAAs associated with BAV or TAV from normal aortic samples (dashed line). Significant differences were observed between the BAV and TAV groups, and between the aneurysm groups and normal aorta.
* p<0.05 from normal aorta, # p<0.05 from BAV
Table 1.
Absolute values for miR expression (no unit) and protein abundance of MMPs and TIMPs (pg/ml) in aortic tissue from normal and TAA patients with BAV or TAV.
| TISSUE ANALYTE | CONTROL | BAV | TAV |
|---|---|---|---|
| microRNA (2−ΔCt) | |||
| miR-1 (1X10−3) | 57.8±2.4 | 54.3±3.3 | 39.4±3.3*# |
| miR-21 (1X10−2) | 169.7±21.4 | 217.7±32.3 | 683.0±191.0*# |
| miR-29a (1X10−2) | 242.4±33.3 | 274.7±17.2 | 220.2±25.8 |
| miR-133a (1X10−3) | 80.3±5.2 | 81.7±5.8 | 69.2±5.1 |
| miR-143 (1X10−2) | 830.9±37.9 | 703.7±45.9 | 560.6±49.1* |
| miR-145 (1X10−2) | 771.0±37.9 | 910.8±72.1 | 715.0±75.6 |
| MMPs (pg/mL) | |||
| MMP-1 | 3.4±1.0 | 15.7±4.0 | 11.3±2.8 |
| MMP-2 (1X102) | 71.6±13.0 | 64.1±7.7 | 56.9±5.4 |
| MMP-3 | 292.9±45.5 | 165.2±22.4 | 160.6±20.2 |
| MMP-7 (1X101) | 8.8±2.9 | 14.6±3.8 | 24.0±7.2 |
| MMP-8 (1X101) | 294.1±128.8 | 121.7±29.3 | 128.5±49.8 |
| MMP-9 (1X102) | 74.9±49.6 | 28.3±7.1 | 26.0±7.9 |
| MMP-12 | ND | ND | ND |
| MMP-13 | ND | ND | ND |
| TIMPs (pg/mL) | |||
| TIMP-1 (1X102) | 113.6±13.7 | 100.2±5.9 | 111.3±6.1 |
| TIMP-2 (1X102) | 127.9±17.1 | 114.2±7.5 | 116.3±6.5 |
| TIMP-3 (1X102) | 22.2±3.0 | 16.5±1.5 | 18.8±2.2 |
| TIMP-4 | 141.9±33.7 | 49.8±5.7* | 43.6±4.6* |
|
| |||
| Sample Size (n) | 10 | 21 | 21 |
p<0.05 vs. Control
p<0.05 vs. BAV
Data Analysis
All analytes were subjected to a Shapiro-Wilk test for normality. For the unequally distributed analytes, the absolute values were log transformed and subjected to an one-way analysis of variance (prcompw module) with Tukey’s wholly significant difference post-hoc analysis for separation of means to determine differences between the referent controls, BAV, and TAV groups.
Table 2.
Absolute values for miR expression (no unit) and protein abundance of MMPs and TIMPs (pg/ml) in plasma from patients with normal aortae and TAA patients with BAV or TAV.
| PLASMA ANALYTE | CONTROL | BAV | TAV |
|---|---|---|---|
| microRNA (2−ΔCt) | |||
| miR-1 (1X10−3) | 70.1±15.7 | 70.8±8.2 | 72.7±13.0 |
| miR-21 | 42.5±11.7 | 36.7±5.8 | 32.4±5.6 |
| miR-29a | 7.2±1.4 | 6.1±0.7 | 5.7±0.8 |
| miR-133a (1X10−3) | 45.0±12.7 | 114.5±22.9 | 128.0±30.0 |
| miR-143 (1X10−1) | 14.4±2.5 | 12.2±1.4 | 11.3±1.4 |
| miR-145 (1X10−2) | 94.3±11.4 | 69.8±5.0 | 84.3±11.0 |
| MMPs (pg/mL) | |||
| MMP-1 | ND | ND | ND |
| MMP-2 (1X104) | 53.4±4.7 | 45.6±3.6 | 41.9±3.1 |
| MMP-3 (1X103) | 22.8±3.6 | 16.1±2.9 | 19.7±4.6 |
| MMP-7 (1X102) | 20.8±18.4 | 16.0±6.3 | 35.5±14.5 |
| MMP-8 (1X102) | 91.0±28.9 | 13.3±3.5* | 10.8±1.3* |
| MMP-9 (1X104) | 83.6±21.5 | 25.6±3.5* | 41.8±5.3*# |
| MMP-12 | ND | ND | ND |
| MMP-13 (1X102) | 24.9±15.0 | 11.0±3.6 | 8.5±3.6 |
| TIMPs (pg/mL) | |||
| TIMP-1 (1X103) | 116.4±24.7 | 56.0±3.2* | 63.8±7.2* |
| TIMP-2 (1X103) | 55.2±2.8 | 43.6±1.7* | 46.9±2.4 |
| TIMP-3 (1X102) | 34.2±4.6 | 24.1±2.8 | 36.2±4.6 |
| TIMP-4 (1X102) | 19.4±2.9 | 14.7±1.2 | 13.3±1.1 |
|
| |||
| Sample Size (n) | 10 | 21 | 21 |
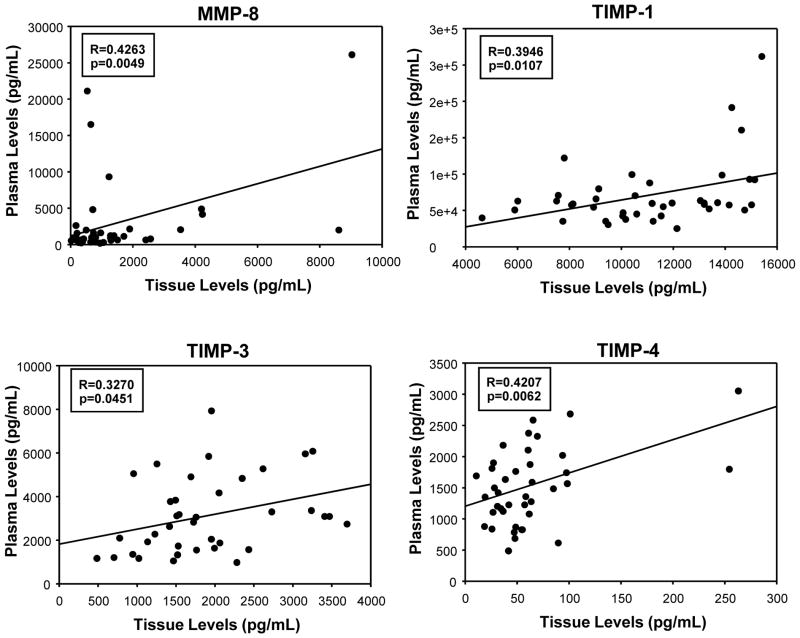
Linear regression analysis, performed to identify significant relationships between tissue and plasma levels of each analyte, revealed significant linear relationships only for MMP-8 and TIMPs -1, -3 and -4. These results are summarized in Figure 2.
Figure 2.
Linear regression analysis identifying significant relationships between analyte tissue and plasma levels. Significant relationships were found for MMP-8, TIMP-1, TIMP-3 and TIMP-4.
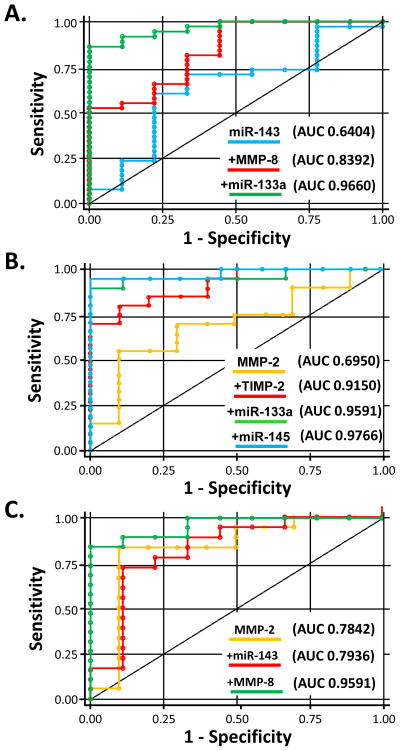
Receiver operator characteristic curve analysis was performed to determine whether plasma levels of the analytes could serve as biomarker(s) for the presence/absence of TAA disease. The AUC values from the univariate analysis are summarized in Table 3. Following this, forward stepwise multivariable receiver operating characteristics analysis was performed, which revealed specific cassettes of analytes predictive of TAA disease, as depicted in Figure 3. For TAA disease overall, the combination of miR-143, MMP-8 and miR-133a maximized AUC values to 0.9660. For TAAs associated with BAV, the combination of MMP-2, TIMP-2, miR -143, miR-133a and miR-145 maximized AUC values to 0.9766. For TAAs associated with TAV, the combination of MMP-2, miR -143 and MMP-8 maximized AUC values to 0.9591.
Table 3.
Area-under-the-curve (AUC) for individual plasma analytes
| All Aneurysms | BAV | TAV | |
|---|---|---|---|
| miR-1 | 0.4571 (0.84) | 0.4795 (0.97) | 0.4306 (0.75) |
| miR-21 | 0.4538 (0.46) | 0.4850 (0.65) | 0.4211 (0.37) |
| miR-29a | 0.4077 (0.31) | 0.4300 (0.41) | 0.3842 (0.35) |
| miR-133a | 0.7654 (<0.01) | 0.7602 (<0.01) | 0.7712 (<0.01) |
| miR-143 | 0.3596 (0.23) | 0.3567 (0.36) | 0.3626 (0.21) |
| miR-145 | 0.3385 (0.21) | 0.2700 (0.02) | 0.4105 (0.65) |
| MMP-1 | Not detectable | ||
| MMP-2 | 0.2615 (0.03) | 0.3050 (0.07) | 0.2158 (0.02) |
| MMP-3 | 0.3231 (0.45) | 0.3350 (0.28) | 0.3105 (0.62) |
| MMP-7 | 0.6071 (0.80) | 0.5769 (0.66) | 0.6333 (0.56) |
| MMP-8 | 0.1321 (<0.01) | 0.1275 (<0.01) | 0.1368 (<0.01) |
| MMP-9 | 0.1667 (<0.01) | 0.0950 (<0.01) | 0.2421 (<0.01) |
| MMP-12 | Not detectable | ||
| MMP-13 | 0.3590 (0.08) | 0.3889 (0.20) | 0.3333 (0.10) |
| TIMP-1 | 0.2231 (<0.01) | 0.1850 (<0.01) | 0.2632 (<0.01) |
| TIMP-2 | 0.2051 (<0.01) | 0.1300 (<0.01) | 0.2842 (0.03) |
| TIMP-3 | 0.3833 (0.44) | 0.2575 (0.04) | 0.5158 (0.75) |
| TIMP-4 | 0.3205 (0.04) | 0.3650 (0.18) | 0.2737 (0.01) |
Values presented as: AUC (p-value) for each individual analyte
Bolded cells indicate AUC values that are statistically significant in univariate analysis
Figure 3.
Receiver operating characteristic curves to assess plasma aneurysm predictability. Inclusion of plasma analytes using forward stepwise variable selection resulted in different combinations for TAA in general (top), BAV-associated TAAs (middle) and TAV-associated TAAs (bottom) providing high area-under-the curve (AUC) values, indicating high sensitivity and specificity.
Logistic regression coefficients and intercepts for biomarkers that were found to be significant predictors of aneurysm development in multivariate analysis were computed.
For all TAA, the equation: ; (r2 = 0.59, p<0.001); yielded a positive predictive value of 0.92, and negative predictive value of 0.58 a sensitivity of 0.87 and a specificity of 0.70.
For the BAV group, the equation: ; (r2: 0.73, p<0.001); resulted a positive predictive value of 0.95, a negative predictive value of 1.00, a sensitivity of 0.95 and a specificity of 1.00.
For the TAV group, the equation: ; (r2: 0.67, p<0.001); yielded a positive predictive value of 0.89, a negative predictive value of 0.80 a sensitivity of 0.89 and a specificity of 0.80.
Discussion
Our knowledge of the pathobiology of TAAs continues to expand and as such, it is becoming more apparent that this information may be used to improve the way we diagnose, track, and treat these serious conditions. Of particular significance is that TAAs of different etiologic subtypes display different biological patterns which may allow for personalized health care strategies. Currently, TAAs are diagnosed serendipitously during routine physical examinations or assessments for other disease conditions. A screening test for TAAs would be very valuable to identify those individuals who have asymptomatic but potentially life threatening aneurysms, necessitating knowledge of plasma biomarker predictors. As such, this study undertook the task of identifying plasma signatures which could be indicative of specific subtypes of ascending aortic aneurysm disease. This study demonstrated that it was possible to measure a variety of different analytes directly relevant to TAA disease in plasma. Second, very little concordance between plasma measurements and aortic tissue measurements of MMPs, TIMPs, and a specific cassette of miRs was observed. Third, it was shown that aneurysms associated with either bicuspid or tricuspid valves displayed different cassettes of tissue and plasma analytes. Finally, it was demonstrated that it was possible to predict with a high degree of specificity and sensitivity the presence of either aneurysm disease in general or specific etiologic subtypes of aneurysm disease in particular using a plasma multi-analyte regression strategy. All of the above carries hope that such technology could eventually be configured to a simple plasma measurement that could aid with the screening of patients and perhaps to use these signatures to predict aneurysm activity or changes in aneurysm size.
In the present study, a significant number of MMPs, TIMPs, and miRs were measured in aortic tissue. The results were consistent to some extent with findings that we3–4 and others1–2 have made before with regards to differential expression of these analytes for aneurysms of different etiologies. This data further supports the concept that different etiologic subtypes of TAAs display measurable biological differences that can be used to distinguish meaningfully between these disease processes.
We showed that a broad range of analytes could be measured in the plasma and that it was possible to generate a different cassette of specific analyte profiles for TAAs associated with bicuspid or tricuspid aortic valves. What was interesting with this study was that in general it was not possible to demonstrate a specific correlation between most tissue and plasma analyte levels. The reasons for this are multifactorial and include the fact that many of the biomarkers measured are primarily interstitial molecules and hence it may be difficult to predict how much measurable spillage into plasma would be observed. Furthermore, the half life of the analytes is variable, making it difficult to correlate plasma and tissue concentrations. Also, tissue and plasma storage and handling carries a significant impact on the ability to accurately measure analytes. Although handling of tissue and plasma was an important and rigorous procedure in the laboratory, it is possible that aberrancies in the storage and processing may have affected the results and decreased the degree of concordance between tissue and plasma levels.
Previous work has underscored the potential for using plasma analytes to predict aortic disease and the results of interventional therapy. Numerous molecules including MMPs, C-reactive protein (CRP) and D-Dimer have shown promise in the prediction of acute aortic dissection.13 A recent meta-analysis implicated measurement of circulating MMP-9 as an indicator of abdominal aortic aneurysm (AAA) disease,14 and plasma measurement of MMP-1 and MMP-9 has been shown to be predictive of aortic rupture.15 Further, composite measurement of high density lipoprotein (HDL), CRP and immunoglobulin G correlates to AAA size.16 In the thoracic aorta, measurement of circulating MMP-9 has been shown to correlate to aortic root dilatation.17 Interestingly, elevated plasma MMP-9 levels have been used to predict endoleaks after endovascular therapy of the abdominal18 and thoracic19 aorta. The present work supports the above results and establishes preliminary algorithms for the screening of patients for ascending aortic aneurysm disease.
An important finding in the study is that measurement of single analytes is not predictive of aneurysm disease to any significant level, but a step-wise combination of multiple analytes produces an algorithm which is highly sensitive and specific. As discussed above, a similar trend has been observed for predictions of aortic dissection and AAA.13, 16 Hence, aneurysms of different etiologies could have specific plasma regression equations containing a composite of analytes that would accurately predict the presence of disease as a screening tool in patients prior to referral for confirmatory imaging.
It must be understood that this study has significant limitations. The sample size of measurement is relatively small and also the panel of MMPs, TIMPs, and miRs tested, while significant in size, is not comprehensive. Further studies must be undertaken testing a larger bank of analytes to assess for any further significance. In addition, it must be realized that the regression equations generated for different aneurysm subtypes must be tested in a larger bioset for validation. Related to this, post-resection plasma samples would be important for validation of the biomarker algorithm. As already discussed, the results of the study may be limited by issues related to obtaining, storing and processing of tissue and plasma within a multicenter biobank such as the aortic biobank housed at the Medical University of South Carolina. Further refinement of storage protocols and processing should decrease the variability associated with measurements. Because the aortic tissue was snap-frozen and analyzed following homogenization, differentiation of biomarker elaboration between cell types was not possible. This latter point is a challenging area, since in addition to release of biomarkers from cells which have undergone apoptosis, some analytes such as miRs are actively and selectively exported by living cells,20 thus significantly confounding the cellular origin issue. Lastly, this observational study does not offer any significant insight regarding which tissue and/or circulating biomarker is a predictor of aortic growth and/or rupture and/or dissection.
The above limitations notwithstanding, the results of this study indicate that specific plasma biosignatures can be generated for aneurysms of different subtypes. These data hold significant importance with regards to the potential advancement of diagnosis, tracking and treatment of thoracic aortic aneurysm disease.
Supplementary Material
Relative proteolytic balance expressed as the ratio of MMP abundance to a composite TIMP score composed of the sum of TIMP-1, TIMP-2, TIMP-3, and TIMP-4. Different profiles of proteolytic balance were observed for the BAV and TAV groups. * p < 0.05 from normal aorta.
Acknowledgments
This study was supported by the following grants: NIH/NIA AG036954, NIH/NHLBI HL102121 and Veterans Affairs Merit Award 1 I01 BX000904-01
Footnotes
Presented in part at the 38th 54 Annual Meeting of the Western Thoracic Surgical Association, Maui, HI
References
- 1.Fedak PW, de Sa MP, Verma S, et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126:797–806. doi: 10.1016/s0022-5223(03)00398-2. [DOI] [PubMed] [Google Scholar]
- 2.LeMaire SA, Wang X, Wilks JA, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–8. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365–70. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 4.Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–36. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 5.Jones JA, Stroud RE, O’Quinn EC, et al. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet. 2011;4:605–13. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–32. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elia L, Quintavalle M, Zhang J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–8. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestdagh P, Van Vlierberghe P, De Weer A, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10:R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer SU, Pfaffl MW, Ulbrich SE. Normalization strategies for microRNA profiling experiments: a ‘normal’ way to a hidden layer of complexity? Biotechnol Lett. 2010;32:1777–88. doi: 10.1007/s10529-010-0380-z. [DOI] [PubMed] [Google Scholar]
- 10.Ikonomidis JS, Ruddy JM, Benton SM, Jr, et al. Aortic dilatation with bicuspid aortic valves: cusp fusion correlates to matrix metalloproteinases and inhibitors. Ann Thorac Surg. 2012;93:457–63. doi: 10.1016/j.athoracsur.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essa EM, Zile MR, Stroud RE, et al. Changes in Plasma Profiles of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of MMPs in Stress-Induced Cardiomyopathy. J Card Fail. 2012;18:487–92. doi: 10.1016/j.cardfail.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ. 1994;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen D, Zhou XL, Li JJ, Hui RT. Biomarkers in aortic dissection. Clin Chim Acta. 2011;412:688–95. doi: 10.1016/j.cca.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Circulating matrix metalloproteinase-9 concentrations and abdominal aortic aneurysm presence: a meta-analysis. Interact Cardiovasc Thorac Surg. 2009;9:437–40. doi: 10.1510/icvts.2009.208835. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WR, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:580–4. doi: 10.1016/j.ejvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Hellenthal FA, Pulinx B, Welten RJ, et al. Circulating Biomarkers and Abdominal Aortic Aneurysm Size. J Surg Res. 2011 doi: 10.1016/j.jss.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Karakaya O, Barutcu I, Esen AM, et al. Relationship between circulating plasma matrix metalloproteinase-9 (gelatinase-B) concentration and aortic root dilatation. Am J Hypertens. 2006;19:361–5. doi: 10.1016/j.amjhyper.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Hellenthal FA, Ten Bosch JA, Pulinx B, et al. Plasma levels of matrix metalloproteinase-9: a possible diagnostic marker of successful endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2012;43:171–2. doi: 10.1016/j.ejvs.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Monaco M, Stassano P, Di Tommaso L, Iannelli G. Response of plasma matrix metalloproteinases and tissue inhibitor of metalloproteinases to stent-graft surgery for descending thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134:925–31. doi: 10.1016/j.jtcvs.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative proteolytic balance expressed as the ratio of MMP abundance to a composite TIMP score composed of the sum of TIMP-1, TIMP-2, TIMP-3, and TIMP-4. Different profiles of proteolytic balance were observed for the BAV and TAV groups. * p < 0.05 from normal aorta.