Summary
Purpose
Extraoperative electrical stimulation mapping (ESM) to identify functional cortex is performed prior to neurosurgical resection at epilepsy surgery programs worldwide. However, the procedure remains unstandardized, with no established clinical guidelines. We sought to determine the current range in ESM practice parameters across established epilepsy surgery centers.
Methods
We developed and distributed a 31 question survey to 220 epilepsy centers worldwide regarding current practice parameters of ESM. Questions addressed preoperative assessment, technical stimulation parameters, language testing protocols, criteria for identification of positive or negative functional sites, management of mapping complications, and postoperative functional outcome.
Key findings
Survey responses were obtained from 56 centers. These revealed marked practice variability in virtually all aspects of the ESM procedure. Importantly, these aspects included critical procedure components such as electrical stimulation settings, the types of language functions tested, the operational definition of a language error, size of surgical resection margin, cortical locations mapped for language, testing in the presence of after discharges, and medical management of mapping complications. Forty-one percent of centers reported at least one persistent adverse language outcome despite preserving all eloquent sites defined by their stimulation mapping procedure.
Significance
The striking variations in practice across centers are likely to influence mapping results, which directly affect the boundaries of cortical resection and, consequently, might worsen either seizure or functional outcomes. Clearly, adverse functional outcomes occur despite mapping procedures that were perceived to be adequate. Investigation of critical technical and procedural aspects of stimulation mapping is warranted, with the ultimate goal of establishing empirically based practice guidelines to improve the safety and efficacy of ESM and resective epilepsy surgery.
Keywords: Electrical stimulation mapping, epilepsy surgery
Introduction
Extraoperative electrical stimulation mapping (ESM) is an invasive procedure performed prior to cortical resection in which electrical stimulation is applied briefly to discrete brain areas to identify regions critical for sensory, motor or cognitive functions. Positive sites identified via ESM are typically spared from resection with the goal of preserving function postoperatively.1 Commonly regarded as the “gold standard” technique for identifying essential functional cortex,2 the procedure is performed in epilepsy surgery programs worldwide.
Despite decades of clinical use and the potentially life altering consequences resulting from ESM based decisions, ESM is unstandardized, with no published guidelines and few empirical studies of procedural parameters. This is concerning, as ESM is a multifaceted, time-constrained procedure, requiring multiple decisions regarding electrophysiological, pharmacological, and cognitive parameters. Certainly, failure to identify eloquent cortex in proposed resection areas has obvious and potentially severe, life-long consequences. However, overestimating the extent of eloquent sites, or incorrectly classifying a site as eloquent, might lead to reduced postsurgical seizure control, also bearing potentially far-reaching adverse consequences. Comprehensive literature review reveals only a limited number of papers addressing isolated aspects of the procedure, mainly from a non-clinical perspective. 2, 3 Only a few investigations have addressed the clinical procedure. 4–7 Given the lack of empirical data to support clinical guidelines, procedures are typically based on institutional tradition, anecdotal experience or simply, personal preferences.
Based on our review of the literature together with our own observations, we suspected that ESM methodology varies widely among surgical centers. We encountered such variability in our attempts to develop clinical research collaborations with other epilepsy surgery programs. As one brief example, whereas one institution restricted stimulation below 10 mA due to concern of current spread, another institution disregarded negative results obtained with stimulation below 10 mA due to the concern of insufficient stimulation levels to disrupt function. On the surface, these diametrically opposed rationales each, taken individually, appear feasible and potentially, scientifically sound. Nevertheless, it is likely that ESM results at these two institutions would differ. Such variability in practice is a concern, as it may compromise the sensitivity and specificity of a procedure in which even a minimal error rate in either direction cannot be tolerated.
We sought to assess the extent of variability in ESM procedures among epilepsy surgery programs that utilize the technique. Toward this end, we developed and distributed an extraoperative neurostimulation mapping survey to gather information regarding current practices in stimulation mapping. This would serve as a first step toward identifying aspects of the procedure that would benefit from empirically determined guidelines.
Methods
Survey development
The authors devised 31 multiple choice or fill-in questions addressing the following topics: mapping personnel (1), preoperative language and motor assessment (3) technical stimulation parameters (15), language protocol (2), criteria for identification of positive and negative functional sites (3), mapping complications and pharmacological management (2), resection boundaries (1), and postoperative functional outcome (4). The complete survey is presented in supplementary material (“Survey”).
The rationale behind many of the survey questions is self-evident; however, for questions that might be less transparent, we provide brief explanations. The first of these is question 2, regarding mapping personnel, which addressed two issues: 1) Different professional specialties bring different types of expertise to the procedure, and 2) the number and type of professionals reflect an allocation of resources to ESM. Two questions addressed pre-operative lateralization (Q4) and localization (Q15), as the decision to conduct ESM is contingent upon the determination that the implanted cerebral hemisphere supports language. Similarly, inconsistency in regions tested could influence ESM results (Q11).
Several questions addressed clinician handling of after discharges (ADs), as these can potentially evolve into seizures. Attempts to avoid ADs can limit stimulation intensity and thus, potentially, the efficacy of the procedure (Q28-Q31). These questions addressed anticonvulsant medication management (Q30, Q31) and testing during a sustained AD (Q29), a topic of considerable controversy. Some clinicians may consider ADs to functionally inactivate the cortical sites stimulated, thereby providing adequate testing conditions. However, there is a concern that ADs may affect areas beyond the electrode sites stimulated, such that alterations in function may not be site-specific. Further, it has been argued that ADs may not inactivate sites as effectively as direct stimulation, resulting in false negative findings.
Because reporting adverse events on a survey is potentially a sensitive topic, we gave centers the option of responding anonymously. When relevant, questions were asked specifically with regard to adult versus pediatric populations. Although ESM is also performed intraoperatively, we limited survey questions to extraoperative practices, as we suspected that intraoperative practices are likely more variable given the constraints of the operating room environment. We presumed that practices were consistent within a given site.
Epilepsy surgery programs were identified via the National Association of Epilepsy Centers website: http:www.naec-epilepsy.org, the ILAE website: http://www.ilae-epilepsy.org and additional internet searching. A questionnaire and cover letter were mailed to each program with instructions that the questionnaire be completed by the individual(s) who routinely conduct stimulation mapping. The cover letter explained that individual centers would remain anonymous in any publications. Two hundred twenty questionnaires were mailed or emailed, 137 to North American sites and 83 to sites in South America, Europe, Asia, Australia and Africa. Five weeks following the initial distribution, and again six months later, reminders for participation were sent via email.
Results
Fifty-six epilepsy surgery programs returned questionnaires. Sixty-nine percent of responding programs were in North America, 16% were in Europe, with the remainder in other countries. Among these centers, 75% conduct ESM in individuals ages 18–60, 36% in ages 10–17, 13% in ages 6–9, 11% in patients under age 5, and 11% over age 60. Sixty-one percent of responders identified their center in their returned survey. For adult ESM, response rates per question were > 90% in nearly all cases (mean = 96%). For the four questions that addressed both adult and pediatric practices, the response rate for the pediatric component ranged from 41–52%; therefore, these results are not reported here. Below we present a summary of the adult survey results. Complete response information, including pediatric results, is provided in supplementary material (“Survey Results”).
Motor mapping
Prior to mapping, 70% of responding centers estimate the location of hand motor/sensory areas. Some sites differentiated primary from associative motor areas by assessing for induced weakness (36%), or entrainment of evoked movements to 1 Hz stimulation pulses8 (11%).
Language mapping
Of the 52 centers that provided a total number of language mappings in the prior year, 310 language mappings were conducted in patients aged 18–60, 39 language mappings in patients aged 10–17, 19 were in 6–9 year olds, 6 were in patients under age 5, and 10 were conducted in individuals over age 60. Most centers conduct some form of preoperative baseline language testing, although type and extent vary. Standardized visual object naming assessment was reported by 66%, verbal/semantic fluency by 46%, auditory description naming by 6%, token test by 7%, and aphasia batteries by 9%; 11% reported using other tests Approximately half of responders also reported using additional, non-standardized techniques (e.g., informal baseline testing utilizing ESM language stimuli, or informal conversation). Most centers reported using one or more presurgical lateralization procedures. Wada testing was the most frequently used technique, with 43% of responders performing Wada tests in most (i.e., 76–100%) patients, and only 9% not using Wada testing at all. fMRI was used frequently (i.e., in 76–100% of surgical candidates) by 31% of responders, with 24% not using fMRI at all. Nine percent reported using MEG on a regular basis.
One main point of agreement involved personnel, with 96% of responders including an epileptologist or neurophysiologist. Inclusion of an EEG technologist was reported by 64% of responders. Inclusion of a neuropsychologist was indicated by only 48%.
Responders were queried about specific language tasks used during ESM. For ease of reporting, these are grouped according to function: speech production is tested at 76% of centers, comprehension at 68%, naming at 89% and reading at 75%. It is quite striking that not one of these basic language functions is uniformly tested across all centers. Moreover, only 29 centers (52%) consistently test all four of these basic language functions. Sixteen centers (29%) test three functions, 10 (18%) test two functions, and one center tests only one language function.
The operational definition for what constitutes a language error during stimulation is a crucial factor in the identification of a positive site. Although there was considerable agreement (93–95%) that non-responses, anomic responses and paraphasic responses represent errors, responders were essentially split with regard to whether hesitations (59%) and perseverations (52%) were considered errors. The minimum number of trials per task administered at each site ranged from 1–10 with a mean of 2.6 (SD 1.7). The proportion of errors required to consider a site positive for language, also a parameter with critical bearing for deciding whether to preserve or resect a particular cortical site, varied markedly. Responses were evenly distributed over 25%, 50%, 67%, 75%, and 100% error rates.
Interference from ADs likely also influences language findings. The definition of an AD varied across centers: 28% defined an AD as one discharge following stimulation, 32% as a run of two or more discharges, and 45% as a sustained train of discharges lasting at least one second. One center identified ADs on a case by case basis. When a language function is disrupted during electrical stimulation that is followed by an AD, the site is considered positive for that function at 34% of centers. Sixty-four percent of responders reported testing function during a sustained AD, following termination of stimulation. If function remains intact during the AD, 43% would consider the site sufficiently tested and classify the site as negative. Fourteen percent of responders would consider the site positive if function was disrupted after stimulation yet during the AD.
Mapping results depend not only on the tests and criteria utilized, but also on the locations typically investigated. Whereas 79% reported testing for language in the anterolateral temporal region, 56% map the basal temporal region, and 21% map insular cortex. When tongue motor or sensory sites are detected, 45% routinely test language at these sites, and an additional 31% test for language function only when the motor finding is subtle. The remaining 25% perform no language testing at these sites.
Neurostimulation
A critical component of ESM is the choice of settings for the user-controlled technical parameters of the electrical stimulation itself: current, frequency, pulse width, stimulation duration, and electrode pairing strategy. To our knowledge, all clinical stimulators used are constant-current, i.e. voltage is automatically stepped to maintain the set current. Both monophasic and biphasic stimulators were reported (monophasic 40%, biphasic 46%, unknown/other 15%).
Most centers reported using a strategy in which frequency and pulse width are fixed, and the current setting is gradually increased at each site to functional threshold, AD threshold, or a pre-determined maximum. At 9% of centers, current and pulse width are increased jointly.4
There was broad variability in most electrical settings across centers (Table 1). The most common frequency range was 50 Hz or above, with 70% using this range for motor mapping and 75% for language mapping. Frequencies of 1–15 Hz were used for motor mapping at 33% of centers, and for language mapping at 29% of centers. The minimum current setting required to clear a test site demonstrated marked variability, with 28% (motor) and 26% (language) of centers reporting current thresholds of either less than 6 mA or greater than 15 mA (Table 2).
Table 1.
Summary of stimulation parameters for language and motor mapping
| Language | mA | Hertz | Stimulation Duration (seconds) | Pulse Width (milliseconds) |
|---|---|---|---|---|
| Mean | 11.3 | 44.6 | 6.5 | 0.5 |
| SD | 3.9 | 12.8 | 3.5 | 0.4 |
| Range | 3–17 | 14–60 | 2–20 | 0.1–2.0 |
| Mode | 10 | 50 | 5.0 | 0.3 |
| Motor | ||||
| Mean | 10.4 | 43.6 | 5.5 | 0.5 |
| SD | 4.3 | 15.3 | 3.5 | 0.4 |
| Range | 1.2–20 | 1–60 | 2–20 | 0.1–2.0 |
| Mode | 10 | 50 | 5.0 | 0.3 |
mA = milliamps
Table 2.
Minimum settings to classify a site as negative
| LANGUAGE | MOTOR | |||
|---|---|---|---|---|
|
| ||||
| mA | Hz | mA | Hz | |
| Mean | 12.2 | 44.1 | 11.3 | 41.7 |
| SD | 4.9 | 14.0 | 5.1 | 17.0 |
| Range | 1–17 | 5–50 | 0.8–20 | 1–50 |
| Mode | 10.0 | 50 | 10.0 | 50 |
mA = milliamps
Regarding electrode pairing strategies, 49% of centers test adjacent electrode pairs, i.e., electrodes located next to each other on a subdural grid or strip, or depth electrode array. At 19% of centers, electrodes were tested against a distant electrode determined to be negative for function. Thirty-two percent of centers employed a mix of strategies, first screening all sites of interest by testing adjacent pairs, then re-testing all potentially positive electrodes by pairing with a cleared distant electrode.
After discharges and medical management
Because ADs can potentially limit ESM efficacy, we included questions addressing their management. The most common methods reported were: waiting 1–5 minutes between stimulations (78% of centers), pausing the procedure to give medications (53%), and reducing pulse train duration (45%). Less frequently reported were reducing pulse width (22%) and lowering the frequency setting (24%).
The use of anticonvulsants to raise AD thresholds varied widely. Nearly half (48%) of responders perform ESM procedures with reduced anticonvulsant medication levels, while 39% restart oral medications the day before mapping. Twenty-nine percent reported pretreating with an IV anticonvulsant, and as we have noted above, 53% administer medications during the procedure to control ADs. The most common IV anticonvulsant is lorazepam (42%), followed by fosphenytoin (32%) and levetiracetam (24%).
Regarding duration of mapping sessions, 22% of centers report placing no limits on duration, whereas fixed time limits were reported by 51% (one hour or less, 6%; two hours, 38%; three hours, 7%). The remainder use functional indicators to decide when to terminate a session (decline in baseline function, 25%; increased focal EEG slowing, 13%; observed patient fatigue, 13%).
Influence on surgical margins and postoperative outcome
Responses regarding the minimal acceptable distance between an ESM-identified positive site and the resection margin were highly variable. One centimeter is the most common margin (60%), while the remaining centers vary between margins of 0–2 cm, or rely on anatomical boundaries (i.e., sparing the gyrus containing the eloquent site).
Function-specific, persistent postoperative deficits were reported after resecting visual naming sites in anterior, superior, or basal temporal regions (13% of centers). This is particularly interesting, as, to our knowledge, with the exception of basal temporal sites, there are no published reports regarding removal of positive visual naming sites. With regard to motor findings, induced motor weakness (7% of centers), and lower face motor (4% of centers) deficits occurred after resecting motor sites.
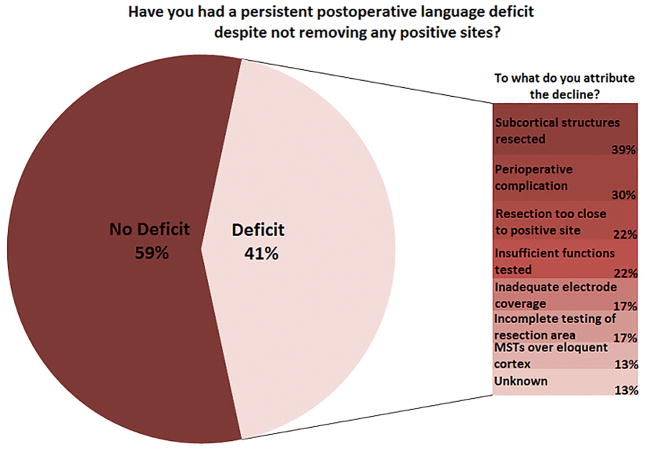
Approximately 40% of centers reported having experienced a persistent postoperative language deficit in at least one case despite preserving all ESM-identified positive sites. Speculations as to the reason for the declines included both procedural and nonprocedural issues (Figure 1).
Figure 1.
Persistent postoperative language deficits after ESM-guided resection. The proportion of centers reporting at least one such case is shown, along with the factors to which the adverse outcomes were attributed by the respondents
Discussion
Results of this survey bring to light the nature and extent of variability in virtually all aspects of the ESM procedure, as it is currently employed in clinical practice. Importantly, many of the variations are not trivial; indeed, these are points of practice that would likely generate disparate results, thereby differentially influencing the resection boundaries and likely affecting both postoperative seizure control and complication rates. Further, results suggest that ESM, as currently practiced, can fail to prevent adverse functional outcomes.
Language mapping
Whereas identification of motor and sensory cortex often involves stimulation evoked positive responses, identification of language cortex requires that the patient engage in a language task, and that stimulation disrupt the performance of that task.9 It is well established that at a given cortical site, stimulation-induced language deficits can be function specific.7, 10–13 For example, stimulation might disrupt reading but not naming, or vice versa. Testing limited to the non-disrupted function would yield a false negative result. Thus, the particular function(s) tested at each site is critical to identification of language cortex.
Given the anatomical dissociability of language functions, the most striking aspect of the language results is that all four basic language functions (i.e., speech production, comprehension, naming, reading) are tested in only about half of responding centers. Despite its importance, task selection has received minimal attention.14, 15 Wellmer and colleagues7 conducted a comprehensive analysis to determine the most economical set of tasks needed to identify eloquent cortex. Depending on location, visual naming and token test (comprehension) were recommended as most likely to identify language cortex. However, the authors caution that they omitted some potentially important tasks (e.g., auditory naming). Most importantly, incomplete postoperative follow up prevented analysis of functional outcome.
Survey results revealed some consistency regarding the operational definition of language errors, yet considerable variability regarding the proportion of errors required to consider a site “positive for language.” We are aware of no studies that have examined this empirically; however, it has been suggested that inconsistent responses reflect “graded,” unessential language areas that can be removed safely.16 Distance between positive sites and the resection margin have been examined retrospectively in small studies, with initial reports indicating 2 cm as a safe distance10, and more recent studies reporting 1 cm as sufficient.17 The current results showed just over half of centers (60%) endorsing 1 cm.
Survey responses also revealed variability in the cortical regions where language is mapped (e.g. basal temporal sites), which would influence findings and hence, surgical resection decisions.
Neurophysiology
Our survey revealed marked variability in stimulation settings, which perhaps reflects the lack of investigation into optimal setting combinations for maximizing functional effects while minimizing the risk of evoking after discharges and seizures. A strategy of jointly stepping current and pulse width to keep total charge delivered close to the theoretical chronaxie was proposed over two decades ago 4; however, this is practiced at only 9% of responding centers. A recent retrospective study comparing 50 to 20 Hz stimulation revealed that while AD risk would theoretically be reduced with the lower setting18, several eloquent language sites would have been missed.19 These studies indicate that even small variations in stimulation parameters can alter ESM results to a degree that increases the risk of adverse clinical events.
Often, the maximum current setting at a given cortical site is limited by elicited ADs. If the AD threshold at a given site is less than the minimum settings at which the site can be declared free of essential function, then that site cannot be adequately tested, potentially yielding a false negative result. Our survey revealed wide variability in the management of ADs, and little agreement regarding the value of testing during an AD. A false negative finding may occur due to incomplete function disruption by the AD, while a false positive finding may result from greater spatial spread of the disruptive effects.
Decline in baseline functioning and patient fatigue are recognized time-dependent effects of electrocortical stimulation, although this has not been formally studied. Fatigue can reduce patient cooperation, and baseline decline can result in false positive results if the patient is not continually reassessed. Notably, mapping session duration is not controlled at 22% of centers.
ESM and surgical decision-making
Given the numerous inconsistencies in the clinical practice of ESM, it is unlikely that all the variations are equally effective, and thus we would predict that the impact of the ESM results on the final resection choice may be practice-dependent to some degree, with errors induced in both directions. In some cases, resections would be too conservative, i.e., in the case of false positives, portions of the epileptogenic zone are left intact, possibly worsening seizure outcome. In the case of false negatives, essential functional sites may be inadvertently resected.
Potentially related to false negative errors, 41% of responding centers reported cases of persistent postoperative language decline despite preserving positive language sites (i.e., as defined by individual centers). Of these centers, 56% attributed this to failure of the mapping procedure to identify critical language sites, including 17% who cited inadequate electrode coverage. Ideally, electrode coverage would always be planned with consideration of language mapping needs, as alternatives to ESM are not readily available.
Although postoperative functional decline can provide evidence of false negative results, false positives are much more difficult to ascertain. This would require a way to test whether a site identified as positive and thus spared from resection, is in fact, not eloquent for the function tested. Instead, the survey addressed several potential sources of false positive findings. These included the spread of current away from the stimulation site (Q29, testing during ADs), high stimulation intensity (Q20, 21, 23), distant electrode pairing (Q26), confusion of primary with associative motor areas (Q16), and criteria for classifying sites as positive for language (Q6-Q8; see Language Mapping section).
We caution that false positive and negative errors can occur in any rigorously standardized diagnostic procedure. Thus, errors in ESM cannot be solely attributable to variations in the procedure. Nevertheless, lack of standardization and the resulting large variations in practice would likely increase the error rate. In any case, the detailing of ESM practice variations should be valuable to clinicians who may appreciate an understanding of the points in which they are in agreement with their peers, and those in which there is little agreement.
Limitations
Common among mail surveys, one limitation of this investigation is self-selection sampling bias, as a substantial number of epilepsy surgery centers failed to respond. Our initial intent was to restrict survey distribution to epilepsy surgery centers that perform subdural monitoring and extraoperative mapping on a regular basis; however, this level of detail was generally unavailable. We reasoned that the results of the survey would be more representative if we erred on the side of inclusiveness, rather than potentially fail to include surgery centers that would be appropriate for the study. Nevertheless, this strategy likely contributed to the reduced response rate. Additionally, with regard to postoperative language outcome, we asked only whether an adverse event had occurred at least once to the responder’s knowledge, and did not require that the results be based on systematic, formal postoperative testing or systematic chart review, as this would likely have substantially reduced the response rate. We therefore acknowledge that these numbers are not empirically based, and might limit the quality of the data on postoperative morbidity.
Closing comments and future directions
Similar to other longstanding medical procedures, ESM came into clinical use at a time when standards for acceptance were less rigorous than they are currently. Given the often dramatic effects of cortical stimulation, it is understandable that the procedure was perceived as straightforward, without need for refinement or validation. Consequently, there has been a paucity of data on which to base procedural and technical decisions. It is within this context that results from this survey revealed unsubstantiated procedural variability as well as postoperative morbidity across surgery centers.
Findings from this survey highlight the need for empirical studies addressing several aspects of ESM, including neurostimulation parameters, selection of language tasks and criteria for positive site identification. By identifying components of the procedure in need of attention, the current survey results serve as a first step toward developing appropriate investigations that would provide valid and reliable data from which to base empirically derived guidelines.
One could conceivably argue that with advances in technology and neuroscience, efforts to improve an invasive method such as ESM might not be the best use of time and resources. Inarguably, the development of noninvasive techniques to replace ESM is a worthy goal. However, it is unlikely that newer methods will replace ESM in the near future. Most alternative techniques (e.g., fMRI18, MEG 20, EEG analysis21) do not differentiate between sites that activate during functional processing, and sites that are critical for functional processing. Further, these techniques are subject to threshold error due to the need to select arbitrary computational criteria for labeling a site as positive. The only other technique that specifically employs local cortical disruption is TMS,22 which is limited by the inability to access certain cortical regions, and also lacks the spatial specificity of ESM. Moreover, for any new technique, ESM will serve as the gold standard for comparison. Thus, without optimizing ESM, newer techniques will be held to a suboptimal standard.
We believe ESM inherently has value; however, it is not clear that this value has been maximized, rendering it a disservice to patients, and falling short in elucidating structure-function relations. Efforts would be well spent investigating critical technical and procedural aspects of ESM so that ultimately, empirically based guidelines can be proposed and utilized.
Supplementary Material
Acknowledgments
We gratefully acknowledge our colleagues for their time and effort in completing the survey. This work was supported by NIH/KM1 CA156709 and NIH/R01 NS35140 (MH).
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose.
Ethical Publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Hamberger MJ. Cortical mapping. In: Caplan B, Kreutzer J, Deluca J, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer Science; 2011. [Google Scholar]
- 2.Borchers S, Himmelbach M, Logothetis N, et al. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nature Reviews, Neuroscience. 2011;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 3.McCreery DB, Agnew WF. Changes in extracellular potassium and calcium concentration and neural activity during prolonged electrical stimulation of the cat cerebral cortex at defined charge densities. Exp Neurol. 1983;79:371–396. doi: 10.1016/0014-4886(83)90220-0. [DOI] [PubMed] [Google Scholar]
- 4.Jayakar P, Alvarez L, Duchowny M, et al. A safe and effective paradigm to functionally map the cortex in childhood. Journal of Clinical Neurophysiology. 1992;9:288–293. doi: 10.1097/00004691-199204010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Chitoku S, Otsubo H, Harada Y, et al. Extraoperative cortical stimulation of motor function in children. Pediatric Neurology. 2001;24:344–350. doi: 10.1016/s0887-8994(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 6.Schevon CA, Carlson C, Zaroff CM, et al. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48:539–545. doi: 10.1111/j.1528-1167.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 7.Wellmer J, Weber C, Mende M, et al. Multitask electrical stimulation for cortical language mapping: hints for necessity and economic mode of application. Epilepsia. 2009;50:2267–2275. doi: 10.1111/j.1528-1167.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 8.Nii Y, Uematsu S, Lesser RP, et al. Does the central sulcus divide motor and sensory functions? Cortical mapping of human hand areas as revealed by electrical stimulation through subdural grid electrodes. Neurology. 1996;46:360–367. doi: 10.1212/wnl.46.2.360. [DOI] [PubMed] [Google Scholar]
- 9.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychology Review. 2007;4:477–489. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 10.Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behavioral Brain Research. 1983;6:189–230. [Google Scholar]
- 11.Schwartz TH, Devinsky O, Doyle W, et al. Function-specific high-probability “nodes” identified in posterior language cortex. Epilepsia. 1999;40:575–583. doi: 10.1111/j.1528-1157.1999.tb05559.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamberger MJ, Goodman RR, Perrine K, et al. Anatomical dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56:56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Malow BA, Blaxton TA, Susumu S, et al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37:245–252. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Ojemann GA. Electrical stimulation and the neurobiology of language. The Behavioral and Brain Sciences. 1983;2:221–230. [Google Scholar]
- 15.Hamberger MJ, Seidel WT, McKhann GM, et al. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742–2749. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- 16.Whitaker H, Ojemann GA. Graded localization of naming from electrical stimulation mapping of left cerebral cortex. Nature. 1977;270:51–51. doi: 10.1038/270050a0. [DOI] [PubMed] [Google Scholar]
- 17.Haglund M, Berger M, Shamseldin M, et al. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Gaillard WD, Balsamo L, Xu B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- 19.Zangaladze A, Sharan A, Evans J, et al. The effectiveness of low-frequency stimulation for mapping cortical function. Epilepsia. 2008;49:481–487. doi: 10.1111/j.1528-1167.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. Journal of Neurosurgery. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- 21.Crone NE, Hao L, Hart J, et al. Electrographic gamma activity during word production in spoken and sign language. Neurology. 2001:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 22.Picht T, Krieg SM, Sollmann N, et al. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery. 2013;5:808–819. doi: 10.1227/NEU.0b013e3182889e01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.