Summary
Objective
Birth weight is an important indicator of prenatal environment and subtle variations of birth weight within the normal range have been associated with differential risk for cognitive and behavioral problems. Therefore, we aimed to determine if there are differences in birth weight between full term children with uncomplicated new/recent-onset epilepsies and typically-developing healthy controls. We further examined the relationships between birth weight and childhood/adolescent cognition, behavior, and academic achievement.
Methods
108 children with new/recent-onset epilepsy and 70 healthy controls underwent neuropsychological assessment. All participants were born full-term (>37 weeks) without birth complications. Parents were interviewed regarding their child's gestation, birth and neurodevelopmental history.
Results
Birth weight of children with epilepsy was significantly lower than healthy controls (p=0.023). Whereas birth weight (covaried with age, sex, handedness, and mother's education) was significantly associated with cognition in controls in multiple domains (intelligence, language, aspects of academic achievement), this relationship was absent in children with epilepsy. Birth weight was not associated with clinical epilepsy variables (age of onset, epilepsy syndrome) and was not predictive of a variety of other academic or psychiatric comorbidities of epilepsy.
Significance
Although the origin of lower birth weight in children with epilepsy is unknown, these findings raise the possibility that abnormal prenatal environment may impact childhood-onset epilepsy. Furthermore, the positive relationship between birth weight and cognition evident in healthy controls was disrupted in children with epilepsy. However, birth weight was not related to academic and psychiatric comorbidities of childhood epilepsy.
Keywords: Birth weight, cognition, academic achievement, new-onset epilepsy, localization-related epilepsy, idiopathic generalized epilepsy
Introduction
There is now substantial evidence indicating that neurobehavioral comorbidities of childhood-onset epilepsy can be evident at or prior to the time of epilepsy onset, 1-6 but the neurodevelopmental origins of these cognitive and behavioral complications are unknown. Human and non-human animal studies have shown that variance in cognitive and behavioral maturation can be traced to fetal development.7; 8 Birth weight has been established as a sensitive indicator of prenatal environment and even subtle differences within the normal range of birth weight have been associated with differential risks for cognitive problems and psychopathology in later life.9; 10 Specifically, in children with normal birth weight for gestational age, lower birth weight was significantly associated with poorer cognitive function in later life, with predictive significance beyond that predicted by socio-economic status.10 More recently, twin studies have shown modest but significant effects of birth weight on later cognitive ability in full term children.11; 12
Extremely low birth weight (less than 1000g), premature birth, and concomitant pre- and perinatal complications have been associated with a variety of neurological disorders, including autism 13 and cerebral palsy 14 as well as poor neurodevelopmental outcomes broadly defined.15; 16 Abnormally low birth weight for gestational age and concomitant pre- and peri-natal complications has also been found in individuals with epilepsy.17; 18 Therefore, it would be expected that these children with complicated births and severe epilepsies would have poor cognitive outcomes. However, an important but unanswered question is whether children born full term with uncomplicated epilepsies will have perturbed prenatal environment as indexed by lower birth weight but within the normal range for gestational age. Given that a majority of children with epilepsy have uncomplicated births, close examination of this issue may have public health implications by raising the possibility of very early neurodevelopmental origin of childhood-onset epilepsies. Therefore, our study aimed to compare birth weight in children with uncomplicated epilepsies, normal gestation, delivery, and normal birth weight for gestational age to birth weight of healthy controls. Further, neurobehavioral comorbidities of epilepsy including abnormalities in academics, cognition, emotion, and social function, are frequently observed in children with epilepsy.6; 19-22 Thus, we investigated the relationship between variations in birth weight and risk for subsequent cognition, social, and psychiatric complications in the children with epilepsy.
Methods
Participants
The initial sample comprised 203 participants aged 8-18 including 124 children with new/recent-onset epilepsy (CWE) and 79 typically-developing healthy controls (HC). All CWE were followed for active epilepsy by a neurologist. Inclusion criteria were a diagnosis of epilepsy within the past 12 months, no other developmental disabilities or neurological disorders, normal neurological examinations and normal neuroimaging results. A board-certified pediatric neurologist (blinded to neuropsychological and interview data) confirmed that each patient had an idiopathic epilepsy diagnosis and provided independent confirmation of syndrome diagnosis. HC participants were age- and gender-matched first-degree cousins of epilepsy participants. HC participants presented with no history of seizures, early initial precipitating injuries (e.g., febrile convulsions), other developmental or neurological disease, or loss of consciousness greater than 5 minutes.
Given that the focus of the current study is on normal birth weight and full-term children, further we applied the following exclusion criteria determined through clinical interview for both healthy controls and completion of an abbreviated version of the Yale Neuropsycho-educational Assessment Scales 23 and review of medical records included children with epilepsy: 1) preterm birth (<37 weeks); 2) low birth weight (at or below 10th percentile for normal gestation period, assessed separately for males and females following published birth weight norms 24); or significant pre-/peri-/postnatal difficulties (e.g., hypoxia/ischemia, cerebral hemorrhage, need for supplemental oxygen or intubation, use of incubator). Finally, one control child was excluded based on a full-scale IQ < 70. In Data for the exclusion criteria were obtained through clinical interview and completion of an abbreviated version of the Yale Neuropsycho-educational Assessment Scales 23 and review of medical records. Exclusion rates were not significantly different between groups (p=0.749): in the epilepsy group, 16/124 (12.9%) of children in the initial sample were excluded, while 9/79 (11.4%) of children in the control group were excluded. Thus our final normal birth weight (NBW) sample consisted of 108 CWE and 70 typically-developing HC. Demographic and clinical epilepsy characteristics of the sample are given in Table 1.
Table 1.
Participant characteristics by group (means and standard deviations).
| Variable | Children with Epilepsy (n=108) |
Healthy Controls (n=70) |
|---|---|---|
| Age (years) | 12.33 (3.24) | 12.63 (3.16) |
|
| ||
| Gender (#/% female) | 49 (45.4%) | 35 (50%) |
|
| ||
| Birthweight (grams)a | 3440.46 (486.25) | 3620.84 (550.89) |
|
| ||
| FSIQa | 102.92 (12.77) | 107.67 (11.59) |
|
| ||
| Academic Problems(+/-)ab | 44/63 | 14/53 |
|
| ||
| Mother's Educational Level (4-year college graduate: yes/no)c | 37/71 | 28/36 |
|
| ||
| Age of seizure onset (years) | 11.45 (3.35) | -- |
| Epilepsy duration (months) | 8.59 (3.78) | -- |
| Epilepsy Syndrome (IGE/LRE)b | 48/58 | -- |
| Number of antiepileptic drugs (0/1/2+) | 21/81/6 | -- |
p≤0.05
Information regarding academic problems was not available for 3 healthy controls and 1 child with epilepsy.
Mother's educational level not available for 6 participants.
2 children with epilepsy were not classifiable as IGE or LRE but were included in all analyses.
FSIQ: Full-scale intelligence quotient
IGE: Idiopathic generalized epilepsy
LRE: Localization-related epilepsy
Standard protocol approvals, registrations, and patient consents
Research approval was obtained from the Health Sciences Institutional Review Board at the University of Wisconsin Medical School. Written informed consent was obtained from legal guardians of participating children and adolescents, written informed consent was obtained from participants over age 18, and written informed assent was obtained from participants age 8-17. All procedures were consistent with the Declaration of Helsinki.25
Procedures
Children underwent comprehensive neuropsychological testing. Parents underwent a clinical interview and completed questionnaires including the abbreviated Yale Neuropsycho-educational Assessment Scales 23 to characterize gestation, delivery, neurodevelopment, and seizure. All pertinent medical records were obtained after parents signed a release of information. Parents were questioned through a structured interview about their child's school progress, including any academic problems and any special educational services provided in order to address those problems. This interview was conducted blind to cognitive and behavioral results. Past and current psychiatric status was determined via a semi-structured interview, the Kiddie-Schedule for Affective Disorders and Schizophrenia – Present and Lifetime Version.26 Rates of anxiety, depression, and attention deficit hyperactivity disorder (ADHD) were assessed within each group (HC, CWE). Finally, each participating parent completed the Child Behavior Checklist for children aged 6-18 (CBCL/6-18) from the Achenbach System of Empirically Based Assessment.27
Neuropsychological assessment and analysis
All participants were administered a comprehensive test battery that included measures of intelligence, academic achievement, language, verbal memory, executive function, and speeded fine motor dexterity. Table 2 gives a complete list of tests by domain. Independent samples t-tests were used to compare CWE and HC groups on birth weight as well as demographic and cognitive variables. Partial correlations were used to examine the relations between birth weight and raw neuropsychological test scores, using age, gender, handedness, and mother's educational level (completion of 4- year college degree: yes/no) as covariates.
Table 2.
Test scores and test score – birth weight partial correlations by functional domain for CWE and HC.
| Domain | Ability | Test | CWE Mean (SD) | CWE Partial r BW * Test Score a | HC Mean (SD) | HC Partial r BW * Test Score a |
|---|---|---|---|---|---|---|
| Intelligence | Full scale | WASI | 102.92 (12.77)b | 0.05 | 107.67 (11.59) | 0.40c |
| Verbal | WASI (verbal IQ) | 103.17 (12.33)b | 0.04 | 107.14 (12.91) | 0.26c | |
| Performance | WASI (performance IQ) | 101.62 (13.97)b | 0.05 | 106.53 (12.29) | 0.39c | |
| Academic Achievement | Letter/word recognition | WRAT-3 (reading) | 102.60 (12.60) | 0.09 | 105.59 (10.79) | 0.12 |
| Letter/word writing | WRAT-3 (spelling) | 101.94 (14.19) | 0.03 | 104.93 (12.91) | 0.23 | |
| Basic arithmetic | WRAT-3 (arithmetic) | 98.14 (12.95)b | 0.11 | 107.06 (11.04) | 0.30c | |
| Language | Confrontation naming | Boston Naming Test | 11.50 (2.17)b d | 0.02 | 12.33 (1.68) | 0.27c |
| Expressive naming | Expressive Vocabulary Test | 98.21 (13.84)b | -0.05 | 103.46 (13.54) | 0.27c | |
| Receptive language | Peabody Picture Vocabulary Test-III | 106.86 (13.15)b | 0.04 | 110.73 (10.13) | 0.29c | |
| Memory | Immediate verbal memory | CMS (word lists learning) | 8.69 (3.05)b e | 0.11 | 9.80 (2.59) | 0.01 |
| Delayed verbal memory | CMS (word lists delayed) | 9.06 (3.23)e | 0.05 | 9.93 (2.85) | -0.03 | |
| Executive Function | Problem-solving | D-KEFS (confirmed correct sorts) | 9.06 (2.61)b e | 0.02 | 10.13 (1.97) | 0.24 |
| Response inhibition | D-KEFS (color-word interference test-Inhibition) | 9.79 (2.78)b e | -0.03 | 10.96 (2.47) | -0.17 | |
| Motor Function | Speeded fine motor dexterity | Grooved Pegboard | 85.10 (20.88)b d | -0.16 | 72.54 (16.00) | 0.23 |
| Psychomotor speed | WISC-III (digit symbol-coding) | 8.24 (2.65)b d | -0.02 | 10.39 (2.96) | 0.05 |
Covariates: age, gender, handedness, parent education
CWE<HC (p≤0.05)
p≤0.05
Raw score
Standardized score: M = 10, SD = 3
CWE: Children with epilepsy
HC: Healthy controls
CWE: Children with epilepsy
WRAT-3: Wide Range Achievement Test - 3
WASI: Wechsler Abbreviated Scale of Intelligence
D-KEFS: Delis-Kaplan Executive Function System
CMS: Children's Memory Scale
WISC-III: Wechsler Intelligence Scale for Children – III
Results
Demographic and cognitive differences between groups
As shown in Table 1, at the time of testing CWE had lower full-scale IQ scores (M = 102.92, SD = 12.77) than did HC (M = 107.67, SD = 11.59), p=0.013, although it should be noted that the IQ of CWE was within the normal or average range. CWE were also more likely than HC to have received special services in school (41.12% versus 20.90%), p=0.008. Groups did not differ in age, gender, or mother's educational level. Clinical characteristics of the epilepsy group are given in Table 1. The average duration of epilepsy was 8.59 months (SD = 3.78), with an average age of onset of 11.45 years (SD = 3.35). CWE performed significantly worse than HC on 12/15 neuropsychological tests of general IQ, academic achievement, memory, language, psychomotor function, and executive function. The only tests in which performance of CWE was not significantly worse than performance of HC were in academic achievement (reading and spelling) and delayed verbal memory. Means and standard deviations of all test scores are presented by group in Table 2.
Birth weight
CWE had significantly lower birth weights (M = 3440.46 grams, SD = 486.25) than HC (M = 3620.84, SD = 550.89), p=0.023. The distributions of birth weight for both CWE and HC are shown in Figure 1. Kurtosis statistics were normal for each group (CWE: .561, SE = .461; HC: -.002, SE = .566). Likewise, the HC group skewness statistics was in the normal range (.232, SE = .287). However, the CWE group distribution was somewhat positively skewed (.483, SE = .233). Note that lower birth weight in CWE compared to HC reported here excluded individuals who were born pre-term, significantly underweight, or with severe pre-/perinatal complications. In both groups, birth weight was unassociated with current weight or head circumference. Additionally, current weight at time of testing was not different between groups.
Figure 1.
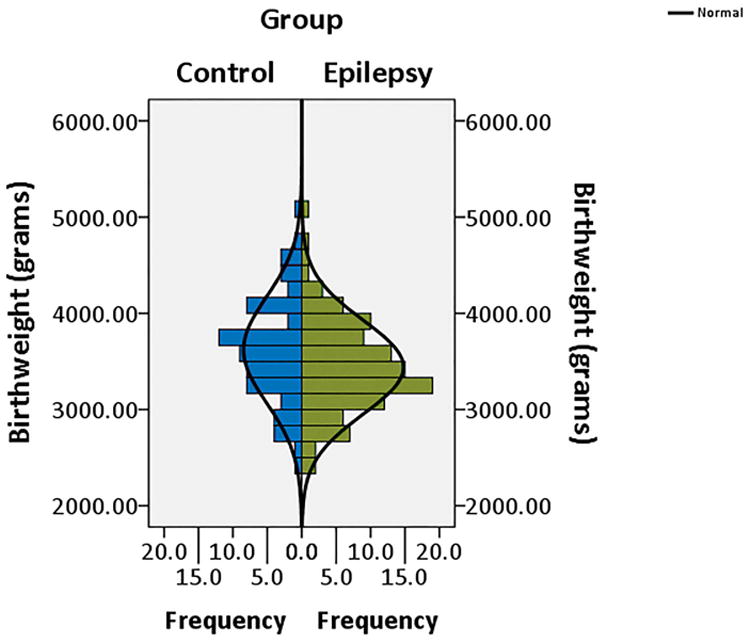
Distribution of birth weight by participant group (children with epilepsy; typically-developing healthy controls).
Birth weight and cognition
Partial correlations were computed to assess relations within each group (CWE, HC) between birth weight and raw cognition scores, controlling for age, gender, handedness, and mother's level of education. For 7/15 tests, birth weight in HC was significantly positively correlated with better performance (r's ranging from 0.26 – 0.40). Notably, these cognitive correlates of birth weight were most evident in domains of general intelligence (full-scale IQ, verbal IQ, performance IQ) and language abilities (confrontation naming, expressive naming, and receptive language), with the exception of an arithmetic test of academic achievement. Other cognitive domains including executive function, memory, motor function, and remaining academic achievement (reading, spelling) were not associated with birth weight in HC, although trend level positive correlations (p's between 0.05 and 1.00) were found for speeded fine motor dexterity (Grooved Pegboard—dominant hand) and problem-solving skills (D-KEFS correct sorts); see Table 2. Conversely, CWE showed no significant relations between birth weight and test scores from any functional domain (r's ranging from -0.16 – 0.11). The differential relationships with birth weight and cognition between CWE and HC, are exemplified in Figure 2 and 3 with side-by-side group comparisons of these partial correlations for full-scale IQ and expressive naming, respectively.
Figure 2.
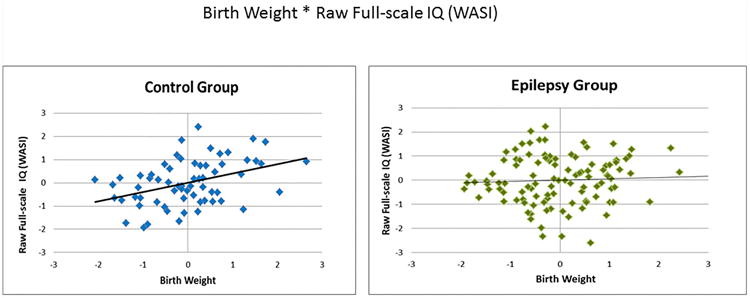
Birth weight by Full-scale IQ partial correlations. Both variables are presented as standardized residuals (covariates: age, gender, handedness, mother's education level.)
WASI: Wechsler Abbreviated Scale of Intelligence
Figure 3.
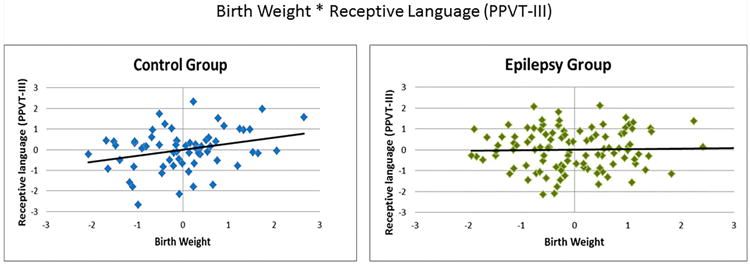
Birth weight by Receptive language partial correlations. Both variables are presented as standardized residuals (covariates: age, gender, handedness, mother's education level.)
PPVT-III: Peabody Picture Vocabulary Test-III
Birth weight and common comorbidities of epilepsy
In CWE, birth weight was not associated with presence of academic difficulties including ADHD, receiving special services in school, having a specific educational plan in place, and presence of learning problems before diagnosis of epilepsy (all p's>0.60). Birth weight was also unassociated with presence of anxiety and depression, both at the time of testing and prior to epilepsy diagnosis (p's>0.70). Furthermore, in children with epilepsy birth weight was not associated with duration of epilepsy, age at diagnosis, epilepsy syndrome (IGE/LRE), or current number of anti-epilepsy medications (p's>0.16).AED's),all p's>0.16. To further examine the possible effects of AED's on birth weight and cognition, we then reran our correlations between birth weight and cognitive variables separately for those children with epilepsy on 0 AED's versus those on 1 or more AED's. We found that these correlations remained non-significant across groups with and without AED exposure, all p's>0.30. Finally, birth weight was not associated with CBCL scales of Total Competence, Total Problems, Internalizing, or Externalizing in either group (p's>0.60).
Discussion
Three primary findings were evident from this investigation. First, children with uncomplicated idiopathic localization-related and generalized epilepsies exhibited significantly lower birth weight compared to healthy controls. It is important to note that this comparison was made using only those participants who were of normal birth weight, who were not born preterm, and whose mothers did not have pregnancy complications. Second, increasing birth weight was related to significantly better cognitive abilities across multiple functional domains later in childhood/adolescence in healthy controls, while this relationship was completely absent in children with epilepsy. Third, contrary to expectations, birth weight in children with epilepsy was predictive neither of common problematic comorbidities including academic difficulties and psychiatric complications, nor of clinical epilepsy features such as duration of epilepsy, age of disease onset, and broad epilepsy syndrome. Each of these points is reviewed in turn below.
Birth weight in children with epilepsy
This is among the first demonstrations that birth weight is significantly lower in children with epilepsy compared to healthy controls. Importantly, the distribution of birth weight was normal in both groups indicating that the effect was not driven by outliers (see Figure 1). Furthermore, excluded from both the epilepsy and control groups were children who were identified as extremely low birth weight, preterm deliveries, or documented pre-/peri-natal complications, rates of which were comparable in the epilepsy (12.9%) and control (11.4%) groups. In children born full term, the average birth weights for both groups fell in the normal range, but were significantly lower in the children with epilepsy regardless of whether they were followed for localization-related or idiopathic generalized syndromes.
Although the origin of lower birth weight in CWE is unknown, these findings raise the possibility that abnormal prenatal environment may impact childhood-onset epilepsy. The brain undergoes rapid growth during in utero and the dynamic of neurodevelopment significantly influences eventual birth weight. For example, Graca and colleagues examined cerebral volumes during the first post-natal week in 128 infants and found brain volumes are highly correlated with birth weight.28 Further, fetal growth as documented by ultrasound during the first 29 and second 30 trimesters has been shown to influence post-natal birth weight. Given the important association between prenatal brain development and postnatal birth weight, our findings of modest birth weight reduction in CWE may provide the earliest indicator for the origin of abnormal neurodevelopment. Future studies should focus on factors that contribute to fetal growth and birth weight in childhood onset epilepsies, which may provide targets for early intervention.
Birth weight and cognition
Similar to findings from prior investigations of normally-developing children and adults, 10-12 our healthy control participants demonstrated a significant association between birth weight and neuropsychological status. Consistent with those results, 10 these relationships were most evident across broad measures of intelligence (full-scale IQ, verbal IQ, performance IQ), as well as one area of academic achievement (arithmetic). We also found relations between birth weight and multiple language-dependent abilities (confrontation naming, expressive naming, receptive language). These analyses, and comparable analyses in the children with epilepsy, controlled for known important covariates including age, gender, handedness, and mother's educational level. 10; 11; 31 In contrast, the relationship between birth weight and cognition was completely absent among the children with epilepsy, with no significant relationship between birth weight and measures of intelligence, language, academic achievement, memory, executive function, or cognitive/psychomotor speed. In summary, our study demonstrated that the presence of childhood epilepsy, even uncomplicated idiopathic epilepsy, is associated with disruption of normal birth weight—cognition associations. These results are consistent with other findings from our group, a recent example being a significantly larger difference in IQ between parent and child IQ in CWE dyads relative to their HC counterparts. 32 Taken as a whole, this pattern of findings suggests that some typical determinants of variance in cognitive maturation of normally-developing children may be weaker or absent altogether in CWE.
Birth weight and the comorbidities of childhood epilepsy
It is now widely appreciated that cognitive, academic, behavioral and psychiatric comorbidities of childhood epilepsy can be identified at or near the time of the first recognized seizure and diagnosis, often prior to initiation of drug treatment, and even prior to the recognition of seizures and the diagnosis of epilepsy. 1; 2; 5; 6 The antecedent factors responsible for such findings largely remain to be characterized. There has been demonstration of family aggregation of some comorbidities, 33-37 and it would be reasonable to hypothesize that factors related to pregnancy, birth and development might be pertinent as they have been shown to be in the general population. 11 Surprisingly, birth weight was not only unrelated to cognition as discussed above, but unrelated to other problematic comorbidities of childhood epilepsy including academic complications (e.g., learning problems, need for extra educational services, etc.) and psychiatric diagnoses of ADHD, anxiety, and mood disorders. These findings were not anticipated. While birth weight is reduced in children with epilepsy, it appears that other factors, yet to be identified, account for variance in later cognitive ability and other neurobehavioral comorbidities.
Limitations and future directions
There are limitations associated with this study. First, our sample was not population-based, although participants were recruited from medical centers covering broad portions of the state of Wisconsin. Second, our sample size precluded analysis of the relation between birth weight and cognition in specific syndromes of IGE and LRE (e.g., Juvenile Myoclonic, Absence, BECTS). We continue to recruit participants in an attempt to make such analyses possible. Third, we did not include some features of the socioeconomic status (SES) of our participating families (e.g., total family income). However, mother's education level—an important factor when assessing SES 38—was used as a covariate in all of partial correlation analyses. Within the healthy control group, birth weight was still significantly associated with cognition later in life even after controlling for mother's education level. Additionally, our sample of healthy controls is presumably similar in SES to participants with epilepsy, given that controls participants were first-degree cousins of participants with epilepsy. Despite these limitations, our study provides additional evidence that childhood-onset epilepsies are associated with early neurodevelopmental complications. These antecedent factors might have a negative impact on cognitive development by altering normal positive associations between birth weight and cognition. Note that the current study examined cognitive correlates of birth weight near the diagnosis of epilepsy. The cognitive impact of birth weight may accrue over time and thus inspection of longer term outcomes will be informative. Another pertinent question is whether lower birth weight in CWE is predictive of abnormal brain development. Therefore, future studies will examine relationships between birth weight and prospective changes in gray/white matter volumes and cortical thickness.
Supplementary Material
Acknowledgments
All phases of this study were supported by NINDS 2RO1-44351 and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. We thank Raj Sheth MD and Monica Koehn MD for study participation and subject recruitment. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
Study funding: Supported by NIH (NINDS 3ROI 44351), which provided funding for study design, data collection, and data analysis. JJL is also supported by NIH grant (K23 NS060993). Funder was not involved in manuscript preparation or publication decisions. DCJ, KLC and BPH wrote the first draft of this manuscript. No authors received any honorarium, grant, or other form of payment for producing this manuscript.
Footnotes
Disclosures: DCJ, KLC, and AKJ report no disclosures. JJL has received speaker's honorariums from UCB-Pharma and is supported by NIH (K23 NS060993). DAH, JEJ, and MS receive research support from NIH (NINDS 3RO1 NS44351 [coinvestigators]). DAH receives research support from the Batterman Family Foundation. CES has received compensation as a consultant for Questcor Pharmaceuticals (2011), serves on the Scientific Board of The Charlie Foundation, serves as Chief Editor for Basic Science of Epilepsy Currents, has received royalties from publication of the books: (1) Epilepsy and the Ketogenic Diet, and (2) Epilepsy: Mechanisms, Models and Translational Perspectives, and received support from NIH (NINDS 3RO1 NS44351 [coinvestigator]). BPH serves as an Associate Editor for Epilepsy & Behavior and receives research support from the NIH (NINDS 3RO1 NS44351 [PI], RO1 AG027161 [coinvestigator], P50AG3314 [coinvestigator], 1RO1NS064034 [coinvestigator], and RO1AG031790 [coinvestigator]).
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Austin JK, Harezlak J, Dunn DW, et al. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Ludvigsson P, Olafsson E, et al. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- 4.Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 5.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, et al. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 6.Lin JJ, Riley JD, Hsu DA, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–685. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rooij SR, Wouters H, Yonker JE, et al. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci U S A. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schull WJ, Norton S, Jensh RP. Ionizing radiation and the developing brain. Neurotoxicol Teratol. 1990;12:249–260. doi: 10.1016/0892-0362(90)90096-u. [DOI] [PubMed] [Google Scholar]
- 9.Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923–930. doi: 10.1001/archgenpsychiatry.2010.100. [DOI] [PubMed] [Google Scholar]
- 10.Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychol Bull. 2004;130:989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- 11.Raznahan A, Greenstein D, Lee NR, et al. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci U S A. 2012;109:11366–11371. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torche F, Echevarria G. The effect of birthweight on childhood cognitive development in a middle-income country. Int J Epidemiol. 2011;40:1008–1018. doi: 10.1093/ije/dyr030. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YS, Weng SF, Cho CY, et al. Higher prevalence of autism in Taiwanese children born prematurely: a nationwide population-based study. Res Dev Disabil. 2013;34:2462–2468. doi: 10.1016/j.ridd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks' gestational age. Pediatrics. 2011;127:62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii N, Kono Y, Yonemoto N, et al. Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics. 2013;132:62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 16.Mercier CE, Dunn MS, Ferrelli KR, et al. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 2010;97:329–338. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falchi M, Palmas G, Pisano T, et al. Incidence of epilepsy in extremely low-birthweight infants (<1,000 g): a population study of central and southern Sardinia. Epilepsia. 2009;50(1):37–40. doi: 10.1111/j.1528-1167.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa T, Kishi S, Inukai K, et al. Subsequent epilepsy in very-low-birthweight infants: a long-term follow-up study from birth. Epilepsia. 1995;36:435–439. doi: 10.1111/j.1528-1157.1995.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 19.Berg AT, Langfitt JT, Testa FM, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones JE, Austin JK, Caplan R, et al. Psychiatric disorders in children and adolescents who have epilepsy. Pediatr Rev. 2008;29:e9–14. doi: 10.1542/pir.29-2-e9. [DOI] [PubMed] [Google Scholar]
- 21.Hoie B, Mykletun A, Sommerfelt K, et al. Seizure-related factors and non-verbal intelligence in children with epilepsy. A population-based study from Western Norway. Seizure. 2005;14:223–231. doi: 10.1016/j.seizure.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Rutter M, Graham P, Yule W. A neuropsychiatric study in childhood. S.I.M.P./William Heineman Medical Books; London: 1970. [Google Scholar]
- 23.Shaywitz SE. The Yale Neuropsycho-educational Assessment Scales. Schizophr Bull. 1982;8:360–424. doi: 10.1093/schbul/8.2.360. [DOI] [PubMed] [Google Scholar]
- 24.Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association Declaration of Helsinki. The Journal of Law, Medicine & Ethics. 1991;19:264–265. [PubMed] [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age forms and profiles. University of Vermont, Research Center for Children, Youth and Families; Burlington, VT: 2001. [Google Scholar]
- 28.Graca AM, Cardoso KR, da Costa JM, et al. Cerebral volume at term age: comparison between preterm and term-born infants using cranial ultrasound. Early Hum Dev. 2013;89:643–648. doi: 10.1016/j.earlhumdev.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Salomon LJ, Hourrier S, Fanchin R, et al. Is first-trimester crown-rump length associated with birthweight? BJOG. 2011;118:1223–1228. doi: 10.1111/j.1471-0528.2011.03009.x. [DOI] [PubMed] [Google Scholar]
- 30.Fox NS, Saltzman DH, Schwartz R, et al. Second-trimester estimated fetal weight and discordance in twin pregnancies: association with fetal growth restriction. J Ultrasound Med. 2011;30:1095–1101. doi: 10.7863/jum.2011.30.8.1095. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Lynch J, Susser ES, et al. Birth weight and cognitive ability in childhood among siblings and nonsiblings. Pediatrics. 2008;122:e350–358. doi: 10.1542/peds.2007-3851. [DOI] [PubMed] [Google Scholar]
- 32.Walker NM, Jackson DC, Dabbs K, et al. Is lower IQ in children with epilepsy due to lower parental IQ? A controlled comparison study. Dev Med Child Neurol. 2013;55:278–282. doi: 10.1111/dmcn.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke T, Strug LJ, Murphy PL, et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2258–2265. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53:301–307. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudson JI, Javaras KN, Laird NM, et al. A structural approach to the familial coaggregation of disorders. Epidemiology. 2008;19:431–439. doi: 10.1097/EDE.0b013e31816a9de7. [DOI] [PubMed] [Google Scholar]
- 36.Levav M, Mirsky AF, Herault J, et al. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- 37.Smith AB, Kavros PM, Clarke T, et al. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 2012;53:705–711. doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


