Abstract
We previously demonstrated that Amblyomma americanum tick serine protease inhibitor 6 (AamS6) was secreted into the host during tick feeding and that both its mRNA and protein were ubiquitously and highly expressed during the first 3 days of tick feeding. This study demonstrates that AamS6 is a cross-class inhibitor of both serine- and papain-like cysteine proteases that has apparent antihaemostatic functions. Consistent with the typical inhibitory serpin characteristics, enzyme kinetics analyses revealed that Pichia pastoris-expressed recombinant (r) AamS6 reduced initial velocities of substrate hydrolysis (V0) and/or maximum enzyme velocity (Vmax) of trypsin, chymotrypsin, elastase, chymase, and papain in a dose–response manner. We speculate that rAamS6 inhibited plasmin in a temporary fashion in that while rAamS6 reduced V0 of plasmin by up to ~53%, it had no effect on Vmax. Our data also suggest that rAmS6 has minimal or no apparent effect on V0 or Vmax of thrombin, factor Xa, and kallikrein. We speculate that AamS6 is apparently involved in facilitating blood meal feeding in that various amounts of rAamS6 reduced platelet aggregation by up to ~47% and delayed plasma clotting time in the recalcification time assay by up to ~210 s. AamS6 is most likely not involved with the tick’s evasion of the host’s complement defense mechanism, in that rAamS6 did not interfere with the complement activation pathway. Findings in this study are discussed in the context of expanding our understanding of tick proteins that control bloodmeal feeding and hence tick-borne disease transmission by ticks.
Keywords: Amblyomma americanum, serine protease inhibitors (serpins), blood coagulation, complement activation pathway, platelet aggregation
Introduction
Ticks represent the most successful vectors of human and animal disease agents (Sonenshine, 1993). In the tropics and sub-tropical areas of the world, ticks and tick-borne diseases represent the major source of economic loss, estimated at billions of dollars annually in the livestock industry (De Castro, 1997; Jongejan & Uilenberg, 2004; Gratz, 2006; Nicholson et al., 2009). In public health, ticks are second to mosquitoes in terms of impact of transmitted disease agents and they surpass any other known vector arthropod in terms of diversity of the transmitted disease agents (Sonenshine, 1993). In the USA, the majority of the reported human vector-borne diseases are tick-borne (Bratton & Corey, 2005; Fish & Childs, 2009).
In our laboratory, we are studying Amblyomma americanum as a model animal to understand the molecular basis of tick feeding physiology. A. americanum is among the most aggressive pests of both humans and livestock (James et al., 2001; Childs & Paddock, 2003; Paddock & Yabsley, 2007). This tick occurs in the Southeast, South-Central United States and along the Atlantic Coast up to New York and Maine (James et al., 2001; Nicholson et al., 2009). Although previously considered a nuisance tick, A. americanum is now recognized as a vector for multiple human tick-borne disease agents including Francisella tularensis, Theileria cervi, Ehrlichia chaffensis, Ehrlichia ewingii and the suspected agent for southern tick-associated rash illness (Childs & Paddock, 2003; Goddard & Varela-Stokes, 2009; Nicholson et al., 2009).
Ticks are pool feeders and accomplish feeding by lacerating host tissue to create the tick feeding lesion from which they suck blood. This feeding style is expected to trigger the tissue repair and immune responses such as inflammation, haemostasis and complement activation. To evade host-to-tick feeding, ticks inject numerous tick saliva proteins into the host. Without successful feeding, ticks cannot cause damage to their host, acquire and transmit disease agents. Thus, with a goal of finding ways to block successful tick feeding, the discovery of tick saliva proteins as a means to understand molecular mechanisms that regulate tick feeding has been aggressively pursued. The design and objectives of these experiments has been influenced by assumptions of how the host may respond to tick feeding behaviour and how the tick may evade host defences to tick feeding activity. To this effect, multiple tick saliva proteins that have anticoagulant (Lai et al., 2004; Nazareth et al., 2006; Prevot et al., 2006; Gao et al., 2011; Chmelar et al., 2012), anticomplement (Schroeder et al., 2007; Tyson et al., 2007; Gillet et al., 2009; Barratt-Due et al., 2011; Schuijt et al., 2011), antiplatelet (Kazimírová et al., 2002; Mans et al., 2002, 2003; Prevot et al., 2006; Chmelar et al., 2011; Assumpcao et al., 2012) and anti-inflammatory (Ribeiro et al., 1985; Déruaz et al., 2008; Prevot et al., 2009; Vancová et al., 2010) functions have been discovered.
We are interested in understanding the roles of tick saliva serine protease inhibitors (serpins) in regulating tick feeding physiology. Important host defence reactions to tick feeding such as inflammation, haemostasis and complement activation are controlled by serpins (Gettins, 2002; Huntington, 2006; Rau et al., 2007). Given the significance of serpins in normal vertebrate host homeostasis, the working hypothesis for research on tick serpins is that ticks use serpins to disrupt serine protease-mediated defence mechanisms and facilitate bloodmeal feeding which in turn facilitates the acquisition and transmission of tick-borne disease agents (Mulenga et al., 2001, 2003, 2007, 2009). In the 10 years that followed the publication of a ‘food for thought’ paper on the potential of serpins as target antigens for tick vaccine development (Mulenga et al., 2001), serpin-encoding cDNAs were cloned from several tick species including A. americanum (Mulenga et al., 2007), Amblyomma variegatum, Amblyomma maculatum (Karim et al., 2011), Dermacentor variabilis (Sonenshine et al., 2011), Rhipicephalus appendiculatus (Mulenga et al., 2003), Rhipicephalus microplus (Rodriguez-Valle et al., 2012), Haemaphysalis longicornis (Sugino et al., 2003; Imamura et al., 2006, 2008), Ixodes scapularis (Ribeiro et al., 2006; Mulenga et al., 2009), and Ixodes ricinus (Prevot et al., 2006; Chmelar et al., 2011). The release of the I. scapularis genome sequence data revealed that, similar to other organisms, the serpin protein family in ticks is large (Mulenga et al., 2009). Comparative modelling (Mulenga et al., 2007) and empirically resolved secondary structures (Kovářová et al., 2010; Chmelar et al., 2011) have shown that tick-encoded serpins retain the consensus secondary structure of 7–9 α-helices and 3-β sheets (Gettins, 2002; Huntington, 2006). A limited number of functional analyses studies have demonstrated that some tick-encoded serpins are functional inhibitors of serine protease activity with antihaemostatic functions (Chmelar et al., 2011; Prevot et al., 2006, 2009; Kovářová et al., 2010). In other studies, the feeding efficiency of ticks that fed on recombinant serpin immunized animals was significantly reduced, demonstrating that, similar to other organisms, serpins play important roles in tick feeding physiology (Imamura et al., 2005, 2006, 2008; Prevot et al., 2007; Jittapalapong et al., 2010).
In proposing that ticks use serpins to mediate the tick’s evasion of host defence mechanisms, the assumption is that these proteins are injected into the host during tick feeding. In most reported studies, serpins were considered as being putatively secreted into the host during tick feeding on the basis of expression of the candidate serpin in the salivary gland and possessing the signal peptide (Mulenga et al., 2007; Chmelar et al., 2011). In our laboratory we have developed a protocol to validate the injection of immunogenic tick saliva serpins into the host during tick feeding (Chalaire et al., 2011). In the present study, the objective was to functionally characterize inhibitor functions, and to gain insight into the role(s) of A. americanum tick saliva serpin 6 (AamS6) at the tick feeding site. AamS6 was previously discovered among 17 serpins that were expressed in salivary glands and midguts of partially fed A. americanum ticks using serpin degenerate primers (Mulenga et al., 2007). At both the mRNA and protein levels, AamS6 was shown to be ubiquitously and strongly expressed in unfed ticks and in ticks that were fed up to 72 h (Mulenga et al., 2007; Chalaire et al., 2011). Native AamS6 protein was demonstrated to be injected into the host during tick feeding as revealed by rabbit antibodies to Escherichia coli-expressed recombinant (r) AamS6 that specifically bound to the expected mature AamS6 protein band on Western blots of pilocarpine-induced tick saliva (Chalaire et al., 2011). Likewise, antibodies to 48 h A. americanum tick saliva proteins also specifically bound to rAamS6 (Chalaire et al., 2011). In this study, we show that Pichia pastoris-expressed rAamS6 is a functional cross-class inhibitor of serine and cysteine proteases. Our data also demonstrate the potential for AamS6 to be part of the tick’s saliva protein complex that mediates the tick’s evasion of the host’s haemostasis defence response to tick feeding activity. We have discussed our findings in the context of advancing our knowledge of tick molecular biology.
Results
Pichia pastoris-expressed recombinant Amblyomma americanum serpin 6 is N-glycosylated
Previous Western blotting analyses experiments validated that AamS6 was injected into the host during tick feeding (Chalaire et al., 2011). Previous efforts to find putative proteases that may be regulated by rAamS6 failed, in that an E. coli-expressed insoluble rAamS6 (Chalaire et al., 2011) that was refolded in vitro was consumed as a substrate for the test protease (Mulenga et al., unpublished data); thus, in order successfully to characterize functions of AamS6, we expressed rAamS6 in the P. pastoris expression system using pPICZα plasmids, which produces soluble proteins as summarized in Fig. 1. Figure 1A summarizes the cumulative daily expression levels of rAamS6 in yeast-spent media, and Fig. 1B shows validation of affinity purification of rAamS6. Based on electrophoresis gel shown in Fig. 1B, affinity purified rAamS6 used in the present study had minimal or no contaminating yeast proteins. Primary sequence analysis predicted that AamS6 contained at least three potential N-glycosylation sites (Mulenga et al., 2007). To validate if molecular predictions of N-glycosylation sites in AamS6 were functional, we treated rAamS6 with the PngaseF enzyme as summarized in Fig. 1C. The observed ~5 kDa molecular weight shift between treated and nontreated rAamS6 demonstrated that the predicted N-glycosylation sites were functional (Fig. 1C). The calculated molecular mass for mature rAamS6 was ~46 kDa, which includes ~42 kDa from AamS6 mature protein backbone and ~3.5 kDa fusion from the pPICZAα vector. Based on the deglycosylation assay in Fig. 1C, post-translation N-glycosylation accounted for ~5 kDa to the observed ~53/54 kDa molecular mass for rAamS6.
Figure 1.
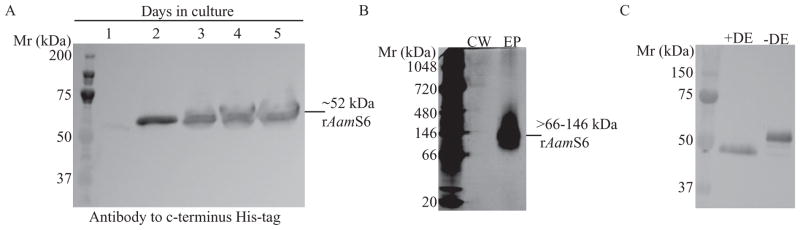
Expression and affinity purification of recombinant (r) Amblyomma americanum tick saliva serine protease inhibitor (AamS6) in Pichia pastoris. The mature AamS6 protein cDNA was cloned into the pPICZα plasmid and elctroporated into X33 P. pastoris strain as described. Positive transformants were selected for methanol utilization on yeast agar plates. Selected colonies were grown in culture to A600 of 1 before inducing rAamS6 expression by daily feeding of cultures with methanol to 5% final concentration. Panel A = daily (lanes 1–5) rAamS6 expression detection by Western blotting using the antibody to c-terminus histidine tag. Panel B = Affinity purified rAamS6 electrophored on a 4–16% gradient native acrylamide gel and silver stained. CW = column wash, EP = column eluted protein. Panel C = Validation of N-glycosylation posttranslational modification of rAamS6. + DE = rAamS6 treated with the deglycosylating PngaseF enzyme, −DE = non-treated rAamS6.
Recombinant Amblyomma americanum serpin 6 s a cross-class inhibitor of serine- and papain-like cysteine proteases
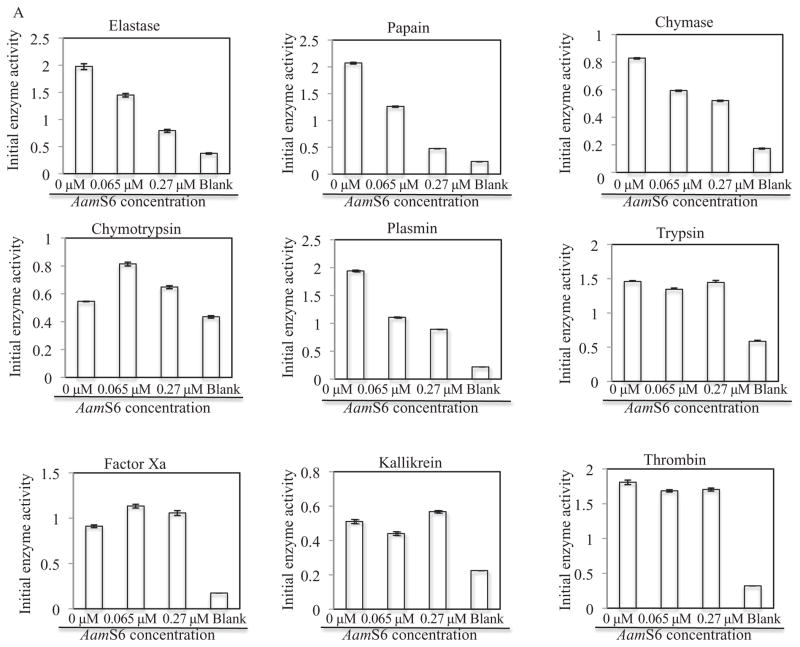
Although on the basis of sequence features, AamS6 was similar to previously characterized functional inhibitory serpins (Gettins, 2002), we sought to determine the functionality of rAamS6 using previously described progress curve methods (Schechter & Plotnick, 2004; Askew et al., 2007; Huang et al., 2008). To determine putative protease targets of AamS6, enzymatic activities of 10 mammalian proteases were assayed in the presence of 0.065 μM and 0.27 μM (0.34 μg and 1.4 μg) affinity purified rAamS6. In order to quantify the effect of rAamS6 on enzymatic activity of proteases, we estimated the initial velocities of substrate hydrolysis (V0. μM/S), and maximum enzyme velocities (Vmax. μM/S), using non-linear regression in Graphpad software to fit the integrated second order polynomial and Michaelis–Menten equations onto the progress-curve data (Fig. 2) respectively (Michaelis et al., 2011). Figure 2A summarizes the effects of rAamS6 on V0. Pre-incubating proteases with 0.065 μM and 0.27 μM rAamS6 reduced V0 of plasmin, papain, elastase and chymase in a dose–response manner by 43 and 53%, 39 and 77%, 27 and 59% and 28 and 37% respectively (Fig. 2A). Fig. 2A also shows that rAamS6 minimally affected or did not affect V0 of trypsin, thrombin, kallikrein, factor Xa and chymotrypsin. Consistent with its effects on V0, rAamS6 reduced Vmax of papain, elastase and chymase in a dose–response manner by ~5 and 58%, 15 and 55% and 16 and 18%, respectively (Fig. 2B), except for plasmin where rAamS6 had no effect on Vmax. It was also notable that, while rAamS6 did not affect the V0 values of trypsin and chymotrypsin, it reduced Vmax of these proteases in a dose–response manner by 17 and 23% and 26 and 28%, respectively (Fig. 2B). Similar to our data in Fig. 2A, rAamS6 had minimal or no apparent effect on Vmax of thrombin, factor Xa, and kallikrein (Fig. 2B). It is also interesting to note that overall, rAamS6 had high inhibitory activity against V0 than Vmax, which could be explained by differences in the two equations used in our analysis.
Figure 2.
Protease inhibitor function profiling of rAamS6. Indicated candidate proteases (500 ng) were co-incubated with affinity purified, 0.34 μg and 1.4 μg or 0.066 μM and 0.27 μM for 15 min at 25 °C. After incubation, appropriate peptide substrates were added to final concentrations indicated in materials and methods. Subsequently the release of the chromophore, p-nitroanilide as proxy for substrate hydrolysis was monitored at A410 using the VersaMax microplate reader. The observed A410 were converted to released μM concentrations of digested peptides as described in materials and methods. Non-linear regression in Graphpad software was used to fit the second order polynomial and the Michaelis and Menten equations on our data to respectively estimate (A) the initial velocities of substrate hydrolysis, and (B) the maximum velocity of substrate hydrolysis.
Recombinant Amblyomma americanum serpin 6 inhibits platelet aggregation in a dose response manner
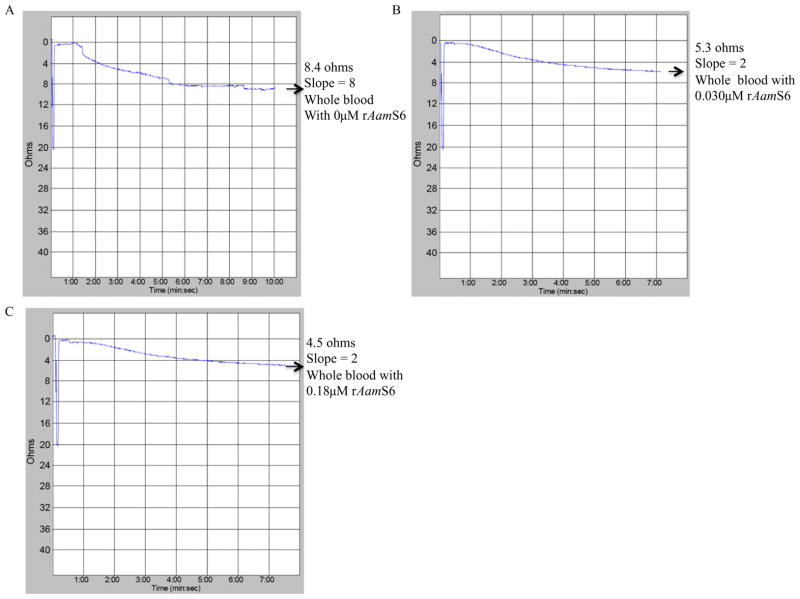
Given that AamS6 is secreted into the host during tick feeding (Chalaire et al., 2011), we wanted to investigate its effects on platelet aggregation function. The platelet aggregation function is one of the first lines of anti-tick defence by the host, as it is the first step in the sequence of reactions towards stopping further bleeding in an injured blood vessel as occurs during tick feeding (Ribeiro et al., 1985). The effect of rAamS6 on platelet aggregation was investigated using the whole-blood approach (Fig. 3), which assays platelet function in a near physiological state in that it does not involve platelet purification. In the whole-blood platelet aggregation method used in the present study, increased platelet aggregation is correlated with an increase in electrical resistance (ohms; Ω). Data summarized in Fig. 4 show that co-incubation of 0.08 μM or 0.4 μM (2 and 10 μg) of affinity-purified rAamS6 with cattle whole blood reduced electrical resistance in a dose–response manner by 37 (5.3/8.4) and 47% (4.5/8.4) Ω (Fig. 3B and C).
Figure 3.
Effect of recombinant Amblyomma americanum tick saliva serine protease inhibitor (rAamS6) on platelet aggregation: Citrated cattle whole diluted 1:1 with normal saline was co-incubated without (A) or with (B and C) rAamS6, 2 and 12 μg or 0.08 and 0.27 μM at 37 °C for 10 min. Subsequently platelet aggregation was induced by adding adenosine diphosphate to final 20 μM concentration. Platelet aggregation was monitored using the whole blood platelet aggregometer as described under materials and methods.
Figure 4.
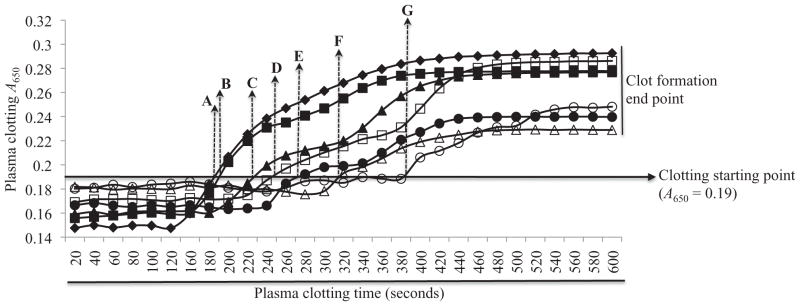
Effect of affinity purified on plasma clotting time: Citrated human plasma depleted of platelets was co-incubated with various amounts (0.48, 0.40, 0.32, 0.24, 0.16 and 0.08 μg or 0.13, 0.11, 0.088, 0.066, 0.044 and 0.022 μM) of rAamS6 at 37 °C for 10 min. Adding 25 mM calcium chloride triggered plasma clotting. Clot formation was monitored at A650 using the VersaMax microplate reader. The solid line arrowhead at A650 indicates plasma clotting starting point. The broken line arrowheads, A, B, C, D, E, F and G mark the plasma clotting start times at 180, 190, 220, 250, 270, 310 and 390 s when plasma was co-incubated with 0, 0.022, 0.044, 0.066, 0.088, 0.11 and 0.13 μM affinity purified rAamS6, respectively.
Recombinant Amblyomma americanum serpin 6 delays plasma clotting time but does not inhibit complement activation pathway
One challenge faced by ticks in their quest for a bloodmeal is to prevent blood from clotting at the tick feeding site. We successfully investigated the effect of rAamS6 on plasma clotting using in vitro assays that measure the functional integrity of the blood clotting system in a holistic way [recalcification time assay (RCT)], intrinsic [activated prothrombin time (APPT)] and extrinsic [prothrombin time (PT)] blood clotting activation pathways as well as the common pathway [thrombin time (TT)], which leads to formation of the fibrin clot. Except for the RCT assay where delayed plasma clotting time was demonstrated (Fig. 4), rAamS6 had minimal or no effect on plasma clotting time in the PT, TT and APPT assays (not shown). As shown in Fig. 4, the point when the A650 begin to rise above background has been designated as the start of plasma clotting (denoted with broken arrowhead in Fig. 4). In the absence of rAamS6 (arrowhead A in Fig. 4), plasma started to clot at ~180 s, while in the presence of rAamS6 plasma clotting start was delayed in a dose–response manner. In the presence of 0.022, 0.044, 0.066, 0.088, 0.11 and 0.13 μM rAamS6, (arrowheads B, C, D, E, F and G in Fig. 4) plasma clotting was delayed in a dose–response manner by ~10, 40, 70, 90, 130 and 210 s respectively (Fig. 4). In the RCT assay firmness of the plasma clot is correlated to observed end A650 values. Based on endpoint A650 values, the plasma clot firmness in presence of the various amounts of rAamS6 was less firm by ~2–22% (not shown). In addition to plasma clotting assays, we investigated the effect of rAamS6 effects on the complement activation cascade. In our protocol we found that rAamS6 did not have any effect on the production of terminal complement complexes (TCCs) also called membrane attack complex (not shown) via the classical complement activation pathway.
Discussion
Transcript and protein profiling during the first 5 days of A. americanum tick feeding showed that both AamS6 mRNA and protein were ubiquitously expressed, reached highest abundance at the 72-h feeding timepoint, and that the AamS6 protein was secreted into host during tick feeding (Mulenga et al., 2007; Chalaire et al., 2011). These data, which suggested an apparent association of the AamS6 protein with regulating early-stage tick feeding events such as tick attachment onto host skin and creation of the tick feeding lesion, prompted the present follow-up study. Although molecular prediction provisionally identified AamS6 as a serpin on the basis of consensus sequence features, it was imperative that we validate its functionality. One of the limitations in functional proteomics is the availability of candidate recombinant or native proteins with appropriate post-translational modifications. To gain functional insight into putative mediator molecules of tick feeding, the expression of recombinant proteins is important. Thus, it was significant to note that consistent with molecular analysis predictions of N-glycosylation sites (Mulenga et al., 2007), rAamS6 expressed in this study was N-glycosylated. This suggests that rAamS6 was correctly folded with appropriate post-translational modifications and that findings in this study were likely to be consistent with events in vivo. Given that AamS6 was ubiquitously expressed and secreted into the host during tick feeding (Chalaire et al., 2011), there was a possibility that this protein functions both in the tick and the tick–host interface. Our study was biased towards understanding the role(s) of AamS6 at the tick–host interface.
Although originally identified as inhibitors of serine proteases and hence the name (Gettins, 2002; Huntington, 2006), serpins with cross-class inhibitor functions against both serine and cysteine proteases (Schick et al., 1998; Hwang et al., 2002; Herrera-Mendez et al., 2009), and those without inhibitor functions have since been described (Carrell et al., 2011). Data in the present study demonstrate that AamS6 is a cross-class inhibitory serpin of serine- and papain-like cysteine proteases. Inhibitory serpins are so-called ‘trapping inhibitors’ in that they form complexes with and destroy their target protease (Gettins, 2002). The net effect of this interaction is the reduction of enzymatic activity of candidate proteases in a dose-dependent manner (Schechter & Plotnick, 2004). Thus, the observation that rAamS6 reduced both the initial velocities of substrate hydrolysis (V0) and/or maximum enzyme velocity (Vmax) or residual enzyme activity of papain, trypsin, chymotrypsin, elastase, and chymase in a dose-dependent manner supports our conclusion that rAamS6 is an inhibitory serpin. Another interesting observation in this study was that, while rAamS6 reduced V0 of plasmin enzymatic activity by ~53%, it had no effect on Vmax of plasmin. This observation may suggest the possibility of rAamS6 forming transient complexes and not necessarily destroying its target proteases, which could mean that rAamS6 in this study was not conforming to behaviour of other characterized serpins that form stable complexes with their target proteases without disassociating (Gettins, 2002). The apparent unstable association of rAamS6 with plasmin has been observed in other serpin–protease interactions. For instance, protein Z-dependent protease inhibitor did not apparently irreversibly inhibit factor Xa (Han et al., 2000; Rezaie et al., 2005). Whether this was the case in the present study is subject to follow-up investigation. It is also important to note that they were discrepancies on the levels of effects of rAamS6 on V0 and Vmax. We are of the opinion that these discrepancies could be explained by the fact that we used two different equations to estimate V0 and Vmax. What was notable, however, is that in both equations the trend was consistent, except for plasmin.
It is important to note that the inhibitory profiles of rAamS6 presented here are based on in vitro data, and thus may not necessarily represent in vivo events at the tick–host interface and/or in the tick itself; however, to a limited extent we can use these data to gain insight into probable functions of AamS6 at the tick–host interface. The tick’s feeding style of creating a wound and then sucking up the blood from the haematoma is expected to provoke a tissue repair response that begins with an inflammation response (Eming et al., 2007). Immunohistochemical studies have documented that cellular mediators of inflammation (Harvima & Nilsson, 2011; Knol & Olszewski, 2011; Siracusa et al., 2011; Amin, 2011; Arita, 2012; Kovach & Standiford 2012; Isobe et al., 2012), macrophages, neutrophils, eosinophils and basophils infiltrate the tick feeding site (Schleger et al., 1976; Nosek et al., 1978; Brown & Askenase, 1982; Brown et al., 1982; Brown et al., 1983; Ushio et al., 1995; Szabó & Bechara, 1999; Lima e Silva et al., 2004) In other studies, cellular mediators of inflammation at sites of injury have been shown to secrete protease mediators of inflammation, including cathepsin G, chymase and elastase (Korkmaz et al., 2008; Heutinck et al., 2010; Kessenbrock et al., 2011). Although there is no direct evidence documenting the secretion of protease mediators of inflammation at the tick feeding site, there is potential that this may indeed be the case. Thus, the observation in the present study that rAamS6 inhibited proteolytic activity of chymase and elastase may suggest a role for AamS6 in mediating the tick’s anti-inflammation function at the tick feeding site. Similarly, papain-like cysteine proteases are associated with cellular surfaces of cellular mediators of inflammation, macrophages and neutrophils (Jevnikar et al., 2012; Sun et al., 2012; Vendramini-Costa & Carvalho, 2012). Thus, there is also a possibility that AamS6 might interfere with the inflammation response by blocking cysteine protease function. Plasmin is mostly known for its role in fibrinolysis (Syrovets et al., 2012). The consequence of this that blood will resume flowing. Logically this should be in the best interest of the tick in that plasmin function will sustain blood flow to the feeding site. Thus the observed inhibition of plasmin enzymatic activity by rAamS6, albeit temporary, could be viewed as counter-intuitive. Plasmin has now been shown to play significant roles in regulating the host defence mechanisms, including regulating normal functioning of immune cells such as monocytes, macrophages and dendritic cells, and controlling inflammation response (Syrovets et al., 2012). Thus, from the perspective of plasmin roles in regulating host’s innate immune systems, the observed inhibition of plasmin by rAamS6 could signal the role for this protein in mediating the tick’s ability to evade the host’s immune defence mechanisms by affecting the plasmin function.
The inhibitory mechanism of a serpin is initiated by the target protease, which attempts to cleave the reactive centre loop of the serpin protein via its P1 amino acid (aa) residue (Gettins, 2002; Huntington, 2006). In the process, the serpin traps and destroys the protease, effectively removing it from the reaction (Gettins, 2002). While the predicted P1 aa residue of a serpin may not be enough to predict the target protease of a candidate serpin (Gettins, 2002), it is notable that consistent with previously characterized cross-class inhibitors of serine and cysteine proteases, Endopin 2 (Hwang et al., 2002) and the squamous cell carcinoma antigen 1 (Masumoto et al., 2003; Askew et al., 2004; Kanaji et al., 2007), both of which have a ‘Ser’ aa residue at their P1 sites, AamS6 also has a ‘Ser’ aa residue at the P1 site (Mulenga et al., 2007). Most reported studies reveal that serpins that have inhibitory functions against trypsin or chymotrypsin have basic (Arg and Lys) or aromatic (Phe, Tyr and Trp) aa residues at the P1 site, (Gettins, 2002) respectively. Thus, the observation in the present study that rAamS6, which has a neutral aa residue ‘Ser’ at the P1 site (Mulenga et al., 2007), inhibited both trypsin and chymotrypsin is interesting though not unusual. Similar observations were reported for other serpins such as rainbow trout (Oncorhynchuss mykiss) and common carp (Cyprinus carpio) α1-antiprotease inhibitor 1 (Mickowska, 2009) that also have polar neutral ‘Met’ at the P1 site. The apparent consistency of findings in the present study with other studies provides a measure of confidence in our data.
Blocking haemostasis to keep host blood in a fluid state at both the tick feeding site and in the gut is key to the tick’s successful feeding and acquiring of and transmitting tick-borne disease agents. Thus, the demonstration in this study that rAamS6 delayed plasma clotting and inhibited platelet aggregation suggests there is potential for native AamS6 to be part of the tick saliva protein complex that mediates A. americanum tick’s antihaemostatic function. Anti-blood clotting, anticomplement, and antiplatelet aggregation functions have all been demonstrated in crude tick saliva (Ribeiro, 1987; Reck et al., 2009; Chmelar et al., 2012). Data in the present study demonstrate that AamS6 is potentially among tick saliva proteins that regulate tick saliva effects against blood clotting and platelet aggregation function. Following injury to blood vessels, as occurs during tick feeding, platelets aggregate and form the initial plug around the injured site to stop bleeding (Nurden, 2011). Thus, the effect of rAamS6 against platelet aggregation, if physiologically consistent, could imply a role for native AamS6 in facilitating tick feeding regulation, as it would participate in preventing formation of the initial platelet plug. Based on data in this study, specific targets that were affected by rAamS6 to exert antiplasma clotting and antiplatelet aggregation functions are unknown. While rAamS6 delayed plasma clotting in the RCT assay, which measures the functional integrity of blood clotting factors in their entirety, our experiments to determine whether or not rAamS6 affected plasma clotting by inhibiting factors that regulate the extrinsic, intrinsic, or common blood clotting activation pathways were inconclusive. The discrepancy between rAamS6 delaying plasma clotting in the RCT assay, but not on extrinsic, intrinsic and common blood clotting activation assays can be explained by differences in detection methods. In the RCT assay, formation of the fibrin clot was progressively measured. In this way, the formation of the fibrin clot at a slow rate in the presence of rAamS6 was detected. Conversely, in the PT, APPT and TT assays, appearance and not the quality of the fibrin clot were measured. In this way the quality of the fibrin clot was not accounted for. To date, few studies on I. ricinus have reported serpins with anticoagulant functions (Prevot et al., 2006, 2009; Chmelar et al., 2011). Additionally a tick salivary gland serpin in I. ricinus was shown to inhibit both platelet aggregation and inflammation (Chmelar et al., 2011); however, the present study is first to show that an experimentally validated tick saliva serpin (Chalaire et al., 2011) is a putative anticoagulant and inhibitor of platelet aggregation.
While the data in this study on the potential for AamS6 to be involved in tick feeding regulation is exciting, we would like to caution that there is potential that observations here could be artifacts. Similar to vertebrates, in arthropods the display of haemocyte aggregation function as an immune response mechanism (Garcia et al., 2004; Nakatogawa et al., 2009) and haemolymph coagulation as a tissue repair response (Dushay, 2009) have been demonstrated. Although similar pathways remain to be elucidated in ticks, there is a possibility that native AamS6 is a regulatory protein that functions within the tick and not at the tick feeding site. From this perspective, the observed antiplasma clotting functions of rAamS6 could be artefacts and not consistent with tick physiological events. However, we are confident that this is not the case, in that AamS6 is an empirically validated tick saliva protein (Chalaire et al., 2011). Thus the chances of AamS6 functioning at the tick feeding site are high. Also notable in this study is that we did not observe complete blockage of function by rAamS6. This may be explained by several factors, e.g. ex vivo experimental conditions not being optimum when compared to in vivo conditions, and the possibility that optimum function may require yet unknown cofactors or that insufficient amounts of rAamS6 were added to the reaction mixtures. Another reason could be that AamS6 is not the sole mediator of tick antihaemostasis function in A. americanum tick saliva. Previous studies have reported the presence of at least two other anticoagulants in A. americanum, 12- and 16-kDa tick salivary gland proteins that affect blood clotting by inhibiting thrombin (Zhu et al., 1997a) and factor Xa (Zhu et al., 1997b), respectively.
Although we demonstrated that rAamS6 was glycosylated, we are yet to determine if glycosylation was important to rAamS6 function. The ideal experiment to investigate this would be comparative functional analyses assays between the glycosylated yeast-expressed protein and the non-glycosylated E. coli-expressed protein. Our efforts to do this experiment failed because in E. coli rAamS6 was expressed as an insoluble fraction (Chalaire et al., 2011). Additionally, our efforts to use an in vitro refolded rAamS6 were unsuccessful in that the refolded protein was digested by the test protease (unpublished data). There are reported studies where serpins expressed as soluble fractions in E. coli showed inhibitory functions (An et al., 2012; Mameri et al., 2012), which may suggest that glycosylation may not be important to serpin function. In conclusion, data reported in the present study advance our knowledge of the understanding of tick feeding physiology regulation, and provide some insight into the putative roles of AamS6 at the tick feeding site. The next phase of the research is to determine native host factors that interact with AamS6 at the tick feeding site and to examine the consequences of blocking or eliminating those interactions on tick feeding success and tick-borne disease transmission.
Experimental procedures
Chemicals and proteases
Proteases: trypsin, chymotrypsin and thrombin (from bovine pancreas), elastase and kallikrein (porcine pancreas), chymase, and plasmin (human plasma), cathepsin G (human neutrophils) were purchased from Sigma (St. Louis, MO, USA). Two other proteases, papain (papaya) and factor Xa (human plasma) were purchased from Spectrum (Gardena, CA, USA) and Fisher Scientific (Middletown, VA, USA) respectively. P-nitroanilide (PNA) labelled peptide substrate for trypsin (Arg- p-nitroanilide), chymotrypsin (Ala-Ala-Val-Ala- p-nitroanilide), papain (Glu-Phe-Leu-p-nitroanilide), plasmin and factor Xa (Gly-Arg-p-nitroanilide), elastase (Pro-Val- p-nitroanilide), chymase (Ala-Ala-Pro-Phe-p-nitroanilide), thrombin and kallikrein (Pro-Phe-Arg-p-nitroanilide) were purchased from Sigma. Reagents for plasma clotting time assays, PT, APPT and TT, as well as accompanying normal reference human plasma were purchased from Pacific Hemostasis through Fisher Scientific. Adenosine diphosphate (ADP) was obtained from Chrono-Log Corp. (Harvetown, PA, USA). Cattle whole blood was collected Texas A & M University meat processing plant.
Expression and affinity purification of recombinant rAamS6 in Pichia pastoris
To construct expression plasmids, forward (GAATTC GAGAC CGACGATGCACTGC TGG) and the reverse (GCGGCCGCAA GACTCTGGACTTCACCGATAA) primers were used to sub-clone the AamS6 mature protein-coding domain into EcoRI/NotI pPICZαA cloning site. With parameters set to ~1500 kV, 25 μF and 200 Ω, the recombinant pPICZαA-AamS6 plasmid was used to transform X-33 P. pastoris strain (Invitrogen, Carlsbad, CA, USA) by electroporation using the BTX ECM 630 electroporator (Harvard apparatus, Holliston, MA, USA). Transformants selected for methanol utilization according to instructions in the user manual, were cultured at 28 °C to A600 of 1 before inducing protein expression by daily feeding of yeast cultures with 5% methanol for 5 days. The pPICZα-A plasmid secretes the recombinant protein into media. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis with Coommassie blue staining or Western blotting analysis using the antibody to the c-terminal histidine tag (Invitrogen) confirmed the expression and secretion of rAamS6 into media. To purify rAamS6, we first precipitated out off culture medium by ammonium sulphate precipitation. This was done by adding 525 g of ammonium sulphate per litre of culture supernatant and then the protein was allowed to precipitate overnight at 4 °C with stirring. Following the overnight incubation, the protein precipitate was centrifuged and the pellet dissolved into and dialysed against phosphate-buffered saline buffer (PBS, pH 7.4). For affinity purification, the recombinant protein in PBS was diluted in 2X native column biding buffer and then affinity purified under native conditions using the NiCl2 charged HiTrap™ Chelating HP column (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA). The affinity-purified rAamS6 was dialysed against 0.1 M or 0.01 M HEPES buffer containing 150 mM NaCl2+ (pH 7.4) or normal saline (0.9% NaCl in sterile distilled water) and stored −80 °C until used in assays below. Affinity-purified protein in respective buffers was concentrated using Jumbosep centrifugal spin filter devices with a 30-kDa cut-off point (Pall Life Sciences, Port Washington, NY, USA) or ammonium sulphate precipitation. Protein quantification was done using the Bradford assay according to instructions by the manufacturer (Thermo Scientific, Barrington, IL, USA).
Recombinant Amblyomma americanum serpin 6 inhibitor function profiling
Inhibitor function profiling of rAmS6 was investigated using the progress curve method under continuous conditions according to other authors (Schechter & Plotnick, 2004; Askew et al., 2007; Huang et al., 2008). All enzyme inhibition assays were conducted in 100 mM HEPES buffer (pH 7.4) containing 150 mM sodium chloride, pH 7.4. In a 100 μL reaction volume, 0.34 μg and 1.4 μg or 0.065 μM and 0.27 μM of affinity purified rAmS6 was pre-incubated with candidate proteases at room temperature (25 °C) for 15 min. Amounts of proteases used in reactions were 500 ng for trypsin, chymotrypsin, factor Xa and papain, 460 ng for plasmin, ~454 ng for chymase, 412 ng for kallikrein, 400 ng elastase, and 250 ng thrombin. After the 15 min room temperature pre-incubation, PNA calorimetric peptide substrates: 25 μM for trypsin, plasmin, thrombin and kallikrein, 50 μM for papain, chymotrypsin, cathepsin G, chymase and elastase and 100 μM factor Xa was added to the reaction mix. Subsequently peptide hydrolysis was monitored continuously every 20 s for 20 min at A410 using the VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA) set to 37 °C. To estimate the amount of released PNA as proxy for digested peptide substrate, the A410 values were converted to μM concentrations of PNA using the formula: released PNA (μM) = A410 X reaction volume/PNA molar extinction coefficient (8800) X volume of added enzyme X Reaction time (seconds) for the assay as previously published (Barrett, 1981).
To estimate the effect of rAamS6 on protease kinetics, non-regression analysis in Graphpad PRISM software package version 6 (Graphpad software, La Jolla, CA) was used to fit the second-order polynomial and the Micahelis–Menten equations onto the progress curve data (Michaelis et al., 2011). The second-order polynomial equation estimated the initial velocity of substrate hydrolysis (V0, μM/S), while the Micahelis–Menten equation estimated maximum velocity of substrate hydrolysis (Vmax, μM/S) as proxy for residual enzyme activity. Estimated V0 values represented enzymatic activity immediately after pre-incubation of the protease with rAamS6. To calculate percent inhibition levels enzymatic activity inhibition levels of rAamS6, V0 and Vmax estimates in presence of rAamS6 were expressed as a percentage of the V0 and Vmax in the absence (0 μM) of rAamS6. This was then subtracted from the assumed 100% enzyme activity in the absence of rAamS6 reactions.
Effect of recombinant Amblyomma americanum serpin 6 on platelet aggregation
The effect of rAamS6 on platelet aggregation was investigated using the whole-blood approach. This method measures platelet aggregation as a function of change in electrical impedance of whole blood. Cattle whole blood was collected from the Texas A & M University slaughterhouse and mixed with sodium citrate in 9:1 ratio to prevent clotting. Citrated cattle blood was then used in the assays within 3 h of collection. Citrated blood (500 μl) diluted with equal volume of normal saline (0.9% NaCl) in a 1:1 ratio was pre-incubated for 10 min at 37 °C with or without 0.08 μM or 0.27 μM (4 and 14 μg) of affinity purified rAamS6. Addition of ADP to 20 μM final concentration triggered platelet aggregation. Platelet aggregation as a function of increased electrical resistance (ohms; Ω) was monitored and recorded using a whole-blood platelet aggregometer (Chrono-Log Corp.).
Effect of recombinant Amblyomma americanum serpin 6 on blood clotting time
The effect of rAamS6 on plasma clotting was investigated using routine plasma clotting assays, RCT, PT, APPT and TT, as previously described (Nazareth et al., 2006; Decrem et al., 2009, Liao et al., 2009; Gao et al., 2011). The RCT assay measures plasma clotting time when calcium (Ca2+), the blood-clotting cofactor is re-supplied to citrated plasma (Liao et al., 2009; Gao et al., 2011). In this assay, the effect of rAamS6 on plasma clotting was investigated by pre-incubating 20 μl of citrated human plasma (Fisher scientific) at 37 °C for 10 min without or with serially diluted rAamS6 (0.48, 0.40, 0.32, 0.24, 0.16 and 0.08 μg or 0.13, 0.11, 0.088, 0.066, 0.044 and 0.022 μM) in a 70 μl 100 mM HEPES plus 150 mM sodium chloride buffer pH 7.4. Adding 30 μl of 25 mM CaCl2 to the reaction mix triggered clotting of plasma. Progression of plasma clotting was monitored at A650 at 20-s intervals over 10 min using the DUB 640 spectrophotometer (Beckman Coulter, Brea, CA, USA) set to 37 °C. The expectation in this assay is that, as plasma clots and turbidity increases, resistance to the light path will increase.
The effect of rAamS6 on factors that mediate the extrinsic blood clotting activation pathway was investigated using the PT assay as previously described (Davie et al., 1991, Lefkowitz, 2006). 100 μL of citrated human plasma was pre-incubated for 10 min at 37 °C with twofold serial dilutions in (0.48, 0.40, 0.32, 0.24, 0.16 and 0.08 μg or 0.092, 0.077, 0.062, 0.046, 0.031 and 0.015 μM) of affinity-purified rAamS6 in 10 mM HEPES and 150 mM sodium chloride at pH 7.4. Subsequently, the PT reagent was added to the reaction mix and continued to incubate at 37 °C for an additional 3 min to activate the reaction. Addition of 100 μL 25 mM CaCl2+ to the reaction to mixture triggered clotting. Plasma clotting time was immediately monitored and determined using the KC1 DELTA coagulometer (Trinity Biotech, Parsippany, NJ, USA). Alternatively, the assay was repeated and plasma clotting time, monitored at A650 using the VersaMax microplate reader set to 37 °C.
The effect of rAamS6 on factors that mediate the intrinsic blood clotting activation pathway was investigated using the APPT assay as previously published (Davie et al., 1991, Lefkowitz, 2006). Citrated plasma was incubated with affinity-purified rAamS6 as described above in the PT assay. Subsequently, 100 μL of the APPT reagent was added and incubation continued for 3 min to activate the reaction. Plasma clotting was triggered by the addition of 100 μL 25 mM CaCl2+. Plasma clotting time was subsequently monitored as described in the PT assay.
The extrinsic and intrinsic blood clotting pathways converge onto the common pathway, during which activated thrombin converts fibrinogen to fibrin to form the clot (Davie et al., 1991; Lefkowitz, 2006). To investigate if rAamS6 interfered with the common pathway, 100 μL of the TT reagent containing thrombin was pre-incubated with affinity-purified rAamS6 as described above. The addition of 200 μL prewarmed plasma triggered conversion of fibrinogen to fibrin followed by clot formation. Alternatively, the assay was repeated by pre-incubating 200 μL plasma with rAamS6 as described above and then triggering clot formation by adding the TT reagent. Clotting time was determined as described above in the PT assay.
Measuring anticomplement function of recombinant Amblyomma americanum serpin 6
The effect of gauge the potential of native AamS6 to interfere with the complement activation pathway, affinity purified rAamS6 was subjected to anticomplement function analysis using the Micro-Vue CH50 ELISA kit (Quidel, San Diego, CA, USA). This kit quantifies the amount of TCCs that are formed when the complement system is activated via the classical complement activation pathway (Kojouharova et al., 2010). Human serum (14 μl) supplied with the kit was pre-incubated at 37 °C for 15 min without (positive control) or with twofold serial dilutions in (0.48, 0.40, 0.32, 0.24, 0.16 and 0.08 μg or 0.513, 0.427, 0.342, 0.256, 0.171 and 0.085 μM) of affinity-purified rAamS6. Subsequently, the serum rAamS6 reaction mixture was then incubated with 86 μl of the complement activator solution for 1 h at 37 °C. To quantify the amount of formed TCC, the reaction mix diluted 1:200 was bound to human anti-TCC antibodies coated onto microwells. After appropriate washing, a reaction was induced in the bound TCC with horseradish peroxidase (HRP)-conjugated antibody to the human TCC that was supplied with the kit. Subsequently the chromogenic HRP substrate was added to the wells to quantify the bound antibody as proxy for the amount of TCC that was produced. The intensity of colour development, which directly represented the amount of formed TCCs was quantified by reading optical density 450 nm using the VersaMax microplate reader.
Acknowledgments
The authors acknowledge research support to AM from NIH/NIAID (NIHRO1AI093858). AMGI was an international visiting student supported by a CNPq scholarship (201690/2010-1) from the Brazilian government.
References
- Amin K. The role of mast cells in allergic inflammation. Respir Med. 2011;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- An C, Hiromasa Y, Zhang X, Lovell S, Zolkiewski M, Tomich JM, et al. Biochemical characterization of Anopheles gambiae SRPN6, a malaria parasite invasion marker in mosquitoes. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0048689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M. Mediator lipidomics in acute inflammation and resolution. J Biochem. 2012;152:313–319. doi: 10.1093/jb/mvs092. [DOI] [PubMed] [Google Scholar]
- Askew DJ, Askew YS, Kato Y, Luke CJ, Pak SC, Brömme D, et al. The amplified mouse squamous cell carcinoma antigen gene locus contains a serpin (Serpinb3b) that inhibits both papain-like cysteine and trypsin-like serine proteinases. Genomics. 2004;84:166–175. doi: 10.1016/j.ygeno.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Askew DJ, Cataltepe S, Kumar V, Edwards C, Pace SM, Howarth RN, et al. SERPINB11 is a new noninhibitory intracellular serpin. Common single nucleotide polymorphisms in the scaffold impair conformational change. J Biol Chem. 2007;282:24948–24960. doi: 10.1074/jbc.M703182200. [DOI] [PubMed] [Google Scholar]
- Assumpcao TC, Ribeiro JM, Francischetti IM. Disintegrins from hematophagous sources. Toxins (Basel) 2012;4:296–322. doi: 10.3390/toxins4050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt-Due A, Thorgersen EB, Lindstad JK, Pharo A, Lissina O, Lambris JD, et al. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J Immunol. 2011;187:4913–4919. doi: 10.4049/jimmunol.1101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Cathepsin G. Methods Enzymol. 1981;80(Pt. C):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323–2330. [PubMed] [Google Scholar]
- Brown SJ, Askenase PW. Blood eosinophil and basophil responses in guinea pigs parasitized by Amblyomma americanum ticks. Am J Trop Med Hyg. 1982;31:593–598. doi: 10.4269/ajtmh.1982.31.593. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol. 1982;129:790–796. [PubMed] [Google Scholar]
- Brown SJ, Worms MJ, Askenase PW. Rhipicephalus appendiculatus: larval feeding sites in guinea pigs actively sensitized and receiving immune serum. Exp Parasitol. 1983;55:111–120. doi: 10.1016/0014-4894(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Carrell R, Qi X, Zhou A. Serpins as hormone carriers: modulation of release. Methods Enzymol. 2011;501:89–103. doi: 10.1016/B978-0-12-385950-1.00006-7. [DOI] [PubMed] [Google Scholar]
- de Castro JJ. Sustainable tick and tick-borne diseases control in livestock improvement in developing countries. Vet Parasitol. 1997;7:77–97. doi: 10.1016/s0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 issecreted into tick saliva during tick feeding. J Exp Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011;117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelar J, Calvo E, Pedra JH, Francischetti IM, Kotsyfakis M. Tick salivary secretion as a source of anti-hemostatics. J Proteomics. 2012;75:3842–3854. doi: 10.1016/j.jprot.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Decrem Y, Rath G, Blasioli V, Cauchie P, Robert S, Beaufays J, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, et al. Ticks produce highly selective chemokine binding proteins with anti-inflammatory activity. J Exp Med. 2008;205:2019–2031. doi: 10.1084/jem.20072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay MS. Insect hemolymph clotting. Cell Moll Life Sci. 2009;66:2643–2650. doi: 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Fish D, Childs JE. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: background and rationale. Vector Borne Zoonotic Dis. 2009;9:357–364. doi: 10.1089/vbz.2009.0022. [DOI] [PubMed] [Google Scholar]
- Gao X, Shi L, Zhou Y, Cao J, Zhang H, Zhou J. Characterization of the anticoagulant protein Rhipilin-1 from the Rhipicephalus haemaphysaloides tick. J Insect Physiol. 2011;57:339–343. doi: 10.1016/j.jinsphys.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Garcia ES, Machado EM, Azambuja P. Inhibition of hemocyte micro-aggregation reactions in Rhodnius prolixus larvae orally infected with Trypanosoma rangeli. Exp Parasitol. 2004;107:31–38. doi: 10.1016/j.exppara.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Gettins PGW. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4803. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Gillet L, Schroeder H, Mast J, Thirion M, Renauld JC, Dewals B, et al. Anchoring tick salivary anti-complement proteins IRAC I and IRAC II to membrane increases their immunogenicity. Vet Res. 2009;40:51. doi: 10.1051/vetres/2009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet Parasitol. 2009;160:1–12. doi: 10.1016/j.vetpar.2008.10.089. [DOI] [PubMed] [Google Scholar]
- Gratz N, editor. Vector-and Rodent-Borne Diseases in Europe and North America. Cambridge University Press; Cambridge: 2006. Tick-borne disease of the USA and Canada; pp. 185–188. [Google Scholar]
- Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000;96:3049–3055. [PubMed] [Google Scholar]
- Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm Venereol. 2011;91:644–650. doi: 10.2340/00015555-1197. Review. [DOI] [PubMed] [Google Scholar]
- Herrera-Mendez CH, Becila S, Blanchet X, Pelissier P, Delourme D, Coulis G, et al. Inhibition of human initiator caspase 8 and effector caspase 3 by cross-class inhibitory bovSERPINA3-1 and A3-3. FEBS Lett. 2009;583:2743–2748. doi: 10.1016/j.febslet.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Heutinck KM, ten Berge IJ, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47:943–955. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Huang X, Swanson R, Broze GJ, Jr, Olson ST. Kinetic characterization of the protein Z-dependent protease inhibitor reaction with blood coagulation factor Xa. J Biol Chem. 2008;283:29770–29783. doi: 10.1074/jbc.M805214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington JA. Shape-shifting serpins – advantages of a mobile mechanism. Trends Biochem Sci. 2006;31:427–435. doi: 10.1016/j.tibs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Hwang SR, Steineckert B, Toneff T, Bundey R, Logvinova AV, Goldsmith P, et al. The novel serpin endopin 2 demonstrates cross-class inhibition of papain and elastase: localization of endopin 2 to regulated secretory vesicles of neuroendocrine chromaffin cells. Biochemistry. 2002;41:10397–10405. doi: 10.1021/bi020088o. [DOI] [PubMed] [Google Scholar]
- Imamura S, Junior I, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. 2005;23:1301–1311. doi: 10.1016/j.vaccine.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Imamura S, Namangala B, Tajima T, Tembo ME, Yasuda J, Ohashi K, et al. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine. 2006;24:2230–2237. doi: 10.1016/j.vaccine.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Imamura S, Konnai S, Vaz Ida S, Yamada S, Nakajima C, Ito Y, et al. Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus. Jpn J Vet Res. 2008;56:85–98. [PubMed] [Google Scholar]
- Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect Dis. 2001;183:1810–1814. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- Jevnikar Z, Mirković B, Fonović UP, Zidar N, Svajger U, Kos J. Three-dimensional invasion of macrophages is mediated by cysteine cathepsins in protrusive podosomes. Eur J Immunol. 2012;42:3429–3441. doi: 10.1002/eji.201242610. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S, Kaewhom P, Pumhom P, Canales M, Fuente J, Stich RW. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound Emerg Dis. 2010;57:103–106. doi: 10.1111/j.1865-1682.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S1–S12. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kanaji S, Tanaka Y, Sakata Y, Takeshita K, Arima K, Ohta S, et al. Squamous cell carcinoma antigen 1 is an inhibitor of parasite-derived cysteine proteases. FEBS Lett. 2007;581:4260–4264. doi: 10.1016/j.febslet.2007.07.072. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS ONE. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimírová M, Jancinová V, Petríková M, Takác P, Labuda M, Nosál R. An inhibitor of thrombin-stimulated blood platelet aggregation from the salivary glands of the hard tick Amblyomma variegatum (Acari: Ixodidae) Exp Appl Acarol. 2002;28:97–105. doi: 10.1023/a:1025398100044. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Dau T, Jenne DE. Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med (Berl) 2011;89:23–28. doi: 10.1007/s00109-010-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol EF, Olszewski M. Basophils and mast cells: underdog in immune regulation? Immunol Lett. 2011;138:28–31. doi: 10.1016/j.imlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Kojouharova M, Reid K, Gadjeva M. New insights into the molecular mechanisms of classical complement activation. Mol Immunol. 2010;47:2154–2160. doi: 10.1016/j.molimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- Kovářová Z, Chmelar J, Sanda M, Brynda J, Mareš M, Rezáčová P. Crystallization and diffraction analysis of the serpin IRS-2 from the hard tick Ixodes ricinus. Acta Crystallograph Sect F Struct Biol Cryst Commun. 2010;66:1453–1457. doi: 10.1107/S1744309110032343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R, Takeuchi H, Jonczy J, Rees HH, Turner PC. A thrombin inhibitor from the ixodid tick, Amblyomma hebraeum. Gene. 2004;342:243–249. doi: 10.1016/j.gene.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Lefkowitz JB. Coagulation Pathway and Physiology. JB Lippincott Co; Philadelphia, PA: 2006. [Google Scholar]
- Liao M, Zhou J, Gong H, Boldbaatar D, Shirafuji R, Battur B, et al. Hemalin, a thrombin inhibitor isolated from a midgut cDNA library from the hard tick Haemaphysalis longicornis. J Insect Physiol. 2009;55:164–173. doi: 10.1016/j.jinsphys.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lima e Silva MF, Szabó MP, Bechara GH. Microscopic features of tick-bite lesions in anteaters and armadillos: Emas National Park and the Pantanal region of Brazil. Ann N Y Acad Sci. 2004;1026:235–241. doi: 10.1196/annals.1307.036. [DOI] [PubMed] [Google Scholar]
- Mameri H, Denery-Papini S, Pietri M, Tranquet O, Larré C, Drouet M, et al. Molecular and immunological characterization of wheat Serpin (Tri a 33) Mol Nutr Food Res. 2012;56:1874–1883. doi: 10.1002/mnfr.201200244. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Louw AI, Neitz AW. Disaggregation of aggregated platelets by savignygrin, a alphaIIbeta3 antagonist from Ornithodoros savignyi. Exp Appl Acarol. 2002;27:231–239. doi: 10.1023/a:1021613001297. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Louw AI, Neitz AW. The influence of tick behavior, biotope and host specificity on concerted evolution of the platelet aggregation inhibitor savignygrin, from the soft tick Ornithodoros savignyi. Insect Biochem Mol Biol. 2003;33:623–629. doi: 10.1016/s0965-1748(03)00047-x. [DOI] [PubMed] [Google Scholar]
- Masumoto K, Sakata Y, Arima K, Nakao I, Izuhara K. Inhibitory mechanism of a cross-class serpin, the squamous cell carcinoma antigen 1. J Biol Chem. 2003;278:45296–45304. doi: 10.1074/jbc.M307741200. [DOI] [PubMed] [Google Scholar]
- Michaelis L, Menten ML, Johnson KA, Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickowska B. Purification and characterization of alpha (1)-proteinase inhibitor and antithrombin III: major serpins of rainbow trout (Oncorhynchuss mykiss) and carp (Cyprinus carpio) blood plasma. Fish Physiol Biochem. 2009;35:231–240. doi: 10.1007/s10695-008-9204-7. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugino M, Nakajima M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J Vet Med Sci. 2001;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Tsuda A, Onuma M, Sugimoto C. Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization. Insect Biochem Mol Biol. 2003;33:267–276. doi: 10.1016/s0965-1748(02)00240-0. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Blandon MA. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J Exp Biol. 2007;210:3188–3198. doi: 10.1242/jeb.006494. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics. 2009;10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa S, Oda Y, Kamiya M, Kamijima T, Aizawa T, Clark KD, et al. A novel peptide mediates aggregation and migration of hemocytes from an insect. Curr Biol. 2009;19:779–785. doi: 10.1016/j.cub.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Nazareth RA, Tomaz LS, Ortiz-Costa S, Atella GC, Ribeiro JM, Francischetti IM, et al. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Sonenshine DE, Lane RS, Uilenberg G. Ticks (Ixodida) In: Mullen GR, Durden LA, editors. Medical and Veterinary Entomology. 2. Academic Press; San Diego, CA: 2009. pp. 493–541. [Google Scholar]
- Nosek J, Rajcáni J, Kozuch O. Reaction of the host to the tick bite III. The bite of viruliferous Ixodes ricinus female. Zentralbl Bakteriol Orig A. 1978;242:141–147. [PubMed] [Google Scholar]
- Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105 (Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, et al. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J Biol Chem. 2006;281:26361–22639. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine. 2007;25:3284–3292. doi: 10.1016/j.vaccine.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, et al. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 2009;276:3235–3246. doi: 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost. 2007;5 (Suppl 1):102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck J, Jr, Berger M, Marks FS, Zingali RB, Canal CW, Ferreira CA, et al. Pharmacological action of tick saliva upon haemostasis and the neutralization ability of sera from repeatedly infested hosts. Parasitol. 2009;136:1339–1349. doi: 10.1017/S0031182009990618. [DOI] [PubMed] [Google Scholar]
- Rezaie AR, Manithody C, Yang L. Identification of factor Xa residues critical for interaction with protein Z-dependent protease inhibitor: both active site and exosite interactions are required for inhibition. J Biol Chem. 2005;280:32722–32728. doi: 10.1074/jbc.M505517200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64:347–353. doi: 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985;161:332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Valle M, Vance M, Moolhuijzen PM, Tao X, Lew-Tabor AE. Differential recognition by tick-resistant cattle of the recombinantly expressed Rhipicephalus microplus serine protease inhibitor-3 (RMS-3) Ticks Tick Borne Dis. 2012;3:159–169. doi: 10.1016/j.ttbdis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Schechter NM, Plotnick MI. Measurement of the kinetic parameters mediating protease-serpin inhibition. Methods. 2004;32:159–168. doi: 10.1016/s1046-2023(03)00207-x. [DOI] [PubMed] [Google Scholar]
- Schick C, Pemberton PA, Shi GP, Kamachi Y, Cataltepe S, Bartuski AJ, et al. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry. 1998;37:5258–5266. doi: 10.1021/bi972521d. [DOI] [PubMed] [Google Scholar]
- Schleger AV, Lincoln DT, McKenna RV, Kemp DH, Roberts JA. Boophilus microplus: cellular responses to larval attachment and their relationship to host resistance. Aust J Biol Sci. 1976;29:499–512. doi: 10.1071/bi9760499. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9:247–250. doi: 10.1016/j.micinf.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe. 2011;10:136–146. doi: 10.1016/j.chom.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–177. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Oxford University Press; Oxford: 1993. [Google Scholar]
- Sonenshine DE, Bissinger BW, Egekwu N, Donohue KV, Khalil SM, Roe RM. First transcriptome of the testis-vas deferens-male accessory gland and proteome of the spermatophore from Dermacentor variabilis (Acari: Ixodidae) PLoS ONE. 2011;6:e24711. doi: 10.1371/journal.pone.0024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino M, Imamura S, Mulenga A, Nakajima M, Tsuda A, Ohashi K, et al. Serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine. 2003;21:2844–2851. doi: 10.1016/s0264-410x(03)00167-1. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu Z, Hayashi Y, Peters C, Tsuda M, Inoue K, et al. Microglial Cathepsin B Contributes to the Initiation of Peripheral Inflammation-Induced Chronic Pain. J Neurosci. 2012;32:11330–11342. doi: 10.1523/JNEUROSCI.0677-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- Szabó MP, Bechara GH. Sequential histopathology at the Rhipicephalus sanguineus tick-feeding site on dogs and guinea pigs. Exp Appl Acarol. 1999;23:915–928. doi: 10.1023/a:1006347200373. [DOI] [PubMed] [Google Scholar]
- Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- Ushio H, Hirota S, Jippo T, Higuchi S, Kawamoto K, Kitamura Y, et al. Mechanisms of eosinophilia in mice infested with larval Haemaphysalis longicornis ticks. Immunology. 1995;84:469–475. [PMC free article] [PubMed] [Google Scholar]
- Vancová I, Hajnická V, Slovák M, Nuttall PA. Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Vet Parasitol. 2010;167:274–278. doi: 10.1016/j.vetpar.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Vendramini-Costa DB, Carvalho JE. Molecular Link Mechanisms between Inflammation and Cancer. Curr Pharm Des. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bowman AS, Brigham DL, Essenberg RC, Dillwith JW, Sauer JR. Isolation and characterization of americanin, a specific inhibitor of thrombin, from the salivary glands of the lone star tick Amblyomma americanum (L.) Exp Parasitol. 1997a;87:30–38. doi: 10.1006/expr.1997.4175. [DOI] [PubMed] [Google Scholar]
- Zhu K, Sauer JR, Bowman AS, Dillwith JW. Identification and characterization of anticoagulant activities in the saliva of the lone star tick, Amblyomma americanum (L.) J Parasitol. 1997b;83:38–43. [PubMed] [Google Scholar]