Abstract
Objectives
To develop and test a method for measuring the relationship between the rise in intra-abdominal pressure and sagittal plane movements of the anterior and posterior vaginal walls during Valsalva in a pilot sample of women with and without prolapse.
Methods
Mid-sagittal MRI images were obtained during Valsalva while changes in intra-abdominal pressure were measured via a bladder catheter in 5 women with cystocele, 5 women with rectocele, and 5 controls. The regional compliance of the anterior and posterior vagina wall support systems were estimated from the ratio of displacement (mm) of equidistant points along the anterior and posterior vaginal walls to intra-abdominal pressure rise (mmHg).
Results
The compliance of both anterior and posterior vaginal wall support systems varied along different regions of vaginal wall for all three groups, with the highest compliance found near the vaginal apex and the lowest near the introitus. Women with cystocele had more compliant anterior and posterior vaginal wall support systems than women with rectocele. The movement direction differs between cystocele and rectocele. In cystocele, the anterior vaginal wall moves mostly toward the vaginal orifice in the upper vagina, but in a ventral direction in the lower vagina. In rectocele, the direction of the posterior vaginal wall movement is generally toward the vaginal orifice.
Conclusions
Movement of the vaginal wall and compliance of its support is quantifiable and was found to vary along the length of the vagina. Compliance was greatest in the upper vagina of all groups. Women with cystocele demonstrated the most compliant vaginal wall support.
Keywords: Vaginal wall support, Compliance, MR imaging, Prolapse, Cystocele, Rectocele
Introduction
Pelvic organ prolapse affects 24 % of American women [1] and results in 200,000 surgical repairs each year in the United States [2], and of these women 25 to 29 % will undergo re-operation [3, 4]. Thus, a better understanding of the pathomechanics of pelvic floor support in women with prolapse would help to provide insights that might lead to improved surgical success rates.
Excessive caudal movement of the pelvic organs is the sine qua non of pelvic organ prolapse. In simple terms, for a given load, one might guess that excessive movement is caused by a loss of support to one (or both) vaginal walls. This loss of support impairs the ability of that support to resist caudal forces that include gravity, inertial forces, and intra-abdominal pressure, without incurring excessive movement. In the biomechanics field, compliance describes the flexibility or pliability of an object or structure; it is defined as the deformation of the structure divided by the change in force that caused the deformation. If the deformation increases for the same change of force, then the compliance is increased. The portion of the vagina or uterus that prolapses determines how it is classified and determines treatment in the clinic. Experienced clinicians know that the pattern of uterovaginal prolapse in each woman is unique and our understanding is based on the hypothesis that different locations of injury determine different types of prolapse [5]. The POP-Q system, which allows quantification of the location of different points along the vaginal wall, is helpful in objectively quantifying pelvic organ prolapse. However, it only tracks a few locations on the vaginal walls. Furthermore, because the hymen, which is used as the reference landmark, also moves caudally with Valsalva, the POP-Q can underestimate the true movement of the vaginal walls during Valsalva because all measurements are made relative to that moving landmark. Thus, a measurement technique that quantifies the direction and magnitude of vaginal wall movement under known loading conditions might help advance our understanding of the mechanisms that underlie the changes in vaginal wall movement and support in women with prolapse.
The goal of this study was to develop a measurement technique to evaluate the regional compliance of the anterior and posterior vaginal wall support system in vivo. We then wanted to test proof of concept in a pilot sample of subjects to demonstrate the feasibility of testing hypotheses regarding regional compliance of vaginal support. To evaluate the capability of this system we used it to test the null hypotheses that compliance of anterior vaginal support in women with cystocele is similar to the compliance of posterior vaginal support in women with rectocele, and that compliance of the nonprolapsed compartment in women with cystocele and rectocele are the same as in women with normal support. Furthermore, we sought to quantify the trajectory of the anterior and posterior vaginal walls in women with cystocele, rectocele and in women with normal support during a Valsalva maneuver.
Materials and methods
Magnetic resonance images (MRI) from 15 women (5 with cystocele-predominant prolapse, 5 with rectocele-predominant prolapse, and 5 controls; Table 1) were selected from an ongoing University of Michigan institutional review board-approved (IRB #1999-0395) case–control study of pelvic organ prolapse. All subjects were symptomatic, not pregnant, had not undergone previous hysterectomy or other surgery for pelvic floor disorders,, were able to perform a satisfactory Valsalva maneuver, and had a Ba or Bp pelvic organ prolapse quantification (POP-Q) value at least 1 cm beyond the hymenal ring on clinical examination. The subjects with cystocele-predominant prolapse had the anterior vaginal wall extend at least 1 cm lower than the most dependent part of the posterior wall or cervix and vice versa for rectocele-predominant cases. Women in whom the cervix was the leading point of prolapse were excluded. All women in the control group were recruited by newspaper and radio advertisement for healthy volunteers who were asymptomatic based on Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaires, not pregnant, had negative full-bladder stress tests, had not had previous surgery for pelvic floor disorders, and were able to perform a satisfactory Valsalva maneuver with all points of the vaginal wall at least 1 cm above the hymenal ring.
Table 1.
Demographic and (mean ± SD) POP-Q information for all subjects
| Demographic | AV Prolapse N=5 | Control N=5 | PV Prolapse N=5 |
|---|---|---|---|
| Age (years) | 53.6±12.2 | 60.4±11.3 | 60.2±9.3 |
| Height (cm) | 164.1±6.9 | 165.6±9.1 | 161.0±7.4 |
| BMI (kg/m2) | 25.8±3.6 | 23.4±3.8 | 27.8±6.7 |
| Parity | 2.8±0.8 | 2.4±1.1 | 2.4±0.9 |
| Aa (cm) | 1.2±1.1 | −1.9±0.5 | −1.4±0.9 |
| Ba (cm) | 2.2±1.6 | −1.9±0.5 | −1.4±0.9 |
| C (cm) | −2.8±2.8 | −7.3±2.6 | −5.4±1.1 |
| D (cm) | −7.4±1.1 | −9.5±2.7 | −7.2±0.8 |
| Ap (cm) | −0.6±1.7 | −2.3±0.4 | 1.4±0.5 |
| Bp (cm) | −0.4±1.8 | −2.3±0.4 | 1.4±0.5 |
| Max POP-Q (cm) | 2.2±1.6 | −0.9±0.5 | 1.4±0.5 |
MR imaging technique
In preparation for imaging, a 12-Fr Foley catheter was placed in the bladder and connected to an MRI-compatible pressure monitoring device (Datascope, Montvale, NJ, USA) to measure intra-abdominal pressure. The use of a Foley catheter to measure intra-abdominal pressure has previously been validated against other intra-abdominal pressure devices [6]. Ultrasound gel was placed in the vagina prior to scanning to facilitate visualization of the vaginal walls on MR images. With subjects in the supine position, dynamic 3-T mid-sagittal MRIs (Philips Medical Systems, The Netherlands) were obtained during Valsalva using a six-channel, torso, phased array coil. Images were acquired approximately every 1.4 s over 30 s using a single-shot turbo spin echo sequence (TR, approximately 1,300 ms; TE, 105 ms; slice thickness, 6 mm; field of view 34 cm; matrix, 256×90; and 1 NSA). Twenty images were obtained per scan. Patients were coached to gradually increase their abdominal pressure by a technician who instructed them to push minimally, moderately, and maximally while relaxing their pelvic floor muscles to allow for a graded Valsalva response. Scans were repeated at least three times for each individual and the images compared with clinical POP-Q examination data to ensure that the maximal extent of the prolapse seen during pelvic examination was reproduced in the scanner [7]. Images were excluded if they were of poor quality.
MR image analysis to determine vector displacement and compliance
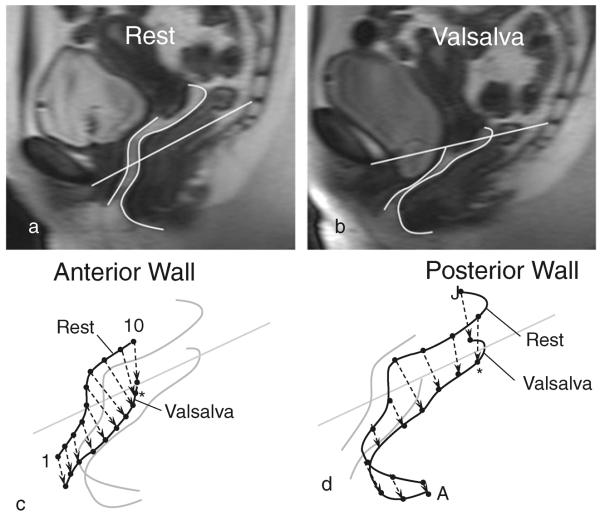
The Valsalva attempt that resulted in the greatest descent of the vaginal wall using a multiple push strategy was selected for analysis. The anterior vaginal wall (AVW) and posterior vaginal wall (PVW) were traced onto each MR image. On the posterior wall, the tracing was continued over the perineal body as far as the anterior margin of the external anal sphincter muscle, so that movement of this portion of the posterior supports could be evaluated. A reference line connecting the origin at the inferior pubic point and the sacro-coccygeal junction (SCIPP line) was used to align images from all subjects to correct for differences in pelvic inclination that arise from patients varying their positioning in the scanner. A custom program was developed using MATLAB v7.0 (MathWorks Natick, MA, USA) to analyze the displacement of the vaginal wall and compliance of the vaginal wall support system relative to the reference line. All anterior and posterior vaginal wall tracings were aligned using the SCIPP line and then 10 equally-spaced virtual landmarks were placed on each scan of a vaginal wall, from rest to maximum Valsalva. The magnitude and direction of the displacement of those landmarks were calculated for each frame during the Valsalva maneuver. Displacement vectors then represented the displacement for each location along the anterior and posterior vaginal wall from rest to maximal Valsalva (Fig. 1). We designated the vaginal wall locations with numbers 1 through 10 for the anterior vaginal wall and letters A through J for the posterior wall, because the two walls have different lengths and these locations therefore occur at different points along the length of the vaginal canal.
Fig. 1.
MRI of a normal subject with tracing of the inferior pubic point and the sacro-coccygeal junction (SCIPP) line of the anterior vaginal wall (AVW) and posterior vaginal wall (PVW) Panel a at rest and Panel b at maximum Valsalva. Derivation of the displacement direction for the Panel c anterior and Panel d posterior wall. Asterisks represent points 9 and I respectively, which are the points used to demonstrate the calculation of vaginal support compliance in Fig. 2
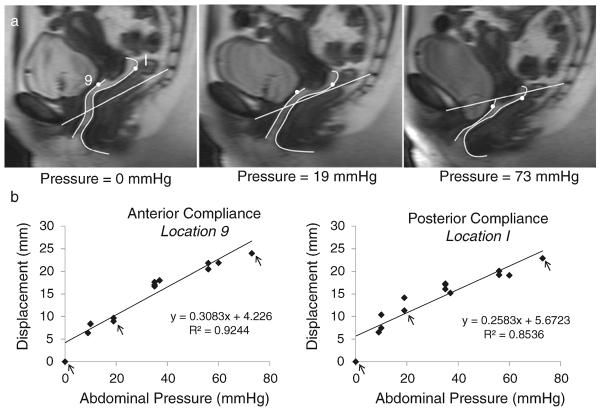
The strategy for assessing vaginal wall support compliance is shown for sample points on the anterior and posterior vaginal walls in Fig. 2. The intra-abdominal pressure for each MR image was determined by synchronizing dynamic MR images with bladder catheter pressure readings. To determine the localized compliance of the anterior and posterior vaginal wall support system at each of the 10 locations for each subject, the abdominal pressure and vaginal wall displacement were plotted and a best-fit line was obtained by using linear regression. The regression line slope is a measure of compliance. We have chosen to report compliance rather than its inverse, stiffness, because a larger value reflects greater movement. Repeated measures ANOVA, post hoc multiple comparisons, and standard t test were performed using SPSS 12.0 to test for differences in compliance among the three different subject groups by vaginal wall location.
Fig. 2.
Quantification of vaginal wall support compliance. Panel a, sequential MR images from a single individual with normal anatomy were taken with pressure ranging from rest to maximum Valsalva. The SCIPP line was drawn and anterior and posterior vaginal walls were traced. Panel b, example of the calculation of the anterior and posterior vaginal wall support compliance using points 9 (AVW) and I (PVW) on the vaginal wall. A linear relationship between the vaginal wall displacement and the intra-abdominal pressure was found. Arrows in the plots indicate the example shown in panel a, pointing out the measurements of points 9 (AVW) and I (PVW) on the selected MR images. In these examples, the anterior wall support compliance and the posterior wall support compliance at these points were 0.308 mm/mmHg and 0.258 mm/mmHg respectively
Results
Patient population
The demographics of subjects in the control, cystocele, and rectocele groups were similar (Table 1). There were no statistically significant differences among the three groups. All subjects were Caucasian, and their average parity was 2.5. The maximum POP-Q of the AV prolapse group and the PV prolapse group were not statistically significantly different (p=0.33; Table 1).
Compliance of the vaginal wall support system
The average compliance of the anterior and posterior vaginal wall support systems in women with normal support, women with cystocele, and women with rectocele is shown in Table 2, calculated by averaging the compliance values for each of the 10 locations on each wall. In general, women with cystocele had the most compliant anterior vaginal wall support systems (P=0.02). For the posterior vaginal wall, there was a trend toward posterior vaginal wall support in women with rectocele being more compliant than in women with normal support, but less compliant than in women with cystocele, although this did not reach statistical significance at this sample size (P=0.076). The compliance of anterior vaginal wall support in cystocele is significantly larger than the compliance of posterior vaginal wall support in rectocele (P<0.001). The nonprolapse wall in cystocele (P<0.001) and rectocele (P=0.045) is significantly more compliant than that of women with normal support. Therefore, we rejected our primary and secondary null hypotheses.
Table 2.
Mean ± SD compliance of vaginal wall support system in women with normal support, cystocele, and rectocele
| Compliance (mm/mmHg) | Normal (n=5) | Cystocele (n=5) | Rectocele (n=5) |
|---|---|---|---|
| Anterior wall support | 0.33±0.02 | 0.70±0.05 | 0.36±0.02 |
| Posterior wall support | 0.29±0.03 | 0.58±0.03 | 0.40±0.02 |
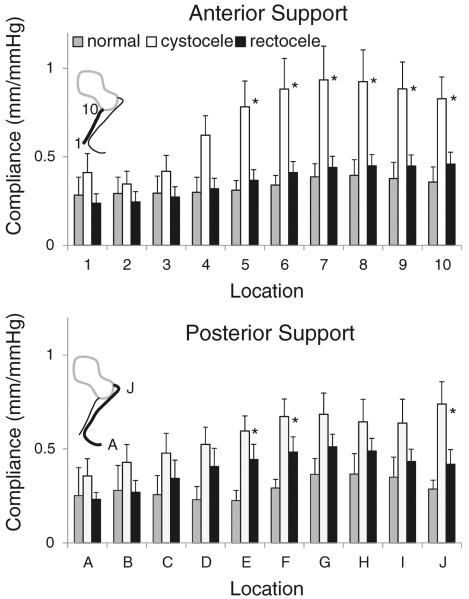
There were regional differences in compliance (Fig. 3). Repeated ANOVA analysis showed that location was a significant factor for differences in regional compliance of AVW support (P<0.001) and PVW support (P=0.001), that is, compliance differed among the 10 locations on the anterior vaginal wall and also the posterior vaginal wall, indicating that there was a different compliance at different locations. Each of the three groups studied demonstrated these differences in the regional compliance of both anterior and posterior vaginal wall support systems. The regional compliance was highest near the vaginal apex and lowest near the introitus. Anterior wall support in women with cystocele had the highest compliance in the upper third of the vagina at location 7 (0.93 mm/mmHg) and the lowest compliance in the lower third of the vagina at location 2 (0.35 mm/mmHg); this constitutes a 2.7-fold difference between the points of minimum and maximum compliance. The posterior vaginal wall support of women with rectocele was also most compliant in the upper third at location G (0.51 mm/mmHg) and least compliant in the lower third at location A (0.23 mm/mmHg); this constitutes a 2.2-fold difference between the points of minimum and maximum compliance. The differences in compliance among the three groups were largest near the apex.
Fig. 3.
Mean regional compliance (mm/mmHg) measured at the 10 locations along the anterior (top) and posterior wall (bottom). Error bars represent standard error. Light gray denotes women with normal support, white denotes women with anterior wall-predominant prolapse, and black denotes women with posterior wall-predominant prolapse. Asterisks show the locations at which the compliance among groups is significantly different (p<0.05)
Direction and magnitude of vaginal wall displacement
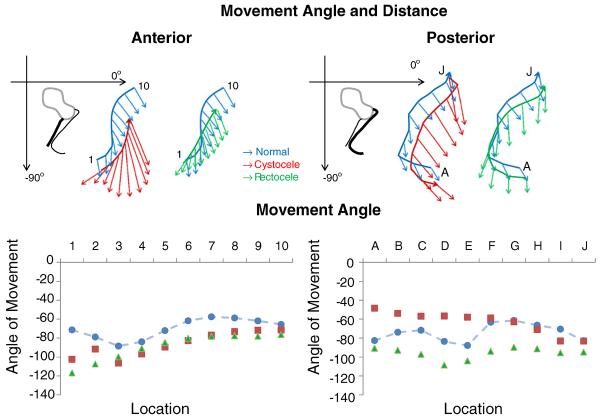
The vaginal wall displacement direction differed among the three groups and along the length of the vagina (Fig. 4). In both women with cystocele and women with rectocele, the vaginal walls start below those in the women with normal support. In women with normal support (blue), the movement angle for the upper portions of the anterior and posterior vaginal walls is posterior toward the rectum and slightly caudal, while the lower portions of the anterior and posterior walls move mostly caudally with Valsalva. In women with cystocele (red) the entire anterior vaginal wall exhibits a movement angle that is more of a caudal translation than the anterior vaginal wall of controls. Additionally, from wall locations 1–5, the anterior vaginal wall moves ventrally, while it moves caudally and slightly dorsally in the controls. The posterior wall of women with rectocele (green) also exhibits a movement direction that is more caudal translation and less dorsal movement than in the controls. Also, in women with rectocele, the middle section of the posterior vaginal wall begins to move ventrally, but much of the movement of the lower third of the posterior vaginal wall is caudad, which is different from that in normal women and women with cystocele. Surprisingly, in women with rectocele and in women with cystocele, the anterior vaginal wall has a similar direction of movement.
Fig. 4.
Top Direction and magnitude (mm) of vaginal wall displacement of the anterior and posterior support system. Bottom Angle of displacement (degrees from the horizontal) in normal women, women with cystocele, and women with rectocele
The average displacement of the vaginal apex in the cystocele and rectocele groups was similar. The average displacement of the anterior fornix was 15 mm in subjects with normal support, 30 mm in subjects with rectocele, and 39 mm in subjects with cystocele. The average displacement ranged from 10.9 to 18.2 mm in normal subjects, 17.5 to 42.6 mm in subjects with cystocele, and 15.3 to 30.2 mm in subjects with rectocele.
Discussion
This study reports the development of a novel in vivo method of quantifying the regional variations in vaginal wall movements, as well as the compliance of the vaginal wall support system based on dynamic MRI analysis during Valsalva. The feasibility of this technique is demonstrated in a pilot study of women with cystocele, rectocele, and normal support allowing the comparisons of both anterior and posterior vaginal wall measurements.
The difference between displacement and compliance is critical when considering the magnitude of pressure change causing a vaginal wall displacement. While displacement describes only movement, compliance describes the amount of movement produced by a given pressure, which reflects a structural property. This technique is useful because it allows the geometric compliance of a system to be assessed that contains different tissues, including muscle, connective tissue and viscera; as such, the compliance represents the integrity of a system, rather than a single structure. This technique should permit a better understanding of structural failure in pelvic floor support when it occurs, because failure would naturally give rise to a large localized or regional compliance value.
As expected the overall compliance of anterior and posterior vaginal wall support systems is different for women with cystocele, rectocele, and normal support, as expected, and our technique allows the location, magnitude, and direction of these differences to be measured. Importantly, our data demonstrate that there is a great deal of vaginal wall compliance in normal women during a Valsalva. This emphasizes that the goal of operative management should be to restore normal vaginal wall movement, not render the vagina immobile. Women with cystocele had the greatest compliance in the anterior and posterior walls of all groups. At first, it seemed counterintuitive that in women with cystocele, the posterior vaginal wall compliance should be greater than that for rectocele. However, on further reflection, this is plausible. The anterior wall moves backward and pushes the posterior wall with it. Because of the great degree of vaginal movement in cystocele, compliance of the posterior vaginal wall is greater.
Even though it is not yet possible to make specific inferences about which anatomical structure is responsible for the detected differences, a strength of the present method is that it is able to determine the location along the vaginal wall where a change of support compliance has occurred. For example, this pilot study demonstrated the locations at which women with cystocele and rectocele differ from women with normal support. Furthermore, we notice that the compliance of the anterior vaginal support system varies along both the anterior and the posterior vaginal walls. In general, vaginal wall support was most compliant near the apex and least compliant near the introitus in all groups. This is somewhat in contrast to a clinician's impression that the distal vaginal movement is most obvious because the upper vagina is not always apparent on clinical examination because it is hidden within the pelvis or distorted by the introduction of a speculum.
Vaginal wall movement direction is a key factor in whether a cystocele or rectocele is present. All groups exhibited some general caudal displacement; however, women with cystocele and rectocele had more motion. Some of the general dorsal and caudal movement is probably related to displacement of the levator plate by the intra-abdominal pressure rise in women with levator impairment. The levator ani muscle creates a sling that wraps around the rectum, vagina, and bladder neck, and is in a state of tonic contraction to maintain continence and keep the uterus elevated. The muscle may relax for a short period of time without sequelae, but if the muscle is permanently damaged, other structures that support these organs may start to fail, causing incontinence and prolapse [8]. The levator ani runs perpendicular to the axis of the vagina; thus, it makes sense that women with weak or damaged muscles would have more caudal translation than women with intact muscles, which was demonstrated in our study.
This study extends the existing literature on the biomechanics of pelvic organ prolapse in three ways. It is an in vivo test method with the capability of quantitatively evaluating the overall vaginal support system rather than a single structure at different regions along the vagina axis. Hsu et al. [9] demonstrated that overall compliance of a single point on the anterior vaginal support system in vivo is 67 % more compliant in women with anterior wall prolapse than it is in normal women, but little is known regarding posterior vaginal wall support in women with rectocele and whether the compliance is the same at the apex and the distal end of the vagina. In the present study we extend the results of Hsu et al. by evaluating the full length of both the anterior and posterior vaginal wall support system in women with cystocele, rectocele, and normal support. Our findings indicate that women with cystocele have the most compliant anterior and posterior vaginal wall support systems of the three groups. Also, we found that the apical portion of the vagina support system has greater compliance than the distal portion. Previous MR imaging and modeling studies demonstrated the ability to study vaginal wall deformation at maximum Valsalva loading in living women with cystocele and rectocele [10–12]. The present study extends their work on the qualitative changes in vaginal wall location by providing the first quantitative measurements of the movements during Valsalva. This complements data obtained regarding specific structures involved in support. Cosson et al. [13, 14] have used in vitro testing methods such as a uniaxial tensiometer to test the properties of pelvic floor tissue biopsy samples and found large variations in the rupture strength and elongation prior to rupture both in cadaver pelvic ligaments and in vaginal tissue from prolapse patients. These data are important to the understanding of the tissue properties. Whether or not the loading condition is likely beyond the range of stress or strain experienced during everyday activities remains to be clarified, and the testing sample condition, such as hydration, would have a significant impact on the testing result [15]. Epstein et al. [16] used a vaginal suction device in vivo and found women with prolapse to have more extensible lateral vaginal skin and a lower stiffness index compared with age-matched controls. While this overcomes some of the difficulties with in vitro testing, it only tests a relatively small area of the vagina. Testing the entire support system takes into account the complicated interactions among the various connective tissue components such as the ligaments and the vaginal wall properties. Our in vivo technique can complement existing methods in order to better understand the vaginal support system.
There are several factors that are important to keep in mind when interpreting the results of this study. Its primary purpose was to develop a technique and to evaluate the feasibility of using it in different subject groups. These preliminary data could then be used for sample size calculations in the future. Even with a small sample of 15 women, AVP and PVP exhibited characteristic displacements and we were able to detect statistically significant differences in direction of the movement and compliance. However, larger studies are needed to assess trends that were not statistically significant at this sample size and to represent a full spectrum of the structural abnormalities seen in prolapse. For example, no enteroceles were included in this first analysis because they are rare with the uterus in situ. Second, it should be noted that our displacement measurements were made in the mid-sagittal plane; therefore, we are not able to infer the 3D behavior of the system such as distinguishing between central and lateral defects. Also, as in our previous studies, supine MR images were obtained that may limit the descent of the pelvic floor, although clinical POPQ examination is performed in a supine position as well, and earlier studies do not document differences when compared with images obtained in the seated position in open MRI scanners [17, 18]. Furthermore, the regional vaginal wall movement is estimated by tracking equally spaced virtual landmarks placed on each scan of the vaginal wall. The anatomical landmarks reliably identifiable on MRI are limited to the most inferior points (1 at the distal vagina and A at the distal perineum) and most superior point (10 at the anterior vaginal fornix and J at the posterior vaginal fornix). Even though these equidistant virtual landmarks do not correspond with specific anatomical features, it is reasonable to assume a correspondence in successive MR frames as a first degree approximation. Third, intravesical pressure was used as a proxy for intra-abdominal pressure instead of measuring it directly. We chose to measure the pressure this way because it is much less invasive and it has been validated as a way of measuring intra-abdominal pressure [6, 19], while bladder volume has been held constant at 100 cm3 water in the study. Fourth, the ultrasound gel that was placed in the vagina may affect the shape of the vaginal wall. Because excess ultrasound gel was expelled, rather than redistributed, during Valsalva, we do not believe that it had a substantial influence on the compliance or movement calculations. All patients had ultrasound gel placed prior to examination; thus, this would not introduce bias by one group. Furthermore, our findings cannot be widely generalized because all of the individuals in our study group were Caucasian between the ages of 39 and 76 years. Last, this methodology is developed based on MRI measurements and ultrasound examination was not performed; the utility of the method on ultrasound images has not been tested.
This study serves as a pilot for a new method of determining localized displacement and compliance of the vaginal wall in vivo, and a basis on which a larger study can be conducted to verify that our results are representative of the changes associated with prolapse and not simply unique to this small sample. After these findings have been verified in a larger sample, the next step is to elucidate the mechanisms underlying the motions that we observed. While it is logical that defects in the levator ani muscle would cause the downward translation that we observed, this hypothesis has not been directly tested. For instance, with the use of virtual three-dimensional biomechanical models based on MR images [20], we can vary the amount of support given by the levator ani muscle and determine if the changes we observed in this study are replicated. Finding an explanation for the patterns of movement and differences in compliance will lead to a more complete understanding of the pathophysiology of prolapse, and could lead to improved surgical interventions. Our method will also allow for evaluation of vaginal wall movements after surgery, and could be used to quantitatively compare different operative strategies.
Acknowledgements
Funding This study is funded by: RO1 HD 38665, P50 HD 44406 and K12 HD004438. This work has been funded by NIH RO1 HD 38665, P50 HD 44406 and K12 HD004438. The University of Michigan has received payment in partial support for Dr. DeLancey and Dr. Ashton-Miller's salaries from American Medical Systems, Kimberly-Clark, and Proctor and Gamble not related to this research.
Footnotes
Conflicts of interest None.
References
- 1.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ. Pelvic Floor Disorders Network (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–115. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 4.Fialkow MF, Newton KM, Lentz GM, Weiss NS. Lifetime risk of surgical management of pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:437–440. doi: 10.1007/s00192-007-0459-9. [DOI] [PubMed] [Google Scholar]
- 5.Richardson AC, Lyons JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–573. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 6.Malbrain ML, De Laet I, Viaene D, Schoonheydt K, Dits H. In vitro validation of a novel method for continuous intra-abdominal pressure monitoring. Intensive Care Med. 2008;34:740–745. doi: 10.1007/s00134-007-0952-0. [DOI] [PubMed] [Google Scholar]
- 7.Tumbarello JA, Hsu Y, Lewicky-Gaupp C, Rohrer S, DeLancey JO. Do repetitive Valsalva maneuvers change maximum prolapse on dynamic MRI? Int Urogynecol J. 2010;21(10):1247–1251. doi: 10.1007/s00192-010-1178-1. doi:10.1007/s00192-010-1178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 9.Hsu Y, Chen L, Tumbarello J, Ashton-Miller JA, DeLancey JO. In vivo assessment of anterior compartment compliance and its relation to prolapse. Int Urogynecol J. 2010;21:1111–1115. doi: 10.1007/s00192-010-1154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J. 2010;21:1103–1109. doi: 10.1007/s00192-010-1161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson KA, Luo J, Guire KE, Chen L, Ashton-Miller JA, DeLancey JO. 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal, and apical defects. Int Urogynecol J. 2012;23(3):285–293. doi: 10.1007/s00192-011-1586-x. doi:10.1007/s00192-011-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Larson KA, Fenner DE, Ashton-Miller JA, DeLancey JO. Posterior vaginal prolapse shape and position changes at maximal Valsalva seen in 3-D MRI-based models. Int Urogynecol J. 2012;23(9):1301–1306. doi: 10.1007/s00192-012-1760-9. doi:10.1007/s00192-012-1760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosson M, Lambaudie E, Boukerrou M, Lobry P, Crépin G, Ego A. A biomechanical study of the strength of vaginal tissues results on 16 post-menopausal patients presenting with genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2004;112:201–205. doi: 10.1016/s0301-2115(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 14.Cosson M, Boukerrou M, Lacaze S, Lambaudie E, Fasel J, Mesdagh H, Lobry P, Ego A. A study of pelvic ligament strength. Eur J Obstet Gynecol Reprod Biol. 2007;109(1):80–87. doi: 10.1016/s0301-2115(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 15.Zimmern PE, Eberhart RC, Bhatt A. Methodology for biomechanical testing of fresh anterior wall vaginal samples from postmenopausal women undergoing cystocele repair. Neurourol Urodyn. 2009;28(4):325–329. doi: 10.1002/nau.20657. [DOI] [PubMed] [Google Scholar]
- 16.Epstein LB, Graham CA, Heit MH. Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. Am J Obstet Gynecol. 2007;197(2):165.e1–165.e6. doi: 10.1016/j.ajog.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223(2):501–508. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 18.Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR Am J Roentgenol. 1998;171(6):1607–1610. doi: 10.2214/ajr.171.6.9843296. [DOI] [PubMed] [Google Scholar]
- 19.Fusco MA, Martin RS, Chang MC. Estimation of intra-abdominal pressure by bladder pressure measurement: validity and methodology. J Trauma. 2001;50:297–302. doi: 10.1097/00005373-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Ashton-Miller JA, DeLancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42(10):1371–1377. doi: 10.1016/j.jbiomech.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]