Abstract
Historically understudied, cholesterol in the retina is receiving more attention now because of genetic studies showing that several cholesterol-related genes are risk factors for age-related macular degeneration (AMD) and because eye pathology studies showing high cholesterol content of drusen, aging Bruch's membrane, and newly found subretinal lesions. The challenge before us is determining how the cholesterol-AMD link is realized. Meeting this challenge will require an excellent understanding these genes’ roles in retinal physiology and how chorioretinal cholesterol is maintained. In the first half of this review, we will succinctly summarize physico-chemical properties of cholesterol, its distribution in the human body, general principles of maintenance and metabolism, and differences in cholesterol handling in human and mouse that impact on experimental approaches. This information will provide a backdrop to the second part of the review focusing on unique aspects of chorioretinal cholesterol homeostasis, aging in Bruch's membrane, cholesterol in AMD lesions, a model for lesion biogenesis, a model for macular vulnerability based on vascular biology, and alignment of AMD-related genes and pathobiology using cholesterol and an atherosclerosis-like progression as unifying features. We conclude with recommendations for the most important research steps we can take towards delineating the cholesterol-AMD link.
Keywords: Cholesterol, lipoproteins, retina, age-related macular degeneration, drusen
1.0 Introduction
Cholesterol is often viewed as a deleterious compound, mainly because its excess in systemic circulation is a risk factor for cardiovascular and Alzheimer's diseases (2002; Solomon et al., 2009; Zambon et al., 2010). Yet cholesterol is involved in many physiological processes and thus a lipid essential for normal human development, growth and physiology.
Studies of cholesterol will be moving to the forefront of vision research because of accumulating data implicating cholesterol homeostasis in the pathogenesis of age-related macular degeneration (AMD), the leading cause of irreversible vision loss and blindness in the elderly of industrialized world (Pascolini et al., 2004). Evidence linking cholesterol and AMD emerged more than a decade ago when cholesterol has been discovered to accumulate with age in human Bruch's membrane (BrM) (Curcio et al., 2001). Subsequent studies also established that esterified (EC) and unesterified cholesterol (UC) are significant components of the lipid-rich lesions associated with AMD (basal linear deposits, BLinD, and soft drusen) and comprise >40% of hard druse volume (Curcio et al., 2011a). The cholesterol-AMD link was confirmed when variants in the cholesterol-related genes were found to be associated with AMD by genome-wide association studies (GWAS) that suggested that these variants may play important roles in early AMD (Chen et al., 2010; Fritsche et al., 2013; Neale et al., 2010; Yu et al., 2012). As a result of all these developments, the investigation of the impact of cholesterol and lipoproteins for AMD, inflammation and angiogenesis has been included in the 2012 report of the National Eye Institute “Vision Research: Needs, Gaps and Opportunities” (http://www.nei.nih.gov/strategicplanning/), which represents a part of the institute strategic planning.
Hopefully, the delineation of the cholesterol-AMD link will follow a more rapid time course than the cholesterol-cardiovascular disease saga (Steinberg, 2004, 2005a, b, 2006a, b). Indeed, it took more than 70 years for the “lipid hypothesis” of atherosclerosis to become widely accepted after it was first introduced by Nikolai Anitschkow in 1913 in his classic work on cholesterol-fed rabbits (Anitschkow, 1913). Even in the middle of 1940's, most physicians considered atherosclerosis as an inevitable disease of aging (Steinberg, 2004). Now we have a good understanding of how cholesterol homeostasis in maintained in a whole body and many organs (Bjorkhem and Meaney, 2004; Brown and Goldstein, 2009; Kalaany and Mangelsdorf, 2006; Norlin and Wikvall, 2007; Russell, 2008), effective and safe cholesterol-lowering drugs, and public awareness of the benefits of a healthy life style and diet. These advances, along with those in cardiovascular surgery, has led to a continued decline (by a total of 43% from 1999 to 2010) in mortality rates for cardiovascular disease (http://www.cdc.gov/nchs/healthy_people/hp2010/hp2010_final_review.htm), an inspiring example for researchers and clinicians combating AMD. The expertise gained in that great public health success is now available to be applied to AMD.
There seems to be increased interest in chorioretinal cholesterol within the last several years as reflected by the thematic review series in the Journal of Lipid Research (Curcio et al., 2010; Fliesler, 2010a; Fliesler and Bretillon, 2010; Rodriguez and Larrayoz, 2010) and review articles on this topic in the specialized eye publications including this journal (Curcio et al., 2009a; Curcio et al., 2011a; Javitt and Javitt, 2009; van Leeuwen et al., 2004). For a detailed overview of cholesterol-related research in the vision field, readers are advised to refer to excellent and comprehensive previous reviews (Albert and Boesze-Battaglia, 2005; Curcio et al., 2010; Curcio et al., 2009a; Curcio et al., 2011a; Fliesler, 2010b; Fliesler and Bretillon, 2010; Fourgeux et al., 2011; Rodriguez and Larrayoz, 2010). The goal of the present paper is to summarize the most recent findings and provide a general view of where we stand in our current knowledge of chorioretinal cholesterol. This view represents the combined expertise of the two investigators, IAP and CAC, with relevant biochemistry and pathology publications. In writing this chapter we wish to recognize and express gratitude for the singular contributions of investigators who pioneered the study of retinal cholesterol and gave us our first glimpses of its localization, regulation, trafficking, toxic by-products, and potential for therapeutic intervention (Boesze-Battaglia et al., 1989; Fliesler and Schroepfer, 1982; Rodriguez and Larrayoz, 2010; Tserentsoodol et al., 2006a)
We begin by providing the background information on cholesterol and outlining how cholesterol homeostasis is maintained in a whole body with particular emphasis on cholesterol-related genes implicated in AMD. Then we will move to the major theme of this paper, chorioretinal cholesterol, and present our data and thoughts on how cholesterol homeostasis is maintained in the neural retina (NR), retinal pigment epithelium (RPE) and BrM, and the cholesterol-AMD link could be realized. We will conclude by pointing to the most important, in our opinion, directions of cholesterol-related research in vision field.
2.0 Cholesterol and lipoprotein particles
2.1. Physico-chemical properties, physiological roles, and distribution in a human body
Cholesterol is a lipid composed of a four-ring system flanked by a hydroxyl group at carbon 3 of the ring A and a branched hydrocarbon side chain at carbon 17 of the ring D (Fig. 1A). The fused ring system is rigid and flat, whereas the side chain is flexible and puckered (Fig. 1B). Most of the molecule is hydrophobic, except the 3β-hydroxyl group, which is hydrophilic, making cholesterol an amphipathic lipid. In addition to polarity, the 3β-hydroxyl renders cholesterol the ability to form esters, usually with fatty acids abundant in a human body. Esterification results in a molecule of greater size and hydrophobicity and a different shape. Hence, the physiological roles of EC, sometimes referred to as cholesteryl esters, are different from those of unesterified, or free cholesterol1. The two forms of cholesterol, however, can be enzymatically interconverted to one another, therefore, a sum of free cholesterol and EC represents a pool of a body's total cholesterol. EC, accounting for ~70% of total cholesterol in a human body, is mainly used for storage and transport. The physiological roles of UC are more diverse. At a cellular level cholesterol is mainly associated with membranes, hence its major and most general role is maintenance of the integrity of cell membranes. Due to its planarity and amphipathic nature, cholesterol readily intercalates between phospholipids (PL) in lipid bilayers and increases the ordering of neighboring lipids, thereby modulating membrane fluidity and permeability. Physical properties of membranes affect in turn the activity of membrane proteins. Cholesterol distribution in cell membranes is not uniform. Cholesterol is found at increased concentrations in lipid rafts, distinct liquid-ordered regions of membranes that are also rich in sphingolipids. Lipid rafts serve as platforms for protein-lipid interactions and transient formation of protein complexes and are implicated in the regulation of many cellular processes such as signal transduction, membrane trafficking, ligand binding, and receptor recycling (George and Wu, 2012; Hicks et al., 2012; Lemaire-Ewing et al., 2012; Pike, 2006). In addition to playing a structural role in cell membranes, cholesterol is involved in many biochemical reactions and serves as the sole precursor for steroid hormones, vitamin D, and bile acids. Cholesterol also regulates gene transcription and is important for nerve conduction and the formation of neuronal synapses (Brown and Goldstein, 2009; Burglin, 2008; Lee et al., 2006; Mauch et al., 2001; Saher et al., 2011).
Fig. 1. Two different views of cholesterol.
A. Chemical structrure and numbering of atoms. B. planarity of the molecule. The oxygen atom in the 3β-hydroxyl is shown in red.
In addition to 2 chemical forms, cholesterol can adopt 3 physical forms, classically described in the physical chemistry of atherosclerosis: oily droplets, lamellar membranes, and monohydrate crystals (Small, 1988) (Table 1). These forms differ in their relative proportions of UC, EC and PL and appear during different stages of the lesion formation in cardiovascular disease and AMD (Section 4).
Table 1.
Forms of cholesterol
| Morphology | % UC/ EC/ PL Mole % | UC/ PL Molar ratio | Size |
| Small droplets | 22 / 69 / 9 | 2.5 | 100 nm |
| Lamellar membranes | 59 / 17 / 24 | 2.6 | 40-200 nm |
| Cholesterol monohydrate crystals | 100 / 0 / 0 | -- | ~4 μm |
UC, unesterified cholesterol; EC, esterified cholesterol; PL, phospholipid Percentages are normalized to the sum of UC, EC, and PL, not total lipid Information sources (Guyton and Klemp, 1996; Kruth, 1997; Small, 1988)
To be transported through systemic circulation and interstitial fluid, i.e. aqueous medium, cholesterol and EC form multimolecular complexes with apolipoproteins, PL, and triglycerides (TG) called lipoproteins (Fig. 2). In these particles, hydrophobic lipids (EC and TG) form a core surrounded by a shell of apolipoproteins and amphipathic lipids (cholesterol and PL). The relative composition of the constituents may vary in these lipoprotein complexes or lipoproteins giving rise to different classes of particles, in which the density (a ratio of mass to volume) is inversely related to the particle diameter. As the density and protein content increase, the particle diameter decreases. In systemic circulation, the major classes of lipoproteins are chylomicrons (CM), very low density (VLDL), intermediate density (IDL), low density (LDL) and high density (HDL) lipoproteins. These classes were isolated by ultracentrifugation since the late 1940’s. Section 3.5 will focus on lipoproteins isolated from human BrM in 2005. Besides density, lipoprotein particles differ in protein composition. Apolipoprotein A-1 (apoA-1) is the major protein constituent of HDL. Apolipoprotein B-100 (apoB-100) is the principal component of LDL and is present in significant amounts in VLDL and IDL. Apolipoprotein B-48 (apoB-48), a truncated variant of apoB-100, is the major protein component of CM.
Fig. 2. Lipoprotein essentials.
A lipoprotein particle (upper left) is a multimolecular assembly that solubilizes oil droplets rich in esterified cholesterol (EC) and triglyceride (TG) for transport through an aqueous environment within a thin surface of phospholipid, unesterified cholesterol (UC), and apolipoproteins that are recognized by receptors and serve as cofactors for enzymes. Lipoproteins are secreted by the liver, intestine, brain, heart, placenta, kidney, and RPE. BrM-LP produced by the RPE represents a distinct class of lipoproteins as compared to the particles present in systemic circulation (CM, VLDL, LDL, and HDL). BrM-LP is composed of apolipoproteins B, E, and AI, and is large like VLDL, yet rich in EC like LDL. BrM-LP provides abundant cholesterol and apolipoproteins (including apolipoproteins B, E, A-I, C-I, C-II) to aging BrM and drusen. Adapted with permission from (Curcio et al., 2011a).
A 70-kg body of an average human male contains ~143 g of total cholesterol, of which 10.8 g (or 8%) is found in the blood and the rest is distributed unevenly among different organs (Sabine, 1977). The largest amounts of cholesterol are contained in the brain and nervous system (32 g, or 22%), connective tissues including adipose and body fluids (31.3 g, or 21.8%) and muscles (30 g, or 21%). The lowest amounts are present in the heart (0.6 g, or 0.42%) and spleen (0.5 g, or 0.35%). The liver contains 5.1 g, or 3.6% of total body cholesterol (Sabine, 1977). When normalized per gram of wet tissue, the content of cholesterol is still among the highest in the central nervous system (~20 mg/g wet weight) as well as in the adrenals (25-150 mg/g wet weight), and is the lowest in the heart (0.9-1.8 mg/g wet weight) (Sabine, 1977). Data on absolute concentrations and cholesterol content per gram of wet tissue in different parts of the eye are scarce and have been reported so far only for bovine specimens as ~2% of dry retinal weight (Fliesler and Anderson, 1983) or 3 mg/g wet tissue in neural retina and 2.6 mg/g wet tissue in the RPE (Bretillon et al., 2007). For comparison, human liver contains ~3 mg of total cholesterol per gram of wet weight (Sabine, 1977).
2.2. Homeostasis in humans and genes associated with AMD
Cholesterol maintenance in a whole body and specific organs is well described in many textbooks on biochemistry and clinical chemistry. Therefore, references will be given primarily to the most recent data or data that are not always included in the textbooks. Further, in this section, we will provide only a brief summary of cholesterol bio-transformations to serve as a framework for a description of the genes pertinent to AMD and proteins discussed in the next sections. Of importance is that the variants of cholesterol-related genes (CETP, ABCA1, LIPC and APOE) that are associated with AMD yet are not correlated with plasma HDL levels in AMD patients. This suggests that some of the gene effects on the disease are independent of the plasma lipoprotein profile and relate to cholesterol homeostasis in the retina, where these (Anderson et al., 2001; Neale et al., 2010; Tserentsoodol et al., 2006a) and many other proteins related to cholesterol homeostasis are expressed (Section 3.3). The functions of CETP, ABCA1, LIPC and apoE are well studied in non-ocular systems. Hence we summarize their functions here to inform the search for their roles in retina.
Every nucleated cell in a human body can synthesize cholesterol (Dietschy and Turley, 2004). Accordingly, humans do not have a dietary requirement for cholesterol and can satisfy all their needs for cholesterol by internal biosynthesis. Yet cholesterol is present in many foods, which represent a source of additional cholesterol. Dietary cholesterol (300-500 mg in an individual on a Western diet) is absorbed in the proximal small intestine (the duodenum and jejunum) along with cholesterol delivered with bile (800-1300 mg) with a total of 1200 to 1700 mg of cholesterol entering the lumen of the small intestine every day (Turley and Dietschy, 2003). Of this amount, only ~50% of cholesterol is then absorbed and further processed by enterocytes (Ostlund et al., 1999; Sudhop et al., 2002); the rest is excreted in feces.
In the enterocyte, cholesterol is esterified by the enzyme acyl-CoA:cholesterol acyltransferase (ACAT) and incorporated, along with UC and TG, into nascent CM, which are released into lymph and reach the systemic circulation via the thoracic duct (Fig. 3). While in lymph and blood, CM obtain some apolipoproteins from HDL and deliver TG to adipose tissues and muscles where TG are hydrolyzed by lipoprotein lipase to free fatty acids (FFA), which are used as energy substrates. After losing most of its TG content, CM interact with HDL again. Cholesteryl ester transfer protein (CETP) is an important enzyme in this process as it transfers EC from HDL to CM in exchange for TG. A gene variant of CETP (the A allele of the single nucleotide polymorphism (SNP) rs3764261) increases the levels of HDL, but is a risk factor for AMD (Chen et al., 2010; Fritsche et al., 2013; Neale et al., 2010; Yu et al., 2011). Interactions with HDL convert CM into CM remnants, which are delivered to the liver and taken up by hepatic parenchymal cells.
Fig. 3. Lipoprotein bio-transformations and trafficking in human circulation.
Lipoprotein particles are shown as circles of different color with the cirlcle diameter reflecting a particle size (not at a scale). Receptor (LDL-R), transporter (ABCA1), and enzymes pertinent to lipoproteins (LIPC, ACAT, CETP, and lipoprotein lipase) are also indicated. CHOL, either esterified or unesterified cholesterol or both; EC, esterified cholesterol; FFA, free fatty acids; PL, phospholipids; TG, triglycerides.
In the liver, cholesterol from CM remnants along with cholesterol from endogenous synthesis and uptake of other lipoprotein particles can be packaged in VLDL and exported into the blood. Similar to CM, VLDL deliver TG to different tissues, mainly between meals. At the same time, UC, PL and apolipoproteins are released from VLDL and taken up by HDL. As the content of plasma VLDL changes, these particles are transformed into IDL and then LDL, the principal carriers of cholesterol through the blood in humans. LDL have the highest cholesterol content of all lipoprotein particles and supply cholesterol to many organs for acquisition via receptor-mediated endocytosis. In the cell, LDL are degraded in the lysosomes, and UC is released. Non-hepatic cells expressing the receptors for LDL (LDL-R) acquire only a part of circulating LDL; the majority (~70%) is removed by the liver, the chief organ for processing whole body cholesterol. LDL are also taken up by macrophages, but via different receptors (SRA and CD36). In the arterial wall, the uptake process is enhanced when LDL concentrations are increased and LDL is modified by oxidative and non-oxidative mechanisms (Tabas et al., 2007). Accumulation of cholesterol in arterial wall macrophages is an important event in the pathogenesis of atherosclerosis. Macrophages and some other tissues also have a receptor-independent mechanism of cholesterol acquisition called fluid-phase pinocytosis, when cells engulf extracellular fluid and any material such as LDL present in that fluid (Kruth, 2011). This mechanism, established only recently, accounts for up to 42% of total tissue uptake of LDL (Kruth, 2011).
Extra-hepatic cells utilize cholesterol provided by LDL delivery and by endogenous synthesis for their specific needs and remove the unused portion primarily through reverse transport by HDL. Lipid-poor apoA-1 is produced in the liver and intestine and released in the blood, where it becomes nascent, disk-shaped HDL. In the circulation, nascent HDL accepts cholesterol and PL from cholesterol-laden cells of the extra-hepatic tissues, CM and VLDL and becomes lipidated. This process of lipidation is mediated by the ATP-binding cassette transporter A1 (ABCA1), which is located in plasma membranes and effluxes cholesterol and PL out of many cells. Complete deficiency of ABCA1 due to inactivating mutations significantly reduces plasma HDL and leads to Tangier's disease (Rust et al., 1999). Functional variants of ABCA1 are also known. The C allele of rs1883025 increases plasma HDL levels, whereas the T allele of rs1883025 has an opposite effect. The C allele increases the risk for AMD, whereas the T allele is protective (Chen et al., 2010; Fritsche et al., 2013; Neale et al., 2010; Yu et al., 2011; Yu et al., 2012). After lipidation, UC within nascent HDL is esterified by lecithin:cholesterol acyltransferase (LCAT), and discoidal nascent HDL becomes spherical mature HDL. HDL provides EC to different lipoprotein particles in exchange for TG, and the remnant HDL particles are delivered to the liver. HDL is recognized by the SR-BI receptors present on the surface of several organs including the adrenal glands and gonads. Unlike LDL, HDL do not contribute to atherosclerosis but instead play an anti-atherogenic role by continuously removing excess cholesterol from tissues and returning this cholesterol to the liver. In addition, HDL have several other functions including activation of signaling pathways in vascular endothelium and carrying antioxidant and complement pathway proteins to preserve vascular health (Prosser et al., 2012; Vaisar et al., 2007). Thus, cardioprotective effects of HDL involve cholesterol-dependent and cholesterol-independent mechanisms (Rye and Barter, 2014).
HDL-mediated reverse cholesterol transport represents the major but not the only pathway whereby non-hepatic organs rid excess cholesterol. Many cells metabolize cholesterol to more soluble oxysterols, which rapidly diffuse out of the cell to the systemic circulation and are quickly delivered to the liver for degradation to bile acids (Meaney et al., 2002). 27- and 24-hydroxycholesterols are the major oxysterols in human plasma (Dzeletovic et al., 1995) 27-Hydroxycholesterol is secreted by the cells containing cytochrome P450 27A1 (CYP27A1), a ubiquitous cholesterol 27-hydroxylase (Cali and Russell, 1991). The source of 24-hydroxycholesterol is different as this oxysterol is the product of catalytic activity of cytochrome P450 46A1 (CYP46A1), present mainly in the brain (Lund et al., 1999). Cholesterol 24-hydroxylation by CYP46A1 is the major pathway for cholesterol removal from the brain because the brain is separated from the systemic circulation by the blood brain barrier, which impedes cholesterol exchange with the circulating lipoprotein particles (Lund et al., 2003; Lutjohann et al., 1996). Metabolism of cholesterol by CYPs 27A1 and 46A1 serves dual roles: to generate transport forms of cholesterol and to activate (in some tissues) liver X receptors (LXRs) (Janowski et al., 1996). LXRs are transcription factors that control the expression of many cholesterol-related genes (e.g., ABCA1 and CETP) and integrate the pathways of cholesterol transport by lipoproteins and removal by cytochrome P450 enzymes (Kalaany and Mangelsdorf, 2006). In specific cells including macrophages, LXRs also participate in suppression of inflammatory and immune responses (Zelcer and Tontonoz, 2006). The role of cholesterol in steroidogenic tissues is different, namely to be utilized for the production of pregnenolone, the precursor of all steroid hormones. Cytochrome P450 11A1 (CYP11A1) uses cholesterol as a substrate and converts it to pregnenolone via the three-step reaction involving the formation of 22R-hydroxycholesterol and 20α,22R-dihyroxycholesterol. This reaction represents the first and rate-limiting step in the synthesis of all steroid hormones.
Irrespective of how and in what form cholesterol is eliminated from non-hepatic organs, it is ultimately delivered to the liver, playing a central role in the maintenance of whole body cholesterol homeostasis. Through coordinate regulation of the pathways of cholesterol input and output, the liver balances cholesterol acquisition from food and endogenous synthesis (~1200 mg/day) by cholesterol elimination. The latter is accomplished via cholesterol degradation to bile acids (~500 mg/day) initiated and controlled by the liver-specific cytochrome P450 enzyme 7A1 (CYP7A1), secretion in bile (600 mg/day), production of steroid hormones (50 mg/day), and losses due to cell sloughing (85 mg/day). Accordingly, cholesterol content in our body does not change significantly even if we have a marked daily variation in the intake of dietary cholesterol. Metabolism of lipoprotein particles in the liver requires a number of enzymes including hepatic lipase (LIPC) that hydrolyzes lipoprotein TG and PL and plays an important role in remodeling of LDL and HDL. In addition, LIPC participates in the recognition of CM remnants and VLDL; this ligand binding role does not require catalytic activity (Brunzell et al., 2012; Deeb et al., 2003). Complete deficiency in LIPC is characterized by elevated levels of plasma total cholesterol and TG and the presence of large, buoyant TG- and PL-enriched LDL and HDL along with large VLDL. Conversely, high LIPC activity makes LDL and HDL smaller and denser. Three functional variants of LIPC (due to the rs493258, rs10468017, and rs920915 SNPs) were found to be associated with AMD. The T alleles of rs493258 and rs10468017 raise HDL and confer protection against AMD, whereas the C allele of rs920915 having unknown effect on HDL is a risk factor for the disease (Cheng et al., 2004; Fritsche et al., 2013; Neale et al., 2010). APOE encoding apolipoprotein E (apoE) is another gene associated with AMD (Klaver et al., 1998; McKay et al., 2011; Souied et al., 1998). ApoE is present in CM, VLDL, IDL and HDL, where it acts as a ligand for LDL-R present of the surface of many cells. The ε2 isoform of apoE has reduced affinity for LDL-R as compared to the ε3 and ε4 isoforms; hence less cholesterol is provided to cells via the ε2 isoform. This isoform increases the risk for AMD, whereas the ε4 isoform plays a protective role (Klaver et al., 1998; McKay et al., 2011; Souied et al., 1998). This is in sharp contrast to the role of the ε2 and ε4 isoforms in cardiovascular disease and Alzheimer disease (Albert and Boesze-Battaglia, 2005), where ε4 increases risk. Resolution of this so-called ApoE Paradox could provide important mechanistic insights into three major diseases. Further, the alleles associated with plasma HDL do not all have the same effect on AMD risk. The contrast between plasma HDL's strongly protective effect on cardiovascular disease and its equivocal role in AMD is stark, and it constitutes an important clue to the significance of these genes. Lack of association of AMD with any measure of systemic lipoproteins or plasma cholesterol, either atherogenic or anti-atherogenic (Dashti et al., 2006), in concert with intra-ocular expression of all these genes (Section 3.3) gives credence to the predominance of local regulation of cholesterol homeostasis.
2.3. Homeostatic differences between humans and mice
The same general principles govern cholesterol maintenance in mammals. Yet homeostatic responses to availability of cholesterol are species- and sometimes gender-specific (e.g. mice) and vary, quantitatively and qualitatively, in animals from different and the same taxonomic ranks (e.g., mice and humans; mice and hamsters) (Dietschy and Turley, 2002; Dietschy et al., 1993; Turley et al., 1998; Turley et al., 1995). Herein we describe homeostatic differences between mice and humans because mice are a popular animal model and an example of rodents highly resistant to atherosclerosis even when fed high-cholesterol diet (Breslow, 1993). We will also comment on cholesterol-containing diets to help vision researchers design studies that are of physiological relevance.
Mice have a 16-fold higher rate of the whole body cholesterol biosynthesis than humans (160 mg/day/kg vs 10 mg/day/kg) with the contribution of the liver to this rate being ~40% in mice and only 10-12% in humans (Dietschy and Turley, 2002). Accordingly, mice deal with a dietary cholesterol challenge much better than humans because down-regulation of hepatic cholesterol biosynthesis is a mechanism to compensate for increased load of absorbed cholesterol (Spady et al., 1985). In addition, mice are more efficient than humans in cholesterol removal as their rate of hepatic LDL clearance is 40-fold greater than that in humans (~500 ml/day/kg vs ~12 ml/day/kg) (Dietschy and Turley, 2002). The rate of hepatic LDL clearance is also a factor determining plasma levels of atherogenic LDL, which are much lower in mice as compared to humans (~7 mg/dl vs ~120 mg/dl) (Dietschy and Turley, 2002). Finally, when challenged with dietary cholesterol, mice but not humans can markedly up-regulate their bile acid biosynthesis and thereby dispose more cholesterol upon increased dietary load than humans. This response reflects interspecies differences in transcriptional regulation of CYP7A1 catalyzing the rate-limiting step in bile acid biosynthesis. In mice, Cyp7a1 contains the LXR-responsive sequence absent in the human gene (Russell, 2008).
To overcome resistance to atherosclerosis, mice and other small rodents are often fed diets containing 1-3% cholesterol. These concentration are >10-times higher than those necessary for a rigorous cholesterol challenge (0.1-0.3% of dietary cholesterol) determined as a cholesterol intake 5-10 times higher than the daily rate of the whole animal cholesterol biosynthesis (Kris-Etherton and Dietschy, 1997). Cholesterol-rich diets (1-3%) are thus highly non-physiological but produce a sustained increase in plasma cholesterol to the 200-300 mg/dl levels (~80 mg/dl in a mouse on a regular chow) and increase in cholesterol in the non-HDL lipoprotein fractions (Breslow, 1993). If the goal of a study is to model normal intake of cholesterol in humans, supply of dietary cholesterol must be significantly decreased and equal to ~50% of the whole body cholesterol biosynthesis in a given animal species (Kris-Etherton and Dietschy, 1997).
Besides homeostatic differences, mouse resistance to atherosclerosis is determined by high plasma levels of anti-atherogenic HDL, the principal carrier of plasma cholesterol (~90% of total plasma cholesterol) in this species. This is because the activity of CETP is not detectable in mouse plasma (Breslow, 1993). In contrast, humans carry most of their plasma cholesterol in LDL (~75% of total plasma cholesterol). In addition, rodents synthesize apoB-48 in hepatocytes as well as enterocytes (Sparks et al., 1981; Zak et al., 2002).
3.0 NR, RPE and BrM
The term “retina” is often referred to the structure comprised of the three principally different components: NR, RPE and choroid (Fig. 4), with the latter two constituting the support system of the photoreceptors (PR). It is essential to understand the layers, compartments, and regions affected by AMD, because AMD pathology itself shows precise laminar and topographic predilections. Of major significance are the two physiological universes of the retina: inside the blood-retina barrier and outside this barrier, within the systemic circulation.
Fig. 4. Chorioretinal layers and major cell types.
Modified from (Zheng et al., 2012). The the neurosensory retina has nine distinct layers (from top to bottom): inner limiting membrane (ILM), nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), external limiting membrane (ELM), photoreceptor inner segments (IS), and photoceptor outer segments (OS). The tenth layer, the retinal pigment epithelium (RPE), lies outside the neurosensory retina but is considered a part of the retina. The major retinal cell types are ganglion cells (G), diffuse amacrine cells (DA), amacrine cells (Am), Müller cells (M), bipolar cells (B), horizontal cells (H), rods (R), and cones (C).
3.1. Distinctive structure and function of retina and its supporting tissues
The NR is the only site in the human body mediating the transmission of the visual signal to the brain. Vision is initiated in the light-sensitive rod and cone PR, unique retinal cells, and undergoes complex processing by other neurons. There are ~ 55 separate neuronal types in the NR in five major neuronal classes (PR, horizontal cell, bipolar cells, amacrine cells, and ganglion cells) (Masland, 2001). In addition, the NR contains glial cells: Müller cells spanning almost the entire thickness of the retina as well as astrocytes and microglial cells (Bringmann et al., 2006). Numerous cells in the NR are spatially organized and form distinct layers. The nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL) form the inner retina, whereas the outer plexiform layer (OPL), outer nuclear layer (ONL), and bacillary layer of the PR (inner and outer segments) form the outer retina (Distler and Dreher, 1996). In humans, the 6 mm diameter macula (area responsible for central vision) contains a 0.8 mm-diameter cone-dominated fovea specialized for high acuity vision (Curcio et al., 1990). GCL, IPL, and INL are absent at the fovea, and PR sweep laterally to connect to bipolar cells are interleaved by Müller cells, forming a thick Henle fiber layer (Curcio et al., 2011b; Drasdo et al., 2007). Adjacent to the outer retina is the RPE, a polarized monolayer, and BrM, a pentilaminar extracellular matrix that includes basement membranes of the RPE and choriocapillary endothelium (Rudolf et al., 2005) and functions as a vessel wall. The choroid has the highest blood flow per unit volume and lowest oxygen extraction in the body in order to maintain high oxygen tension at the PR inner segments (IS). The outermost component of the blood-retina barrier is the junctional complexes uniting the RPE monolayer, with apical RPE inside this barrier, and basolateral RPE facing the systemic circulation in the choroid. The layer of choriocapillaries facing the RPE has openings (fenestrae) that enable delivery of nutrients from the systemic circulation directly to the RPE and removal of waste products directly from the RPE. The inner retina has a distinct and spatially separated blood supply originating form the central retinal artery. Branches from the central retina artery dive in the NR and form three plexi that extend from the GCL to the INL with normally no blood vessels being found in the OPL. Unlike the choroidal blood vessels, the intraretinal blood vessels do not have fenestrae and their endothelium has tight junctions. These junctions as well as Müller cells and astrocytes that wrap retinal blood vessels establish the blood-retinal barrier that prevents the movement of large molecules in or out of the retinal vessels (Anand-Apte and Hollyfield, 2011).
3.2. Distribution of cholesterol in the NR
Similar to other species (e.g. mice and cows), the majority of cholesterol in human NR (~90%) is unesterified. Moreover, UC is present at concentrations several orders of magnitude higher than those of other sterols (Fliesler and Anderson, 1983; Fliesler and Schroepfer, 1982; Mast et al., 2011; Omarova et al., 2012). Accordingly, the distribution of UC in the NR is detectable by staining with filipin, a fluorescent antibiotic interacting specifically with the free 3β-hydroxyl group of UC and other unesterified sterols (Castanho et al., 1992). Filipin staining shows that in humans UC is ubiquitously distributed the retina in layer-specific patterns consistent with cellular membranes (Fig. 5) (Curcio et al., 2005a). The only layer that shows very weak filipin staining is the PR outer segments (OS). In this layer, UC forms a gradient with a higher concentration in the region bordering the PR IS and lower concentration at the distal tip of the rods embraced by the RPE apical processes (Albert and Boesze-Battaglia, 2005; Boesze-Battaglia et al., 1989).
Fig. 5. Cholesterol distribution in human macula, localized by filipin.
This staining came from a set of experiments described in (Curcio et al., 2005a). Labeling of retinal layers is the same as in Fig. 4. Syn/ped, synapses of photoreceptors with post-receptoral neurons (inner), and layer of cone pedicles (outer); He, Henle fiber layer. Arrowheads point to cone photoreceptor outer segments.
Advances in mass spectrometry (MS) and availability of the laser capture micro-dissection technique made it possible to recently investigate rod and cone OS cholesterol content in human retinal samples (Table 2). The data obtained so far suggest that cone OS are more enriched in UC than rod OS. Hence more UC is present in the relatively cone-enriched macula than peripheral retina. While certainly worthy of further exploration, this finding provides important insight into the pathobiology of AMD-specific lesions (Section 4).
Table 2.
OS cholesterol content in human retina
| Total cholesterol, pmol | |||
|---|---|---|---|
| Gender | Age | Macula* | Periphery |
| M | 50 | 290 (1.26) | 230 |
| M | 59 | 229 (1.39) | 164 |
| M | 63 | 250 (1.19) | 210 |
| M** | 64 | 100 (0.70) | 142 |
| M | 63 | 67 (2.09) | 32 |
| M | 78 | 213 (1.25) | 171 |
| M | 85 | 232 (1.74) | 133 |
| M | 87 | 100 (0.32) | 310 |
| F | 57 | 60 (1.87) | 32 |
| F | 88 | 83 (1.93) | 43 |
Quantified by isotope-dilution gas-chromatography mass spectrometry as described (Mast et al., 2011) using deuterated cholesterol as internal standard. OS were obtained by laser-capture microdissection using 700 laser shots. Combined efforts of Saida Omarova, Casey D. Charvet, and Natalia Mast in the laboratory of IAP. Retinal sections were provided by CAC.
Number in parentheses represents fold difference relative to periphery
Material from 600 laser shots
Ten eyes with unremarkable maculas from donors 50-88 yr of age were used. Macular and peripheral samples of each eye were analyzed to permit within-eye comparison, using published values for rod and cone densities in these regions (Curcio et al., 1993; Curcio et al., 1990). We first computed the percentage of total photoreceptors in macula and periphery that were cones, where the macula was defined as a 6 mm diameter area centered on the fovea and periphery was centered at 13 mm temporal. In young adult macula, 9.3% of photoreceptors are cones, in older adult macula, 13.25% of photoreceptors are cones, and in peripheral retina of both ages. For periphery, the corresponding numbers are 3.7% and 3.1%. By assuming that laser shots captured rods and cones in proportion to their spatial densities, rod OS were dominated by disks containing 10% UC, and cone OS were dominated by plasma membrane containing 30% UC, we predict a macula/periphery (M/P) ratio for OS layer UC content in these assays of 1.09-1.18, depending on the age of the eye. In 8/10 eyes, the M/P ratio of total cholesterol was 1.19-2.19, thus exceeding this prediction and suggesting that cone OS are indeed enriched in UC relative to rods. There are limitations to this analysis. Cone OS are shorter than rod OS, thus effectively lowering the proportion of cone in the sample. More information about the membrane density of cone and rod OS (mm2/mm3) would strengthen this analysis. This question may also be answerable by comparing OS layer UC content in retinas from species that are more cone-dominated than human (e.g., pig, tree shrew, sand rat, NRL-knockout mouse).
3.3. Cholesterol homeostasis in the NR
Our knowledge of cholesterol homeostasis in the NR is still very limited as compared to intensive investigations in the brain, the organ sharing several important similarities with the retina. Like the retina, the brain contains neurons and glial cells with the cholesterol content in both organs being mainly in an unesterified form. In addition, both the brain and retina are separated from the systemic circulation by the blood-brain or blood-retina barrier. These similarities justify the structure of this section, in which the pathways of cholesterol input and output in the retina will be presented in the context of how cholesterol maintenance is realized in the brain. The major emphasis will be on the most recent findings in vision field that were not included in the comprehensive previous reviews published several years ago (Albert and Boesze-Battaglia, 2005; Fliesler and Bretillon, 2010; Fourgeux et al., 2011; Rodriguez and Larrayoz, 2010). Where we deem appropriate, we will also provide relevant information about the RPE. A comprehensive picture of RPE cholesterol homeostasis will be presented in the next section.
In the brain, cholesterol supply comes almost exclusively from local synthesis with no evidence of cholesterol transfer from the systemic circulation (Dietschy and Turley, 2001). The NR also synthesizes cholesterol as demonstrated by intravitreal injections of radioactive cholesterol precursors to rats (Fliesler et al., 1993; Fliesler and Keller, 1995) and immunohistochemistry localization of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR), the rate-limiting enzyme in cholesterol biosynthesis. HMGCR was found to be abundant in rat Müller cells and rod IS (Fliesler and Bretillon, 2010). Recent immunostainings of human NR also detected HMGCR in PR IS and in addition in the GCL, INL and ONL (Fig. 6), the layers containing Müller cells as well as other cell types (Zheng et al., 2012). The retina requires endogenous cholesterol biosynthesis to achieve normal structure and function. This is indicated by studies utilizing rats with pharmacologically inhibited reduction of 7-dehydrocholesterol, the last step in cholesterol biosynthesis (Fliesler, 2010b; Fliesler and Bretillon, 2010). The blockage of this last step leads to a significant accumulation of a cholesterol precursor 7-dehydrocholesterol and reduced cholesterol levels in the retina as well as other organs and bodily fluids. A progressive retinal degeneration is observed. While the underlying reasons for this degeneration are currently unknown, an important insight recently came from a demonstration that accumulated 7-dehydrocholesterol in rat brain and retina leads to the sterol oxidation to a variety of metabolites. (Xu et al., 2011; Xu et al., 2012). The known biological effects of these metabolites suggest that alone or in combination, they could evoke retinal degeneration (Xu et al., 2012)
Fig. 6. Immunohistochemistry localizations of cholesterol-related proteins in human retina and RPE.
SREBPs, SCAP, Insigs control the expression of HMGCR and LDLR playing key roles in cellular cholesterol input: HMGCR is the rate-limiting enzyme in cholesterol biosynthesis, whereas LDLR uptakes cholesterol-rich LDL. LXRs regulate the expression of ABCA1, a cholesterol efflux transporter, as well as many other genes involved in cellular cholesterol output and other cellular processes. CYPs 27A1, 46A1, and 11A1 are the only three enzymes that initiate the quantitatively significant pathways of cholesterol metabolism in non-hepatic organs including the retina and RPE. Phase contrast images (on the left of each panel) are given for comparison. Nuclei were stained by DAPI (blue), and immunoreactivity was detected by DyLight 649 conjugated secondary Abs (red). Staining with serum from non-immunized animal (rabbit or goat) served as a negative control. Labeling of retinal layers is the same as in Fig. 4. Scale bars are 30 μm. Taken from (Zheng et al., 2012).
Local biosynthesis is not the only source of cholesterol for the retina as cholesterol from the systemic circulation can cross the RPE and reach the NR. This was established in the studies using systemic injections of fluorescently-labeled lipoprotein particles and subsequent fluorescence imaging of retinal cross sections (Elner, 2002; Tserentsoodol et al., 2006b). In one study, monkeys were perfused over 20 min with fluorescently-labeled LDL: intense fluorescence appeared in the RPE, suggesting the uptake of blood-borne LDL (Elner, 2002), and was accompanied by less pronounced labeling in the NR. In a different study, rats were injected either with LDL or HDL containing cholestatrienol, a fluorescent cholesterol analog. Intense fluorescence from cholestatrienol-containing LDL was detected in the RPE and throughout the retina within 2 hr. A much less intense fluorescence from cholestatrienol-containing HDL was mainly observed in the RPE. These results confirmed that the RPE and NR readily and rapidly uptake lipoprotein particles from the systemic circulation and suggested that LDL is the major carrier of cholesterol to the retina (Tserentsoodol et al., 2006b), at least in rats. Feeding animals high-cholesterol diet also leads to an increase in retinal cholesterol (~2.7-fold) as demonstrated in experiments utilizing a rat model of Smith-Lemli-Opitz syndrome, where the retina and other tissues are cholesterol-deficient (Fliesler et al., 2007). Thus, unlike the blood-brain barrier, the outer blood-retina barrier is permeable for cholesterol. This is due in part to fenestrae present in the choriocapillaries forming the outer blood-retina barrier, and absent in capillary network supplying the brain, enabling the RPE-directed transit of cholesterol-containing lipoprotein particles from the systemic circulation. The RPE contains the receptors for LDL (LDL-R) and HDL (SR-BI and SR-BII) at the basolateral side facing the choroid (Duncan et al., 2009; Tserentsoodol et al., 2006a; Tserentsoodol et al., 2006b) and likely uptakes the blood-borne lipoprotein particles. Consistent with a receptor-mediated mechanism, mice lacking LDL-R accumulate lipids in BrM (Rudolf et al., 2005). Perhaps blood-borne cholesterol enters the neurosensory retina not only from the RPE but also from the inner blood-retina barrier involving the inner retinal blood vessels (Wu et al., 2008). Yet this mechanism is currently speculative and requires additional studies. Thus, in contrast to the brain, the NR has endogenous (local synthesis) and exogenous (delivery from the systemic circulation) sources of cholesterol, and the primary route of access seems to be the RPE. Nevertheless, the steady-state levels of cholesterol in human retina are much lower than those in the brain (41-55 vs 250-350 nmol/mg total protein in gray matter of temporal lobe or 307-405 nmol/mg total protein in gray matter of cerebellum) (Mast et al., 2011), possibly because of a lower content of cholesterol-rich myelin, which in the brain contains up to 80% of total cholesterol (Dietschy and Turley, 2004).
Brain cholesterol may be synthesized by both neurons and glial cells (Dietschy and Turley, 2004). Yet the neuronal soma is questioned as a source of the additional cholesterol necessary for synapse formation, because of its distance from the site of synthesis. Therefore, to meet this need, astrocytes synthesize and secrete apoE-associated cholesterol that is taken up by the axons via one of the LDL-R family of receptors (Dietschy and Turley, 2004). Cerebral neurons were even suggested to outsource cholesterol synthesis to astrocytes and deal only with the elimination of cholesterol excess via CYP46A1-mediated catabolism to 24-hydroxycholesterol (Pfrieger, 2003b). Is a similar mechanism operative in the NR? Acquisition of the blood-borne lipoproteins also raises a question of how these particles are transported within the RPE and NR. Partial answers to these questions were obtained through immunochemical localizations of the proteins related to cholesterol transport. These studies, often conducted in conjunction with quantifications by RT-PCR, documented the expression in the NR of the most important proteins necessary for efflux, assembly, maturation and uptake of lipoprotein particles (ABCA1, apoE, apoA-1, LCAT, CETP, SR-BI, SR-BII, and CD36) (Amaratunga et al., 1996; Claudepierre et al., 2010; Duncan et al., 2002; Duncan et al., 2009; Hayes et al., 1989; Kuhrt et al., 1997; Kurumada et al., 2007; Li et al., 2005a; Tserentsoodol et al., 2006a; Zheng et al., 2012). Collectively, these investigations, undertaken in different laboratories and on different mammalian species including humans, suggest that indeed, there is a lipoprotein-mediated cholesterol transport in the retina and that Müller cells, the major glial cells in the retina, as well as the RPE can provide APOE for this transport. HDL-like particles are proposed to mediate cholesterol transport in the retina (Tserentsoodol et al., 2006a) summarized in the only currently available model of how cholesterol traffics across the subretinal space. This model is also consistent with AMD pathology described in Section 4. Interestingly, apoB and microsomal triglyceride transfer protein (MTP) necessary for assembly of VLDL are expressed in retinal ganglion cells (Li et al., 2005b). The significance of this finding remains to be established. Cholesterol trafficking was also studied inside retinal cells by characterizing flies (Drosophila) and mice lacking Niemann-Pick type C1 (NPC1) protein necessary for the exit of lipoprotein-derived cholesterol from lysosomes (Claudepierre et al., 2010; Phillips et al., 2008). In both species, ablation of NPC1 expression led to age-progressive retinal degeneration. In mice, NPC1 was immunolocalized to the NFL, GCL, OPL, OS, and RPE. NPC1 loss impaired visual function and caused degeneration of OS, disruption of synaptic layers, and accumulation of cholesterol in the RPE. There was an up-regulation of apoA-1 and Abca1 in some of the NPC1-containing retinal layers, suggesting a compensatory response to increased cholesterol levels (Claudepierre et al., 2010). Studies of Npc1−/− mice highlighted the point that intracellular events following LDL-R-mediated cholesterol uptake are important for specific retinal cell types and therefore must be incorporated in studies of overall chorioretinal cholesterol maintenance. On the other hand, the fact that UC was not detected in the NR of wild-type mice in this study raises questions about the cholesterol accumulation seen in Npc1−/− mice.
In the brain, cholesterol elimination is mainly accomplished via CYP46A1-mediated catabolism to 24-hydroxycholesterol, accounting for ~75% and ~40% of cholesterol elimination in humans and mice, respectively (Bjorkhem et al., 1998; Lund et al., 2003). Cerebral expression of CYP46A1 is mainly confined to multiple neuron types with the enzyme being bound to the endoplasmic reticulum and distributed throughout neuronal cell bodies and dendrites (Ramirez et al., 2008). Several laboratories studied whether CYP46A1 is expressed in the retina using different antibody types (anti-peptide, monoclonal and polyclonal) for immunolocalization, as well as different species. In rat retina, the enzyme was found to be expressed in the cell bodies of neurons (in the GCL and INL) with no expression in layers containing axons and synapses (IPL and OPL), PR OS and RPE (Bretillon et al., 2007). In mice, CYP46A1 was detected in the GCL, some cells at the edge of the INL, and some cells in the RPE layer (Ramirez et al., 2008). In a different study also on mice, CYP46A1 was similarly found in the GCL and the IPL/INL interface and in addition in the OPL (Zheng et al., 2012). In human samples, signal for CYP46A1 was present in the GCL, IS, and RPE (Fig. 6) (Zheng et al., 2012). Thus, despite interspecies/inter-laboratory differences in CYP46A1 immunolocalizations, there is consensus that CYP46A1 is expressed in the neuron-containing GCL. Retinal CYP46A1 expression is supported by detection in the NR of the CYP46A1 product 24-hydroxycholesterol. Two laboratories conducted these quantifications and used highly accurate and sensitive isotope-dilution gas chromatography-MS (GC-MS). The data obtained are within the same order of magnitude, which is a good correspondence for a femtomolar level of detection: in the range of 0.04-0.1 pmol (Mast et al., 2011) and 0.1-0.5 pmol of 24-hydroxycholesterol per nmol of cholesterol in human NR (Fliesler and Bretillon, 2010); and 0.3 pmol (Mast et al., 2011) and 3.1 pmol of 24-hydroxycholesterol per nmol of cholesterol in bovine NR (Bretillon et al., 2007).
CYP46A1 is not the only cholesterol hydroxylase present in the NR. Mitochondrial enzymes CYP27A1 and CYP11A1, which metabolize cholesterol to 27-hydroxycholesterol and 5-cholestenoic acid (CYP27A1) and pregnenolone (CYP11A) are also expressed in the retina as documented by immunostainings (Guarneri et al., 1994; Jaliffa et al., 2005; Lee et al., 2006; Zheng et al., 2012). Retinal localizations of CYP27A1 were assessed in monkey and human samples using anti-peptide and polyclonal antibodies to human CYP27A1, respectively. Studies on monkey sections detected the predominant enzyme expression in the PR IS and a lesser expression in Müller cells, ganglion cells, and RPE (Lee et al., 2006). Immunostaining of human specimens showed a broader pattern of CYP27A1 expression with the strong signal not only in the photoreceptor IS but also in the GCL, INL, ONL, and RPE (Fig. 6) (Zheng et al., 2012), consistent with ubiquitous nature of CYP27A1 (Andersson et al., 1989). Immunolocalizations of CYP11A1 were conducted in rat, hamster and human retina. In rats and hamsters, CYP11A1 was detected only in the cells of GCL and INL (Guarneri et al., 1994; Jaliffa et al., 2005). In human retina, CYP11A1 was found in almost every retinal layer except the IPL and OS (Fig. 6) (Zheng et al., 2012). Thus, three different cholesterol-metabolizing CYPs are present in the NR, raising the question, which of the three is the most important for retinal cholesterol metabolism and retinal function. To address this question, three aspects of retinal cholesterol metabolism were investigated in the laboratory of author IAP. First, retinal protein and metabolite levels of all three cholesterol hydroxylases were determined and compared, within retina and also to the brain (Liao et al., 2011; Liao et al., 2010; Wang et al., 2012). Second, a mechanism of the retina-specific and age-dependent deterioration of CYP27A1 activity was studied and established as well as a novel strategy to combat this mechanism (Charvet et al., 2011; Charvet et al., 2013a; Charvet et al., 2013b). Third, the role of CYP27A1 and CYP46A1 in mice was evaluated by ophthalmic characterization of Cyp27a1−/− (Omarova et al., 2012) and Cyp27a1−/−Cyp46a1−/− animals (manuscript in preparation).
Quantification of cholesterol-hydroxylating enzymes was particularly challenging, because they are membrane-bound proteins and present at much lower levels than abundant vision-associated proteins (e.g., rhodopsin or RPE65). Such low protein levels in the NR and RPE could be detected only by a highly sensitive and accurate MS technique called multiple reaction monitoring (MRM) (Kitteringham et al., 2009). To adapt this technique to absolute quantifications of low abundant membrane-bound proteins, the procedures for the quantitative CYP46A1, CYP27A1 and CYP11A1 extractions from the membranes and enrichment of the extracted samples were established. The best possible internal standards (isotopically-labeled full-length recombinant CYP27A1, CYP46A1, and CYP11A1) were prepared and purified. Purified protein preparations were used to identify the signature peptides for each of the studied CYPs and transitions to be monitored by MRM. These transitions were then found in samples of human retina, and the proteins were quantified (Table 3) based on the calibration curves, rather than a single concentration of the internal standard (Liao et al., 2011; Liao et al., 2010; Wang et al., 2012). Measuring the CYP sterol products by GC-MS were more straightforward but required developing a protocol for simultaneous measurements of multiple cholesterol metabolites (Mast et al., 2011). Unexpectedly, these studies revealed that the major CYP27A1 metabolite in human NR was 5-cholestenoic acid, not 27-hydroxycholesterol, the most typical CYP27A1 product, which could then be oxidized two times by CYP27A1 and converted to 5-cholestenoic acid (Pikuleva et al., 1998). The production of 5-cholestenoic acid outside the eye is limited to only alveolar macrophages and vascular endothelial cells, and determined by high CYP27A1 expression, cholesterol availability, and the presence of an acceptor (albumin) in the medium (Babiker et al., 1999; Babiker et al., 1997; Reiss et al., 1997). Enzyme assays modeling CYP27A1 environment in the NR suggested that retinal formation of 5-cholestenoic acid is also determined by the enzyme expression levels and cholesterol availability and in addition, by the specific composition of retinal PLs containing high amounts of n-3 polyunsaturated fatty acids (Heo et al., 2012). The physiological necessity for 5-cholestenoic production in the NR is, however, unclear and remains to be established.
Table 3.
Comparison of protein and sterol metabolite levels of different cholesterol-metabolizing CYPs in human NR, RPE, and brain
| Donor | CYP27A1, fmol/mg protein |
27-COOHa, pmol/mg protein |
CYP46A1, fmol/mg protein |
24-OHa, pmol/mg protein |
CYP11A1, fmol/mg protein |
Pregnenolone, fmol/mg protein |
|---|---|---|---|---|---|---|
| Human Neural Retina | ||||||
| 17 | 570 ± 51 | 25b | 63 ± 5 | 2 | NDd | |
| 20 | 533 ± 63 | 51 | 59 ± 6 | 3 | ND | |
| 12 | 464 ± 38 | 130 | 58 ± 7 | 4 | ND | |
| 13 | 509 ± 51 | 29 | 59 ± 5 | 1 | 6c | ND |
| 8 | NDd | 75 | ND | 2 | 3 | |
| 9 | ND | 125 | ND | 1 | 2 | |
| 11 | ND | 37 | ND | 4 | 4 | |
| Human RPE | ||||||
| 17 | 1,170 ± 200 | ND | ND | ND | ND | ND |
| 20 | 1,570 ± 190 | ND | ND | ND | ND | ND |
| 12 | 1,460 ± 240 | ND | ND | ND | ND | ND |
| 13 | 2,010 ± 180 | ND | ND | ND | ND | ND |
| 8 | ND | 2 | ND | <1e | ND | |
| 9 | ND | 10 | ND | ND | <1e | |
| 11 | ND | 3 | ND | ND | ||
| Human Brain (gray matter temporal lobe) | ||||||
|---|---|---|---|---|---|---|
| Donor | CYP27A1, fmol/mg protein |
27-OHa, pmol/mg protein |
CYP46A1, fmol/mg protein |
24-OH, pmol/mg protein |
CYP11A1 | Pregnenolone, fmol/mg protein |
| 1 | 121 ± 19 | ND | 385 ± 45 | ND | ND | |
| 2 | 117 ± 11 | 22 ± 1 | 326 ± 35 | 1,294 ± 42 | 16 ± 5f | 14 ± 2 |
| 4 | 114 ± 16 | 14 ± 1 | 356 ± 34 | 1,339 ± 45 | 14 ± 1 | |
| 3 | 107 ± 19 | 11 ± 1 | 381 ± 37 | 1,160 ± 56 | 9 ± 1 | |
27-COOH, 5-cholestenoic acid; 24-OH, 24-hydroxycholesterol, 27-OH, 27-hydroxycholesterol.
Lack of error bars indicates single measurement; all other results represent the mean ± SD of triplicate measurements.
Bovine NR.
ND, not determined.
Limit of detection.
Donor 5.
Information sources (Heo et al., 2011; Liao et al., 2011; Liao et al., 2010; Mast et al., 2011; Wang et al., 2012)
The quantifications of sterols and cholesterol-catabolizing CYPs in human samples (Table 3) clearly identified CYP27A1 as the principal cholesterol hydroxylase in human NR and RPE. The average protein levels of CYP27A1 in the NR were much higher than the levels of CYP46A1, the second most abundant cholesterol hydroxylase (464-570 vs 58-63 fmol/mg of total retinal protein, respectively). Similarly, the concentrations of the CYP27A1 metabolite 5-cholestenoic acid were much higher than the concentrations of the CYP46A1 product 24-hydroxycholesterol (25-130 vs 1-4 pmol of sterol /mg of total retinal protein). The profile of CYP expression and cholesterol metabolite content in the brain (gray matter of temporal lobe) was the opposite, consistent with the role of CYP46A1 as the enzyme responsible for the majority of cholesterol elimination from this organ (Bjorkhem et al., 1998). Thus, different enzymes are important for cholesterol disposal in the brain and retina and different cholesterol products dominate in the retinal and cerebral sterol profiles. CYP27A1, the main retinal cholesterol-metabolizing enzyme, is detected in multiple retinal cell types, hence, unlike the brain, the NR does not have specific cell types (neurons) that are solely responsible for cholesterol elimination.
Simultaneous measurements of the protein and metabolite levels of cholesterol-metabolizing CYPs in the same human samples led to another important finding. Retinal concentrations of 5-cholestenoic acid were discovered to vary >5-fold in different individuals and did not correlate with the CYP27A1 protein levels (Liao et al., 2011). This discrepancy, absent in the brain, suggested retina-specific post-translational modification of CYP27A1 and was supported by another finding that one of the CYP27A1 peptides was consistently underrepresented in the MRM measurements (Liao et al., 2009). The retina contains high quantities of polyunsaturated fatty acids (Gulcan et al., 1993) and has a highly oxidative environment. Hence, CYP27A1 modification with isolevuglandins (isoLGs), oxidation products of arachidonic acid, highly abundant in the retina, was investigated, and the isoLG-modified CYP27A1 peptide was found in the aged human retina of the donor affected by AMD (Charvet et al., 2011). IsoLGs, which are much more reactive than most other lipid peroxidation products (e.g., malondialdehyde and 4-hydroxynonenal), bind to proteins and other biomolecules (phospholipids and DNA) non-specifically and affect in many cases their function (Salomon, 2005). The identification of the isoLG-protein adduct in human NR established a mechanism whereby aging and the associated oxidative stress could lead to disturbances in retinal cholesterol metabolism. The later, indirectly confirmed in a separate study demonstrating that the isoLG modification impairs CYP27A1 activity in vitro (Charvet et al., 2013a), gave impetus to the pharmacologic treatments with pyridoxamine, former dietary supplement, now an investigational new pharmaceutical, as the FDA has changed its regulatory status in 2009. Pyridoxamine, efficient scavenger of lipid peroxidation products including isoLGs (Caldes et al., 2011), was administered to mice after they were exposed to bright light to induce the formation of isoLGs (Charvet et al., 2013b). This treatment tested whether pharmacologic targeting of the downstream steps in the oxidative injury cascade, e.g. oxidation of lipids, reduces deleterious consequences of the oxidative stress. The idea behind this approach was to scavenge lipid peroxidation products before they bind and damage biomolecules. The pyridoxamine treatment indeed mitigated isoLG-associated retinal effects as assessed by increased proportion of mitochondria with normal morphology in the IS and decreased number of disrupted and fragmented mitochondria (Charvet et al., 2013b). These data suggest that scavengers of lipid peroxidation products should be considered for inclusion in antioxidant formulations for eye diseases that currently primarily target only the first step in the oxidative injury cascade, the production of reactive oxygen species. In support of this notion are the results of a randomized, double-blind placebo-controlled trial, in which 5442 females at high risk for cardiovascular diseases were treated with a combination of folic acid, cyanocobalamine and pyridoxine, a form of vitamin B6 that can be converted to pyridoxamine and other B6 vitamers. The data obtained suggested that this supplementation may reduce the risk for AMD (Christen et al., 2009).
The importance of CYP27A1 implicated by the characterization of human retinal samples is corroborated by ocular phenotype of people lacking CYP27A1, which develop cerebrotendinous xanthomatosis (CTX). CTX is characterized by multiple non-ocular (tendon xanthomas, neurological dysfunction, premature atherosclerosis and osteoporosis) and ocular manifestations. The latter include juvenile bilateral cataracts, cholesterol-like crystals in the vitreous, premature retinal senescence with drusen and retinal vessel sclerosis, cholesterol-like deposits along the vascular arcades, RPE defects on fluorescein angiography and optic disc pallor (Bjorkhem et al., 1995; Cruysberg et al., 1995; Dotti et al., 2001; Morgan et al., 1989). CTX is, however, a very rare disease, and thus difficult to study in humans, especially when the affected individuals have bilateral cataracts, one of the CTX manifestations. Therefore, a comprehensive ophthalmic evaluation of Cyp27a1−/− mice was carried (Omarova et al., 2012). In agreement with the enzyme importance for retinal cholesterol maintenance, the loss of CYP27A1 led to dysregulation of retinal cholesterol homeostasis, including unexpected up-regulation of retinal cholesterol biosynthesis. The Cyp27a1−/− retina was hypoxic, had activated Müller cells and focal deposits of cholesterol beneath the RPE associated with retinal-choroidal anastomoses. Blood vessel leakage was noted in the areas of retinal-choroidal anastomoses (Omarova et al., 2012). These abnormalities suggested the link between cholesterol metabolism in the retina and status of retinal vasculature, consistent with observed sclerosis of retinal blood vessels in CTX patients (Dotti et al., 2001). However, Cyp27a1−/− mice have limitations as a model of CTX because their systemic manifestations of CYP27A1 deficiency are less severe than in humans (Rosen et al., 1998). This seemed to be the case with the manifestations in the retina; therefore, Cyp27a1−/−Cyp46a1−/− mice were generated to mostly block retinal cholesterol metabolism. Vascular abnormalities in Cyp27a1−/−Cyp46a1−/− mice were more pronounced than in the Cyp27a1−/− line and included (besides retinal-choroidal anastomoses) additional types of lesions: arteriovenous shunts, localized vascular deformations as well as vascular leakage, dilation, non-perfusion and capillary degeneration (submitted for publication). Such a wide variety of vascular lesions provided further support for the notion that normal structure and function of retinal vasculature requires cholesterol metabolism to oxysterols. The caveat is that oxysterols may play a dual role in the retina as well as in any other organ (Bjorkhem, 2009; Pannu et al., 2012). CYP27A1- and CYP46A1-generated oxysterols represent the means by which cholesterol excess is transported to the liver for further degradation to bile acids. Simultaneously, these oxysterols may act as bioactive molecules interacting with insulin-induced gene (INSIG) protein and LXRs, one of the key proteins regulating cholesterol homeostasis (Brown and Goldstein, 2009; Kalaany and Mangelsdorf, 2006). The investigation of the mechanisms whereby the oxysterol products of cholesterol metabolism contribute to the health of retinal vasculature is under the investigation.
Unlike CYP27A1, no humans have been found so far to have CYP46A1 deficiency. CYP46A1 is, however, a highly polymorphic gene with the most frequent SNPs being in the introns or gene promoter regions. One of these SNPs (rs754203) was evaluated for association with primary open-angle glaucoma and AMD. The rs754203 was found to be a risk factor for the former but not the latter (Fourgeux et al., 2012; Fourgeux et al., 2009). The association with primary angle glaucoma was not, however, confirmed in a different study (Mossbock et al., 2011).
Pathways of cholesterol input and output in non-ocular organs are usually tightly coordinated to maintain cholesterol balance (Brown and Goldstein, 2009). An investigation of the capacity for cholesterol regulation in grossly normal peripheral retinas from 6 different human donors was carried out (Zheng et al., 2012). A PCR microarray was first used to profile retinal expression of 84 major genes involved in the biosynthesis and uptake of cholesterol from systemic circulation; intracellular cholesterol processing, trafficking, storage and regulation; and cholesterol elimination via metabolism and lipoproteins. Then three groups of genes (13 total) were assessed by qRT-PCR. The first group pertained to the regulation of cholesterol biosynthesis (SREBPs 1 and 2, SCAP, and INSIG1 and 2) and regulation of cholesterol elimination (LXRs α and β). The second group involved the genes of cholesterol input (HMGCR, LDL-R and ABCA1) as well as enzymatic removal (CYPs 27A1, 46A1, and 11A1). The third group comprised receptors participating in reverse cholesterol transport (SR-BI, SR-BII, and CD36). Protein expression of six of these 13 genes (SREBP, SCAP, INSIG, LXRs α and β, LDL-R, ABCA1, CYP27A1, CYP46A1 and CY11A1) was characterized by immunohistochemistry and correlated with histochemical cholesterol localizations conducted on adjacent retinal sections. The major findings are as follows.
First, every gene in the “Lipoprotein signaling and cholesterol metabolism” PCR microarray was detected in human NR and RPE (Zheng et al., 2012) suggesting that cholesterol homeostasis at these locations could be relatively independent from the rest of the body, consistent with the presence of the blood-retina barrier. Interestingly, in human NR, genes related to cholesterol biosynthesis were in general more abundant than genes from other functional groups. Despite this pattern, the expression of APOE (cholesterol transport) was the highest among cholesterol-related genes and similar to that of the housekeeping genes. These PCR array data are in a good agreement with the results of a recent study that carried out whole transcriptome expression analysis by RNA sequencing of different retinal regions from eight human donor eyes with normal maculas (Li et al., 2013). Overall, this comprehensive sequencing study showed that 80% of the transcriptome is expressed in the posterior layers of the eye (Li et al., 2013), and in that context, expression of the all cholesterol and lipoprotein genes detected by the PCR microarray were also detected, with a strong positive correlation in quantitative expression levels.
Second, immunolocalizations of the proteins involved in the regulation of cholesterol homeostasis (SREBP, SCAP, INSIG, LXRs α and β) as well as cellular cholesterol acquisition (LDL-R) and elimination (ABCA1, CYP27A1, CYP46A1 and CY11A1) suggested the presence of the pathways of cholesterol input and output in cell bodies of retinal neurons and axons of the ganglion cells and a strong transcriptional control of cholesterol levels at these locations by the SREBP/SCAP/INSIG complex and LXRβ (Fig. 6). This is in contrast to absent SREBP regulation of cholesterol input in the RPE, where cholesterol homeostasis seems to be regulated only by LXRβ at the level of cholesterol output (Dwyer et al., 2011; Zheng et al., 2012). Such regulation, combined with significant inter-individual variability in the RPE expression of LXRβ, also established in this study, points to the poor control of cholesterol input to the RPE. This finding offers an explanation for the previous in vivo investigation in rats suggesting constant, unregulated uptake of blood-borne LDL by the retina (Tserentsoodol et al., 2006b) and cell culture studies showing internalization of large amounts of LDL by RPE cells (Gordiyenko et al., 2004; Hayes et al., 1989). If indeed true, weak regulation of cholesterol input in the RPE could be one of the factors underlying the development of cholesterol-containing deposits leading to AMD in some but not all individuals.
Third, unexpectedly, cholesterol maintenance in the PR OS was found to be significantly different from that in other retinal layers as the OS had weak or absent signal for most of the studied proteins and also for cholesterol as assessed by filipin staining (Zheng et al., 2012). Low cholesterol content and apparent lack of the key proteins involved in cholesterol biosynthesis, uptake, metabolism, efflux, and regulation suggest OS-specific mechanism(s) of cholesterol input and output. Cholesterol input could involve either intracellular transport or passive diffusion from the IS because IS have a higher cholesterol content than OS. Cholesterol diffusion from the IS would also explain cholesterol gradient in the OS with the highest sterol concentration in the region bordering the IS, declining towards the OS tip (Albert and Boesze-Battaglia, 2005; Boesze-Battaglia et al., 1990; Boesze-Battaglia et al., 1989). Cholesterol removal from the OS could also rely on passive diffusion or mechanisms unrelated to ABCA1 efflux or CYP-mediated metabolism, for example, SR-BI/SR-BII-mediated flux. Previously, OS were suggested to acquire lipids from HDL-like particles via these receptors (Tserentsoodol et al., 2006a). We propose that OS could also use SR-BI/R-BII to offload cholesterol, with the net result being cholesterol elimination rather than acquisition, a proposed pathway with implications for AMD pathology (Section 4). Further studies are required to clarify how cholesterol is delivered and removed from the OS.
Fourth, the physiological role of low cholesterol content and unusual cholesterol maintenance in the OS extends beyond cholesterol gradient in the rod OS. About 10% of the rod OS are phagocytized every day by the RPE (Bok and Young, 1979), and this process could lead to significant cholesterol losses by the NR. If the OS were cholesterol-rich, more cholesterol would have to be replenished in the NR either via endogenous biosynthesis and/or cholesterol delivery from systemic circulation. Both processes are energy consuming (DeBose-Boyd, 2008; Pfrieger, 2003a). Cholesterol biosynthesis, for example, requires 18 molecules of ATP and 29 molecules of NADPH to synthesize one molecule of cholesterol. Therefore, OS is probably cholesterol-poor to utilize the energy saved for other processes, possibly visual cycle. Low cholesterol content in the OS is also in agreement with experimental data showing that high-cholesterol environment in the basal OS disks reduces the efficiency of the phototransduction cascade (Albert and Boesze-Battaglia, 2005), the key event in the vision process, at that location. Now that the unusual cholesterol maintenance in OS is identified, the specifics of its maintenance should be delineated. Since PR are unique neurons, this knowledge will expand our general understanding of cholesterol homeostasis in different cell types and will help to start putting together the general picture of the NR cholesterol homeostasis that accommodates and compensates for the daily phagocytosis-induced cholesterol loss, a pathway of cholesterol disposal unique to the NR.
3.4 Distribution of cholesterol in the RPE/BrM
Unlike the NR, the samples of human RPE/choroid region were shown to contain abundant EC (57% of total cholesterol) (Bretillon et al., 2008a) consistent with the earlier histochemical filipin stainings (Curcio et al., 2001) (Fig. 7). The cholesterol content of BrM is unique and relevant to AMD, and will be discussed in Section 4.
Fig. 7. Localization of cholesterol in human BrM and isolated drusen.
All drusen shown are considered hard. Drusen in E-F are mechanically isolated. Bars in C and F are 20 μm. A, Oil red O (ORO) binds to lipids in BrM and presumed retinoids in RPE lipofuscin. B. Filipin staining reveals intense fluorescence for EC, the predominant component of BrM lipid per direct assay of isolated lipoproteins (Curcio et al., 2009b). RPE lipofuscin is slightly autofluorescent at ultraviolet excitation wavelengths used for filipin visualization. C. Cholesterol localizes to lipoproteins in BrM and membranes of RPE and choroidal cells. RPE fluorescence is due to lipofuscin plus additional signal due to intracellular cholesterol. D. In drusen ORO binding EC shows a scalloped pattern with EC-poor cores at the base of many lesions, i.e., near BrM. E. A similar pattern is visible by filipin staining, plus dots signifying EC-rich lakes. F. In contrast, UC is particularly prominent in cores at druse base, perhaps signifying extracellular neutral pH cholesterol esterase activity that hydrolyzes EC, leaving cholesterol behind.
3.5. Cholesterol homeostasis in RPE/BrM: lifelong physiology, uncovered by aging
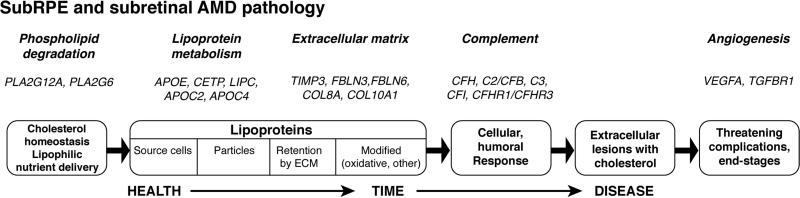
Key insight into chorioretinal cholesterol homeostasis was obtained through the study of aging human eye, motivated by the fact that aging is the largest risk factor for AMD (Jonasson et al., 2011; Rudnicka et al., 2012; Smith et al., 2001). One route to pathobiology insight is to ask if aging retina reveals biologic processes that serve as anatomical, physiological, and molecular predictors for disease initiation and advancement. Focus on BrM proved fruitful, because it is the compartment for AMD-specific lesions (drusen and BLinD), thus imparting great potential for direct involvement in lesion biogenesis. For the purpose of this section, it is sufficient to know that soft drusen and BLinD are two forms (lump and thin layer) of the same AMD-specific lesion, and both are on the inner surface of the inner collagenous layer of BrM (details in section 4.1).
Early electron microscopists described aged BrM as filled with debris, including amorphous electron dense material, membrane fragments, vesicles, and calcification (Bairaiti and Orzalesi, 1963; Nakaisumi et al., 1964). The involvement of lipids in this material was suggested by the earliest descriptions of drusen as lipid globules (Donders, 1854; Wedl, 1854)). The famous eye pathologist Verhoeff speculated that calcification of aging BrM might follow lipoidal deposition (Verhoeff and Sisson, 1926), perhaps by analogy with experimental atherosclerosis studies (Anitschkow, 1913). Later investigators described BrM as sudanophilic (i.e., containing histochemically detectable lipid) (Streeten, 1961; Wolter and Falls, 1962).
Clinical observations on fluid-filled RPE detachments in older adults led to Bird and Marshall's hypothesis (Bird and Marshall, 1986) that a lipophilic barrier in BrM blocked a normal, choroid-directed fluid efflux from the RPE. This hypothesis prompted a seminal 1990 study by Pauleikhoff et al (Pauleikhoff et al., 1990) that demonstrated material binding the histochemical stain oil red O localized exclusively to BrM of normal human eyes (Fig. 7), focusing attention on compounds bound by this stain (EC, TG, and FFA). This lipid staining was absent at the age <30 years, variably present in 31-60 year-old individuals, and abundant in ≥61 year-old individuals. Follow-up biochemical studies confirmed the strongly age-related nature of the deposition (Holz et al., 1994; Sheraidah et al., 1993). Pioneering mid-1990's studies by Marshall employed BrM explants to test the concept that BrM lipid deposition could impair the transport across this tissue of molecules necessary for outer retinal health (Moore et al., 1995; Starita et al., 1995; Starita et al., 1997). Thus a large, previously unknown, and constitutive process of outer retinal physiology was introduced.
The oil red O-binding material proved to be EC, which with UC accumulates markedly in BrM (Fig. 7), in 7-fold higher quantities in macula than periphery (Curcio et al., 2001; Haimovici et al., 2001). Among lipids, EC is confined exclusively to BrM whereas UC and phospholipids additionally localize to nearby cellular membranes (Rudolf and Curcio, 2009), focusing attention on lipoproteins, the only means by which EC is released by cells. Further evidence established RPE, not hepatocytes or enterocytes, as the source of apoB-containing lipoproteins. RPE expresses apoB gene and protein, along with MTP, required for apoB lipidation and secretion. Secretion of full-length apoB was demonstrated by two laboratories using metabolic labeling and immunoprecipitation in rat-derived and human-derived RPE cell lines (Wang et al., 2009b; Wu et al., 2010). The apoB-MTP combination marks RPE as a constitutive lipoprotein secretor. Further, genetic disorders signify that lipoprotein assembly and secretion is essential for retinal health. Lack of functional MTP and apoB is the basis of abetalipoproteinemia and hypobetalipoproteinemia, respectively, two rare inherited disorders that include pigmentary retinopathies, historically attributed to impaired dietary delivery of lipophilic vitamins by plasma apoB-containing lipoproteins but not reversible by long-term supplementation (Chowers et al., 2001).
Lipid-preserving ultrastructure and analytic biochemistry support the concept of an EC-rich, apoB/apoE-containing large lipoprotein secreted by RPE. Profiles historically called vesicles proved to be solid, space-filling particles, 60-80 nm in diameter. When viewed with lipid-preserving quick-freeze deep etch, these particles have a characteristic surface-and-core morphology resembling lipoproteins (Curcio et al., 2011a). Particles of comparable diameter with lipoprotein-like flotation properties and spherical shapes indicating neutral lipid cores are isolable from normal human BrM (Li et al., 2005a; Wang et al., 2009b). These fractions include apolipoproteins B, A-I, and E. BrM cholesterol is EC-enriched (EC/total cholesterol = 0.56), as replicated by five different assay methods (Bretillon et al., 2008b; Curcio et al., 2001; Li et al., 2005a; Wang et al., 2009b). BrM neutral lipid is EC-enriched as compared to triglycerides (EC/TG = 4-11), unlike hepatic VLDL, of similar diameter, which are TG-rich. An early report of TG-enriched BrM (Holz et al., 1994) was not replicated. Thus, BrM lipoproteins are unusual apoB-lipoproteins because they are large like VLDL and EC-rich like atherogenic LDL (Fig. 2). A natural history of BrM lipid deposition obtained with quick-freeze deep etch show lipoprotein particles first gathering among fibrils of the BrM elastic layer in early adulthood (Huang et al., 2007; Ruberti et al., 2003). This accumulation then extends toward RPE to fill the inner collagenous layer (ICL), eventually leading to a new layer between the ICL and RPE basal lamina in many older eyes. Initially called a Lipid Wall, this layer is now recognized as pre-BLinD, the immediate precursor to BLinD (Section 4) (Huang et al., 2007). The pattern of age-related lipid deposition in BrM, whereby particles first appear in the elastic layer and fill in towards the RPE, is consistent with an RPE origin. (Huang et al., 2007)
An attractive and long-standing hypothesis that debris in aging BrM might represent disposed OS membranes phagocytosed by RPE (Hogan, 1972) was directly tested through fatty acid profiling of EC and other lipid classes in isolated BrM lipoproteins (Bretillon et al., 2008b; Wang et al., 2009b). This question was approachable because of the distinctively high concentration of docosahexaenoate in OS membrane phospholipids (Fliesler and Anderson, 1983). Studies in two laboratories showed that all lipid classes in isolated particles are dominated by the fatty acid linoleate (abundant in plasma) with little docosahexaenoate (abundant in retina). This result implicates plasma lipoproteins and not photoreceptors as a major upstream source of the fatty acids. BrM lipid deposition, shown as steps 1-4 and 6 in Fig. 8 is thus proposed as a recycling system in which plasma lipoprotein delivering dietary essentials (perhaps even vitamin A) are stripped of cargo destined for PR, and excess fatty acids and cholesterol is repackaged for secretion to BrM and eventual choroidal clearance. This logic is analogous to finding milk cartons in neighborhood curbside recycling bins (BrM) and deducing that milk had been consumed by the householders. The paucity of docosahexaenoate in BrM is consistent with the lack of immunoreactive rhodopsin in BrM (Feeney-Burns et al., 1988) and is inconsistent with models of AMD pathogenesis invoking docosahexaenoate by-products as primary instigating agents (Hollyfield et al., 2008).
Fig. 8. Proposed RPE lipid inputs-outputs and a model for AMD lesion formation.
BLinD/soft drusen and SDD are localized in two different compartments (below and above RPE, respectively). Normal aging RPE is at the left and center. AMD is at right. The input-output pathways are suggested based on available data. These pathways serve as a basis for the model (2L2C model) that identifies RPE-based lipid recycling pathways for rods and cones as biologic processes that drive the formation of AMD extracellular lesions. Different cholesterol content of rod and cone OS membranes is of key importance The sub-RPE lesions are plausibly formed when passage of constitutively secreted products from RPE are either overproduced or retained instead of being efficiently cleared. The evidence for this mechanism is good and involves the following steps: 1) Plasma lipoproteins delivering lipophilic essentials, including vitamins E, A, lutein, and cholesterol (UC), enter basolateral RPE (Tserentsoodol et al., 2006b). 2) ApoB,E lipoproteins secreted basolaterally by RPE (Johnson et al., 2011) (gold circles) are assembled from multiple lipid sources. Fatty acids in lipoproteins isolated from BrM are dominated by linoleate, implicating internalized plasma lipoproteins (from step 1) as a major source. UC from all sources is esterified to EC. 3) Lipoproteins are retained by interacting with BrM extracellular matrix and accumulate throughout adulthood, creating pre-BLinD on BrM's inner surface. 4) Reactive oxygen species from nearby mitochondria promote appearance of pro-inflammatory and toxic moieties. Lipoproteins fuse and form lipid pools and UC-rich liposomes within BLinD/soft drusen, rendering them biomechanically unstable, pro-inflammatory, and cytotoxic. 5) Disks in rod OS lose UC and gain docosahexaenoate in transit from OS base to tip (Albert and Boesze-Battaglia, 2005) (shown as loss of white). OS-derived docosahexaenoate stored as triacylglycerol in RPE after phagocytosis return to OS (Rodriguez de Turco et al., 1999). HDL particles cycling between RPE and photoreceptors (Tserentsoodol et al., 2006a) could handle both transfers as part of a vectorial lipid flow retainable within interphotoreceptor matrix as UC-containing SDD, especially under rod-rich perifovea. 6) Cone OS maintain high UC content along their length, because their disks are comb-like projections of plasma membrane (Albert and Boesze-Battaglia, 2005). Cone OS UC enters RPE via disk shedding, lysosomal uptake, and acid lipase activity (Elner, 2002). UC is released for intracellular transfer, esterification, and assembly into basolaterally-secreted lipoproteins, especially under cone-rich fovea. The mechanism for the sub-retinal lesion (SDD) formation is not yet known. Adapted with permission from (Curcio et al., 2013).
If diet rather than OS phagocytosis drives RPE lipoprotein secretion into BrM, and OS are not required for this process, then cells in culture medium alone should be able to create deposits. A landmark 2011 study by Johnson et al confirmed these predictions (Johnson et al., 2011), demonstrating that highly differentiated and polarized human fetal RPE maintained only in culture medium secreted apoE-immunoreactive particles into Transwell insert pores in 2 morphologies, one resembling BrM lipoproteins seen in situ and the other an electron-dense amorphous material. Particulate apoE bound exogenously supplied complement components, allowing detailed study of plasma and intra-ocular contributors to drusen for the first time.
While the source of cholesterol in BrM lipoproteins has not been determined by published studies to date, endogenous synthesis, taken-up plasma lipoproteins, and phagocytosed OS are three obvious choices. Despite low cholesterol concentration in rod OS membranes, in the aggregate they are now a strong possibility for source, due to the discovery of cholesterol-bearing AMD lesions with a rod-like topography in the subretinal space (see Section 4.1). Further, quantitative filipin histochemistry (Curcio et al., 2005a) revealed that cholesterol in drusen and BLamD was much more concentrated than could be accounted for by pass-through of unprocessed OS membranes into BrM, suggesting an enrichment process, additional sources of cholesterol, or both.
Finally, it is informative to contrast BrM lipid deposition with the systemic process of perifibrous lipid, the background process that gives rise to atherosclerosis in large arteries. Extracellular oil red O-binding lipids increase with age in other normal human connective tissues, including arterial intima (Smith, 1974)], sclera (Broekhuyse, 1972), and cornea (Gaynor et al., 1996). The source of extracellular EC in these locations is LDL translocated from plasma. EC-enriched particles are thought to arise from smaller LDL particles by extracellular matrix-mediated trapping of LDL, followed by degradation of LDL protein and/or PL components, and fusion of the remaining lipid components (Kruth, 1997). The evidence that lipid deposition in aging BrM is a distinct phenomenon dictated by needs of the photoreceptors and not simply an ocular manifestation of plasma-LDL-driven perifibrous lipid is compelling. However, the commonality of cholesterol-rich lipoproteins in a vessel wall is a rationale for seeking guidance in cardiovascular disease for AMD pathogenesis and treatments.
4.0 Cholesterol and the specific lesions of AMD
4.1 Drusen, BLinD, and SDD
AMD can be productively considered a multifactorial disease of the PR support system, involving the RPE and BrM. AMD has vascular, metabolic, and inflammatory components, with a secondary neurodegeneration of the PR. Thus AMD is distinguished from the many monogenic diseases affecting primarily photoreceptors. PR degeneration and loss is the basis of visual dysfunction in AMD. These occur in the setting of a thick and stereotypic deposition of extracellular material, interposed between outer retinal cells and their blood supply and providing a vascular insufficiency component to this disease. AMD can be compared in many ways to atherosclerotic cardiovascular disease, which also features extracellular lipoprotein deposition in a vessel wall of the systemic circulation and end-stages including calcification and neovascularization (Curcio et al., 2010). Thus the investigations described below were guided by the conceptual framework known as the Response-to-Retention hypothesis of atherosclerosis (Curcio et al., 2009a; Williams and Tabas, 1995). We will describe four types of AMD-specific lesions that all contain cholesterol (drusen, BLinD, basal laminar deposit (BLamD), and subretinal drusenoid deposit (SDD)).
Drusen are focal extracellular deposits that lie in the sub-RPE space, bounded internally by the basal lamina of the RPE and externally by the internal collagenous layer of BrM. That lipids were a major druse component was recognized by their discoverers (Wedl, 1854; Wolter and Falls, 1962). Every druse has histochemically detectable EC and cholesterol (Fig. 7) (Curcio et al., 2001; Curcio et al., 2005a), and 40% of hard druse volume is Folch-extractable lipid, with EC and phosphatidylcholine predominant (Wang et al., 2010). The essential ultrastructural descriptions of drusen were provided by Shirley and John Sarks (Bressler et al., 2006; Sarks et al., 1988, 1994; Sarks et al., 1980). The multiple layers of AMD pathology were defined by the Sarks and Green (Green and Enger, 1993) (Fig. 9) and standardized by Marshall (Marshall et al., 1998). Large or soft drusen are major risk factors for AMD progression (Klein et al., 2007). Hard drusen are especially numerous in peripheral retina (Friedman et al., 1971; Lengyel et al., 2004; Rudolf et al., 2008a). Although some BLinD can be present peripherally, both it and high-risk soft drusen are found preferentially in the central macula (Rudolf et al., 2008a) (Yehoshua et al., 2011), with BLinD thickest under the fovea (Curcio et al., 2013). The principal component of soft drusen was originally called membranous-debris by the Sarks (Sarks et al., 1988) and renamed lipoprotein derived debris by one of us (CAC) to reflect an origin that better explained the high EC content in these lesions, without excluding the possibility that shed cellular membranes do appear in drusen (Wang et al., 2009a). This debris was seen in 5 places - soft drusen, BLinD, tracks or aggregations within BLamD, within RPE, and in the subretinal space. The latter is now called SDD (Rudolf et al., 2008b), and it is recognized as a lesion distinct from drusen (see below).
Fig. 9. Layers of AMD pathology.
Histology of the outer retina shows distinctive AMD pathology on either aspect of the retinal pigment epithelium (RPE). SDD is subretinal drusenoid deposit (bright yellow), an extracellular lesion surrounded by delicate RPE apical processes containing melanosomes. The RPE is at stage of degeneration featuring melanosomes and lipofuscin granules shed into underlying BLamD (pale yellow). BLamD is basal laminar deposit, a stereotypically thickened RPE basal lamina, often containing basal mounds. The latter are an aggregation of soft druse/BLinD material in transit from the RPE to BrM. Pre-BLinD is layer of lipoprotein particles internal to the inner collagenous layer of BrM and an immediate precursor to BLinD (basal linear deposit). BLinD (not shown here) is pooled lipoprotein-derived debris, in a thin layer. A lump large enough to elevate the RPE is a soft druse. BrM is the inner surface of the choroidal vasculature, and thus a vessel wall in addition to a stratum for RPE attachment. It has inner and outer collagenous layers that are calcified and glassy-appearing in this example. ChC, choriocapillaries with fenestrated endothelium, are partly atrophied in this example. Other layers of the neurosensory retina: OPL, outer plexiform layer; HFL, Henle fibers; ONL, outer nuclear layer; IS, inner segments; OS, outer segments. From a 85 yr old Caucasian male with geographic atrophy, 3 mm from the foveal center, 0.8 μm section, osmium tannic acid paraphenylenediamine post-fixation, toluidine blue stain. With permission from the Annual Review of Genomics and Human Genetics, Volume 15 © 2014 by Annual Reviews, http://www.annualreviews.org (Fritsche et al., 2014).
Soft drusen and BLinD are two forms, i.e., a lump and thin layer, respectively, of the same AMD-specific lesion containing lipoprotein-derived debris. These forms may be interconvertible, as multiple imaging technologies reveal that drusen are dynamic (Acton et al., 2011; Sarks et al., 1994; Yehoshua et al., 2011). This debris consists of native and fused lipoprotein particles, exhibiting the physical forms of cholesterol seen in the lipid rich core of atherosclerotic plaque with the notable exception of cholesterol crystals (Curcio et al., 2005b; Guyton and Klemp, 1989; Small, 1988), a mark of atheroma maturity. Soft drusen contains polygonal lakes of EC up to several micrometers in length, as well as membranous profiles. Lipoprotein particles within hard drusen are dispersed within a proteinaceous matrix, sometimes forming a rim around the druse exterior (Anderson et al., 2004; Li et al., 2006; Wang et al., 2009b). Circumstantial evidence suggests that soft drusen are more highly cholesterol-enriched and biomechanically fragile than hard drusen. All drusen contain cholesterol, yet macular drusen are less frequently immunoreactive for apoB and apoE than hard drusen, consistent with a higher lipid:protein ratio (Malek et al., 2003). Further, only 20% of isolated macular soft drusen survive with complete contents (Rudolf et al., 2008a). Histopathology of intact tissues shows detachment at 75% of sites of BLinD vs none at sites of BLamD (Curcio et al., 2013). The presence of 15 μm-diameter cores poor in EC and rich in UC, even in the smallest drusen, suggest ongoing lipase activity and remodeling in the extracellular space (Norlin and Wikvall, 2007; Rudolf et al.).
A model of soft druse/BLinD formation proposes that constituently secreted apoB/apoE-containing lipoprotein from RPE are retained by an increasingly less passable BrM, until an oily layer forms on BrM's surface, with oxidation or other modification followed by fusion of individual lipoproteins over time to form BLinD. The process has been likened to an oil spill on aging BrM (Curcio et al., 2011a). The net effect is to promote inflammation and type I choroidal neovascularization in a narrow cleavage plane between the RPE basal lamina and the inner collagenous layer of BrM, as proposed early (Spaide et al., 2003). Compounds capable of these side effects are linoleate hydroperoxide and 7-ketocholesterol, by-products of lipoproteins found in atherosclerotic plaque (Upston et al., 2002) and also in aging BrM (Moreira et al., 2009; Spaide et al., 1999) and drusen (I. Rodriguez and C.A. Curcio, work in progress) and confirmed as deleterious in vivo (Amaral et al., 2013; Baba et al., 2010). Thus RPE-secreted apoB/apoE lipoproteins are a source of peroxidizable lipids immediately subjacent to the RPE, in the compartment where choroidal neovascularization occurs. Reactive oxygen species from basolaterally localized RPE mitochondria are thus just micrometers away from these lipoprotein particles.
The abundance of BLamD, a diffuse and stereotypic thickening of RPE basal lamina, is a marker of disease severity, likely reflecting RPE stress level (Sarks et al., 2007; Sarks, 1976). BLamD is ultrastructurally basement membrane in early and late forms (Sarks et al., 1988), with 120 nm periodicity long spacing collagen (type VI) (Knupp et al., 2000). BLamD contains histochemically detectable cholesterol along the basal aspect next to BrM (Curcio et al., 2005b; Lommatzsch et al., 2008) and is crossed by linear tracks of lipoproteins stretching between the RPE and BrM. The orderliness of this disposition perhaps signifies guiding of transiting lipoproteins by tethering proteins (Milam et al., 2000). A thick BLamD with ultrastructural signatures suggesting similar lipid tracks has been demonstrated in at least 4 engineered mouse models (Esteve-Rudd et al., 2012; Garland et al., 2013; Marmorstein et al., 2007; Wavre-Shapton et al., 2013). These findings may mean that mouse RPE also secretes lipoproteins that are revealed when retained by the appropriate matrix. Next steps include determining lipid content of these lesions with lipid-preserving ultrastructural techniques and determining molecular commonality between mouse and human BLamD.
A century and half after drusen were discovered, a new layer of AMD pathology in the subretinal space was recognized - reticular pseudodrusen and their leading histological correlate, SDD (Zweifel et al., 2010). The prevalence of these biomicroscopic fundus signs, first seen in 1990 (Mimoun et al., 1990), varied with imaging technique and patient population (review (Curcio et al., 2013)) with estimates up to 90% of geographic atrophy patients (Schmitz-Valckenberg et al., 2011). A distinct patterning of multifocal, regularly distributed lesions was suggestive of an inflammatory component, like a skin pox (Smith et al., 2006). Initially thought to be located in the choroid (Arnold et al., 1995), histopathology and high-resolution imaging have combined to put these solid-space filling lesions firmly in the subretinal space (Curcio et al., 2013; Mrejen et al., 2013; Querques et al., 2012; Sarks et al., 2011; Zweifel et al., 2010). The smallest lesions are the size of individual RPE cells, and the largest ≥50 μm tall. Superjacent PR have deflected, shortened, or missing OS and IS. Because SDD are adjacent to PR, they are potentially directly cytotoxic in addition to interposing a diffusion barrier. SDD accumulation is associated with progression to type 3 neovascularization (retinal angiomatous proliferation) (Ueda-Arakawa et al., 2012). A lesion life cycle observed via longitudinal clinical imaging includes substantial atrophy of outer retina as SDD is degraded (Spaide, 2013). Initial reports indicate that vision function over SDD can be markedly impaired, even more so than for drusen (Mrejen et al., 2013; Querques et al., 2013). Of major pathogenic import is the finding that histologically detected SDD is abundant in the rod-rich perifovea, in contrast to BLinD, which is thickest under the fovea (Curcio et al., 2013). Of major public health significance, SDD are misclassified or omitted from grading systems utilizing color fundus photography (Bird et al., 1995; Davis et al., 2005; Ferris et al., 2013; 1991; Seddon et al., 2006). These systems have provided the basis of disease prevalence estimates and gene association studies for over two decades.
SDD are associated with inherited, acquired, and experimental dysfunction in retinoid delivery pathways (Aleman et al., 2013; Berson, 1973; Genead et al., 2010; Querques et al., 2009; Schatz et al., 2010), yet are not clinically autofluorescent (Schatz et al., 2010; Spaide and Curcio, 2010), suggesting a lack of bisretinoids while not excluding retinoids fluorescing at other wavelengths. Limited compositional data to date suggest that SDD excludes markers for outer retinal cells and contains proteins CFH, vitronectin, apoE, and CD59 plus cholesterol, with little evidence for the EC of intra-ocular lipoprotein origin that dominates conventional drusen (Curcio et al., 2005a; Ebrahimi et al., 2012; Oak et al.; Rudolf et al., 2008b). Thus SDD are not simply drusen in the wrong place but distinctive lesions that merit investigation on their own terms.
Of relevance to SDD are variants in CETP and LIPC that modify AMD risk independent of plasma HDL levels (Chen et al., 2010; Neale et al., 2010; Yu et al., 2011). ApoE, CETP, LIPC, LCAT, and SRB-II immunoreactivity, along with PLTP activity, localize to the subretinal space, likely interphotoreceptor matrix (Dudley and Anderson, 1978; Tserentsoodol et al., 2006a). ApoE is secreted by RPE and Müller cells, appearing in aspirates from rhegmatogenous retinal detachments (Anderson et al., 2001; Ishida et al., 2004; Schneeberger et al., 1997; Shanmugaratnam et al., 1997; Wong et al., 2000). SDD contains complement cascade components and regulators (Ebrahimi et al., 2012; Rudolf et al., 2008b). Thus numerous molecules with well-known HDL associations are present in the subretinal space and could participate in SDD biogenesis.
4.2 2-lesion, 2-compartment model of AMD lesion biogenesis (2L2C)
At the time of previous review articles (Curcio et al., 2010; Curcio et al., 2009a; Curcio et al., 2011a), the importance of SDD was only just being realized. Three findings suggest that the specific cholesterol-containing lesions of AMD can be incorporated into one comprehensive 2-lesion, 2-compartment model (Fig. 8). First, both SDD and BLinD/soft drusen contain apoE and cholesterol, suggesting that these lesions had together uncovered a larger system of outer retinal lipid recycling involving more than just BrM. Second, the finding that SDD and BLinD topography mirrored the topography of rods and cones, respectively, in human macula (Curcio et al., 1990) suggested that differential aspects of rod and cone physiology, such as cholesterol homeostasis, were being revealed. There is a precedent in bifurcate delivery of retinoids to these photoreceptor types (Garlipp and Gonzalez-Fernandez, 2013; Mata et al., 2002). Third, the biological processes in the proposed model can together account for 19 of 24 genes highlighted by a recent GWAS meta-analysis of the AMD Consortium (Fig. 10). These genes can be incorporated in an atherosclerosis-like schema that accentuates lesion formation and its sequelae, and both sub-retinal and sub-RPE compartments are necessary.
Fig. 10. Genes and pathobiology align.
Shown at the top are biological processes encompassing genetic associations of AMD recently published by the AMD Gene Consortium Meta-analysis (Fritsche et al., 2013) comprising 17,000 late AMD cases. In the boxes is an atherosclerosis-like “response to retention of lipoproteins” theory of pathogenesis (Tabas et al., 2007; Williams and Tabas, 1995, 1998) that has been re-tooled for sub-RPE AMD pathology involving BrM, soft drusen, and BLinD, and neovascularization (Curcio et al., 2009a; Spaide et al., 2003). Evidence supporting the pathobiology model was recently called compelling (Miller, 2013). Newly characterized AMD pathology in the subretinal space (SDD) (Curcio et al., 2013) can be accommodated within this multi-pathway scheme if cholesterol and lipoproteins are considered the unifying features. Although a very large genetic association ARMS2/HTRA1 is not yet included, because the function of its encoded gene product is uncertain, 19/24 genes identified by this comprehensive meta-analysis (Fritsche et al., 2013) can be assigned to biological processes along this scheme. The processes are weighted towards formation and sequelae of the extracellular lesions, and including both sub-RPE and sub-retinal compartments accounts for the most number of genes.
As highlighted above, a pathogenic model for BLinD and soft drusen (Curcio et al., 2009a; Curcio et al., 2011a) borrows mechanisms from nutrition and atherosclerotic cardiovascular disease with an intra-ocular lipoprotein source as the proximal disease instigator rather than plasma LDL (summarized as steps 1-4 in Fig. 8). Similar processes may occur in the sub-retinal space in the formation of SDD (Zweifel et al., 2010). The model in Fig. 8 invokes the differential cholesterol content of rod and cone OS membranes to explain why SDD accumulates in the subretinal space under rod-rich perifovea and BLinD accumulates in the sub-RPE space under cone-rich fovea (Curcio et al., 2013). Central to this hypothesis is the idea that the RPE is a polarized and bi-directional secretor of lipoproteins that serve both photoreceptor and RPE physiology.
Rod OS disks pinch off from the plasma membrane near the inner segment. They become internal membranes, which unlike plasma membranes, are low in cholesterol content (10% vs 30-35%) (Boesze-Battaglia et al., 1990; Dowhan and Bogdanov, 2002). In transit from OS base to tip, (Albert and Boesze-Battaglia, 2005) disks reduce cholesterol and increase the fatty acid docosahexaenoate within phospholipids (step 5, Fig. 8). These changes enable the conformational flexibility of rhodopsin required by single-photon sensitivity. OS-derived docosahexaenoate stored in RPE after disk shedding and phagocytosis is recycled back to inner segments (Bazan et al., 1992; Rodriguez de Turco et al., 1999) by an as-yet unspecified mechanism. HDL particles cycling between RPE and photoreceptors, proposed for intra-retinal lipid transfer to inner segments (Tserentsoodol et al., 2006a), could move both UC from, and docosahexaenoate to, OS disks progressing toward the RPE. In contrast (step 6, Fig. 8), cone OS disks are comb-like projections of plasma membrane and are believed to maintain high cholesterol content along their length (Albert and Boesze-Battaglia, 2005). Cone OS cholesterol enters RPE via disk shedding and lysosomal uptake. This cholesterol is released for intracellular transfer, esterification, and assembly into basolaterally-secreted apoB,E-containing lipoproteins, especially under cone-dominant fovea, where they form the basis of BLinD (Step 3, 4, Fig. 8). Using perturbation of cholesterol homeostasis and lipid transfer as unifying mechanisms, it may be thus possible to explain the formation of SDD in areas enriched with rods and BLinD under the cone-dominant fovea, with downstream negative consequences such as inflammation, neovascularization, and cytotoxicity, specific to both compartments.
The 2-lesion, 2-compartment model is both speculative and testable, and it accounts for more data than other extant models of lesion formation, e.g., involving models amyloid deposition or RPE lipofuscin and its components or adducts. The precise laminar and regional specificity of AMD lesion formation are features that other theories invoking inflammation and phototoxicity are yet to incorporate.
4.3 Why the macula?
The predilection of macula for AMD is an enduring and critical question that can be approached by examining outer retinal anatomy and physiology for large differences between macula and periphery. Although light exposure over the lifespan is often mentioned as contributing to oxidative damage in AMD, illuminance at the retina is almost uniform across the fundus (Kooijman, 1983; Pflibsen et al., 1988). The distribution of esterified cholesterol in age-normal BrM, however, has a very strong macula to periphery gradient (7:1) (Curcio et al., 2001). Although PR, RPE, and choroid form a tightly integrated neurovascular unit, the distribution of PR does not obviously account for a gradient in BrM. We suggest a model based on blood flow, that could combine with differential photoreceptor (cones vs rods) OS content to produce this effect. We will again analogize BrM to a vascular intima (inner wall of artery) (Curcio et al., 2001), and seek guidance from atherosclerotic cardiovascular disease (CVD), which also features extracellular lesions (plaques) made of accumulated apoB-lipoproteins.
Atherosclerotic plaques occur at stereotypic sites in the vascular tree, i.e., at sites of subendothelial lipid accumulation. Although CVD risk factors are systemic, the vulnerability to lipid accumulation is local and related to blood vessel bifurcation points. The arterial intima thickens physiologically at these points with normal aging (Giddens et al., 1993; Malek et al., 1999; Stary, 1992; Stary et al., 1992; VanderLaan et al., 2004), an adaptive process secondary to mechanical stress due to variations in physiological blood flow. Hemodynamics thus “prime the soil” in which lipoproteins accumulate and lesions develop. In CVD the “soil primed” may be extracellular matrix, endothelial cells, or smooth muscle cells, or all three.
Analogous processes occur in BrM, whose thickening of extracellular matrix with age may be the “primed soil” into which RPE-secreted lipoproteins lodge and eventually form drusen. Blood flow in choriocapillaries is 7-11X greater in macula than periphery (Alm and Bill, 1973; Nork et al., 2006), and the reason for this huge difference is not understood. It is not accounted for by either increased photoreceptor density (2.7 for rods, 1.9 for cones (Curcio et al., 1990) or increased outer retinal thickness (2.3; CAC, unpublished data) in the macula. Limited data suggest that species lacking maculas also lack a large central-to-peripheral gradient in blood flow (Ahmed et al., 2001). High blood flow suggests that macular cells have unique physiological and anatomical requirements, likely dictated by high-acuity vision (Provis et al., 2013). One specialization is the Henle fibers. These comprise extraordinarily long fibers belonging to two cell types: photoreceptor axons that transmit graded potentials to inner retinal cells and Müller cell processes that are interleaved between the photoreceptors (Curcio et al., 2011b; Drasdo et al., 2007; Polyak, 1941). Another is the relative enrichment of cones (9:1, rods:cones, in macula, vs 20:1 for the entire retina (Curcio et al., 1990). Cone IS have abundant mitochondria, one of the most metabolically active organelles, and in cones, mitochondria may have optical functions as well (Hoang et al., 2002; Spaide and Curcio, 2011). It will be interesting to test this vascular hypothesis by determining regional differences in choroidal blood flow in a species with an area of high cone density but lacking a foveal pit and Henle fibers (Beltran et al., 2014).
Whatever biological processes are involved in creating SDD in the subretinal space will also have differences between macula and periphery that are yet to be defined. The topography of this lesion is not yet fully characterized and may extend well into mid-peripheral retina. The relative number of cones and rods and their lipid homeostasis requirements including cholesterol (Table 2) and retinoids are likely players, and more factors await discovery. Clearly AMD has opened a vast window into outer retinal biology waiting to be explored.
5.0 Future directions
We offer a Top Twelve list of current pressing questions about the role of cholesterol in the retina, organized into groups pertinent to biochemistry/nutrition of chorioretinal region, AMD pathobiology, and translational research.
5.1 Chorioretinal biochemistry and nutrition
5.1.1. GWAS GENES CHORIORETINAL LOCALIZATION AND FUNCTION
The direction of unquestionable importance is the delineation of chorioretinal significance of the cholesterol-related genes identified by GWAS as risk factors for AMD (CETP, ABCA1, LIPC and APOE). The function of the proteins encoded by these genes outside the eye is well established as are their localization in the retina. We do yet know how different variants of these genes affect chorioretinal cholesterol homeostasis and function of the retina, and we do not know how the localizations may vary with among individuals or stage of disease.
5.1.2. PATHWAYS OF CHOLESTEROL INPUT AND OUTPUT
Knowledge of the quantitative significance of different pathways of cholesterol input and output in the chorioretinal region and specific cell types continues to be an outstanding question in the field. The importance of these studies has been stated in the previous review (Fliesler and Bretillon, 2010); yet only limited progress has been made in this direction within the last three years, mainly pertinent to acknowledging specific cholesterol maintenance in the PR (Claudepierre et al., 2010; Zheng et al., 2012) and spatial distribution of the retinal transcriptome (Li et al., 2013). Knowledge of which pathways are the most important for chorioretinal cholesterol homeostasis, particularly with PR will facilitate prioritizing our research directions. Rapid progress in cardiovascular field following the delineation of the major principles of the whole body cholesterol maintenance nicely illustrates this point and is a good example to follow.
5.1.2. REGULATION OF CHOLESTEROL HOMEOSTASIS
We need to have a better understanding of how cholesterol homeostasis is regulated in the retina and how to shift the cholesterol input↔output equilibrium toward cholesterol elimination when there is the lipid deposition in the RPE/BrM region (Curcio et al., 2011a; Curcio et al., 2013; Oak et al., 2014). Knowledge of the most important regulatory pathways of cholesterol homeostasis in the retina is of great medical relevance (section 5.3.3) and will help us to understand, among other approaches, whether to focus on cholesterol-lowering drugs statins that reduce cholesterol biosynthesis, agents that affect the apoB and MTP production, or LXR agonists that enhance cholesterol elimination, both non-enzymatic (by lipoproteins) and enzymatic (by metabolism) (section 5.3.3).
5.1.3. CHORIORETINAL NUTRITION
Above we hypothesized that a force driving the assembly and secretion of BrM lipoproteins is dietary delivery of nutrients, with phagocytosed OS an important determinant of the final EC-rich composition. What dietary nutrients are being delivered, and by which plasma lipoproteins? Retinoid supply for phototransduction seems to take multiple parallel pathways, (Ruiz et al., 2012; Vogel et al., 2002) – could plasma LDL deliver vitamin A and be recycled into BrM lipoproteins? What about plasma HDL delivering lutein (Loane et al., 2010)? Better understanding of plasma lipoprotein-mediated outer retinal nutrition also has clinical importance, as dietary anti-oxidant supplementation will be important for AMD treatment for years to come, thanks to large clinical trials establishing efficacy (2001; 2013). Conversely, the finding of retinyl palmitate in BrM lipoproteins (Wang et al., 2009b) suggests an interaction with the retinoid system (Qtaishat et al., 2003), as it does in the intestine (O'Byrne and Blaner, 2013), where chylomicrons are loaded with retinyl palmitate for systemic delivery.
5.2 AMD pathobiology
5.2.1. MOLECULAR COMPOSITION OF AMD LESIONS
Lesion composition will continue to reveal previously underexplored biology. SDD natural history, precursors, molecular composition, physical structure, and interaction with cells bordering the subretinal space are now a priority, so as to bring understanding of this lesion to the same level as sub-RPE drusen. These questions are important, because evidence points to the subretinal space as a prime site of action for the HDL genes implicated by the AMD GWAS. Molecularly informed clinical imaging of lesion ultrastructure and composition is now approaching reality through high resolution imaging utilizing adaptive optics ophthalmoscopy and spectral domain optical coherence tomography (Meadway et al.; Mukkamala et al., 2012). Here clinical ophthalmology and cardiovascular medicine may find synergy in the imaging of cholesteryl linoleate-rich atherosclerotic plaques and drusen (Fleming et al., 2013), both formed by deposited apoB-lipoproteins.
5.2.2. CYTOTOXIC MOLECULES IN LESIONS
An important question about lesion composition is the site, source, and action of cytotoxic molecules that derive from lipoproteins and other lipid sources, with capability for forming adducts of proteins, other lipids, and DNA. These molecules include linoleate hydroperoxide (Spaide et al., 1999), carboxyethylpyrrole (Crabb et al., 2002), oxidized phosphatidylcholine (Suzuki et al., 2007), 4-hydroxynonenal (Shen et al., 2007), malondialdehyde (Weismann et al., 2011), isoLGs (Charvet et al., 2013b), and 7-ketocholesterol (Moreira et al., 2009). Of these, 7-ketocholesterol (Rodriguez and Curcio, work in progress) and isoLGs (Charvet et al., 2013b) has been shown specific to drusen, as they are minimally present in other layers. Others are present in drusen/BrM and are either also present in other layers (carboxyethylpyrrole, 4-hydroxynonenal, oxidized phosphatidylcholine) or have not yet been tested for presence in other layers (linoleate hydroperoxide, malondialdehyde) so whether lesions are preferentially enriched in these toxic compounds are unknown. SDD remains unexplored in this respect. Model systems utilizing cultured RPE to create lesion components should be tested for these compounds (Johnson et al., 2011).
5.2.3. INTERACTION WITH OTHER PATHWAYS
AMD specific lesions rich in cholesterol also contain considerable immunoreactivity for proteins of the complement cascade. Thus the interaction of toxic molecules with cellular and humoral response system, including complement (Rohrer et al., 2011) is a priority for linking up two heretofore-unlinked aspects of the disease. Similar interactions have been described for complement in atherosclerotic artery walls (Oksjoki et al., 2007). How toxic lipids interact with and cleared by surveying dendritic cells (Bobryshev, 2010; Choi et al., 2011; Weber and Noels, 2011) and macrophages (Sene et al., 2013) is potentially addressable in culture systems and relevant to mobilizing these activities to clear lesions in AMD patients.
5.2.4. IMPROVED MODEL SYSTEMS
Widespread adoption of highly polarized and differentiated RPE for experimentation is critically essential for understanding how this versatile and hard-working cell contributes to distinct AMD pathology on its apical and basal aspects. The limitations of cell lines for uncovering essential physiology is now recognized (Beebe, 2013), and investigators should endeavor to use polarized cells and in vivo model systems to provide maximal in vivo relevance of their findings. Culture conditions for RPE have been rationalized and standardized (Hu and Bok, 2001; Johnson et al., 2011; Maminishkis et al., 2006; Sonoda et al., 2009), with human fetal RPE emerging as a standard. Retinoid processing in culture has now been achieved for both human fetal cells (Ablonczy et al., 2011) and cells originated from induced pluripotential stem cells (Maeda et al., 2013; Muniz et al., 2013), suggesting that high-fidelity cell culture systems suitable for study of cholesterol processing will be soon available.
5.3 Translating new cholesterol science to the clinic
5.3.1 IMPLEMENTING THE OIL SPILL STRATEGIES
In 2011 one of us (CAC) likened BLinD/soft drusen to an Oil Spill on BrM that could be approached therapeutically just like the real-world disasters, using strategies to detoxify or remove lipoproteins in BrM, slow down the outflow of lipoproteins from RPE, and prevent the accumulation of lipoproteins in BrM by modifying upstream dietary pathways. The discovery of SDD suggests that an analogous set of strategies may be required for future treatments targeting the subretinal space. Because sub-RPE lesions are so strongly associated with type I choroidal neovascularization (Freund et al., 2010), which constitutes the majority that are currently treated in AMD patients today, these strategies continue to have merit.
5.3.2 DETOXIFY/REMOVE DRUSEN
Such steps are pertinent to treatments like replacement cell transplants on a refurbished BrM or synthetic substitute (Hu et al., 2012; Treharne et al., 2012). Apolipoprotein mimetics can sequester oxidized lipids avidly (Rudolf et al., 2010; Van Lenten et al., 2009; Zhang et al., 2009). A different approach is compounds (e.g., pyridoxamine (Charvet et al., 2013b)), which also bind oxidized lipids. Yet these compounds scavenge oxidized lipids not only in the sub-RPE space but also in the RPE and neurosensory retina and do this before oxidized lipids exert their deleterious effects. Thus, unlike AREDS formulations, which are aimed at scavenging reactive oxygen species, pyridoxamine-like compounds act downstream of AREDS vitamins. Perhaps additional approached could be identified if we establish how the pathways of cholesterol homeostasis interact with other pathways for lipophilic essentials in retina (retinoids, carotenoids, polyunsaturated fatty acids). Overall, the more we study chorioretinal cholesterol maintenance, the more chance to find new AMD therapeutics.
5.3.3 RETARD DRUSEN FORMATION
One way to retard drusen formation is to reduce the outflow of apoB,E-containing lipoproteins from RPE into BrM. Statins are widely and successfully HMGCR inhibitors that reduce plasma LDL levels by up-regulating LDL receptors throughout the body and especially at the liver. In addition, statins can also directly reduce hepatic apoB secretion (Funatsu et al., 2001) so it is possible that statins could similarly affect RPE (Wu et al., 2010).To date there is little evidence that statins are beneficial for AMD (Gehlbach et al., 2012; Tsao and Fong, 2013). However, these studies were largely retrospective or population-based in design, included patients of variably defined AMD status, used systemically delivered statins of different molecular mechanisms (Wu et al., 2010), and pre-dated concepts of intra-ocular cholesterol and lipoprotein homeostasis described herein. In vitro studies have shown that RPE-derived cells can respond differently to various statins (Wu et al., 2010). Re-visiting statins (e.g. (Barathi et al., 2014; Guymer et al., 2013)) with new knowledge about chorioretinal cholesterol and informed choices of model system, patients, compounds, and delivery route may prove very informative.
Another approach is nucleic acid–based therapies originally intended to control hepatic VLDL. These can target apoB directly with anti-sense to apoB (Mipomersen), currently approved for familial hypercholesterolemia and in phase 3 trials for LDL reduction in patients at high risk for cardiovascular events (Thomas et al., 2013; Thomas and Ginsberg, 2010). Another target is MTP, required for apoB lipidation. Pharmacologic MTP inhibitors proved unsuitable for systemic use due to associated hepatic steatosis (fatty liver) and plasma transaminases signifying liver injury (Wierzbicki et al., 2009). miRNAs are small noncoding RNAs that post-transcriptionally and potently regulate gene expression through mRNA translational repression or destabilization. Of these, microRNA-30c via one region in its sequence markedly reduces MTP expression and apoB secretion. By another region, it reduces de novo synthesis of fatty acids (Soh et al., 2013). Both sequences combine to lowering plasma cholesterol and slow atherosclerosis, while maintaining liver health. MicroRNA-30c could be an attractive target for both liver and RPE.
A third approach to retarding drusen formation is synthetic agonists to LXR stimulating cellular cholesterol removal. A caveat about these potent compounds is that they can exert side effects upon systemic administration including a marked increase in hepatic and serum triglyceride content (Li et al., 2010; Schultz et al., 2000). Of concern, could also be the upregulation of Vegf, an LXR target (Walczak et al., 2004), and hence a stimulatory effect on angiogenesis (Chen et al., 2009). Yet inhibitory effects on angiogenesis are also observed (Noghero et al., 2012; Sene et al., 2013) with one being a significant and dose-dependent reduction of laser induced choroidal neovascularization used as an AMD-relevant model (Sene et al., 2013). Side effects of available LXR agonists could be resolved by changing their route of administration; use of oxysterols, endogenous LXR ligands, as an alternative; or by pharmacologic stimulation of the cholesterol-metabolizing enzymes that produce these oxysterols (Mast et al., 2013). Animal studies comprehensively evaluating the effects of LXR agonists on retinal gene expression, retinal structure and function are required as well (ongoing in the IAP laboratory) to gain a better understanding of the potential of LXR agonists to retard drusen formation.
As these and other treatments are tested and brought to market for cardiovascular disease, they bear watching by ophthalmologists for both negative and positive reasons: negative, because these drugs may have retinal consequences because they interact with intra-ocular lipoprotein and cholesterol pathways, including apoB-MTP pathway, and positive, because demonstration of such consequences constitutes in vivo pathway validation in human patients.
5.3.4 PREVENT DRUSEN FORMATION
Lipoprotein outflow into BrM could be modulated through dietary manipulation of the upstream lipid sources and lipase activity (Elner, 2002), fulfilling a long-anticipated potential of diet for AMD risk reduction (Chiu et al., 2009; Cho et al., 2001; Chong et al., 2009; SanGiovanni et al., 2008; Seddon et al., 1994). That a balanced diet is overall beneficial for most conditions of aging is common sense.
6.0 Conclusions
Studies of chorioretinal cholesterol have reached an exciting stage when accumulated knowledge can newly inform pre-clinical/clinical studies. Many open questions remain, so additional human and capital resources will certainly be required. Yet we can take heart in one of medicine's greatest public health successes. A decades-long decline in cardiovascular disease and stroke was propelled by risk factor reduction and drugs precisely targeted to blunt the effects of lipid-rich lesions in vessel walls and preserve cardiac and neuronal function. This success involved inquiry and intervention into the many of same biological pathways that we now bring to the retina. It is energizing to realize the wealth of expertise, tools, and experience now available for tackling a public health concern as significant as AMD. Thus return on effort and investment in this area should be substantial.
Article highlights.
Interest in chorioretinal cholesterol was increased within the last several years.
Chorioretinal cholesterol maintenance began to be delineated.
The specific lesions of age-related macular degeneration are cholesterol-rich.
Genes modulating AMD risk encode cholesterol-handling proteins.
Testable hypotheses are proposed to test this link.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (EY018383 to IAP and EY06109 to CAC). IAP is a recipient of the Jules and Doris Stein Professorship from the Research to Prevent Blindness.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ACAT
acyl-CoA:cholesterol acyl-transferase
- AMD
age-related macular degeneration
- apoA-1
apolipoprotein A1, apoE, apolipoprotein E, apo B-48, apolipoprotein B-48
- apo B-100
apolipoprotein B-100
- BLamD
basal laminar deposit
- BLinD
basal linear deposits, BrM, Bruch's membrane
- CETP
cholesteryl ester transfer protein
- CM
chylomicrons
- CTX
cerebrotendinous xanthomatosis
- CVD
cardiovascular disease
- CYP
cytochrome P450 EC, esterified cholesterol
- FFA
free fatty acids
- GCL
ganglion cell layer
- GC-MS
gas chromatography-mass spectrometry
- GWAS
genome-wide association studies
- HDL
high density lipoprotein
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- ICL
inner collagenous layer
- IDL
intermediate density lipoprotein
- INL
inner nuclear layer
- INSIG
insulin-induced gene protein
- IPL
inner plexiform layer
- IS
inner segments
- isoLGs
isolevuglandins
- LCAT
lecithin:cholesterol acyltransferase
- LDL
low density lipoprotein
- LDL-R
low density lipoprotein receptor
- LIPC
hepatic lipase
- LXR
liver X receptor
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- NPC1
Niemann-Pick type C1
- NFL
nerve fiber layer
- NR
neural retina
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- OS
outer segments
- PL
phospholipid
- PR
photoreceptors
- RPE
retinal pigment epithelium
- SDD
subretinal drusenoid deposit
- SNP
single nucleotide polymorphism
- TG
triglycerides
- UC
unesterified cholesterol
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We use the term cholesterol to mean unesterified (also called free) cholesterol, and EC to mean esterified cholesterol. The abbreviation UC for unesterified cholesterol is also used to match the terminology in previous publications.
References
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS Report No. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. J. Amer. Med. Assoc. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- Ablonczy Z, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investigative ophthalmology & visual science. 2011;52:8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton JH, et al. Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol. 2011;89:e404–411. doi: 10.1111/j.1755-3768.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- Ahmed J, et al. Measurement of blood flow through the retinal circulation of the cat during normoxia and hypoxemia using fluorescent microspheres. Microvasc Res. 2001;62:143–153. doi: 10.1006/mvre.2001.2321. [DOI] [PubMed] [Google Scholar]
- Albert AD, Boesze-Battaglia K. The role of cholesterol in rod outer segment membranes. Prog. Lipid. Res. 2005;44:99–124. doi: 10.1016/j.plipres.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman TS, et al. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc. Ophthalmol. 2013;127:239–243. doi: 10.1007/s10633-013-9403-0. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp. Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Amaral J, et al. 7-Ketocholesterol induces inflammation and angiogenesis in vivo: a novel rat model. PloS one. 2013;8:e56099. doi: 10.1371/journal.pone.0056099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga A, et al. Apolipoprotein E is synthesized in the retina by Muller glial cells, secreted into the vitreous, and rapidly transported into the optic nerve by retinal ganglion cells. J. Biol. Chem. 1996;271:5628–5632. doi: 10.1074/jbc.271.10.5628. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. In: Dart DA, Dana R, D'amore P, Niederkorn JY, editors. Immunology, inflammation and diseases of the eye. Academic Press; San Diego, CA: 2011. pp. 186–192. [Google Scholar]
- Anderson DH, et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am. J. Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- Anderson DH, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Andersson S, et al. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Anitschkow N. Ueber die veranderungen der kaninchenaorta bei experimenteller cholesterinsteatose. Beitr. Pathol. Anat. 1913;56:379–404. [Google Scholar]
- Arnold JJ, et al. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–191. [PubMed] [Google Scholar]
- Baba T, et al. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide Injection. Am. J. Pathol. 2010;176:3085–3097. doi: 10.2353/ajpath.2010.090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker A, et al. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J. Lipid Res. 1999;40:1417–1425. [PubMed] [Google Scholar]
- Babiker A, et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 1997;272:26253–26261. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- Bairaiti A, Orzalesi N. The ultrastructure of the pigment epithelium and of the photoreceptor-pigment epithelium interface. J. Ultrastruct. Res. 1963;9:484–496. doi: 10.1016/s0022-5320(63)80080-5. [DOI] [PubMed] [Google Scholar]
- Barathi VA, et al. Effects of simvastatin on retinal structure and function of a high-fat atherogenic mouse model of thickened Bruch's membrane. Invest Ophthalmol Vis Sci. 2014;55:460–468. doi: 10.1167/iovs.13-11636. [DOI] [PubMed] [Google Scholar]
- Bazan NG, et al. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv Exp Med Biol. 1992;318:295–306. doi: 10.1007/978-1-4615-3426-6_26. [DOI] [PubMed] [Google Scholar]
- Beebe DC. The use of cell lines to “model” ocular tissues: cautionary tales. Investigative ophthalmology & visual science. 2013;54 doi: 10.1167/iovs.13-12873. [DOI] [PubMed] [Google Scholar]
- Beltran WA, et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PloS one. 2014;9:e90390. doi: 10.1371/journal.pone.0090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL. Experimental and therapeutic aspects of photic damage to the retina. Investigative ophthalmology & visual science. 1973;12:35–44. [PubMed] [Google Scholar]
- Bird AC, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Bird AC, Marshall J. Retinal pigment epithelial detachments in the elderly. Trans. Ophthalmol. Soc. UK. 1986;105:674–682. [PubMed] [Google Scholar]
- Bjorkhem I. Are side-chain oxidized oxysterols regulators also in vivo? J. Lipid Res. 2009;50(Suppl):S213–218. doi: 10.1194/jlr.R800025-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, et al. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver AL, Beaudet AL, Sly WS, Valle D, Childs B, editors. The metabolic and molecular bases of inherited disease. 7th ed. McGraw-Hill; New York: 1995. pp. 2073–2099. [Google Scholar]
- Bjorkhem I, et al. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab. Invest. 2010 doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, et al. Relationship of cholesterol content to spatial distribution and age of disk membranes in retinal rod outer segments. J. Biol. Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, et al. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J. Biol. Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- Bok D, Young RW. Phagocytic properties of the retinal pigment epithelium. Harvard University Press Cambridge; MA: 1979. [Google Scholar]
- Breslow JL. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc. Natl. Acad. Sci. U S A. 1993;90:8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SB, et al. Age-related macular degeneration: nonneovascular early AMD, intermediate AMD, and geographic atrophy. In: Ryan SJ, editor. Retina. Fourth ed. Mosby, St Louis: 2006. pp. 1041–1074. [Google Scholar]
- Bretillon L, et al. ApoB100,LDLR−/− mice exhibit reduced electroretinographic response and cholesteryl esters deposits in the retina. Investigative ophthalmology & visual science. 2008a;49:1307–1314. doi: 10.1167/iovs.07-0808. [DOI] [PubMed] [Google Scholar]
- Bretillon L, et al. Cholesterol-24S-hydroxylase (CYP46A1) is specifically expressed in neurons of the neural retina. Curr. Eye Res. 2007;32:361–366. doi: 10.1080/02713680701231857. [DOI] [PubMed] [Google Scholar]
- Bretillon L, et al. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res. 2008b;87:521–528. doi: 10.1016/j.exer.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Bringmann A, et al. Muller cells in the healthy and diseased retina. Prog. Retin. Eye. Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Broekhuyse RM. Lipids in tissues of the eye. VII. Changes in concentration and composition of sphingomyelins, cholesterol esters and other lipids in aging sclera. Biochim. Biophys. Acta. 1972;280:637–645. [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 2009;50(Suppl):S15–27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell JD, et al. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim. Biophys. Acta. 2012;1821:365–372. doi: 10.1016/j.bbalip.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burglin TR. The Hedgehog protein family. Genome Biol. 2008;9:241. doi: 10.1186/gb-2008-9-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldes C, et al. Phenol group in pyridoxamine acts as a stabilizing element for its carbinolamines and Schiff bases. Chem. Biodivers. 2011;8:1318–1332. doi: 10.1002/cbdv.201000296. [DOI] [PubMed] [Google Scholar]
- Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reactions in bile acid biosynthesis. J. Biol. Chem. 1991;266:7774–7778. [PubMed] [Google Scholar]
- Castanho MA, et al. Absorption and fluorescence spectra of polyene antibiotics in the presence of cholesterol. J. Biol. Chem. 1992;267:204–209. [PubMed] [Google Scholar]
- Charvet C, et al. Isolevuglandins and mitochondrial enzymes in the retina: mass spectrometry detection of post-translational modification of sterol-metabolizing CYP27A1. J. Biol. Chem. 2011;286:20413–20422. doi: 10.1074/jbc.M111.232546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CD, et al. Posttranslational modification by an isolevuglandin diminishes activity of the mitochondrial cytochrome P450 27A1. J. Lipid Res. 2013a;54:1421–1429. doi: 10.1194/jlr.M035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CD, et al. Pretreatment with pyridoxamine mitigates isolevuglandin-associated retinal effects in mice exposed to bright light. J. Biol. Chem. 2013b;288:29267–29280. doi: 10.1074/jbc.M113.498832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke; a journal of cerebral circulation. 2009;40:2532–2538. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, et al. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. U S A. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, et al. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br. J. Ophthalmol. 2009;93:1241–1246. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 2001;73:209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- Choi JH, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Chong EW, et al. Fat consumption and its association with age-related macular degeneration. Arch. Ophthalmol. 2009;127:674–680. doi: 10.1001/archophthalmol.2009.60. [DOI] [PubMed] [Google Scholar]
- Chowers I, et al. Long-term assessment of combined vitamin A and E treatment for the prevention of retinal degeneration in abetalipoproteinaemia and hypobetalipoproteinaemia patients. Eye. 2001;15:525–530. doi: 10.1038/eye.2001.167. [DOI] [PubMed] [Google Scholar]
- Christen WG, et al. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women's Antioxidant and Folic Acid Cardiovascular Study. Arch. Intern. Med. 2009;169:335–341. doi: 10.1001/archinternmed.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudepierre T, et al. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Mol. Cell. Neurosci. 2010;43:164–176. doi: 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Crabb JW, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruysberg JR, et al. Ocular and systemic manifestations of cerebrotendinous xanthomatosis. Am. J. Ophthalmol. 1995;120:597–604. doi: 10.1016/s0002-9394(14)72206-8. [DOI] [PubMed] [Google Scholar]
- Curcio CA, et al. Apolipoprotein B-containing lipoproteins in retinal aging and age-related maculopathy. J. Lipid Res. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, et al. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Progress in retinal and eye research. 2009a;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, et al. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol. 2011a;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, et al. Human chorioretinal layer thicknesses measured using macula-wide high resolution histological sections. Investigative ophthalmology & visual science. 2011b;52:3943–3954. doi: 10.1167/iovs.10-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, et al. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investigative ophthalmology & visual science. 1993;34:3278–3296. [PubMed] [Google Scholar]
- Curcio CA, et al. Accumulation of cholesterol with age in human Bruch's membrane. Investigative ophthalmology & visual science. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, et al. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005a;81(6):731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Curcio CA, et al. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp. Eye Res. 2005b;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Curcio CA, et al. Histochemistry and lipid profiling combine for insights into aging and age-related maculopathy. In: Armstrong D, editor. Methods in Molecular Biology/ Lipomics. Humana Press; New York: 2009b. pp. 267–281. [DOI] [PubMed] [Google Scholar]
- Curcio CA, et al. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dashti N, et al. Plasma apolipoproteins and risk for age-related maculopathy. Br. J. Ophthalmol. 2006;90:1028 – 1033. doi: 10.1136/bjo.2006.093856. (Erratum Br J Ophthalmol. 2007 Mar;1091(1023):1403.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell. Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb SS, et al. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J. Lipid Res. 2003;44:1279–1286. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J. Biol. Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, et al. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- Distler C, Dreher Z. Glia cells of the monkey retina -- II. Müller cells. Vision Res. 1996;36:2381–2394. doi: 10.1016/0042-6989(96)00005-3. [DOI] [PubMed] [Google Scholar]
- Donders F, Donders F. Beitrage zur pathologischen Anatomie des Auges. Archiv. Fur Ophthalmologie. 1854;1:106–118. [Google Scholar]
- Dotti MT, et al. Cerebrotendinous xanthomatosis: heterogeneity of clinical phenotype with evidence of previously undescribed ophthalmological findings. J. Inherit. Metab. Dis. 2001;24:696–706. doi: 10.1023/a:1012981019336. [DOI] [PubMed] [Google Scholar]
- Dowhan W, Bogdanov M. Functional roles of lipids in membranes. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier; Amsterdam: 2002. pp. 1–35. [Google Scholar]
- Drasdo N, et al. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vis. Research. 2007;47:2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley PA, Anderson RE. Phospholipid transfer protein from bovine retina with high activity towards retinal rod disc membranes. FEBS letters. 1978;95:57–60. doi: 10.1016/0014-5793(78)80051-9. [DOI] [PubMed] [Google Scholar]
- Duncan KG, et al. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun. 2002;292:1017–1022. doi: 10.1006/bbrc.2002.6756. [DOI] [PubMed] [Google Scholar]
- Duncan KG, et al. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br. J. Ophthalmol. 2009;93:1116–1120. doi: 10.1136/bjo.2008.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer MA, et al. Research resource: nuclear receptor atlas of human retinal pigment epithelial cells: potential relevance to age-related macular degeneration. Mol. Endocrinol. 2011;25:360–372. doi: 10.1210/me.2010-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeletovic S, et al. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- Ebrahimi KB, et al. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol. 2012;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner VM. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans. Am. Ophthalmol. Soc. 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- Esteve-Rudd J, et al. AMD-like Pathology in Klc1−/− Mice. Investigative ophthalmology & visual science. 2012;53:4756. [Google Scholar]
- Feeney-Burns L, et al. The fate of immunoreactive opsin following phagocytosis by pigment epithelium in human and monkey retinas. Investigative ophthalmology & visual science. 1988;29:708–719. [PubMed] [Google Scholar]
- Ferris FL, 3rd, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming CP, et al. Depth resolved detection of lipid using spectroscopic optical coherence tomography. Biomed. Opt. Express. 2013;4:1269–1284. doi: 10.1364/BOE.4.001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ. Lipids and lipid metabolism in the eye. J. Lipid Res. 2010a;51:1–3. doi: 10.1194/jlr.E003533-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ. Retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome: thinking beyond cholesterol deficiency. Adv. Exp. Med. Biol. 2010b;664:481–489. doi: 10.1007/978-1-4419-1399-9_55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Bretillon L. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 2010;51:3399–3413. doi: 10.1194/jlr.R010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, et al. In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett. 1993;335:234–238. doi: 10.1016/0014-5793(93)80736-e. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Keller RK. Metabolism of [3H]farnesol to cholesterol and cholesterogenic intermediates in the living rat eye. Biochem. Biophys. Res. Commun. 1995;210:695–702. doi: 10.1006/bbrc.1995.1715. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Schroepfer GJ., Jr. Sterol composition of bovine retinal rod outer segment membranes and whole retinas. Biochim. Biophys. Acta. 1982;711:138–148. doi: 10.1016/0005-2760(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Fourgeux C, et al. 24S-hydroxycholesterol and cholesterol-24S-hydroxylase (CYP46A1) in the retina: from cholesterol homeostasis to pathophysiology of glaucoma. Chem. Phys. Lipids. 2011;164:496–499. doi: 10.1016/j.chemphyslip.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Fourgeux C, et al. Single nucleotide polymorphism in the cholesterol-24S-hydroxylase (CYP46A1) gene and its association with CFH and LOC387715 gene polymorphisms in age-related macular degeneration. Investigative ophthalmology & visual science. 2012;53:7026–7033. doi: 10.1167/iovs.12-9652. [DOI] [PubMed] [Google Scholar]
- Fourgeux C, et al. Primary open-angle glaucoma: association with cholesterol 24S-hydroxylase (CYP46A1) gene polymorphism and plasma 24-hydroxycholesterol levels. Investigative ophthalmology & visual science. 2009;50:5712–5717. doi: 10.1167/iovs.09-3655. [DOI] [PubMed] [Google Scholar]
- Freund KB, et al. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- Friedman E, et al. XXI Concilium Ophthalmologicum Mexico. Elsevier; 1971. Pathogenesis of senile disciform degeneration of the macula; pp. 454–458. [Google Scholar]
- Fritsche LG, et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013;45:433–439. 439e431–432. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, et al. Age-related macular degeneration: genetics and biology coming together. Annual Review of Genomics and Human Genetics. 2014 doi: 10.1146/annurev-genom-090413-025610. in press 1/30/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T, et al. Prolonged inhibition of cholesterol synthesis by atorvastatin inhibits apo B-100 and triglyceride secretion from HepG2 cells. Atherosclerosis. 2001;157:107–115. doi: 10.1016/s0021-9150(00)00714-0. [DOI] [PubMed] [Google Scholar]
- Garland DL, et al. Mouse genetics and proteomic analyses demonstrate a critical role for complement in a model of DHRD/ML, an inherited macular degeneration. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlipp MA, Gonzalez-Fernandez F. Cone photoreceptor and Muller cell pericellular matrices are binding domains for interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Gaynor PM, et al. Cholesterol accumulation in human cornea: evidence that extracellular cholesteryl ester-rich lipid particles deposit independently of foam cells. J. Lipid Research. 1996;37:1849–1861. [PubMed] [Google Scholar]
- Gehlbach P, et al. Statins for age-related macular degeneration. Cochrane database of systematic reviews. 2012;3:CD006927. doi: 10.1002/14651858.CD006927.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genead MA, et al. Spectral-domain optical coherence tomography and fundus autofluorescence characteristics in patients with fundus albipunctatus and retinitis punctata albescens. Ophthalmic Genet. 2010;31:66–72. doi: 10.3109/13816810903584971. [DOI] [PubMed] [Google Scholar]
- George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicol. Appl. Pharmacol. 2012;259:311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddens DP, et al. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- Gordiyenko N, et al. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest Ophthalmol Vis Sci. 2004;45:2822–2829. doi: 10.1167/iovs.04-0074. [DOI] [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Guarneri P, et al. Neurosteroidogenesis in rat retinas. J. Neurochem. 1994;63:86–96. doi: 10.1046/j.1471-4159.1994.63010086.x. [DOI] [PubMed] [Google Scholar]
- Gulcan HG, et al. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Investigative ophthalmology & visual science. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Guymer RH, et al. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PloS one. 2013;8:e83759. doi: 10.1371/journal.pone.0083759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton JR, Klemp KF. The lipid-rich core region of human atherosclerotic fibrous plaques. Prevalence of small lipid droplets and vesicles by electron microscopy. Am. J. Pathol. 1989;134:705–717. [PMC free article] [PubMed] [Google Scholar]
- Guyton JR, Klemp KR. Development of the lipid-rich core in human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- Haimovici R, et al. The lipid composition of drusen, Bruch's membrane, and sclera by hot stage polarizing microscopy. Investigative ophthalmology & visual science. 2001;42:1592–1599. [PubMed] [Google Scholar]
- Hayes KC, et al. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Investigative ophthalmology & visual science. 1989;30:225–232. [PubMed] [Google Scholar]
- Heo GY, et al. Conversion of 7-ketocholesterol to oxysterol metabolites by recombinant CYP27A1 and retinal pigment epithelial cells. J. Lipid Res. 2011;52:1117–1127. doi: 10.1194/jlr.M014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo GY, et al. Features of the retinal environment which affect the activities and product profile of cholesterol-metabolizing cytochromes P450 CYP27A1 and CYP11A1. Arch. Biochem. Biophys. 2012;518:119–126. doi: 10.1016/j.abb.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks DA, et al. Lipid rafts and Alzheimer's disease: protein-lipid interactions and perturbation of signaling. Front. Physiol. 2012;3:189. doi: 10.3389/fphys.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang Q, et al. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Visual Neuroscience. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1972;76:64–80. [PubMed] [Google Scholar]
- Hollyfield JG, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, et al. Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch. Ophthalmol. 1994;112:402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- Hu Y, et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic. Res. 2012;48:186–191. doi: 10.1159/000338749. [DOI] [PubMed] [Google Scholar]
- Huang J-D, et al. Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch. Exp. Eye Res. 2007;85:202–218. doi: 10.1016/j.exer.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida BY, et al. Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J. Lipid Res. 2004;45:263–271. doi: 10.1194/jlr.M300306-JLR200. [DOI] [PubMed] [Google Scholar]
- Jaliffa CO, et al. Effect of neurosteroids on the retinal gabaergic system and electroretinographic activity in the golden hamster. J. Neurochem. 2005;94:1666–1675. doi: 10.1111/j.1471-4159.2005.03321.x. [DOI] [PubMed] [Google Scholar]
- Janowski BA, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Javitt NB, Javitt JC. The retinal oxysterol pathway: a unifying hypothesis for the cause of age-related macular degeneration. Curr. Opin. Ophthalmol. 2009;20:151–157. doi: 10.1097/ICU.0b013e32832af468. [DOI] [PubMed] [Google Scholar]
- Johnson LV, et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson F, et al. Prevalence of age-related macular degeneration in old persons: Age, Gene/environment Susceptibility Reykjavik Study. Ophthalmology. 2011;118:825–830. doi: 10.1016/j.ophtha.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kitteringham NR, et al. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:1229–1239. doi: 10.1016/j.jchromb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Klaver CC, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmol. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- Klein R, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Knupp C, et al. Structure of abnormal molecular assemblies (collagen VI) associated with human full thickness macular holes. J. Struct. Biol. 2000;129:38–47. doi: 10.1006/jsbi.1999.4202. [DOI] [PubMed] [Google Scholar]
- Kooijman AC. Light distribution on the retina of a wide-angle theoretical eye. Journal of the Optical Society of America. 1983;73:1544–1550. doi: 10.1364/josa.73.001544. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Dietschy J. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am. J. Clin. Nutr. 1997;65:1590S–1596S. doi: 10.1093/ajcn/65.5.1590S. [DOI] [PubMed] [Google Scholar]
- Kruth HS. The fate of lipoprotein cholesterol entering the arterial wall. Curr. Opin. Lipidology. 1997;8:246–252. doi: 10.1097/00041433-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Kruth HS. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr. Opin. Lipidol. 2011;22:386–393. doi: 10.1097/MOL.0b013e32834adadb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhrt H, et al. Changes in CD44 and ApoE immunoreactivities due to retinal pathology of man and rat. J. Hirnforsch. 1997;38:223–229. [PubMed] [Google Scholar]
- Kurumada S, et al. Stage-specific association of apolipoprotein A-I and E in developing mouse retina. Investigative ophthalmology & visual science. 2007;48:1815–1823. doi: 10.1167/iovs.06-0902. [DOI] [PubMed] [Google Scholar]
- Lee JW, et al. Expression and localization of sterol 27-hydroxylase (CYP27A1) in monkey retina. Exp. Eye Res. 2006;83:465–469. doi: 10.1016/j.exer.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire-Ewing S, et al. Lipid rafts: a signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis. 2012;221:303–310. doi: 10.1016/j.atherosclerosis.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Lengyel I, et al. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Investigative ophthalmology & visual science. 2004;45:2886–2892. doi: 10.1167/iovs.03-1083. [DOI] [PubMed] [Google Scholar]
- Li C-M, et al. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Investigative ophthalmology & visual science. 2006;47:3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- Li CM, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Investigative ophthalmology & visual science. 2005a;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Li CM, et al. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J. Lipid Res. 2005b;46:628–640. doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- Li M, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum. Mol. Gen. 2013 doi: 10.1093/hmg/ddu114. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Liver X receptor modulators: a review of recently patented compounds (2007 - 2009). Expert Opin. Ther. Pat. 2010;20:535–562. doi: 10.1517/13543771003621269. [DOI] [PubMed] [Google Scholar]
- Liao W-L, et al. Quantification of cholesterol-metabolizing P450s CYP27A1 and CYP46A1 in neural tissues reveals a lack of enzyme-product correlations in human retina but not human brain. J. Proteome Res. 2011;10:241–248. doi: 10.1021/pr1008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WL, et al. Steroid and protein ligand binding to cytochrome P450 46A1 as assessed by hydrogen-deuterium exchange and mass spectrometry. Biochemistry. 2009;48:4150–4158. doi: 10.1021/bi900168m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WL, et al. Optimizing the conditions of a multiple reaction monitoring assay for membrane proteins: quantification of cytochrome P450 11A1 and adrenodoxin reductase in bovine adrenal cortex and retina. Anal. Chem. 2010;82:5760–5767. doi: 10.1021/ac100811x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane E, et al. The respective relationships between lipoprotein profile, macular pigment optical density, and serum concentrations of lutein and zeaxanthin. Investigative ophthalmology & visual science. 2010;51:5897–5905. doi: 10.1167/iovs.09-4878. [DOI] [PubMed] [Google Scholar]
- Lommatzsch A, et al. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Lund EG, et al. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, et al. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, et al. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.518571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek AM, et al. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Malek G, et al. Apolipoprotein B in cholesterol-containing drusen and basal deposits in eyes with age-related maculopathy. Am. J. Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Investigative ophthalmology & visual science. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, et al. Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice: a model for the early pathogenic course of macular degeneration. Hum. Mol. Genet. 2007;16:2423–2432. doi: 10.1093/hmg/ddm199. [DOI] [PubMed] [Google Scholar]
- Marshall J, et al. Aging and Bruch's membrane. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium: Function and Disease. Oxford University Press; New York: 1998. pp. 669–692. [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat. Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Mast N, et al. Pharmacologic stimulation of cytochrome P450 46A1 and cerebral cholesterol turnover in mice. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.532846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, et al. Cholestenoic acid is an important elimination product of cholesterol in the retina: comparison of retinal cholesterol metabolism with that in the brain. Investigative ophthalmology & visual science. 2011;52:594–603. doi: 10.1167/iovs.10-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, et al. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McKay GJ, et al. Evidence of association of APOE with age-related macular degeneration: a pooled analysis of 15 studies. Hum. Mutat. 2011;32:1407–1416. doi: 10.1002/humu.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadway A, et al. Microstructure of sub-retinal drusenoid deposits revealed by adaptive optics imaging. Biomed. Opt. Express. 2014 doi: 10.1364/BOE.5.000713. 2/6/14 accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney S, et al. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J. Lipid Res. 2002;43:2130–2135. doi: 10.1194/jlr.m200293-jlr200. [DOI] [PubMed] [Google Scholar]
- Milam AH, et al. Dominant late-onset retinal degeneration with regional variation of sub-RPE deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000;107:2256–2266. doi: 10.1016/s0161-6420(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Miller JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2013;155:1–35. e13. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Mimoun G, et al. [Macular drusen]. J. Fr. Ophtalmol. 1990;13:511–530. [PubMed] [Google Scholar]
- Moore DJ, et al. Age-related variation in the hydraulic conductivity of Bruch's membrane. Investigative ophthalmology & visual science. 1995;36:1290–1297. [PubMed] [Google Scholar]
- Moreira EF, et al. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implication in the induction of VEGF and CNV formation. Investigative ophthalmology & visual science. 2009;50:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SJ, et al. Case of cerebrotendinous xanthomatosis. I: Unusual ophthalmic features. Br. J. Ophthalmol. 1989;73:1011–1014. doi: 10.1136/bjo.73.12.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossbock G, et al. Role of cholesterol 24S-hydroxylase gene polymorphism (rs754203) in primary open angle glaucoma. Mol. Vis. 2011;17:616–620. [PMC free article] [PubMed] [Google Scholar]
- Mrejen S, et al. Assessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft drusen in vivo using adaptive optics imaging. Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkamala SK, et al. Optical coherence tomographic imaging of sub-retinal pigment epithelium lipid. Arch. Ophthalmol. 2012;130:1–7. doi: 10.1001/archophthalmol.2012.2491. [DOI] [PubMed] [Google Scholar]
- Muniz A, et al. Retinoid uptake, processing and secretion in human iPS-RPE support the visual cycle. Investigative ophthalmology & visual science. 2013 doi: 10.1167/iovs.13-11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaisumi Y, et al. The ultrastructure of Bruch's membrane. III. The macular area of the human eye. Arch. Ophthalmol. 1964;72:395–400. doi: 10.1001/archopht.1964.00970020395018. [DOI] [PubMed] [Google Scholar]
- Neale BM, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. U. S. A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noghero A, et al. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler. Thromb. Vasc. Biol. 2012;32:2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- Nork TM, et al. Measurement of regional choroidal blood flow in rabbits and monkeys using fluorescent microspheres. Archives of Ophthalmology. 2006;124:860–868. doi: 10.1001/archopht.124.6.860. [DOI] [PubMed] [Google Scholar]
- Norlin M, Wikvall K. Enzymes in the conversion of cholesterol into bile acids. Curr. Mol. Med. 2007;7:199–218. doi: 10.2174/156652407780059168. [DOI] [PubMed] [Google Scholar]
- O'Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J. Lipid Res. 2013;54:1731–1743. doi: 10.1194/jlr.R037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak ASW, et al. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014 doi: 10.1097/IAE.0000000000000121. accepted 12/24/13. [DOI] [PubMed] [Google Scholar]
- Oksjoki R, et al. Function and regulation of the complement system in cardiovascular diseases. Front Biosci. 2007;12:4696–4708. doi: 10.2741/2419. [DOI] [PubMed] [Google Scholar]
- Omarova S, et al. Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. J. Clin. Invest. 2012;122:3012–3023. doi: 10.1172/JCI63816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund RE, Jr., et al. Cholesterol absorption efficiency declines at moderate dietary doses in normal human subjects. J. Lipid Res. 1999;40:1453–1458. [PubMed] [Google Scholar]
- Pannu PS, et al. Oxysterol generation and liver X receptor-dependent reverse cholesterol transport: Not all roads lead to Rome. Mol. Cell. Endocrinol. 2012 doi: 10.1016/j.mce.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Pascolini D, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic. Epidemiol. 2004;11:67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, et al. Aging changes in Bruch's membrane: a histochemical and morphological study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- Pflibsen KP, et al. Retinal illuminance using a wide-angle model of the eye. Journal of the Optical Society of America. A, Optics and image science. 1988;5:146–150. doi: 10.1364/josaa.5.000146. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 2003a;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003b;25:72–78. doi: 10.1002/bies.10195. [DOI] [PubMed] [Google Scholar]
- Phillips SE, et al. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J. Neurosci. 2008;28:6569–6582. doi: 10.1523/JNEUROSCI.5529-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pikuleva IA, et al. Activities of recombinant human cytochrome P450c27 (CYP27) which produce intermediates of alternative bile acid biosynthetic pathways. J. Biol. Chem. 1998;273:18153–18160. doi: 10.1074/jbc.273.29.18153. [DOI] [PubMed] [Google Scholar]
- Polyak SL. The Retina. University of Chicago; Chicago: 1941. [Google Scholar]
- Prosser HC, et al. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr. Opin. Lipidol. 2012;23:182–189. doi: 10.1097/MOL.0b013e328352c4dd. [DOI] [PubMed] [Google Scholar]
- Provis JM, et al. Adaptation of the central retina for high acuity vision: Cones, the fovea and the avascular zone. Progress in retinal and eye research. 2013;35:63–81. doi: 10.1016/j.preteyeres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qtaishat NM, et al. Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice. Investigative ophthalmology & visual science. 2003;44:1435–1446. doi: 10.1167/iovs.02-0679. [DOI] [PubMed] [Google Scholar]
- Querques G, et al. High-definition optical coherence tomographic visualization of photoreceptor layer and retinal flecks in fundus albipunctatus associated with cone dystrophy. Arch. Ophthalmol. 2009;127:703–706. doi: 10.1001/archophthalmol.2009.87. [DOI] [PubMed] [Google Scholar]
- Querques G, et al. Impact of reticular pseudodrusen on macular function. Retina. 2013 doi: 10.1097/IAE.0b013e3182993df1. [DOI] [PubMed] [Google Scholar]
- Querques G, et al. Analysis of progression of reticular pseudodrusen by spectral domain optical coherence tomography. Invest. Ophthal. Vis. Sci. 2012;53:1264–1270. doi: 10.1167/iovs.11-9063. [DOI] [PubMed] [Google Scholar]
- Ramirez DM, et al. Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J. Comp. Neurol. 2008;507:1676–1693. doi: 10.1002/cne.21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AB, et al. Sterol 27-hydroxylase: expression in human arterial endothelium. J. Lipid Res. 1997;38:1254–1260. [PubMed] [Google Scholar]
- Rodriguez de Turco EB, et al. Selective retinal pigment epithelial cell lipid metabolism and remodeling conserves photoreceptor docosahexaenoic acid following phagocytosis. J. Neurosci. Res. 1999;57:479–486. [PubMed] [Google Scholar]
- Rodriguez IR, Larrayoz IM. Cholesterol oxidation in the retina: Implications of 7-ketocholesterol formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 2010;51:2847–2862. doi: 10.1194/jlr.R004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, et al. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol Immunol. 2011;48:e1–8. doi: 10.1016/j.molimm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, et al. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J. Biol. Chem. 1998;273:14805–14812. doi: 10.1074/jbc.273.24.14805. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane. Investigative ophthalmology & visual science. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Rudolf M, et al. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Investigative ophthalmology & visual science. 2008a;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, et al. ApoA-I mimetic peptide reduces lipid deposition in murine Bruch's membrane after intravitreal injection. Investigative ophthalmology & visual science. 2010;51:2984. [Google Scholar]
- Rudolf M, Curcio CA. Esterified cholesterol is highly localized to Bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid. J. Histochem. Cytochem. 2009;57:731–739. doi: 10.1369/jhc.2009.953448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, et al. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp. Eye Res. 2008b;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, et al. Internal structure consistent with remodeling in very small drusen, revealed by filipin histochemistry for esterified cholesterol. Br. J. Ophthalmol. doi: 10.1136/bjophthalmol-2013-304226. [DOI] [PubMed] [Google Scholar]
- Rudolf M, et al. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch's membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 2005;89:1627–1630. doi: 10.1136/bjo.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Investigative ophthalmology & visual science. 2012;53:3027–3039. doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW. 50 years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2008:S120–125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust S, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- Rye KA, Barter PJ. Cardioprotective functions of HDLs. J. Lipid Res. 2014;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine JR. Cholesterol. Marcel Dekker, Inc.; New York and Basel: 1977. [Google Scholar]
- Saher G, et al. Cholesterol: a novel regulatory role in myelin formation. Neuroscientist. 2011;17:79–93. doi: 10.1177/1073858410373835. [DOI] [PubMed] [Google Scholar]
- Salomon RG. Levuglandins and isolevuglandins: stealthy toxins of oxidative injury. Antioxid. Redox Signal. 2005;7:185–201. doi: 10.1089/ars.2005.7.185. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch. Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks J, et al. Evolution of reticular pseudodrusen. Br. J. Ophthalmol. 2011;95:979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- Sarks JP, et al. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Sarks JP, et al. Evolution of soft drusen in age-related macular degeneration. Eye. 1994;8:269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- Sarks S, et al. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Investigative ophthalmology & visual science. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br. J. Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks SH, et al. Softening of drusen and subretinal neovascularization. Trans. Ophthalmol. Soc. U. K. 1980;100:414–422. [PubMed] [Google Scholar]
- Schatz P, et al. Lack of autofluorescence in fundus albipunctatus associated with mutations in RDH5. Retina. 2010;30:1704–1713. doi: 10.1097/IAE.0b013e3181dc050a. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest. Ophthal. Vis. Sci. 2011;52:5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- Schneeberger SA, et al. Apolipoprotein E in the subretinal fluid of rhegmatogenous and exudative retinal detachments. Retina. 1997;17:38–43. doi: 10.1097/00006982-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. J. Am. Med. Assn. 1994;272:1413–1420. [PubMed] [Google Scholar]
- Seddon JM, et al. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113:260–266. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sene A, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell. Metab. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaratnam J, et al. Retinal Muller glia secrete apolipoproteins E and J which are efficiently assembled into lipoprotein particles. Mol. Brain Res. 1997;50:113–120. doi: 10.1016/s0169-328x(97)00176-9. [DOI] [PubMed] [Google Scholar]
- Shen JK, et al. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- Sheraidah G, et al. Correlation between lipids extracted from Bruch's membrane and age. Ophthalmology. 1993;100:47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Smith EB. The relationship between plasma and tissue lipids in human atherosclerosis. Adv. Lipid Res. 1974;12:1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Smith RT, et al. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Investigative ophthalmology & visual science. 2006;47:5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W, et al. Risk factors for age-related macular degeneration. Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Soh J, et al. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med. 2013;19:892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, et al. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat. Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, et al. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am. J. Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- Spady DK, et al. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J. Lipid. Res. 1985;26:465–472. [PubMed] [Google Scholar]
- Spaide R, Curcio CA. Anatomic correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide R, et al. Characterization of peroxidized lipids in Bruch's membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]
- Spaide RF, et al. Continuing medical education review: choroidal neovascularization in age-related macular degeneration--what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CE, et al. Hepatic and intestinal contribution of two forms of apolipoprotein B to plasma lipoprotein fractions in the rat. Can. J. Biochem. 1981;59:693–699. doi: 10.1139/o81-096. [DOI] [PubMed] [Google Scholar]
- Starita C, et al. Decreasing hydraulic conductivity of Bruch's membrane: relevance to photoreceptor survival and lipofuscinoses. Am. J. Med. Genet. 1995;57:235–237. doi: 10.1002/ajmg.1320570224. [DOI] [PubMed] [Google Scholar]
- Starita C, et al. Localization of the site of major resistance to fluid transport in Bruch's membrane. Investigative ophthalmology & visual science. 1997;38:762–767. [PubMed] [Google Scholar]
- Stary HC. Composition and classification of human atherosclerotic lesions. Virchows Archiv A Pathol Anat. 1992;421:277–290. doi: 10.1007/BF01660974. [DOI] [PubMed] [Google Scholar]
- Stary HC, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. Circulation. 1992;85:391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I. J. Lipid Res. 2004;45:1583–1593. doi: 10.1194/jlr.R400003-JLR200. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis: an interpretive history of the cholesterol controversy, part III: mechanistically defining the role of hyperlipidemia. J. Lipid. Res. 2005a;46:2037–2051. doi: 10.1194/jlr.R500010-JLR200. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 2005b;46:179–190. doi: 10.1194/jlr.R400012-JLR200. [DOI] [PubMed] [Google Scholar]
- Steinberg D. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part IV: the 1984 coronary primary prevention trial ends it--almost. J. Lipid Res. 2006a;47:1–14. doi: 10.1194/jlr.R500014-JLR200. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J. Lipid Res. 2006b;47:1339–1351. doi: 10.1194/jlr.R600009-JLR200. [DOI] [PubMed] [Google Scholar]
- Streeten BW. The sudanophilic granules of the human retinal pigment epithelium. Arch. Ophthalmol. 1961;66:391–398. [Google Scholar]
- Sudhop T, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- Suzuki M, et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol. Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- Tabas I, et al. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Thomas GS, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013;62:2178–2184. doi: 10.1016/j.jacc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ginsberg H. Development of apolipoprotein B antisense molecules as a therapy for hyperlipidemia. Curr Atheroscler Rep. 2010;12:58–65. doi: 10.1007/s11883-009-0078-7. [DOI] [PubMed] [Google Scholar]
- Treharne AJ, et al. Developing methacrylate-based copolymers as an artificial Bruch's membrane substitute. J. Biomed. Mat. Res. Part A. 2012 doi: 10.1002/jbm.a.34178. [DOI] [PubMed] [Google Scholar]
- Tsao SW, Fong DS. Do statins have a role in the prevention of age-related macular degeneration? Drugs Aging. 2013;30:205–213. doi: 10.1007/s40266-013-0061-4. [DOI] [PubMed] [Google Scholar]
- Tserentsoodol N, et al. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 2006a;12:1319–1333. [PubMed] [Google Scholar]
- Tserentsoodol N, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006b;12:1306–1318. [PubMed] [Google Scholar]
- Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 2003;14:233–240. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- Turley SD, et al. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 1998;28:1088–1094. doi: 10.1002/hep.510280425. [DOI] [PubMed] [Google Scholar]
- Turley SD, et al. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J. Lipid Res. 1995;36:67–79. [PubMed] [Google Scholar]
- Ueda-Arakawa N, et al. Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am. J. Ophthalmol. 2012;155:260–269. e262. doi: 10.1016/j.ajo.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Upston JM, et al. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Ameri. J. Pathol. 2002;160:701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisar T, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen R, et al. Cholesterol and age-related macular degeneration: is there a link? Am. J. Ophthalmol. 2004;137:750–752. doi: 10.1016/j.ajo.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Van Lenten BJ, et al. Apolipoprotein A-I mimetic peptides. Curr. Atheroscler. Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLaan PA, et al. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Verhoeff FH, Sisson RJ. Basophilic staining of Bruch's membrane. Arch. Ophthalmol. 1926;55:125–127. [Google Scholar]
- Vogel S, et al. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry. 2002;41:15360–15368. doi: 10.1021/bi0268551. [DOI] [PubMed] [Google Scholar]
- Walczak R, et al. Transcription of the vascular endothelial growth factor gene in macrophages is regulated by liver X receptors. J. Biol. Chem. 2004;279:9905–9911. doi: 10.1074/jbc.M310587200. [DOI] [PubMed] [Google Scholar]
- Wang AL, et al. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PloS one. 2009a;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Abundant lipid and protein components of drusen. PloS one. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Lipoprotein particles of intra-ocular origin in human Bruch membrane: an unusual lipid profile. Investigative ophthalmology & visual science. 2009b;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. Sample prefractionation for mass spectrometry quantification of low-abundance membrane proteins. Anal. Chem. 2012;84:5186–5191. doi: 10.1021/ac300587v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wavre-Shapton ST, et al. Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PloS one. 2013;8:e57769. doi: 10.1371/journal.pone.0057769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Wedl C. In: Grundzüge der pathologischen Histologie. Busk G, editor. Carl Gerold & Sohn; Vienna: 1854. [Google Scholar]
- Weismann D, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AS, et al. Future challenges for microsomal transport protein inhibitors. Curr Vasc Pharmacol. 2009;7:277–286. doi: 10.2174/157016109788340703. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Wolter JR, Falls HF. Bilateral confluent drusen. Arch. Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- Wong P, et al. Clusterin protein diversity in the primate eye. Mol. Vis. 2000;6:184–191. [PubMed] [Google Scholar]
- Wu M, et al. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Investigative ophthalmology & visual science. 2008;49:2679–2685. doi: 10.1167/iovs.07-1440. [DOI] [PubMed] [Google Scholar]
- Wu T, et al. Apolipoprotein B100 secretion by cultured ARPE-19 cells is modulated by alteration of cholesterol levels. J. Neurochem. 2010;114:1734–1744. doi: 10.1111/j.1471-4159.2010.06884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta. 2012;1821:877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehoshua Z, et al. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118:2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, et al. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Investigative ophthalmology & visual science. 2011;52:4663–4670. doi: 10.1167/iovs.10-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, et al. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Investigative ophthalmology & visual science. 2012;53:1548–1556. doi: 10.1167/iovs.11-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak Z, et al. Expression of simian CETP in normolipidemic Fisher rats has a profound effect on large sized apoE-containing HDL. J Lipid Res. 2002;43:2164–2171. doi: 10.1194/jlr.m200253-jlr200. [DOI] [PubMed] [Google Scholar]
- Zambon D, et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am. J. Med. 2010;123:267–274. doi: 10.1016/j.amjmed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H866–873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, et al. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PloS one. 2012;7:e37926. doi: 10.1371/journal.pone.0037926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel SA, et al. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. e.301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]