Abstract
Although functional MRI traditionally has been applied mainly to study changes in task-induced brain function, evolving acquisition methodologies and improved knowledge of signal mechanisms have increased the utility of this method for studying responses to pharmacological stimuli, a technique often dubbed “phMRI”. The proliferation of higher magnetic field strengths and the use of exogenous contrast agent have boosted detection power, a critical factor for successful phMRI due to the restricted ability to average multiple stimuli within subjects. Receptor-based models of neurovascular coupling, including explicit pharmacological models incorporating receptor densities and affinities and data-driven models that incorporate weak biophysical constraints, have demonstrated compelling descriptions of phMRI signal induced by dopaminergic stimuli. This report describes phMRI acquisition and analysis methodologies, with an emphasis on data-driven analyses. As an example application, statistically efficient data-driven regressors were used to describe the biphasic response to the mu-opioid agonist remifentanil, and antagonism using dopaminergic and GABAergic ligands revealed modulation of the mesolimbic pathway. Results illustrate the power of phMRI as well as our incomplete understanding of mechanisms underlying the signal. Future directions are discussed for phMRI acquisitions in human studies, for evolving analysis methodologies, and for interpretative studies using the new generation of simultaneous PET/MRI scanners.
Keywords: phMRI, fMRI, general linear model, remifentanil, dopamine, PET/MR
1. Introduction
Functional MRI (fMRI) has become a ubiquitous tool for studying task-induced changes in human brain activity associated with sensory, motor, or cognitive stimuli. Within the context of neuropharmacology, most fMRI studies in human subjects employ blood oxygen level dependent (BOLD) signal and incorporate an acute drug challenge in order to modulate the response induced by a traditional task design, which may involve alternating periods of sensory stimuli (e.g., pain) or cognitive load in order to ascertain the modulatory effects of the drug. From a methodological viewpoint, these pharmacological fMRI studies are slight modifications of routine fMRI procedures and employ well-developed acquisition and analysis techniques (Poline and Brett, 2012). Averaging many stimuli within each session enables the within-subject detection power that is required in order to make inferences about pharmacological neuromodulation of task-induced activity within or across groups. fMRI analyses are highly stereotyped: neurons respond much more quickly than the blood supply, so a timing diagram of the stimulus paradigm, which is convolved with a hemodynamic response function to account for the delay of the blood response, provides a generally accurate model of the experimental time course during analysis. Infusion of a drug may modify task-induced response magnitudes, and inferences are made as differential comparisons in task-induced activation without evidence of how the drug directly affects function.
Another type of study attempts to measure direct effects of acute drug challenges upon the brain; this method will be labeled “phMRI” within this article (Chen et al., 1997). Although the use of these two terms – fMRI versus phMRI – may seem like a distinction without a difference, experimental designs and analyses have important differences that alter detection power and information content. Unlike fMRI stimuli, most pharmacological challenges induce cerebral responses that are long in duration and refractory in nature, placing limits on the ability to average multiple stimuli or to resample the baseline in order to monitor signal drift that is unrelated to the physiological response. Thus, optimizing the inherent detection power of the phMRI method is critical for successful neuroimaging of changes in brain function.
Drug stimuli also pose challenges for analyses. Many drugs perturb systemic physiology, and so a component of the hemodynamic response may not reflect the neurovascular coupling of interest. Moreover, drug infusions evoke neural responses that are slow compared to the response of the blood supply, so that temporal models of the response are either post-hoc empirical descriptions of data or attempts to model the complex CNS response with very limited information. On the other hand, the temporal response can aid interpretation of the underlying pharmacology by providing insight into the neural response. This insight into neural function through the temporal response of the blood supply is relevant for phMRI but not usually for fMRI studies. Vascular delays and dispersion largely determine regional differences in temporal responses for fMRI (Lee et al., 1995), whereas drug infusions evoke changes in neural function that evolve very slowly over a time scale of many seconds to many minutes.
As an example of the temporal information carried by phMRI signal, one of the earliest phMRI studies reported that amphetamine-induced phMRI signals in rat striatum closely matched the time evolution of extracellular dopamine as measured by microdialysis (Chen et al., 1999). Innumerable studies now have demonstrated that phMRI signal carries relevant information about changes in brain neurochemistry. However, many pharmacological stimuli influence multiple presynaptic and postsynaptic processes, producing complex responses that can be difficult to interpret in the absence of additional information about underlying neurochemical mechanisms enabled by other techniques or by a series of studies using targeted antagonists or agonists. Nevertheless, significant progress has been made in understanding the phMRI response in many instances, such as for the dopaminergic response in basal ganglia during development (Chen et al., 2010), across species (Mandeville et al., 2011), and at different levels of evoked dopamine (Ren et al., 2009), even including explicit physiological models of the sign and shape of the dopamine-induced temporal response (Mandeville et al., 2013). Thus, the complexity of phMRI signal does not represent an intractable problem by any means, and the richness of the response enables some capabilities, such as efficient whole-brain assessment of functional connectivity in vivo, that are difficult to conceive in the absence of this method.
Continuing developments in multimodal imaging and animal models will further expand interpretative studies and help clarify the nature of neurovascular coupling in the context of pharmacological stimuli, thereby enabling phMRI signal to serve as a surrogate biomarker for neurochemical responses. For instance, a D2 antagonist induces phMRI signal in rough proportion to receptor occupancy, but subregions of basal ganglia are differentiated much better by phMRI than by binding potentials, suggesting a sensitivity to basal levels of dopamine, a clinically important marker (Sander et al., 2013). If our understanding of dopamine-induced phMRI signal is accurate, then phMRI responses in primate striatum should largely reflect the temporal response of dopamine release and D2 binding (Mandeville et al., 2013), providing an alternative (or adjunct) to PET as a means to study dopamine efflux in human brain.
This report describes and discusses basic methods for robust experimental design, acquisition, and analysis in phMRI studies. An example application illustrates the general approach: dopaminergic and GABAergic antagonists are employed to reveal coordinated activity within the mesolimbic dopamine system induced by the synthetic mu-opioid agonist, remifentanil. Future directions are discussed for near-term acquisition methods, data analysis strategies, and interpretive studies.
2. Data Acquisition
2.1 The magnitude of phMRI signal changes
Detectable fMRI signal responses in human subjects correspond to changes in cerebral blood flow (CBF) that may be 10% in prefrontal cortex for a working memory task (Kim et al., 2006) to 100% or more for a robust visual or motor task (Chiarelli et al., 2007). Using a common clinical field strength like 3 Tesla, these CBF changes translate into BOLD signal changes that range from a few tenths of one percent (Wagner et al., 2001) up to 3–4% (Chiarelli et al., 2007), excluding large signal changes in prominent draining veins.
A common misconception about phMRI is that changes in CBF or BOLD signal due to injected drugs, and particularly those drugs that produce very large elevations in a neurotransmitter or exogenous agonist, must be much larger than those commonly observed in standard fMRI studies. This generally is untrue. One reason for small phMRI signal changes is that neuroreceptor subtypes can be positively or negatively coupled to function, so that elevation of even a single neurotransmitter generally produces competing functional influences. Moreover, functional responses can be down-regulated dynamically on a time scale of minutes by desensitization through mechanisms like receptor internalization (Goodkin et al., 2005, Guo et al., 2010).
Perhaps due to these mechanisms, phMRI signal changes generally are no larger than fMRI signal changes. Using specific examples for selected drugs, a large bolus of cocaine (0.5 mg/kg) decreases CBF in human basal ganglia by 20–30% (Wallace et al., 1996, Johnson et al., 1998), and a similar dose decreases cerebral blood volume (CBV) in the basal ganglia of non-human primates by 10–15% (Mandeville et al., 2011), values which are concordant with the human data when using the commonly applied power-law relationship between CBF and CBV (Grubb et al., 1973). In rodent models, even very large doses of psycho-stimulants produce changes in CBV that rarely exceed 20% in magnitude (Jenkins, 2012). For such stimuli, BOLD signal changes at 2 Tesla are small (< 3%) except in draining veins and superficial cortex (Mandeville et al., 2001) and increase sublinearly with magnetic field strength, because one generally reduces the echo time (TE) at high field strengths to mitigate artifacts in BOLD-weighted signal acquisitions.
2.2 Responses mediated by systemic physiology
Drug-induced perturbations to systemic physiology affect all types hemodynamic responses, but changes in respiration produce particularly strong influences on phMRI signal, and especially when using BOLD signal. Although CBF and CBV also change versus the level of arterial CO2, CO2-mediated changes in BOLD signal are about twice as large as metabolically-mediated changes in BOLD signal for matched changes in CBF (Hoge et al., 1999). This result occurs because BOLD signal represents a cancelation of hemodynamic responses with oxygen utilization, so that dilation in the absence of oxygen utilization (e.g., due to CO2) produces very large BOLD signal changes. For this reason, respiration should be controlled or monitored, and non-BOLD contrast mechanisms should be considered when respiratory variation is unavoidable.
Conversely, changes in blood pressure have relatively less effect on hemodynamics (Kalisch et al., 2001) as long as mean arterial blood pressure is restricted to the autoregulatory range for CBF. This claim may be counterintuitive for CBV, because arterial resistance compensates for changes in blood pressure to maintain CBF, but the post-arterial component of CBV is expected to follow CBF under such circumstances (Mandeville et al., 1998, Zaharchuk et al., 1999).
2.3 IRON phMRI for data acquisition
Given generally small phMRI responses and a restricted ability to average within-subject stimuli, how can we increase the magnitude of phMRI signal changes? In animal models, the simplest way is inject an exogenous iron oxide contrast prior to the functional challenge in order to increase blood magnetization (Mandeville, 2012). We refer to this methodology as the “IRON” (increased relaxation for optimized neuroimaging) method (Chen et al., 2001, Leite and Mandeville, 2006) in order to differentiate it from other MRI variants that also are sensitive to CBV. Suitable contrast agents have 1) a very blood half-life in order to enable signal changes to be associated with changes in CBV, rather than changes in agent blood concentration, and 2) a strong effect on transverse signal relaxation, so that baseline signal can be reduced about 2-fold in order to optimize detection power and minimize competing BOLD influences.
The IRON technique affords several advantages. The method produces a physiological index – the percentage changes in CBV – that is much easier to understand and to relate to the literature than percent changes in BOLD signal, which are idiosyncratic to acquisition methods and difficult to relate to underlying physiology. Other advantages can be viewed as various aspects of improved global or regional contrast to noise ratios (CNR). Tissue signal changes become much greater in absolute magnitude. Additionally, signal from large vessels is suppressed, and large vessels areas are readily identified following injection of agent; conversely, signal in draining veins can be prominent using the BOLD method. The IRON echo time can be lowered without compromising CNR (but with an appropriate adjustment in iron dose), reducing dropout artifacts that arise near interfaces between tissue and bone/air.
In rodent studies, we typically employ very short echo times in order to reduce susceptibility artifacts and then use large iron doses to compensate for the loss of detection power that would accompany this strategy using BOLD contrast. In fact, IRON detection power is highest at short echo times and high dose, and accurate measurements of percentage changes in CBV can be obtained even at high magnetic fields under these conditions (Mandeville et al., 2004). At 9.4 Tesla, which slowly has become more widely available as a small-bore investigation tool in many imaging laboratories, one can expect a 2-fold increase in CNR from the use of iron oxide; at clinical field strengths, the CNR boost relative to BOLD signal is much larger. In theory, an optimal iron dose drops baseline signal to about 37% (1/e) of the pre-iron baseline level, but CBV varies regionally, so the signal drop also is regionally specific. In practice, we employ a combination of iron dose and echo time that drops global brain signal by a factor of about 2. At an echo time of 5 ms in rodents, a suitable iron dose is 30–40 mg/kg. Note that the use of short echo times and at high iron doses also mitigates the problem of BOLD contamination in CBV-weighted signal, an effect that could reduce detection power and degrade accuracy in percent CBV calculations at high magnetic fields using lower doses of contrast agent (Lu et al., 2007).
For repeated phMRI in non-human primates, we employ more conservative iron doses of about 10 mg/kg and we also apply periodic treatment by an iron chelator in order to limit the rate of iron accumulation in repeated studies. A somewhat longer echo time (typically 20 ms) is consistent with this iron dose (Leite et al., 2002). In order to achieve an isotropic resolution approaching 1 mm in non-human primates in conjunction with this echo time, parallel imaging is required in order to shorten the long EPI acquisition time. Single-shot imaging is particularly useful for awake non-human primate studies, where motion artifacts degrade the quality of multi-shot methods. Regardless of the imaging variant employed, a temporal resolution better than 10–15 sec per brain volume generally is desirable in order to follow potentially rapid changes in function. Even for a drug like amphetamine, which produces prolonged changes in CBV in rodent and non-human primate models at high doses of drug (Chen et al., 1997, Jenkins et al., 2004), the temporal dynamics at lower doses can be remarkably fast – much faster than the dynamics of dopamine release presumably due to competition between stimulation of D1-like and D2-like receptors (Mandeville et al., 2013) – and so rapid temporal sampling generally is a good idea unless very high spatial resolution is required to target a specific area.
The IRON technique has not been employed for phMRI studies in human subjects to date. Ferumoxytol contrast agent (Feraheme, AMAG Pharmaceuticals, Inc., Lexington MA) has been employed off-label as an MRI contrast agent in numerous human studies at doses that should prove effective for phMRI (Hamilton et al., 2011, Christen et al., 2012, Yilmaz et al., 2012, D’Arceuil et al., 2013), and one study reported a 2-fold improvement in the CNR for fMRI at 3 Tesla (Qiu et al., 2012) using a lower dose of iron than that used in animal studies, a result in agreement with preclinical predictions for this agent and dose (Mandeville, 2012).
2.4 Gradient echoes versus spin echoes
Spin echoes images refocus static magnetic field inhomogeneities to reduce localized signal dropout and thus improve the aesthetic quality of MR images, but spin echoes generally represent a poor choice for BOLD or IRON phMRI. In the functional regime, spin echoes have been suggested to bestow a degree of spatial specificity lacking in gradient echo acquisitions by increasing the relative weighting of capillary contributions. This argument derives from early estimates of the relative capillary weighting of the extravascular component of signal induced by paramagnetic contrast agents (Boxerman et al., 1995). In fact, a majority of BOLD spin-echo signal arises from the intravascular space at common magnetic field strengths (Oja et al., 1999), nullifying arguments of capillary specificity. Moreover, spin-echoes refocus a significant fraction of the microscopic susceptibility-induced changes in magnetic field that drive the functional response, thereby reducing BOLD CNR by a factor of 3–4 at clinical field strengths (Bandettini et al., 1994) and by a factor of about 2 even at 7 Tesla (Yacoub et al., 2005).
When using the IRON method, an increase in the dose of contrast agent presents a way to compensate for a portion of the CNR lost by choosing a spin-echo acquisition. However, spin-echoes cannot achieve a detection power comparable with gradient echoes using the IRON method (Mandeville et al., 2007). Furthermore, it is unclear whether spin echoes offer any improvement in spatial specificity relative to gradient echoes, because large-vessel signal already is attenuated with gradient-echo IRON acquisitions.
2.5 Awake versus anesthetized phMRI in animal studies
Functional neuroimaging is intolerant to motion, as the goal is to repeatedly interrogate various aspects of local brain function as a function of time without variations in space. Compliant human subjects voluntarily curtail movement, but suppressing motion in animal models presents a conundrum. Motion-reduction methods for animal imaging include anesthesia or physical restraint coupled with intensive training. These alternative strategies present a series of tradeoffs that must be considered within the context of the goals and logistics of each study.
Imaging of animals in the conscious state removes confounds associated with anesthesia and enables studies of behavior and cognition. Restraint and training techniques depend upon species and upon the amount of effort dedicated to each subject. Alternative strategies include the use of ear and mouth bars that mimic the stereotaxic frames often used for targeted injections in anesthetized animals (Ferris et al., 2011), implanted head posts following established procedures developed for electrophysiological recordings in awake animals (Vanduffel et al., 2001), custom molded helmets for each subject (Silva et al., 2011), or even no restraint at all in the case of well-trained dogs (Berns et al., 2012). In our experience using non-human primates (e.g., (Vanduffel et al., 2001, Leite et al., 2002, Mandeville et al., 2011), the essential ingredient for effective neuroimaging is intensive training over a period of several months. Studies that rely only or mainly upon restraint should expect confounds of stress and arousal, which have been suggested to make responses less specific and interpretable (Peeters et al., 2001), and altered behavioral states like learned helplessness, which has been shown to modulate multiple neurotransmitter systems, including dopamine, serotonin and norepinephrine (Pryce et al., 2011).
Potential downsides to awake imaging for phMRI studies generally include a limited number of subjects due to the high investment in each subject, a reduced ability to monitor physiology and control respiration, increased motion artifact, and restricted pharmacological flexibility due to adverse behavioral consequences at some drug doses. For pharmacological stimuli, infusion-correlated motion artifacts in awake animals can be problematic due either to direct brain motion or to changes in magnetic field associated with body motion (Pfeuffer et al., 2007). Effects of body motion can be reduced through the use of high-powered gradients and parallel imaging (Kolster et al., 2007) in order to reduce pixel shifts associated with changes in the magnetic field. Repeated studies further reduce motional artifacts in the average response.
For phMRI studies of acute or chronic effects of drug in the absence of behavioral correlates, anesthesia represents an alternative path that improves physiological monitoring and removes behavioral confounds but cannot completely eliminate all uncertainties in interpretation. It is well known that some anesthetics are particularly bad for some applications. For instance, alpha-chloralose often is used to preserve fMRI signals that arise due to sensorimotor stimuli, but dopaminergic function is far more depressed under that anesthetic than under the volatile agents (Nieoullon and Dusticier, 1980). According, we found that cocaine-induced fMRI responses to dopaminergic stimuli were severely blunted under alpha-chloralose relative to volatile agents (Chen et al., 2000), and cocaine-induced optical imaging signals from cortex differed between agents as well (Du et al., 2009). Additionally, one should expect modified pharmacokinetics, due at least to altered systemic metabolism. An example is shown in Fig. 2c, where the more prolonged response to cocaine in the anesthetized state probably reflects a longer plasma half-life; similarly prolonged responses have been shown following ethanol infusion in anesthetized rodents (Luo et al., 2007).
Fig. 2.
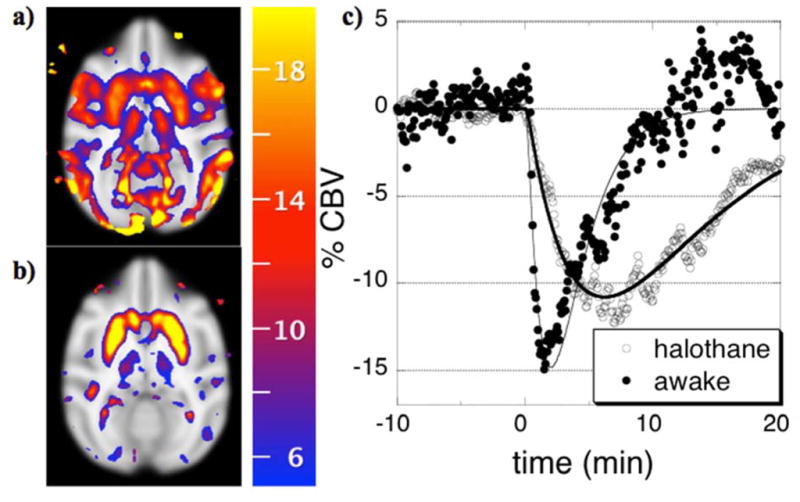
Percentage reductions in CBV (color scale) correlated with simple gamma-variate regressors that model responses to 0.5 mg/kg cocaine in single sessions in the same non-human primate that is either awake (a) or anesthetized by 1% halothane (b), and the corresponding temporal responses from putamen (c).
Because anesthetic mechanisms are poorly understood, one generally cannot exclude the possibilities of interactions anesthetics and drug challenges, and so a necessary strategy is to validate some functional readout in the particular anesthetic protocol versus awake studies for the neurotransmitter system under investigation, using methods that may or may not include neuroimaging (e.g., microdialysis, glucose utilization, CBF, …). An illustrative awake-anesthetized comparison is shown in Figure 2, in which 0.5 mg/kg was administered to the same animal in the awake state (Fig. 2a) or under 1% halothane (Fig. 2b). In each case, cocaine decreased CBV throughout basal ganglia by similar magnitudes. In the awake animal, additional decreases in CBV throughout motor and visual cortex probably reflect behavioral consequences secondary to the direct effects of cocaine, due to a loss of visual fixation and reduced body motion following administration of this cocaine dose. Hence, in order to interpret the pharmacological/behavioral response to cocaine administration in awake animals, we have employed methods that closely monitor behavior, use moderate doses of drug, and attempt to temporally segment cue-induced responses from cocaine-induced phMRI signal (Mandeville et al., 2011, Nelissen et al., 2012).
In summary, studies in awake animals are necessary for investigating behavioral consequences of drug reward and for direct comparisons with results obtained in human subjects. Conversely, it is unclear that awake animal models are necessary or sufficient for understanding the direct effects that result from the binding of a drug to targets in the central nervous system, and so anesthetized studies will continue to play a role in studies that evaluate drugs or seek to understand brain function using tools like phMRI or PET.
2.6 How long should I scan?
Sampling only a portion of the response to a drug infusion incurs several penalties. Firstly, one cannot determine a pharmacodynamics profile for the drug without viewing the full wash-in and wash-out kinetics, so short scans limit the information content that is accessible. Secondly, drug responses often evolve slowly and therefore exhibit covariance with baseline drift, an inescapable factor in phMRI studies; longer periods of sampling can reduce this covariance. To address this second issue, imagine that we inject a drug at a time point one third of the way through a functional run, and we ask how long we should image in order to detect a response that reaches a peak after time τ and then slowly returns to baseline. Short responses will be easy to distinguish from baseline drift, whereas longer responses will have a greater area (assuming a similar response magnitude) but will be more difficult to distinguish from baseline drift.
One way to approach this problem is to analyze experimental designs with a general linear model (GLM). Data analysis will require a model for the drug-induced response plus a description of variations in the “baseline” signal. Suppose we use three terms to model the baseline – a constant term, a linear drift, and a quadratic drift. The four regressors form a design matrix (X), and data (Y) as a function of time (t) is analyzed with the GLM as
| [1] |
CNR for a gamma-variate response is the magnitude of the gamma-variate function divided by the standard error, which includes a contribution from sample noise relative to the model and another contribution that depends only upon the design matrix (Poline and Brett, 2012):
| [2] |
where the subscripts designate the gamma-variate regressor index.
This formulation enables a simple calculation of CNR for each value of the time-to-peak relative to the end of the run. As shown in Fig. 1a, CNR initially increases for longer responses, assuming each response has the same magnitude. This is as we expect – CNR should increase as the cumulative area of the response increases. However, the CNR function starts to drop for responses that do not resolve prior to the end of the scan. In such cases, it becomes harder to distinguish slowly varying responses from signal drift in the baseline. Figure 1b shows three responses with the same CNR, based upon the analysis of Eq. 2. Responses that do not resolve prior to the end of run offer no better CNR than much shorter responses, as illustrated by a comparison of the two gray lines in Fig. 1b. Each of the responses indicated by the gray lines must be twice as large as the response shown in black in order to produce the same CNR, based simply upon the sample covariance of the design matrix shown in Eq. 2. In summary, better sensitivity (and less artifact) results from sampling throughout the entire pharmacological response to reduce the covariance between baseline drift and the drug model.
Fig. 1.
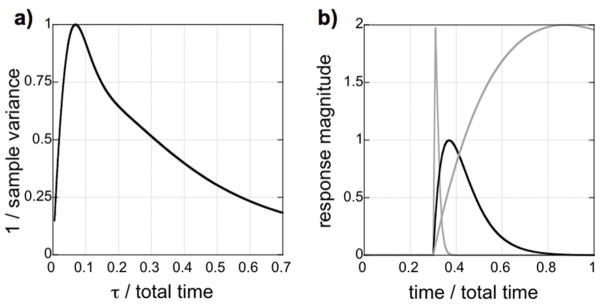
a) The inverse of the sample covariance for a gamma-variate regressor in a design matrix including drift terms. Initially, CNR increases as the area of the response increases, but CNR decreases for long values of the time-to-peak (τ) as the response becomes more difficult to separate from baseline drift. b) Three response shapes with the same CNR based upon differences in sample variance. Optimal sampling continues until the response returns to baseline (dark solid line). If sampling is terminated during the response, then a long response will have no larger CNR than a very short response (gray solid lines).
Note that although this assessment of baseline drift employed one particular statistical framework (GLM), the implications are independent of the analysis strategy: slow changes in brain activity induced by pharmacological stimuli are intrinsically difficult to distinguish from drift. Methods that can help disambiguate drug-induced changes from signal drift include a sufficient duration of sampling, as addressed in this section, and amplification of drug-induced signals using appropriate methods (e.g., IRON contrast, and gradient-echo rather than spin-echo imaging), as discussed previously.
Finally, one additional method has been mentioned in the literature as a way to control drift artifact. Arterial spin labeling (ASL) forms flow-sensitive images through a pairwise subtraction of image volumes with and without labeling of proximal blood water. As such, some drift contributions will be removed in the differential signal, which should exhibit less sensitivity to low-frequency signal fluctuations, particularly for stimuli that cannot be cycled in order to resample the baseline (Aguirre and Detre, 2012). Although within-subject detection power generally is small compared to BOLD signal, some evidence suggests that inter-subject variance is reduced relative to BOLD signal in areas that can be detected by ASL within individual subjects (Tjandra et al., 2005). These considerations suggest that ASL may play a useful role in human phMRI studies.
3. Data Analysis
3.1 Cross-subject normalization
Cross-subject registration is a standard and requisite method in the neuroimaging literature. However, some of the standard human analysis tools, like automated brain identification (“skull stripping”), are not well developed even in non-human primate species (Rohlfing et al., 2012), let alone rodents. Our strategy for preclinical imaging has been to analyze all data in a standardized space, generally corresponding to a published stereotaxic atlas. By only matching voxels within the region defined as brain tissue in the standard space, one eliminates the need to define brain tissue in each native space. Our strategy employs a mutual information cost function (Pluim et al., 2003) after reducing variations in the RF response field by dividing both target and source volumes by low-resolution versions of themselves. Initial registration includes automated adjustment of up to 12 affine parameters at multiple resolution steps, followed by automated adjustment of multi-resolution distortion fields encoded by spatial basis functions (e.g., (Ashburner and Friston, 1999)) to refine registration. The use of a mutual information cost function is advantageous for the last step, so that deformations, which often occur in functional images but are dramatically reduced by most high-resolution anatomical imaging methods, can be reduced by refining the registration of the functional images relative to a conventional non-distorted anatomical target. Further documentation of our methods and software are publicly available (http://www.nitrc.org/projects/jip).
3.2 Analyses within the general linear model (GLM)
GLM has become the standard analysis technique for fMRI applications (Poline and Brett, 2012), because it combines parameter estimation and statistical testing within a flexible and efficient framework. Moreover, the framework is designed to represent data using multiple regressors that overlap in time. GLM views each temporal data vector as a simple summation of multiple temporal responses, each with local scaling factors, which evolve simultaneously throughout the scan. Purely from a neuroscience viewpoint, GLM has an intuitive appeal in that we often think of local brain processes as summations of activity generated from various neurochemical processes and neural projections.
Many drugs produce functional response with several temporal components (Liu et al., 2007, Choi et al., 2010, Chen et al., 2011, Mandeville et al., 2013). In principle, different temporal components can represent systemic versus local effects, multiple receptor systems with different affinities, or adaptive processes like agonist-mediated receptor internalization. Additionally, these responses occur simultaneous with other regressors of less interest, such as signal drift arising from multiple sources including motion. The ability to model all these overlapping responses, or drug interactions in basic paradigms like antagonist-agonist experiments, is so critical that one might think of GLM as the “general overlap model”.
GLM enables a multitude of ways to represent a response to a drug, but not all methods are statistically efficient or offer insight into the underlying biology. One simple and common way to analyze a response is to divide the time domain into periods before and after drug infusion. However, boxcar regressors do not account for the slow rise of responses; such methods are statistically inefficient (Schwarz et al., 2007) and are analogous to fMRI analyses that ignore hemodynamic delays. Moreover, simple boxcar regressors might not even detect biphasic responses, as can occur due to stimulation of multiple post-synaptic receptor subtypes. At the opposite extreme, another GLM implementation tests each point after drug infusion as an independent observation; this method exacerbates the multiple comparisons problem and has limited detection power.
Ultimately, there is no getting around the main issue: one has to provide some sort of model for the phMRI response that sufficiently captures the complexity of the data while limiting the number of regressors. We can motivate an approximate functional form as the starting point for a basis set, but it’s generally not possible to define the form very specifically from simple principles. From first-order pharmacological principles in a two-compartment plasma-tissue model, we can approximate delivery of a drug to the CNS space as the difference of two exponential functions. Many drugs will modify neurotransmitter concentrations by binding to transporters or autoreceptors, adding another layer of dynamics. The specific binding of drugs or neurotransmitters to receptors or transporters are subject to saturation according to Michaelis-Menten kinetics (Michaelis et al., 2011). Receptor trafficking – in particular agonist-induced receptor endocytosis as a homeostatic down-regulatory mechanism – adds a further layer of complexity to the dynamics of the functional response. In the end, any presumed fMRI model will be an approximation of the actual neural response, but the essential form looks like a gamma-variate function representing the wash-in, binding, and wash-out of drug. The first real attempt to provide a physiologically motivated analysis model for phMRI data employed a waveform model of this type (Bloom et al., 1999).
For simple responses that empirically exhibit a single temporal response shape across all brain regions, there is no ambiguity: the response model should be a regressor that approximates the shape of the data in order to maximize detection power, following the concept of the matched filter in engineering. In our work, typically we use simple alternative forms of phMRI regressors to model the response to a drug: gamma-variate functions are applied to analyze responses that reach a peak and resolve toward baseline during the measurement period, and sigmoidal functions represent responses that show no or little return toward baseline during the measurement period. In fact, the exact form of regressors is not particularly important, as all regressors within the model covary with baseline polynomial terms that serve both to describe drift and to diminish imperfections in the drug model. As in other fMRI analyses, additional regressors can be included to account for effects of motion or changes in systemic physiology.
On the other hand, drugs that evoke responses that vary regionally present an inherent ambiguity in the best way to proceed for analysis, because there are multiple ways that an analysis can achieve good fits across multiple regions. One could choose to employ multiple gamma-variate functions, linear combinations of those functions, or even biphasic functions (e.g., a gamma-variate function and its derivative) to form a basis set of analysis regressors that can adequately represent data across multiple brain regions. The main question is this: what does one wish to accomplish with a data-driven phMRI analysis? The following considerations suggest an approach:
Response shapes should be mono-phasic (e.g., simple gamma-variate). Clearly, this is not a requirement for GLM, but rather a biological choice based upon the idea that individual receptor subtypes are G-protein coupled to tissue function positively or negatively, so we want regressors that are clearly signed in order to attempt to identify spatiotemporal phMRI profiles with receptor stimulations. For example, dopamine D1/D5 receptors are positively coupled to function, and D2/D3/D4 receptors are negatively coupled to function as measured by molecular biology (Stoof and Kebabian, 1981) or phMRI (Choi et al., 2006). Biphasic responses can occur from dopaminergic stimuli like amphetamine (Mandeville et al., 2013), but presumably these responses represent the competing effects of D1 and D2 receptor stimulation, rather than biphasic effects at either receptor subtype.
In order to detect responses in all regions that do exhibit a response, defined as any variation in signal that is reproducible across injections and subjects, one needs an analysis basis set that adequately captures regional variations in shape. Regional response shapes can only be represented in a linear model analysis with two or more regressors. Obviously, multiple regressors enable a type of pattern analysis based upon the temporal domain. Optimally, the goal is to associate temporal responses and regions with underlying receptor concentrations and pathways.
Too many regressors will degrade statistical power through covariance (and to smaller extent, a loss of degrees of freedom). As a practical matter, we have found that the number of regressors that can be assigned to a single drug infusion generally is limited to two in practice (assuming regional variation of the response), although we have used as many as three separate regressors in some cases (Liu et al., 2007).
-
Even after fixing the number of regressors and the basic functional form (e.g., gamma-variate functions), some ambiguity still remains, but not all choices are equally efficient from the viewpoint of within-subject or across-subject statistical power. Ways to resolve the ambiguity in analysis regressors include considerations of biology or statistics.
A “strong hypothesis” approach based upon a biophysical model (e.g., (Mandeville et al., 2013)) in principle can provide a full description of the temporal profile, or a division of the response into physiologically-motivated temporal regressors if the responses are observed to vary regionally.
A “weak hypothesis” approach can define at least one regressor based upon a reference region known to contain, or conversely to not contain, neural targets of the drug of interest, with other regressors defined by minimizing the variance or standard error of the model fit across regions.
A statistical approach minimizes the standard error of the model fit after accounting for covariance; this latter approach is illustrated in analysis of remifentanil data reported here.
Finally, it should be clear that the analysis model must be fixed across all subjects, treatments, and groups, because each regressor magnitude (βc terms in Eq. 1) that is fit within the GLM depends upon the explicit form of the drug model. One should not, for instance, alter the form of a model due to application of an antagonist (i.e., do not use different regressors shapes for the drug response prior to the antagonist versus after the antagonist), because then the comparison of amplitudes with and without antagonist pretreatment would be meaningless.
3.3 Across-subject analyses
Methods for cross-subject analyses for phMRI studies do not differ in any way from routine fMRI analyses. Because variance across subjects is larger than would be predicted from the first-level GLM variance within a subject, an analysis that averages or concatenates the time points from every subject and analyzes data using a first-level GLM risks false positive results (Type I errors) driven by a single subject or a few subjects. Conversely, a pure “random effects” analysis that ignores first-level variance and degrees of freedom is quite conservative, resulting in Type II error. A mixed-effect model offers a good compromise between “efficiency, generality, validity” (Worsley et al., 2002). Error bars from the first level influence the relative weights for each subject, the effects of outliers can be reduced significantly compared to a fixed-effects model, the random-effects variance is regularized using the more extensive variance data acquired at the first level, and the multiple comparisons problem for neuroimaging can be addressed with smaller populations than required in a pure random-effects analysis.
Neuroimaging studies require an analysis of cost versus benefit, and so some judgment must be made about the relative effect sizes of interest versus the number of subjects to be scanned. For many studies, especially preclinical mechanistic or exploratory studies, an analysis based upon a priori regions of interest (ROIs) may be appropriate. For instance, a raw p-value threshold of 10−3 will support 50 ROIs at a corrected p-value threshold of 0.05, but it is not adequate is to support a full voxel-wise map. In our experience when using a reasonably reproducible pharmacological stimulus and about 6 subjects, a mixed-effects analysis following the Worsley method with a raw p-value threshold of 10−3 produces a functional map that is qualitatively similar to a fixed-effects analysis using a strictly corrected p-value threshold, and this analysis should prove amenable to a true random-effects ROI-based analysis with about 50 ROIs.
Finally, it’s important to keep in mind a general statistical truth about differential comparisons: paired tests are far more powerful than pooled tests. When possible, differential comparisons should be performed within each subject so that the differential condition can be compared directly across subjects. For instance, the effects of selective antagonist on a drug can be evaluated using one experiment with an infusion order of drug-antagonist-drug, as long as one can demonstrate reproducibility of the drug’s response in a drug-vehicle-drug paradigm.
3.4 Alternatives to the General Linear Model
Data analyses can be confirmatory of a hypothesized model, including a GLM specification, or exploratory in nature. The latter method replaces explicit models with constraints on the form of candidate models and then defines a set of analysis regressors based upon an optimization subject to the constraints. Blind source separation methods, especially independent component analysis (ICA), have become popular for spatially segmenting resting state fMRI data into regions with highly correlated temporal responses (Beckmann et al., 2005). ICA attempts to explain variance in data and is particularly good for detecting artifacts (outliers) in fMRI data. A probabilistic independent component analysis (PICA) approach incorporates a noise model to reduce over-fitting problem and enable statistical inference (Beckmann and Smith, 2004).
ICA is used to segment a spatiotemporal dataset into a series of spatial components with temporally distinct features. Because if offers no insight into the nature of the temporal vectors, ICA functions primarily as an exploratory tool to identify regressors for subsequent analysis using GLM or other hypothesis-driven (confirmatory) methods. For instance, ICA can identify spatially independent resting-state networks, and these regions then can provide a series of time vectors for use in a multivariate linear analysis in order to identify group-wise differences (Filippini et al., 2009). ICA has been applied less commonly to analyze phMRI data; in one example, analysis of CBF temporal responses during amphetamine administration in human subjects using ICA identified several components that appeared to be related to drug infusion (Schouw et al., 2013).
4. An example application
As an example application to illustrate a methodological approach to phMRI acquisition and analysis, we employed the mu-opioid receptor (MOR) agonist remifentanil, which we previously showed to produce a complex pattern of signal changes with mono-phasic responses in some brain regions and multi-phasic response in others (Liu et al., 2007). Remifentanil’s biological action is multi-fold, as MOR is heterogeneously but widely distributed throughout rat brain (Tempel and Zukin, 1987), and MOR agonists produce regional neuronal inhibition and disinhibition, as well as modulation of several different neurotransmitters including dopamine (Mansour et al., 1995). In this study, acquisition employed the IRON method to achieve high detection power. Data were collected with sufficient temporal resolution to clearly define regional response shapes. Analysis employed multiple regressors within a GLM framework, followed by a second-level GLM analysis across subjects. Application of dopaminergic and GABAergic antagonists explored mechanisms using within-subject blocking designs. All experiments were performed at the Massachusetts General Hospital according to NIH and Institutional animal care guidelines.
4.1 Animal groups and data acquisition
Data were collected in paralyzed and mechanically ventilated halothane-anesthetized rats (n=16 total) using previously reported methods for animal preparation, monitoring, and scanning (Liu et al., 2007). In each animal, we infused 10 ug/kg remifentanil over 2 minutes using an infusion pump, and infusions were repeated four times at 15-minute intervals. To investigate opioid-induced activation of the mesolimbic dopaminergic pathway mediated by GABAergic influences, we divided animals into three groups to test effects of three antagonists. In each group, we repeated the first functional scan during continuous infusion of one of these antagonists: a) the D1 antagonist SCH-23390 (n=5), b) the GABA-A antagonist bicuculline (n=5), or c) the GABA-B antagonist CPG-35348 (n=6). SCH-23390 was delivered as a slow bolus of 0.4 mg/kg at a time 10 min into the scan followed by continuous infusion at a rate of 0.4 mg/kg/hr. Bicuculline and CPG-25348 were infused at a rate of 0.25 mg/kg/min.
4.2 Data Exploration
Data from individual subjects (n=16) were averaged in four dimensions and subjected to probabilistic independent component analysis using Melodic software (Beckmann and Smith, 2004). Figure 3 shows the first three spatiotemporal components from one analysis of this dataset. Two of these components were nearly identical in the temporal domain, and in fact many components were nearly collinear in time except for slight variations in drift or noise contributions. One of the first three components (red) was strongly biphasic, a characteristic that is not constrained by ICA. Results proved to be remarkably sensitive to the amount of noise in the dataset, such that small differences in the mask used to isolate brain tissue produced large differences in the resulting factorization. Overall, ICA proved to have some utility as an exploration tool in order to identify patterns as a step in forming hypothesis for subsequent analysis.
Fig. 3.
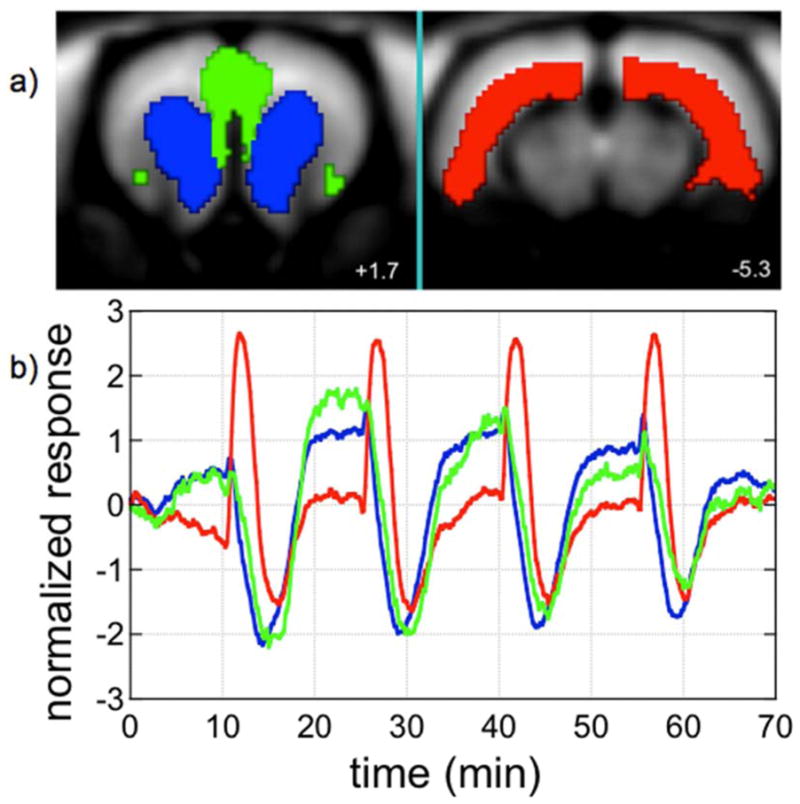
Three spatio-temporal components, coordinated by color, were identified by independent component analysis of a phMRI series with repetitive remifentanil infusions. Slices 1.7 mm anterior and 5.3 mm posterior to bregma are shown.
4.3 Data Analysis Strategy by GLM
The mu-opioid agonist remifentanil induces a regionally variable response pattern that we previously analyzed as a sum of gamma-variate temporal components after including a slight correction for the extended infusion interval (Liu et al., 2007). This prescription was found to adequately describe regional variations in response profiles and to implicate function connections reported by electrophysiology, including those between hippocampus, amygdala, and piriform cortex. To analyze this dataset, we employed modified procedures using two gamma-variate functions plus a three-parameter polynomial description of baseline drift.
There is an inherent ambiguity in choosing multiple regressors to model regional variations in phMRI signals; for instance, for any choice of two regressors, some linear combination of those regressors could represent an equally valid choice in terms of simply minimizing the goodness of fit, based upon a criteria like the chi-squared per degree of freedom. However, given a choice of functions (e.g., gamma-variates) for a basis set, covariance dictates a statistical preference. To illustrate this, we assessed automated methods for defining two gamma-variate regressors by minimizing a global fitting cost function across many regions of interest, given a series of known injection times. One strategy minimizes the global variance and yields the best fit across all tested regions. Another strategy minimizes the standard error of parameter estimations (denominator in Eq. 2) including both the variance and the covariance between parameters. Note that the covariance penalty essentially regularizes fits and prevents nearly redundant regressors that fit well through large cancelations of relative signal. To account for the estimation of multiple parameters, one can define estimation efficiency (ε) as (Dale, 1999, Liu et al., 2001):
| [3] |
This definition of efficiency assumes that we are equally interested in each of the specified parameters determined from GLM, and not in linear combinations (e.g., differences) in those parameters.
To minimize the global variance and standard error, we reduced the dimensionality by averaging results across 50 regions of interest, rather than all voxels in the volume. We included two gamma-variate functions of the form t/τ*exp(-t/τ) in the GLM, so that the problem was reduced to determining two time constants: τslow and τfast. We then performed a two-dimensional grid search in which all GLM parameters, including baseline parameters, were fit across all regions for all values of each time constant. Note that all dataset were registered to a standardized space, the Paxinos-Watson rat brain atlas (Paxinos and Watson, 1998), based upon a multi-subject (n=20) multi-contrast MRI template registered to the digital atlas using the methods described in Section 3.1.
4.4 Results: Analysis of time constants
Fig. 4 shows results obtained by globally optimizing two different GLM fitting cost functions using two gamma-variate regressors and a second-degree polynomial description of the baseline signal. Maximizing the efficiency, defined by Eq. 3, produced time constants that were more widely separated and had a smaller standard deviation across animals than the method of optimizing the global goodness of fit without taking account of parameter covariance. Panels 2b and 2c show regressors corresponding to average shapes determined using each method. Panel 2d illustrates that both methods can provide a good fit to data, focusing on dorsal hippocampus in the figure, but more similar regressors fit the data by large cancelations between components. Not surprisingly, regressors obtained by optimizing the efficiency also produced a higher contrast-to-noise ratio across animals in a second-order GLM analysis, due to better stability in the fits. All further results employ the efficient regressors of Fig. 4b to describe the phMRI response to remifentanil consistently in each animal.
Fig. 4.
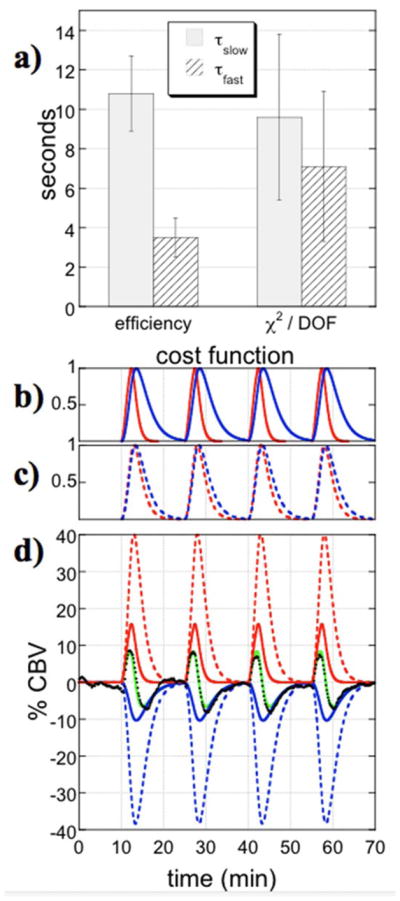
a) Time constants and standard deviations across animals (n=16) using two cost functions for global fits. Regressors derived from efficiency (b) or chi2/DOF (c) each result in good fits (green line, d) to data (black points), although the efficient regressors produce coefficients with less dispersion across animals.
Fig. 5 illustrates across-animal results for the fast and slow regressors in a second-level GLM analysis to demonstrate the stability of the temporal segmentation. Figure 5a shows the fast and slow components across animals (n=16) in nucleus accumbens and dorsal hippocampus. Within-subject error bars were calculated as the denominator in Eq. 2, and lines represent the average response across animals. Fig. 5b shows maps of the percentage change in CBV associated with slow components at slice levels corresponding to nucleus accumbens and dosal hippocampus, and Fig. 5c depicts CBV changes associated with the fast component for both slices. For each voxel, the magnitude represents the average value from the second-level analysis, depicted in selected regions in the graphs. Functional maps were subjected to a statistical threshold of 0.01 after correction for multiple comparisons across the entire brain volume, corresponding to a raw statistical threshold of about 10−6 in the second-level analysis.
Fig. 5.
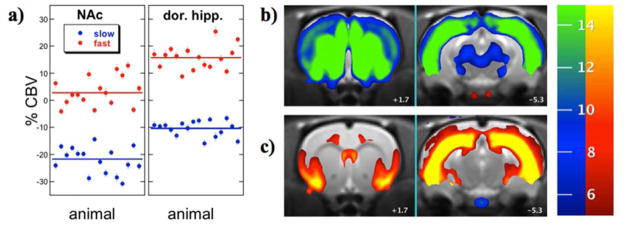
a) Individual responses (points) and average response (lines) for slow (blue) and fast (red) temporal components in nucleus accumbens (left) and dorsal hippocampus (right). b) Maps of the slow temporal response in coronal slices at the level of nucleus accumbens (left, +1.7 mm from bregma) and hippocampus (right, −5.3 mm from bregma). c) Maps of the fast temporal response in identical slices. The colorbar shows magnitude changes in CBV, with negative changes on the blue-green scale and positive changes on the red-yellow scale.
This analysis of remifentanil data in this report incorporated some simple biologically motivated constraints, in terms of the functional form of regressors, but otherwise did not highly constrain the final model. The spatial maps corresponding to each separate regressor suggest that temporal segmentations of this type implicate functional connectivity, and this conclusion is not particular sensitive to which choice of alternative regressors in Fig. 4. If we compare functional maps produced using the efficient regressors of Fig. 4b with the regressors that minimized the chi-squared of the fit (Fig. 4c) across whole brain (not shown), the results are qualitatively similar, although the response magnitudes differ between the two analyses, and the efficient regressors produce somewhat better detection power. In each case, however, the pattern of functional maps closely matches previously published results (Liu et al., 2007). As discussed in the earlier publication, the map of the slow response is associated almost exclusively with reductions in CBV and corresponds well spatially with the known distribution of mu-opioid receptors, while the map of the fast response is associated primarily with increases in CBV and may reflect disinhibition through GABAergic interneurons in the hippocampus and connected structures.
4.5 Results: Effects of Antagonists
The abuse potential for MOR agonists suggests a role for dopamine, which is released in ventral striatum when opiates disinhibit GABAergic neurons in the ventral midbrain (Hyman et al., 2006). Microdialysis in rats revealed that repeated infusions of remifentanil induce repeated elevations of dopamine within the striatum (Crespo et al., 2005). Thus, remifentanil-induced phMRI signal in striatum presumably reflects a competition between opioid-induced decreases in CBV and dopamine-induced increases in CBV.
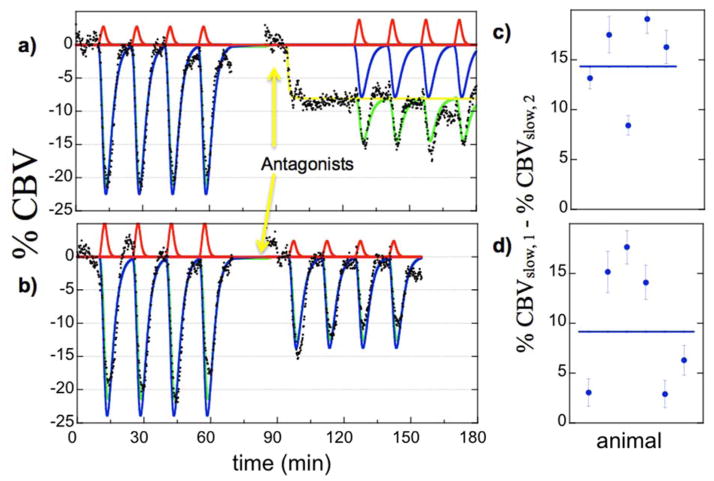
Figure 6 illustrates the subject-averaged data in nucleus accumbens for the SCH-23390 group (a, n=5) and the CPG-25348 group (b, n=6). The D1 antagonist SCH-23390 inhibited CBV in accumbens (yellow curve), a well-known result in the basal ganglia of rodents (Marota et al., 2000, Choi et al., 2006). Because the GABA-B antagonist CPG-25348 did not produce a robust functional response in preliminary studies, we began infusion prior to the run; the direct effects of CPG-25348 were not assessed in this study. However, each antagonist produced inhibition of the subsequent response to remifentanil. Effects of antagonism were assessed within the GLM model at the first and second levels as the differential response of the slow regressor before infusion of antagonist versus the corresponding regressor during infusion of antagonist, with the subject-specific results for the respective antagonists shown in the second-level analysis of Fig. 6 (c, d).
Fig. 6.
Subject-averaged temporal responses in nucleus accumbens in the SCH-23390 group (a, n=5) and the CPG-25349 group (b, n=6). Antagonism produced a significant effect on the slow temporal response (blue regressors) but not on the faster response (red). Subject-specific results for the differential slow response are shown in panels c and d for the two antagonists.
Figure 7 shows functional maps of the effects of antagonism on remifentanil-induced changes in CBV. Data are shown as percentage changes in CBV for the differential response between slow regressors before and during infusion of antagonist. A raw statistical threshold of p<0.001 was applied to the data, which does not sufficiently account for multiple comparisons across all voxels; however, this threshold supports a corrected p-value of 0.05 for 50 regions of interest. For each antagonist (SCH-23390, CPG-25348, bicuculline), differential responses between slow regressors highlighted regions associated with mesolimbic dopaminergic pathways. Significant differential responses were observed in nucleus accumbens and most aspects of medial prefrontal cortex, including cingulate, prelimbic, and infralimbic cortex in the Paxinos-Watson terminology (Fig. 7), as well as hippocampus and amygdala (not shown). For this analysis, differential responses between fast regressors did not achieve significance in any of 50 regions tested.
Fig. 7.
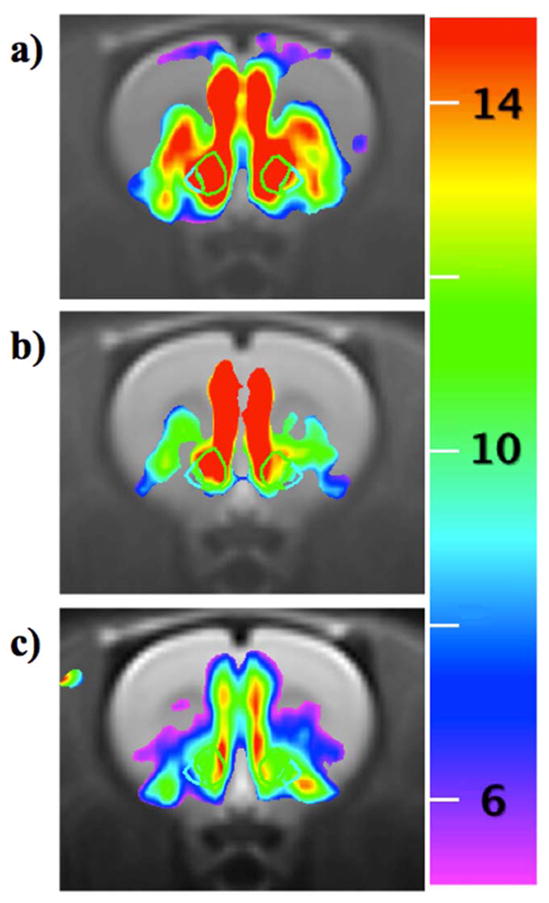
Differential effects of antagonism on remifentanil-induced percent change in CBV (color scale) at the level of nucleus accumbens, outlines by wire frames. a) SCH-23390, b) CPG-25348, c) bicuculline.
4.6 Discussion: Advantages and limitations, as illustrated by this example application
This example paradigm using antagonism of remifentanil illustrates both advantages and drawbacks of phMRI. Among the advantages, both the temporal segmentation and the differential response to antagonists illustrate ways to elucidate functional connectivity. Antagonism can demonstrate neurochemical components of the composite phMRI response. phMRI studies of this type can successfully achieve statistical significance for differential comparisons in ROI-based analyses even with subject cohorts as small as n=5, and mixed-effects analyses provide a good way to visualize differences with only moderate bias from Type I error.
Although this data-driven analysis offers more insight into the response than blind source separations like principle or independent component analyses, the information provided by a few such experiments nevertheless is not particularly specific. For instance, there are several potential mechanisms for producing the different time constants that are required in the analysis: remifentanil could have different affinities for mu receptor subtypes; this putative mu-opioid agonist might also have affinity for kappa or delta receptors (Jeong et al., 2012); agonist-mediated receptor internalization could abbreviate the phMRI response associated with GABA-mediated disinhibition (Liu et al., 2007).
The result of the antagonist experiments is somewhat easier to interpret, but some uncertainties remain. D1 antagonism alone attenuates striatal CBV, suggesting that a component of the differential remifentanil response can be attributed to prior inhibition of D1-innervated medium spiny neurons. Other aspects of the antagonist experiments are more difficult to interpret. Certainly, one hypothesis is that the GABAergic antagonists and SCH-23390, which increase dopamine efflux (Imperato and Di Chiara, 1988, Yan, 1999), also function to enhance remifentanil-mediated dopamine efflux, producing a positive contribution to the predominantly mu-mediated inhibition. Further experiments would be needed to provide a more complete picture of the mechanisms underlying these antagonists, as well as the composite response to remifentanil.
5. Future Directions
5.1 Data Acquisition
In human subjects, BOLD sensitivity at standard clinical field strengths presents a limitation for studies of the acute effects of drug on the brain. For instance, human fMRI studies of cocaine action using a magnetic field of 1.5 Tesla do not consistently agree on the sign or spatial pattern of the response (Breiter et al., 1997, Kufahl et al., 2005, Risinger et al., 2005). However, recent and near-term developments promise to significantly improve phMRI capabilities in human subjects. Higher magnetic field strengths and advanced RF technology, such as multichannel receive coils, continue to improve the magnitude and stability of fMRI signal changes using endogenous contrast mechanisms. BOLD signal magnitudes increase in an approximately linear manner with magnetic field strength (Gati et al., 1997), so the new generation of 7 Tesla human MRI scanners promises more than a 4-fold increase in the size of BOLD signal changes relative to 1.5 Tesla scanners. Because averaging improves detection power only as the square root of the number of subjects, about 20 times more subjects would need to be scanned at 1.5 Tesla than at 7 Tesla to match results in a fixed-effects analysis. In fact, results using drugs like cocaine suggest that the signal response is so hard to track within individual subjects that within-subject variance may even be as important as across-subject variance for pharmacological stimuli at low magnetic fields like 1.5 Tesla.
Exogenous contrast agent, as routinely used for phMRI in animal models, potentially also will find phMRI applications in human subjects. As discussed in Section 2.2, IRON fMRI already has been employed in human subjects in the USA with predictable increases in detection power given the injected iron dosage (Qiu et al., 2012). Like the analysis for 7 Tesla BOLD signal above, IRON fMRI in human subjects using the approved 510 mg dose promises about a 4-fold increase in the absolute magnitude of phMRI signal changes relative to 1.5 Tesla BOLD signals, an increase that would dramatically improve phMRI capabilities in human subjects. However, the safety profile for ferumoxytol-based MRI, an off-label application of an iron supplement designed to treat iron anemia, remains somewhat unclear. Although high iron doses even up to double the approved dose appear to be well tolerated generally (Auerbach et al., 2013), post-marketing experience has suggested the potential for acute hypersensitive reactions in a small percentage of patients (Pai and Garba, 2012), mandating caution and close patient monitoring in MRI studies using ferumoxytol.
5.2 Data Analysis
Drugs evoke functional responses through a complex series of physiological events, potentially including binding to presynaptic and postsynaptic targets and modulation of synaptic neurotransmitter levels. However, theoretical and empirical considerations suggest a reasonable simplification: phMRI responses can be viewed as summations of responses at post-synaptic receptors. It has been argued that post-synaptic currents dominate energy expenditures due to task-induced function, although flow and metabolism may be coupled indirectly through parallel feed-forward mechanisms (Attwell and Iadecola, 2002). Using selective dopamine receptor agonists and antagonist, induced phMRI responses appear to mimic the effects of these same ligands on adenylate cyclase and cyclic-AMP (Jenkins, 2012). Of course, descriptions of phMRI responses in vivo also must account for effects of endogenous neurotransmitters (displacement, modulation by presynaptic autoreceptors), which are not a consideration in most in vitro model systems. Moreover, better empirical data is needed to firmly establish the primacy of post-synaptic events in driving the hemodynamic response.
A post-synaptic, receptor-based analysis framework appears to provide a good working model to describe the phMRI response to dopaminergic stimuli in the basal ganglia. Viewing phMRI signal as a competition between excitatory effects of the D1 receptor family and inhibitory effects from the D2 receptor family can explain consistently the sign and magnitude of the phMRI response versus species and the level of evoked dopamine (Jenkins, 2012, Mandeville et al., 2013). In principle, similar models can be reconstructed for other neurotransmitter systems like serotonin, where receptor-specific functional coupling has been investigated extensively in model systems (Alex and Pehek, 2007), if not in vivo.
While explicit physiological models provide an interpretive basis for experimental design and hypothesis testing, they may not be optimal as data analysis models, depending upon how they are used. They may overly constrain analyses or suggest temporal components that are only subtly different (e.g., due to different receptor-ligand affinities) with large mutual covariance. Imperfections in our knowledge of underlying processes means that most analyses will continue to be based upon data-driven observations, rather than a priori models. Nevertheless, improvements in our understanding of neurovascular coupling mechanisms and neurochemical function will continue to be integrated into analysis frameworks that seek both statistical robustness and biological insight.
5.3 PET/fMRI
Recent efforts by biomedical manufacturers have succeeded in conjoining fMRI and positron emission tomography (PET) within a single scanner, enabling simultaneous measurements of neurochemical and neurovascular function for a wide range of pharmacological stimuli (Judenhofer et al., 2008, Catana et al., 2012). This new modality provides an efficient means of evaluating phMRI signal in relation to the local densities of receptors and transporter and in relation to neuroreceptor occupancies, which may be driving the phMRI response. Simultaneous PET/phMRI studies facilitate receptor-based models of the neurovascular response and enable fundamentally new ways of assessing aspects of neurophysiology, like basal receptor occupancies (Sander et al., 2013), that are not easily measured by either modality alone.
Although many future PET/phMRI studies rightly will focus on elucidating the nature of the neurovascular response in relation to the underlying neurochemistry, phMRI adds tremendous value by capturing elements of the response that are not easily visualized by PET. phMRI offers a means to evaluate CNS responses for neural targets that lack a PET ligand, and PET/phMRI enables studies of functional connectivity along neural pathways to projections of neurochemical targets.
Moreover, a consistent theme from PET displacement studies is that radioligands do not accurately reflect synaptic receptor occupancies when using agonist stimuli. For instance, D1 and some D2 ligands appear to be unaffected by dopamine release, and the D2 ligand raclopride can be displaced from D2 receptors for a time period that far exceeds the known duration of dopamine release (Laruelle, 2000). Conversely, antagonists easily displace any of these ligands. Similar results are observed using opioid PET ligands, which are easily displaced by antagonist but not agonists (Melichar et al., 2005). Speculation about these observations centers on agonist-induced receptor internalization (Chugani et al., 1988, Laruelle, 2000), a homeostatic mechanism that shifts receptors to an intracellular compartment that is inaccessible to endogenous neurotransmitter but accessible to PET ligands with altered affinities (Guo et al., 2010). When using agonist drugs, phMRI may prove to be the best way to evaluate the CNS response and to understand the functionality of PET ligands under these conditions. The ability now to simultaneously measure neurochemistry and function should open wide a window on the neurobiology underlying the phMRI and PET responses.
Highlights.
We review methods for pharmacological MRI acquisition and analysis.
Antagonism of a mu-opioid agonist illustrates methods and capabilities.
Data-driven general linear model analyses combine efficiency and interpretability.
High magnetic fields and exogenous contrast agent will improve phMRI capabilities.
Advances in simultaneous PET/MRI offer exciting prospects for future studies.
Acknowledgments
This research was supported by NIH grants P41RR14075, P20DA026002, and R01-EB001782.
Footnotes
The authors declare no conflicts of interest associated with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Aguirre GK, Detre JA. The development and future of perfusion fMRI for dynamic imaging of human brain activity. Neuroimage. 2012;62:1279–1285. doi: 10.1016/j.neuroimage.2012.04.039. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Auerbach M, Strauss W, Auerbach S, Rineer S, Bahrain H. Safety and efficacy of total dose infusion of 1020 mg of ferumoxytol administered over 15 minutes. Am J Hematol. 2013 doi: 10.1002/ajh.23534. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Jesmanowicz A, Hinks RS, Hyde JS. Spin-echo and gradient-echo EPI of human brain activation using BOLD contrast: a comparative study at 1.5 T. NMR Biomed. 1994;7:12–20. doi: 10.1002/nbm.1940070104. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berns GS, Brooks AM, Spivak M. Functional MRI in awake unrestrained dogs. PLoS One. 2012;7:e38027. doi: 10.1371/journal.pone.0038027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AS, Hoffmann RG, Fuller SA, Pankiewicz J, Harsch HH, Stein EA. Determination of drug-induced changes in functional MRI signal using a pharmacokinetic model. Hum Brain Mapp. 1999;8:235–244. doi: 10.1002/(SICI)1097-0193(1999)8:4<235::AID-HBM7>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Breiter H, Gollub RL, Weisskoff RM, Kennedy D, Makris N, Berke J, Goodman J, Kantor H, Gastfriend D, Riorden J, Mathew T, Rosen B, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. J Nucl Med. 2012;53:1916–1925. doi: 10.2967/jnumed.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Galpern WR, Brownell AL, Mathews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–398. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- Chen YI, Brownell AL, Galpern W, Isacson O, Bogdanov M, Beal MF, Livni E, Rosen BR, Jenkins BG. Detection of dopaminergic cell loss and neural transplantation using pharmacological MRI, PET and behavioral assessment. Neuroreport. 1999;10:2881–2886. doi: 10.1097/00001756-199909290-00001. [DOI] [PubMed] [Google Scholar]
- Chen YI, Choi JK, Xu H, Ren J, Andersen SL, Jenkins BG. Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev Neurosci. 2010;32:125–138. doi: 10.1159/000286215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Famous K, Xu H, Choi JK, Mandeville JB, Schmidt HD, Pierce RC, Jenkins BG. Cocaine self-administration leads to alterations in temporal responses to cocaine challenge in limbic and motor circuitry. Eur J Neurosci. 2011;34:800–815. doi: 10.1111/j.1460-9568.2011.07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Mandeville JB, Marota JJA, Nguyen TV, Green AR, Jenkins BG. Int Soc Magn Reson Med. Denver: 2000. Anesthetic filters for eliciting specific neurotransmitter effects in pharmacologic MRI; p. 967. [Google Scholar]
- Chen YI, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG. Improved Mapping of Pharmacologically Induced Neuronal Activation using the IRON Technique with Superparamagnetic Iron Blood Pool Agents. J Magn Reson Imaging. 2001;14:517–524. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med. 2007;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Choi JK, Mandeville JB, Chen YI, Grundt P, Sarkar SK, Newman AH, Jenkins BG. Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists. Psychopharmacology (Berl) 2010;212:59–72. doi: 10.1007/s00213-010-1924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen T, Ni W, Qiu D, Schmiedeskamp H, Bammer R, Moseley M, Zaharchuk G. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol. Magn Reson Med. 2012 doi: 10.1002/mrm.24500. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Ackermann RF, Phelps ME. In vivo [3H]spiperone binding: evidence for accumulation in corpus striatum by agonist-mediated receptor internalization. J Cereb Blood Flow Metab. 1988;8:291–303. doi: 10.1038/jcbfm.1988.64. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. Simultaneous intra-accumbens remifentanil and dopamine kinetics suggest that neither determines within-session operant responding. Psychopharmacology (Berl) 2005;183:201–209. doi: 10.1007/s00213-005-0180-7. [DOI] [PubMed] [Google Scholar]
- D’Arceuil H, Coimbra A, Triano P, Dougherty M, Mello J, Moseley M, Glover G, Lansberg M, Blankenberg F. Ferumoxytol enhanced resting state fMRI and relative cerebral blood volume mapping in normal human brain. Neuroimage. 2013;83:200–209. doi: 10.1016/j.neuroimage.2013.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal Experimental Design for Event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo Z, Koretsky AP, Benveniste H. Differential effects of anesthetics on cocaine’s pharmacokinetic and pharmacodynamic effects in brain. Eur J Neurosci. 2009;30:1565–1575. doi: 10.1111/j.1460-9568.2009.06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Smerkers B, Kulkarni P, Caffrey M, Afacan O, Toddes S, Stolberg T, Febo M. Functional magnetic resonance imaging in awake animals. Reviews in the neurosciences. 2011;22:665–674. doi: 10.1515/RNS.2011.050. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK. Experimental Determination of the BOLD Field Strength Dependence in Vessels and Tissue. Magn Reson Med. 1997;38:296–302. doi: 10.1002/mrm.1910380220. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Phelps ME, Raichle ME, Ter-Pogossian MM. The effects of arterial blood pressure on the regional cerebral blood volume by X-ray fluorescence. Stroke. 1973;4:390–399. doi: 10.1161/01.str.4.3.390. [DOI] [PubMed] [Google Scholar]
- Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, Slifstein M, Abi-Dargham A, Laruelle M, Javitch JA, Rayport S. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Nesbit GM, Dosa E, Gahramanov S, Rooney B, Nesbit EG, Raines J, Neuwelt EA. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1- and T2-weighted MRI in neuroimaging. AJR Am J Roentgenol. 2011;197:981–988. doi: 10.2214/AJR.10.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol. 1988;156:385–393. doi: 10.1016/0014-2999(88)90284-1. [DOI] [PubMed] [Google Scholar]
- Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage. 2012;62:1072–1085. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24:9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Kim SJ, Jeong C, Lee S, Jeong H, Lee J, Yoo KY. Neuroprotective effects of remifentanil against transient focal cerebral ischemia in rats. Journal of neurosurgical anesthesiology. 2012;24:51–57. doi: 10.1097/ANA.0b013e3182368d70. [DOI] [PubMed] [Google Scholar]
- Johnson B, Lamki L, Fang B, Barron B, Wagner L, Wells L, Kenny P, Overton D, Dhother S, Abramson D, Chen R, Kramer L. Demonstration of dose-dependent global and regional cocaine-induced reductions in brain blood flow using a novel approach to quantitative single photon emission computerized tomography. Neuropsychopharmacology. 1998;18:377–384. doi: 10.1016/S0893-133X(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Elbel GK, Gossl C, Czisch M, Auer DP. Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 Tesla: implications for pharmacologic mri. Neuroimage. 2001;14:891–898. doi: 10.1006/nimg.2001.0890. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. Neuroimage. 2006;31:376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Wald LL. Int Soc Magn Reson Med. Berlin, Germany: 2007. Methodology for Sub-Millimeter Resolution fMRI in Awake Monkeys at 7T. [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lee AT, Glover GH, Meyer CH. Discrimination of large venous vessels in time-course spiral blood- oxygen-level-dependent magnetic-resonance functional neuroimaging. Magn Reson Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- Leite FP, Mandeville JB. Characterization of event-related designs using BOLD and IRON fMRI. Neuroimage. 2006;29:901–909. doi: 10.1016/j.neuroimage.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI Using Iron Oxide Contrast Agent in Awake, Behaving Macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Liu CH, Greve DN, Dai G, Marota JJ, Mandeville JB. Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation. Neuroimage. 2007;34:1042–1053. doi: 10.1016/j.neuroimage.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. Neuroimage. 2001;13:759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4T. Magn Reson Med. 2007;58:616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, Ferris CF. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. J Magn Reson Imaging. 2007;26:557–563. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]
- Mandeville JB. IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W. FMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology. 2011;36:1187–1198. doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, Chen YC, Choi JK, Kim YR, Belen D, Liu C, Kosofsky BE, Marota JJ. Exogenous contrast agent improves sensitivity of gradient-echo functional magnetic resonance imaging at 9.4 T. Magn Reson Med. 2004;52:1272–1281. doi: 10.1002/mrm.20278. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJA. Regional Sensitivity and Coupling of BOLD and CBV Changes during Stimulation of Rat Brain. Magn Reson Med. 2001;45:443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Leite FP, Marota JJ. Spin-echo MRI underestimates functional changes in microvascular cerebral blood plasma volume using exogenous contrast agent. Magn Reson Med. 2007;58:769–776. doi: 10.1002/mrm.21380. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Zaharchuk G, Ayata C, Davis TL, Moskowitz MA, Weisskoff RM, Rosen BR. Int Soc Magn Reson Med. Sydney, Aus: 1998. Evidence of a Cerebral Post-arteriole Windkessel; p. 1144. vol. In Press. [Google Scholar]
- Mandeville JB, Sander CY, Jenkins BG, Hooker JM, Catana C, Vanduffel W, Alpert NM, Rosen BR, Normandin MD. A receptor-based model for dopamine-induced fMRI signal. Neuroimage. 2013;75:46–57. doi: 10.1016/j.neuroimage.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Marota JJA, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine Activation Discriminates Dopamergic Projections by Temporal Response: an fMRI Study in Rat. NeuroImage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- Melichar JK, Hume SP, Williams TM, Daglish MR, Taylor LG, Ahmad R, Malizia AL, Brooks DJ, Myles JS, Lingford-Hughes A, Nutt DJ. Using [11C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J Pharmacol Exp Ther. 2005;312:309–315. doi: 10.1124/jpet.104.072686. [DOI] [PubMed] [Google Scholar]
- Michaelis L, Menten ML, Johnson KA, Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Jarraya B, Arsenault J, Rosen BR, Wald LL, Mandeville JB, Marota JJ, Vanduffel W. Neural correlates of the formation and retention of cocaine-induced stimulus-reward associations. Biol Psychiatry. 2012;72:422–428. doi: 10.1016/j.biopsych.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Dusticier N. Effects of alpha-chloralose on the activity of the nigrostriatal dopaminergic system in the cat. Eur J Pharmacol. 1980;65:403–410. doi: 10.1016/0014-2999(80)90344-1. [DOI] [PubMed] [Google Scholar]
- Oja JM, Gillen J, Kauppinen RA, Kraut M, van Zijl PC. Venous blood effects in spin-echo fMRI of human brain. Magn Reson Med. 1999;42:617–626. doi: 10.1002/(sici)1522-2594(199910)42:4<617::aid-mrm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Pai AB, Garba AO. Ferumoxytol: a silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? Journal of blood medicine. 2012;3:77–85. doi: 10.2147/JBM.S29204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peeters RR, Van Camp N, Van der Linden A. Int Soc Magn Reson Med. Glasgow, U.K: 2001. BOLD and CN100150 Contrast Enhanced fMRI of the cortex and cerebellum of the rat during electrical stimulation of the paw; p. 1333. [Google Scholar]
- Pfeuffer J, Shmuel A, Keliris GA, Steudel T, Merkle H, Logothetis NK. Functional MR imaging in the awake monkey: effects of motion on dynamic off-resonance and processing strategies. Magn Reson Imaging. 2007;25:869–882. doi: 10.1016/j.mri.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Pluim JP, Maintz JB, Viergever MA. Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging. 2003;22:986–1004. doi: 10.1109/TMI.2003.815867. [DOI] [PubMed] [Google Scholar]
- Poline JB, Brett M. The general linear model and fMRI: does love last forever? Neuroimage. 2012;62:871–880. doi: 10.1016/j.neuroimage.2012.01.133. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Qiu D, Zaharchuk G, Christen T, Ni WW, Moseley ME. Contrast-enhanced functional blood volume imaging (CE-fBVI): Enhanced sensitivity for brain activation in humans using the ultrasmall superparamagnetic iron oxide agent ferumoxytol. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Xu H, Choi JK, Jenkins BG, Chen YI. Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse. 2009;63:764–772. doi: 10.1002/syn.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A. The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Frontiers in neuroinformatics. 2012;6:27. doi: 10.3389/fninf.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, Vanduffel W, Rosen BR, Mandeville JB. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci U S A. 2013;110:11169–11174. doi: 10.1073/pnas.1220512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouw ML, Kaag AM, Caan MW, Heijtel DF, Majoie CB, Nederveen AJ, Booij J, Reneman L. Mapping the hemodynamic response in human subjects to a dopaminergic challenge with dextroamphetamine using ASL-based pharmacological MRI. Neuroimage. 2013;72:1–9. doi: 10.1016/j.neuroimage.2012.12.056. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Whitcher B, Gozzi A, Reese T, Bifone A. Study-level wavelet cluster analysis and data-driven signal models in pharmacological MRI. J Neurosci Methods. 2007;159:346–360. doi: 10.1016/j.jneumeth.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Silva AC, Liu JV, Hirano Y, Leoni RF, Merkle H, Mackel JB, Zhang XF, Nascimento GC, Stefanovic B. Longitudinal functional magnetic resonance imaging in animal models. Methods in molecular biology. 2011;711:281–302. doi: 10.1007/978-1-61737-992-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra T, Brooks JC, Figueiredo P, Wise R, Matthews PM, Tracey I. Quantitative assessment of the reproducibility of functional activation measured with BOLD and MR perfusion imaging: implications for clinical trial design. Neuroimage. 2005;27:393–401. doi: 10.1016/j.neuroimage.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RBH, Orban GA. Visual Motion Processing Investigated using Contrast-enhanced fMRI in Awake Behaving Monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wallace EA, Wisniewski G, Zubal G, vanDyck CH, Pfau SE, Smith EO, Rosen MI, Sullivan MC, Woods SW, Kosten TR. Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology (Berl) 1996;128:17–20. doi: 10.1007/s002130050104. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Van De Moortele PF, Shmuel A, Ugurbil K. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24:738–750. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Yan Q. Focal bicuculline increases extracellular dopamine concentration in the nucleus accumbens of freely moving rats as measured by in vivo microdialysis. Eur J Pharmacol. 1999;385:7–13. doi: 10.1016/s0014-2999(99)00699-8. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rosch S, Klumpp S, Klingel K, Kandolf R, Helluy X, Hiller KH, Jakob PM, Sechtem U. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs366. [DOI] [PubMed] [Google Scholar]
- Zaharchuk G, Mandeville JB, Bogdonov AA, Jr, Weissleder R, Rosen BR, Marota JJA. Cerebrovascular dynamics of autoregulation and hypotension: an MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke. 1999;30:2197–2205. doi: 10.1161/01.str.30.10.2197. [DOI] [PubMed] [Google Scholar]