Abstract
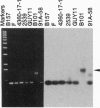
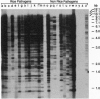
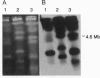
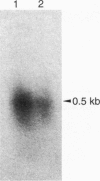
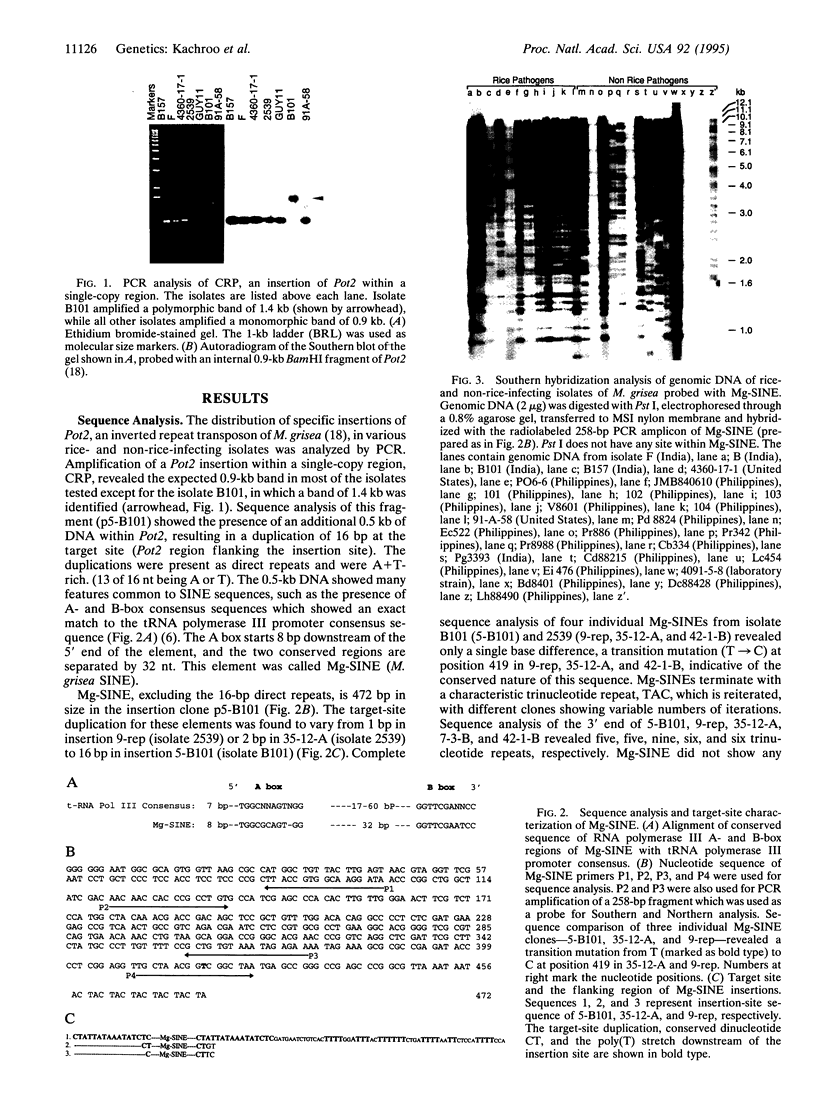
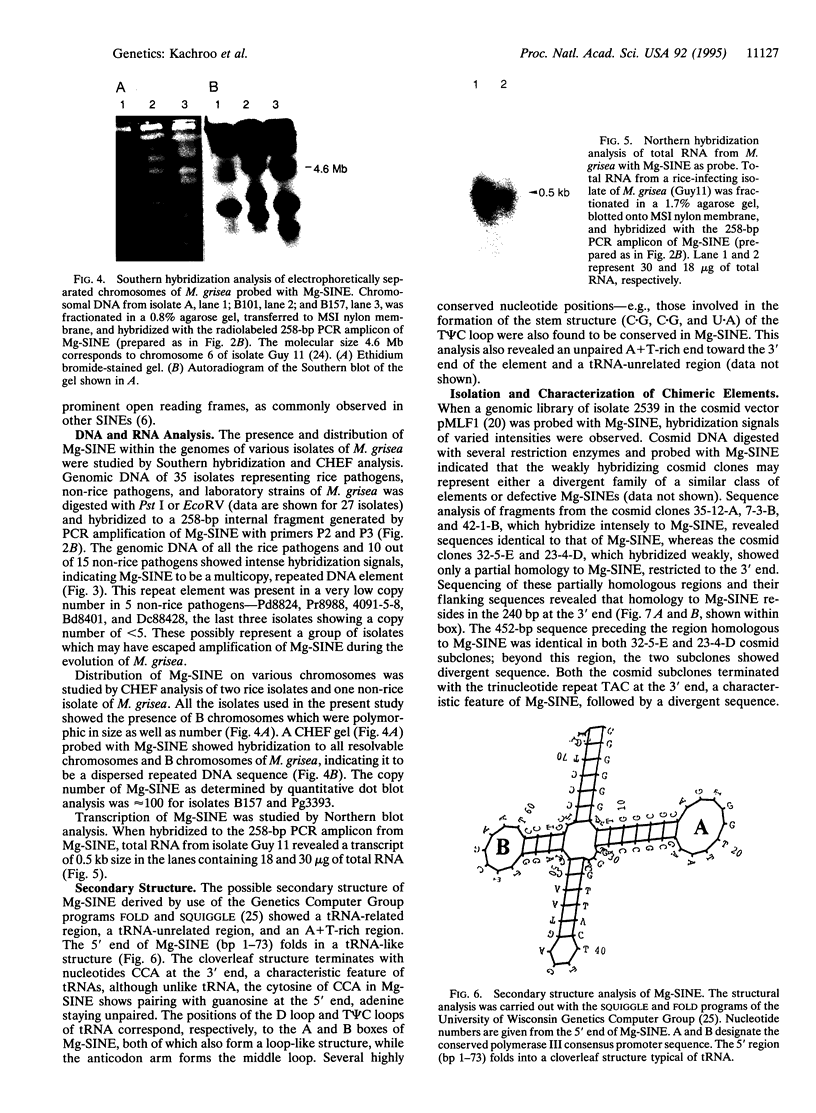
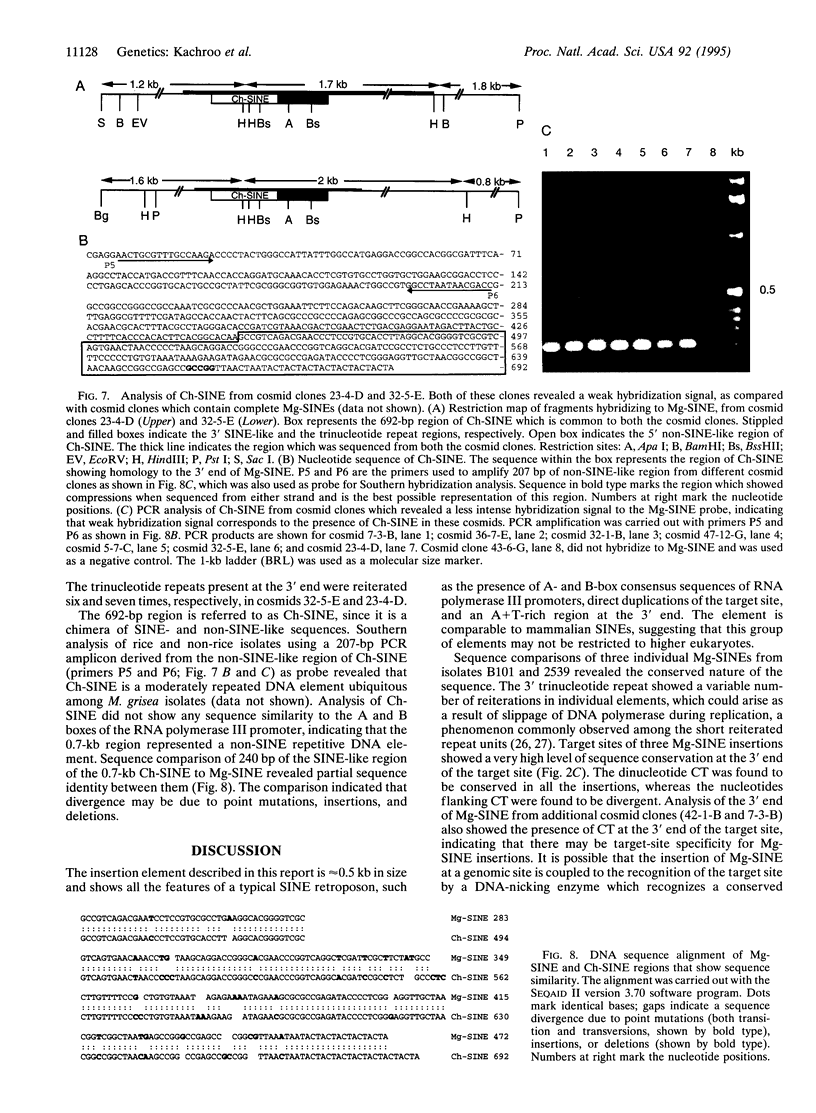
A short interspersed nuclear element, Mg-SINE, was isolated and characterized from the genome of the rice blast fungus, Magnaporthe grisea. Mg-SINE was isolated as an insertion element within Pot2, an inverted-repeat transposon from M. grisea and shows typical features of a mammalian SINE. Mg-SINE is present as a 0.47-kb interspersed sequence at approximately 100 copies per haploid genome in both rice and non-rice isolates of M. grisea, indicating a common evolutionary origin. Secondary structure analysis of Mg-SINE revealed a tRNA-related region at the 5' end which folds into a cloverleaf structure. Genomic fusions resulting in chimeric Mg-SINEs (Ch-SINEs) composed of a sequence homologous to Mg-SINE at the 3' end and an unrelated sequence at its 5' end were also isolated, indicating that this and other DNA rearrangements mediated by these elements may have a major effect on the genomic architecture of this fungus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels G. R., Deininger P. L. Characterization of a third major SINE family of repetitive sequences in the galago genome. Nucleic Acids Res. 1991 Apr 11;19(7):1649–1656. doi: 10.1093/nar/19.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman S. A., Deininger P. L., LaPorte P., Friedmann T., Geiduschek E. P. Analysis of transcription of the human Alu family ubiquitous repeating element by eukaryotic RNA polymerase III. Nucleic Acids Res. 1981 Dec 11;9(23):6439–6456. doi: 10.1093/nar/9.23.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y. P., Rommens J. M., Andrew S. E., Hutchinson G. B., Lin B., Theilmann J., Graham R., Glaves M. L., Starr E., McDonald H. Identification of an Alu retrotransposition event in close proximity to a strong candidate gene for Huntington's disease. Nature. 1993 Mar 25;362(6418):370–373. doi: 10.1038/362370a0. [DOI] [PubMed] [Google Scholar]
- Hamer J. E., Farrall L., Orbach M. J., Valent B., Chumley F. G. Host species-specific conservation of a family of repeated DNA sequences in the genome of a fungal plant pathogen. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9981–9985. doi: 10.1073/pnas.86.24.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kachroo P., Leong S. A., Chattoo B. B. Pot2, an inverted repeat transposon from the rice blast fungus Magnaporthe grisea. Mol Gen Genet. 1994 Nov 1;245(3):339–348. doi: 10.1007/BF00290114. [DOI] [PubMed] [Google Scholar]
- Köhrer K., Domdey H. Preparation of high molecular weight RNA. Methods Enzymol. 1991;194:398–405. doi: 10.1016/0076-6879(91)94030-g. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Oliviero S., Monaci P. RNA polymerase III promoter elements enhance transcription of RNA polymerase II genes. Nucleic Acids Res. 1988 Feb 25;16(4):1285–1293. doi: 10.1093/nar/16.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L., Gale K. C. Catalytic function of DNA topoisomerase II. Bioessays. 1991 Jun;13(6):269–273. doi: 10.1002/bies.950130603. [DOI] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Slagel V. K., Deininger P. L. In vivo transcription of a cloned prosimian primate SINE sequence. Nucleic Acids Res. 1989 Nov 11;17(21):8669–8682. doi: 10.1093/nar/17.21.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F., Mougneau E., Glaichenhaus N., Vaigot P., Darmon M., Cuzin F. Coordinated posttranscriptional control of gene expression by modular elements including Alu-like repetitive sequences. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):208–212. doi: 10.1073/pnas.90.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]