Abstract
Protein secretion from acinar cells of the pancreas and parotid glands is controlled by G-protein coupled receptor activation and generation of the cellular messengers Ca2+, diacylglycerol and cAMP. Secretory granule (SG) exocytosis shares some common characteristics with nerve, neuroendocrine and endocrine cells which are regulated mainly by elevated cell Ca2+. However, in addition to diverse signaling pathways, acinar cells have large ˜1 ! m diameter SGs (˜30 fold larger diameter than synaptic vesicles), respond to stimulation at slower rates (seconds versus milliseconds), demonstrate significant constitutive secretion, and in isolated acini, undergo sequential compound SG-SG exocytosis at the apical membrane. Exocytosis proceeds as an initial rapid phase that peaks and declines over 3 min followed by a prolonged phase that decays to near basal levels over 20-30 min. Studies indicates the early phase is triggered by Ca2+ and involves the SG proteins VAMP2 (vesicle associated membrane protein2), Ca2+-sensing protein synatotagmin 1 (syt1) and the accessory protein complexin 2. The molecular details for regulation of VAMP8-mediated SG exocytosis and the prolonged phase of secretion are still emerging. Here we review the known regulatory molecules that impact the sequential exocytic process of SG tethering, docking, priming and fusion in acinar cells.
In spite of considerable advances made in understanding the Ca2+-sensing mechanisms that control secretory granule (SG) or synaptic vesicle (SV) exocytosis in endocrine and nerve cells, comparatively less is known about this process in acinar cells of pancreatic and salivary glands. This is due at least in part to the complexity of intracellular signaling pathways that mediate acinar exocytosis which unlike nerve and many endocrine cells, do not express voltage-regulated Ca2+ channels or undergo exocytosis in response to membrane depolarization. Neural and endocrine secretion is primarily controlled by elevated intracellular Ca2+. Acinar secretion is mediated by G-protein coupled receptor activation inducing inositol trisphosphate (IP3)-mediated Ca2+ release, diacylglycerol (DAG) formation, and elevated cAMP. Moreover, these signaling molecules have diverse roles in different acinar cell types. Pancreatic acinar cell exocytosis is dependent mainly on elevated Ca2+ and DAG but is significantly potentiated by cAMP [1, 2]. Conversely, exocytosis in parotid and submandibular acini is triggered mainly by cAMP and is potentiated by elevated Ca2+ [3, 4]. This review will address what is known regarding Ca2+-sensing pathways in acinar cells; however due to the interrelationships of these signaling events in shaping the secretory response it will also briefly address knowledge of how cAMP and DAG participate in the process.
Following formation at the trans-Golgi, immature secretory granules undergo a poorly defined process of maturation and the resulting mature SGs (termed zymogen granules in pancreas) move either by diffusion or on microtubules to the apical cytoplasm where they undergo exocytosis over the next 24 hours in response to a meal. To reach the apical plasma membrane, SGs must traverse a terminal web of actin filaments involving myosin motor proteins and actin rearrangements. The canonical process of regulated exocytosis in secretory cells is thought to involve sequential mechanisms of SG/SV tethering, docking and priming at the plasma membrane (docking and priming may represent a single step) (Fig 1). The final step of SG/SV fusion with the plasma membrane is triggered exclusively in response to elevated Ca2+ in neural and neuroendocrine cells [5, 6]. It is now well accepted that docking and fusion requires specific heterotrimeric interactions between membrane-associated SNARE proteins (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) however each step of the process is impacted by specific accessory proteins, few of which are directly modulated by changes in cellular Ca2+ (reviewed in [52]).
Figure 1.
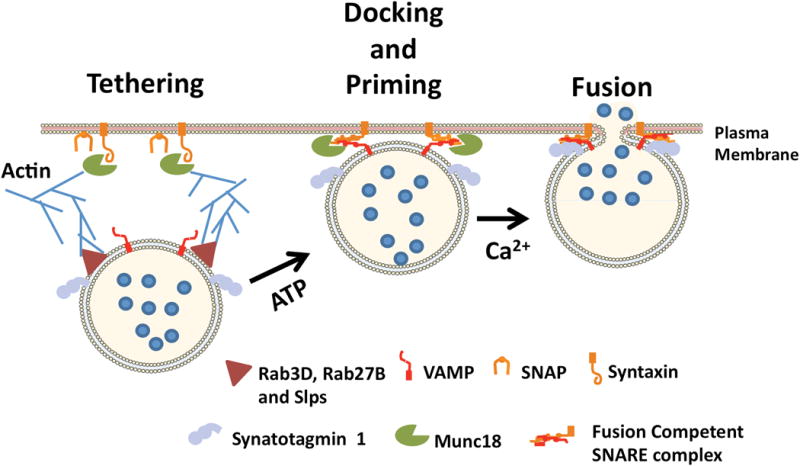
Secretory granule exocytosis proceeds through tethering, docking, priming and fusion steps. Putative acinar SG tethering factors include Rab3D, Rab27B and Slps. Docking and priming involves SNAREpin formation and in acinar cells is facilitated by Munc18-b and Munc 18-c interactions with syntaxins. Synaptotagmin 1 and complexin 2 (not shown) trigger VAMP2-mediated granule fusion in response to elevated Ca2+.
Acinar Cell SNARE proteins
SNAREs are characterized as belonging to the syntaxin, SNAP 25 (25 kDa synaptosome-associated protein) and vesicle associated membrane protein (VAMP) families (reviewed in[7]). The syntaxins and VAMPs have C-terminal transmembrane domains and extended cytoplasmic regions containing helical SNARE interaction segments. SNAP 25 has two SNARE interaction domains and is associated with membrane by palmitoylation. Functionally, SNAREs are classified as either v-SNAREs (VAMPs) or t-SNAREs (syntaxins and SNAP25) according to their vesicle or target membrane localization, respectively. Biochemically they are classified as Q-SNAREs (syntaxin or SNAP 25) or R-SNAREs (VAMPs) based on the presence of highly conserved glutamine or arginine residues within the SNARE motif. When brought in close proximity, trans-SNARE interactions between a v-SNARE and cognate t-SNAREs on opposing membranes promote the formation a heterotrimeric SNAREpin complex which underlies SG docking and ultimately provides the driving force for membrane fusion [7-9].
In nerve endings, SNAREs constitute approximately 1% of total protein whereas in acinar cells SNAREs account for approximately < 0.1% of total protein (unpublished results). Several SNARE proteins have been identified in pancreatic and salivary acinar cells and shown to play important roles in SG exocytosis (Table 1). Both VAMP2 (also known as synaptobrevin 2) and VAMP8 (also known as endobrevin) were identified on acinar SGs and evidence in pancreas supports that there are at least two populations of SGs based on VAMP2 or 8 expression levels [8, 10-13]. Three syntaxin isoforms have been implicated in acinar secretion; syntaxin 2 on the apical plasma membrane, syntaxin 3 on SGs and apical membranes and syntaxin 4 on both the apical and basolateral plasma membranes [10, 14, 15]. SNAP23, a ubiquitous isoform of SNAP25, has also been shown on apical and basolateral membranes as well as SGs [10, 16]. In pancreas the SNAP25 isoform SNAP29 was shown to be associated with SGs and apical membrane although its secretory function is uncertain [10, 17].
Table 1.
Localization of SNARE proteins in acinar cells. SG, secretory granule; AM, apical membrane; BM, basolateral membrane.
| SNARE Protein | Localization | Ref |
|---|---|---|
| VAMP2 | SG | 10-13 |
| VAMP8 | SG | 8, 10 |
| Syntaxin 2 | AM | 10, 14 |
| Syntaxin 3 | SG, AM | 10, 14, 15 |
| Syntaxin 4 | BM, AM | 10, 14, 20 |
| SNAP 23 | BM, AM, SG | 10, 16 |
| SNAP 29 | AM, SG | 10, 17 |
The specific v- and t-SNARE combinations that mediate SG exocytosis in pancreatic and parotid acinar cells have been studied by GST-pull down assays and coimmunoprecipitations but for the most part have proved difficult to detect, potentially due to their low abundance. Cosen-Binker, et al. [18] provided the most comprehensive profile of complete SNAREpin complexes involving SGs in pancreatic acini by coimmunoprecipitations with specific anti-syntaxin antibodies and purified membrane fractions from isolated acinar cells (Table 2). They concluded that SG-apical membrane exocytosis was mediated by syntaxin 2/SNAP23/VAMP2, whereas SG-SG compound exocytosis involved syntaxin 3/SNAP23/VAMP8. A third complex of syntaxin 4/SNAP23/VAMP8 was predicted to mediate aberrant SG fusion with the basolateral membrane during acute pancreatitis. A similar mechanism was later described for aberrant basolateral exocytosis in submandibular glands of patients with Sjogren's Syndrome [19]. Other studies in pancreatic [10] and parotid [20] acini reported syntaxin 4 is present on the apical membrane and dominant negative constructs omitting its transmembrane domain inhibited Ca2+-stimulated secretion supporting a physiological role for syntaxin 4 in SG exocytosis.
Table 2.
SNAREpin complexes involved in acinar secretion. SG, secretory granule; AM, apical membrane; BM, basolateral membrane.
| SNAREpin Complex | Fusion Compartments | Ref |
|---|---|---|
| VAMP2/syntaxin2/SNAP23 | SG-AM | 18 |
| VAMP8/syntaxin3/SNAP23 | SG-SG | 18 |
| VAMP8/syntaxin4/SNAP23 | SG-BM, SG-AM | 18, 10 |
SG tethering to the plasma membrane
Functionally, tethering is regarded as a long range interaction between a vesicle and target membrane that is initiated at too great a distance for SNARE complex assembly [6]. Consensus differs on whether tethering involves cytoskeletal interactions. This is especially complicated in acinar cells which have a highly developed actin web immediately below the apical plasma membrane (Fig 1). In general, tethering factors act by facilitating coordinated interactions between Rabs, phosphoinositides and SNARE proteins to ultimately promote trans-SNARE formation marking the docking/priming steps. A number of potential tethering proteins are present on acinar SGs including Rab3D [21], Rab27B and the synaptotagmin-like proteins (see below). The molecular details of tethering complexes for constitutive membrane fusion in endosomal and secretory pathways are still emerging with some such as the exocyst, HOPS, and CORVET involving large multimeric protein complexes [6, 22, 23]. A recent study in parotid acini identified subunits of the multimeric exocyst tethering complex along the apical membrane and provided the first evidence for a role of a multimeric tethering complex in SG exocytosis by demonstrating the inhibition of isoproterenol-induced amylase secretion following introduction of subunit-specific antibodies into permeabilized acinar cells [24]. Recent evidence also suggests that the vesicle priming factors of the Munc13/CAPS (Ca2+-activated protein for secretion) protein family may have a tethering function for regulated secretion [6]. CAPS contains a PH domain that interacts with plasma membrane phosphatidylinositol 4,5-bisphosphate (PI4,5P2) and a Munc homology domain that interacts with Q- and R-SNAREs to promote SNAREpin formation. Studies examining potential roles for the CAPs/Munc13 proteins in acinar secretion are lacking.
Actin filaments and associated regulatory proteins
A potential role for the actin cytoskeleton in directly modulating SG exocytosis was first indicated by results that introduction of low levels of actin depolymerizing agents into permeabilized pancreatic acinar cells was capable of triggering constitutive SG exocytosis whereas high concentrations inhibited agonist-stimulated secretion suggesting actin filaments prevent high rates of basal secretion but are necessary for a full secretory response [25]. Valentjian et al. using electron microscopy and the actin filament disrupting agent cytochalasin D revealed that cytoskeleton disassembly did not inhibit stimulated SG exocytosis but rather that actin filament contractile forces were essential to expel SG contents and concluded that the filaments play an important role in membrane retrieval [26]. More recently the small G-proteins RhoA and Rac1 have also been shown to regulate actin rearrangements supporting acinar secretion [21, 27, 28].
A number of studies have shown that SGs become coated with actin during acinar stimulation [29-31] and although most indicate that SG coating occurs post-fusion, the precise role of this process is uncertain. SG coating was reported to be important for maintaining SG shape and providing structural support for sequential compound SG-SG fusion [31]. Others indicate actin coating mainly regulates fusion pore dynamics [32, 33]. Finally a recent study using intravital microscopy of parotid gland in vivo failed to detect sequential SG-SG compound exocytosis at the apical membrane and provided evidence that F-actin and nonmuscle myosin II promote the gradual collapse of SGs into the plasma membrane thereby facilitating expulsion of SG content into the acinar lumen [34].
Synaptotagmin-like Proteins (SLPs) and Rab27
SLPs are a family of molecules that include Slp1, SLP2-a, 3-a, 4-a, Slp5 and rabphilin (reviewed in[35]) each containing C-terminal C2A and C2B domains characteristic of synaptotagins (syt) (see below). The Slps typically are associated with SGs and act as Rab27A and/or Rab27B effectors in various cell types including acinar cells. Their role in the secretory pathway appears to be in SG trafficking to promote tethering at the plasma membrane rather than controlling Ca2+-triggered membrane fusion as is identified for syt. Unlike syt, SLPs are peripheral membrane proteins that contain a unique N-terminal Rab27 binding domain termed the SLP homology domain (SHD) that is shared by a number of additional proteins including the Slac (SLP homology lacking C2 domains) proteins and Noc2, although these later molecules which lack C2 domains share no homology with syt. Of the Slp proteins, only Slp3-a and rabphilin were shown to undergo Ca2+-dependent interactions with phospholipids in vitro; the significance of this activity for their function is uncertain. Slp 1 and 4 are present in pancreas and genetic deletion of Slp1 results in an accumulation of SGs [36-38]. Slp4 was also shown to interact with syntaxin 2 and play a significant role in isoproterenol-stimulated amylase secretion from parotid acinar cells [38]. Of the Rab27 effectors that do not contain C2 domains, Noc2 is present in parotid and pancreatic acinar cells and Noc2-/- acini likewise accumulate SGs but appear to have defects in secretagogue-induced intracellular Ca2+ release [39]. Parotid acinar cells also express Slac2-c which was shown to interact with Rab27B moreover, inhibition of that interaction with Rab27B-specific antibodies inhibited isoproterenol-stimulated amylase secretion [37]. Further supporting a role SLPs and Rab27 in secretion, pancreatic, parotid and lacrimal acinar cells all express Rab27B on SGs and expression of dominant negative and constitutively active forms of the protein significantly inhibit or enhance secretory activity, respectively [40-42].
SG Docking
Sec1/Munc18 proteins
Members of the Sec1/Munc18-like protein family play a critical role in forming and stabilizing SNARE complexes in cells primarily through dynamic interactions with the Q-SNARE syntaxin [43]. The most well characterized neural protein Munc18-1 interacts with syntaxin 1 in part to chaperone it to the plasma membrane. In vitro, Munc18-1 binds syntaxin 1 in a closed configuration initially preventing trans-SNARE formation but in the presence of preincubated SNAP25 and VAMP2 switches to a facilitative role promoting SNAREpin formation. Parotid and pancreatic acinar cells express Munc18-b which interacts with syntaxin 2 [38, 44]. Moreover, the Munc18-b/syntaxin 2 interaction was shown to facilitate binding of Slp4-a to syntaxin 2 suggesting these proteins form a SG tethering complex. Munc18-c interacts with syntaxin 4 on basolateral membranes in pancreatic acini [45] and apical membranes of parotid acini [46]. Treatment with high-dose CCK or isoproterenol was shown to displace Munc18-c to the cytosol by its PKC-! -dependent phosphorylation [18, 47]. The significance of Munc18-c displacement to the cytosol in controlling SNARE formation/exocytosis is uncertain as the Munc18-c/syntaxin 4 interaction was shown to exclusively promote SNAREpin formation and membrane fusion but unlike Munc18-1, had no inhibitory effect on SNARE formation [48].
Cab45b as a Ca2+-sensor for Munc18-b-mediated SNARE formation
Cab45 was originally identified as an intra-Golgi localized Ca2+-binding protein that contains 6 EF-hand motifs [49]. A yeast 2 hybrid screen for Munc18-b interacting proteins revealed a cytosolic splice variant Cab45b containing 3 EF-hand motifs with a single EF-hand showing Ca2+ binding in vitro. Cab45b interaction with Munc18-b is enhanced in the presence of Ca2+, and coimmunoprecipitation from acinar lysates demonstrated complexes containing Cab45b/Munc18-b/syntaxin 2 and syntaxin 3. Strikingly, incubation of permeabilized acini with affinity purified Cab45b antibodies dose-dependently inhibited Ca2+-stimulated amylase secretion by greater than 90%. Similar results for Cab45b were also seen using membrane capacitance studies in pancreatic beta-cells where EF-hand mutations that interrupt Cab45b/Munc18-b interactions strongly inhibited exocytosis [50]. Further studies employing reconstituted SNAREs in liposome fusion assays will be necessary fully understand how Cab45b impacts docking, priming and fusion.
SG priming
In regulated exocytosis the process of SG priming involves formation of the SNAREpin complex prior to Ca2+-triggered exocytosis, however because tethering and docking also ultimately supports SNARE complex assembly, these processes are closely linked and functionally overlapping. Originally, the concept of ATP-dependent SG priming was described using permeabilized PC12 cells and shown to involve the disassembly of heterotrimeric cis-SNARE complexes on the plasma membrane by the chaperone NSF (N-ethylmaleamide sensitive fusion protein) [51, 52]. Presumably SNAREpin disassembly following exocytosis acts to free SNAREs for subsequent rounds of fusion. Others indicated the ATP-dependent localized synthesis of PI4,5P2 is essential for the formation of a dimeric Q-SNARE complex between syntaxin 1 and SNAP 25 on the plasma membrane [53]. Formation of dimeric Q-SNAREs greatly facilitates interaction with VAMP on SGs thereby promoting full SNAREpin formation [54]. Subsequently additional priming factors for regulated exocytosis were identified which, although they do not directly utilize ATP, are essential to facilitate SNAREpin assembly thereby promoting the final stage of fusion. These include the Munc13 and CAPS family of proteins which share conserved Munc-homology domains and similar to syt and SLPs contain C2 domains [6]. Of particular interest, Munc13-4 was recently shown to undergo Ca2+-dependent interactions with SNARE proteins and phospholipids and to facilitate Ca2+-sensitive SNARE-mediated liposome fusion in vitro [55]. The ability of the Munc13/CAPS proteins to promote opposing membrane association and facilitate SNARE interactions suggests they may also impart a tethering mechanism to support fusion.
Although it appears none of the above mechanisms for SG priming have been investigated in acinar cells, functional studies conducted with isolated pancreatic acinar cells have provided evidence that Ca2+-dependent SG exocytosis indeed proceeds through docking/ATP-dependent priming and Ca2+-dependent fusion steps. Analysis of amylase secretion from a single group of acinar cells over time using a flow-through system revealed that CCK induces an initial rapid phase of secretion peaking and declining over approximately 2-3 minutes followed by a second extended phase that decays to near basal levels over 20-30 min (Fig 2) [56]. Padfield and Panesar [57] utilized alpha-toxin permeabilized acini, which produces plasma membrane pores of approximately 1 kDa to limit the loss of cytosolic proteins while allowing the careful control of intracellular Ca2+ and ATP levels, to demonstrate that the initial rapid phase triggered by Ca2+ is independent of Mg-ATP but the prolonged phase required ATP-dependent priming. They reasoned that the initial rapid phase represented SGs already primed and poised to undergo exocytosis at the plasma membrane whereas further rounds of exocytosis required one or more ATP-dependent steps to achieve that status. Of note, in a later study they showed that the characteristic secretory inhibition seen in response to supramaximal levels of CCK selectively inhibited the ATP-dependent priming step thereby allowing only docked and primed SGs to exocytose but inhibiting subsequent rounds of fusion [58]. Studies in parotid acini, where cAMP exerts a major role in SG exocytosis, have shown similarly that elevated Ca2+ induces the early rapid phase response whereas cAMP causes a larger sustained secretory response [59].
Figure 2.
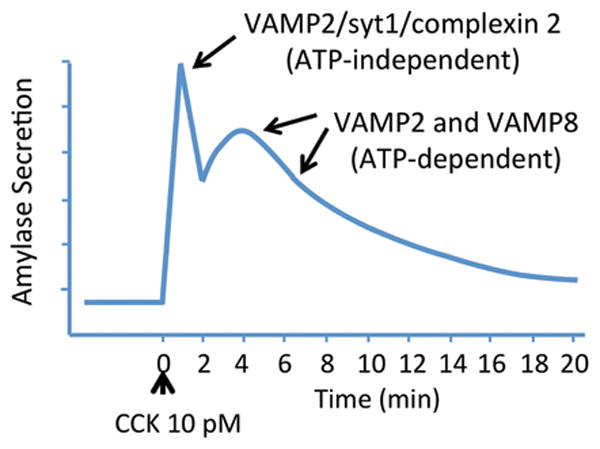
Secretion from pancreatic acinar cells is marked by an initial rapid phase which peaks and declines over 2-3 min followed by a second prolonged phase that decays toward basal levels over 20 min. Evidence supports that the initial phase involves a post-priming step and is therefore independent of ATP. The early phase is Ca2+-dependent and regulated by synaptotagmin 1 (syt1) and complexin 2. Molecular details of regulatory control of exocytosis in the second phase involving both VAMP2 and VAMP8 are unclear.
Thus in both pancreatic and salivary acinar cells elevated Ca2+ triggers an early rapid phase of secretion likely involving SGs that have undergone SNAREpin formation. Although acinar early phase Ca2+-dependent exocytosis retains some functional characteristics in common with neural and neuroendocrine cells, the mechanisms by which Ca2+, cAMP and DAG impact the second prolonged phase of secretion are unclear. A final consideration is that acinar cells undergo significant constitutive secretion which involves both endosomal vesicles [60] and SGs [61]. Similar to constitutive fusion reactions between intracellular compartments, this process also progress through tethering, docking/priming and fusion. The possibility that Ca2+, DAG and cAMP signaling differentially modulate components of the constitutive SG pathway to induce the prolonged phase of secretion has not been explored.
Regulated SG fusion with the apical membrane
Synaptotagmins (Syts) and Ca2+-dependent secretion
Syts are a family of integral membrane proteins composed of 17 known mammalian isoforms distributed widely in neuronal and nonneuronal tissues (for comprehensive reviews see ref [62-64]. Family members share a basic structure consisting of a short N-terminal intravesicular domain followed by a transmembrane domain and a large C-terminal cytoplasmic segment that contains two tandem C2 domains termed C2A and C2B. These C2 domains provide homology among family members and in a subset of syts function as Ca2+ binding sites that trigger Ca2+-dependent interactions with phospholipids and SNARE proteins. Due to the absence of key acidic residues within their C2 domains, only 8 isoforms show Ca2+-dependent phospholipid binding activity – 1, 2, 3, 5, 6, 7, 9 and 10. Syt1, the most well characterized isoform, is thought to be the primary Ca2+ sensing protein for the final stage (membrane fusion) of exocytosis of SVs in neurons but has also been shown to regulate vesicle docking and priming [65]. In comparison, dense core vesicles in neuroendocrine PC12 cells express syt1, 7 and 9 as well as syt4, a unique isoform that acts to inhibit exocytosis. Although syt1 and 9 play primary roles in Ca2+-stimulated PC12 cell secretion [66], recent evidence indicates that different sized vesicles express different proportions of these four isoforms thereby providing functional distinctions in Ca2+-sensitivity, fusion pore dynamics and inhibition by syt4 [67].
A number of studies have reported syt isoform expression in acinar cells. Syt1, 3, 6 and 7 were demonstrated in isolated pancreatic acinar cells by RT-PCR [68], whereas mouse parotid acinar cells express mRNAs to syt1, 2, 3, 4, 6 and 7 [69-71]. Levius et al. [71] first isolated and sequenced syt1 from rat parotid acini; these results were later confirmed in mouse and rat parotid, submandibular and pancreatic acinar cells by immunoblotting [70, 72, 73]. A functional role for syt1 in pancreatic acinar secretion is supported based on results that introduction of an inhibitory truncation-construct omitting the transmembrane domain and containing only the C2 domains into permeabilized acinar cells partially inhibited Ca2+-dependent exocytosis whereas similar constructs of syt 3, which localizes to endo-lysosomes, had no effects [73]. Syt1 immunoreactivity was detected in both SG-rich apical cytoplasm and purified SG fractions where it significantly colocalized with VAMP2. Finally cell surface labeling of syt1 in intact acini with an antibody specific to the syt1 intraluminal domain demonstrated localization exclusively along the apical membrane and its presence was strongly enhanced over the first 5 min of secretagogue stimulation indicating syt1 insertion into the apical membrane. Although these findings support a role for syt1 as a Ca2+-sensor for exocytosis, the incomplete secretory inhibition achieved with syt1 inhibitory constructs strongly supports additional Ca2+-sensing mechanisms are present. Further genetic studies using acinar-specific syt1, 3, 6 and 7 manipulations will be necessary to better understand their potential roles in secretion.
VAMP2/Syt1/complexin 2 mediates the early phase of acinar secretion
Colocalization studies using lysine-fixable dextran to label sites of SG exocytosis and VAMP2 or 8 antibodies to identify VAMP-specific SG populations indicate that VAMP2-positive SGs occupy the most apical aspects of acinar cells and are the first to undergo exocytosis upon CCK-8 stimulation followed by VAMP8-positive SGs [10] which unlike VAMP2 also mediates SG-SG compound exocytosis [74]. Falkowski et al. subsequently reported VAMP2 interacts with both syt1 and complexin 2 on acinar SGs. In neurons complexin is an accessory protein for the final stage of Ca2+-stimulated SG fusion acting both to stabilize SNAREpin complexes in a prefusion state and together with syt1 facilitating membrane fusion following cell stimulation [73, 75]. Further supporting a concerted role for VAMP2, syt1 and complexin 2 in secretion, inhibition of their function in permeabilized acini using tetanus toxin to specifically cleave VAMP2 or syt1 and complexin 2 inhibitory constructs all showed similar inhibition (˜40%) of Ca2+-stimulated secretion [13, 73, 75]. Together these results suggest that consistent with its role in neural and endocrine cells, VAMP2-mediated Ca2+-stimulated exocytosis is regulated by syt1 and accounts for the early rapid phase of secretion (Fig 2). These results also suggest that the second prolonged phase of secretion likely involves both VAMP2 and VAMP8-positive SGs however the regulatory mechanisms controlling VAMP8-mediated stimulated secretion in acinar cells are not clear.
Rap1 coordinates Ca2+, DAG and cAMP signaling for secretion
Rap1 is a member of the Ras family of small G-proteins and has been implicated in diverse cellular functions including growth, metabolism and secretion [76]. Rap1 function is controlled by guanine-exchange factors (Rap1-GEFs) that modulate its activity (Fig 3). Two Ca2+ and DAG sensitive GEFs, CALDAG-GEFI and III are reversibly activated in response to elevation of these cellular messengers. Two additional cAMP-sensitive GEFs which function independently of PKA are termed EPAC1 and EPAC2 (exchange protein directly activated by cAMP). Of these, CALDAG-GEFIII and Epac1 are expressed in pancreatic acini [77]. In acini Rap1 was originally identified by proteomic studies of acinar SG membranes [17]. Sabbatini et al. later demonstrated Rap1 activation in response to both Ca2+/DAG- and cAMP- generating secretagogues and pharmacological agents [77]. A functional role for Rap1 in regulating digestive enzyme secretion was demonstrated by expressing a GTPase activating protein (Rap1-GAP) to inhibit Rap1 activity resulting in 60% and 40% inhibition of secretion in response to the cAMP-generating vasoactive intestinal peptide and the Ca2+-mobilizing secretagogues CCK and carbachol, respectively. Rap1 was also shown to be expressed and play a secretory role in parotid acini [78]. Although these studies provide important mechanistic evidence for cAMP-mediated effects on secretion, use of EPAC-specific analogs of cAMP and PKA inhibitors indicate that PKA activation also exerts and important regulatory role on acinar secretion [79, 80]. Given the ability of Rap1 to coordinate the effects of diverse cellular signaling messengers, investigation of its down-stream effects on SG trafficking and exocytosis should provide key insight into acinar secretion.
Figure 3.
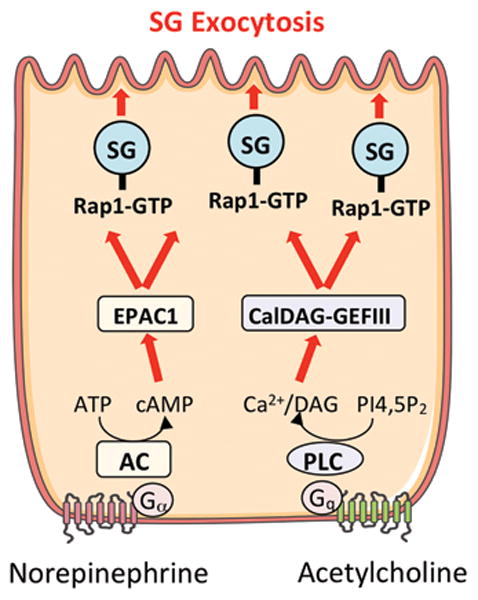
Acinar secretion is mediated by G-protein coupled receptor activation resulting adenylyl cyclase (AC) mediated cAMP production and/or elevation of Ca2+ and DAG levels via phospholipase C (PLC) activation. The small G-protein Rap1 is present on secretory granules and activated by the guanine exchange factors EPAC1 and CalDAG-GEFIII which respond to cAMP and Ca2+/DAG, respectively. Rap1 inhibition was shown to significantly inhibit both cAMP- and Ca2+-stimulated amylase secretion.
Summary
Many studies in acinar cells aimed at understanding SG exocytosis have been based on exocytic mechanisms that occur in more well characterized nerve and neuroendocrine cells. These studies have revealed that exocytosis in acinar cells from pancreas and parotid glands involves specific interactions between cognate SNARE proteins present on SGs and plasma membrane as well as a number of accessory proteins for tethering, docking, priming and fusion that are either identical or homologous to those in nerve and neuroendocrine cells . Ca2+-stimulated acinar secretion triggers an early rapid phase of exocytosis that is mediated by VAMP2-specific SNARE interactions and is likely controlled by the Ca2+-sensing protein syt1 and accessory protein complexin 2. The second prolonged phase of secretion likely involves additional regulatory mechanisms that in parotid acini are controlled mainly by cAMP and involve Rap1 and PKA-dependent events. Whether cAMP potentiates the second prolonged phase of secretion in pancreatic acini where Ca2+ plays a major role in secretion is not yet known.
The diversity of signaling molecules (Ca2+, DAG and cAMP) which shape the acinar secretory response clearly support that either additional regulatory proteins or yet to be identified reversible covalent and noncovalent protein modifications are involved in controlling acinar exocytosis. As these cellular messengers have diverse roles in modulating kinase and phosphatase enzymes, small-G proteins, cytoskeletal motor proteins etc., more detailed molecular studies of specific functional interactions between the acinar-specific secretory proteins are needed. Progress in this area is hampered by the difficulty of modifying protein expression with RNA silencing technologies or rapid protein expression in highly differentiated acinar cells immediately following their isolation. The best alternative is pancreas-specific and inducible gene manipulations using CRE-LOX recombination in mice which is both time consuming and a considerable expense. Reductionist approaches utilizing liposome fusion systems containing specific SNARE proteins and other accessory molecules have advanced much of what is understood of Ca2+-regulated exocytosis. As more details of the acinar tethering, docking and fusion proteins emerge, use of that technology to understanding the molecular and biophysical details of Ca2+, DAG and cAMP mediated membrane fusion should prove useful.
Acknowledgments
G.E. Groblewski was supported by NIH DK07088, USDA/HATCH WIS01583 and a UW Graduate School Vilas Associate Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Pont JJ, Fleuren-Jakobs AM. Synergistic effect of A23187 and a phorbol ester on amylase secretion from rabbit pancreatic acini. FEBS letters. 1984;170:64–68. doi: 10.1016/0014-5793(84)81369-1. [DOI] [PubMed] [Google Scholar]
- 2.Collen MJ, Sutliff VE, Pan GZ, Gardner JD. Postreceptor modulation of action of VIP and secretin on pancreatic enzyme secretion by secretagogues that mobilize cellular calcium. The American journal of physiology. 1982;242:G423–428. doi: 10.1152/ajpgi.1982.242.4.G423. [DOI] [PubMed] [Google Scholar]
- 3.Quissell DO, Watson E, Dowd FJ. Signal transduction mechanisms involved in salivary gland regulated exocytosis. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1992;3:83–107. doi: 10.1177/10454411920030010701. [DOI] [PubMed] [Google Scholar]
- 4.Scott J, Baum BJ. Involvement of cyclic AMP and calcium in exocrine protein secretion induced by vasoactive intestinal polypeptide in rat parotid cells. Biochimica et biophysica acta. 1985;847:255–262. doi: 10.1016/0167-4889(85)90028-x. [DOI] [PubMed] [Google Scholar]
- 5.Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9:1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 6.James DJ, Martin TF. CAPS and Munc13: CATCHRs that SNARE Vesicles. Frontiers in endocrinology. 2013;4:187. doi: 10.3389/fendo.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nature reviews. Molecular cell biology. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 8.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Developmental cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 10.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. The Journal of biological chemistry. 2007;282:9635–9645. doi: 10.1074/jbc.M611108200. [DOI] [PubMed] [Google Scholar]
- 11.Braun JE, Fritz BA, Wong SM, Lowe AW. Identification of a vesicle-associated membrane protein (VAMP)-like membrane protein in zymogen granules of the rat exocrine pancreas. The Journal of biological chemistry. 1994;269:5328–5335. [PubMed] [Google Scholar]
- 12.Gaisano HY, Sheu L, Grondin G, Ghai M, Bouquillon A, Lowe A, Beaudoin A, Trimble WS. The vesicle-associated membrane protein family of proteins in rat pancreatic and parotid acinar cells. Gastroenterology. 1996;111:1661–1669. doi: 10.1016/s0016-5085(96)70030-6. [DOI] [PubMed] [Google Scholar]
- 13.Gaisano HY, Sheu L, Foskett JK, Trimble WS. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. The Journal of biological chemistry. 1994;269:17062–17066. [PubMed] [Google Scholar]
- 14.Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Molecular biology of the cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen NJ, Antonin W, Edwardson JM. Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell. The Journal of biological chemistry. 1999;274:22871–22876. doi: 10.1074/jbc.274.32.22871. [DOI] [PubMed] [Google Scholar]
- 16.Gaisano HY. A hypothesis: SNARE-ing the mechanisms of regulated exocytosis and pathologic membrane fusions in the pancreatic acinar cell. Pancreas. 2000;20:217–226. doi: 10.1097/00006676-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Molecular & cellular proteomics : MCP. 2006;5:306–312. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Cosen-Binker LI, Lam PP, Binker MG, Gaisano HY. Alcohol-induced protein kinase Calpha phosphorylation of Munc18c in carbachol-stimulated acini causes basolateral exocytosis. Gastroenterology. 2007;132:1527–1545. doi: 10.1053/j.gastro.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Barrera MJ, Sanchez M, Aguilera S, Alliende C, Bahamondes V, Molina C, Quest AF, Urzua U, Castro I, Gonzalez S, Sung HH, Albornoz A, Hermoso M, Leyton C, Gonzalez MJ. Aberrant localization of fusion receptors involved in regulated exocytosis in salivary glands of Sjogren's syndrome patients is linked to ectopic mucin secretion. Journal of autoimmunity. 2012;39:83–92. doi: 10.1016/j.jaut.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Imai A, Nashida T, Yoshie S, Shimomura H. Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane. Archives of oral biology. 2003;48:597–604. doi: 10.1016/s0003-9969(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 21.Williams JA, Chen X, Sabbatini ME. Small G proteins as key regulators of pancreatic digestive enzyme secretion. American journal of physiology. Endocrinology and metabolism. 2009;296:E405–414. doi: 10.1152/ajpendo.90874.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger CM, Klute MJ, Dacks JB. Comparative genomic analysis of multi-subunit tethering complexes demonstrates an ancient pan-eukaryotic complement and sculpting in apicomplexa. PloS one. 2013;8:e76278. doi: 10.1371/journal.pone.0076278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai A, Yoshie S, Haga-Tsujimura M, Nashida T, Shimomura H. Exocyst subunits are involved in isoproterenol-induced amylase release from rat parotid acinar cells. European journal of oral sciences. 2012;120:123–131. doi: 10.1111/j.1600-0722.2012.00952.x. [DOI] [PubMed] [Google Scholar]
- 25.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. The Journal of cell biology. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentijn KM, Gumkowski FD, Jamieson JD. The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. Journal of cell science. 1999;112(Pt 1):81–96. doi: 10.1242/jcs.112.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Bi Y, Williams JA. A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. American journal of physiology Cell physiology. 2005;289:C22–32. doi: 10.1152/ajpcell.00395.2004. [DOI] [PubMed] [Google Scholar]
- 28.Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. American journal of physiology. Gastrointestinal and liver physiology. 2005;289:G561–570. doi: 10.1152/ajpgi.00508.2004. [DOI] [PubMed] [Google Scholar]
- 29.Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turvey MR, Thorn P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflugers Archiv : European journal of physiology. 2004;448:552–555. doi: 10.1007/s00424-004-1288-z. [DOI] [PubMed] [Google Scholar]
- 31.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. The Journal of biological chemistry. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 32.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cellular and molecular life sciences : CMLS. 2013;70:2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang Y, Soekmadji C, Mitchell JM, Thomas WG, Thorn P. Real-time measurement of F-actin remodelling during exocytosis using Lifeact-EGFP transgenic animals. PloS one. 2012;7:e39815. doi: 10.1371/journal.pone.0039815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14:949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 36.Saegusa C, Kanno E, Itohara S, Fukuda M. Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Archives of biochemistry and biophysics. 2008;475:87–92. doi: 10.1016/j.abb.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Imai A, Fukuda M, Yoshie S, Nashida T, Shimomura H. Redistribution of Rab27-specific effector Slac2-c, but not Slp4-a, after isoproterenol-stimulation in rat parotid acinar cells. Archives of oral biology. 2009;54:361–368. doi: 10.1016/j.archoralbio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda M, Imai A, Nashida T, Shimomura H. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18-2-dependent manner. The Journal of biological chemistry. 2005;280:39175–39184. doi: 10.1074/jbc.M505759200. [DOI] [PubMed] [Google Scholar]
- 39.Ogata S, Miki T, Seino S, Tamai S, Kasai H, Nemoto T. A novel function of Noc2 in agonist-induced intracellular Ca2+ increase during zymogen-granule exocytosis in pancreatic acinar cells. PloS one. 2012;7:e37048. doi: 10.1371/journal.pone.0037048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. Journal of cell science. 2004;117:1945–1953. doi: 10.1242/jcs.01048. [DOI] [PubMed] [Google Scholar]
- 41.Chiang L, Ngo J, Schechter JE, Karvar S, Tolmachova T, Seabra MC, Hume AN, Hamm-Alvarez SF. Rab27b regulates exocytosis of secretory vesicles in acinar epithelial cells from the lacrimal gland. American journal of physiology. Cell physiology. 2011;301:C507–521. doi: 10.1152/ajpcell.00355.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochemical and biophysical research communications. 2004;323:1157–1162. doi: 10.1016/j.bbrc.2004.08.212. [DOI] [PubMed] [Google Scholar]
- 43.Hong W, Lev S. Tethering the assembly of SNARE complexes. Trends in cell biology. 2014;24:35–43. doi: 10.1016/j.tcb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Dolai S, Liang T, Lam PP, Fernandez NA, Chidambaram S, Gaisano HY. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology. 2012;143:832–843. doi: 10.1053/j.gastro.2012.06.011. e831-837. [DOI] [PubMed] [Google Scholar]
- 45.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, Tamori Y, Trimble WS, Salapatek AM. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. The Journal of clinical investigation. 2001;108:1597–1611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takuma T, Arakawa T, Tajima Y. Interaction of SNARE proteins in rat parotid acinar cells. Archives of oral biology. 2000;45:369–375. doi: 10.1016/s0003-9969(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 47.Imai A, Nashida T, Shimomura H. Roles of Munc18-3 in amylase release from rat parotid acinar cells. Archives of biochemistry and biophysics. 2004;422:175–182. doi: 10.1016/j.abb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Yu H, Rathore SS, Lopez JA, Davis EM, James DE, Martin JL, Shen J. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3271–3280. doi: 10.1073/pnas.1311232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam PP, Hyvarinen K, Kauppi M, Cosen-Binker L, Laitinen S, Keranen S, Gaisano HY, Olkkonen VM. A cytosolic splice variant of Cab45 interacts with Munc18b and impacts on amylase secretion by pancreatic acini. Molecular biology of the cell. 2007;18:2473–2480. doi: 10.1091/mbc.E06-10-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Kang YH, Chang N, Lam PP, Liu Y, Olkkonen VM, Gaisano HY. Cab45b, a Munc18b-interacting partner, regulates exocytosis in pancreatic beta-cells. The Journal of biological chemistry. 2009;284:20840–20847. doi: 10.1074/jbc.M109.017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee A, Barry VA, DasGupta BR, Martin TF. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. The Journal of biological chemistry. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 52.Holz RW, Bittner MA, Peppers SC, Senter RA, Eberhard DA. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. The Journal of biological chemistry. 1989;264:5412–5419. [PubMed] [Google Scholar]
- 53.Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 54.Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, Mullar S, Diederichsen U, Fasshauer D, Grubmuller H, Hell SW, Eggeling C, Kuhnel K, Jahn R. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nature structural & molecular biology. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boswell KL, James DJ, Esquibel JM, Bruinsma S, Shirakawa R, Horiuchi H, Martin TF. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. The Journal of cell biology. 2012;197:301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imamura K, Wakasugi H, Shinozaki H, Ibayashi H. Dynamic analysis of secretagogue-induced amylase secretion from rat pancreatic acini studied by perifusion system. The Japanese journal of physiology. 1983;33:687–698. doi: 10.2170/jjphysiol.33.687. [DOI] [PubMed] [Google Scholar]
- 57.Padfield PJ, Panesar N. MgATP acts before Ca2+ to prime amylase secretion from permeabilized rat pancreatic acini. The American journal of physiology. 1997;273:G655–660. doi: 10.1152/ajpgi.1997.273.3.G655. [DOI] [PubMed] [Google Scholar]
- 58.Padfield PJ, Panesar N. Cholecystokinin octapeptide inhibits Ca2+-dependent amylase secretion from permeabilized pancreatic acini by blocking the MgATP-dependent priming of exocytosis. The Biochemical journal. 1998;330(Pt 1):329–334. doi: 10.1042/bj3300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshimura K, Fujita-Yoshigaki J, Murakami M, Segawa A. Cyclic AMP has distinct effects from Ca(2+) in evoking priming and fusion/exocytosis in parotid amylase secretion. Pflugers Archiv : European journal of physiology. 2002;444:586–596. doi: 10.1007/s00424-002-0844-7. [DOI] [PubMed] [Google Scholar]
- 60.Messenger SW, Thomas DD, Falkowski MA, Byrne JA, Gorelick FS, Groblewski GE. Tumor protein D52 controls trafficking of an apical endolysosomal secretory pathway in pancreatic acinar cells. American journal of physiology. Gastrointestinal and liver physiology. 2013;305:G439–452. doi: 10.1152/ajpgi.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arvan P, Castle JD. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. The Journal of cell biology. 1987;104:243–252. doi: 10.1083/jcb.104.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annual review of biochemistry. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 63.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Current opinion in cell biology. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moghadam PK, Jackson MB. The Functional Significance of Synaptotagmin Diversity in Neuroendocrine Secretion. Frontiers in endocrinology. 2013;4:124. doi: 10.3389/fendo.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Liu H, Gu Y, Chapman ER. Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. The Journal of cell biology. 2011;195:1159–1170. doi: 10.1083/jcb.201104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch KL, Martin TF. Synaptotagmins I and IX function redundantly in regulated exocytosis but not endocytosis in PC12 cells. Journal of cell science. 2007;120:617–627. doi: 10.1242/jcs.03375. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Wu Y, Wang Z, Dunning FM, Rehfuss J, Ramanan D, Chapman ER, Jackson MB. Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Molecular biology of the cell. 2011;22:2324–2336. doi: 10.1091/mbc.E11-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependent exocytosis in SERCA2(+/-) mice. The EMBO journal. 2001;20:2680–2689. doi: 10.1093/emboj/20.11.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imai A, Nashida T, Shimomura H. mRNA expression of membrane-fusion-related proteins in rat parotid gland. Archives of oral biology. 2001;46:955–962. doi: 10.1016/s0003-9969(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 70.Jo H, Byun HM, Kim JH, Kim MS, Kim SH, Hong JH, Seo JT, Lee SI, Shin DM, Son HK. Expression of Ca2+-dependent synaptotagmin isoforms in mouse and rat parotid acinar cells. Yonsei medical journal. 2006;47:70–77. doi: 10.3349/ymj.2006.47.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levius O, Feinstein N, Linial M. Expression and localization of synaptotagmin I in rat parotid gland. European journal of cell biology. 1997;73:81–92. [PubMed] [Google Scholar]
- 72.Choi JH, Jo H, Hong JH, Lee SI, Shin DM. Alteration of expression of Ca2+ signaling proteins and adaptation of Ca2+ signaling in SERCA2+/- mouse parotid acini. Yonsei medical journal. 2008;49:311–321. doi: 10.3349/ymj.2008.49.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falkowski MA, Thomas DD, Messenger SW, Martin TF, Groblewski GE. Expression, localization, and functional role for synaptotagmins in pancreatic acinar cells. American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G306–316. doi: 10.1152/ajpgi.00108.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behrendorff N, Dolai S, Hong W, Gaisano HY, Thorn P. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. The Journal of biological chemistry. 2011;286:29627–29634. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falkowski MA, Thomas DD, Groblewski GE. Complexin 2 modulates vesicle-associated membrane protein (VAMP) 2-regulated zymogen granule exocytosis in pancreatic acini. The Journal of biological chemistry. 2010;285:35558–35566. doi: 10.1074/jbc.M110.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends in cell biology. 2011;21:615–623. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. The Journal of biological chemistry. 2008;283:23884–23894. doi: 10.1074/jbc.M800754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimomura H, Imai A, Nashida T. Evidence for the involvement of cAMP-GEF (Epac)pathway in amylase release from the rat parotid gland. Archives of biochemistry and biophysics. 2004;431:124–128. doi: 10.1016/j.abb.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:G1403–1410. doi: 10.1152/ajpgi.00478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu CY, DiJulio DH, Jacobson KL, McKnight GS, Watson EL. The contribution of AKAP5 in amylase secretion from mouse parotid acini. American journal of physiology. Cell physiology. 2010;298:C1151–1158. doi: 10.1152/ajpcell.00382.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


