Abstract
Excitatory amino acid transporters or EAATs are the major transport mechanism for extracellular glutamate in the nervous system. This family of five carriers not only displays an impressive ability to regulate ambient extracellular glu concentrations but also regulate the temporal and spatial profile of glu after vesicular release. This dynamic form of regulation mediates several characteristic of synaptic, perisynaptic, and spillover activation of ionotropic and metabotropic receptors. EAATs function through a secondary active, electrogenic process but also possess a thermodynamically uncoupled ligand gated anion channel activity, both of which have been demonstrated to play a role in regulation of cellular activity. This review will highlight the inception of EAATs as a focus of research, the transport and channel functionality of the carriers, and then describe how these properties are used to regulate glutamatergic neurotransmission.
Keywords: Glutamate, Transporters, Ion channels, Synapse, Receptors, Plasticity
1. INTRODUCTION
L-glutamate (glu) is the primary excitatory amino acid in the central nervous system. Dysfunction of glutamatergic signaling is related to many debilitating diseases (1), and therefore proper coordination and fidelity of release, activation, and reuptake of this neurotransmitter is paramount for total system homeostasis. Excitatory amino acid transporters (EAATs) are secondary active, electrogenic transport systems that couple the accumulation of glu into the cytoplasm to downhill movement of co-transported ions along their concentration gradient. Alteration of these ion gradients, such as during anoxic depolarization, halts or even reverses glu transport and can contribute to excitotoxic conditions (2-5). The roles of the EAATs have been studied intensely for the last 30 years and much insight has been gathered into their structure, function, localization, and how they regulate neurotransmission. EAATs regulate glutamatergic neurotransmission but the mechanism by which they accomplish this process is by a dynamic coupling of bioenergetics of the transport process and the localization of the transporters themselves. The consequence of this coupling is the creation of complex spatio-temporal profiles for extracellular glu. Here we will review background information on the SLC1 family of transporters including their function and structure and how these transporters regulate neurotransmission.
1.1. Isoforms and Localization
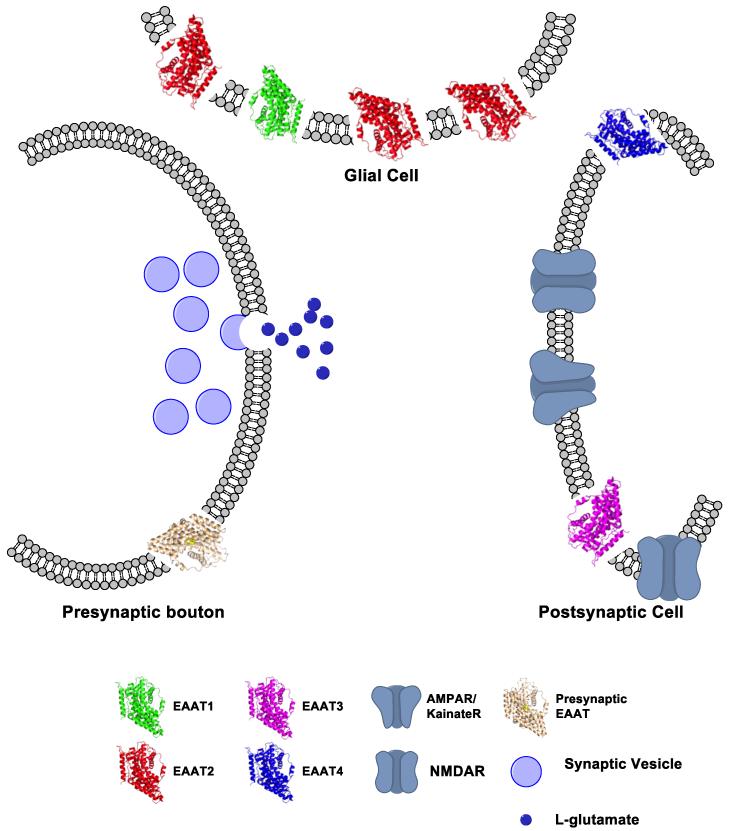
The solute carrier 1 (SLC1) family of neurotransmitter transporters is comprised of several solute carriers including the excitatory amino acid transporter (EAATs). The initial cloning of a glu carrier in the SLC1 family was performed in 1992 with the isolation of a 60 kDa protein from rat brain termed the glutamate/aspartate transporter (GLAST) (6). One month later, glutamate transporter 1 (GLT-1) from rat, and excitatory amino acid carrier 1 (EAAC1) from rabbit were both cloned (7,8). All of these carriers were described as Na+ and K+ dependent, SLC1 family members that accumulate glu and L- or D-aspartate (asp). Subsequently two novel human isoforms were cloned from the cerebellum and retina, excitatory amino acid transporter 4 (EAAT4) and EAAT5, respectively (9,10). Human isoforms of GLAST, GLT-1, and EAAC1 were also cloned and renamed EAAT1-3 to denote their human species of origin (11). EAAT1-5 share an approximate 65% primary sequences homology between them. The transporters can roughly be divided into two classes - astrocytic or neuronal (Figure 1). EAATs 1 and 2 are found predominantly in astrocytes while EAAT3, EAAT4, and EAAT5 are neuronal. EAAT1 is enriched in cerebellar astrocytes but also found in astrocytes throughout the brain (6,12). EAAT2 is the most abundant glu transporter found in the brain and, by some estimates, accounts for ~90% of the total glu uptake in the brain (13,14). EAAT3 is most often described as a postsynaptic neuronal carrier with expression ranging throughout the brain. EAAT4, like EAAT3, is also a neuronal transporter (15). While the Purkinje cell localization of EAAT4 is dramatic, this carrier is also found in other neurons at low levels (15,16). Expression of EAAT5 is exclusively in the retina (10). Throughout this paper will refer to general properties of the carriers using their EAAT nomenclature unless specifically in reference to the non-human isoforms. Although basic properties of the various isoforms are largely similar, minor differences in their kinetics, localization, and regulation dramatically affect glutamatergic neurotransmission.
Figure 1. Localization of EAATs in the synapse.
Model of a basic glutamatergic synapse illustrating the localization of EAATs 1-4. EAAT1 (green) and EAAT2 (red) are localized to glial membranes. EAAT3 (blue) and EAAT4 (magenta) are localized to post-synaptic membranes. Expression and function of a presynaptic carrier (grey) is unclear, but EAAT2 and EAAT3 isoforms have both been implicated. Postsynaptic ionotropic glu receptors are depicted with a generic structure in blue. Structures are modeled as monomers of an archeal homolog of a glu transporter from Pyrococcus horikoshii (GltPh) and were modified from (72). The GltPh pdb file 1FXH was modified using Pymol vs 1.3. Model was built using ChemBioDraw vs 13.0 (Perkin Elmer).
2. MECHANISM OF TRANSPORT
2.1. Characterization of Glu Transport
Initial functional studies of glu translocation were done in rat brain synaptosomes and the translocation were described as Na+ and K+ dependent processes (17). Experiments controlling the transmembrane potential in synaptosomes demonstrated that accumulation of glu was also an electrogenic process. Initial electrophysiological recordings of glu carriers described the electrogenic nature of the transport cycle and supported the basic findings of earlier biochemical studies. A large, rectifying inward current, between −160 and +80 mV, in response to extracellular glu addition was generated in whole cell patch recording of Müller glial cells (18). These currents were voltage and Na+ dependent and only responded to applications of D- and L-asp and L-glu but not D-glu, congruent with the initial characterizations of glu transport performed by Kanner’s group. Early electrophysiological reports also corroborated conclusions from biochemical data when the dependence of transport on intracellular K+ was investigated by whole cell patch recordings from glia (19).
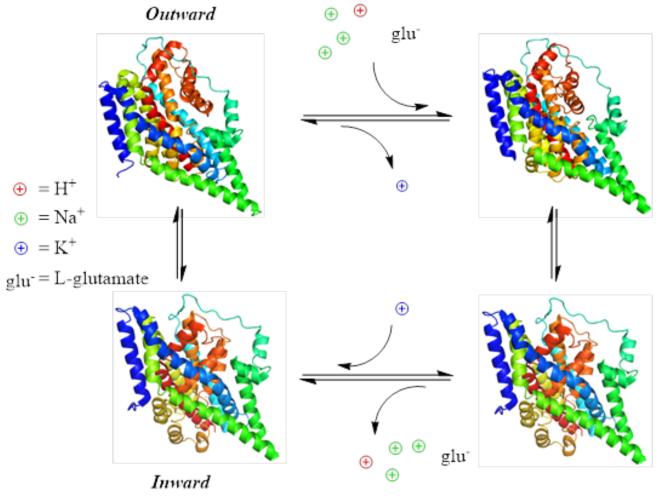
The translocation of glu is a reversible cycle, and homoexchange of glu was characterized before the molecular identity of the EAATs were known (20,21). All experiments pointed to a relatively high affinity carrier which could translocate substrates in the low micromolar range. Work done on a bacterial homolog glu transporter from Bacillus stearothermophilus, GLTBs, demonstrated that electrogenic glu uptake was accompanied by a proton as well as a sodium ion (22), although confirmation of this proton co-transport was contentious and the possibility of OH− counter transport as the source of pH change associated with glu transport arose (23,24). The most accurate estimation of stoichiometry was demonstrated using a series of experiments controlling ion concentrations and measuring reversal potentials of transport as well as pre-steady state kinetic measurements using substrate concentration jumps (25-27). From data presented in these studies, it can be concluded that EAATs likely function by transporting 1 glu with 3 Na+, 1 H+, and the counter transport of 1 K+ (Figure 2).
Figure 2. EAATs are secondary, electrogenic transporters.
4-state transport diagram depicting coupled stoichiometry of glu transport and ion movement. Transporters are displayed to represent the outward open state (upper left), the outward bound occluded state (upper right), the inward occluded state (lower right), and the inward open state (lower left). The structures used are adapted from the 2NWW, 1FXH, and 3KBC pdb files, respectively (72,82,83).
Initial investigations into the transport kinetics revealed that hyperpolarization of the membrane stimulated transport in a manner that was dependent on glu concentration. This led authors to propose that glu translocation was the rate limiting step of the transport cycle (28,29). It wasn’t until pre-steady state kinetics were investigated using a photo-releasable glu analog that a detailed study of initial glu binding and translocations steps were possible (26). Glu release on a sub-millisecond time scale revealed an initial transient electrogenic component which rapidly decayed down to a steady state level. This voltage and concentration dependent decay process is associated with the initial translocation of glu. Furthermore, these data can only be reconciled with a transport cycle with a rate limiting step not related to glu translocation but to the K+ mediated reorientation process as a rate limiting glu translocation would not produce the same transient components (30). Additionally, replacement of internal K+ with Cs+ dramatically affected cycling times, support that K+ interaction and reorientation are the late limiting steps of the transport cycle (31). The translocation of glu is dependent on the binding and co-transport of several cationic species. Na+ binding to the empty transporter has been shown to be a voltage dependent process, generating measureable capacitive currents (32,33). Since the steady state affinity of EAATs for glu is voltage independent although glu translocation generates net inward charge movements, then another voltage dependent process has to produce an equal and opposite charge movement. K+ reorientation has, therefore, been hypothesized to neutralize more than −1 elementary charge (30). To further support the hypothesis co-transported cations are used to neutral the endogenous charges present in the empty transporter (34), a combination of pre-steady state concentration and voltage jump experiments with computational modeling were used. These data led to the conclusion that the negative charge generated by the movement of the empty transport is, indeed, in part compensated by the binding and translocation of Na+, H+, and K+ ions (35). The compensation of endogenous transporter charges by ion binding, in addition to a defocused electric field and distribution of electrogenic steps, allows for a tightly regulated and energetically reasonable transport mechanism (Figure 2).
2.2. Physiological Regulation of Transport
The transport of glu against its concentration gradient is coupled to the downhill movement of co-transported ions with their gradient. Alterations of the ion gradients for Na+, H+, and K+ would, therefore, impact the ability for the EAATs to transport substrates. Evidence for reverse transport came early when Kanner and Marva used vesicles from rat brain which were loaded with high internal Na+ and high extracellular K+ to efflux radiolabeled glu which had been loaded into the vesicle (21). In whole cell patch recording of Müller glial cells of the salamander retina, dialysis of the internal solution using a pipette solution containing Na+ and glu drove glu transporters in the reverse direction with the addition of extracellular K+ (36). The reversal of the transport cycle, or glutamate mediated efflux, has been demonstrated in a variety of glu transporters isoforms such as EAAT1 or EAAC1 (rat EAAT3), suggesting that reverse transport capability is intrinsic to the SLC1 family (37,38). During normal physiological conditions, it is unlikely that reverse transport occurs frequently enough to have a physiological consequence (39), yet during anoxic depolarization it has been suggested that glu efflux mediated by reversal of the EAAT transport cycle can mediate excitotoxic conditions (4). So how do the bioenergetics of transport influence glutamatergic neurotransmission? First, the coupled movement of three sodium ion indicates that glu can be concentrated inside a neuron or astrocyte with a 106 fold glu concentration difference or keep extracellular glu levels down to the low nanomolar concentration (25). Additionally, under ambient conditions, the low affinity for Na+ and glu at the intracellular face of the transporter means that reverse transport would occur with a negligible frequency (33). During ischemic conditions, however, in which the Na+ and K+ concentrations and transmembrane voltage are disturbed, a significant amount of glu has been demonstrated to be released through an EAAT mediated reverse transport process (4). Thus, the bioenergetics of glu transport tightly controls the rates and amount of neurotransmitter removal from the synaptic space. Because cessation of excitatory neurotransmission is dependent on the efficient removal of extracellular glu, the bioenergetics of EAAT transport contributes to the termination of glutamatergic neurotransmission in an essential manner.
3. LIGAND-GATED ANION CHANNELS
3.1. Characterization of Channel Activity
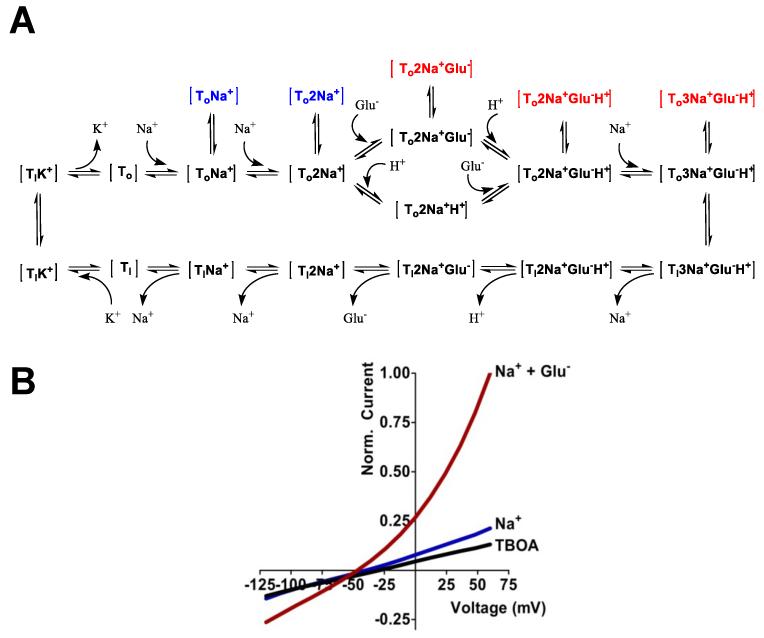
In 1994, electrophysiological characterization of a novel EAAT cloned from cerebellar mRNA of humans, revealed a remarkable property of glu transporters (9). An EAAT-mediated Cl− flux which was Na+ and glu dependent but thermodynamically independent of the transport process was demonstrated (Figure 3). These data were the first to unambiguously reveal anion channel activity in the EAATs. Initial voltage clamp studies of the EAAT anion channel revealed several basic properties. First, the anion channel is promiscuous, with a permeability sequence generally following SCN− > ClO4− > NO3− > I− > Br− > Cl− > F− (40-42). Second, the interaction of substrates with EAATs elicits a robust anion current, but this anion flux occurs, with diminished amplitude, in the absence of substrates as well. This “leak” current was originally revealed by the loss of a tonic cellular conductance in the presence of inhibitors (43). Anion conductances in the absence of extracellular glu were substantiated by several groups (37,44,45). It was demonstrated that the leak current is actually dependent on extracellular Na+ (46) and indeed gated by Na+ interaction with the EAATs in the absence of glu (31,47). Stationary and non-stationary noise analyses have been used to characterize channel properties mediated by Na+ and glu interaction (37,48-50). These data revealed that Na+ interaction with the EAATs mediates a low open probability and the open probability of the channel is increased upon glu binding subsequent to Na+, without a significant change in low (0.5-500 fS) single channel conductance. The physiological role or impact of these tonic anion fluxes in the absence of active transmission has yet to be examined in detail.
Figure 3. EAATs mediate anion flux through multiple open states.
A) 20 step state diagram depicting stable conformations of glu transport (black) and open channel states gated by either Na+ interaction (blue) or Na+ and glu interaction (red) associated with the outward facing conformations of the transport cycle. Inward facing open channel states have been removed for clarity. B) Demonstrative current-voltage relationship for an EAAT in response to extracellular application of Na+ + DL-threo-β-benzyloxyaspartate (DL-TBOA; black), Na+ alone (blue), or Na+ + 500 μM glu (red). Currents are representative of NO3 flux through EAAT4.
The mechanism by which substrate interaction with the EAATs gates the channel is still under investigation. Initial work by Vandenberg’s group using a series of point mutations between residues R90 and K114 of EAAT1 demonstrated that the D112A mutation significantly reduced glu gated anion current amplitudes yet strongly enhanced Na+ gated currents (51). These data suggest that D112 is involved in the ability of substrate to gate that the anion channel. Similarly, when R388 in transmembrane domain 8 (TM8) is mutated to an acidic amino acid residue, substrate gated anion channel activity is completely abolished while current amplitudes in the presence of Na+ alone are enhanced several fold (Delany Torres-Salazar; personal communication). Other residues have been implicated in the gating mechanism in addition to D112 and R388. Mutations of D272 (TM5), K384, and R385 (TM7) in hEAAT1 have all been demonstrated to increase channel activity after mutation to alanine (52). Only a handful of mutations have been demonstrated to alter channel activity, however non-mutational data have also contributed to our understanding of the mechanism of channel gating. Pre-steady state kinetics has been a powerful technique to study the initial steps in substrate interaction and channel activity. Laser pulse photolysis of caged glu was employed for a series of investigations to measure the initial kinetics of substrate binding, translocation, and substrate gating. These elegant experiments have lent great insight in the mechanism of transport and channel activity and have been thoroughly reviewed elsewhere (53,54).
3.2. Role of the Chloride Channel in Regulating Synaptic Transmission
The physiological role of a low conductance anion channel present in a family of neurotransmitter transporters remains elusive. One possible role for this ligand-gated channel is the stabilization of the membrane potential to offset the positive ion influx associated with electrogenic transport of substrates during neurotransmission (41). Veruki et al. (2006) used whole cell and paired recordings from rod bipolar cells and AII amacrine cells to demonstrate that glutamatergic activation of EAAT5 causes a membrane hyperpolarization which reduces subsequent transmitter release (55). These data demonstrate that anion flux through the EAATs is capable of regulating cellular excitability. Additional work demonstrated that EAAT5 mediated a large inhibitory current in response to bipolar cell depolarization, confirming a role for the EAAT mediated anion conductance in shaping neurotransmission (56).
4. STRUCTURE OF THE SLC1 FAMILY
4.1. EAAT Topology
Upon cloning of the first members of the SLC1 family, hydropathy plots gave initial insight into the secondary structure of these carriers. A bacterial SLC1 family member, the glutamate-proton co-transporter (GltP) from E. coli, displayed 12 putative transmembrane domains (TM) with a cytoplasmic C-terminus (57). Topology models of GLT-1 and EAAC1 displayed 8 and 10 transmembrane domains respectively, but both models contained a large glycosylated loop between TM3 and 4, an intracellular N-terminus, and a long, intracellular C-terminal tail (7,8). Structural experiments were used extensively by several groups in order to further refine the topology of the transporter during different conformational states. Rat membrane vesicles containing GLT-1 were probed for their sensitivity to trypsin digestion in the presence and absence of the GLT-1 inhibitor dihydrokainate (DHK) as well as the substrate glu. The difference in trypsin induced fragments with bound substrates and antagonist indicated at least two conformational states representing inhibitor stabilized, outward facing states and substrate induced, transporting states of GLT-1 (58). Additional topology models were proposed over the next few years including a refined 4 TM domain C-terminal region (59) and a possible β-sheet structure in the C-terminal transmembrane domains (60).
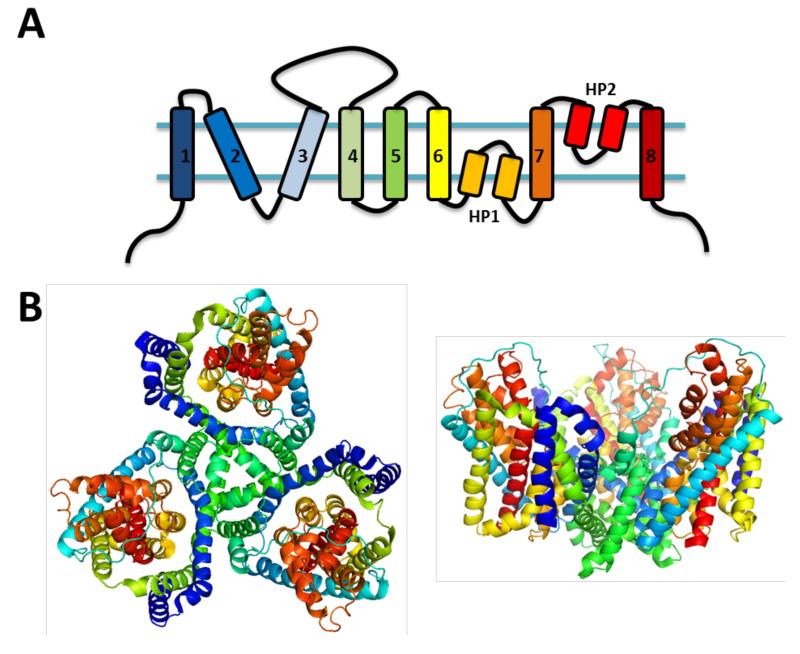
In order to increase the resolution of the structural topology data, a method originally used to screen pore lining domains using modification of single cysteine residues was employed (61-63). Initial experiments employing cysteine mutagenesis based methods revealed a topology for GLT-1 with 8 TM domains and a reentrant loop (HP2) between TM 7 and 8 (64). Additional data using substituted cysteine accessibility method (SCAM) supported the presence of two reentrant loops, although disagreeing on their location in the c-terminal half of the transporter (65-67). The first accurate report of EAAT membrane topology came when Grunewald et al. (2000) performed SCAM experiments across residues 347 to 393 (68). The ability for only residues A364C and G365C (GLT-1) to be labeled by the extracellular application of the non-permeable MTSET, depicted another reentrant loop (HP1) located upstream of TM7. Currently the model for EAAT topology is 8 TM domains and 2 hairpin loops (HP1 and HP2); HP1 located between TM 6 and 7, and HP2 located between TM7 and 8 (Figure 4A).
Figure 4. General structure of the SLC1 family of transporters.
A. Two-dimensional representation of the topology of the GltPh monomer colored with a rainbow pattern. B. (Left) Top-down view of the homotrimeric GltPh crystal from pdb file # 1FXH. Each monomer was colored with the same rainbow pattern as in A) using Pymol version 1.3. (Right) View of the same trimeric representation of the GltPh crystal structure from a horizontal or membrane view.
4.2. The Crystal Structure of an Archeal Homolog of Mammalian EAATs
Work investigating the oligomerization of EAATs was incipient with exposing liposomes reconstituted from rat forebrain and cerebellum with chemical crosslinking reagents (69). These experiments, along with similar data from assays using heterologous expression of EAATs, support a homotrimeric organization of EAATs (70). However, the homotrimeric, quaternary structure of EAATs was disputed by freeze-fracture electron microscopy data which suggested a pentameric assembly (71). The first definitive picture of tertiary and quaternary structure came in 2004 when the crystal structure of an archeal homolog to the EAATs, GltPh was solved (72). The crystal architecture displayed 8 transmembrane domains and two hairpin loops located between TM6-7 and TM7-8, as previously described (68). The organization of the transporter in the membrane was that of a homotrimer with the subunit interface comprised of TM 2, 4, and 5 regions (Figure 4B). The trimer shape of the GltPh crystal formed a bowl or extracellular solvent-accessible basin and the co-crystallization of bound substrates confirmed the location of the hypothesized binding site. The binding site is adjacent to TM7, at the tips of HP2 and HP1 and the substrate was coordinated by several residues previously demonstrated to have a role in substrate interactions (27,73-81). The conformational state that seemed to be displayed by this snapshot was the outward conformation with the binding site occluded by HP2. Although the mutated archeal transporter used for crystallized was inactive, the resulting crystal structure was in strong congruence with structure-function data and served well to refine lingering questions in the field. To date, three more crystal structures have been solved using the archeal asp carrier GltPh. The location of the substrate-binding site was confirmed with investigations demonstrating that D394 in TM8 coordinates the amino group of asp or glu and R397 interacts with the side chain carboxyl group. Additional structures demonstrated DL-TBOA bound states with slight differences in the location of HP2 suggested possible outward open states (82) (see also Figure 2). One of the next structural developments depicted a possible inward-facing state. Through crosslinking of distal residues in HP2 and TM2, crystals were obtained and a transport mechanism was proposed to explain the transition of the previously described outward facing structures to the HP2/TM2 cross-linked structure. The model includes a substantial inward shift of TM3, TM6, HP1, TM7, HP2, and TM8, which was termed the translocation domain as it contained the HP2 and HP1 occluded, substrate binding site. This piston-like movement leaves both the translocation domain and TM1, TM2, TM4, and TM5, termed the scaffold domain, largely similar in conformation, when considered individually, albeit over an 18 Å inward shift of the substrate binding site (83).
4.3. Structural Influences of the Trimeric Organization of EAATs in Glu Uptake
Although many of the functional and structural features of the EAATs have been resolved, there may be other aspects of the structure which can influence carrier activity in unforeseen ways. Indeed it has been proposed that the bowl-like shape of the trimer may play a role in restricting glu diffusion after initial binding, thus helping to reduce effective glu release after initial binding events during synaptic transmission (84). Future work may further reveal aspect of how the quaternary structure of EAATs support their function roles in regulating excitatory neurotransmission.
5. THE ROLE OF EAATS IN SYNAPTIC TRANSMISSION
5.1. The Roles of EAATs in Glutamatergic Neurotransmission in the Hippocampus
The role of EAATs is to control extracellular glu levels, both ambient and in response to exocytosis of neurotransmitter due to neuronal activity. Levels of ambient glu have been estimated as low as 25 nM and blocking the carriers with a specific antagonist causes a non-linear rise in ambient levels, supporting the classic role of EAATs in maintaining low extracellular glu concentrations (85). Synaptic concentrations of glu, after vesicular release from dissociated hippocampal neurons, were initially estimated to be in the millimolar range, approximately 1.0 – 1.5 mM (86). Early studies looked at the effect of EAAT blockade, or competition at the carrier binding site, on activation of post-synaptic ionotropic glu receptors (iGluRs) and indicated that loss of active transporters could increase the concentration of neurotransmitter in the synapse, observed as a slight, transient increase in mEPSC amplitudes (87) or as a decrease in decay rates of EPSCs (88). Through the delicate manipulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) kinetics with various pharmacology, it was demonstrated that in the first 100 μsec of glu release from the presynaptic terminal, EAATs act to reduce mEPSC amplitude and shorten rise times of the transient post synaptic currents (89). Since the transport cycle is significantly slower than this timing, approximately 15 – 90 s−1 (30,32), it was proposed that glu transporters act to buffer synaptic glu with a relatively high affinity binding interaction, yet the slow kinetics of transport allows for some unbinding of substrate before a substantial amount of glu can be transported (30). To support the hypothesis of rapid buffering, EAAT expression near the synapse would need to be at remarkably high levels. Indeed estimates for EAAT2 concentrations in hippocampal astrocytes are as high as 12,000/μm3 and EAAC1 neuronal levels were estimated to be 130/μm3 (13,90). Thus, EAATs can modulate the concentration of synaptic glu to some degree but also act to control the temporal profile of postsynaptic receptor activation and diffusion to perisynaptic regions as glu binds and unbinds rapidly to a high number of carriers.
Diffusion accounts for much of the rapid decrease in synaptic levels of the neurotransmitter and elevated perisynaptic concentrations of glu can be detected for a sustained period of time (>10 msec). This sustained activation of peri-synaptic transporters has been demonstrated for EAAT expressing astrocytes in the CA1 region of the hippocampus with stimulation of Schaffer collaterals synapsing on CA1 pyramidal cells (45). These data demonstrate that rapid clearance of synaptic glu is accomplished mainly through diffusion but a significant level of glu persists at the perisynaptic regions. This persistence of neurotransmitter is not due to heteroexchange functions of the EAATs (glu in - glu out) since application of the competitive transportable substrate threo-hydroxyaspartate (THA; a substrate which could induce heteroexchange for cytoplasmic glu) results in the same activation of iGluRs as does application of the non-transportable competitive inhibitor DL-threo-β-hydroxybenzoylaspartate (DL-TBOA; 91). The influence of EAAT-mediated glu buffering and transport capacity on hippocampal circuitry during high frequency stimulation was investigated (92). Synaptically evoked transporter currents (STCs) from CA1 region astrocytic carriers (EAAT1 and EAAT2) were not significantly altered after a series of stimulations at near-physiological temperatures arguing for a very capacious glu uptake system in the hippocampus. To further clarify the mechanism of glu removal surrounding the synapse (perisynaptic regions), Schaffer collatorals-CA1 pyramidal cells synapses were exposed to a low affinity N-methyl-D-aspartate receptor (NMDAR) antagonist and the effects of TBOA blockade on NMDAR mediated EPSCs were measured (93). Results indicated that TBOA inhibition of the perisynaptic EAATs resulted in an increase in non-synaptically located, or distal, NMDAR activation. Additionally, replacement of internal cations in a post-synaptic neuron for NMDG, effectively blocking transport, also significantly altered the activation of NMDARs suggesting a role for post-synaptic neuronal EAATs in limiting spillover activation from adjacent synapses, a role which was previously attributed predominately to the glial EAAT isoforms. Investigations using EAAC1 (mouse EAAT3) knock-out transgenic models refined the role of post-synaptic EAAC1 by speeding the decay constant of NMDAR activation in the presence of a low affinity antagonist, supporting the role these carrier play in modulating NDMAR activation, especially NR2B containing receptors, in perisynaptic spaces (94). This study, however, did not support the role of EAAC1 in limiting glu spillover to adjacent synapses. From these data, it is clear that EAATs not only modulate post-synaptic receptor activation but can regulate activation of ionotropic receptors located at sites beyond the synaptic cleft. The distance that synaptically released glu can influence activity supports the need for a diverse and widely localized family of carriers.
5.2. The Role of EAATs in the Cerebellum
The role of glu transporters is similar for many brain regions although differences in expression pattern, synaptic architecture, and general features of circuitry can influence glutamatergic neurotransmission. Due to the high concentration of EAAT4 (a higher affinity yet lower turnover rate isoform) expressed in the cerebellum, Otis et al. (1997) (95) investigated buffering of released glu from climbing fiber-Purkinje cell synapses (CF-PC). Results indicated some synaptic glu transporters as well as perisynaptic EAATs could rapidly buffer upwards of 22% of glu released from a single vesicle. Likewise, at PF-PC synapses, PC cells with lower endogenous expression of EAAT4 had larger glial AMPAR-mediated current amplitudes suggesting that the expression of EAAT4 could influence activation of extrasynaptic receptors (96). In the cerebellar cortex, EAAT4 mimics the actions of EAAT3 in the hippocampus by rapidly buffering synaptic glu. Although both EAAT3 and EAAT4 are expressed in CF synapses, investigations using EAAT3 and EAAT4 transgenic knockouts into the relative contribution of these two carriers in the CF synapse revealed a dominate role for EAAT4 as compared to EAAT3 in this system (97). To investigate the role of glial carriers (predominately EAAT1) in cerebellar excitatory neurotransmission, climbing fibers were stimulated and caused a rapid onset of EAAT transport currents in Bergmann glial cells which persisted for a prolonged period of time, supporting the notion of rapid escape of glu from the synapse and slow re-uptake once diffused to extrasynaptic regions (98).
One of the most striking features of the CF-PC synapse is the concentrations of glu that are released following CF stimulation. CF-PC synapses have been thoroughly demonstrated to mediate multivesicular release and moderate firing rates induce a significant glu spillover (99). Spillover of glu causes activation of molecular layer interneurons (MLIs) and was observed in the cerebellum during activation of CF-PC synapses (100). This spillover mechanism is so profound that excitation of MLIs can mediate feed-forward inhibition and alter activity patterns in neighboring interneurons and Purkinje cells (101). Thus at the synaptic cleft, perisynaptic space, and even at adjacent synapses glu transporters play vital roles in regulating the concentration of extracellular glu.
5.3. EAATs Influence Activation of Metabotropic Glu Receptors and Synaptic Plasticity
It is clear that glu transporters in conjunction with diffusion regulate the temporal and spatial pattern of glu after various modes of release. How does this spatial-temporal profile effect perisynaptic receptor activation and downstream processes such as synaptic plasticity? It has been observed that EAAC1 activity regulates NMDAR activation in the hippocampus but the consequences of ionotropic activation go beyond basic excitatory transmission. It is well demonstrated that activation of NMDAR (and some Ca2+ permeable AMPARs) can lead to several forms of synaptic plasticity (102-104). NMDAR activation is a key component to many alterations of synaptic strength. This alteration has been demonstrated to take various forms such as presynaptic alteration, typically reported as a difference in quanta, as well as postsynaptic alterations such as changes in AMPAR surface expression (105-107). The extent to which EAAT3 limits activation of perisynaptic NMDARs is great enough to influence both LTP and LTD in the CA1 region of the hippocampus (94). Metabotropic GluRs (mGluRs) have a significant role in changes in synaptic strength in the CA1 region of the hippocampus (104,108). As we have described here, it is clear that EAATs mediate the ability for glu to activate perisynaptic receptors and as the affinity of many of the mGluRs for glu is lower as compared to iGluRs, the role of EAATs in regulating mGluR activation is pronounced. Indeed, inhibition of EAATs in the CA1 region of the hippocampus caused an enhancement of mGluR1α mediated currents in O-LM interneurons in that region (109). Likewise, application of TBOA to rat hippocampal slices altered the activation of mGluR5 and group III mGluRs supporting a general mechanism of EAATs regulating glu accessibility to any perisynaptically localized glu receptor (110). Thus, in the hippocampus, EAAT mediated regulation of receptor activation is a critical process that modulates not only fast ex citatory neurotransmission but ionotropic and metabotropic regulation of synaptic function and strength.
The role of EAATs in modulating synaptic plasticity is not constrained to the hippocampus as demonstrated by investigations into the cerebellum. EAAT4 expression in the cerebellum is localized adjacent to the post-synaptic density (PSD) which overlaps with that of metabotropic glutamate receptors (mGluRs). Inhibition of post-synaptic EAAT4 (and EAAT3 to some extent) causes a significant enhancement of long-term depression (LTD) in PF-PC synapses through an mGluR dependent process (111,112). Additionally, in CF-PC synapses the presence of a group 1 mGluR component is only observed when EAATs are inhibited (112,113). This mGluR activation has profound roles in CF associated LTD but also in synaptic pruning of multiple climbing fiber inputs to the PC early in development, and in general provides a CF-PF homosynaptic form of regulation tightly mediated by glu release and EAAT activity. Thus, one of the many roles of EAATs is to influence activation of mGluRs and subsequent synaptic functioning. Regional differences in the ability of Purkinje cells to be regulated through mGluR dependent activity are strongly controlled by the expression pattern of EAAT4 in the cerebellum (114). Another study demonstrated that LTD of PCs synapses results in a decrease in postsynaptic AMPAR expression and a concomitant persistent increase of post-synaptic EAAT4 currents (115). Thus, as synaptic strength is decreased by AMPAR trafficking, EAAT4 levels likely increase to compensate for the decreased number of receptors by enhanced rapid buffering of glu and regulation of perisynaptic glu concentrations. Overall, these data presented here, and elsewhere, strongly support the role of glu carriers in shaping several aspects of neuronal functioning.
6. CONCLUSION
Glu transporters or EAATs regulate extracellular glu dynamics throughout the nervous system. The role of neuronal isoforms has expanded to included such processes as cysteine transport for glutathione synthesis (116,117) and glu uptake for GABA synthesis (118), but the prevailing hypothesis is that glial isoforms mediate the bulk of glu transport and have the greatest degree of influence on extrasynaptic receptor activation. Excess glutamatergic signaling can lead to excitotoxic cell death and during ischemic conditions the transporters themselves become potent releasers of glu. The activities of EAATs are indicated in almost every process of neurotransmission and we are still trying to understand the roles of the chloride channel activity these transporters mediate. It is likely that the channel function of the EAATs can indeed regulate cellular activity either through offsetting inward positive charge movement associated with a high capacity transport mechanism or through acting as a classic ionotropic Cl− receptor. Indeed it is an exciting time to study this fascinating family of carriers and their roles in shaping glutamatergic neurotransmission.
ACKNOWLEDGMENTS
This work was supported by the NIMH Department of Intramural Research (S.M.U.) and NIH grant MH080726 (C.B.D). The authors would like to thank Jon W. Johnson for his thoughtful review and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 2.Djali S, Dawson LA. Characterization of endogenous amino acid efflux from hippocampal slices during chemically-induced ischemia. Neurochemical research. 2001;26:135–143. doi: 10.1023/a:1011094728469. [DOI] [PubMed] [Google Scholar]
- 3.Phillis JW, Smith-Barbour M, Perkins LM, O’Regan MH. Characterization of glutamate, aspartate, and GABA release from ischemic rat cerebral cortex. Brain research bulletin. 1994;34:457–466. doi: 10.1016/0361-9230(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 4.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 5.Seki Y, Feustel PJ, Keller RW, Jr., Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke; a journal of cerebral circulation. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- 6.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 8.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 9.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 10.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The Density of EAAC1 (EAAT3) Glutamate Transporters Expressed by Neurons in the Mammalian CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 15.Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat Neurosci. 1998;1:105–113. doi: 10.1038/355. [DOI] [PubMed] [Google Scholar]
- 16.Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y. High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. The Journal of comparative neurology. 2008;511:155–172. doi: 10.1002/cne.21823. [DOI] [PubMed] [Google Scholar]
- 17.Kanner BI, Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- 18.Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- 19.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanner BI, Bendahan A. Binding order of substrates to the sodium and potassium ion coupled L-glutamic acid transporter from rat brain. Biochemistry. 1982;21:6327–6330. doi: 10.1021/bi00267a044. [DOI] [PubMed] [Google Scholar]
- 21.Kanner BI, Marva E. Efflux of L-glutamate by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1982;21:3143–3147. doi: 10.1021/bi00256a017. [DOI] [PubMed] [Google Scholar]
- 22.de Vrij W, Bulthuis RA, van Iwaarden PR, Konings WN. Mechanism of L-glutamate transport in membrane vesicles from Bacillus stearothermophilus. J Bacteriol. 1989;171:1118–1125. doi: 10.1128/jb.171.2.1118-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360:471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- 24.Amato A, Ballerini L, Attwell D. Intracellular pH changes produced by glutamate uptake in rat hippocampal slices. J Neurophysiol. 1994;72:1686–1696. doi: 10.1152/jn.1994.72.4.1686. [DOI] [PubMed] [Google Scholar]
- 25.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 26.Watzke N, Rauen T, Bamberg E, Grewer C. On the mechanism of proton transport by the neuronal excitatory amino acid carrier 1. J Gen Physiol. 2000;116:609–622. doi: 10.1085/jgp.116.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grewer C, Watzke N, Rauen T, Bicho A. Is the glutamate residue Glu-373 the proton acceptor of the excitatory amino acid carrier 1? J Biol Chem. 2003;278:2585–2592. doi: 10.1074/jbc.M207956200. [DOI] [PubMed] [Google Scholar]
- 28.Kanai Y, Stelzner M, Nussberger S, Khawaja S, Hebert SC, Smith CP, Hediger MA. The neuronal and epithelial human high affinity glutamate transporter. Insights into structure and mechanism of transport. J Biol Chem. 1994;269:20599–20606. [PubMed] [Google Scholar]
- 29.Kanai Y, Nussberger S, Romero MF, Boron WF, Hebert SC, Hediger MA. Electrogenic properties of the epithelial and neuronal high affinity glutamate transporter. J Biol Chem. 1995;270:16561–16568. doi: 10.1074/jbc.270.28.16561. [DOI] [PubMed] [Google Scholar]
- 30.Grewer C, Watzke N, Wiessner M, Rauen T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc Natl Acad Sci U S A. 2000;97:9706–9711. doi: 10.1073/pnas.160170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergles DE, Tzingounis AV, Jahr CE. Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J Neurosci. 2002;22:10153–10162. doi: 10.1523/JNEUROSCI.22-23-10153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Tao Z, Gameiro A, Barcelona S, Braams S, Rauen T, Grewer C. Transport direction determines the kinetics of substrate transport by the glutamate transporter EAAC1. Proc Natl Acad Sci U S A. 2007;104:18025–18030. doi: 10.1073/pnas.0704570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson HP, Tzingounis AV, Koch HP, Kavanaugh MP. Fluorometric measurements of conformational changes in glutamate transporters. Proc Natl Acad Sci U S A. 2004;101:3951–3956. doi: 10.1073/pnas.0306737101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grewer C, Zhang Z, Mwaura J, Albers T, Schwartz A, Gameiro A. Charge compensation mechanism of a Na+-coupled, secondary-active glutamate transporter. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.364059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 37.Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watzke N, Grewer C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 2001;503:121–125. doi: 10.1016/s0014-5793(01)02715-6. [DOI] [PubMed] [Google Scholar]
- 39.Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- 40.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 41.Eliasof S, Jahr CE. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc Natl Acad Sci U S A. 1996;93:4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J Neurosci. 1996;16:6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenberg RJ, Arriza JL, Amara SG, Kavanaugh MP. Constitutive ion fluxes and substrate binding domains of human glutamate transporters. J Biol Chem. 1995;270:17668–17671. doi: 10.1074/jbc.270.30.17668. [DOI] [PubMed] [Google Scholar]
- 44.Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz EA, Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J Physiol. 1990;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watzke N, Bamberg E, Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J Gen Physiol. 2001;117:547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson HP, Picaud SA, Werblin FS, Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996;70:733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melzer N, Biela A, Fahlke C. Glutamate modifies ion conduction and voltage-dependent gating of excitatory amino acid transporter-associated anion channels. J Biol Chem. 2003;278:50112–50119. doi: 10.1074/jbc.M307990200. [DOI] [PubMed] [Google Scholar]
- 50.Torres-Salazar D, Fahlke C. Neuronal Glutamate Transporters Vary in Substrate Transport Rate but Not in Unitary Anion Channel Conductance in. The Journal of Biological Chemistry. 2007 doi: 10.1074/jbc.M704118200. [DOI] [PubMed] [Google Scholar]
- 51.Ryan RM, Mitrovic AD, Vandenberg RJ. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem. 2004;279:20742–20751. doi: 10.1074/jbc.M304433200. [DOI] [PubMed] [Google Scholar]
- 52.Huang S, Vandenberg RJ. Mutations in transmembrane domains 5 and 7 of the human excitatory amino acid transporter 1 affect the substrate-activated anion channel. Biochemistry. 2007;46:9685–9692. doi: 10.1021/bi700647f. [DOI] [PubMed] [Google Scholar]
- 53.Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol. 2005;203:1–20. doi: 10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T. Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia IUBMB. Life. 2008;60:609–619. doi: 10.1002/iub.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nature neuroscience. 2006;9:1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- 56.Wersinger E, Schwab Y, Sahel JA, Rendon A, Pow DV, Picaud S, Roux MJ. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. The Journal of physiology. 2006;577:221–234. doi: 10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolner B, Poolman B, Wallace B, Konings WN. Revised nucleotide sequence of the gltP gene, which encodes the proton-glutamate-aspartate transport protein of Escherichia coli K-12. J Bacteriol. 1992;174:2391–2393. doi: 10.1128/jb.174.7.2391-2393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grunewald M, Kanner B. Conformational changes monitored on the glutamate transporter GLT-1 indicate the existence of two neurotransmitter-bound states. J Biol Chem. 1995;270:17017–17024. doi: 10.1074/jbc.270.28.17017. [DOI] [PubMed] [Google Scholar]
- 59.Slotboom DJ, Lolkema JS, Konings WN. Membrane topology of the C-terminal half of the neuronal, glial, and bacterial glutamate transporter family. J Biol Chem. 1996;271:31317–31321. doi: 10.1074/jbc.271.49.31317. [DOI] [PubMed] [Google Scholar]
- 60.Wahle S, Stoffel W. Membrane topology of the high-affinity L-glutamate transporter (GLAST-1) of the central nervous system. J Cell Biol. 1996;135:1867–1877. doi: 10.1083/jcb.135.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akabas MH, Karlin A. Identification of acetylcholine receptor channellining residues in the M1 segment of the alpha-subunit. Biochemistry. 1995;34:12496–12500. doi: 10.1021/bi00039a002. [DOI] [PubMed] [Google Scholar]
- 62.Javitch JA, Fu D, Chen J, Karlin A. Mapping the binding-site crevice of the dopamine D2 receptor by the substituted-cysteine accessibility method. Neuron. 1995;14:825–831. doi: 10.1016/0896-6273(95)90226-0. [DOI] [PubMed] [Google Scholar]
- 63.Wilson G, Karlin A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci U S A. 2001;98:1241–1248. doi: 10.1073/pnas.031567798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grunewald M, Bendahan A, Kanner BI. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron. 1998;21:623–632. doi: 10.1016/s0896-6273(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 65.Seal RP, Amara SG. A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron. 1998;21:1487–1498. doi: 10.1016/s0896-6273(00)80666-2. [DOI] [PubMed] [Google Scholar]
- 66.Zarbiv R, Grunewald M, Kavanaugh MP, Kanner BI. Cysteine scanning of the surroundings of an alkali-ion binding site of the glutamate transporter GLT-1 reveals a conformationally sensitive residue. The Journal of biological chemistry. 1998;273:14231–14237. doi: 10.1074/jbc.273.23.14231. [DOI] [PubMed] [Google Scholar]
- 67.Seal RP, Leighton BH, Amara SG. A model for the topology of excitatory amino acid transporters determined by the extracellular accessibility of substituted cysteines. Neuron. 2000;25:695–706. doi: 10.1016/s0896-6273(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 68.Grunewald M, Kanner BI. The accessibility of a novel reentrant loop of the glutamate transporter GLT-1 is restricted by its substrate. J Biol Chem. 2000;275:9684–9689. doi: 10.1074/jbc.275.13.9684. [DOI] [PubMed] [Google Scholar]
- 69.Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- 70.Yernool D, Boudker O, Folta-Stogniew E, Gouaux E. Trimeric subunit stoichiometry of the glutamate transporters from Bacillus caldotenax and Bacillus stearothermophilus. Biochemistry. 2003;42:12981–12988. doi: 10.1021/bi030161q. [DOI] [PubMed] [Google Scholar]
- 71.Eskandari S, Kreman M, Kavanaugh MP, Wright EM, Zampighi GA. Pentameric assembly of a neuronal glutamate transporter. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8641–8646. doi: 10.1073/pnas.97.15.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 73.Tao Z, Grewer C. Cooperation of the conserved aspartate 439 and bound amino acid substrate is important for high-affinity Na+ binding to the glutamate transporter EAAC1. J Gen Physiol. 2007;129:331–344. doi: 10.1085/jgp.200609678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teichman S, Qu S, Kanner BI. Conserved asparagine residue located in binding pocket controls cation selectivity and substrate interactions in neuronal glutamate transporter. J Biol Chem. 2012;287:17198–17205. doi: 10.1074/jbc.M112.355040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosental N, Gameiro A, Grewer C, Kanner BI. A conserved aspartate residue located at the extracellular end of the binding pocket controls cation interactions in brain glutamate transporters. The Journal of biological chemistry. 2011;286:41381–41390. doi: 10.1074/jbc.M111.291021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teichman S, Kanner BI. Aspartate-444 is essential for productive substrate interactions in a neuronal glutamate transporter. J Gen Physiol. 2007;129:527–539. doi: 10.1085/jgp.200609707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scopelliti AJ, Ryan RM, Vandenberg RJ. Molecular determinants for functional differences between alanine-serine-cysteine transporter 1 and other glutamate transporter family members. J Biol Chem. 2013;288:8250–8257. doi: 10.1074/jbc.M112.441022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bastug T, Heinzelmann G, Kuyucak S, Salim M, Vandenberg RJ, Ryan RM. Position of the third Na+ site in the aspartate transporter GltPh and the human glutamate transporter, EAAT1. PLoS One. 2012;7:e33058. doi: 10.1371/journal.pone.0033058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tao Z, Rosental N, Kanner BI, Gameiro A, Mwaura J, Grewer C. Mechanism of cation binding to the glutamate transporter EAAC1 probed with mutation of the conserved amino acid residue Thr101. The Journal of biological chemistry. 2010;285:17725–17733. doi: 10.1074/jbc.M110.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosental N, Kanner BI. A conserved methionine residue controls the substrate selectivity of a neuronal glutamate transporter. The Journal of biological chemistry. 2010;285:21241–21248. doi: 10.1074/jbc.M109.087163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsson HP, Wang X, Lev B, Baconguis I, Caplan DA, Vyleta NP, Koch HP, Diez-Sampedro A, Noskov SY. Evidence for a third sodium-binding site in glutamate transporters suggests an ion/substrate coupling model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13912–13917. doi: 10.1073/pnas.1006289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 83.Reyes N, Ginter C, Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462:880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leary GP, Holley DC, Stone EF, Lyda BR, Kalachev LV, Kavanaugh MP. The central cavity in trimeric glutamate transporters restricts ligand diffusion. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14980–14985. doi: 10.1073/pnas.1108785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 87.Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 88.Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 89.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- 93.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Otis TS, Kavanaugh MP, Jahr CE. Postsynaptic glutamate transport at the climbing fiber-Purkinje cell synapse. Science. 1997;277:1515–1518. doi: 10.1126/science.277.5331.1515. [DOI] [PubMed] [Google Scholar]
- 96.Tsai MC, Tanaka K, Overstreet-Wadiche L, Wadiche JI. Neuronal glutamate transporters regulate glial excitatory transmission. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:1528–1535. doi: 10.1523/JNEUROSCI.5232-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE. Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci. 2004;24:103–111. doi: 10.1523/JNEUROSCI.4473-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 100.Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- 101.Coddington LT, Rudolph S, Vande Lune P, Overstreet-Wadiche L, Wadiche JI. Spillover-mediated feedforward inhibition functionally segregates interneuron activity. Neuron. 2013;78:1050–1062. doi: 10.1016/j.neuron.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kauer JA, Malenka RC, Nicoll RA. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988;334:250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- 103.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 104.Nicoll RA, Oliet SH, Malenka RC. NMDA receptor-dependent and metabotropic glutamate receptor-dependent forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neurobiol Learn Mem. 1998;70:62–72. doi: 10.1006/nlme.1998.3838. [DOI] [PubMed] [Google Scholar]
- 105.Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992;355:50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- 106.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 107.Madison DV, Malenka RC, Nicoll RA. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- 108.Xiao MY, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 109.Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selkirk JV, Naeve GS, Foster AC. Blockade of excitatory amino acid transporters in the rat hippocampus results in enhanced activation of group I and group III metabotropic glutamate receptors. Neuropharmacology. 2003;45:885–894. doi: 10.1016/s0028-3908(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 111.Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 112.Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochemistry international. 2004;45:537–544. doi: 10.1016/j.neuint.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 113.Dzubay JA, Otis TS. Climbing fiber activation of metabotropic glutamate receptors on cerebellar purkinje neurons. Neuron. 2002;36:1159–1167. doi: 10.1016/s0896-6273(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 114.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- 115.Shen Y, Linden DJ. Long-term potentiation of neuronal glutamate transporters. Neuron. 2005;46:715–722. doi: 10.1016/j.neuron.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 116.Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 118.Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]