Spondyloarthritis (SpA) represents a group of immune-mediated inflammatory diseases that exhibit overlapping clinical features, genetic predisposition, and pathogenic mechanisms (1), and affect 0.5–1.5% of the population (2). Disease can be undifferentiated, or manifest as reactive arthritis, psoriatic arthritis (PsA), arthritis associated with inflammatory bowel disease (IBD), or ankylosing spondylitis (AS). Enthesitis is an important pathologic feature of SpA, and contributes to both axial and peripheral arthritis, although synovitis also occurs. Structural damage in AS is dominated by new bone formation that can result in spinal fusion and marked functional limitation. Trabecular bone loss is also prominent, and paradoxically, occurs in close proximity to vertebral osteoproliferation. It can take years from the onset of symptoms to fulfill modified New York criteria for AS. Consequently, newer classification systems have been developed to identify individuals with early axial SpA (3,4) who may benefit from aggressive treatment.
Tumor necrosis factor inhibitors (TNFi) have had a major impact on the treatment of SpA (5). Their ability to reduce new bone formation in AS continues to be debated (6), although recent results are encouraging (7). TNFi are not beneficial in all patients, and thus there is a need for greater understanding of pathogenic mechanisms and translation of this knowledge into more effective therapies. Over the last decade our knowledge of the role of IL-23 and IL-17 cytokine pathways in immunity and immune-mediated inflammatory diseases, from multiple sclerosis (MS) to IBD, arthritis, and psoriasis has grown exponentially (8–11). For SpA, there has been a striking convergence of evidence from genetic studies (12–15), animal models (16–19), translational studies (20–23), and now a therapeutic trial (24) firmly implicating the IL-23/IL-17 axis in pathogenesis (25). In this review we outline key evidence accumulated over the last several years that forms the basis for this conclusion, and provides a background on which to build and refine models of pathogenesis, from inflammation to dysregulated bone formation. These developments, together with the availability of biologics and small molecules that target the IL-23/IL-17 axis, provide an unprecedented opportunity for advances in the treatment of SpA.
The IL-23/IL-17 Axis
Th17 T Cells
The view of CD4+ T cell mediated immunity as a balance between distinct lineages of T helper 1 (Th1) and Th2 cells changed dramatically with the discovery of ‘Th17’ T cells (26,27). The Th1/Th2 paradigm, which placed IFN-γ-producing Th1 cells at the center of many autoimmune diseases, began to erode as it became clear in animal models of MS that IFN-γ-deficient mice were more susceptible to disease (28). IL-12, a key cytokine driving Th1 development, shares a common subunit (IL-12p40) with IL-23. In the MS model, eliminating the specific IL-12p35 protein failed to prevent disease, whereas IL-23p19 was critical for disease progression (29). IL-23 is now recognized to be essential for the proliferation and terminal differentiation of CD4+ Th17 T cells, maintaining IL-17 production, and ultimately driving the pathogenicity of these cells in multiple autoimmune models (11,30).
Of the six highly conserved IL-17 family members (IL-17A-F), IL-17A and IL-17F are secreted by Th17 T cells and represent the principal players studied to date (reviewed in (10)). IL-17 (IL-17A) has a host of biological effects, including induction of IL-6, IL-8, TNF, chemokines, matrix metalloproteinases, and RANK ligand in a variety of target cells from fibroblasts to epithelial and endothelial cells, macrophages, dendritic cells (DCs), chondrocytes, and osteoblasts. Through these actions IL-17 serves a protective role in mucosal immunity to bacteria and fungi, but also promotes inflammation and bone and cartilage destruction when expressed chronically and in inappropriate locations. In addition to IL-17, Th17 T cells produce other pro-inflammatory cytokines including IL-6, TNF, IL-22, and IL-21 (10). There is also heterogeneity and plasticity amongst Th17 T cells (31). For example, Th17 cells derived ex vivo begin to express IFN-γ in vivo after adoptive transfer (32), and double positive (IL-17+/IFN-γ+) Th17 T cells can be found in vivo (33,34) and have been implicated in inflammatory disease (35). Indeed, an emerging view is that local cytokines and conditions influence the precise T cell phenotype (36,37).
Innate IL-17-Producing Cells
Most of the IL-17 produced in vivo comes from innate immune cells, rather than Th17 T cells (38,39). Although the list may be incomplete, IL-17 producers include γδ T cells, certain natural killer cells (NKp46+), invariant NKT (iNKT) cells, intestinal Paneth cells, mast cells, lymphoid tissue inducer cells, and other myeloid cells including neutrophils (reviewed in (9)). Most of these cell types are activated by IL-23, which can be produced in large amounts by DCs and macrophages exposed to microbial products. Innate T cells such as γδ T and iNKT cells, are particularly potent producers of IL-17 when activated by IL-1 and IL-23 independent of T cell receptor engagement by MHC molecules (39). Innate IL-17-producing cells are found in the skin, gut mucosal tissues, and lung, and are considered important sentinels for the immune system. They play a role in preserving barrier function through regulation of antimicrobial peptides, and epithelial integrity.
Genetic Susceptibility
The relationship between HLA-B27 and AS, first recognized 40 years ago, is one of the strongest associations of a single gene variant in a complex genetic disease. The search for additional susceptibility genes based on candidates or family-based linkage studies was largely uninformative. However, major advances in genomic methods and information technology have enabled focused and genome-wide association studies (GWAS) that have yielded remarkable results (12–14,40–42). Forty-three genes or genetic regions have now been associated with AS susceptibility (reviewed in (43,44)). In many instances, due to extended haplotypes, the associated gene has not been definitively identified. Where there is more certainty, the sequence variant(s) responsible for risk/protection may not be known, and there may be rare as yet unrecognized sequence variants that affect expression and/or function of the gene product. Nevertheless, GWAS have proven invaluable in identifying immune pathways deserving further study. A comprehensive discussion of these regions and their potential role in pathogenesis is beyond the scope of this review. Rather, we will focus on several findings with relevance to the IL-23/IL-17 axis.
One of the earliest discoveries that implicated IL-23 signaling in AS was an association with variants in the gene encoding one subunit of the IL-23 receptor (IL23R) (12), a gene previously associated with IBD (45) and psoriasis (46). This finding implicated IL23R as a common susceptibility factor in diseases considered part of the ‘seronegative’ SpA spectrum. Interestingly, the minor allele of IL23R associated with protection changes Arg at amino acid 381 to Gln (R381Q) and impairs IL-23-induced Th17 responses (47). While the association was confirmed in subsequent studies of individuals of European descent (13), the IL23R association was not found in Korean (48) or Chinese populations (41,49). However, a recent study identified several rare IL23R variants in a Chinese population, including a non-synonymous SNP predicted to reduce function that is associated with protection from AS (50).
Subsequent work has revealed associations between AS and several additional genes whose products may directly or indirectly influence the production of, and/or response to IL-23, including the development of Th17 T cells (14,40,42,51) (Figure 1). These genes include CARD9, IL12B, IL6R, IL27, TYK2, and STAT3. CARD9 can influence the production of IL-23 as well as other cytokines. It is an adaptor protein involved in transducing signals from extracellular microbial products from bacteria and fungi that interact with cell surface Toll-like receptors or the C-type lectin receptor Dectin-1 (CLEC7A), and intracellular microbial products through NOD2 (52). Dectin-1 signaling results in DC maturation and production of IL-6, IL-1β, and IL-23, which promote Th17 differentiation from CD4+ T cells (53). Dectin-1 and CARD9 also mediate TNF-α production from macrophages that have been primed with IFN-γ (54). IL12B encodes IL-12p40, the common subunit of IL-23 and IL-12. IL-6 binds to the IL-6 receptor (encoded by IL6R), which in turn binds to gp130 and triggers downstream IL-6 signaling. TYK2 encodes a protein kinase that associates with the cytoplasmic domain of several cytokine receptors including gp130, which is a common component of IL-6 and IL-27 receptors, the receptor for type I interferons (IFN-α/β), and IL-12Rβ1, the common chain of heterodimeric receptors for IL-12 and IL-23 (55). IL-27 is a counter-regulatory cytokine that represses the development of Th1, Th2, and Th17 T cells (56,57). IL-27 promotes secretion of IL-10, which is important in regulating the balance between protective and pathogenic T cell responses (57). STAT3 is a transcription factor that mediates signaling through a variety of receptors (55), and is critical for many aspects of the immune response, including Th17 development (58). Thus, while these gene products are be involved in multiple immunological pathways, the IL-23/IL-17 axis is one common denominator.
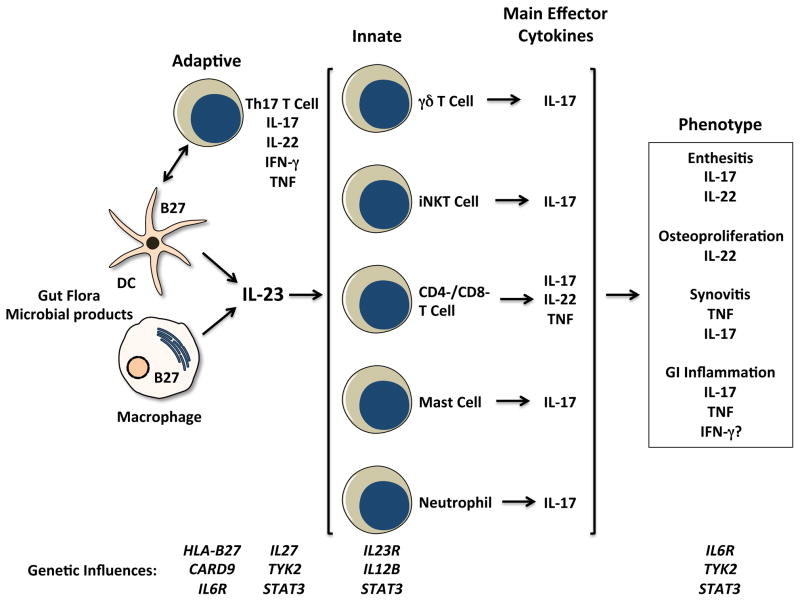
Figure 1.
Schematic depicting cells, effector cytokines, and possible contributions to spondyloarthritis (SpA) pathogenesis. Dendritic cells (DC) and macrophages are major sources of IL-23, which is produced in response to microbes and their products signaling through pattern recognition receptors. Consequences of HLA-B27 misfolding and ER stress can augment IL-23 and IL-1α production, and CARD9 variants could affect production of this cytokine as well. Cells of the adaptive and innate immune response are implicated in SpA pathogenesis. CD4+ Th17 T cell expansion has been reported, KIR3DL2+ cells may be triggered by surface HLA-B27 dimers to produce IL-17. Th17 development and survival may be influenced by variants of the IL-23 and IL-6 receptors (IL23R, IL6R), as well as IL-27 and TYK2- and STAT3-mediated cytokine responses. Tissue inflammation in SpA involves innate immune IL-17-expressing mast cells (synovium), neutrophils (facet joints), and CD3+CD4-CD8- entheseal-resident T cells. The latter produce IL-22, which can drive osteoproliferation in rodent models. Variants in IL6R, TYK2, and STAT3 may affect tissue responses to cytokines.
Despite the large number of genetic susceptibility regions identified, current estimates are that only about 29% of AS heritability has been determined. HLA-B27 comprises approximately 25% of heritability, with the additional 42 genes contributing just over 4% (42), and more than 70% remaining to be elucidated. Such scenarios are common in complex genetic diseases, and the nature of what is referred to as ‘missing heritability’ is unclear. Lander and colleagues have suggested that a large proportion could be due to gene-gene interactions that remain to be identified (59). Other possibilities include additional common alleles with small effect sizes that will require even larger studies (>10,000 patients) or relaxed statistical cut-offs for genome-wide significance, and rare variants that remain to be discovered (60). Nevertheless, the recent genetic discoveries provide a rich and fertile ground for generating, testing, and refining hypotheses that will further our understanding of pathogenic mechanisms in SpA.
IL-23/IL-17 Axis Dysregulation in SpA
A number of studies have documented dysregulation of the IL-23/IL-17 axis in human subjects with AS both in peripheral blood and affected tissues.
Peripheral Blood
Some of the earliest evidence for activation of the IL-23/IL-17 axis in SpA came from reports of increased levels of IL-17 in serum and synovial fluid of patients with active AS, undifferentiated SpA, and PsA (61,62). These results have been confirmed and extended to include elevated IL-23, but correlations with disease activity, severity and response to TNF-inhibitors have been variable (61,63–66), and one study reported that serum levels of IL-23 were more strongly correlated with disease activity in RA than SpA (63). Consistent with serum cytokine elevations, others have reported increases in the number or frequency of circulating CD4+ Th17 T cells in AS, PsA and ReA (67–70), and in some cases more IL-17 production when cells are stimulated ex vivo. There is also evidence for a specific increase in killer immunoglobulin receptor (KIR) KIR3DL2+ CD4+ Th17 cells in peripheral blood and synovial fluid of patients with AS (20). While KIR3DL2+ cells represented about 15% of CD4+ T cells in the peripheral blood, they were responsible for 70% of the increase in Th17 seen in patients. KIR3DL2+ Th17 cells have an interesting connection to HLA-B27, which will be discussed below. In contrast to these results, others found no difference in conventional IL-17-expressing CD4+ T cells in the peripheral blood of AS patients (21,71), with one group reporting an increase in γδ T cells expressing the IL-23 receptor (IL-23R+) (71). The γδ T cells secreted IL-17 in response to IL-23 or antigen-independent activation with antibodies against CD3 and CD28. Although these innate lymphoid cells are a quantitatively minor subset of T cells, they are important in mucosal and epithelial tissues such as the gut and the skin where they help bridge innate and adaptive immunity. Interestingly, in contrast to increases in Th17 and γδ T cells in AS, decreases in Th17 and Th1 cells in the peripheral blood of patients with non-radiographic axial SpA have recently been reported (72). These seemingly discordant observations need to be resolved. Variables such as the form of SpA, disease duration, disease activity, and concurrent medications need to be carefully considered in comparing different results.
Since IL-23 is a key driver of Th17 survival and IL-17 expression, an important question is whether its production is dysregulated in SpA. Macrophages derived in vitro from the peripheral blood of AS patients produce more IL-23 in response to LPS than healthy controls (73). In addition, peripheral blood monocyte-derived DCs from AS patients produced more IL-23 than cells from rheumatoid arthritis (RA) patients, but differences between AS patients and healthy controls were not found, suggesting that the RA patient responses were abnormal (74).
Inflamed Tissue
Events occurring at sites of inflammation are equally or perhaps even more important to consider. Several studies have documented increased expression of IL-17 and IL-23 from various cell types. In contrast to the T cell focus in peripheral blood, there is evidence for strong IL-17 expression by innate immune cells, with the source varying by tissue. For example, there is striking IL-17 production by mast cells in peripheral synovial tissue from SpA patients (23), whereas facet joints from AS patients exhibit strong IL-17 expression by myeloperoxidase-positive (MPO+) CD15+ segmented neutrophils, and some mononuclear cells (21). In the former study, 3 of the 4 cohorts with SpA (defined by European Spondyloarthropathy Study Group criteria) had median disease duration of 6–10 years, indicating that evolution to AS had not occurred despite a significant proportion of patients having long-standing disease. Consequently, these patients could differ from individuals with AS.
There is also evidence for increased IL-23 expression in inflamed tissues including the gut and bone marrow. In the gut of SpA patients, IL-23 has been localized to infiltrating monocytes as well as Paneth cells (75). Interestingly, IL-17 was not significantly upregulated in AS, nor were IL-6 or IL-1β, whereas these cytokines were overexpressed in subjects with Crohn’s disease. The reason for the lack of IL-17 upregulation was not clear, but subsequent work revealed increases in IL-22-expressing NKp44+ cells in AS patients (76). These cells and IL-22 are thought to protect from IBD (77–79) leading to the suggestion that they might be responsible for preventing progression from sub-clinical to symptomatic inflammation in AS (76). In spinal facet joints obtained from AS patients undergoing corrective surgery, IL-23 was overexpressed in bone marrow cells compared to facet joints from osteoarthritis patients, and localized predominantly in MPO+ CD15- myeloid precursors and to a lesser extent in CD68+ or CD163+ macrophages (22). IL-12 was also increased, but to a much lesser extent. Interestingly, in fibrous areas within the marrow, IL-23 was more prominent in CD68+ macrophages than MPO+ cells in AS, and both CD68+ and CD163+ macrophages in OA, but overall there were more IL-23+ cells in OA than AS. This result was surprising, but could support an association between IL-23 and excess bone formation in both diseases.
Genotype to Immunological Phenotype
One of the great challenges in dissecting pathogenic mechanisms is to link the genetic variants to alterations in immune function and ultimately disease phenotype. Toward this end, a recent study of diverse SpA patients examined several AS-associated single nucleotide polymorphisms (SNPs) in IL-23/IL-17 pathway genes, and differences in peripheral blood CD4+ T cell phenotype as assessed by expression of Th1 and Th17 cytokines, cytokine receptors, and differentiation-regulating transcription factors (80). The protective IL23R variant (R381Q) showed the most robust association with decreased expression of Th17 and Th1 genes, with IL12B and CCR6 (a chemokine receptor on Th17 T cells) SNPs exerting similar but less significant effects. Furthermore, as a ‘combinatorial effect’, SpA patients with greater numbers of IL-23/IL-17 pathway risk SNPs expressed higher levels of Th17 and Th1-associated mRNAs. The coordinate increase in expression of Th1 and Th17 genes was ascribed, at least in part, to close proximity of IL23R and IL12B genes in the human genome. However, the role of dual cytokine producing cells, and even Th1 cells in AS pathogenesis remains unclear. For example, increased IFN-γ from Th1 cells and double positive (IFN-γ+/IL-17+) Th17 cells has been implicated in animal models (described below), but analysis of individual cell types obtained from peripheral blood cells of AS subjects by gene expression microarray and flow cytometry suggest low Th1 (IFN-γ) expression (81,82). More studies investigating the influence of disease-associated gene variants on immune function in human subjects are needed.
Lessons from Animal Models
HLA-B27 and the IL-23/IL-17 Axis
Genetic susceptibility to AS is dominated by an MHC class I molecule recognized by CD8+ cytotoxic T cells as part of the adaptive immune response. For many years the role of HLA-B27 in SpA has been hypothesized to be a consequence of arthritogenic self-peptides targeted by autoreactive CD8+ T cells (83). However, from a series of observations beginning in the 1990s it has become evident that SpA-like disease that develops in HLA-B27 transgenic (HLA-B27-Tg) rats, is mediated by CD4+ rather than CD8+ T cells (84–86). The lack of a requirement for CD8+ T cells was confirmed by knocking out expression of the Cd8a gene, which eliminated CD8αβ T cells but had no significant effect on SpA (87). From these experiments it is clear that classical CD8+ T cell recognition of HLA-B27, and by inference arthritogenic peptides, are not required for rat SpA. Furthermore, in the rat model the mere overexpression of HLA-B27 (and human β2-microglobulin) is sufficient to result in CD4+ Th17 T cell activation and accumulation in the gut (16) and affected joints and draining lymph nodes (17). CD4+ T cells co-expressing IFN-γ and IL-17A in the gut (RA Colbert, unpublished observations), and TNF-α and IL-17A in lymph nodes (17) are also present, along with less prominent Th1 expansion and IFN-γ overexpression (16,85). In addition to CD4+ T cells and HLA-B27, the gut disease in HLA-B27-Tg rats begins shortly after weaning (16) and is dependent on gut flora (88,89) raising the question of whether changes in the gut microbiome are influencing activation of the IL-23/IL-17 axis (90). This is an important area for future investigation.
Activation of the IL-23/IL-17 axis in HLA-B27-Tg rats raises the question of how these seemingly disparate pathways intersect. The answer to this conundrum may be related to unusual properties of HLA-B27, including its tendency to misfold, and to form disulfide-linked dimers on the cell surface (91,92). These topics and their potential links to SpA pathogenesis have been reviewed recently (93,94). Briefly, HLA-B27 misfolding generates ER stress, which can activate pathways such as the unfolded protein response (UPR) (95,96) that restore ER homeostasis or trigger apoptotic cell death (reviewed in (97)). Components of the UPR are required for full expression of cytokines such as IL-6 and TNF-α (98), and when upregulated converge with innate immune signaling pathways to synergistically induce IL-23 and IFN-β (16,99–103), with potential effects on IL-1α as well (104). Concomitant UPR activation and IL-23 overexpression is apparent in myeloid cells isolated from the gut of HLA-B27-Tg rats, suggesting that HLA-B27 misfolding may drive activation of the IL-23/IL-17 axis and gut inflammation in this animal model (16). As noted earlier, IL-23 upregulation in the gut of patients with AS is not associated with overexpression of IL-17 (or other pro-inflammatory cytokines), consistent with the lack of clinical symptoms but also highlighting differences between rodents and humans. Interestingly, unfolded and/or misfolded class I heavy chains are prominent in ileal tissue sections from AS patients, and co-localize with HRD1 (SYVN1) a ubiquitin ligase involved in ER-associated degradation of misfolded MHC class I proteins (105). Autophagy pathways were activated in the AS patient samples, but not the UPR, raising the question of whether the response to HLA-B27 misfolding may be different in human samples (93).
Dimers of HLA-B27 (B27-dimers) have been shown to promote survival of subsets of natural killer (NK) (106) and CD4+ Th17 T cells (20) expressing KIR3DL2. The B27-dimer-KIR3DL2 interaction has been demonstrated using tetramers of B27-dimers as well as HLA-B27-transfected cells overexpressing B27-dimers, and antibodies against KIR3DL2 or HLA-B27 can block the interaction (107). KIR3DL2+ CD4+ Th17 T cells that encounter B27-dimers exhibit greater survival and increased IL-17 secretion (20). Thus, B27-dimers could be an important in vivo stimulus promoting IL-17 production and its inflammatory consequences. KIR3DL2 does not exist in rats, but rodent paired immunoglobulin-like receptors (PIRs) are homologous to KIRs, and have been shown to recognize B27-dimers, and could play a role in rat SpA (108). In addition, DCs from HLA-B27-Tg rats have been reported to promote Th17 development (17,109). Although the mechanism is not clear, impaired synapse formation with naïve T cells (110,111) and enhanced apoptotic loss of tolerogenic CD103+ DCs have been observed (109).
Entheseal-Resident T Cells and the Spondyloarthritis Phenotype
Entheseal inflammation where tendons and ligaments attach to bone is a unifying pathologic characteristic of spondyloarthritis (112). The question of how IL-23 might drive enthesitis and the phenotype associated with AS was recently addressed in mice. A reporter mouse expressing a green fluorescent protein in IL-23R+ cells, revealed a previously unrecognized population of CD3+ entheseal T cells that expressed neither CD4 nor CD8, but were positive for the Th17 transcription factor RORγt (18). The IL-23R+RORγt+CD3+CD4-CD8- T cells were present in the entheses as well as other anatomical sites that can be affected in spondyloarthritis including the aortic root and the uvea, and were present in normal healthy mice (18). When IL-23 was expressed systemically these cells were activated, driving local expression of IL-17A and IL-17F, IL-6, IL-22, and chemokines, resulting in robust entheseal inflammation in both peripheral and axial locations. After 2–3 weeks inflamed entheses exhibited evidence of osteoblast and chondrocyte proliferation, with periosteal osteoid and new bone formation. Osteoproliferative changes were IL-22-dependent, and could be reproduced with systemic IL-22 expression, bringing this cytokine into focus as a potential target to reduce new bone formation in AS. These results have profound implications for our understanding of how the unique AS phenotype can develop, essentially independent of tissue or organ-specific autoreactivity.
While systemic IL-23 clearly can produce a SpA phenotype by driving enthesitis, previous studies had described IL-23-driven hyperplastic and inflamed synovium in mice (113). Although synovitis can result from extension of chronic enthesitis into the synovium, there is evidence from MRI studies of patients with early peripheral arthritis that enthesitis occurs with a similar frequency in SpA as in early RA, and synovitis can be prominent in both (114). It is also worth noting that in IL-23 driven enthesitis in mice, increased expression of TNF-α was noted, but TNF blockade had only a modest benefit, with IL-17A and IL-22 blockade being most effective, particularly when administered in combination (18). In mouse models of TNF overexpression, the primary joint pathology is synovitis rather than enthesitis (115). Thus, the situation in humans is likely to be complex and represent a combination of pathologic processes where TNF, IL-23, IL-17A, and IL-22 all represent viable targets.
Summary and Implications for Treatment
Compelling evidence from genetics, translational research, and animal models implicates the IL-23/IL-17 axis and related cytokines in the pathogenesis of SpA. Predisposing genes including HLA-B27 may contribute to excess innate immune activation and IL-23 production, altered IL-23 responses, and increased production of IL-17 and related cytokines. Recognition of the importance of these pathways has spawned the development of biologics and small molecules that are offering unique opportunities to advance the treatment of several immune-mediated diseases (10,116,117). For example, ustekinumab, which blocks IL-23 and IL-12 by binding to the common p40 subunit, is beneficial for plaque psoriasis (118,119) psoriatic arthritis (120,121), and TNFi-refractory Crohn’s disease (122). However, trials targeting IL-17 have yielded mixed results. While there is clear evidence that IL-17 inhibition is effective in psoriasis (123,124), there was a surprising lack of benefit in Crohn’s disease (125,126). For RA and PsA results are promising but primary endpoints of these studies were not met (127,128). The first trial of IL-17 inhibition in SpA has shown evidence of benefit in active AS after 6 weeks of treatment (24). This is encouraging and consistent with evidence of IL-23/IL-17 axis activation, but larger studies of longer duration are needed (129). There may be several reasons for varying efficacy of IL-17 inhibition in immune-mediated diseases where there is evidence of IL-23/IL-17 dysregulation. The majority of IL-17 production is downstream of IL-23, and there is heterogeneity in the IL-17-producing cells that may underlie differences in pathogenicity (130). In addition, there may be tissue-dependent differences in the effects of cytokines in this axis. For example, IL-22 can be protective in the gut (77,78), but promote bone formation in skeletal tissue (18). While there is considerable optimism that these insights into pathogenesis will translate into better treatments for patients with AS and related diseases, there is also cause to proceed with caution.
Acknowledgments
Financial Support: This work was supported by the Rheumatology Research Foundation (JAS), and the National Institute of Arthritis Musculoskeletal and Skin Diseases (NIAMS) Intramural Research Program, Z01 AR041184 (RAC).
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–37. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 2.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthr Care & Res. 2012;64:905–10. doi: 10.1002/acr.21621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 4.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 5.Sieper J. Developments in therapies for spondyloarthritis. Nat Rev Rheumatol. 2012;8:280–7. doi: 10.1038/nrrheum.2012.40. [DOI] [PubMed] [Google Scholar]
- 6.Maksymowych WP, Elewaut D, Schett G. Motion for debate: the development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum. 2012;64:1713–9. doi: 10.1002/art.34442. [DOI] [PubMed] [Google Scholar]
- 7.Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The Impact of TNF-inhibitors on radiographic progression in Ankylosing Spondylitis. Arthritis Rheum. 2013 doi: 10.1002/art.38070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 9.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 10.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Disc. 2012;11:763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 11.Zuniga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 12.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–7. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–7. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013 doi: 10.1038/ng.2667. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–43. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glatigny S, Fert I, Blaton MA, Lories RJ, Araujo LM, Chiocchia G, et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 2012;64:110–20. doi: 10.1002/art.33321. [DOI] [PubMed] [Google Scholar]
- 18.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 19.Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. beta-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–22. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 20.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–80. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, et al. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. 2011;13:R95. doi: 10.1186/ar3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appel H, Maier R, Bleil J, Hempfing A, Loddenkemper C, Schlichting U, et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 2013;65:1522–9. doi: 10.1002/art.37937. [DOI] [PubMed] [Google Scholar]
- 23.Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64:99–109. doi: 10.1002/art.33396. [DOI] [PubMed] [Google Scholar]
- 24.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61134-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Layh-Schmitt G, Colbert RA. The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr Opin Rheumatol. 2008;20:392–7. doi: 10.1097/BOR.0b013e328303204b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–51. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 29.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 30.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends in Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–87. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 35.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirahara K, Poholek A, Vahedi G, Laurence A, Kanno Y, Milner JD, et al. Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. The Journal of Allergy and Clin Immunol. 2013;131:1276–87. doi: 10.1016/j.jaci.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Danoy P, Pryce K, Hadler J, Bradbury LA, Farrar C, Pointon J, et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Gen. 2010;6:e1001195. doi: 10.1371/journal.pgen.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Z, Bei JX, Shen M, Li Q, Liao Z, Zhang Y, et al. A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat Genet. 2012;44:73–7. doi: 10.1038/ng.1005. [DOI] [PubMed] [Google Scholar]
- 42.Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, et al. International Genetics of Ankylosing Spondylitis C. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013;45:730–8. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reveille JD. Genetics of spondyloarthritis--beyond the MHC. Nature reviews Rheumatology. 2012;8:296–304. doi: 10.1038/nrrheum.2012.41. [DOI] [PubMed] [Google Scholar]
- 44.Robinson PC, Brown MA. The genetics of ankylosing spondylitis and axial spondyloarthritis. Rheum Dis Clin North Am. 2012;38:539–53. doi: 10.1016/j.rdc.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One. 2011;6:e17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung IH, Kim TH, Bang SY, Kim TJ, Lee B, Peddle L, et al. IL-23R polymorphisms in patients with ankylosing spondylitis in Korea. J Rheumatol. 2009;36:1003–5. doi: 10.3899/jrheum.081121. [DOI] [PubMed] [Google Scholar]
- 49.Davidson SI, Wu X, Liu Y, Wei M, Danoy PA, Thomas G, et al. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 2009;60:3263–8. doi: 10.1002/art.24933. [DOI] [PubMed] [Google Scholar]
- 50.Davidson SI, Jiang L, Cortes A, Wu X, Glazov EA, Zheng Y, et al. Brief report: high-throughput sequencing of IL23R reveals a low-frequency, nonsynonymous single-nucleotide polymorphism that is associated with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 2013;65:1747–52. doi: 10.1002/art.37976. [DOI] [PubMed] [Google Scholar]
- 51.Pointon JJ, Harvey D, Karaderi T, Appleton LH, Farrar C, Stone MA, et al. Elucidating the chromosome 9 association with AS; CARD9 is a candidate gene. Genes Immun. 2010;11:490–6. doi: 10.1038/gene.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Micro. 2011;14:392–9. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 53.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 54.Goodridge HS, Shimada T, Wolf AJ, Hsu YM, Becker CA, Lin X, et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182:1146–54. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 57.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 58.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci U S A. 2012;109:1193–8. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Gen. 2013;14:661–73. doi: 10.1038/nrg3502. [DOI] [PubMed] [Google Scholar]
- 61.Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–5. doi: 10.1016/j.jbspin.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Singh R, Aggarwal A, Misra R. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J Rheumatol. 2007;34:2285–90. [PubMed] [Google Scholar]
- 63.Melis L, Vandooren B, Kruithof E, Jacques P, De Vos M, Mielants H, et al. Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann Rheum Dis. 2010;69:618–23. doi: 10.1136/ard.2009.107649. [DOI] [PubMed] [Google Scholar]
- 64.Chen WS, Chang YS, Lin KC, Lai CC, Wang SH, Hsiao KH, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc. 2012;75:303–8. doi: 10.1016/j.jcma.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheum. 2011;30:269–73. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 66.Xueyi L, Lina C, Zhenbiao W, Qing H, Qiang L, Zhu P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-alpha therapy. Journal of Clin Immunol. 2013;33:151–61. doi: 10.1007/s10875-012-9774-0. [DOI] [PubMed] [Google Scholar]
- 67.Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–17. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 68.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 69.Shen H, Goodall JC, Gaston JS. Frequency and phenotype of T helper 17 cells in peripheral blood and synovial fluid of patients with reactive arthritis. J Rheumatol. 2010;37:2096–9. doi: 10.3899/jrheum.100146. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. doi: 10.1371/journal.pone.0031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64:1420–9. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 72.Bautista-Caro MB, Arroyo-Villa I, Castillo-Gallego C, de Miguel E, Peiteado D, Puig-Kroger A, et al. Decreased Th17 and Th1 cells in the peripheral blood of patients with early non-radiographic axial spondyloarthritis: a marker of disease activity in HLA-B27(+) patients. Rheumatology (Oxford) 2013;52:352–62. doi: 10.1093/rheumatology/kes267. [DOI] [PubMed] [Google Scholar]
- 73.Zeng L, Lindstrom MJ, Smith JA. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 2011;63:3807–17. doi: 10.1002/art.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prevosto C, Goodall JC, Hill Gaston JS. Cytokine secretion by pathogen recognition receptor-stimulated dendritic cells in rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2012;39:1918–28. doi: 10.3899/jrheum.120208. [DOI] [PubMed] [Google Scholar]
- 75.Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–65. doi: 10.1002/art.24389. [DOI] [PubMed] [Google Scholar]
- 76.Ciccia F, Accardo-Palumbo A, Alessandro R, Rizzo A, Principe S, Peralta S, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012;64:1869–78. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 77.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–57. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology. 2010;139:882–92. 92 e1–3. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 80.Coffre M, Roumier M, Rybczynska M, Sechet E, Law HK, Gossec L, et al. Combinatorial control of Th17 and Th1 cell functions by genetic variations in genes associated with the interleukin-23 signaling pathway in spondyloarthritis. Arthritis Rheum. 2013;65:1510–21. doi: 10.1002/art.37936. [DOI] [PubMed] [Google Scholar]
- 81.Smith JA, Barnes MD, Hong D, DeLay ML, Inman RD, Colbert RA. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008;58:1640–9. doi: 10.1002/art.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudwaleit M, Siegert S, Yin Z, Eick J, Thiel A, Radbruch A, et al. Low T cell production of TNFα and IFN γ in ankylosing spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis. 2001;60:36–42. doi: 10.1136/ard.60.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamin RJ, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–42. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 84.Breban M, Fernandez-Sueiro JL, Richardson JA, Hadavand RR, Maika SD, Hammer RE, et al. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol. 1996;156:794–803. [PubMed] [Google Scholar]
- 85.Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–23. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 86.May E, Dorris ML, Satumtira N, Iqbal I, Rehman MI, Lightfoot E, et al. CD8ab T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003;170:1099–105. doi: 10.4049/jimmunol.170.2.1099. [DOI] [PubMed] [Google Scholar]
- 87.Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, et al. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009;60:1977–84. doi: 10.1002/art.24599. [DOI] [PubMed] [Google Scholar]
- 88.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, et al. Normal luminal bacteria, especially bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human b2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosenbaum JT, Davey MP. Time for a gut check: evidence for the hypothesis that HLA-B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum. 2011;63:3195–8. doi: 10.1002/art.30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mear JP, Schreiber KL, Munz C, Zhu X, Stevanovic S, Rammensee H-G, et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–70. [PubMed] [Google Scholar]
- 92.Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–8. [PubMed] [Google Scholar]
- 93.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2013 doi: 10.1016/j.molimm.2013.07.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaw J, Hatano H, Kollnberger S. The biochemistry and immunology of non-canonical forms of HLA-B27. Mol Immunol. 2013 doi: 10.1016/j.molimm.2013.05.243. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Turner MJ, Delay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: Implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 2007;56:215–23. doi: 10.1002/art.22295. [DOI] [PubMed] [Google Scholar]
- 96.Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–48. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 97.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 98.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–8. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233:181–202. doi: 10.1111/j.0105-2896.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol. 2008;38:1194–203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–30. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu YP, Zeng L, Tian A, Bomkamp A, Rivera D, Gutman D, et al. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol. 2012;189:4630–9. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Layh-Schmitt G, Yang EY, Kwon G, Colbert RA. HLA-B27 alters the response to TNFalpha and promotes osteoclastogenesis in bone marrow monocytes from HLA-B27 transgenic rats. Arthritis Rheum. 2013 doi: 10.1002/art.38001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 2005;52:3586–95. doi: 10.1002/art.21395. [DOI] [PubMed] [Google Scholar]
- 107.Kollnberger S, Bird LA, Sunm M-Y, Retiere C, Braud VM, McMichael A, et al. Cell surface expression and immune receptor recogntion of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–82. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 108.Kollnberger S, Bird LA, Roddis M, Hacquard-Bouder C, Kubagawa H, Bodmer HC, et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173:1699–710. doi: 10.4049/jimmunol.173.3.1699. [DOI] [PubMed] [Google Scholar]
- 109.Utriainen L, Firmin D, Wright P, Cerovic V, Breban M, McInnes I, et al. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondylarthritis. Arthritis Rheum. 2012;64:3199–209. doi: 10.1002/art.34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hacquard-Bouder C, Chimenti MS, Giquel B, Donnadieu E, Fert I, Schmitt A, et al. Alteration of antigen-independent immunologic synapse formation between dendritic cells from HLA-B27-transgenic rats and CD4+ T cells: selective impairment of costimulatory molecule engagement by mature HLA-B27. Arthritis Rheum. 2007;56:1478–89. doi: 10.1002/art.22572. [DOI] [PubMed] [Google Scholar]
- 111.Dhaenens M, Fert I, Glatigny S, Haerinck S, Poulain C, Donnadieu E, et al. Dendritic cells from spondylarthritis-prone HLA-B27-transgenic rats display altered cytoskeletal dynamics, class II major histocompatibility complex expression, and viability. Arthritis Rheum. 2009;60:2622–32. doi: 10.1002/art.24780. [DOI] [PubMed] [Google Scholar]
- 112.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med and Biol. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 113.Adamopoulos IE, Tessmer M, Chao CC, Adda S, Gorman D, Petro M, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol. 2011;187:951–9. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paramarta JE, van der Leij C, Gofita I, Yeremenko N, van de Sande MG, de Hair MJ, et al. Peripheral joint inflammation in early onset spondyloarthritis is not specifically related to enthesitis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203155. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 115.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. The EMBO J. 1991;10:4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sherlock JP, Cua DJ. Interleukin-23: a promising therapeutic target in seronegative spondyloarthropathy. Curr Opinion Pharmacol. 2013;13:445–8. doi: 10.1016/j.coph.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Wada Y, Cardinale I, Khatcherian A, Chu J, Kantor AB, Gottlieb AB, et al. Apilimod inhibits the production of IL-12 and IL-23 and reduces dendritic cell infiltration in psoriasis. PLoS One. 2012;7:e35069. doi: 10.1371/journal.pone.0035069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–84. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 119.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 120.Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–40. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 121.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 122.Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–28. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 123.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 124.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–9. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 125.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raine T, Kaser A. Seventeen in Crohn’s disease: less prime than we thought? Gut. 2012;61:1653–4. doi: 10.1136/gutjnl-2012-302525. [DOI] [PubMed] [Google Scholar]
- 127.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–9. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 128.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 129.Colbert RA, Ward MM. 17 and 23: prime numbers for ankylosing spondylitis? Lancet. 2013 doi: 10.1016/S0140-6736(13)61913-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–9. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]