Summary
The striking morphology of the Golgi complex has fascinated cell biologists since its discovery over 100 years ago. Yet, despite intense efforts to understand how membrane flow relates to Golgi form and function, this organelle continues to baffle cell biologists and biochemists alike. Fundamental questions regarding Golgi function, while hotly debated, remain unresolved. While Golgi function is historically described from a protein-centric point of view, we now appreciate that conceptual frameworks for how lipid metabolism is integrated with Golgi biogenesis and function are essential for a mechanistic understanding of this fascinating organelle. It is from a lipid-centric perspective that we discuss the larger question of Golgi dynamics and membrane trafficking. We review the growing body of evidence for how lipid metabolism is integrally written into the engineering of the Golgi system, and highlight questions for future study.
The Golgi apparatus is a central station for the sorting and transport of protein and lipids that transit the secretory pathway. This organelle also serves as a biochemical factory where anterograde cargo is subject to serial post-translational modifications before being sorted at the trans-Golgi network (TGN) for delivery to the appropriate destinations. As such, the Golgi system plays a central role in eukaryotic cell biology. At steady-state, Golgi membranes are typically organized in a stack of flattened cisternae with dilated rims [1]. Such an organization has been argued to reflect the logic for ordering the biochemical activities of the system. That is, one simply generates stable compartments in the context of the cisternal arrangement. This steady-state morphology is deceiving, however. The Golgi complex is a dynamic organelle subject to enormous membrane flux in its capacity as an intermediate station between the endoplasmic reticulum (ER) and the distal compartments of the secretory pathway. These fluxes are bidirectional as the Golgi system directs retrograde trafficking pathways for purposes of retrieval and recycling of Golgi and ER components, and receives cargo from the PM and endosomes [1].
The structural plasticity of the Golgi system is evident at multiple levels. In mammalian cells, this organelle disassembles in mitosis, and subsequently reassembles into a functional unit upon completion of cell division [2]. Golgi structural plasticity is also on display when the system is subjected to a variety of perturbations [3]. Disruption of a morphologically proper Golgi system interferes with the modification, sorting and delivery of proteins, and with larger cellular processes such as ciliogenesis, cell polarity, cell migration, stress responses and apoptosis [4–6]. Thus, the forces that shape Golgi morphology exert unexpectedly broad effects on cell physiology. Perhaps reflective of these larger cellular functions, individual Golgi stacks are often laterally inter-connected to form a reticular ribbon positioned in the perinuclear region in proximity to the vertebrate centrosome.
Yet, the Golgi system is resilient. It exhibits remarkable capacities for self-organization which allow it to recover from catastrophic structural derangements. As example, brefeldin A-induced collapse of the Golgi system into the ER is followed by re-formation of a functional organelle upon drug removal [7]. Thus, the steady-state form of the Golgi system portrays an illusion of compartmental stability. The very existence of the organelle is balanced on a knife’s edge of competing forces which create it and those that would consume it. It is the remarkable dynamics of the Golgi system that has, over the past decade, fueled a re-evaluation of the fundamental nature of this organelle, and maturation concepts now supplant stable compartment models as favored mechanisms for Golgi biogenesis and function [8].
Lipid Metabolism and the Golgi System
Initial studies of Golgi membrane trafficking and dynamics were exclusively protein-centric [9, 10]. It is now appreciated that lipid metabolism is integrally written into the fabric of the transport carrier cycle and of Golgi function. Since the first demonstrations to this effect in permeabilized cell systems and in yeast [11–14], we now understand that the interface of lipid metabolism with membrane trafficking is complex. This interface is a major factor in controlling Golgi morphology and dynamics. It also involves a large cast of interesting proteins and enzymes including: lipid transfer proteins [11, 12, 15–18], lipid kinases and phosphatases [19–22], phospholipases D and A2 [23–25], phospholipid acyl-transferases [26, 27], and amino-phospholipid flippases which harness their ATPase activities for topological control of lipid distribution between bilayer leaflets [28–30].
Lipid metabolism interfaces with membrane trafficking in several general ways. First, it helps create platforms for protein recruitment to, and activation at, appropriate sites on membrane surfaces. The reduced dimensionality achieved by recruiting soluble factors to a surface has a powerful concentrating effect that promotes effective biochemistry in systems governed by modest affinities. In these capacities, lipid metabolism plays signaling roles. Second, it facilitates the structural deformations of membranes that accompany vesicle budding, fusion and tubulation. Third, it effects a lateral segregation of molecules, and this partitioning contributes to Golgi function. For example, regulation by lateral segregation is the underlying principle of a rapid partitioning model proposed to account for cargo export kinetics from the Golgi complex [31]. The model is based on a continuous two-phase system; one that can readily be generated by lipid segregation into fluid and relatively less fluid domains. While the continuous two-phase partitioning model is overly simplistic, and some of its basic tenets are at odds with known properties of the Golgi complex [32], the concept illustrates how self-organizing principles linked to lipid metabolism/composition might give rise to complex Golgi functions. Studies suggesting that the trans-membrane domains of resident proteins are matched to the physical properties of the membranes in which these reside, also support partitioning concepts [33]. For the remainder of this review, we organize the discussion from the perspective of classes of lipids and how these molecules modulate Golgi functions.
PtdIns-4-phosphate and TGN Function
Involvements of phosphatidylinositol (PtdIns), and its phosphorylated derivatives (the phosphoinositides), were the first established cases for lipids playing active roles in regulating membrane trafficking [44–48]. PtdIns-4-phosphate (PtdIns-4-P) is an important phosphoinositide in operation of the Golgi system [34]. That biologically sufficient production of PtdIns-4-P is integrated with phosphatidylcholine (PtdCho) metabolism provides a striking demonstration of the cross-talk between lipid metabolism and Golgi secretory function [11, 12, 35, 36]. The issue of cross-talk is discussed below in the context of lipid transfer protein function.
Mammalian Golgi membranes harbor two types of PtdIns 4-OH kinases -- PI4KIIIβ and PI4KIIα. Their respective yeast cognates are Pik1 and Lsb6, and Pik1 is localized to yeast Golgi membranes [37]. The PI4KIIIβ enzymes are best understood and function as heterodimers with a myristoylated Ca2+-binding non-catalytic subunit [38, 39]. These PtdIns 4-OH kinases also engage in direct interactions with the vesicle biogenic machinery as mammalian PI4KIIIβ homes to Golgi membranes by binding the GTP-bound form of Arf1 [40, 41], whereas the yeast ortholog Pik1 targets to Golgi membranes by binding an ARF-GEF [42].
PtdIns 4-OH kinase catalytic activity is clearly important for Golgi function. Acute inactivation of yeast Pik1 kinase activity [21, 22], or evoked degradation of PtdIns-4-P to PtdIns in mammalian Golgi [43], induces trafficking defects. Inactivation of PtdIns-binding proteins (e.g. Sec14), which potentiate PtdIns 4-OH kinase activities by presenting PtdIns to the enzyme for efficient modification, also compromises Golgi membrane trafficking [11, 36]. The lipid kinase activity is not the sole essential property of PtdIns 4-OH kinase with respect to Golgi function, however. The Drosophila PI4KIIIβ binds to a small Rab GTPase (Rab11) in the TGN and executes a scaffolding function independent of its catalytic activity [44].
How does PtdIns-4-P potentiate Golgi secretory functions? First, PtdIns-4-P contributes to the recruitment of peripheral membrane proteins important for transport carrier biogenesis (Figure 1). These include Golgi adaptors for clathrin binding such as AP-1 [45, 46], and Arf1-GTP effectors such as GGA proteins [47, 48], Rabs and Rab-GEFs [49, 50], and the GBF1 Arf-GEF [51]. Oxysterol binding-related proteins interface with PtdIns-4-P signaling [16–18], and other lipid binding/transfer proteins which further remodel Golgi membrane lipid composition, are also PtdIns-4-P effectors (see below).
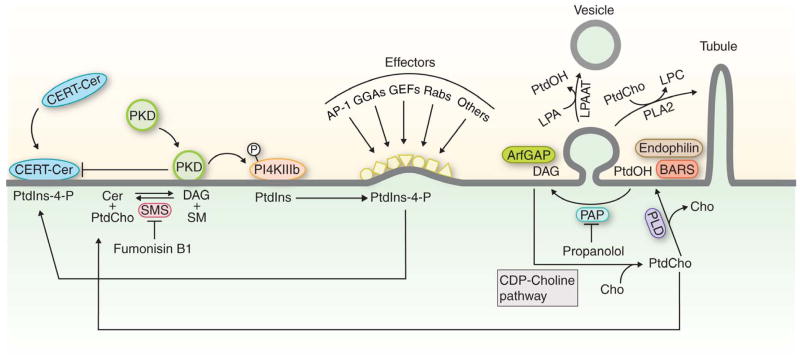
Figure 1. Lipid metabolism and formation of transport carriers.
This diagram highlights existing views of the interface between lipid metabolism and components of the protein machinery which drives formation of vesicles or tubular transport carriers in mammals. No obvious CERT- or PKD activities are present in yeast, and yeast do not exhibit obvious counterparts for BARS, endophilin or LPAATs. The dissonance between this diagram and in vivo readouts for PLD, BARS and CERT function in mammals is discussed in the text. Abbreviations: Cer, ceramide; LPC, lyso-PtdCho; PAP, PtdOH phosphatase; SM, sphingomyelin; SMS, sphingomyelin synthase.
Second, PtdIns-4-P modulates protein activities by direct binding mechanisms. One example is the yeast amino-phospholipid flippase Drs2, a Type-IV integral membrane ATPase, which translocates phosphatidylserine (PtdSer) and phosphatidylethanolamine (PtdEtn) from the lumenal- to the cytosolic-leaflets of TGN/endosomal membranes [28–30]. Drs2 flippase activity is stimulated by binding both to PtdIns-4-P and to an Arf1-GEF [52]. Another example of PtdIns-4-P binding regulating activity of a protein component of the trafficking machinery is described by the coincidence-detection mechanism for yeast Sec2 function. Sec2 is a GEF for the Rab GTPase Sec4. PtdIns-4-P binding quenches the ability of Sec2 to nucleate assembly of a multi-protein complex (the exocyst) required for the interaction of secretory vesicles with the plasma membrane [52, 53]. By discouraging premature Sec2-mediated recruitment of the exocyst to TGN membranes, PtdIns-4-P helps preserve the compartmental distinction between transport intermediates and the Golgi system.
Recent studies of the yeast PtdIns-binding protein Sec14 and PtdIns-4-P binding protein Vps74 indicate that retrograde membrane flow from endosomes to the TGN and retention of glycosyltransferases within the Golgi system is PtdIns-4-P dependent, respectively [54, 55]. PtdIns-4-P binding by Vps74 coordinates the interactions of this protein with the cytosolic tails of glycosyltransferases and with the coatomer complex [55]. Vps74 is an ortholog of the mammalian GOLPH3 which collaborates with the nonconventional myosin MYO18A to control Golgi morphology [55]. In addition, GOLPH3 interacts with the retromer complex which potentiates retrograde membrane trafficking from endosomes [56]. These data suggest GOLPH3 also functions in cargo sorting and retrieval in mammals.
The Yin and Yang of Lipid Transfer Proteins and TGN Functions
The extensive lipid involvement in regulating Golgi function demands close coordination of lipid metabolism with PtdIns-4-P signaling. Lipid transfer proteins are the coupling devices through which this coordination is executed, and PtdIns-transfer proteins (PITPs) provide outstanding examples. The major yeast PITP (Sec14) coordinates PtdIns-4-P function in the trans-Golgi network with the activity of a DAG-consuming pathway for PtdCho biosynthesis [11–13, 57, 58]. The remarkable structural design for how Sec14 differentially binds PtdIns and PtdCho is central to how Sec14 is posited to use heterotypic phospholipid exchange to effect a ‘PtdCho-primed’ PtdIns presentation to PtdIns 4-OH kinases [35, 36]. Such an elaborate presentation mechanism is essential for phosphoinositide homeostasis in vivo because PtdIns 4-OH kinases are biologically inadequate interfacial enzymes when asked to modify membrane-incorporated PtdIns – i.e. the presumed natural mode of presentation. By this view, Sec14-stimulated PtdIns-4-P synthesis is primed in response to PtdCho metabolic cues [35, 36]. Indeed, mammalian disease mutations forecast such presentation functions are general properties of Sec14-like proteins [35, 59]. A physical interaction between Sec14 and PtdIns 4-OH kinases has not been shown, and such an interaction may not be necessary [15, 16]. Details for how a PtdIns-presentation mechanism works remain to be elucidated, and other evidence suggests that some mammalian Sec14-domains involved in vesicle trafficking bind both lipids and proteins [60].
The pro-secretory activities of yeast Sec14 are opposed by Kes1 (Osh4) -- a member of an unrelated class of lipid transfer proteins (the oxysterol binding related proteins -- ORPs) [15, 16]. Kes1 binds PtdIns-4-P, this activity is essential for Kes1 biological activity as a trafficking ‘brake’ [15, 16], and the Kes1/Sec14 antagonism plays itself out in the context of PtdIns-4-P signaling [16, 18, 61]. How this occurs is not clear. Some evidence suggests ORPs stimulate phosphoinositide phosphatases that degrade PtdIns-4-P [e.g. Sac1, see below; [18]. Other data indicate Kes1 competes with pro-secretory factors for PtdIns-4-P binding [62]. Kes1 exhibits two PtdIns-4-P binding sites – one on the protein surface [16], and the second involves the hydrophobic cavity and overlaps the sterol binding site [63]. PtdIns-4-P binding is essential for Kes1 localization to TGN/endosomal membranes. Missense substitutions in either PtdIns-4-P binding site render Kes1 incompetent for targeting to TGN/endosomes [16, 62].
With regard to sterol binding, it is now clear that sterol-binding defects enhance Kes1 biological activity as TGN/endosomal trafficking ‘brake’ [62, 64]. These findings are in direct contradiction to a prominent claim that sterol-binding is required for Kes1 function in vivo [65]. The dual PtdIns-4-P/sterol-binding activities of Kes1 cooperate in a rheostat mechanism where the interplay between sterol- and PtdIns-4-P binding controls the amplitude of the Kes1-imposed PtdIns-4-P clamp on TGN/endosomal trafficking [62] (Figure 2). The discovery that the Kes1 sterol-binding site overlaps with a PtdIns-4-P binding site neatly accounts for how sterol tunes Kes1-mediated inhibition of PtdIns-4-P signaling. When coupled with the demonstration that Kes1 and other ORPs are collectively dispensable for non-vesicular sterol transfer in yeast [66], the data indicate Kes1 is not a sterol transfer protein in vivo; as has been argued [67, 68].
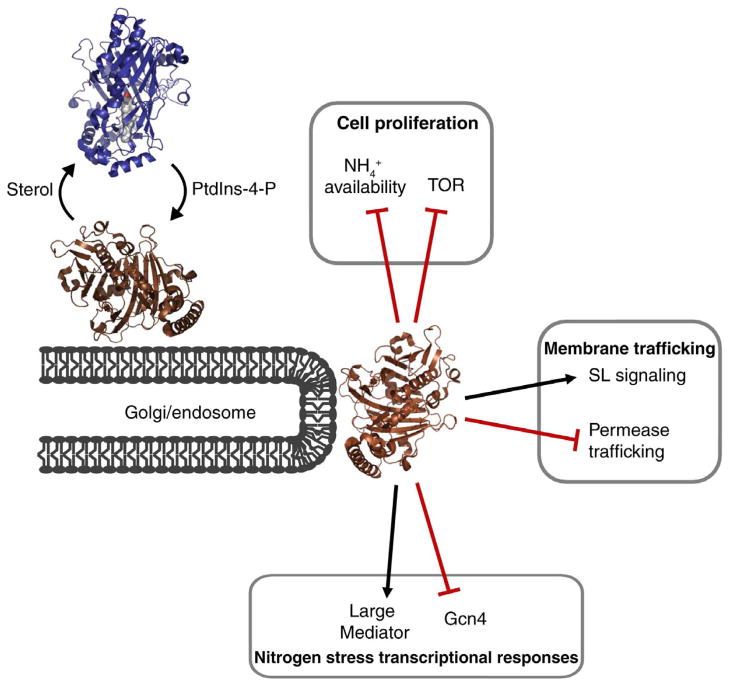
Figure 2. Kes1 integrates PtdIns-4-P signaling, sterols, TGN trafficking control, and larger cellular physiological responses.
Kes1 is recruited to Golgi membranes by virtue of its ability to bind PtdIns-4-P where it clamps availability of this phosphoinositide and functions as a trafficking ‘brake’ [62]. Sterol binding at the TGN releases Kes1 from the membrane, thereby releasing the trafficking brake. Larger consequences of this negative regulation of membrane trafficking include TGN/endosomal sphingolipid metabolism-mediated control of cell proliferation, TOR signaling, and execution of nitrogen stress transcriptional responses.
The Kes1 rheostat has larger physiological involvements as it sets the gain of endosomal sphingolipid signaling, modulates TOR activation by amino acids, regulates nuclear activity of the major transcriptional activator of the general amino acid control pathway, and administrates a coherent exit from proliferative programs to quiescent states (Figure 2). The transcriptional arm of this novel endosome/nuclear axis involves the cyclin-dependent kinase module of the ‘large Mediator’ complex [62]. Whether mammalian PITPs and ORPs play similarly opposing functions is a question for future inquiry. The idea that a PITP/ORP ‘tug-of war’ fine-tunes cell growth regulation and metabolic control as a function of TGN/endosomal trafficking flux has interesting implications for cell entry into post-mitotic fates, and for tissue biogenesis [62].
Metazoan Lipid Transfer Proteins and the Golgi System
The Steroidogenic Acute Regulatory Protein-related Lipid Transfer (StART)-like mammalian PITPs are structurally unrelated to Sec14-like PITPs, and functional depletion of specific isoforms (i.e. PITPβ) is reported to compromise COP1-mediated retrograde Golgi to ER transport [69]. This defect purportedly comes without compromise of anterograde ER to Golgi trafficking -- a curious result given retrograde transport is essential for recycling of v-SNARES for anterograde ER to Golgi transport. With regard to vertebrate PITPβ, zebrafish with strongly reduced PITPβ levels develop normally, although these exhibit defects in outer segment biogenesis and/or maintenance in double cone photoreceptor cells [70]. These data argue against housekeeping roles for PITPβ in retrograde Golgi to ER trafficking, although such functions might be important in specialized contexts that require high-capacity membrane flux.
Other StART-like PITPs are reported to play roles similar to yeast Sec14 in coordinating Golgi PtdCho and DAG metabolism with PtdIns-4-P signaling [17]. Nir2 is a multi-domain PITP reported to collaborate with two other StART-domain lipid transfer proteins, an oxysterol binding protein (OSBP) and a ceramide transfer protein (CERT), in forming a membrane contact site (MCS). The MCS is hypothesized to bridge TGN and ER membranes – a concept that couples TGN activities with those of the ER [71]. For purposes of discussion, the hypothetical MCS is illustrated in Figure 3. Both OSBP and CERT are PtdIns-4-P binding proteins and both are PKD substrates [72]. In this model, CERT supplies the TGN with ceramide via a mechanism where PtdIns-4-P binding mediates CERT interaction with TGN membranes, and CERT phosphorylation by PKD releases CERT from the TGN [73] (Figure 3). Current models propose CERT fuels sphingomyelin synthase-driven DAG production in the Golgi at the expense of ceramide. DAG recruits PKD which then activates PI4KIIIβ [71]. Nir2, a large protein for which the PITP domain accounts for ca. 20% of the polypeptide, is posited to co-assemble into the CERT/OSBP MCS for the purpose of transferring PtdIns from the ER to the Golgi to sustain PI4KIIIβ activity [71]. In sum, this specific MCS model assigns essential roles for CERT, Nir2 and OSBPs in promoting membrane trafficking from the Golgi complex [74].
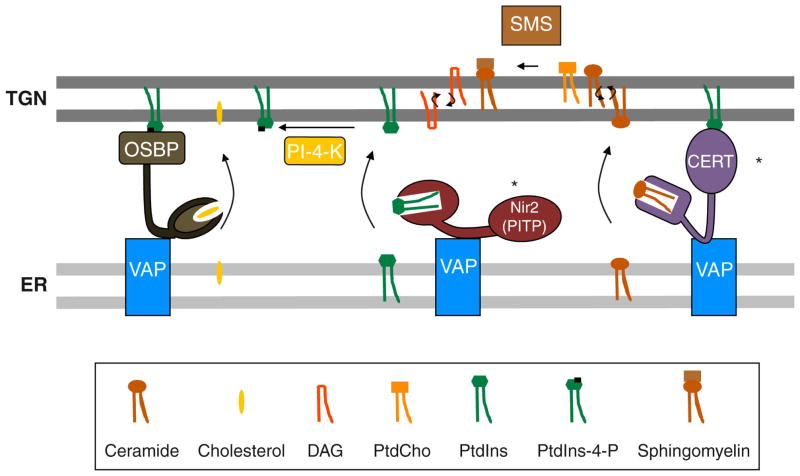
Figure 3. Lipid transfer proteins and a hypothetical membrane contact site.
CERT, OSBP and Nir2 are proposed to co-assemble into a TGN/ER membrane contact site (MCS) where these proteins catalyze ER to TGN trafficking of ceramide, sterol and PtdIns-4-P, respectively. CERT and OSBP interact with the TGN by virtue of their PtdIns-4-P-binding activities. Nir2 is proposed to supply the TGN with PtdIns from the ER for sustained PtdIns-4-P production. The MCS is envisioned to be held together by integral membrane proteins of the ER (the VAPs) which bind the FFAT motifs of CERT, OSBP and Nir2, and the lipid transfer activities are proposed to be essential for membrane trafficking from the Golgi complex. The concepts highlighted by asterisks are not supported by available in vivo data (see text).
CERT and Nir2 gene ablation data fail to support the basic hypotheses, however. CERT-less Drosophila develop normally and reach adulthood. Although cert0/0 flies age prematurely due to oxidative plasma membrane damage, these animals are otherwise remarkably unaffected [75]. By contrast, cert0/0 mice expire at embryonic day 11.5 from failure in cardiac organogenesis. However, cert0/0 embryonic fibroblasts are viable, and these too are prone to accelerated senescence [76]. Thus, the evidence consistently highlights CERT involvements in oxidative stress management. While CERT requirements in trafficking of unusual cargos required for organogenesis remain formal possibilities, genetic data argue against CERT involvements in core Golgi trafficking functions. It is also yet to be demonstrated that CERT bridges ER and Golgi membranes in vivo. This is a critical tenet of the MCS model (Figure 3).
Are Nir2-like PITPs obligatorily required for function of metazoan Golgi -- as proposed [71]? Available evidence does not support this hypothesis either. Drosophila mutants ablated for their single Nir2 ortholog (RdgB) survive through adulthood. Nir2-less flies do suffer a rapid light-accelerated retinal degeneration caused by inability of photoreceptors to terminate the photo-response. This degeneration is cured by expression of the isolated RdgB PITP domain which presumably does not efficiently assemble into an MCS, and the rescue comes with a restored photo-response – even under saturating light conditions [77]. This is an impressive outcome given the enormous phosphoinositide flux demanded by this signaling cascade. Mammals employ Nir2 differently than do flies, however, as evidenced by the demonstration that nir20/0 mice suffer pre-implantation lethality [78]. The terminal nir20/0 phenotype in mice is uncharacterized so it is unknown whether lethality stems from Golgi trafficking defects, or not. There is also no direct evidence to indicate Nir2 functions in a PtdIns-supply capacity – even though this is the common interpretation. Whether StART-like PITPs operate in cells as PtdIns-presenting scaffolds, or as PtdIns carriers, is difficult to determine in vertebrate systems. These two modes of action differ in fundamental respects and are discussed from alternative points of view [71, 79]. However, vertebrate StART-like PITPs resemble their yeast and plant Sec14-like PITP counterparts in having the intrinsic capacity to potentiate PtdIns 4-OH kinase activities under conditions where PtdIns supply requirements are moot [70]. Moreover, the discovery that PtdIns-synthase mobilizes from ER to sites adjacent to plasma membrane and other organelles, to replenish phosphoinositide signaling pools in those membranes, challenges the fundamental assumption for why cells would even require bona fide PtdIns-transfer proteins [80].
Sac1 Phosphoinositide Phosphatases and the Golgi System
Maintenance of phosphoinositide homeostasis requires balanced control over the biosynthetic and the degradative aspects of their metabolism. Phosphoinositide turnover is the domain of lipid phosphatases (e.g. synaptojanins, the oculocerebrorenal Lowe’s Syndrome OCRL protein, PTEN, myotubularins, etc). These enzymes are particularly interesting because of their association with a variety of diseases [81–84]. The mixed specificity phosphoinositide phosphatase Sac1 localizes to ER and Golgi membranes, and is unique amongst the inositol lipid phosphatases in that it is an integral membrane protein [19, 85]. This enzyme is incapable of utilizing phosphoinositides with vicinal phosphate groups as substrates [20]. Sac1 executes larger roles in cell physiology on the basis of its modulation of PtdIns-4-P signaling in both yeast and mammals. Under conditions of extreme nutrient or growth factor insufficiency, the Sac1 PtdIns-4-P phosphatase redistributes from the ER to the Golgi complex [86]. Presumably, regulated trafficking of the phosphatase discourages cell proliferation by clamping activity of the distal secretory pathway (via degradation of Golgi PtdIns-4-P) under suboptimal growth conditions.
Sac1 dysfunction also alleviates the normally essential requirement for PtdIns-transfer protein activity for Golgi secretory function in yeast [19, 85]. This outcome reflects Sac1 constituting the major activity for PtdIns-4-P degradation in yeast [20, 58]. Paradoxically, this enzyme specifically consumes the PtdIns-4-P produced by the plasma membrane-localized Stt4 PtdIns 4-OH kinase, and not the Golgi-localized Pik, in yeast [87, 88]. It is unknown whether this pool specificity translates to mammals. While Sac1-insufficiencies do not evoke large derangements in bulk PtdIns-4-P mass in mammalian cells, these do result in pre-implantation lethality, morphological derangements of the Golgi, defects in mitotic spindle organization, mis-sorting of Golgi glycosyltransferases, and aberrant protein glycosylation [89, 90].
Diacylglycerol and the Transport Carrier Cycle
In addition to PtdIns-4-P, to which we assign primarily a signaling role, a number of other lipids also have key involvements in Golgi functions. Some of these lipids likely play both signaling and structural roles. Diacylglycerol (DAG), a neutral lipid with unusual physical properties, is one of these. The extreme inverted cone shapes assumed by DAG (due to its small headgroup to acyl chain axial area ratio) facilitate adoption of the non-bilayer configurations that lipid molecules assume in strongly deformed membrane regions. Such deformations accompany both vesicle budding and scission [91, 92]. Accordingly, DAG regulates vesicle budding at multiple steps in the exocytic pathway. These include transport from the yeast and mammalian TGN [17, 57, 93–95], and formation of mammalian COP1-vesicles for retrograde trafficking from early Golgi cisternae to the ER [65,66].
In some cases, DAG directly regulates the activity of protein components of the trafficking machinery. For example, DAG potentiates Arf-GTPase activating protein (Arf-GAP) function in both yeast and mammals [93, 95], and DAG exhibits at least two execution points in mammalian COP1-dependent vesicle biogenesis. One is at an early step in formation of buds/tubules when the membrane is first deformed, and another at the scission step where the nascent vesicle is released from its donor membrane [96, 97] (Figure 1). DAG involvement in scission requires ArfGAP1 activity -- suggesting DAG potentiates scission both by activating ArfGAP1 and by facilitating formation of non-bilayer membrane structures which characterize fission intermediates [96]. DAG-activated PKD also displays vesicle scission execution points in the TGN [98, 99]. Although there is as yet no evidence for obligate DAG involvements in early stages of the yeast secretory pathway, roles for DAG in yeast TGN are documented [96].
Compartment-specific requirements imply DAG primarily functions in a signaling capacity. In that regard, DAG recruits protein kinase D (PKD) isoforms to mammalian TGN membranes [94]. PKD activation serves as the nexus of a larger signaling hub which connects DAG metabolism to downstream lipid metabolic events required for optimal membrane trafficking from the TGN [98–100] (Figure 1). This larger hub includes the recruitment of PI4KIIIβ -- which generates PtdIns-4-P in the TGN with the pro-trafficking sequelae detailed above. DAG recruits protein kinases C, Ras guanosine nucleotide release proteins [101–104], and PKCή (which phosphorylates PKD and activates the enzyme) to TGN membranes as well [105].
Phosphatidic Acid Metabolism and Golgi Membrane Trafficking
Pools of DAG generated from phosphatidic acid (PtdOH) by the action of PtdOH phosphatases are required for membrane trafficking through yeast and mammalian Golgi [96, 97]. That PtdOH itself executes pro-secretory functions was suggested by demonstrations that phospholipase D (PLD), an enzyme which hydrolyzes PtdCho to PtdOH and choline, is activated by PtdIns(4,5)P2 and Arf-GTP [23, 25]. Mammals express two PLD isoforms – PLD1 and PLD2 – and numerous studies claim obligatory PLD1 and/or PLD2 involvements in producing PtdOH pools essential for membrane trafficking through the mammalian Golgi system [106, 107]. Biochemical studies suggest PtdOH acts in concert with its binding proteins endophilin and BARS in scission of COP1 vesicles whose formation is Arf-GTP-dependent [108, 109] (Figure 1). In vitro studies suggest endophilin and BARS resolve fission intermediates by physically deforming membranes, and that a PtdOH pool generated by PLD2 is required for execution of these functions [110]. In vivo relevance is suggested by siRNA experiments that show PLD2 is necessary for cis-Golgi maintenance, and for KDEL-receptor retrieval from early Golgi cisternae back to the ER [111]. DAG kinases (enzymes which produce PtdOH from DAG) fail as PLD2 surrogates in this system [111], suggesting direct roles for PLD-generated PtdOH pools in Golgi function. Whether PtdOH serves as a DAG precursor in these contexts is unresolved.
PtdOH Remodeling Enzymes and Golgi Dynamics
Phospholipids are subject to two-stage remodeling reactions that convert one molecular species of a particular phospholipid to another. The first reaction involves removal of the sn-2 fatty acid from the glycerol backbone of a phospholipid by a phospholipase A2 to form a lyso-phospholipid with a single acyl chain. The lyso-phospholipid is a substrate for acyltransferases that incorporate another fatty acid at sn-2 to regenerate the original phospholipid, albeit a different molecular species. That PtdOH remodeling enzymes contribute to Golgi dynamics and trafficking was suggested by reports that endophilin and BARS are lyso-PtdOH acyltransferases [108, 109]. More detailed analyses showed these proteins have no such activity, however [112]. The evidence indicates a phospholipase A2/lysoPtdOH acyltransferase (LPAAT) cycle regulates tubulation events which potentiate membrane trafficking and cargo sorting in mammalian Golgi [24, 26, 27, 113]. Phospholipase A2-induced Golgi tubulations are enhanced by secretory cargo, and these tubules consolidate what would otherwise be individual Golgi stacks into a Golgi ribbon [24, 26, 27, 113]. Connecting tubules are suggested to represent the portals through which anterograde cargo passes as it transits from one Golgi cisterna to the next.
COP1/BARS initiate formation of both tubules and vesicles from mammalian Golgi membranes in vitro [114]. Growing tubules are stabilized by cytosolic phospholipase A2 (cPLA2-α) activity on the one hand, and resolved into vesicles by LPAAT-γ on the other. In these assays, tubules score as non-concentrative cargo carriers, while vesicles score as concentrative carriers -- suggesting that anterograde trafficking is a passive process while retrograde trafficking is an active one [114]. Because formation of both tubules and vesicles is COP1-dependent, this idea offers a resolution to the debate of whether COP1-coated membranes define anterograde- or retrograde-carriers by conceptualizing how COP1 might participate in both pathways [32]. How general cPLA2/LPAAT mechanisms are as a core Golgi trafficking strategy is unclear given yeast and worms do not produce obvious LPAATs. However, yeast exhibit a naturally vesiculated Golgi – a feature that might obviate an LPAAT requirement. Also, some organisms might employ monoacylglycerol-acyltransferases, rather than LPAATs, for vesicle scission.
Genetic Models for Phospholipase D Function
Given the pharmacological and biochemical evidence for PLD-generated PtdOH pools in driving multiple aspects of membrane trafficking, it is surprising that mice nullizygous for either the PLD1 or PLD2 structural genes are developmentally normal [115, 116]. The PLD1 null model does reveal a PLD1 requirement in both starvation-induced expansion of autophagosomes, and for clearance of protein aggregates in brain tissue by macro-autophagy [96]. The enzyme relocalizes from endosomes to the outer membrane of autophagosomes in the face of nutrient stress via a mechanism that requires PtdIns 3-OH kinase activity [96]. PLD2-nullizygous mice, while also overtly normal, present enhanced resistance to the neurotoxic effects of amyloid β-peptide [96]. Perhaps most surprising is that PLD1 and PLD2 activities fail to cross-compensate to any significant degree as pld10/0 pld20/0 double mutant mice do not appear to exhibit enhanced phenotypes relative to the respective single mutants (Gilbert DiPaolo; personal communication).
Does DAG-kinase-mediated conversion of DAG to PtdOH compensate for PLD in nullizygous mice and cells? While an unresolved question, compensation by DAG-kinases would necessarily operate in the absence of the numerous physical interactions reported for PLD isoforms with membrane trafficking components and proteins involved in lipid signaling [96]. Such functional compensation would also be inconsistent with conclusions of in vitro experiments that contend PtdOH pools produced by DAG-kinases cannot substitute for those generated by PLD2 – at least not for COP1 vesicle budding [111]. Why the dissonance? In vitro reconstitutions, their power notwithstanding, are inefficient systems. Consequently, these might exhibit non-physiological dependences on particular lipid metabolic pathways for basic operation (i.e. those that preserve a relative robustness in cell-free preparations) – even when the in vitro system faithfully reconstitutes a specific lipid requirement.
The non-essentiality of PLD for core trafficking functions is also the case in fungi as the single PtdCho-specific yeast PLD is dispensable in vegetative cells [117]. PLD catalytic activity is required for membrane trafficking in certain lipid transfer protein-deficient mutants, however [118]. A physiological role for PLD is on display in the developmental reorientation of membrane trafficking from the TGN to the nuclear envelope during sporulation. PLD produces a PtdOH pool which recruits and activates a sporulation-specific t-SNARE of the Sec9/SNAP-25 family (Spo20). This t-SNARE re-directs post-Golgi trafficking to the forming nuclear envelopes at the expense of the plasma membrane, and PLD defects prevent post-Golgi vesicle fusion with nascent nuclear envelopes [119]. Thus, PtdOH generated by PLD landmarks a developmentally-regulated vesicle fusion process -- not vesicle formation or vesicle scission.
Genetic Models for BARS Function
How congruent are in vivo models with in vitro data for endophilin and BARS function as PtdOH effectors in membrane trafficking? In the case of endophilin there is good agreement as this protein is indeed essential for fission and uncoating of clathrin-coated vesicles in neurons [120–122]. BARS is reported to be essential for fragmentation of the Golgi ribbon in cultured cells, and ribbon scission is required for cells to negotiate the G2/M boundary. Interestingly, only cells with Golgi ribbons exhibit BARS-regulated Golgi mitotic checkpoints [123]. These findings emphasize the link between Golgi structure, lipid metabolism, and cell cycle control.
BARS has a curious history, however, as it was first described as a member of the CtBP protein family of transcriptional co-repressors, and is a spliceoform of CtBP1 [124]. BARS/CtBP null (ctbp10/0) mice exhibit various developmental phenotypes associated with defects in body size, vascularization and body patterning. These phenotypes primarily reflect the transcriptional functions of BARS. The ctbp10/0 phenotypes, and the viability of ctbp10/0 embryonic fibroblasts, are neither consistent with essential roles for BARS in Golgi housekeeping functions, nor with obligate requirements for BARS in progression through the G2/M Golgi checkpoint [124]. It is noted that the Golgi system of ctbp10/0 embryonic fibroblasts differs from that of wild-type fibroblasts in that it is not organized as an intact ribbon, and this morphological derangement is argued to relieve ctbp10/0 fibroblasts of a BARS requirement for cell cycle progression [123]. This argument begs the question of how is fragmentation of the Golgi ribbon realized in BARS-less cells? What activity (if any) compensates for BARS in ctbp10/0 cells? Non-neuronal endophilins are candidates, and in vitro data support this notion [125]. But, given the dissonance between in vitro and in vivo readouts, this hypothesis must be tested in a suitable in vivo context.
Amino-phospholipids and Membrane Trafficking
Functional involvements of glycerophospholipids in Golgi secretory function are not limited to PtdIns, phosphonositides, PtdCho, and PtdOH. Roles for amino-phospholipids, such as PtdEtn and PtdSer, in membrane trafficking is amply demonstrated by the important roles amino-phospholipid-flippases (i.e. Drs2) play in controlling membrane trafficking through the yeast TGN/endosomal system [28–30]. These P4-type ATPases translocate PtdSer and PtdEtn from cytosolic- to lumenal- membrane leaflets, and these activities interface with PtdIns-4-P signaling and the Arf pathway as yeast Drs2 flippase activity is stimulated by binding both to PtdIns-4-P and to an Arf1-GEF [52]. Yeast P4-type ATPases are also indirectly subject to regulation by sphingolipids via the Fpk protein kinases that phosphorylate (and activate) the flippases [126].
The complexity of the amino-phospholipid flippase involvement in yeast membrane trafficking is emphasized by the overlapping functional redundancies of multiple Drs2-like flippases [28–30]. A long-standing idea is that amino-phospholipid flippases promote positive membrane curvature (and therefore vesicle budding) by driving local leaflet asymmetries -- both in terms of phospholipid composition and phospholipid distribution between the cytosolic and lumenal TGN/endosomal leaflets [28–30]. While the evidence identifies an interface of Drs2 (and Drs2-like flippases) with Arf and clathrin-dependent functions in yeast [34], it remains to be determined how flippase activities potentiate membrane trafficking. Is removal of PtdEtn/PtdSer from the cytosolic leaflet of functional import? Is enrichment of the lumenal leaflet with PtdEtn/PtdSer the key? Are both outcomes functionally significant?
Trafficking functions for PtdSer disposed on the cytosolic-leaflets of endosomal membranes are also recognized. This amino-phospholipid is required for retrograde membrane trafficking from mammalian recycling endosomes [127]. The primary, and perhaps exclusive, PtdSer effector in this system is evectin-2. This protein harbors a pleckstrin homology (PH) domain that displays an exquisite specificity for PtdSer, and does not bind phosphoinositides. PtdSer binding is required for evectin-2 localization to recycling endosomes and for protein function in cells [127]. How evectin-2 regulates trafficking remains to be elucidated.
Sterols and the Golgi Complex
Membrane sterol content increases progressively through the compartments of the secretory pathway, and this gradient facilitates membrane protein sorting [128]. Sterols organize plasma membrane microdomains that modulate endocytosis and receptor activation and regulate membrane trafficking from the TGN. Biosynthetic trafficking of a subset of yeast plasma membrane proteins is disrupted by defects in sterol biosynthesis [129–131]. A common property of the affected cargos is their incorporation into ergosterol-containing detergent resistant membranes [132]. Interestingly, compromise of late steps in sterol biosynthesis results in missorting of these cargos– even though bulk sterol levels are unchanged under those conditions. The chemical profile of the accumulated sterols is altered, however [133]. These accumulated sterols, while chemically distinct from ergosterol, support formation of detergent resistant membrane microdomains. Yet, integral membrane proteins destined for the plasma membrane are missorted; indicating subtle differences in sterol structure influence trafficking fidelity.
Budding of anterograde vesicles from the TGN is proposed to be driven by the phase separation of sterol and sphingolipids into microdomains where the immiscibility of two liquid phases in lipid bilayers promotes the membrane bending necessary for this process [134]. Indeed, sterols and sphingolipids are enriched in TGN-derived vesicles relative to the bulk composition of the donor organelle [132, 135]. The data suggest that a single lipid-driven sorting process drives biogenesis of TGN-derived vesicles bound for the plasma membrane [132]. This mechanism diverges from that which governs COP1 vesicle budding from bulk Golgi membranes in vitro. Those vesicles exhibit reduced sphingomyelin and cholesterol content relative to the bulk Golgi membranes from which these were formed [136]. Yet, sphingomyelin discharges an important role as cofactor in COP1 vesicle formation. Brügger, Wieland and colleagues report the remarkable discovery that a single molecule of a specific sphingomyelin molecular species binds the transmembrane domain of a COP1 coat subunit (p24), modulates the oligomeric state of p24, and thereby regulates COP1 coat biogenesis [137].
Glycolipid Transfer Proteins
Glycolipid transfer proteins (GLTPs) bind both sphingoid- and glycerol-based glycolipids and mobilize these lipids between membrane bilayers in vitro [138]. The glucosylceramide (GlcCer) transfer protein FAPP2 is recruited to Golgi membranes in a PtdIns-4-P-dependent manner, and is required for production of complex glycosphingolipids for which GlcCer is a precursor. Although FAPP2 is suggested to deliver GlcCer to distal Golgi compartments as a lipid carrier [139], others report FAPP2 promotes retrograde transport of GlcCer from Golgi to the ER [140]. The rationale for the retrograde pathway is that newly synthesized GlcCer, which resides in the cytosolic leaflet of Golgi membranes, is mobilized to ER for the purpose of being flipped into the lumenal ER leaflet (Figure 3). Vesicular trafficking from ER to the Golgi subsequently introduces the lumenally-disposed GlcCer to Golgi-localized glycosyltransferases for maturation into complex glycosphingolipids [140]. A GlcCer-independent role for FAPP2 in TGN trafficking is suggested by FAPP2 forming a curved dimer that tubulates membranes in a PtdIns-4-P-dependent manner [141]. These studies describe a mechanism for how FAPP2 potentiates cargo transport from the TGN to apical surfaces of polarized epithelial cells [142].
Concluding Thoughts
Much progress has been made in understanding the mechanisms that control Golgi dynamics and architecture since discovery of this organelle more than 100 years ago. Lipids, lipid-binding proteins, and lipid metabolism are major contributors to plasticity of the Golgi system. However, we have only a rudimentary understanding of the cross-talk between different arms of the Golgi lipid metabolome. ‘Systems’ approaches to model the landscape of Golgi lipid metabolism will be necessary for detailed description of cross-talk mechanisms. These ‘systems’ approaches also hold the ultimate promise of unifying lipid biochemical principles with function of the organelle.
It still remains unclear why the Golgi adopts its characteristic morphology given secretory activity can be insensitive to dramatic structural derangements of this organelle. The answer must lie in unappreciated levels of physiological regulation associated with how the Golgi is organized, or with the maturation process itself. Insights to this effect are offered by tunable PITP/ORP rheostats as these suggest mechanisms for integrating TGN/endosomal maturation (and lipid signaling) with control of cell proliferation and nuclear responses to stress [62]. These circuits speak to an unappreciated physiological plasticity of Golgi/endosomal maturation programs, and involvements of such rheostats in modulating Golgi plasticity. Such circuits seem ideally suited for chaperoning cell entry into post-mitotic states, or in maintaining post-mitotic cell physiology. Perhaps maturation mechanisms for membrane trafficking evolved, in part, because these afford superior instruments for fine-tuning of cell growth regulation and metabolic control than do stable compartment mechanisms. In this regard, the fidelity of mitotic spindle formation and function is also influenced by Golgi organization, and evidence is building that lipid metabolism has its hand in this circuit as well [92,98,141,142]. We anticipate that studies of Golgi lipid metabolism, in the developmental context of multicellular organisms, will prove a major contributor to the future of Golgi research. The fruits of those studies will undoubtedly yield more surprises from an organelle that has already produced its share.
Acknowledgments
This work was supported by NIH grant GM44530 to VAB. We are grateful to Gilbert Di Paolo for granting us permission to cite unpublished data. We also thank Lora L. Yanagisawa (Univ. Alabama-Birmingham) and three referees for careful review of the work and for their many critical comments. Their input greatly improved the manuscript.
Footnotes
The authors declare no financial conflict.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang D, Mar K, Warren G, Wang Y. Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay. J Biol Chem. 2008;283:6085–6094. doi: 10.1074/jbc.M707715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisel B, Wang Y, Wei JH, Xiang Y, Tang D, Miron-Mendoza M, Yoshimura S, Nakamura N, Seemann J. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008;4:e1000315. doi: 10.1371/journal.pgen.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol. 2004;16:364–372. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman JE. The protein machinery of vesicle budding and fusion. Protein Sci. 1996;5:185–194. doi: 10.1002/pro.5560050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 11.Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- 12.Cleves A, McGee T, Bankaitis V. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- 13.Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 18.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 21.Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- 22.Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 23.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADPribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 24.de Figueiredo P, Drecktrah D, Polizotto RS, Cole NB, Lippincott-Schwartz J, Brown WJ. Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic. 2000;1:504–511. doi: 10.1034/j.1600-0854.2000.010608.x. [DOI] [PubMed] [Google Scholar]
- 25.Ktistakis NT, Brown HA, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci U S A. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drecktrah D, Chambers K, Racoosin EL, Cluett EB, Gucwa A, Jackson B, Brown WJ. Inhibition of a Golgi complex lysophospholipid acyltransferase induces membrane tubule formation and retrograde trafficking. Mol Biol Cell. 2003;14:3459–3469. doi: 10.1091/mbc.E02-11-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci U S A. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthusamy BP, Natarajan P, Zhou X, Graham TR. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim Biophys Acta. 2009;1791:612–619. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emr S, Glick BS, Linstedt AD, Lippincott-Schwartz J, Luini A, Malhotra V, Marsh BJ, Nakano A, Pfeffer SR, Rabouille C, et al. Journeys through the Golgi--taking stock in a new era. J Cell Biol. 2009;187:449–453. doi: 10.1083/jcb.200909011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpe HJ, Stevens TJ, Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142:158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4- kinases. Trends Cell Biol. 2011;21:113–121. doi: 10.1016/j.tcb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bankaitis VA, Mousley CJ, Schaaf G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem Sci. 2010;35:150–160. doi: 10.1016/j.tibs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaaf G, Ortlund EA, Tyeryar KR, Mousley CJ, Ile KE, Garrett TA, Ren J, Woolls MJ, Raetz CR, Redinbo MR, et al. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- 40.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 41.Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- 42.Gloor Y, Schone M, Habermann B, Ercan E, Beck M, Weselek G, Muller-Reichert T, Walch-Solimena C. Interaction between Sec7p and Pik1p: the first clue for the regulation of a coincidence detection signal. Eur J Cell Biol. 2010;89:575–583. doi: 10.1016/j.ejcb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Szentpetery Z, Varnai P, Balla T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. Proc Natl Acad Sci U S A. 2010;107:8225–8230. doi: 10.1073/pnas.1000157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polevoy G, Wei HC, Wong R, Szentpetery Z, Kim YJ, Goldbach P, Steinbach SK, Balla T, Brill JA. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. J Cell Biol. 2009;187:847–858. doi: 10.1083/jcb.200908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15:540–547. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 47.Demmel L, Gravert M, Ercan E, Habermann B, Muller-Reichert T, Kukhtina V, Haucke V, Baust T, Sohrmann M, Kalaidzidis Y, et al. The clathrin adaptor Gga2p is a phosphatidylinositol 4-phosphate effector at the Golgi exit. Mol Biol Cell. 2008;19:1991–2002. doi: 10.1091/mbc.E06-10-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell. 2007;18:2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Graaf P, Zwart WT, van Dijken RA, Deneka M, Schulz TK, Geijsen N, Coffer PJ, Gadella BM, Verkleij AJ, van der Sluijs P, et al. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell. 2004;15:2038–2047. doi: 10.1091/mbc.E03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumaresq-Doiron K, Savard MF, Akam S, Costantino S, Lefrancois S. The phosphatidylinositol 4-kinase PI4KIIIalpha is required for the recruitment of GBF1 to Golgi membranes. J Cell Sci. 2010;123:2273–2280. doi: 10.1242/jcs.055798. [DOI] [PubMed] [Google Scholar]
- 52.Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- 53.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 54.Mousley CJ, Tyeryar K, Ile KE, Schaaf G, Brost RL, Boone C, Guan X, Wenk MR, Bankaitis VA. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol Biol Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009;187:967–975. doi: 10.1083/jcb.200909063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–1090. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p” and inositol auxotrophy. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nile AH, Bankaitis VA, Grabon A. Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clin Lipidol. 2010;5:867–897. doi: 10.2217/clp.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huynh H, Bottini N, Williams S, Cherepanov V, Musumeci L, Saito K, Bruckner S, Vachon E, Wang X, Kruger J, et al. Control of vesicle fusion by a tyrosine phosphatase. Nat Cell Biol. 2004;6:831–839. doi: 10.1038/ncb1164. [DOI] [PubMed] [Google Scholar]
- 61.Fairn GD, Curwin AJ, Stefan CJ, McMaster CR. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc Natl Acad Sci U S A. 2007;104:15352–15357. doi: 10.1073/pnas.0705571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mousley C, Yuan P, Gaur NA, Trettin KD, Nile AH, Deminoff S, Dewar BJ, Wolpert M, MacDonald JM, Herman PK, et al. A sterol binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell. 2012;148:702–715. doi: 10.1016/j.cell.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol. 2011;195:965–978. doi: 10.1083/jcb.201104062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfaro G, Johansen J, Dighe SA, Duamel G, Kozminski KG, Beh CT. The sterolbinding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic. 2011;12:1521–1536. doi: 10.1111/j.1600-0854.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- 65.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK. Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic. 2011;12:1341–1355. doi: 10.1111/j.1600-0854.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta. 2007;1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carvou N, Holic R, Li M, Futter C, Skippen A, Cockcroft S. Phosphatidylinositol-and phosphatidylcholine-transfer activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the endoplasmic reticulum. J Cell Sci. 2010;123:1262–1273. doi: 10.1242/jcs.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ile KE, Kassen S, Cao C, Vihtehlic T, Shah SD, Mousley CJ, Alb JG, Jr, Huijbregts RP, Stearns GW, Brockerhoff SE, et al. Zebrafish class 1 phosphatidylinositol transfer proteins: PITPbeta and double cone cell outer segment integrity in retina. Traffic. 2010;11:1151–1167. doi: 10.1111/j.1600-0854.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Prinz WA. Lipid trafficking sans vesicles: where, why, how? Cell. 2010;143:870–874. doi: 10.1016/j.cell.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao RP, Yuan C, Allegood JC, Rawat SS, Edwards MB, Wang X, Merrill AH, Jr, Acharya U, Acharya JK. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc Natl Acad Sci U S A. 2007;104:11364–11369. doi: 10.1073/pnas.0705049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Rao RP, Kosakowska-Cholody T, Masood MA, Southon E, Zhang H, Berthet C, Nagashim K, Veenstra TK, Tessarollo L, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milligan SC, Alb JG, Jr, Elagina RB, Bankaitis VA, Hyde DR. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J Cell Biol. 1997;139:351–363. doi: 10.1083/jcb.139.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu C, Peng YW, Shang J, Pawlyk BS, Yu F, Li T. The mammalian retinal degeneration B2 gene is not required for photoreceptor function and survival. Neuroscience. 2001;107:35–41. doi: 10.1016/s0306-4522(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 79.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 80.Kim YJ, Guzman-Hernandez ML, Balla T. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell. 2011;21:813–824. doi: 10.1016/j.devcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blero D, Payrastre B, Schurmans S, Erneux C. Phosphoinositide phosphatases in a network of signalling reactions. Pflugers Arch. 2007;455:31–44. doi: 10.1007/s00424-007-0304-5. [DOI] [PubMed] [Google Scholar]
- 82.Clague MJ, Lorenzo O. The myotubularin family of lipid phosphatases. Traffic. 2005;6:1063–1069. doi: 10.1111/j.1600-0854.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 83.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Bankaitis VA. Phosphoinositide phosphatases in cell biology and disease. Prog Lipid Res. 2010;49:201–217. doi: 10.1016/j.plipres.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol. 1993;122:79–94. doi: 10.1083/jcb.122.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blagoveshchenskaya A, Cheong FY, Rohde HM, Glover G, Knodler A, Nicolson T, Boehmelt G, Mayinger P. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J Cell Biol. 2008;180:803–812. doi: 10.1083/jcb.200708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nemoto Y, Kearns BG, Wenk MR, Chen H, Mori K, Alb JG, Jr, De Camilli P, Bankaitis VA. Functional characterization of a mammalian Sac1 and mutants exhibiting substrate-specific defects in phosphoinositide phosphatase activity. J Biol Chem. 2000;275:34293–34305. doi: 10.1074/jbc.M003923200. [DOI] [PubMed] [Google Scholar]
- 89.Cheong FY, Sharma V, Blagoveshchenskaya A, Oorschot VM, Brankatschk B, Klumperman J, Freeze HH, Mayinger P. Spatial regulation of Golgi phosphatidylinositol-4-phosphate is required for enzyme localization and glycosylation fidelity. Traffic. 2010;11:1180–1190. doi: 10.1111/j.1600-0854.2010.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Boukhelifa M, Tribble E, Morin-Kensicki E, Uetrecht A, Bear JE, Bankaitis VA. The Sac1 phosphoinositide phosphatase regulates Golgi membrane morphology and mitotic spindle organization in mammals. Mol Biol Cell. 2008;19:3080–3096. doi: 10.1091/mbc.E07-12-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burger KN. Greasing membrane fusion and fission machineries. Traffic. 2000;1:605–613. doi: 10.1034/j.1600-0854.2000.010804.x. [DOI] [PubMed] [Google Scholar]
- 92.Chernomordik L, Kozlov MM, Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- 93.Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- 94.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 95.Yanagisawa LL, Marchena J, Xie Z, Li X, Poon PP, Singer RA, Johnston GC, Randazzo PA, Bankaitis VA. Activity of specific lipid-regulated ADP ribosylation factor- GTPase-activating proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell. 2002;13:2193–2206. doi: 10.1091/mbc.01-11-0563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asp L, Kartberg F, Fernandez-Rodriguez J, Smedh M, Elsner M, Laporte F, Barcena M, Jansen KA, Valentijn JA, Koster AJ, et al. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell. 2009;20:780–790. doi: 10.1091/mbc.E08-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernandez-Ulibarri I, Vilella M, Lazaro-Dieguez F, Sarri E, Martinez SE, Jimenez N, Claro E, Merida I, Burger KN, Egea G. Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway. Mol Biol Cell. 2007;18:3250–3263. doi: 10.1091/mbc.E07-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 99.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 100.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caloca MJ, Zugaza JL, Bustelo XR. Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J Biol Chem. 2003;278:33465–33473. doi: 10.1074/jbc.M302807200. [DOI] [PubMed] [Google Scholar]
- 102.Lehel C, Olah Z, Jakab G, Szallasi Z, Petrovics G, Harta G, Blumberg PM, Anderson WB. Protein kinase C epsilon subcellular localization domains and proteolytic degradation sites. A model for protein kinase C conformational changes. J Biol Chem. 1995;270:19651–19658. doi: 10.1074/jbc.270.33.19651. [DOI] [PubMed] [Google Scholar]
- 103.Maissel A, Marom M, Shtutman M, Shahaf G, Livneh E. PKCeta is localized in the Golgi, ER and nuclear envelope and translocates to the nuclear envelope upon PMA activation and serum-starvation: C1b domain and the pseudosubstrate containing fragment target PKCeta to the Golgi and the nuclear envelope. Cell Signal. 2006;18:1127–1139. doi: 10.1016/j.cellsig.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Wang QJ, Bhattacharyya D, Garfield S, Nacro K, Marquez VE, Blumberg PM. Differential localization of protein kinase C delta by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J Biol Chem. 1999;274:37233–37239. doi: 10.1074/jbc.274.52.37233. [DOI] [PubMed] [Google Scholar]
- 105.Diaz Anel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2-and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bi K, Roth MG, Ktistakis NT. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- 107.Roth MG. Molecular mechanisms of PLD function in membrane traffic. Traffic. 2008;9:1233–1239. doi: 10.1111/j.1600-0854.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 108.Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV, Witke W, Huttner WB, Soling HD. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- 109.Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 110.Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang JS, Gad H, Lee SY, Mironov A, Zhang L, Beznoussenko GV, Valente C, Turacchio G, Bonsra AN, Du G, et al. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–678. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- 113.de Figueiredo P, Drecktrah D, Katzenellenbogen JA, Strang M, Brown WJ. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci U S A. 1998;95:8642–8647. doi: 10.1073/pnas.95.15.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang JS, Valente C, Polishchuk RS, Turacchio G, Layre E, Moody DB, Leslie CC, Gelb MH, Brown WJ, Corda D, et al. COPI acts in both vesicular and tubular transport. Nat Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dall’Armi C, Hurtado-Lorenzo A, Tian H, Morel E, Nezu A, Chan RB, Yu WH, Robinson KS, Yeku O, Small SA, et al. The phospholipase D1 pathway modulates macroautophagy. Nat Commun. 2010;1:142. doi: 10.1038/ncomms1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, et al. Phospholipase d2 ablation ameliorates Alzheimer’s disease-linked synaptic dysfunction and cognitive deficits. J Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rose K, Rudge SA, Frohman MA, Morris AJ, Engebrecht J. Phospholipase D signaling is essential for meiosis. Proc Natl Acad Sci U S A. 1995;92:12151–12155. doi: 10.1073/pnas.92.26.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci U S A. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neiman AM, Katz L, Brennwald PJ. Identification of domains required for developmentally regulated SNARE function in Saccharomyces cerevisiae. Genetics. 2000;155:1643–1655. doi: 10.1093/genetics/155.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 121.Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 122.Bai J, Hu Z, Dittman JS, Pym EC, Kaplan JM. Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell. 2010;143:430–441. doi: 10.1016/j.cell.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colanzi A, Hidalgo Carcedo C, Persico A, Cericola C, Turacchio G, Bonazzi M, Luini A, Corda D. The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2. Embo J. 2007;26:2465–2476. doi: 10.1038/sj.emboj.7601686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hildebrand JD, Soriano P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol. 2002;22:5296–5307. doi: 10.1128/MCB.22.15.5296-5307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang JS, Zhang L, Lee SY, Gad H, Luini A, Hsu VW. Key components of the fission machinery are interchangeable. Nat Cell Biol. 2006;8:1376–1382. doi: 10.1038/ncb1503. [DOI] [PubMed] [Google Scholar]
- 126.Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J. A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci U S A. 2010;107:34–39. doi: 10.1073/pnas.0912497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uchida Y, Hasegawa J, Chinnapen D, Inoue T, Okazaki S, Kato R, Wakatsuki S, Misaki R, Koike M, Uchiyama Y, et al. Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci U S A. 2011;108:15846–15851. doi: 10.1073/pnas.1109101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaiser HJ, Orlowski A, Rog T, Nyholm TK, Chai W, Feizi T, Lingwood D, Vattulainen I, Simons K. Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc Natl Acad Sci U S A. 2011;108:16628–16633. doi: 10.1073/pnas.1103742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Umebayashi K, Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol. 2003;161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bagnat M, Chang A, Simons K. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol Biol Cell. 2001;12:4129–4138. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bagnat M, Simons K. Cell surface polarization during yeast mating. Proc Natl Acad Sci U S A. 2002;99:14183–14188. doi: 10.1073/pnas.172517799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K. Generic sorting of raft lipids into secretory vesicles in yeast. Traffic. 2011;12:1139–1147. doi: 10.1111/j.1600-0854.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 133.Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, et al. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc Natl Acad Sci U S A. 2005;102:17981–17986. doi: 10.1073/pnas.0509107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lipowsky R. Domain-induced budding of fluid membranes. Biophys J. 1993;64:1133–1138. doi: 10.1016/S0006-3495(93)81479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brugger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–518. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Contreras FX, Ernst AM, Haberkant P, Bjorkholm P, Lindahl E, Gonen B, Tischer C, Elofsson A, von Heijne G, Thiele C, et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature. 2012;481:525–529. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- 138.Brown RE, Mattjus P. Glycolipid transfer proteins. Biochim Biophys Acta. 2007;1771:746–760. doi: 10.1016/j.bbalip.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 140.Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao X, Coskun U, Rossle M, Buschhorn SB, Grzybek M, Dafforn TR, Lenoir M, Overduin M, Simons K. Golgi protein FAPP2 tubulates membranes. Proc Natl Acad Sci U S A. 2009;106:21121–21125. doi: 10.1073/pnas.0911789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vieira OV, Verkade P, Manninen A, Simons K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J Cell Biol. 2005;170:521–526. doi: 10.1083/jcb.200503078. [DOI] [PMC free article] [PubMed] [Google Scholar]