Background: Human lens proteins accumulate pigmented, protein-cross-linked AGE adducts during cataract formation, and the mechanisms of their formation are poorly understood.
Results: UVA-excited kynurenines can oxidize ascorbate under anoxic conditions and promote synthesis of AGEs.
Conclusion: UVA light-excited kynurenines promote AGE synthesis and contribute to cataract formation.
Significance: This study provides a mechanism for UVA light-mediated damage to lens proteins during cataract formation.
Keywords: Aging, Ascorbic Acid, Cataract, Glycation, Lens, Tryptophan
Abstract
Advanced glycation end products (AGEs) contribute to lens protein pigmentation and cross-linking during aging and cataract formation. In vitro experiments have shown that ascorbate (ASC) oxidation products can form AGEs in proteins. However, the mechanisms of ASC oxidation and AGE formation in the human lens are poorly understood. Kynurenines are tryptophan oxidation products produced from the indoleamine 2,3-dioxygenase (IDO)-mediated kynurenine pathway and are present in the human lens. This study investigated the ability of UVA light-excited kynurenines to photooxidize ASC and to form AGEs in lens proteins. UVA light-excited kynurenines in both free and protein-bound forms rapidly oxidized ASC, and such oxidation occurred even in the absence of oxygen. High levels of GSH inhibited but did not completely block ASC oxidation. Upon UVA irradiation, pigmented proteins from human cataractous lenses also oxidized ASC. When exposed to UVA light (320–400 nm, 100 milliwatts/cm2, 45 min to 2 h), young human lenses (20–36 years), which contain high levels of free kynurenines, lost a significant portion of their ASC content and accumulated AGEs. A similar formation of AGEs was observed in UVA-irradiated lenses from human IDO/human sodium-dependent vitamin C transporter-2 mice, which contain high levels of kynurenines and ASC. Our data suggest that kynurenine-mediated ASC oxidation followed by AGE formation may be an important mechanism for lens aging and the development of senile cataracts in humans.
Introduction
Human lens proteins undergo numerous chemical changes with age, and this process is accelerated in cataractogenesis (1–3). One of the most prominent features of this process is pigmentation (1, 2, 4–6). Yellowish and brown pigments accumulate throughout the lens during aging, but they appear denser at the lens nucleus (2, 4, 7). In senile cataractous lenses, these pigments appear even deeper in color. The vast majority of the lens pigments are confined to the water-insoluble protein fraction of the human lens (1, 6). The proteins containing these pigments exist as highly cross-linked, large aggregates capable of scattering visible light impinging on the lens (8, 9). Although numerous previous studies have attempted to elucidate the biochemical pathways that lead to the formation of these pigments, none so far have succeeded.
It is generally accepted that there are two main types of protein-bound pigments in the human aged and cataractous lenses (10–13). The first type is the reaction product of the lens proteins with kynurenines, which are generated by the tryptophan oxidation catalyzed by IDO (14). This reaction produces N-formylkynurenine (Nfk)4 as the first product, which, by subsequent degradation, forms kynurenine (Kyn), 3OH-kynurenine (3OHKyn), and 3OH-kynurenine glucoside as well as several other products, finally yielding NAD. The human lenses contain significant quantities of these kynurenines (15). At physiological pH and temperature, the kynurenines are unstable; they spontaneously lose their α-amino group as ammonia, forming highly reactive α,β-unsaturated ketones that react in turn with Lys, His, and Cys residues to form Michael-type adducts in lens proteins (10, 16–19).
The second class of pigments is the advanced glycation end products (AGEs). These are produced from the reaction of methylglyoxal, glucose, ribose, and ASC with the Lys and Arg side chains in proteins (20). ASC by itself cannot react with lens proteins; it must be oxidized to dehydroascorbate (DHA) by molecular oxygen (21, 22) or reactive oxygen species (ROS) (23) or be formed through photochemical reactions (24). DHA has a very short half-life (from a few min to 6 h) under physiological conditions. If DHA is not reduced by GSH within this time, it undergoes further ring opening to form 2,3-diketogulonate (21), which further degrades under physiological conditions to erythrulose, threose, and other products (21, 22, 25) that are precursors of AGEs. Several AGEs derived from ASC, such as 1-(5-amino-5-carboxypentyl)-4-(5-amino-5-carboxypentyl-amino)-3-hydroxy-2,3-dihydropyridinium, oxalate monoalkylamide, vesperlysine A, pentosidine, CML, and 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate, have been detected in the human lens (6, 9, 24, 26–29). In addition, Ortwerth's group identified more than 100 ASC-derived AGEs in cataractous human lens proteins (1), for which the structures are not known. Thus, ASC is a dominant AGE precursor in the human lens.
Although ASC-derived AGEs are present in relatively large amounts in the lens, the nearly anoxic environment in the human lens, especially in the nucleus (30), is highly prohibitive for ASC oxidation. Additionally, the human lens contains high levels of GSH (2–4 mm) (31), which could inhibit ASC oxidation. Nonetheless, ASC is oxidized in cataractous lenses (32). How ASC is oxidized in such an environment is a conundrum.
Numerous epidemiological studies have identified UVA light exposure as a risk factor for cataractogenesis (33–35). According to Zigman et al. (36) and Neale et al. (37), there is a direct correlation between UVA light (320–400 nm) exposure and nuclear cataract formation. Other studies also support the role of UVA light in nuclear cataractogenesis; in fact, Sliney (38) showed that the UVA component of sunlight that reaches the lens is at least 1,000 times greater than UVB light. Godar et al. (39) and Ortwerth et al. (40) demonstrated that ∼0.8–1.1 mJ/cm2 of UVA can reach the lens surface in the midday sun, but only 0.06–0.08 mJ/cm2 of UVB light reaches the lens under the same conditions (39, 41, 42). Dillon et al. (42) demonstrated that, unlike UVB light, UVA light can penetrate the nucleus of the aged human lens, where most photooxidation has been observed (43, 44).
Both protein-free and protein-bound kynurenines are weak UVA light sensitizers (10, 11, 45–48). In UVA-excited states, they react directly with ASC in the absence of oxygen via a type I photochemical mechanism that results in ASC oxidation (24, 48). Thus, we conducted our studies to evaluate the relative ability of Nfk, Kyn, and 3OHKyn, in free and protein-bound form, to oxidize ASC during UVA photolysis and to establish whether DHA generated by UVA irradiation is capable of producing AGEs in lens proteins. We also examined whether UVA photolysis of human lenses leads to ASC oxidation and AGE formation. Furthermore, we tested kynurenine-mediated ASC oxidation and AGE formation in the lenses of double transgenic mice that overexpressed human indoleamine 2,3-dioxygenase (hIDO) and a human ASC transporter (hSVCT2), where these genetic modifications resulted in elevated levels of ASC and kynurenines in the lens.
EXPERIMENTAL PROCEDURES
l-(+)-Ascorbate (99% pure) was purchased from Acros Organics (Thermo Fisher Scientific). 4,5-Dimethyl-1,2-phenylenediamine (98% pure), kynurenine (≥95.0% pure), 3OHKyn (98% pure), and diethylene triamine pentaacetic acid (DTPA) were obtained from Sigma-Aldrich. Nfk was synthesized as described previously (49). All other chemicals were of analytical grade. De-ionized water (18 megaohms or greater) was used throughout this project. All phosphate buffers employed in this project were treated with Chelex 100 resin (10.0 g/liter, 200–400 mesh, Bio-Rad) to remove transient metal ion contaminants.
Non-cataractous human lenses were obtained from the Midwest Eye Bank (Ann Arbor, MI) and the Missouri Lions Eye Research Foundation (Columbia, MO). Human cataractous lenses were obtained from the Iladevi Cataract and IOL Research Center (Ahmedabad, India). The lenses were processed directly or were stored at −80 °C until use.
Generation of Double Transgenic Mice
Animals were used in accordance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research, and the experiments were approved by the Institutional Animal Care and Use Committee. To generate the double transgenic mice that specifically overexpressed hIDO and hSVCT2 in the lens epithelium and fiber cells, we cross-bred homozygous hIDO with homozygous hSVCT2 transgenic mice, both on a C57BL/6 background. The details for the hIDO mice have been published elsewhere (19). These animals contain high levels of kynurenines in the lenses. The heterozygous transgenic mice for hSVCT2 animals exhibit high levels of ASC in the lens (5, 50). A homozygous hSVCT2 line was derived from breeding hSVCT2 heterozygotes. The offspring from cross-breeding hIDO and hSVCT2 homozygotes were confirmed to be double transgenic by genotyping using the following primers: 5′-TCTTCCGGTGGTGATAAATGGA-3′ (sense) and 5′-GCTCTGCTGTTCCATTGGCA-3′ (antisense) for hSVCT2 and 5′-TCTGAGAGCCTCTGCTGCTC-3′ (sense) and 5′-GGTCCATGGTGATACAAGGGAC-3′ (antisense) for hIDO.
Preparation of Human Lens Low Molecular Weight (LMW) Fraction and Water-soluble (WS) and Water-insoluble (WI) Protein Fractions
Intact human lenses (20–38 years old) were used throughout this study. Each decapsulated lens was homogenized in 2 ml of ice-cold deionized water. The homogenate was centrifuged at 27,000 × g for 30 min at 4 °C. The resulting supernatant was aspirated and further centrifuged at 15,000 × g for 120 min in an Amicon Ultra (cut-off = 3 kDa) centrifugal filter (Millipore Corp., Billerica, MA) at 4 °C. The filtrate (LMW) was collected and placed immediately at −80 °C until use. The supernatant was adjusted to ∼1 ml with 10 mm phosphate buffer (pH 7.0) containing 0.1 mm DTPA, exhaustively dialyzed against the same buffer, and designated as the WS fraction. The protein pellet was washed three times with ice-cold 10 mm phosphate buffer (pH 7.0) containing 0.1 mm DTPA and sonicated with 1.0 ml of the same buffer for 5 min with cooling. A power setting of 4 (∼40 watts) and a 30% duty cycle were used with a Branson Digital Sonifier (model S-450D, Branson Ultrasonics Co., Danbury, CT). The resulting suspension was centrifuged at 21,000 × g for 30 min. The supernatant was designated as the WI fraction. The protein concentrations in the WS and WI fractions were measured by the BCA method using BSA as the standard (Pierce). These fractions were stored at −80 °C until use.
Preparation of Mouse Lens LMW Fraction
Intact mouse lenses (from 1-month-old mice) were used throughout this study. Each decapsulated lens was homogenized in a 1-ml tissue homogenizer with 200 μl of ice-cold deionized water. The homogenate was processed as above to obtain the LMW fraction, which was stored at −80 °C until use.
Modification of WS Protein of Human Lens with Kynurenines
The WS protein at 1 mg/ml in 0.1 m sodium phosphate buffer (pH 7.4) was incubated for 7 days at 37 °C in the presence or absence of a 0.1 mm concentration of one of the following kynurenines: Nfk, Kyn, or 3OHKyn. All reaction mixtures were sterile filtered with a 0.2-μm syringe filter (Millipore Corp.). After incubation, the samples were dialyzed against 2 liters of PBS for 48 h with a change after 24 h, and the protein concentration was determined by the BCA method.
Assessment of Protein-modified Kynurenines by ELISA
Microplate wells were coated with Kyn-modified lens proteins in 0.05 m carbonate buffer (pH 9.7) at a concentration of 1 μg and incubated overnight at 4 °C. The wells were washed three times with PBST before blocking at room temperature for 2 h with 300 μl of 5% non-fat dry milk in PBST. The wells were further washed three times with PBST and incubated with monoclonal antibody for 3OHKyn-modified or Kyn-modified proteins (1:1,000 diluted in 5% non-fat dry milk) for 1 h at 37 °C in a humidified chamber. The washed plates were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Promega Corp., Madison, WI; diluted 1:5,000) for 1 h at 37 °C. The enzyme reaction was assessed by the addition of 100 μl of 3,3,5,5-tetramethylbenzidine (Sigma) followed by the addition of 50 μl of 2 n sulfuric acid and measurement of chromophore absorbance at 450 nm.
Measurement of ASC Degradation (Assay 1)
ASC was measured at λ = 265 nm using an extinction coefficient value of ϵ = 1.65 × 104 at pH 6.8 (51).
Measurement of ASC Degradation (Assay 2)
ASC and DHA were measured by an HPLC assay as described previously (52) with minor modifications. Briefly, aliquots of the samples were derivatized by adding 70 μl of 100 mm sodium phosphate buffer (pH 4.0) and 20 μl of 4,5-dimethyl-1,2-phenylenediamine (1.0 mg/ml in a 10% ethanolic solution of 5 mm H2SO4). After a 30-min incubation at room temperature in the dark, the sample was injected into the high performance liquid chromatograph and separated on a Gemini C18 (5 μ, 4.6 × 250 cm) column (Phenomenex, Los Angeles, CA). The mobile phase was 40% methanol and 40 mm phosphate buffer (final pH 7.3) eluted at 0.75 ml/min. The 4,5-dimethyl-1,2-phenylenediamine derivative was detected in a fluorescent detector set at λex/λem = 360/440 nm (Rt ∼8 min).
ASC Oxidation by UVA-excited Kynurenines
The assay mixture contained ASC (0.125 mm) and a kynurenine (0.01–0.125 mm) in argon-saturated 20 mm phosphate buffer (pH 7.1) supplemented with 0.1 mm DTPA in a final volume of 5.0 ml. The mixtures were equally split between two screw cap 3.5-ml cuvettes, each containing a magnetic stirrer. One cuvette was then irradiated with UVA light, whereas the other cuvette was kept in the dark at the same temperature for the duration of the experiment, serving as a dark control. The experiments were routinely conducted under anoxic conditions after oxygen removal by purging argon into the tightly screw-capped cuvette for 15 min. All irradiations were carried out with a light source consisting of a 1,000-watt mercury/xenon lamp (Newport Industrial Glass, Stanton, CA) with light passing through a dichroic mirror (280 nm ≤ λ ≥ 400 nm) and a 335-nm cut-off filter (Kopp 9335; 2-inch diameter, 3-mm thick; Newport Industrial Glass) with the temperature kept at a constant 20 °C. The UVA light measured was 100 milliwatts/cm2 at the cuvette surface.
ASC Oxidation by Human Lens Protein-bound Pigments
Cataractous and age-matched non-cataractous lenses (age 58–76 years) were homogenized in PBS, dialyzed against PBS, and lyophilized. The lyophilized powder (3 mg/ml) was digested with Pronase E (3.6% of total protein) for 24 h at 37 °C and passed through a 3-kDa molecular mass cut-off filter. The digested material in the filtrate (equal to the original 600 μg of protein) in 1.0 ml of PBS was mixed with 0.1 mm ASC and irradiated with UVA light (100 milliwatts/cm2 for 45 min) in the presence or absence of oxygen. The absorbance at 265 nm was measured to monitor the ASC oxidation.
Measurement of GSH in the Human Lens LMW Fraction
The levels of GSH in the LMW fractions of human lenses were determined by 5,5′-dithiobis-2-nitrobenzoic acid as described by Lou et al. (53).
Measurement of Kynurenines in the Mouse LMW Fraction
The kynurenines in the mouse lens LMW fraction were determined using a GraceVydac C18 column (4.6 × 250 cm; 218TP54) in a high performance liquid chromatograph, which was run using a 0–12% H2O-acetonitrile gradient at a flow rate of 0.8 ml/min with effluent monitoring at λex/λem = 360/480 nm, as described previously (19). Kyn, Nfk, and 3OHKyn standard curves were generated under the same conditions to establish the levels of the kynurenines in the LMW fractions. This method is linear for 0.05–3.9 nmol/ml of kynurenines.
AGE Formation by UVA Light-excited Kynurenine-modified Lens Proteins
Mixtures of 0.2 mm ASC with 1 mg/ml kynurenine-modified protein (either by Nfk, Kyn, or 3OHKyn) in 50 mm phosphate buffer (pH 7.0, containing 0.1 mm DTPA) at a final volume of 2.5 ml were placed in 3.5-ml quartz cuvettes with screw cap tops and septa. These mixtures were then deaerated by carefully bubbling argon for at least 15 min. Two identical mixtures for each kynurenine-modified protein were used at a time. One reaction mixture was irradiated with 100 milliwatts/cm2 of UVA light, whereas the other was kept in the dark for 2 h. Both cuvettes were kept under argon and at 20 °C for the duration of irradiation. At the end of the UVA irradiation, the cuvettes were flushed with argon again for an additional 5 min and injected with 5 μl each of chloroform and toluene through a cap septum. The tops of the cuvettes were dipped in melted wax to block oxygen seepage, and the reaction mixtures were further incubated for 3 days at 37 °C. We should note that this procedure may not have completely blocked oxygen re-entry into the reaction mixture during the follow-up incubation periods. Therefore, our results on AGEs should be interpreted as if they are formed under nearly anoxic conditions (which is in fact the case in the lens nucleus) rather than in total anoxic conditions. At the end of the incubation period, the proteins were dialyzed against 50 mm phosphate (pH 7.0) containing 0.1 mm DTPA. All protein preparations were measured for protein content by the BCA method as described above, and then they were stored at −20 °C. Aliquots of these reaction mixtures were further hydrolyzed with 6 n HCl, dried, reconstituted with distilled water, and analyzed for AGEs (see below).
UVA Irradiation and AGE Measurement in Lens Explants
Young human lenses (matching pairs of 20–36-year-old lenses from donor eyes) were suspended in 0.5 ml of artificial aqueous humor (AAH) (54) in a 3.5-ml quartz cuvette with a screw cap, degassed through a septum with argon for an additional 15 min, and either kept in the dark or subjected to UVA photolysis (320–400 nm; 100 milliwatts/cm2) for the duration described in the legends to Figs. 4 and 6 at 20 °C. At the end of the incubation period, the lenses were homogenized in 1 ml of 50 mm sodium phosphate buffer (pH 4.0) and submitted for ASC, DHA, and GSH determination, or the cuvettes containing the lenses were injected with 5 μl of toluene and 5 μl of chloroform, sealed as above, and placed in an incubator at 37 °C for 7 days.
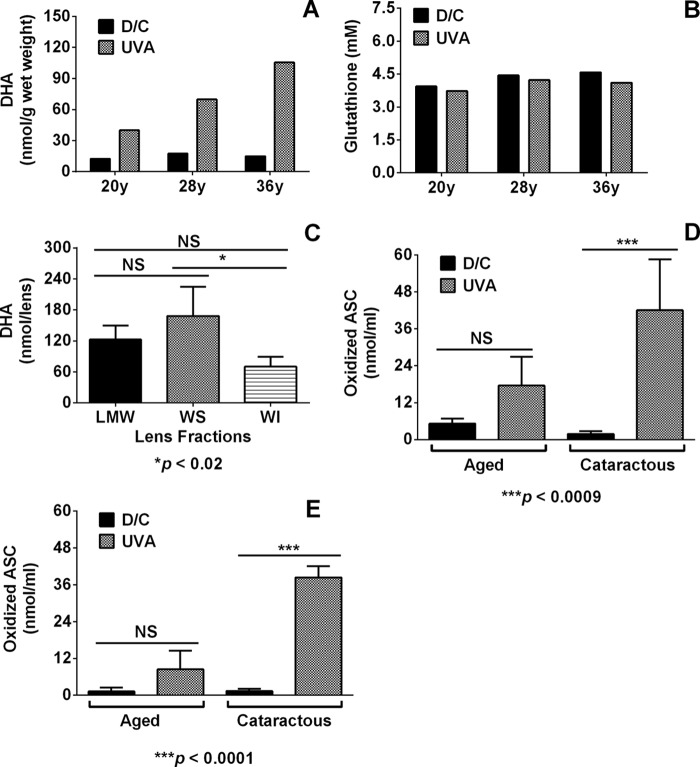
FIGURE 4.
UVA irradiation of human lenses and isolated cataractous lens proteins leads to the oxidation of ASC. A, levels of DHA in the lens kept in the dark (dark control; D/C) and the levels in the matched lens subjected to UVA photolysis (UVA). B, levels of GSH in the same lenses. C, relative ASC oxidation capacity in different fractions of young lenses during a 45-min UVA photolysis. Bars in C, means ± S.D. (error bars) from at least three lenses. Proteins from the cataractous and age-matched (age 58–76 years) lenses were irradiated with UVA light (100 milliwatts/cm2 for 45 min) in the presence of 0.1 mm ASC under aerobic (D) and anoxic (E) conditions.
FIGURE 6.
UVA photolysis of human lenses under anoxic conditions leads to the formation of AGEs. Human lenses in AAH were irradiated with UVA light (100 milliwatts/cm2) for 3 h and incubated for 7 days at 37 °C. The AGEs (A, argpyrimidine and B, pentosidine) were determined by HPLC. Bars, means ± S.D. (error bars) from three independent measurements.
The decapsulated mouse lenses (2–4 months old) from wild type and hSVCT2/hIDO mice were suspended in 0.5 ml of AAH in 3.5-ml quartz cuvettes with screw caps, degassed through the septa with argon for an additional 15 min, and either kept in the dark or subjected to UVA photolysis for 2 h and incubated as above.
At the end of the incubation period, the lenses from the two aforementioned treatments were homogenized in 1 ml of 50 mm sodium phosphate buffer (pH 7.4) and dialyzed against the same buffer. The dialyzed samples were subjected to 6 n HCl hydrolysis and to AGE estimation as described below.
Measurements of AGEs
The protein preparations were subjected to acid hydrolysis in 6 n HCl at 110 °C for 16 h under argon. The samples were evaporated in a vacuum centrifuge, and the dried samples were reconstituted in 250 μl of deionized water and filtered through a 0.45-μ filter. Pentosidine and argpyrimidine were measured by fluorescence HPLC, as described previously (55). CML was quantified by HPLC after precolumn derivatization of the sample with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate, as described previously (56). Synthetic AGEs were used as standards for identification and quantification.
Statistics
The data are presented as the mean ± S.D. of the specific number of experiments indicated in the figure legends. The data were analyzed using StatView software (SAS Institute Inc., Cary, NC). The statistical significance was evaluated with a paired two-tailed t test, and differences were considered significant at p < 0.05.
RESULTS
UVA Light-excited Kynurenines Oxidize ASC under Anoxic Conditions
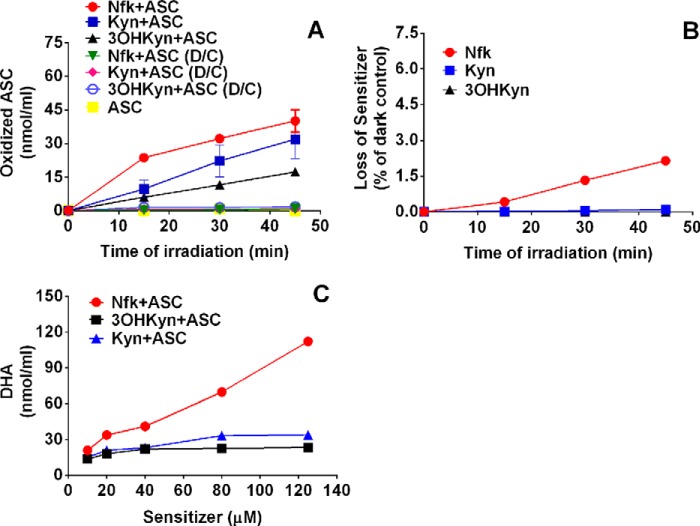
Upon UVA light excitation, all three kynurenines (Nfk, Kyn, and 3OHKyn) were able to oxidize ASC under anoxic conditions. Nfk showed the highest ability to oxidize ASC, yielding 40.1 ± 5.0 nmol/ml of oxidized ASC/ml during the 45-min irradiation (Fig. 1A). Kyn and 3OHKyn showed lower abilities to oxidize ASC, yielding 31.9 ± 8.7 and 17.4 ± 1.0 nmol/ml of oxidized ASC/ml, respectively, during the same time period. During this time period, no appreciable loss in kynurenines (sensitizers) was noted (Fig. 1B). Moreover, the dark controls showed no appreciable ASC oxidation. We did not detect direct photolysis of ASC under our experimental conditions.
FIGURE 1.
Oxidation of ASC by photoexcited kynurenines. A, deaerated 2.5-ml reaction mixtures containing 20 μm kynurenine and 0.1 mm ASC were irradiated over 45 min in 50 mm Chelex100-treated phosphate buffer, pH 7.0, under anoxic conditions, and readings at λ = 265 nm were taken every 15 min. B, photooxidation of the kynurenines under the same conditions with readings taken at λ = 320 nm for Nfk and at λ = 360 nm for Kyn and 3OHKyn. C, 2.5-ml reactions containing different concentrations of the kynurenines and 0.25 mm ASC were irradiated over 45 min in 50 mm Chelex100-treated phosphate buffer, pH 7.0, under anoxic conditions. An aliquot of the reaction mixture was taken for immediate derivatization, and the DHA concentration was measured as described under “Measurement of ASC Degradation (Assay 2).” Each point represents the mean ± S.D. of three independent experiments in A, and for B and C, each point represents the mean of two independent experiments. D/C, dark control.
In the next experiment, we sought to determine whether kynurenine-mediated ASC oxidation led to the formation of DHA. We used 4,5-dimethyl-1,2-phenylenediamine to trap DHA; the reaction produces a fluorescent quinoxaline-like structure, which can be detected by a fluorescence detector. At 20 μm kynurenines, a concentration similar to those found in young human lenses (57), Nfk showed the highest level of DHA after the 45-min photolysis (33.9 nmol/ml) (Fig. 1C), followed by Kyn and 3OHKyn. These two latter kynurenines produced 21.2 and 18.2 nmol/ml DHA, respectively.
GSH Partially Prevents Kynurenine-mediated Photooxidation of ASC
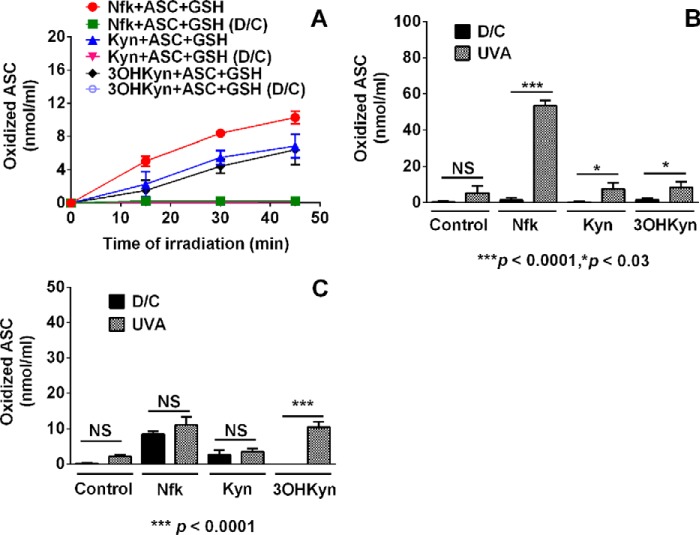
Human lens tissue contains high levels of GSH (31), which is believed to reduce DHA to ASC in the human lens and to bind deaminated kynurenines (58–60). However, Snytnikova et al. (24) showed that the rate of the reaction between the triplet state of kynurenine and GSH is at least 85-fold lower than the reaction of GSH with ASC. This means that in the reaction mixtures containing ASC, kynurenines, and GSH, ASC preferentially reacts with excited kynurenines rather than GSH. To verify this possibility, we used 0.2 mm GSH with 0.1 mm ASC and 20 μm kynurenines for irradiation. The ratio of GSH to ASC chosen for this experiment was similar to the ratio found in human lenses (31, 61). Despite a 2-fold excess of GSH in the reaction mixture relative to ASC, our data showed that after 45 min of UVA irradiation, ASC was oxidized (Fig. 2A).
FIGURE 2.
Oxidation of ASC by photoexcited kynurenines is not prevented by GSH. A, the quantity of residual ASC in the reaction mixtures (both dark control (D/C) and UVA-irradiated) was obtained by measuring the absorbance at λ = 265 nm. Each point represents the mean ± S.D. of three independent experiments. To further determine the influence of the environment of lens nucleus and outer cortex on ASC oxidation, a solution of lens proteins (2.0 mg/ml), ASC (2 mm), kynurenines (50 μm), and glutathione (1 mm) under anoxic conditions (B) or lens proteins (2.0 mg/ml), ASC (2 mm), kynurenines (50 μm), and glutathione (4 mm) under aerobic conditions (C) in 50 mm sodium phosphate buffer, pH 7.2, was irradiated by UVA light for 2 h. Bars, means ± S.D. (error bars) from three independent measurements.
We conducted two additional experiments to further confirm ASC oxidation in the presence of GSH. Under anoxic and less reducing conditions, similar to the ones found in the human lens nucleus (2 mg/ml of lens protein, 1 mm GSH, 2 mm ASC, and 50 μm kynurenine), a 2-h UVA photolysis yielded 53.4 ± 5.3, 7.5 ± 6.1, 8.4 ± 5.5 nmol/ml of oxidized ASC from Nfk, Kyn, and 3OHKyn, respectively (Fig. 2B), which corroborated the data in Fig. 2A. In contrast, under similar conditions that were aerobic with higher concentrations of GSH (4 mm, similar to human lens cortex), slightly lower levels of ASC oxidation were detected with UVA-photolyzed Nfk, 3OHKyn, and Kyn (11.1 ± 4.0, 10.5 ± 2.6, and 3.5 ± 1.3 nmol/ml of oxidized ASC, respectively) (Fig. 2C). Because of the competition between molecular oxygen and ASC for the excited state of UVA-photolyzed kynurenines (24), these data are not surprising. Our data also show that kynurenine-mediated ASC oxidation can occur in the presence of lens proteins. Taken together, these data confirm that ASC oxidation can occur in the presence of GSH and in the presence or absence of molecular oxygen, and they suggest the possibility of persistent ASC oxidation in the human lens under chronic exposure to direct and reflected light.
ASC Oxidation by UVA Photolysis of LMW Fraction of hIDO/hSVCT2 Mouse Lens
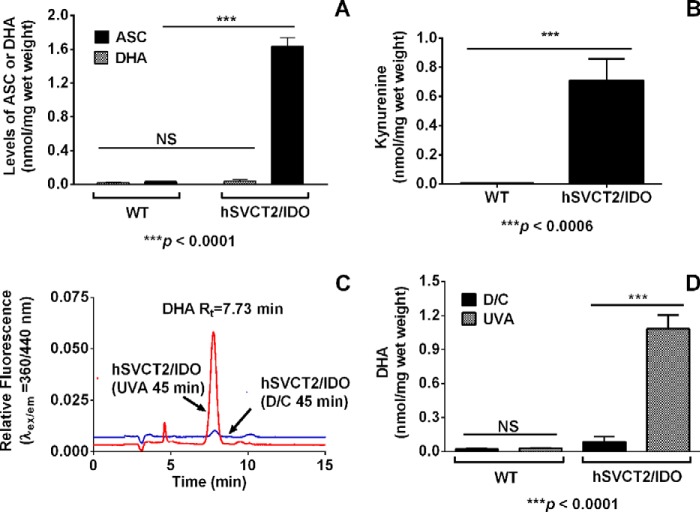
We used the LMW fraction (<3 kDa) from the double transgenic mouse lenses to study the formation of DHA from ASC as a result of UVA photolysis of the intrinsic lens kynurenines. The overexpression of hSVCT2 in the mouse lens led to elevated ASC levels (1.63 ± 0.1 versus 0.035 ± 0.002 nmol/mg wet weight in the wild type mouse lens; Fig. 3A). However, the levels of DHA in the lenses of double transgenic mice were low (0.036 ± 0.017 nmol/mg wet weight). Whereas the Kyn levels in the wild type mouse lens were 0.0028 ± 0.001 nmol/mg wet weight, our data show that it was ∼250-fold higher (0.71 ± 0.14 nmol/mg wet weight) in the lens of the double-transgenic mouse (Fig. 3B), which is similar to the levels of Kyn in human lenses (57, 62).
FIGURE 3.
hIDO/hSVCT2 double transgenic mouse lenses contain high levels of ASC and kynurenine, and UVA irradiation of those lenses leads to oxidation of ASC. The levels of ASC and DHA in the lenses of 1.5-month wild type and hIDO/hSVCT2 double transgenic animal lenses are shown in A. The kynurenine levels in the same lenses are shown in B. The LMW fractions (<3 kDa) of the wild type and double transgenic mouse lenses were incubated under anoxic conditions for 45 min at room temperature in the dark (dark control; D/C) or were UVA-irradiated for 45 min at 100 milliwatts/cm2. C, HPLC profile for DHA in the mouse lenses; D, DHA levels in these lenses. Bars, mean ± S.D. (error bars) from at least three lenses.
Irradiation of the LMW fraction from the double transgenic mouse lens under anoxic conditions for 45 min caused a significant increase in DHA, as revealed by the HPLC chromatogram (Fig. 3C). There was a 12.4-fold increase in the DHA levels from 0.087 ± 0.04 nmol/mg wet weight in the dark control to 1.1 ± 0.1 nmol/mg in the UVA-irradiated LMW fraction (Fig. 3D). These results clearly demonstrate the ability of UVA-excited kynurenine to react directly with ASC in the mammalian lens to form DHA in the absence of oxygen.
Formation of DHA as a Result of UVA Photolysis of the Human Lens
The UVA irradiation of whole human young lens explants under anoxic conditions led to the formation of DHA. UVA photolysis for 45 min resulted in the formation of 40, 69.9, and 105.4 nmol/g wet weight of the lens of DHA in 20-, 28-, and 36-year-old human lenses, respectively (Fig. 4A). During this time, only 5–10% of the GSH was consumed (Fig. 4B). The examination of the relative ability of different lenticular fractions of the 20-year-old lens showed that the UVA-sensitizing activity was predominantly located in the LMW fraction and in the WS proteins, with 122.5 ± 26.6 nmol of ASC/lens transformed to DHA for the LMW fraction and 162.3 ± 56.4 nmol/lens for the WS proteins (Fig. 4C). According to Cheng et al. (2) and Korlimbinis et al. (62, 63), the only UVA-responsive sensitizers present in the young lens are mostly free kynurenines. Thus, it is conceivable that most of the UVA-mediated ASC oxidation in young human lenses occurs as a result of direct interaction between the UVA-excited kynurenines.
Oxidation of ASC by UVA-excited Protein-bound Sensitizers in Human Cataractous Lenses
UVA irradiation of exhaustively digested human lens proteins (600 μg) under aerobic conditions for 45 min in the presence of 0.1 mm ASC led to 42.0 ± 16.5 nmol/ml of oxidized ASC by the cataractous lens proteins, whereas for the age-matched normal lenses, it was 17.5 ± 9.4 nmol/ml (Fig. 4D). A similar pattern of UVA-driven ASC oxidation by protein-bound sensitizers was observed under anoxic conditions of the experiment (Fig. 4E) as well. The dark controls did not show any appreciable ASC oxidation. These data imply that once human lens proteins acquire UVA-responsive protein-bound pigments, they can also oxidize ASC.
Oxidation of ASC under Anoxic Conditions and Formation of AGEs by UVA-excited Protein-bound Kynurenines
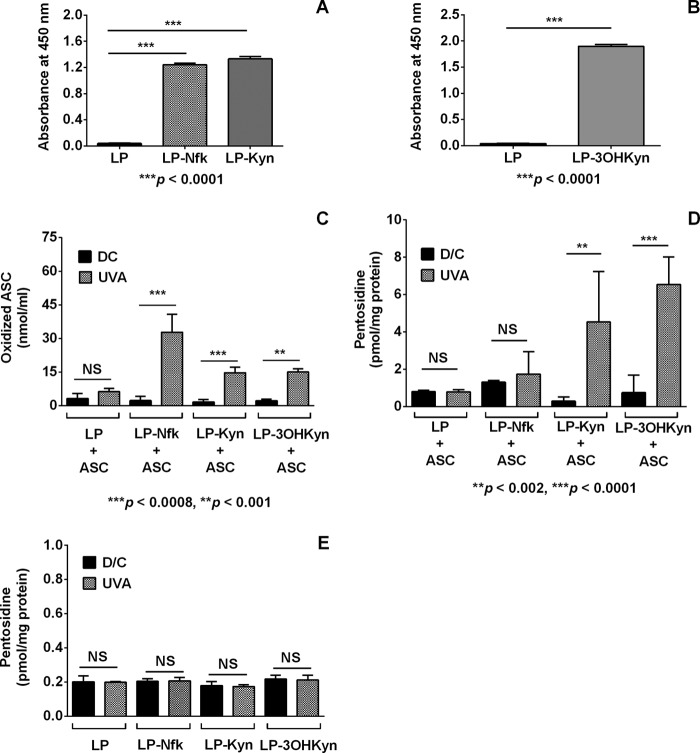
Previous studies have shown that protein-bound kynurenines are also UVA light-responsive sensitizers (10, 11, 64) and therefore have the potential to oxidize ASC under anoxic conditions. ASC degradation products could then react with lens proteins to form AGEs. To explore this possibility, we irradiated kynurenine-modified human lens proteins (in the presence of 0.1 mm ASC) with 100 milliwatts/cm2 of UVA light for 2 h under anoxic conditions. Incubation of WS protein with Nfk, Kyn, or 3OHKyn led to the formation Nfk-, Kyn-, and 3OHKyn-modified proteins (Fig. 5, A and B). Non-irradiated controls along with the photolyzed reaction mixtures were then incubated for 3 days at 37 °C. The 2-h irradiation of the kynurenine-modified proteins in the presence of ASC led to ASC oxidation. We detected 15.0 ± 0.8, 14.7 ± 1.5, and 32.8 ± 4.6 nmol/ml of oxidized ASC with UVA-photolyzed 3OHkyn-, Kyn-, and Nfk-modified lens protein, respectively (Fig. 5C). This is much higher than 1.1, 10.8, and 5.6 nmol/ml of oxidized ASC in the respective dark controls. The incubation of these samples for 3 days at 37 °C led to the formation of pentosidine. Pentosidine was produced at significantly higher levels in the UVA-photolyzed mixtures compared with the non-irradiated samples. 3OHKyn- and Kyn-modified lens proteins showed 27.5-fold (6.5 ± 1.5 pmol/mg protein) and 15.6-fold (4.5 ± 2.7 pmol/mg protein) increases in pentosidine, respectively (Fig. 5D). The Nfk-modified lens proteins showed only a marginal increase in the pentosidine levels (1.7 ± 1.2 nmol/mg protein in the UVA-irradiated sample versus 1.3 ± 0.1 nmol/mg protein in the non-irradiated control). In kynurenine-modified lens proteins irradiated in the absence of ASC, pentosidine levels were very low and similar to the unmodified control samples (Fig. 5E). These data clearly show, for the first time, the ability of UVA-excited protein-bound kynurenines to oxidize ASC in anoxic conditions and to consequently generate AGEs (possibly under the low oxygen environment of our experimental conditions).
FIGURE 5.
Photooxidation of ASC by protein-bound kynurenines leads to the formation of AGEs. Kynurenine modification of WS protein was assessed in ELISAs using monoclonal antibodies to Nfk/Kyn (A) and 3OHKyn modifications (B). During a 120-min UVA photolysis treatment, the kynurenine-modified lens proteins (1 mg/ml of lens proteins with 100 μm each kynurenine) oxidize ASC (0.1 mm) under anoxic conditions (C). Incubation of this reaction mixture for 3 days at 37 °C under anoxic conditions leads to the formation of pentosidine (D). However, under similar conditions, UVA irradiation of kynurenine-modified lens proteins in the absence of ASC does not generate additional pentosidine; the pentosidine levels were similar to those in controls (E). Bars, means ± S.D. (error bars) from at least three independent experiments. LP, lens proteins. LP-Nfk, LP-Kyn, and LP-3OHKyn, lens protein modified by Nfk, Kyn and 3OHKyn, respectively.
Demonstration of AGE Formation in Young Human Lens Explants as a Result of UVA Photolysis
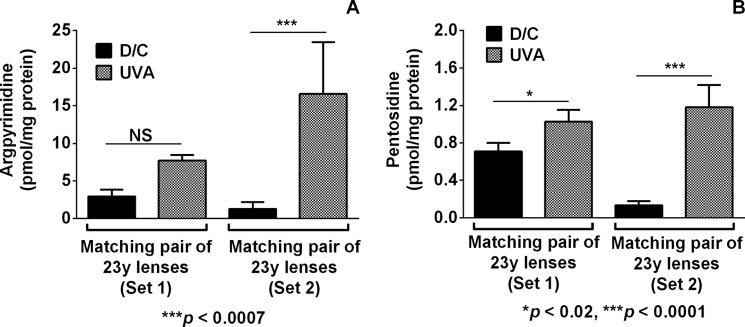
We further investigated whether a relatively short UVA irradiation period of young human lens explants under anoxic conditions followed by incubation in the dark led to AGE formation in lens proteins. Whole lenses (two matching pairs) were irradiated in AAH for 3 h with UVA light under anoxic conditions, followed by 7 days of incubation at 37 °C. In non-irradiated 23-year-old control lenses, the levels of argpyrimidine varied between 1.3 and 2.9 pmol/mg protein (Fig. 6A). UVA photolysis followed by incubation at 37 °C for 7 days showed 2.6–12-fold higher levels than in non-irradiated matching control lenses. The formation of pentosidine occurred in a similar manner (Fig. 6B). In the dark control lenses, the levels of pentosidine were within the range of 0.13–0.7 pmol/mg protein. The UVA irradiation of one lens increased the levels of pentosidine to 1.02 ± 0.13 pmol/mg protein (43% increase; p < 0.0001). In the other lens, however, the pentosidine formation was more dramatic, amounting to 1.18 ± 0.24 pmol/mg protein (an approximately 9-fold increase compared with the dark control; p < 0.02).
AGE Formation in Double Transgenic (hIDO/hSVCT2) Mouse Lens Explants as a Result of UVA Photolysis
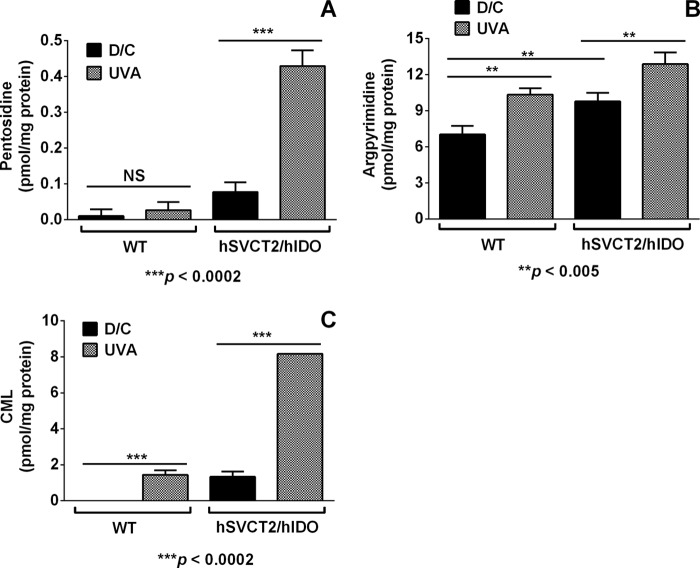
The hIDO/hSVCT2 mouse lenses have low levels of AGEs (Fig. 7A). The UVA irradiation of these lenses for 2 h, followed by a 7-day incubation in the dark, produced a statistically significant 5-fold increase in the levels of pentosidine compared with the non-irradiated lenses (0.43 ± 0.04 pmol/mg protein versus 0.08 ± 0.03 pmol/mg protein; p < 0.0002). Very low levels of pentosidine were detected in the irradiated wild type lenses. We also detected a small but statistically significant increase in the levels of argpyrimidine, amounting to 12.89 ± 0.97 versus 9.78 ± 0.73 pmol/mg protein (32% increase; p < 0.0005), in the UVA-irradiated and dark control lenses, respectively (Fig. 7B). Because the UVA-photolyzed WT lenses (which have negligible levels of Kyn) showed a similar increase in argpyrimidine levels compared with the dark controls (10.35 ± 0.51 versus 7.03 ± 0.7 pmol/mg protein; 42% increase; p < 0.0005), argpyrimidine formation may also occur by a non-UVA-dependent mechanism.
FIGURE 7.
UVA photolysis of hIDO/hSVCT2 transgenic mouse lenses results in AGE formation. The lenses from 1-month-old hIDO/hSVCT2 transgenic or wild type (WT) mice (3 pairs each) in AAH were either UVA-irradiated or kept in the dark (dark control; D/C). Then the lenses were incubated for 7 days at 37 °C. The AGEs (A, pentosidine; B, argpyrimidine; and C, CML) were determined by HPLC. Bars, mean ± S.D. from three independent measurements.
Our results demonstrated a 6-fold increase in the levels of CML in the UVA-irradiated transgenic (hIDO/hSVCT2) mouse lenses compared with the non-irradiated control lenses (8.17 ± 0.01 versus 1.33 ± 0.29 pmol/mg protein; p < 0.0002). The increase in CML levels in the UVA-irradiated wild type lenses (1.44 ± 0.25 versus 0.0 ± 0.0 pmol/mg protein; p < 0.0002) compared with dark control lenses suggests the possibility of an alternate, kynurenine-independent pathway of ASC oxidation leading to the CML precursors.
DISCUSSION
The purpose of this study was to investigate the hypothesis that kynurenine-mediated ASC oxidation leads to AGE formation in the lens proteins. This hypothesis was based on the fact that human lenses are chronically exposed to UVA light from the sun (reflected and direct) and ambient UVA light, and UVA sensitizing kynurenines are present in significant quantities in free and protein-bound forms in the lens.
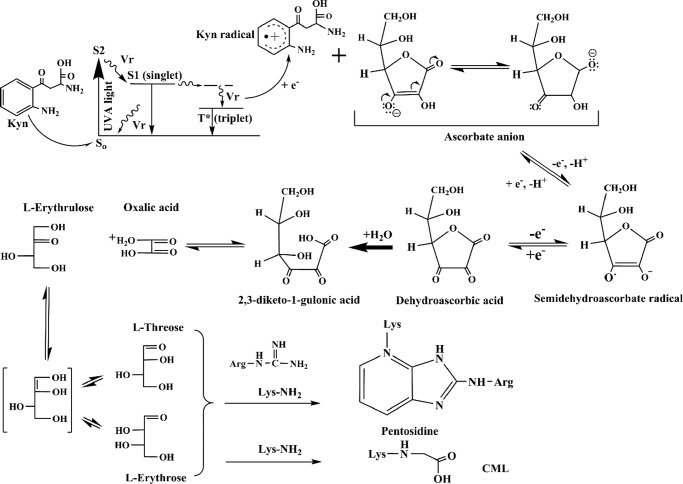
Our hypothesis drew further strength from earlier observations that photoexcited kynurenines can oxidize quenchers, such as ASC (64–67). The rate constant of the reaction of ASC with the triplet states of the UVA-photolyzed kynurenines is 8.5 ± 1.2 × 108 mol−1 s−1 (24), which is comparable with the rate constant of the reaction with molecular oxygen (68). The rate constant of the UVA-photolyzed kynurenines with GSH is at least 85-fold lower than with molecular oxygen (24), indicating that under physiological conditions in the lens, ASC and molecular oxygen predominantly act as natural quenchers of the triplet state of the kynurenines. Based on flash photolysis studies (24, 65), the reaction involves the quenching of the triplet state of the kynurenines by ASC through an electron transfer mechanism (a type I photochemical reaction). This reaction leads to the formation of a kynurenine radical anion and an ASC radical cation. Although UVA-photolyzed Nfk is known to generate singlet oxygen under aerobic conditions that can react in turn with ASC to form DHA (23), this reaction is not likely in the lens because of the low oxygen tension. Instead, the ASC radical can undergo further spontaneous dismutation (τ½ ∼30 s), which leads to the formation of DHA (kobs at pH 7.4 = 1.4 × 105 m−1 s−1; see Fig. 8). This rate constant increases by a factor of ∼10 when phosphate is present (69). Thus, it was conceivable that ASC oxidation by photoexcited kynurenines could occur in the absence of oxygen, and the present study clearly showed that is the case in human lenses.
FIGURE 8.
Proposed mechanism of the UVA photolysis of kynurenines, the oxidation of ASC, and the formation of AGEs. The photoexcited kynurenines react with ASC to form an ASC radical, which can undergo spontaneous dismutation to form DHA. DHA undergoes further degradation to form 2,3-diketogulonate (2,3-DKG) and other products that react with proteins to form the AGE adducts.
Previously, Ortwerth et al. (48) demonstrated that the photoexcited 3OHKyn glucoside and its deaminated product were able to oxidize ASC in the absence of oxygen. We have now extended this observation to show that other major kynurenines of the lens can also induce ASC oxidation under anoxic conditions. More importantly, ASC oxidation by the kynurenines was observed in reaction mixtures containing a 2-fold excess of GSH over ASC. Furthermore, UVA photolysis of the LMW fraction from the double transgenic mouse that is likely to contain significant GSH also promoted ASC oxidation. The results from the UVA-photolyzed young human lenses that contained high levels of GSH also showed significant quantities of DHA. Together, these findings argue that GSH is inefficient at reversing the UVA/kynurenine-mediated oxidation of ASC. Our study also showed that lens proteins do not inhibit the kynurenine-mediated oxidation of ASC.
The DHA produced from this UVA/kynurenine-mediated ASC oxidation could undergo degradation to form strong precursors of AGEs, such as erythrulose and other recently identified carbonyls and dicarbonyls (25). Our study provides strong evidence for such a mechanism. The UVA irradiation of the lens explants under anoxic conditions followed by incubation at 37 °C showed a statistically significant increase in the pentosidine levels over the dark controls. Similar results were obtained with the UVA-irradiated double-transgenic (hIDO/hSVCT2) mouse lenses. As seen in the UVA-irradiated human lenses, the levels of pentosidine in these lenses increased 5-fold, with very low levels of pentosidine formed in the irradiated wild type lenses. Because the major intermediate responsible for the formation of pentosidine is DHA (70) and the major UVA absorbing compounds in the human lens are kynurenines (10, 64, 67, 71), our data strongly suggest that AGE formation occurred from the reaction between the UVA-ASC oxidation products and lens proteins in the presence of high levels of GSH.
Although relatively high quantities of argpyrimidine were formed in the UVA-irradiated human and mouse lens explants, this increase was rather unexpected. The precursor for argpyrimidine is methylglyoxal, and the formation of argpyrimidine from ASC oxidation is a rather sluggish process (72). The fact that a similar, statistically significant increase in the levels of argpyrimidine was observed in both UVA-photolyzed wild type mouse lenses, which have negligible levels of Kyn and ASC, suggests that the formation of argpyrimidine is UVA-driven but mostly independent of ASC oxidation.
Our data also show that not only free kynurenines but also UVA-excited protein-bound kynurenines could oxidize ASC under anoxic conditions. The levels of all free kynurenines decreased at a rate of ∼12%/decade in human lenses (57) as a result of kynurenines binding to proteins. Photochemically, these protein-bound kynurenines are more potent than free kynurenines, and the protein-bound kynurenines have been shown to generate peroxides upon UVA photolysis under aerobic conditions (10, 11). Mechanistic studies by Sherin et al. (67) using Kyn-Lys, Kyn-His, and Kyn-Cys model compounds identical to those present in aged human lens proteins demonstrated that the quantum yields of the photochemical reactions of the Kyn-His and Kyn-Lys triplet states with O2 are higher than those with Kyn or 3OHKyn. The quantum yields for the anoxic UVA photodecomposition of Kyn-Lys, Kyn-His, and Kyn-Cys are 3–8-fold higher than for the protein-free kynurenines. More importantly, DHA formed as a result of ASC photooxidation was able to promote the synthesis of AGEs in proteins. Although this mechanism of AGE formation may not be important in young human lens (young lenses have negligible protein-bound kynurenines), it could be important for aged lenses (Fig. 8). Moreover, although we were not able to establish the relative contribution of free and protein-bound kynurenines in the formation of AGEs, mechanistic studies by Sherin et al. (67) suggest that protein-bound kynurenines have a higher probability of forming these two AGEs.
AGEs themselves are photosensitizers (73). Upon UVA irradiation, they could oxidize ASC and promote the synthesis of AGEs. Thus, a vicious cycle could form in which AGEs promote AGE synthesis and consequently propagate lens protein modification. We have previously shown that 3OHKyn can promote pentosidine synthesis from ASC under aerobic conditions (74). Thus, there may be additional UVA-independent mechanisms for the formation of AGEs in lens proteins.
In our study, irradiation of young human lenses for 1 h at 20 °C yielded 37 nmol of DHA by 840 J of absorbed UVA light/cm2, or ∼0.04 nmol/J. Reflected sunlight exposes lens to about 10 J of UVA light/day (38, 75) and can potentially generate 3.1 nmol of DHA/week or 159 nmol of DHA/year. Given the high reactivity of ASC degradation products toward the ϵ-amino group of Lys and the guanidine group in Arg in lens proteins (1, 2) and the lack of protein turnover in the human lens, the formation of AGEs through kynurenine/ASC is a viable mechanism.
Taken together, our data show that UVA light-excited free and protein-bound kynurenines can oxidize ASC in a molecular oxygen-independent manner, and this oxidation occurs even in the presence of GSH. The kynurenine-mediated ASC oxidation and subsequent AGE formation may contribute to protein cross-linking and aggregation in the nucleus of the aging and cataractous lenses.
Acknowledgments
We are thankful to Heather Butler, Katie Zongolowicz, Dr. Ming-Jin Chang, and Ganna Yakubenko (Visual Sciences Research Center, Case Western Reserve University) for breeding and genotyping the animals. We thank Drs. Sruthi Sampathkumar and Rooban B. Nahomi for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants EY022061, EY023286 (to R. H. N.), P30EY-11373 (Visual Sciences Research Center of Case Western Reserve University), and EY07099 (to V. M. M.). This work was also supported by Research to Prevent Blindness, New York (Case Western Reserve University) and the Ohio Lions Eye Research Foundation.
This paper is dedicated to the memory of our mentor, friend, and colleague Dr. Beryl Ortwerth (deceased November 16, 2013), who contributed immensely to our understanding of ascorbate-mediated glycation in the lens.
- Nfk
- N′-formylkynurenine
- Kyn
- kynurenine
- 3OHKyn
- dl-3-hydroxykynurenine
- ASC
- ascorbate
- AGE
- advanced glycation end product
- hSVCT
- human sodium-dependent vitamin C transporter-2
- IDO
- indoleamine 2,3-dioxygenase
- hIDO
- human IDO
- DHA
- dehydroascorbate
- AAH
- artificial aqueous humor
- LMW
- low molecular weight
- WS
- water-soluble
- WI
- water-insoluble
- CML
- Nϵ-carboxymethyllysine
- ROS
- reactive oxygen species
- DTPA
- diethylene triamine pentaacetic acid.
REFERENCES
- 1. Cheng R., Feng Q., Ortwerth B. J. (2006) LC-MS display of the total modified amino acids in cataract lens proteins and in lens proteins glycated by ascorbic acid in vitro. Biochim. Biophys. Acta 1762, 533–543 [DOI] [PubMed] [Google Scholar]
- 2. Cheng R., Lin B., Ortwerth B. J. (2002) Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim. Biophys. Acta 1587, 65–74 [DOI] [PubMed] [Google Scholar]
- 3. Chen Y. C., Reid G. E., Simpson R. J., Truscott R. J. (1997) Molecular evidence for the involvement of α crystallin in the colouration/crosslinking of crystallins in age-related nuclear cataract. Exp. Eye Res. 65, 835–840 [DOI] [PubMed] [Google Scholar]
- 4. Lou M. F., Dickerson J. E., Jr., Tung W. H., Wolfe J. K., Chylack L. T., Jr. (1999) Correlation of nuclear color and opalescence with protein S-thiolation in human lenses. Exp. Eye Res. 68, 547–552 [DOI] [PubMed] [Google Scholar]
- 5. Fan X., Reneker L. W., Obrenovich M. E., Strauch C., Cheng R., Jarvis S. M., Ortwerth B. J., Monnier V. M. (2006) Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. U.S.A. 103, 16912–16917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng R., Feng Q., Argirov O. K., Ortwerth B. J. (2004) Structure elucidation of a novel yellow chromophore from human lens protein. J. Biol. Chem. 279, 45441–45449 [DOI] [PubMed] [Google Scholar]
- 7. Balasubramanian D. (2000) Ultraviolet radiation and cataract. J. Ocul. Pharmacol. Ther. 16, 285–297 [DOI] [PubMed] [Google Scholar]
- 8. Linetsky M., Shipova E., Cheng R., Ortwerth B. J. (2008) Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim. Biophys. Acta 1782, 22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., Monnier V. M. (1991) High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U.S.A. 88, 10257–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizdrak J., Hains P. G., Truscott R. J., Jamie J. F., Davies M. J. (2008) Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic. Biol. Med. 44, 1108–1119 [DOI] [PubMed] [Google Scholar]
- 11. Parker N. R., Jamie J. F., Davies M. J., Truscott R. J. (2004) Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic. Biol. Med. 37, 1479–1489 [DOI] [PubMed] [Google Scholar]
- 12. Kessel L., Kalinin S., Nagaraj R. H., Larsen M., Johansson L. B. (2002) Time-resolved and steady-state fluorescence spectroscopic studies of the human lens with comparison to argpyrimidine, pentosidine and 3-OH-kynurenine. Photochem. Photobiol. 76, 549–554 [DOI] [PubMed] [Google Scholar]
- 13. Ortwerth B. J., Prabhakaram M., Nagaraj R. H., Linetsky M. (1997) The relative UV sensitizer activity of purified advanced glycation endproducts. Photochem. Photobiol. 65, 666–672 [DOI] [PubMed] [Google Scholar]
- 14. Takikawa O., Truscott R. J., Fukao M., Miwa S. (2003) Age-related nuclear cataract and indoleamine 2,3-dioxygenase-initiated tryptophan metabolism in the human lens. Adv. Exp. Med. Biol. 527, 277–285 [DOI] [PubMed] [Google Scholar]
- 15. Streete I. M., Jamie J. F., Truscott R. J. (2004) Lenticular levels of amino acids and free UV filters differ significantly between normals and cataract patients. Invest. Ophthalmol. Vis. Sci. 45, 4091–4098 [DOI] [PubMed] [Google Scholar]
- 16. Korlimbinis A., Aquilina J. A., Truscott R. J. (2007) Protein-bound and free UV filters in cataract lenses. The concentration of UV filters is much lower than in normal lenses. Exp. Eye Res. 85, 219–225 [DOI] [PubMed] [Google Scholar]
- 17. Staniszewska M. M., Nagaraj R. H. (2005) 3-hydroxykynurenine-mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J. Biol. Chem. 280, 22154–22164 [DOI] [PubMed] [Google Scholar]
- 18. Staniszewska M., Nagaraj R. H. (2007) Detection of kynurenine modifications in proteins using a monoclonal antibody. J. Immunol. Methods 324, 63–73 [DOI] [PubMed] [Google Scholar]
- 19. Mailankot M., Staniszewska M. M., Butler H., Caprara M. H., Howell S., Wang B., Doller C., Reneker L. W., Nagaraj R. H. (2009) Indoleamine 2,3-dioxygenase overexpression causes kynurenine-modification of proteins, fiber cell apoptosis and cataract formation in the mouse lens. Lab. Invest. 89, 498–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagaraj R. H., Linetsky M., Stitt A. W. (2012) The pathogenic role of Maillard reaction in the aging eye. Amino Acids 42, 1205–1220 [DOI] [PubMed] [Google Scholar]
- 21. Simpson G. L., Ortwerth B. J. (2000) The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim. Biophys. Acta 1501, 12–24 [DOI] [PubMed] [Google Scholar]
- 22. Lee K. W., Simpson G., Ortwerth B. (1999) A systematic approach to evaluate the modification of lens proteins by glycation-induced crosslinking. Biochim. Biophys. Acta 1453, 141–151 [DOI] [PubMed] [Google Scholar]
- 23. Kramarenko G. G., Hummel S. G., Martin S. M., Buettner G. R. (2006) Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem. Photobiol. 82, 1634–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snytnikova O. A., Sherin P. S., Kopylova L. V., Tsentalovich Y. P. (2007) Kinetics and mechanism of reactions of photoexcited kynurenine with molecules of some natural compounds. Russ. Chem. Bull. Int. Ed. 56, 732–738 [Google Scholar]
- 25. Smuda M., Glomb M. A. (2013) Maillard degradation pathways of vitamin C. Angew. Chem. Int. Ed. Engl. 52, 4887–4891 [DOI] [PubMed] [Google Scholar]
- 26. Nagaraj R. H., Shamsi F. A., Huber B., Pischetsrieder M. (1999) Immunochemical detection of oxalate monoalkylamide, an ascorbate-derived Maillard reaction product in the human lens. FEBS Lett. 453, 327–330 [DOI] [PubMed] [Google Scholar]
- 27. Tessier F., Obrenovich M., Monnier V. M. (1999) Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 274, 20796–20804 [DOI] [PubMed] [Google Scholar]
- 28. Saxena P., Saxena A. K., Cui X. L., Obrenovich M., Gudipaty K., Monnier V. M. (2000) Transition metal-catalyzed oxidation of ascorbate in human cataract extracts: possible role of advanced glycation end products. Invest. Ophthalmol. Vis. Sci. 41, 1473–1481 [PubMed] [Google Scholar]
- 29. Argirov O. K., Lin B., Ortwerth B. J. (2004) 2-Ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J. Biol. Chem. 279, 6487–6495 [DOI] [PubMed] [Google Scholar]
- 30. McNulty R., Wang H., Mathias R. T., Ortwerth B. J., Truscott R. J., Bassnett S. (2004) Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 559, 883–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giblin F. J. (2000) Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther. 16, 121–135 [DOI] [PubMed] [Google Scholar]
- 32. Tessier F., Moreaux V., Birlouez-Aragon I., Junes P., Mondon H. (1998) Decrease in vitamin C concentration in human lenses during cataract progression. Int. J. Vitam. Nutr. Res. 68, 309–315 [PubMed] [Google Scholar]
- 33. Dolin P. J. (1994) Ultraviolet radiation and cataract: a review of the epidemiological evidence. Br. J. Ophthalmol. 78, 478–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hiller R., Giacometti L., Yuen K. (1977) Sunlight and cataract: an epidemiologic investigation. Am. J. Epidemiol. 105, 450–459 [DOI] [PubMed] [Google Scholar]
- 35. Javitt J. C., Taylor H. R. (1994) Cataract and latitude. Doc. Ophthalmol. 88, 307–325 [DOI] [PubMed] [Google Scholar]
- 36. Zigman S., Datiles M., Torczynski E. (1979) Sunlight and human cataracts. Invest. Ophthalmol. Vis. Sci. 18, 462–467 [PubMed] [Google Scholar]
- 37. Neale R. E., Purdie J. L., Hirst L. W., Green A. C. (2003) Sun exposure as a risk factor for nuclear cataract. Epidemiology 14, 707–712 [DOI] [PubMed] [Google Scholar]
- 38. Sliney D. H. (1987) Estimating the solar ultraviolet radiation exposure to an intraocular eye implant. J. Cataract Refract Surg. 13, 296–301 [DOI] [PubMed] [Google Scholar]
- 39. Godar D. E., Wengraitis S. P., Shreffler J., Sliney D. H. (2001) UV doses of Americans. Photochem. Photobiol. 73, 621–629 [DOI] [PubMed] [Google Scholar]
- 40. Ortwerth B. J., Chemoganskiy V., Mossine V. V., Olesen P. R. (2003) The effect of UVA light on the anaerobic oxidation of ascorbic acid and the glycation of lens proteins. Invest. Ophthalmol. Vis. Sci. 44, 3094–3102 [DOI] [PubMed] [Google Scholar]
- 41. Dillon J. (1999) Sunlight exposure and cataract. JAMA 281, 229; author reply 230 [PubMed] [Google Scholar]
- 42. Dillon J., Zheng L., Merriam J. C., Gaillard E. R. (1999) The optical properties of the anterior segment of the eye: implications for cortical cataract. Exp. Eye Res. 68, 785–795 [DOI] [PubMed] [Google Scholar]
- 43. Giblin F. J., Leverenz V. R., Padgaonkar V. A., Unakar N. J., Dang L., Lin L. R., Lou M. F., Reddy V. N., Borchman D., Dillon J. P. (2002) UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp. Eye Res. 75, 445–458 [PMC free article] [PubMed] [Google Scholar]
- 44. Simpanya M. F., Ansari R. R., Leverenz V., Giblin F. J. (2008) Measurement of lens protein aggregation in vivo using dynamic light scattering in a guinea pig/UVA model for nuclear cataract. Photochem. Photobiol. 84, 1589–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Linetsky M., Ortwerth B. J. (1997) Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem. Photobiol. 65, 522–529 [DOI] [PubMed] [Google Scholar]
- 46. Linetsky M., James H. L., Ortwerth B. J. (1996) The generation of superoxide anion by the UVA irradiation of human lens proteins. Exp. Eye Res. 63, 67–74 [DOI] [PubMed] [Google Scholar]
- 47. Linetsky M., Ortwerth B. J. (1996) Quantitation of the reactive oxygen species generated by the UVA irradiation of ascorbic acid-glycated lens proteins. Photochem. Photobiol. 63, 649–655 [DOI] [PubMed] [Google Scholar]
- 48. Ortwerth B. J., Bhattacharyya J., Shipova E. (2009) Tryptophan metabolites from young human lenses and the photooxidation of ascorbic acid by UVA light. Invest. Ophthalmol. Vis. Sci. 50, 3311–3319 [DOI] [PubMed] [Google Scholar]
- 49. Simat T., Meyer K., Stöver B., Steinhart H. (1996) Oxidation of free and peptide bound tryptophan. Adv. Exp. Med. Biol. 398, 655–659 [DOI] [PubMed] [Google Scholar]
- 50. Fan X., Monnier V. M. (2008) Vitamin C-mediated Maillard reaction in the lens probed in a transgenic-mouse model. Ann. N.Y. Acad. Sci. 1126, 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hewitt E. J., Dickes G. J. (1961) Spectrophotometric measurements on ascorbic acid and their use for the estimation of ascorbic acid and dehydroascorbic acid in plant tissues. Biochem. J. 78, 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tessier F., Birlouez-Aragon I., Tjani C., Guilland J. C. (1996) Validation of a micromethod for determining oxidized and reduced vitamin C in plasma by HPLC-fluorescence. Int. J. Vitam. Nutr. Res. 66, 166–170 [PubMed] [Google Scholar]
- 53. Lou M. F., Dickerson J. E., Jr., Garadi R., York B. M., Jr. (1988) Glutathione depletion in the lens of galactosemic and diabetic rats. Exp. Eye Res. 46, 517–530 [DOI] [PubMed] [Google Scholar]
- 54. Bassnett S. (1990) Intracellular pH regulation in the embryonic chicken lens epithelium. J. Physiol. 431, 445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilker S. C., Chellan P., Arnold B. M., Nagaraj R. H. (2001) Chromatographic quantification of argpyrimidine, a methylglyoxal-derived product in tissue proteins: comparison with pentosidine. Anal. Biochem. 290, 353–358 [DOI] [PubMed] [Google Scholar]
- 56. Ahmed N., Argirov O. K., Minhas H. S., Cordeiro C. A., Thornalley P. J. (2002) Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nϵ-carboxymethyl-lysine- and Nϵ-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 364, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bova L. M., Sweeney M. H., Jamie J. F., Truscott R. J. (2001) Major changes in human ocular UV protection with age. Invest. Ophthalmol. Vis. Sci. 42, 200–205 [PubMed] [Google Scholar]
- 58. Garner B., Vazquez S., Griffith R., Lindner R. A., Carver J. A., Truscott R. J. (1999) Identification of glutathionyl-3-hydroxykynurenine glucoside as a novel fluorophore associated with aging of the human lens. J. Biol. Chem. 274, 20847–20854 [DOI] [PubMed] [Google Scholar]
- 59. Taylor L. M., Andrew Aquilina J., Jamie J. F., Truscott R. J. (2002) UV filter instability: consequences for the human lens. Exp. Eye Res. 75, 165–175 [DOI] [PubMed] [Google Scholar]
- 60. Parker N. R., Korlimbinis A., Jamie J. F., Davies M. J., Truscott R. J. (2007) Reversible binding of kynurenine to lens proteins: potential protection by glutathione in young lenses. Invest. Ophthalmol. Vis. Sci. 48, 3705–3713 [DOI] [PubMed] [Google Scholar]
- 61. Varma S. D. (1987) Ascorbic acid and the eye with special reference to the lens. Ann. N.Y. Acad. Sci. 498, 280–306 [DOI] [PubMed] [Google Scholar]
- 62. Korlimbinis A., Aquilina J. A., Truscott R. J. (2007) Protein-bound UV filters in normal human lenses: the concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest. Ophthalmol. Vis. Sci. 48, 1718–1723 [DOI] [PubMed] [Google Scholar]
- 63. Korlimbinis A., Truscott R. J. (2006) Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochemistry 45, 1950–1960 [DOI] [PubMed] [Google Scholar]
- 64. Sherin P. S., Grilj J., Tsentalovich Y. P., Vauthey E. (2009) Ultrafast excited-state dynamics of kynurenine, a UV filter of the human eye. J. Phys. Chem. B 113, 4953–4962 [DOI] [PubMed] [Google Scholar]
- 65. Reszka K. J., Bilski P., Chignell C. F., Dillon J. (1996) Free radical reactions photosensitized by the human lens component, kynurenine: an EPR and spin trapping investigation. Free Radic. Biol. Med. 20, 23–34 [DOI] [PubMed] [Google Scholar]
- 66. Kopylova L. V., Snytnikova O. A., Chernyak E. I., Morozov S. V., Tsentalovich Y. P. (2007) UV filter decomposition. A study of reactions of 4-(2-aminophenyl)-4-oxocrotonic acid with amino acids and antioxidants present in the human lens. Exp. Eye Res. 85, 242–249 [DOI] [PubMed] [Google Scholar]
- 67. Sherin P. S., Tsentalovich Y. P., Snytnikova O. A., Sagdeev R. Z. (2008) Photoactivity of kynurenine-derived UV filters. J. Photochem. Photobiol. B 93, 127–132 [DOI] [PubMed] [Google Scholar]
- 68. Walrant P., Santus R., Grossweiner L. I. (1975) Photosensitizing properties of N-formylkynurenine. Photochem. Photobiol. 22, 63–65 [DOI] [PubMed] [Google Scholar]
- 69. Bors W., Buettner G. R. (1997) in Vitamin C in Health and Disease (Packer L., Fuchs J., eds) pp. 75–94, Marcel Dekker, Inc., New York [Google Scholar]
- 70. Grandhee S. K., Monnier V. M. (1991) Mechanism of formation of the Maillard protein cross-link pentosidine: glucose, fructose, and ascorbate as pentosidine precursors. J. Biol. Chem. 266, 11649–11653 [PubMed] [Google Scholar]
- 71. Snytnikova O. A., Fursova A. Z., Chernyak E. I., Vasiliev V. G., Morozov S. V., Kolosova N. G., Tsentalovich Y. P. (2008) Deaminated UV filter 3-hydroxykynurenine O-β-d-glucoside is found in cataractous human lenses. Exp. Eye Res. 86, 951–956 [DOI] [PubMed] [Google Scholar]
- 72. Shipanova I. N., Glomb M. A., Nagaraj R. H. (1997) Protein modification by methylglyoxal: chemical nature and synthetic mechanism of a major fluorescent adduct. Arch. Biochem. Biophys. 344, 29–36 [DOI] [PubMed] [Google Scholar]
- 73. de La Rochette A., Birlouez-Aragon I., Silva E., Morlière P. (2003) Advanced glycation endproducts as UVA photosensitizers of tryptophan and ascorbic acid: consequences for the lens. Biochim. Biophys. Acta 1621, 235–241 [DOI] [PubMed] [Google Scholar]
- 74. Nagaraj R. H., Padmanabha S., Mailankot M., Staniszewska M., Mun L. J., Glomb M. A., Linetsky M. D. (2010) Modulation of advanced glycation endproduct synthesis by kynurenines in human lens proteins. Biochim. Biophys. Acta 1804, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sabburg J., Parisi A. V., Wong J. (2001) Effect of cloud on UVA and exposure to humans. Photochem. Photobiol. 74, 412–416 [DOI] [PubMed] [Google Scholar]