Abstract
Histone modifying enzymes catalyze the addition or removal of an array of covalent modifications in histones and non-histone proteins. Within the context of chromatin, these modifications regulate gene expression as well as other genomic functions and have been implicated in establishing and maintaining a heritable epigenetic code that contributes to defining cell identity and fate. Biochemical and structural characterization of histone modifying enzymes has yielded important insights into their respective catalytic mechanisms, substrate specificities, and regulation. In this review, we summarize recent advances in understanding these enzymes, highlighting studies of the histone acetyltransferases (HATs) p300 (also now known as KAT3B) and Rtt109 (KAT11) and the histone lysine demethylases (HDMs) LSD1 (KDM1) and JMJD2A (KDM4A), present overriding themes that derive from these studies, and pose remaining questions concerning their regulatory roles in mediating DNA transactions.
Keywords: Chromatin, Transcription, Histone Modifications, Histone Acetyltransferases, Histone Demethylases
Introduction
Eukaryotic DNA is packaged within a nucleic acid-protein complex termed chromatin. The fundamental repeating unit of chromatin is the nucleosome core particle which is comprised of approximately 150 base pairs of DNA wound around an octameric core of two molecules each of the core histones H2A, H2B, H3 and H4 [1]. In the presence of linker histones or heterochromatin associated-proteins, nucleosomes can be condensed into 30 nm diameter fibers and high-ordered assemblies whose structures are poorly understood. These hierarchical structures form a repressive environment that inhibit the activities of enzymes that require direct access to the DNA template, such as in transcription, DNA repair, replication, and recombination. Chromatin condensation can be modulated through a variety of mechanisms, including covalent modifications of DNA and histones. While DNA undergoes methylation of its cytosine bases within CpG repeats, histones are subject to a myriad of modifications in their random coil N-terminal tails, and to a lesser extent within their C-terminal tails and globular domains [2]. The 1960s and 1970s witnessed the discovery of a multitude of histone modifications including lysine acetylation[3], lysine methylation [3, 4], serine phosphorylation [5, 6], ADP ribosylation [7], and ubiquitination[8, 9]. Although data from many laboratories furnished circumstantial evidence linking modification states to the transcriptional status of genes, the exact biological functions of histone modifications remained enigmatic for several decades.
In 1996, two groups reported the discovery of histone modifying enzymes that were related by sequence homology to previously identified transcriptional regulators in Saccharomyces cerevisiae. Utilizing an affinity matrix, Schreiber and colleagues isolated a mammalian histone deacetylase (HDAC) that harbors 60% sequence identity with the yeast transcriptional repressor Rpd3 [10]. Concurrently, Allis and coworkers purified a histone acetyltransferase (HAT) from Tetrahymena thermophila that is highly homologous to the yeast transcriptional adaptor Gcn5 [11]. The identification of these enzymes represented a milestone in understanding the biological functions underlying histone modifications because they furnished the first direct evidence unequivocally linking histone modification states and transcriptional regulation. The discovery of Gcn5 and Rpd3 presaged the identification and characterization of other families of HATs and HDACs as well as other classes of histone modifying enzymes, including kinases [12, 13], lysine and arginine-specific methyltransferases [14–16], arginine deiminases [17, 18], ubiquitinases [19], deubiquitinases [20–22], and lysine- and arginine-specific demethylases (HDMs) [23–25]. Previous studies had implicated many of these proteins in transcription regulation or other genomic functions, further underscoring the correlation between histone modifications and chromatin-dependent processes. Collectively, these efforts have transformed our understanding of the role of chromatin modifications in governing eukaryotic gene expression and other DNA-dependent functions.
Following their discovery, structural and biochemical studies have played a central role in defining the molecular basis for the substrate specificities and catalytic mechanisms of histone modifying enzymes. This review summarizes current advances in understanding these enzymes, focusing on structures and mechanisms of HATs and HDMs. Specifically, we will focus on the recently described crystal structure of human p300, a member of the metazoan-specific p300/CBP HAT family, and yeast Rtt109, a recently identified fungal-specific histone H3 Lys56 (H3K56)-specific acetyltransferase [26–28]. In addition, we will examine the recently reported structures of the HDMs LSD1 and JMJD2A bound to histone H3 peptides that have furnished new insights into their respective substrate specificities.
Overview of HATs
Since the isolation of the first HAT from Tetrahymena by Allis and coworkers just over a decade ago, [29] many other HATs have been identified from yeast to man. In addition to the cytoplasmic B-type HATs, such as HAT1, that are involved in histone deposition, nuclear or A-type HATs, can be grouped into at least four different families based on sequence conservation within the HAT domain. This includes Gcn5/PCAF (named for its founding member yeast Gcn5 and its human ortholog, PCAF), MYST (named for the founding members MOZ, Ybf2/ Sas3, Sas2 and Tip60), p300/CBP (named for the two human paralogs p300 and CBP) and Rtt109 (named for its initial identification as a regulator of Ty1 transposition gene product 109). While the Gcn5/PCAF and MYST families have homologs from yeast to man, p300/CBP is metazoan-specific, and Rtt109 is fungal-specific. Although other nuclear HAT families have been identified, such as the steroid receptor coactivators (ACTR/AIB1, SRC1) [30], TAF250 [31], ATF-2 [32], and most recently CLOCK [33], their HAT activities have not been studied as extensively as the four major HAT classes.
The Gcn5/PCAF and MYST HATs were the first to be characterized at the biochemical and structural levels, and the reader is directed to one of several reviews summarizing the findings of these earlier studies (for example, see [34, 35]). In brief, the Gcn5/PCAF and MYST HATs do not contain significant sequence homology between them, yet they each adopt a globular α/β fold containing a structurally superimposable central region associated with acetyl-Coenzyme A (Ac-CoA) cofactor binding but structurally more divergent regions flanking this central region (Figures 1a and 1b). In the case of the Gcn5/PCAF family, the N- and C-terminal regions play a direct role in histone substrate binding while the role of these regions in the MYST HATs are implicated to have similar roles and have also been more recently implicated in chromatin targeting [36]. Curiously, although both the Gcn5/PCAF and MYST HAT families have been shown to employ a structurally conserved glutamate residue as a general base for the acetyl-transfer reaction, the Gcn5/PCAF HATs have been shown to employ a ternary bi-bi catalytic mechanism [37, 38], while the yeast Esa1 member of the MYST family of HATs has been demonstrated to use a ping-pong catalytic mechanism employing an acetyl-cysteine protein intermediate [39]. A more recent report demonstrates that Esa1 assembled within a physiologically relevant piccolo NuA4 complex proceeds through a ternary bi-bi catalytic mechanism [40], suggesting that the same HAT enzyme may employ different catalytic mechanisms within different cellular contexts.
Figure 1. Overall structure of nuclear HATs.
(a) Tetrahymena Gcn5 (1PUA.pdb) is shown in complex with CoA (stick representation in CPK coloring) and histone H3 (backbone representation in green coloring) illustrating a representative Gcn5/PCAF HAT. The protein is shown in cartoon representation with the structurally conserved HAT core region highlighted in blue and the more structurally variable HAT regions shown in cyan.
(b) Yeast Esa1 in complex with CoA (1FY7.pdb) illustrates a representative MYST HAT. The coloring is as described in part (a).
(c) Human p300 in complex with the Lys-CoA bisubstrate inhibitor (3BIY.pdb) shows a representative p300/CBP HAT. The coloring is as described in part (a) and the substrate binding loop is highlighted in cartoon representation in red.
(d) Yeast Rtt109 in complex with Ac-CoA (3D35.pdb). The coloring is as described in part (c).
Very recently, structural and associated biochemical and enzymatic studies have been described for the p300/CBP [41] and Rtt109 [42] HATs and these more recent studies will be the focus of the current discussion of HATs.
p300/CBP
The p300 and CBP paralogs (herein called p300/CBP) were first characterized about 15 years ago as activators of cyclic-AMP genes and as adenovirus E1A binding proteins [43]. These proteins were subsequently shown to be metazoan-specific global transcriptional coactivators that function, in part, through the recruitment of many transcription factors including other HATs such as PCAF [44]. In 1996, Bannister and Kouzarides reported that CBP has intrinsic acetyltransferase activity on histones [45], and numerous subsequent reports demonstrated that p300 and CBP not only acetylate the four core histones but also acetylate numerous transcription factor and non-transcription factor targets [46]. Interestingly, the p300/CBP HAT domain shows no sequence homology to other known HATs.
Earlier this year, Liu et al. reported the crystal structure of the p300 HAT domain in complex with a Lys-CoA bisubstrate inhibitor and accompanying biochemical analysis of the enzyme [41]. The structure reveals an elongated globular domain containing a central seven-strand β-sheet surrounded by nine α-helices and several loops (Figure 1c). One side of the Lys-CoA inhibitor is positioned roughly in the middle (between β4 and β5) and against the wide edge of the β-sheet and the C-terminal ends of two helices (α3 and α4). A long ~25 residue loop (L1), called the substrate binding loop, encapsulates the opposite side of the Lys-CoA inhibitor. A comparison with the Gcn5/PCAF and MYST HATs reveals structural conservation within the central core region associated with acetyl-CoA cofactor binding, despite the lack of sequence homology, but significant structural divergence flanking this central core region (Figures 1a–1c). Despite the similarities among the HATs within the domain associated with Ac-CoA cofactor binding, the L1 substrate binding loop is a unique feature of the p300 HAT domain, burying about one-third of the CoA portion of the Lys-CoA inhibitor and mediating about one-half of the CoA interactions.
There are several other structural features of p300 that deviate from the Gcn5/PCAF and MYST HATs that appears to be important for p300-specific HAT function. Most notably, the electrostatic surface proximal to the lysine portion of the Lys-CoA inhibitor is electronegative (Figure 2a), while the corresponding regions of Gcn5/PCAF and MYST HATs are considerably more apolar. In addition, this electronegative surface of p300 contains two pockets separated by a distance corresponding to about 3–4 amino acid residues. Correlating with this observation, an alignment of all known p300/CBP substrates reveals that they all contain a basic amino acid either three or four residues up- or downstream of the target lysine. Liu et al. [41] show that mutagenesis of this electronegative site increases the KM for histone H3 substrate, further supporting the importance of this site for protein substrate binding by p300. Taken together, the presented data is consistent with the more promiscuous substrate binding properties of p300/CBP relative to the Gcn5/PCAF and MYST HATs.
Figure 2. p300/CBP substrate binding site and enzymatic mechanism.
(a) The histone/protein binding site groove is highlighted. An electrostatic surface of p300 is shown with positive, negative, and neutral charged surfaces shown in blue, red, and white, respectively. The two pronounced electronegative p300 pockets that are proposed to participate in protein substrate binding are highlighted with black dotted circles. Lys-CoA is shown in yellow stick representation.
(b) The active site highlighting residues (CPK coloring with carbon atoms in green) that are in position to play catalytic roles.
(c) The Theorell-Chance (hit and run) catalytic mechanism is shown.
(d) A model for regulation of p300/CBP by autoacetylation is shown where it is proposed that the lysine-rich basic activation loop blocks the histone/protein binding site in the hypoacetylated form and is released from this site upon autoacetylation.
Prior to the structure and biochemical analysis of the p300 HAT domain reported by Liu et al. [41], the catalytic mechanism of p300/CBP had been in question. Specifically, while the observation that p300 is inhibited by the Lys-CoA bisubstrate inhibitor suggested a ternary complex mechanism [47], the parallel line pattern observed in the double reciprocal plots for the bisubstrate kinetics was suggestive of a ping-pong mechanism [48]. Interestingly, the p300 crystal structure reveals that there is no glutamate residue that is analogous to those in Gcn5/PCAF and Esa1, which is in position to function as a general base for catalysis. Mutagenesis and kinetic analysis of the residues in the active site that might play a role in catalysis reveal that only two residues show a significant effect on catalysis when mutated (Figure 2b) [41]. A Phe substitution of Tyr1467 shows about a 400-fold reduction in catalytic efficiency (kcat/KM). Similarly, mutation of Trp1436 to an alanine reduces catalytic efficiency 50-fold, while a substitution by Phe shows a more modest effect on catalysis. Based on the position of these residues in the structure, Tyr1467 is proposed to play a role as a general acid for catalysis, while Trp1436 is proposed to orient the target lysine into the active site. Thus, it appears that p300/CBP does not employ a general base for catalysis, in contrast to the Gcn5/PCAF and Esa1 HATs. Taken together with the fact that p300 is inhibited potently by the more primitive Lys-CoA inhibitor but poorly by bisubstrate analogs with longer peptide moieties; and the observation that longer peptides are better substrates for p300 than lysine, is consistent with a “hit-and-run” or Theorell-Chance acetyl transfer mechanism that is distinct from the catalytic mechanisms employed by the Gcn5/PCAF and MYST HAT families (Figure 2c).
An interesting property of the p300/CBP HATs is the presence of a highly basic autoacetylation loop that is hyperacetylated in the active form of the p300/CBP HAT [49] and that has been proteolytically cleaved in the current structure of p300 [41]. It has been demonstrated that this autoacetylation occurs through an intermolecular mechanism [50] and Liu et al. [41] propose that this highly basic loop sits in the electronegative substrate binding site and is released from this site upon autoacetylation (Figure 2d).
Rtt109
Rtt109 was first identified as a budding yeast regulator of Ty1 transposition that also plays an important role in the DNA damage response to genotoxic agents [51, 52]. More recently, genomic and proteomic screens as well as a more directed effort lead to the observation that Rtt109 promotes genome stability and resistance to a variety of DNA-damaging agents through the direct acetylation of H3K56 during S-phase [26–28]. Similar to the p300/CBP family, Rtt109 has no detectable sequence homology to other known HATs. Unlike other HATs, Rtt109 requires the presence of one of two histone chaperone proteins, Asf1 or Vps75, to acetylate H3K56 [53–55].
Tang et al. recently reported the crystal structure of Rtt109 bound to Ac-CoA [42]. The most striking and unexpected aspect of this structure is the overall structural similarity to p300 (Figures 1c and 1d). This includes a structurally conserved central seven stranded β-sheet, surrounding helices and L1 substrate binding loop that plays an analogous role in Ac-CoA interaction. A sequence alignment, based on the structural superposition between p300 and Rtt109 reveals only 7% sequence identity (bordering on the identity shared by two unrelated proteins) that is generally scattered throughout the respective HAT domains.
Despite the structural superposition, Tang et al. [42] present several lines of investigation suggesting that the Rtt109 and p300/CBP HATs diverge significantly at the functional level. First, the Rtt109 substrate binding site is not as electronegative as it is in p300. Indeed the Rtt109 substrate binding site is more apolar and more similar to the Gcn5/PCAF and MYST HATs. In addition, the active site residues of Rtt109 are not conserved in p300, nor are they conserved in Gcn5/PCAF or Esa1 (Figure 3a). Finally, the potent Lys-CoA p300 inhibitor is a poor inhibitor for Rtt109. Taking this data together suggests that Rtt109 employs a catalytic mechanism that is distinct from other HATs, despite its high structural conservation with p300.
Figure 3. Structural details of the Rtt109/Ac-CoA complex.
(a) The active site highlighting residues that are mutationally sensitive for HAT activity. Ac-CoA is shown in CPK coloring and stick representation.
(b) Autoacetylated Lys290 is shown hydrogen bonded to Asp288 and buried within a hydrophobic core of phenylalanine residues.
Analysis of Rtt109 mutations in the context of the structure reveals some preliminary insights into the catalytic mechanism of Rtt109. Asparagine residues 89, 287 and 288 were previously shown to be mutationally sensitive for HAT activity by Rtt109 [27, 56], and the Rtt109/Ac-CoA structure reveals that while Asp89 plays an important role in stabilizing the position of the L1 loop, asparagines residues 287 and 288 may participate in binding the lysine-bearing histone substrate (Figure 3a). The more recent structure-based mutagenesis performed by Tang et al. [42] reveals that Tyr199 and Trp222, which are not conserved in the active site of other HATs, also play important roles in catalysis with likely effects on cofactor or histone substrate binding. Although the precise catalytic mechanism employed by Rtt109 has not been established, an unusual sigmoidal concentration dependence is observed with constant Ac-CoA and varied H3 that fits to a Hill equation with a Hill coefficient of 3, suggesting a high degree of cooperativity of the histone substrate in the acetylation reaction. Together, these results suggest that Rtt109 employs a catalytic mechanism that is distinct from other HATs and is consistent with a more complex nature of protein substrate recognition.
An unusual and unexpected finding of the Rtt109/Ac-CoA structure was the observation of a buried acetyl-lysine residue (Lys290) (Figure 3b) [42]. This acetyl-lysine forms hydrogen bonds with Asp288, a mutationally sensitive Rtt109 residue. Mass spectrometry analysis also shows that this acetylation occurs in vitro as well as in yeast cells. Interestingly, a Lys to Arg mutant of residue 290 does not show a detectable genotoxic agent sensitivity in vivo, suggesting that Lys290 acetylation may have a function other than to facilitate H3K56 acetylation, a histone modification that promotes genomic stability.
Broader implications for the HAT family of proteins
A surprising feature of the HAT enzymes is the significant divergence in sequence between the different HAT families. Despite this sequence divergence, a comparison of representative members of the Gcn5/PCAF, MYST, p300/CBP and Rtt109 HAT families reveals a structurally conserved central core region for Ac-CoA cofactor binding (Figure 1). Interestingly, despite the structural conservation of this central core region, the different HAT families appear to employ different catalytic mechanisms. This highlights the fact that while the templating of the acetyl transfer reaction is conserved, the specific mode of acetyl transfer is not conserved, likely reflecting the fact that acetyltransfer from a thioester to an amine is thermodynamically favorable and can proceed through various mechanisms.
Another interesting aspect that emerged from the studies on the p300 and Rtt109 HATs was that these proteins appear to be autoacetylated. In the case of p300, this autoacetylation is important for stimulating the ability of p300 to acetylate other substrates [49]. In the case of Rtt109, this autoacetylation does not appear to be important for the ability of Rtt109 to acetylate H3K56, but might facilitate Rtt109 acetylation of other substrates. PCAF has also been shown to autoacetylate itself within its nuclear localization signal region, and this autoacetylation has been shown to increase PCAF HAT activity [57]. Taking these observations together, it is possible that autoacetylation may extend to other HATs and may represent a mode of regulation that shares similarities with the phospho-regulation of kinases.
Interacting proteins mediate another level of HAT regulation. In the case of Rtt109, interaction with either the Asf1 or Vps75 histone chaperones is required for HAT activity and, although the mechanism for this cooperation has not yet been established, the fact that Asf1 and Vps75 assemble histone H3/H4 dimers and tetramers, respectively, into chromatin [54, 58] implies that at least one activity of the histone chaperones is to deliver the histone H3 substrate to Rtt109 for acetylation. This histone chaperone requirement for Rtt109 HAT activity may be related to the fact that Rtt109 does not contain other associated domains to interact with other chromatin modifiers. This is in contrast to other HATs, such as the bromodomain containing Gcn5/PCAF HATs, the chromodomain containing MYST HATs and the p300/CBP HATs that contain multiple protein interacting domains [59]. Together, these other domains within HATs may facilitate their targeting to chromatin for histone acetylation.
Human p300/CBP, PCAF, and MOF HATs have been shown to acetylate non-histone substrates such as the p53 tumor suppressor and DNA binding protein [60]. Although other HATs such as Rtt109 and many MYST HATs have not been reported to acetylate non-histone proteins, it is possible that alternative substrates for these enzymes exist. Indeed, given the broad substrate spectrum of the class II HDACs [61], it would not be surprising if more substrates, histone or non-histone, will be identified for the superfamily of HAT enzymes.
Overview of HDMs and their respective mechanisms
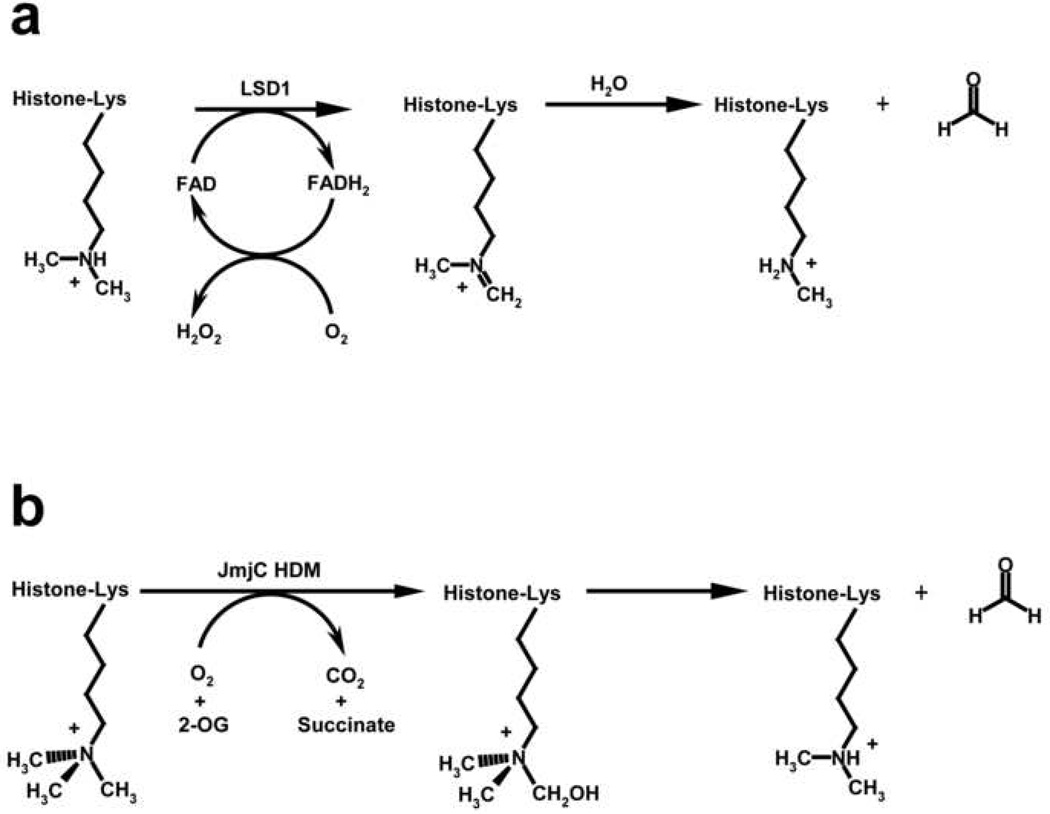
Following the discovery of histone lysine methyltransferases in 2000 [16], there was significant debate concerning the permanence of lysine methyl marks in vivo and whether these modifications are enzymatically reversible by yet unidentified HDMs. Due to the intrinsic strength of the lysine Nε-CH3 covalent bond, most proposed mechanisms of demethylation invoked one or two-electron redox chemistry, resulting in the oxidation of the methyl group and its release as formaldehyde [62, 63]. This controversy was resolved in 2004 when Shi and coworkers reported that an amino oxidase subunit associated with various HDAC corepressor complexes exhibits demethylase activity specific for mono- and dimethylated Lys4 in histone H3 (H3K4me1/2) [24]. They named this enzyme Lysine Specific Demethylase 1 (LSD1) to denote its selectivity for protein methyllysines. LSD1 belongs to the flavin adenine dinucleotide (FAD)-dependent amino oxidase family and is conserved in Schizosaccharomyces pombe through humans. Through an array of biochemical assays, Shi and colleagues demonstrated that demethylation by LSD1 is consistent with an amine oxidase-based mechanism in which the methyllysine Nε-CH3 bond is oxidized to an imine intermediate (Figure 4a). The reducing equivalents are concomitantly transferred to FAD yielding FADH2, which is recycled to its oxidized state through reduction of molecular oxygen to hydrogen peroxide. The methyllysine imine intermediate subsequently undergoes hydrolysis, resulting in the demethylation of the lysine ε-amine group and the release of the byproduct formaldehyde. The reaction mechanism of LSD1 requires a protonatable lysine ε-amine for amine oxidation, furnishing a chemical rationale for its strict specificity for mono- and dimethylated lysines. LSD1’s inactivity toward trimethylated substrates hinted at the possibility that other HDMs exist that employ alternative mechanisms compatible with trimethyllysine demethylation, spurring efforts to identify these enzymes.
Figure 4. Chemical mechanisms of lysine demethylation.
(a) FAD-dependent demethylation of mono- and dimethyllysines by LSD1. The oxidation of the methyl ε-ammonium group forms an imine intermediate that subsequently hydrolyzes to yield the demethylated lysine and a molecule of formaldehyde.
(b) Fe(II)-dependent demethylation of mono-, di-, and trimethyllysines by JmjC HDMs. Using the co-substrates O2 and 2-OG, JmjC enzymes catalyze the hydroxylation of a lysine methyl group via a radical-based mechanism, forming the products succinate and carbon dioxide. The hydroxyl-methyl ε-ammonium hemiaminal intermediate subsequently decomposes to yield the demethylated lysine and formaldehyde.
Shortly after the discovery of LSD1, a second HDM, JHDM1A (also known as FBXL11 and KDM2A) was reported and shown to demethylate H3K36me1/2 [25]. Unlike LSD1, JHDM1 possesses a JmjC catalytic domain that was previously implicated in chromatin-dependent functions [64]. Subsequently, other JmjC enzymes were identified that possess lysine demethylase activity with distinct methylation site and state specificities, including the JHDM2 (H3K9me1/2) [65], JMJD2/JHDM3 (H3K9me2/3 with some homologs exhibiting specificity for H3K36me2/3) [66–69], JARID1 (H3K4me2/3) [70–77], and UTX/JMJD3 (H3K27me2/3) families [78–84]. In addition, human JMJD6 was recently shown to encode an arginine-specific HDM that demethylates H3R2me1/2 and H4R3me1/2 [23]. Many of these proteins are conserved in yeast through humans and have been demonstrated to regulate chromatin methylation states and the transcriptional status of genes in vivo, defining these JmjC enzymes as a new class of protein demethylases.
JmjC enzymes were initially hypothesized to possess demethylase activity based on their sequence homology with the DNA repair enzyme AlkB that catalyzes the demethylation of alkylated DNA bases [25, 85]. Both AlkB and the JmjC HDMs are members of the Cupin superfamily of mononuclear Fe(II)-dependent oxygenases that catalyze hydroxylations and other redox reactions [86]. The reaction mechanism of the JmjC HDMs proceeds via the hydroxylation of the ζ-methyl group of a methyllysine using the co-substrates 2-oxoglutarate (2-OG) and molecular oxygen, which produces hydroxyl-methyllysine intermediate, succinate, and carbon dioxide (Figure 4b) [25]. The hydroxyl-methyl ε-amine intermediate represents an unstable hemiaminal species that decomposes to yield the demethylated lysine and formaldehyde. Unlike LSD1, the hydroxylation-based mechanism of the JmjC HDMs does not require a protonatable lysine ε-amine group, enabling these enzymes to demethylate trimethyllysines as well as mono- and dimethylated substrates. Thus, the JmjC HDMs provide a mechanism for cells to dynamically regulate all degrees of histone lysine methylation.
LSD
LSD1 (also known as KIAA0601, BHC110, and KDM1) was initially identified as a subunit of CtBP [87], Co-REST [88], NRD [89], and BRAF35 HDAC complexes [90]. Consistent with its HDAC-association, LSD1 has been shown to repress gene expression through the demethylation H3K4me1/2, a methylation site frequently associated with transcriptionally poised or active genes. Surprisingly, LSD1 has also been linked to gene activation. Metzger et al. first reported this altered function, demonstrating that LSD1 associates with the androgen receptor (AR) to enhance the expression of AR target genes [91]. Unexpectedly, their results also implicated LSD1 in AR-dependent demethylation of H3K9me1/2, a methylation site enriched in silent chromatin. Recent studies have corroborated these findings, indicating that LSD1 possesses coactivator functions [92, 93]. In addition, LSD1 has been reported to demethylate mono- and dimethylated Lys370 in the regulatory domain of the tumor suppressor p53, precluding the binding of the transcriptional coactivator 53BP1 [94]. Together, these results indicate that the complexes in which LSD1 resides tightly coordinate its gene regulatory functions and also influence its specificity for histone and non-histone substrates.
Recently, two groups have reported crystal structures of LSD1/CoREST complex bound to histone H3 peptides. Yang et al. co-crystallized the enzyme with a suicide inhibitor consisting of a 21-residue histone H3 peptide in which K4 is modified by an N-methylpropargyl group (Figure 5a) [95]. This moiety forms a covalent adduct with the reactive N5 atom of the flavin isoalloxazine ring, permitting the visualization of the first seven residues in the histone H3 peptide (Figure 5b). These residues adopt three successive γ-turns, resulting in an approximately W-shaped conformation of the H3 peptide backbone. The inhibitor and LSD1 interact through a series of main and side chain hydrogen bonds and van der Waals contacts, further stabilizing the compact conformation of the H3 peptide. Notable interactions with the inhibitor include hydrogen bonds to its R2 and Q5 side chains and a salt bridge interaction between the α-amine of A1 and Asp555 in LSD1. Mutations of the residues in LSD1 that interact with the inhibitor generally impair H3K4me1/2 demethylation, illustrating their role in substrate binding [95]. In particular, the addition of acetyl or glycyl blocking groups to the N-terminus of the H3K4me2 peptide or the substitution of the α-amine of A1 with a methyl group disrupts the ionic interaction between Asp555 in LSD1 and the H3 peptide α-amine, diminishing specificity over 20-fold. These data demonstrate that LSD1 prefers an unmodified α-amine three residues preceding the methyllysine in the protein substrate, consistent with its specificity for H3K4me1/2 in vitro.
Figure 5. Structural basis for the substrate specificity of LSD1.
(a) Crystal structure of a human LSD1/Co-REST complex covalently bound to a histone H3 peptide suicide inhibitor (2UXN.pdb). The secondary structure of the SWIRM (blue), AOD (gray), and Tower domains of LSD1 and the linker (magenta) and SANT2 (red) domains of Co-REST are illustrated in cartoon representation. The covalently modified FAD cofactor and H3K4 inhibitor are shown in yellow stick representation.
(b) Structure of LSD1’s active site bound to the H3 peptide methylpropargyl-K4 (K4mp) inhibitor that forms a covalent adduct with FAD (CPK coloring with yellow carbon atoms). Residues in LSD1 that interact with the H3 peptide are shown and labeled in black. Hydrogen bonds are delineated as orange dashed lines.
(c) Structure of LSD1/Co-REST complex bound to an H3K4M peptide inhibitor (cyan carbons) (2V1D.pdb) illustrated as in (b). The C-terminal residues K14, A15, and P16 in the H3 peptide are omitted for clarity.
(d) Superimposition of the H3 peptide inhibitors in (b) and (c) illustrating their distinct binding conformations within the substrate binding cleft of LSD1.
In contrast to employing a suicide inhibitor, Forneris et al. have reported LSD1/CoREST-C complex co-crystallized with a 20-residue histone H3 peptide inhibitor in which Lys4 is mutated to a methionine (H3K4M; Figure 5c) [96]. The overall conformation of the H3K4M peptide is roughly U-shaped, a binding mode that is strikingly different than the γ-turn geometry adopted by the suicide inhibitor (Figure 5d). The peptide’s binding is stabilized by a complex network of intramolecular hydrogen bonds and intermolecular hydrogen bonds and van der Waals contacts with residues comprising the substrate binding cleft of LSD1 (Figure 5c). In particular, multiple hydrogen bonds and salt bridge interactions with the guanidinium groups of R2 and R8 in H3K4M appear to be important in maintaining the peptide’s conformation and interactions with LSD1. This binding mode positions M4, which functions as a methyllysine mimic, into the pocket adjacent to the flavin moiety of FAD. Modeling of K4me2 based on the coordinates of the M4 side chain indicates that the dimethyl ε-amine group is at an appropriate distance for hydride transfer to the N5 atom in the FAD isoalloxazine ring [96].
The structure of the H3K4M complex offers molecular insights into the H3K4 specificity of LSD1. To catalyze efficient demethylation, the enzyme requires H3 peptides at least 16 residues in length [97], consistent with the well-defined electron density corresponding to the first 16 residues of the H3K4M inhibitor [96]. In addition, LSD1 exhibits a strong preference toward H3K4me2 substrates lacking other covalent modifications, including R2me, R8me, S10ph, K9ac, and K14ac [97, 98]. In the H3K4M complex, the side chains of R2, R8, and S10 engage in intra- and intermolecular hydrogen bonds that would be disrupted by covalent modifications [96]. In contrast, the side chains of K9 and K14 are solvent-exposed and do not participate in direct contacts with LSD1. However, acetylation of these lysines would diminish favorable electrostatic interactions between the basic H3 peptide and the acidic substrate binding cleft of the enzyme, consistent with the elevated KM values reported for acetylated H3K4me2 substrate peptides [98].
While the structures of these H3 peptide complexes have yielded new details into the mechanism and specificity of LSD1, several questions remain regarding its ability to demethylate other methylation sites. Specifically, both complexes display selective recognition of the unmodified α-amine of A1 in histone H3 by LSD1 [95, 96], a requirement that cannot be met in H3K9me1/2 and p53K370me1/2 in which the methylation sites are distal from the substrates’ N-terminal α-amine groups. However, the variations in the binding modes of the H3 inhibitors indicates that LSD1 exhibits plasticity in binding peptide substrates, in agreement with its spacious substrate binding cleft that is capable of accommodating substrates in multiple conformations (Figure 5d). In addition, the findings that the androgen receptor and potentially other transcription factors regulate the specificity of LSD1 suggest these proteins may alter the conformation of the enzyme’s substrate binding cleft or may directly interact with the substrate to promote recognition. Future structural studies are required to resolve the mechanisms by which LSD1’s specificity is modulated by its binding partners.
JMJD2A
JMJD2A was the first reported trimethyllysine-specific JmjC HDM [68, 69]. This HDM belongs to the JMJD2 family that is conserved in yeast through mammals with the human genome encoding four paralogs: JMJD2A-D [99]. Human JMJD2A exhibits dual specificity for the trimethylated and, to a lesser extent, the dimethylated forms of H3K9 and H3K36, while other JMJD2 paralogs, such as JMJD2B and JMJD2D, are specific for H3K9me2/3 [66–69]. Initial characterization of JMJD2A implicated this enzyme in transcriptional silencing, owing to its association with the retinoblastoma protein, class I HDACs, and the nuclear corepressor N-CoR [100, 101]. Conversely, recent work has revealed that JMJD2A and its paralog JMJD2D associate with the androgen receptor (AR) to upregulate the expression of AR-dependent genes [102]. Thus, similar to LSD1, the transcriptional functions of JMJD2 enzymes appear to be context-dependent.
To elucidate the molecular basis of its substrate specificity, three groups independently solved crystal structures of JMJD2A in complex with histone H3 peptides bearing different methylated forms of K9 and K36 [103–105] (Figure 6a–6c). To isolate inactivated substrate complexes, the enzyme was either co-crystallized with N-oxalylglycine (NOG), a non-reactive 2-OG analog, or with Ni(II), which substitutes for Fe(II) and inhibits the hydroxylation reaction. In the structures of the H3K9me3 and H3K36me3 complexes, the peptides bind in the same directionality within the substrate binding cleft of JMJD2A, depositing the trimethyllysines into the active site (Figure 6b and 6c). The majority of the interactions between the enzyme and H3 peptides involve hydrogen bond and van der Waals interactions with the backbone atoms in the substrates, explaining JMJD2A’s unusual dual specificity for two sites that possess dissimilar sequences. A superimposition of the H3K9me3 and H3K36me3 peptide complexes reveals that the residues N-terminal to the trimethyllysines adopt a similar β-strand-like conformation, while the C-terminal residues in the peptides adopt distinct binding modes (Figure 6d). The conformation of the two H3 peptides diverge at the +1 position due to a 180° rotation around the amide bond linking K36me3 and K37 relative to the amide bond between K9me3 and S10 [105]. Following the +1 position, the residues in the H3K36me3 peptide assume an extended conformation due to the steric constraints imposed on the peptide backbone by P38 (+2 position), while T11, G12, and G13 (+2 to +4 positions) in H3K9me3 peptide adopt a bent backbone geometry owing to the flexibility of the di-glycine motif at the +3 and +4 positions in the H3K9 sequence.
Figure 6. Dual specificity of JMJD2A for H3K9me3 and H3K36me3.
(a) Crystal structure of the catalytic domain of human JMJD2A bound to an H3K9me3 peptide (2OQ6.pdb). The JmjN domain (blue), mixed region (magenta), JmjC domain (gray), and C-terminal region (green) that comprise the catalytic domain are shown in cartoon representation. The H3K9me3 peptide (CPK coloring with cyan carbon atoms) and NOG (yellow carbons) are rendered in stick representation. The His188–Glu190–His276 triad that coordinates the active site Ni(II) (teal) and the Zn atom (dark gray) that forms the Zn-finger motif between the JmjC domain and C-terminal region are also illustrated.
(b) Substrate binding cleft of JMJD2A bound to the H3K9me3 peptide, NOG, and Ni(II). Residues in JMJD2A that interact with the peptide are colored according to their domain association (a) and labeled in black. Hydrogen bonds are shown as in Figure 4b.
(c) Structure of JMJD2A bound to an H3K36me3 peptide (orange carbons) (2OS2.pdb) illustrated as in (b). Residues H39 and R40 in the H3 peptide are modeled as alanines owing to the ambiguous electron density observed for their side chains.
(d) Superimposition of the H3K9me3 and H3K36me3 peptides bound to JMJD2A, illuminating the similarities and differences in the binding conformations of the two substrates.
The impact of these alternative binding modes on substrate recognition was investigated in a series of biochemical assays. Kinetic analysis of the demethylation of comparable length H3K9me3 and H3K36me3 peptides by JMJD2A revealed an approximately fivefold preference in specificity for the H3K9me3 substrate [104]. This difference is almost entirely due to a higher KM value for the H3K36me3 peptide, suggesting that JMJD2A preferentially recognizes the H3K9me3 site. Further confirmation of this specificity was obtained through mutational analyses, which demonstrated that mutations of the G12-G13 motif abrogate H3K9me3 demethylation by JMJD2A [104, 105]. In a complementary study, introduction of a di-glycine motif at the +3 to +4 positions of the H3K27 sequence, a site which shares sequence homology with the H3K9 sequence, enables JMJD2A to efficiently demethylate H3K27me3 [103].
In addition, the three groups examined the determinants that confer specificity for trimethyllysines, and to a letter extent, dimethyllysines in JMJD2A. Mono-, di-, and trimethyllysines bind within a methylammonium binding pocket adjacent to the Fe(II) and 2-OG binding sites in JMJD2A (Figure 7a-c) [103–105]. This pocket is lined with an array of oxygen atoms that participate in direct contacts with ζ-methyl groups of the trimethylated substrate. Couture et al. noted that the distances between the oxygen atoms and the lysine ζ-methyl groups are consistent carbon-oxygen (CH⋯O) hydrogen bonding, an unusual form of hydrogen bonding between an oxygen atom and a carbon atom that is polarized through its bonding to an electron-withdrawing heteroatom [104]. In structures of the H3K9me3 and H3K36me3 complexes, the ζ-methyl groups are bound in identical orientations within three distinct subsites in the methylammonium pocket of JMJD2A through CH⋯O hydrogen bonding networks (as illustrated for H3K9me3 in Figure 7a) [103–105]. One of these sub-sites is adjacent to the Fe(II) center, positioning the methyl group bound within for hydroxylation and demethylation. However, in the H3K9me2 complex, the dimethyllysyl side chain adopts two alternative binding modes (Figure 7b). In one conformation, one of the ζ-methyl group is bound within the subsite adjacent to Fe(II) for hydroxylation, whereas in the alternative orientation, the two ζ-methyl groups are sequestered in the two subsites distal from the metal center, representing a nonproductive enzyme:substrate complex. These alternative conformations furnish an explanation for JMDJ2A’s diminished specificity toward dimethyllysines compared to trimethylated substrates. Finally, in the H3K9me1 complex, the ζ-methyl group of monomethyllysine occupies a single subsite that is distal to the Fe(II) center (Figure 7c), in accord with previous biochemical data showing that JMJD2 enzymes are unable to efficiently demethylate monomethyllysines.
Figure 7. Methylation state specificity of JMJD2A.
(a) Structure of the active center of JMJD2A bound to K9me3 (CPK coloring with cyan carbon atoms), Ni(II) (teal), and NOG (yellow carbons) (2Q8C.pdb). Key residues that engage in CH⋯O hydrogen bonds with the methyllysine substrates are delineated. The dashed red line denotes the reaction coordinate between the ζ-methyl group positioned for hydroxylation and the water molecule bound in the presumptive O2 coordination site.
(b) The active site of JMJD2A in complex with K9me2 (2OX0.pdb) illustrated as in (a). The alternative conformation of dimethyllysine side chain is shown with pink carbon atoms.
(c) The active site of JMJD2A bound to K9me1 with its methyl group oriented distal from the metal center (2OT7.pdb), precluding hydroxylation and demethylation.
To probe the functions of CH⋯O hydrogen bonding in methylation state specificity, Couture et al. mutated two hydroxyl-group bearing residues within the methylammonium binding pocket of JMJD2A [104]. The phenol side chain of Tyr177 in JMJD2A engages in bifurcated hydrogen bonding with two ζ-methyl groups of trimethyllysine, including the methyl group positioned adjacent to the Fe(II) center (Figure 7a). A mutation of Y117F diminishes demethylation of H3K9me3 and H3K36me3 peptides by approximately tenfold, demonstrating the importance of the tyrosyl hydroxyl group in methyllysine coordination. This tyrosine is highly conserved in the sequences of trimethyllysine-specific HDMs, implying a conserved role in trimethyllysine binding. In contrast, Ser288 in JMD2A participates in CH⋯O hydrogen bonding with methyllysine substrates but is substituted by an alanine in certain JMJD2 enzymes and other trimethyllysine-specific HDMs [104]. Preliminary analysis of a S288A substitution in JMJD2A revealed that this mutant displays enhanced specificity for H3K9me2 and H3K36me2 without altering activity toward trimethyllysines, consistent with the H3K9me2/3 specificity of JMJD2D which possesses an alanine (Ala291) in this position [106]. Kinetic analysis of the JMJD2A S2888A mutant substantiated these findings, demonstrating an approximately 12-fold increase in H3K9me2 specificity versus the native enzyme, whereas the converse A291S mutant in JMJD2D reduced H3K9me2 specificity approximately fivefold [104]. These data suggest that the Ser/Ala position in the methylammonium binding pocket modulates the methylation state specificity of JMJD2 enzymes by altering the propensity of dimethyllysines to assume different conformations within the pocket that either promote or preclude demethylation (Figure 7b).
The cumulative data obtained through these studies have provided a framework for understanding the substrate specificity and catalytic mechanism of JmjC HDMs. Based on the structures of the H3K9me3 and H3K36me3 complexes, it is evident that that conformational recognition is an important determinant of the dual site specificity of JMJD2A, enabling the enzyme to demethylation two site that share no significant sequence homology. However, it remains unclear why JMJD2A is unable to demethylate other sites, notably H3K4 or H4K20, nor it is understood why other JMJD2 enzymes are unable to recognize H3K36me3, warranting further studies into their respective substrate specificities. Moreover, other families of JmjC HDMs exhibit more exquisite selectivity for individual histone methylation sites, requiring structural and functional analysis to elucidate the molecular basis of their respective specificities. Another important aspect of substrate recognition by JmjC HDMs is their methylation state specificities. In JMJD2A, CH⋯O hydrogen bonding within its methylammonium binding pocket confers specificity for trimethyllysines, and it is conceivable that similar interactions define methylation state specificities of other JmjC HDMs. Finally, many JmjC HDMs appear to function in the context of large multimeric complexes that govern their localization, transcriptional functions, and potentially their substrate specificity. In the case of certain JmjC enzymes, these complexes appear to be critical in conferring specificity for nucleosomal substrates [68, 74, 82]. Ongoing efforts to characterize these JmjC-containing HDM complexes will be critical to illuminating their roles in regulating demethylation within chromatin.
Future Directions
The last decade has witnessed tremendous progress in identifying and characterizing histone modifying enzymes and their regulatory roles in gene expression and other chromatin-associated functions. Structural and functional studies have underpinned efforts to elucidate the molecular specificities, mechanisms, and regulation of these enzymes. A consistent theme that has emerged throughout this period is the assembly of histone modifying enzymes into large macromolecular complexes that regulate their nuclear functions through a variety of mechanisms, such as by targeting enzymes to specific gene loci and by altering their substrate specificity. Structural biology will be instrumental in illuminating the structures of these macromolecular complexes and how histone modifying enzymes are regulated within them. Ultimately, these efforts will be crucial to developing therapeutics that target histone modifying enzymes within specific complexes in order to treat human disease.
Acknowledgments
We wish to thank Dan Bochar and Yong Tang for reviewing this manuscript and providing insightful comments. R.M. and R.C.T. are supported by grants from the National Institutes of Health (GM60293 to R.M. and GM073839 to R.C.T.). Structural figures were prepared using PyMOL (http://pymol.sourceforge.net).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luger K, Hansen JC. Nucleosome and chromatin fiber dynamics. Curr Opin Struct Biol. 2005;15:188–196. doi: 10.1016/j.sbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray K. The Occurrence of Epsilon-N-Methyl Lysine in Histones. Biochemistry. 1964;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 5.Kleinsmith LJ, Allfrey VG, Mirsky AE. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966;55:1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ord MG, Stocken LA. Metabolic properties of histones from rat liver and thymus gland. Biochem J. 1966;98:888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishizuka Y, Ueda K, Honjo T, Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968;243:3765–3767. [PubMed] [Google Scholar]
- 8.Goldknopf IL, Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977;74:864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt LT, Dayhoff MO. Amino-terminal sequence identity of ubiquitin and the nonhistone component of nuclear protein A24. Biochem Biophys Res Commun. 1977;74:650–655. doi: 10.1016/0006-291x(77)90352-7. [DOI] [PubMed] [Google Scholar]
- 10.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 11.Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 12.Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 13.Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. Embo J. 1999;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 15.Gary JD, Lin WJ, Yang MC, Herschman HR, Clarke S. The predominant protein-arginine methyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1996;271:12585–12594. doi: 10.1074/jbc.271.21.12585. [DOI] [PubMed] [Google Scholar]
- 16.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, Schneider R, Gregory PD, Tempst P, Bannister AJ, Kouzarides T. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 19.Robzyk K, Recht J, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- 20.Emre NC, Ingvarsdottir K, Wyce A, Wood A, Krogan NJ, Henry KW, Li K, Marmorstein R, Greenblatt JF, Shilatifard A, Berger SL. Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere-proximal Sir2 association and gene silencing. Mol Cell. 2005;17:585–594. doi: 10.1016/j.molcel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Gardner RG, Nelson ZW, Gottschling DE. Ubp10/Dot4p regulates the persistence of ubiquitinated histone H2B: distinct roles in telomeric silencing and general chromatin. Mol Cell Biol. 2005;25:6123–6139. doi: 10.1128/MCB.25.14.6123-6139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry KW, Wyce A, Lo WS, Duggan LJ, Emre NC, Kao CF, Pillus L, Shilatifard A, Osley MA, Berger SL. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation mediated by SAGA-associated Ubp8. Genes Dev. 2003;17:2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 26.Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J, Bajwa P, Johnson FC, Bhaumik SR, Shilatifard A. Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. J Biol Chem. 2006;281:37270–37274. doi: 10.1074/jbc.C600265200. [DOI] [PubMed] [Google Scholar]
- 29.Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog of yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 30.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou JX, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, Omalley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 31.Mizzen CA, Yang X-J, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, Workman J, Wang L, Berger SL, Kouzarides T, Nakatani Y, Allis CD. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki H, Schiltz L, Chiu R, Itakura K, Taira K, Nakatani Y, Yokoyama KK. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195–200. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- 33.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Marmorstein R, Roth SY. Histone acetyltransferases: function structure and catalysis. Curr. Opin. Genet.& Develop. 2001;11:155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 35.Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene. 2007;26:5528–5540. doi: 10.1038/sj.onc.1210619. [DOI] [PubMed] [Google Scholar]
- 36.Holbert MA, Sikorski T, Carten J, Snowflack D, Hodawadekar S, Marmorstein R. The human monocytic leukemia zinc finger histone acetyltransferase domain contains DNA-binding activity implicated in chromatin targeting. Journal of Biological Chemistry. 2007;282:36603–36613. doi: 10.1074/jbc.M705812200. [DOI] [PubMed] [Google Scholar]
- 37.Trievel RC, Rojas JR, Sterner DE, Venkataramani R, Wang L, Zhou J, Allis CD, Berger SL, Marmorstein R. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc. Natl. Acad. Sci. USA. 1999;96:8931–8936. doi: 10.1073/pnas.96.16.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner KG, Trievel RC, Kuo M-H, Howard R, Berger SL, Allis CD, Marmorstein R, Denu JM. Catalytic mechanism and function of invariant glutamic acid-173 from the histone acetyltransferase GCN5 transcriptional coactivator. Biol. J. Chem. 1999;274:18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- 39.Yan Y, Harper S, Speicher DW, Marmorstein R. The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nature Struct. Biol. 2002;9:862–869. doi: 10.1038/nsb849. [DOI] [PubMed] [Google Scholar]
- 40.Berndsen CE, Albaugh BN, Tan S, Denu JM. Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry. 2007;46:623–629. doi: 10.1021/bi602513x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Wang L, Zhao K, Thompson PR, Hwang Y, Marmorstein R, Cole PA. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008;451:846–850. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Holbert MA, Wurtele H, Meeth K, Rocha W, Gharib M, Jiang E, Thibault P, Verrault A, Cole PA, Marmorstein R. Fungal Rtt109 histone acetyltransferase is an unexpected structural homolog of metazoan p300/CBP. Nat Struct Mol Biol. 2008;15:738–745. doi: 10.1038/nsmb.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein RW, Corrigan M, Yaciuk P, Whelan J, Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990;64:4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J. Cell. Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 46.Kalkhoven E. CBP and p300: HATs for different occasions. Biochemical Pharmacology. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 47.Lau OD, Kundu TK, Soccio RE, Ait-Si-Ali S, Khalil EM, Vassilev A, Wolffe AP, Nakatani Y, Roeder RG, Cole PA. HATs off: Selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell. 2000;5:539–595. doi: 10.1016/s1097-2765(00)80452-9. [DOI] [PubMed] [Google Scholar]
- 48.Thompson PR, Kurooka H, Nakatani Y, Cole PA. Transcriptional coactivator protein p300. Kinetic characterization of its histone acetyltransferase activity. J. Biol. Chem. 2001;276:33721–33729. doi: 10.1074/jbc.M104736200. [DOI] [PubMed] [Google Scholar]
- 49.Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, Levrero M, Sartorelli V, Cotter RJ, Cole PA. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308–315. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- 50.Karanam B, Jiang L, Wang L, Kelleher NL, Cole PA. Kinetic and mass spectrometric analysis of p300 histone acetyltransferase domain autoacetylation. Journal of Biological Chemistry. 2006;281:40292–40301. doi: 10.1074/jbc.M608813200. [DOI] [PubMed] [Google Scholar]
- 51.Bennett CB, Lewis LK, Karthikeyan G, Lobachev KS, Jin YH, Sterling JF, Snipe JR, Resnick MA. Genes required for ionizing radiation resistance in yeast. Nat Genet. 2001;29:426–434. doi: 10.1038/ng778. [DOI] [PubMed] [Google Scholar]
- 52.Chang M, Bellaoui M, Boone C, Brown GW. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16934–16939. doi: 10.1073/pnas.262669299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsubota T, Berndsen CE, Erkmann JA, Smith CL, Yang L, Freitas MA, Denu JM, Kaufman PD. Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Molecular Cell. 2007;25:703–712. doi: 10.1016/j.molcel.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Zhou H, Li Z, Xu RM, Zhang Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. Journal of Biological Chemistry. 2007;282:28587–28596. doi: 10.1074/jbc.M702496200. [DOI] [PubMed] [Google Scholar]
- 55.Han J, Zhou H, Li Z, Xu RM, Zhang Z. The Rtt109-Vps75 histone acetyltransferase complex acetylates non-nucleosomal histone H3. Journal of Biological Chemistry. 2007;282:14158–14164. doi: 10.1074/jbc.M700611200. [DOI] [PubMed] [Google Scholar]
- 56.Schneider J, Bajwa P, Johnson FC, Bhaumik SR, Shilatifard A. Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. Journal of Biological Chemistry. 2006;281:37270–37274. doi: 10.1074/jbc.C600265200. [DOI] [PubMed] [Google Scholar]
- 57.Santos-Rosa H, Valls E, Kouzarides T, Martinez-Balbas M. Mechanisms of P/CAF auto-acetylation. Nucleic Acids Research. 2003;31:4285–4292. doi: 10.1093/nar/gkg655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.English CM, Maluf NK, Tripet B, Churchill ME, Tyler JK. ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3-H4 heterotetramer on DNA. Biochemistry. 2005;44:13673–13682. doi: 10.1021/bi051333h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bottomley MJ. Structures of protein domains that create or recognize histone modifications. EMBO Reports. 2004;5:464–469. doi: 10.1038/sj.embor.7400146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang K, Dent SY. Histone modifying enzymes and cancer: going beyond histones. J Cell Biochem. 2005;96:1137–1148. doi: 10.1002/jcb.20615. [DOI] [PubMed] [Google Scholar]
- 61.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109:801–806. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 63.Chinenov Y. A second catalytic domain in the Elp3 histone acetyltransferases: a candidate for histone demethylase activity? Trends Biochem Sci. 2002;27:115–117. doi: 10.1016/s0968-0004(02)02058-3. [DOI] [PubMed] [Google Scholar]
- 64.Clissold PM, Ponting CP. JmjC: cupin metalloenzyme-like domains in jumonji, hairless and phospholipase A2beta. Trends Biochem Sci. 2001;26:7–9. doi: 10.1016/s0968-0004(00)01700-x. [DOI] [PubMed] [Google Scholar]
- 65.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A a JmjC-containing H3K9 demethylase facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 66.Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 67.Fodor BD, Kubicek S, Yonezawa M, O'Sullivan RJ, Sengupta R, Perez-Burgos L, Opravil S, Mechtler K, Schotta G, Jenuwein T. Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev. 2006;20:1557–1562. doi: 10.1101/gad.388206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- 69.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 70.Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Eissenberg JC, Lee MG, Schneider J, Ilvarsonn A, Shiekhattar R, Shilatifard A. The trithorax-group gene in Drosophila little imaginal discs encodes a trimethylated histone H3 Lys4 demethylase. Nat Struct Mol Biol. 2007;14:344–346. doi: 10.1038/nsmb1217. [DOI] [PubMed] [Google Scholar]
- 72.Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG., Jr The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Lee MG, Norman J, Shilatifard A, Shiekhattar R. Physical and functional association of a trimethyl H3K4 demethylase and Ring6a/MBLR a polycomb-like protein. Cell. 2007;128:877–887. doi: 10.1016/j.cell.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Lee N, Zhang J, Klose RJ, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. The trithorax-group protein Lid is a histone H3 trimethyl-Lys4 demethylase. Nat Struct Mol Biol. 2007;14:341–343. doi: 10.1038/nsmb1216. [DOI] [PubMed] [Google Scholar]
- 76.Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seward DJ, Cubberley G, Kim S, Schonewald M, Zhang L, Tripet B, Bentley DL. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol. 2007;14:240–242. doi: 10.1038/nsmb1200. [DOI] [PubMed] [Google Scholar]
- 78.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 79.Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, Ge K. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem. 2007;282:20395–20406. doi: 10.1074/jbc.M701574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 81.Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci U S A. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 83.Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;318:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- 84.Smith ER, Lee MG, Winter B, Droz NM, Eissenberg JC, Shiekhattar R, Shilatifard A. Drosophila UTX is a histone H3 Lys27 demethylase that colocalizes with the elongating form of RNA polymerase II. Mol Cell Biol. 2008;28:1041–1046. doi: 10.1128/MCB.01504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trewick SC, McLaughlin PJ, Allshire RC. Methylation: lost in hydroxylation? EMBO Rep. 2005;6:315–320. doi: 10.1038/sj.embor.7400379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hausinger RP. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 87.Shi Y, Sawada J, Sui G, Affar el B, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 88.You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST-human histone deacetylase complex. Proc Natl Acad Sci U S A. 2001;98:1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 90.Hakimi MA, Bochar DA, Chenoweth J, Lane WS, Mandel G, Shiekhattar R. A core-BRAF35 complex containing histone deacetylase mediates repression of neuronal-specific genes. Proc Natl Acad Sci U S A. 2002;99:7420–7425. doi: 10.1073/pnas.112008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wissmann M, Yin N, Muller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Gunther T, Buettner R, Metzger E, Schule R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 94.Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 95.Yang M, Culhane JC, Szewczuk LM, Gocke CB, Brautigam CA, Tomchick DR, Machius M, Cole PA, Yu H. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nat Struct Mol Biol. 2007;14:535–539. doi: 10.1038/nsmb1255. [DOI] [PubMed] [Google Scholar]
- 96.Forneris F, Binda C, Adamo A, Battaglioli E, Mattevi A. Structural basis of LSD1-CoREST selectivity in histone H3 recognition. J Biol Chem. 2007;282:20070–20074. doi: 10.1074/jbc.C700100200. [DOI] [PubMed] [Google Scholar]
- 97.Forneris F, Binda C, Vanoni MA, Battaglioli E, Mattevi A. Human histone demethylase LSD1 reads the histone code. J Biol Chem. 2005;280:41360–41365. doi: 10.1074/jbc.M509549200. [DOI] [PubMed] [Google Scholar]
- 98.Forneris F, Binda C, Dall'Aglio A, Fraaije MW, Battaglioli E, Mattevi A. A highly specific mechanism of histone H3-K4 recognition by histone demethylase LSD1. J Biol Chem. 2006;281:35289–35295. doi: 10.1074/jbc.M607411200. [DOI] [PubMed] [Google Scholar]
- 99.Katoh M. Identification and characterization of JMJD2 family genes in silico. Int J Oncol. 2004;24:1623–1628. [PubMed] [Google Scholar]
- 100.Gray SG, Iglesias AH, Lizcano F, Villanueva R, Camelo S, Jingu H, Teh BT, Koibuchi N, Chin WW, Kokkotou E, Dangond F. Functional characterization of JMJD2A a histone deacetylase- and retinoblastoma-binding protein. J Biol Chem. 2005;280:28507–28518. doi: 10.1074/jbc.M413687200. [DOI] [PubMed] [Google Scholar]
- 101.Zhang D, Yoon HG, Wong J. JMJD2A is a novel N-CoR-interacting protein and is involved in repression of the human transcription factor achaete scute-like homologue 2 (ASCL2/Hash2) Mol Cell Biol. 2005;25:6404–6414. doi: 10.1128/MCB.25.15.6404-6414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin S, Janknecht R. Activation of androgen receptor by histone demethylases JMJD2A and JMJD2D. Biochem Biophys Res Commun. 2007;359:742–746. doi: 10.1016/j.bbrc.2007.05.179. [DOI] [PubMed] [Google Scholar]
- 103.Chen Z, Zang J, Kappler J, Hong X, Crawford F, Wang Q, Lan F, Jiang C, Whetstine J, Dai S, Hansen K, Shi Y, Zhang G. Structural basis of the recognition of a methylated histone tail by JMJD2A. Proc Natl Acad Sci U S A. 2007;104:10818–10823. doi: 10.1073/pnas.0704525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Couture JF, Collazo E, Ortiz-Tello PA, Brunzelle JS, Trievel RC. Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nat Struct Mol Biol. 2007;14:689–695. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
- 105.Ng SS, Kavanagh KL, McDonough MA, Butler D, Pilka ES, Lienard BM, Bray JE, Savitsky P, Gileadi O, von Delft F, Rose NR, Offer J, Scheinost JC, Borowski T, Sundstrom M, Schofield CJ, Oppermann U. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature. 2007;448:87–91. doi: 10.1038/nature05971. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Simpson M, Mao Q, Pan CH, Dai S, Hagman J, Hansen K, Shi Y, Zhang G. Structural insights into histone demethylation by JMJD2 family members. Cell. 2006;125:691–702. doi: 10.1016/j.cell.2006.04.024. [DOI] [PubMed] [Google Scholar]