Abstract
A complement to the classic three-component Mannich reaction, the redox-Mannich reaction, utilizes the same starting materials but incorporates an isomerization step that enables the facile preparation of ring-substituted β-amino ketones. Reactions occur under relatively mild conditions and are facilitated by benzoic acid.
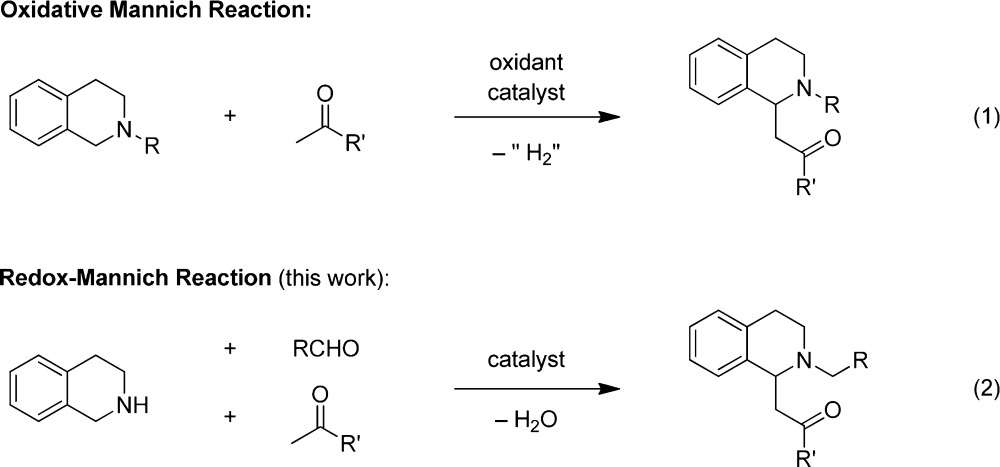
The classic three-component Mannich reaction of a primary or a secondary amine with a nonenolizable aldehyde and a C–H acidic carbonyl compound remains an extremely valuable method for C–C bond construction.1,2 Among the most extensively studied variants are oxidative two-component approaches with tertiary amines that provide access to ring-substituted β-amino ketones, products not available by the classic Mannich approach (eq 1).3 Here we report a redox-neutral alternative that utilizes the same starting materials as the regular Mannich reaction but leads to amine α-C–H bond functionalization (eq 2).
 |
1 |
The oxidative functionalization of amine α-C–H bonds via cross-dehydrogenative coupling reactions (CDC reactions) is an attractive avenue for the generation of more complex amines from simpler starting materials.4 Different oxidants and strategies including photoredox approaches have been utilized, often in the context of functionalizing N-aryl tetrahydroisoquinolines.4,5 Recently, our group has advanced an alternate and redox-neutral concept for amine α-C–H bond functionalization that obviates the need for an oxidant by effectively linking a reductive N-alkylation event to an oxidative α-functionalization.6−8 Unstabilized azomethine ylides9 have been identified as intermediates in these processes. In some instances, in particular for relatively acidic substrates such as phenols, amine α-functionalization can be achieved in the absence of any additives.6k,6m Other redox-transformations with concurrent amine α-C–H bond functionalization are facilitated by carboxylic acids6d,6f,6j,6k,6n or simply by the solvent (e.g., ethanol).6h Because the realization of such a process offered a unique set of challenges (vide infra) and would provide products of synthetic utility, we were intrigued by the possibility of developing an unprecedented redox version of the Mannich reaction.
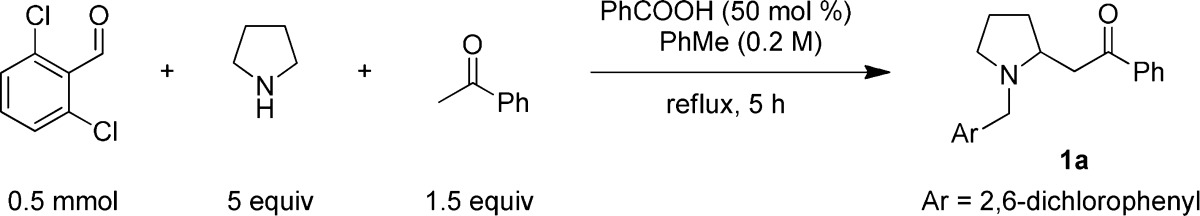
For the purpose of exploring the feasibility of a redox-Mannich reaction, we selected a substrate combination of pyrrolidine, 2,6-dichlorobenzaldehyde, and acetophenone (Table 1). Following extensive experimentation, the most successful conditions that were identified call for the slow addition (5 h) of a toluene solution of a 1:1.5 mixture of 2,6-dichlorobenzaldehyde and acetophenone to excess pyrrolidine (5 equiv), heated under reflux in toluene in the presence of benzoic acid (50 mol %). The reaction was completed (as judged by the disappearance of 2,6-dichlorobenzaldehyde) within <5 min after the slow addition had ended. Under these conditions, β-amino ketone 1a was isolated in 56% yield. The redox-Mannich reaction proved remarkably tolerant to deviation from the optimized conditions. While the presence of a carboxylic acid catalyst was a strict requirement, the nature or the amount of acid was found to be less important. Also, slow addition of ketone and aldehyde or aldehyde alone was not absolutely necessary and the amount of pyrrolidine could be reduced to 2 equiv without a major reduction in yield. Interestingly, the addition of molecular sieves led to a decrease in yield. This is in contrast to other redox processes in which molecular sieves greatly increase substrate conversion and product yields.
Table 1. Reaction Developmenta.
| entry | deviation from optimized conditions | yield (%) |
|---|---|---|
| 1 | none | 56 |
| 2 | no PhCOOH | trace |
| 3 | 100 mol % of PhCOOH | 45 |
| 4 | 20 mol % of PhCOOH | 48 |
| 5 | direct mixing of all reagents, reflux, 1 h | 45 |
| 6 | 2 equiv of pyrrolidine | 45 |
| 7 | with 4 Å MS | 43 |
| 8 | slow addition of aldehyde only | 51 |
| 9 | 2-EHA instead of PhCOOH | 39 |
| 10 | 4-Me2N-C6H4-COOH instead of PhCOOH | 28 |
| 11 | 4-MeO-C6H4-COOH instead of PhCOOH | 42 |
Reactions were performed on a 0.5 mmol scale. A mixture of aldehyde and ketone in 0.5 mL of toluene was added over 5 h to a solution of pyrrolidine and benzoic acid in 2 mL of toluene. All yields correspond to isolated yields. 2-EHA: 2-ethylhexanoic acid.
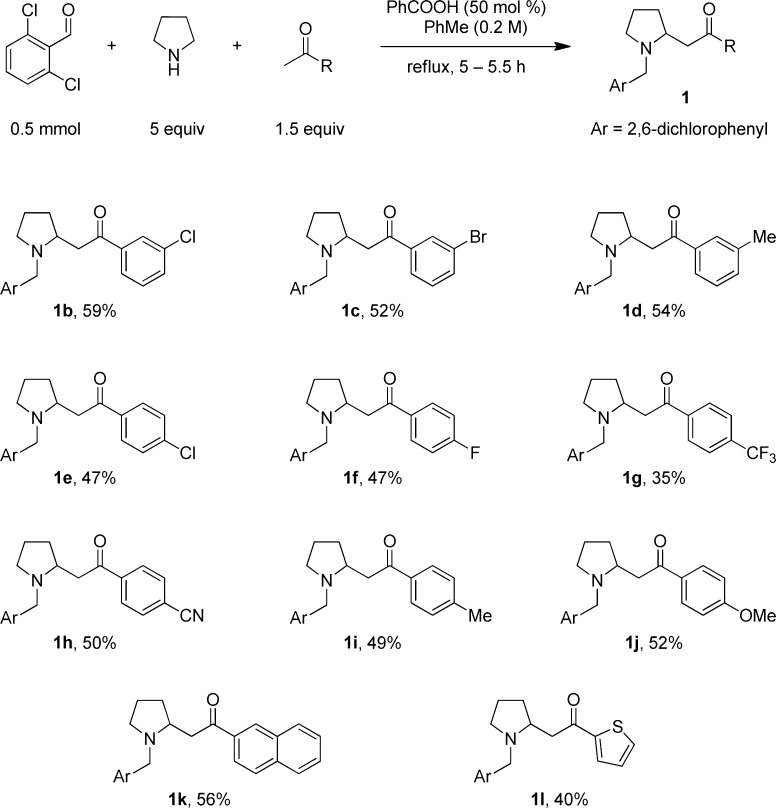
Under the optimized conditions, the pyrrolidine/2,6-dichlorobenzaldehyde pair was found to readily undergo redox-Mannich reactions with a range of aromatic and heteroaromatic methylketones (Scheme 1). Electron-donating and -withdrawing substituents in the m- and p-position of the ring were well tolerated. Interestingly, o-substituted acetophenones failed to undergo the title reaction, possibly due to less favorable keto/enol equilibration.
Scheme 1. Scope of the Redox-Mannich Reaction with Pyrrolidine.
Reactions were performed on a 0.5 mmol scale. A mixture of aldehyde and ketone in 0.5 mL of toluene was added over 5 h to a solution of pyrrolidine and benzoic acid in 2 mL of toluene. All yields correspond to isolated yields.
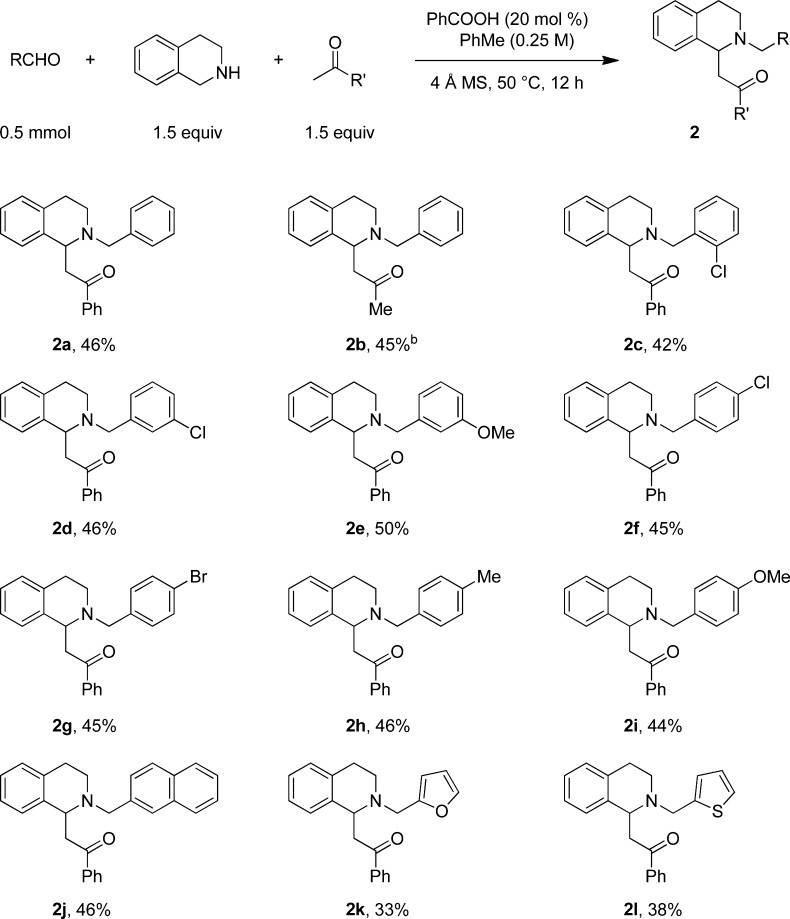
Tetrahydroisoquinoline (THIQ) was found to be an excellent substrate for redox-Mannich reactions (Scheme 2). Consistent with the enhanced reactivity of the benzylic α-C–H bond of THIQ over the α-C–H bond of pyrrolidine, reactions could be performed at a reduced temperature of 50 °C. A 20 mol % loading of benzoic acid was optimal, and slow addition of substrate was unnecessary. Unlike in the case of pyrrolidine, the addition of molecular sieves had a beneficial effect on the reaction outcome. The scope of this transformation was shown to be broad with regard to the aldehyde. Electronically diverse aromatic and heteroaromatic aldehydes with various substitution patterns readily engaged in redox-Mannich reactions with THIQ and acetophenone. Acetone was also shown to act as a competent nucleophile in this process.
Scheme 2. Scope of the Redox-Mannich Reaction with Tetrahydroisoquinoline.
Reactions were performed on a 0.5 mmol scale. The substrates were mixed directly. All yields correspond to isolated yields. b 3 equiv of acetone were used.
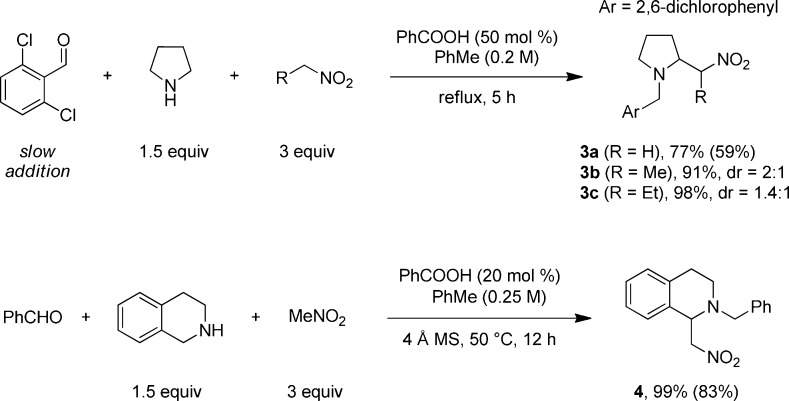
In an effort to further extend the scope of the redox-Mannich reaction, nitroalkanes were evaluated as nucleophiles (Scheme 3). Gratifyingly, the conditions optimized for ketones were directly applicable to this class of nucleophiles and reactions of pyrrolidine and THIQ proceeded in good to excellent yields. It should be noted, however, that products 3 (in particular 3b and 3c) and 4 were found to be relatively unstable to column chromatographic purification.
Scheme 3. Redox-Mannich Reactions with Nitroalkanes.
Reactions were performed on a 0.5 mmol scale. Yields were determined by 1H NMR using an internal standard. Yields in parentheses correspond to isolated yields.
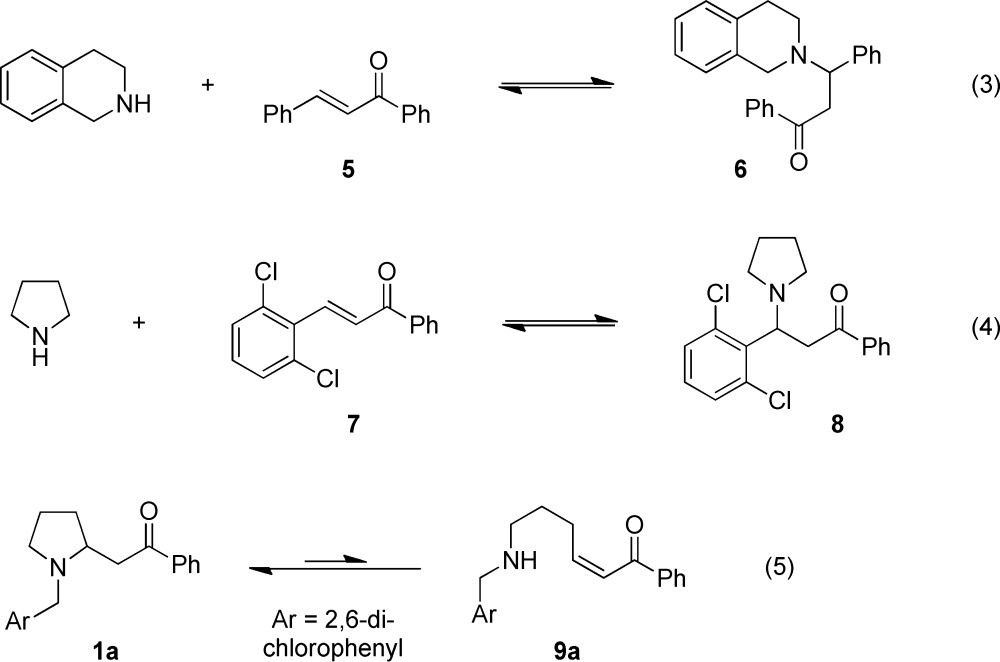
A remarkable feature of the redox-Mannich reactions described herein is that regular Mannich products were never observed as byproducts in any of the reactions. There is probably a rather simple explanation for this observation which, on cursory inspection, might appear rather surprising. While reactions of secondary aliphatic amines with formaldehyde and various ketones readily provide isolable β-amino ketones, as established by Carl Mannich himself,10 the corresponding products from other nonenolizable aldehydes, in particular aromatic aldehydes, are known to be relatively unstable by way of amine elimination under the typically acidic reaction conditions.11 On the other hand, secondary aliphatic amines are known to add to the thus formed α,β-unsaturated ketones under neutral conditions. For instance, THIQ was reported to add to chalcone (5) to give 6 (eq 3).12,13 We have observed that pyrrolidine and α,β-unsaturated ketone 7 form β-amino ketone 8 in equilibrium (eq 4). Both 6 and 8 are unstable to standard chromatography and readily revert to starting materials under acidic conditions. Redox-Mannich products such as 1a and 2a do not suffer from this instability. This is readily rationalized by considering the hypothetical equilibrium between 1a and elusive species 9a that would be expected to favor 1a (eq 5).14 The relative instability of the redox-Mannich products derived from nitroalkanes (Scheme 3) can probably be ascribed to the reversibility of the Mannich addition itself rather than the formation of nitroalkenes.
 |
3 |
It is important to note that the desirable redox-Mannich pathway does not benefit from the instability of the regular Mannich products (e.g., 6 and 8). In fact, the formation and subsequent decomposition of products such as 8 is in direct competition to the desired reaction outcome.15 In order to obtain redox-Mannich products, it is crucial that the reaction is conducted under conditions at which the relative rate of iminium isomerization is sufficiently fast to effectively compete with the classic Mannich pathway. In the case of pyrrolidine, this was achieved with an electron-deficient and sterically demanding aldehyde (e.g., 2,6-dichlorobenzaldehyde). As iminium isomerization is significantly more facile for THIQ, a broader range of aldehydes can be used.16
In summary, we have developed a new variant of the Mannich reaction that provides access to ring-substituted β-amino ketones with unprecedented ease. While the yields for the redox-Mannich products are mostly moderate, the simplicity of this process partially compensates for this current shortcoming.
Acknowledgments
Financial support from the NIH–NIGMS (Grant R01GM101389-01) is gratefully acknowledged.
Supporting Information Available
Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Mannich C.; Krösche W. Arch. Pharm. 1912, 250, 647. [Google Scholar]
- Selected recent reviews on the Mannich reaction:; a Arend M.; Westermann B.; Risch N. Angew. Chem., Int. Ed. 1998, 37, 1044. [DOI] [PubMed] [Google Scholar]; b Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ting A.; Schaus S. E. Eur. J. Org. Chem. 2007, 5797. [Google Scholar]; d Verkade J. M. M.; van Hemert L. J. C.; Quaedflieg P.; Rutjes F. Chem. Soc. Rev. 2008, 37, 29. [DOI] [PubMed] [Google Scholar]; e Arrayas R. G.; Carretero J. C. Chem. Soc. Rev. 2009, 38, 1940. [DOI] [PubMed] [Google Scholar]; f Kobayashi S.; Mori Y.; Fossey J. S.; Salter M. M. Chem. Rev. 2011, 111, 2626. [DOI] [PubMed] [Google Scholar]; g Girling P. R.; Kiyoi T.; Whiting A. Org. Biomol. Chem. 2011, 9, 3105. [DOI] [PubMed] [Google Scholar]; h Noble A.; Anderson J. C. Chem. Rev. 2013, 113, 2887. [DOI] [PubMed] [Google Scholar]; i Karimi B.; Enders D.; Jafari E. Synthesis 2013, 45, 2769. [Google Scholar]
- Recent examples of oxidative Mannich reactions:; a Li Z.; Li C.-J. J. Am. Chem. Soc. 2005, 127, 3672. [DOI] [PubMed] [Google Scholar]; b Li Z.; Li C.-J. Eur. J. Org. Chem. 2005, 3173. [Google Scholar]; c Dubs C.; Hamashima Y.; Sasamoto N.; Seidel T. M.; Suzuki S.; Hashizume D.; Sodeoka M. J. Org. Chem. 2008, 73, 5859. [DOI] [PubMed] [Google Scholar]; d So M.-H.; Liu Y.; Ho C.-M.; Che C.-M. Chem.—Asian J. 2009, 4, 1551. [DOI] [PubMed] [Google Scholar]; e Shen Y.; Li M.; Wang S.; Zhan T.; Tan Z.; Guo C.-C. Chem. Commun. 2009, 953. [DOI] [PubMed] [Google Scholar]; f Sud A.; Sureshkumar D.; Klussmann M. Chem. Commun. 2009, 3169. [DOI] [PubMed] [Google Scholar]; g Shu X. Z.; Xia X. F.; Yang Y. F.; Ji K. G.; Liu X. Y.; Liang Y. M. J. Org. Chem. 2009, 74, 7464. [DOI] [PubMed] [Google Scholar]; h Chu L.; Zhang X.; Qing F.-L. Org. Lett. 2009, 11, 2197. [DOI] [PubMed] [Google Scholar]; i Grigg R.; Somasunderam A.; Sridharan V.; Keep A. Synlett 2009, 97. [Google Scholar]; j Sureshkumar D.; Sud A.; Klussmann M. Synlett 2009, 1558. [Google Scholar]; k Richter H.; García Mancheño O. Eur. J. Org. Chem. 2010, 4460. [Google Scholar]; l Shu X.-Z.; Yang Y.-F.; Xia X.-F.; Ji K.-G.; Liu X.-Y.; Liang Y.-M. Org. Biomol. Chem. 2010, 8, 4077. [DOI] [PubMed] [Google Scholar]; m Zeng T. Q.; Song G. H.; Moores A.; Li C. J. Synlett 2010, 2002. [Google Scholar]; n Zhang G.; Zhang Y.; Wang R. Angew. Chem., Int. Ed. 2011, 50, 10429. [DOI] [PubMed] [Google Scholar]; o Rueping M.; Vila C.; Koenigs R. M.; Poscharny K.; Fabry D. C. Chem. Commun. 2011, 47, 2360. [DOI] [PubMed] [Google Scholar]; p Pan Y.; Kee C. W.; Chen L.; Tan C.-H. Green Chem. 2011, 13, 2682. [Google Scholar]; q Boess E.; Sureshkumar D.; Sud A.; Wirtz C.; Farès C.; Klussmann M. J. Am. Chem. Soc. 2011, 133, 8106. [DOI] [PubMed] [Google Scholar]; r Su W. K.; Yu J. B.; Li Z. H.; Jiang Z. J. J. Org. Chem. 2011, 76, 9144. [DOI] [PubMed] [Google Scholar]; s Hari D. P.; Koenig B. Org. Lett. 2011, 13, 3852. [DOI] [PubMed] [Google Scholar]; t Xie J.; Li H.; Zhou J.; Cheng Y.; Zhu C. Angew. Chem., Int. Ed. 2012, 51, 1252. [DOI] [PubMed] [Google Scholar]; u Zhang J.; Tiwari B.; Xing C.; Chen X.; Chi Y. R. Angew. Chem., Int. Ed. 2012, 51, 3649. [DOI] [PubMed] [Google Scholar]; v Cherevatskaya M.; Neumann M.; Füldner S.; Harlander C.; Kümmel S.; Dankesreiter S.; Pfitzner A.; Zeitler K.; König B. Angew. Chem., Int. Ed. 2012, 51, 4062. [DOI] [PubMed] [Google Scholar]; w Liu Q.; Li Y. N.; Zhang H. H.; Chen B.; Tung C. H.; Wu L. Z. Chem.—Eur. J. 2012, 18, 620. [DOI] [PubMed] [Google Scholar]; x Rueping M.; Zoller J.; Fabry D. C.; Poscharny K.; Koenigs R. M.; Weirich T. E.; Mayer J. Chem.—Eur. J. 2012, 18, 3478. [DOI] [PubMed] [Google Scholar]; y Alagiri K.; Devadig P.; Prabhu K. R. Chem.—Eur. J. 2012, 18, 5160. [DOI] [PubMed] [Google Scholar]; z Boess E.; Schmitz C.; Klussmann M. J. Am. Chem. Soc. 2012, 134, 5317. [DOI] [PubMed] [Google Scholar]; aa Freeman D. B.; Furst L.; Condie A. G.; Stephenson C. R. J. Org. Lett. 2012, 14, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]; ab Zhao G.; Yang C.; Guo L.; Sun H.; Chen C.; Xia W. Chem. Commun. 2012, 48, 2337. [DOI] [PubMed] [Google Scholar]; ac Möhlmann L.; Baar M.; Rieß J.; Antonietti M.; Wang X.; Blechert S. Adv. Synth. Catal. 2012, 354, 1909. [Google Scholar]; ad Rueping M.; Vila C.; Bootwicha T. ACS Catal. 2013, 3, 1676. [Google Scholar]; ae Zhang G.; Wang S.; Ma Y.; Kong W.; Wang R. Adv. Synth. Catal. 2013, 355, 874. [Google Scholar]; af Zhang G.; Ma Y.; Wang S.; Kong W.; Wang R. Chem. Sci. 2013, 4, 2645. [Google Scholar]; ag Ratnikov M. O.; Xu X. F.; Doyle M. P. J. Am. Chem. Soc. 2013, 135, 9475. [DOI] [PubMed] [Google Scholar]; ah Liu X.; Sun B.; Xie Z.; Qin X.; Liu L.; Lou H. J. Org. Chem. 2013, 78, 3104. [DOI] [PubMed] [Google Scholar]; ai Xue Q. C.; Xie J.; Jin H. M.; Cheng Y. X.; Zhu C. J. Org. Biomol. Chem. 2013, 11, 1606. [DOI] [PubMed] [Google Scholar]; aj Nobuta T.; Tada N.; Fujiya A.; Kariya A.; Miura T.; Itoh A. Org. Lett. 2013, 15, 574. [DOI] [PubMed] [Google Scholar]; ak Dhineshkumar J.; Lamani M.; Alagiri K.; Prabhu K. R. Org. Lett. 2013, 15, 1092. [DOI] [PubMed] [Google Scholar]; al Bergonzini G.; Schindler C. S.; Wallentin C.-J.; Jacobsen E. N.; Stephenson C. R. J. Chem. Sci. 2014, 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]; am Tanoue A.; Yoo W.-J.; Kobayashi S. Org. Lett. 2014, 16, 2346. [DOI] [PubMed] [Google Scholar]
- Selected reviews on CDC reactions:; a Li C.-J. Acc. Chem. Res. 2009, 42, 335. [DOI] [PubMed] [Google Scholar]; b Scheuermann C. J. Chem.—Asian J. 2010, 5, 436. [DOI] [PubMed] [Google Scholar]; c Yoo W. J.; Li C. J. Top. Curr. Chem. 2010, 292, 281. [DOI] [PubMed] [Google Scholar]; d Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. [DOI] [PubMed] [Google Scholar]; e Liu C.; Zhang H.; Shi W.; Lei A. W. Chem. Rev. 2011, 111, 1780. [DOI] [PubMed] [Google Scholar]; f Cho S. H.; Kim J. Y.; Kwak J.; Chang S. Chem. Soc. Rev. 2011, 40, 5068. [DOI] [PubMed] [Google Scholar]; g Klussmann M.; Sureshkumar D. Synthesis 2011, 353. [Google Scholar]; h Zhang C.; Tang C. H.; Jiao N. Chem. Soc. Rev. 2012, 41, 3464. [DOI] [PubMed] [Google Scholar]; i Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. [DOI] [PubMed] [Google Scholar]
- Other selected reviews on amine C–H functionalization, including redox-neutral approaches:; a Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443. [Google Scholar]; b Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2625. [Google Scholar]; c Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. [DOI] [PubMed] [Google Scholar]; d Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem.—Eur. J. 2010, 16, 2654. [DOI] [PubMed] [Google Scholar]; e Wendlandt A. E.; Suess A. M.; Stahl S. S. Angew. Chem., Int. Ed. 2011, 50, 11062. [DOI] [PubMed] [Google Scholar]; f Sun C. L.; Li B. J.; Shi Z. J. Chem. Rev. 2011, 111, 1293. [DOI] [PubMed] [Google Scholar]; g Jones K. M.; Klussmann M. Synlett 2012, 23, 159. [Google Scholar]; h Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem.—Eur. J. 2012, 18, 10092. [DOI] [PubMed] [Google Scholar]; j Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357. [Google Scholar]; k Peng B.; Maulide N. Chem.—Eur. J. 2013, 19, 13274. [DOI] [PubMed] [Google Scholar]; l Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551. [Google Scholar]; m Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137. [Google Scholar]; n Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. [DOI] [PubMed] [Google Scholar]
- a Zhang C.; De C. K.; Mal R.; Seidel D. J. Am. Chem. Soc. 2008, 130, 416. [DOI] [PubMed] [Google Scholar]; b Deb I.; Seidel D. Tetrahedron Lett. 2010, 51, 2945. [Google Scholar]; c Zhang C.; Das D.; Seidel D. Chem. Sci. 2011, 2, 233. [Google Scholar]; d Deb I.; Das D.; Seidel D. Org. Lett. 2011, 13, 812. [DOI] [PubMed] [Google Scholar]; e Zhang C.; De C. K.; Seidel D. Org. Synth. 2012, 89, 274. [Google Scholar]; f Ma L.; Chen W.; Seidel D. J. Am. Chem. Soc. 2012, 134, 15305. [DOI] [PubMed] [Google Scholar]; g Das D.; Sun A. X.; Seidel D. Angew. Chem., Int. Ed. 2013, 52, 3765. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Dieckmann A.; Richers M. T.; Platonova A. Y.; Zhang C.; Seidel D.; Houk K. N. J. Org. Chem. 2013, 78, 4132. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Richers M. T.; Deb I.; Platonova A. Y.; Zhang C.; Seidel D. Synthesis 2013, 45, 1730. [PMC free article] [PubMed] [Google Scholar]; j Das D.; Seidel D. Org. Lett. 2013, 15, 4358. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Chen W.; Wilde R. G.; Seidel D. Org. Lett. 2014, 16, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Seidel D. Org. Chem. Front. 2014, 1, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Chen W.; Kang Y.; Wilde R. G.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5179. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Richers M. T.; Breugst M.; Platonova A. Y.; Ullrich A.; Dieckmann A.; Houk K. N.; Seidel D. J. Am. Chem. Soc. 2014, 136, 6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examples of related amine redox transformations:; a Oda M.; Fukuchi Y.; Ito S.; Thanh N. C.; Kuroda S. Tetrahedron Lett. 2007, 48, 9159. [Google Scholar]; b Zheng L.; Yang F.; Dang Q.; Bai X. Org. Lett. 2008, 10, 889. [DOI] [PubMed] [Google Scholar]; c Pahadi N. K.; Paley M.; Jana R.; Waetzig S. R.; Tunge J. A. J. Am. Chem. Soc. 2009, 131, 16626. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Mao H.; Xu R.; Wan J.; Jiang Z.; Sun C.; Pan Y. Chem.—Eur. J. 2010, 16, 13352. [DOI] [PubMed] [Google Scholar]; e Ghavtadze N.; Narayan R.; Wibbeling B.; Wuerthwein E. U. J. Org. Chem. 2011, 76, 5185. [DOI] [PubMed] [Google Scholar]; f Xue X. S.; Yu A.; Cai Y.; Cheng J. P. Org. Lett. 2011, 13, 6054. [DOI] [PubMed] [Google Scholar]; g Zheng Q.-H.; Meng W.; Jiang G.-J.; Yu Z.-X. Org. Lett. 2013, 15, 5928. [DOI] [PubMed] [Google Scholar]; h Lin W.; Cao T.; Fan W.; Han Y.; Kuang J.; Luo H.; Miao B.; Tang X.; Yu Q.; Yuan W.; Zhang J.; Zhu C.; Ma S. Angew. Chem., Int. Ed. 2014, 53, 277. [DOI] [PubMed] [Google Scholar]; i Haldar S.; Mahato S.; Jana C. K. Asian J. Org. Chem. 2014, 3, 44. [Google Scholar]; j Rahman M.; Bagdi A. K.; Mishra S.; Hajra A. Chem. Commun. 2014, 50, 2951. [DOI] [PubMed] [Google Scholar]; k Li J.; Wang H.; Sun J.; Yang Y.; Liu L. Org. Biomol. Chem. 2014, 12, 2523. [DOI] [PubMed] [Google Scholar]; l Lin W.; Ma S. Org. Chem. Front. 2014, 1, 338. [Google Scholar]
- Selected recent examples of redox-neutral amine α-functionalization via hydride shifts:; a Barluenga J.; Fananas-Mastral M.; Aznar F.; Valdes C. Angew. Chem., Int. Ed. 2008, 47, 6594. [DOI] [PubMed] [Google Scholar]; b Polonka-Balint A.; Saraceno C.; Ludányi K.; Bényei A.; Matyus P. Synlett 2008, 2846. [Google Scholar]; c Zhang C.; Murarka S.; Seidel D. J. Org. Chem. 2009, 74, 419. [DOI] [PubMed] [Google Scholar]; d Murarka S.; Zhang C.; Konieczynska M. D.; Seidel D. Org. Lett. 2009, 11, 129. [DOI] [PubMed] [Google Scholar]; e Mori K.; Ohshima Y.; Ehara K.; Akiyama T. Chem. Lett. 2009, 38, 524. [Google Scholar]; f Ruble J. C.; Hurd A. R.; Johnson T. A.; Sherry D. A.; Barbachyn M. R.; Toogood P. L.; Bundy G. L.; Graber D. R.; Kamilar G. M. J. Am. Chem. Soc. 2009, 131, 3991. [DOI] [PubMed] [Google Scholar]; g Cui L.; Peng Y.; Zhang L. J. Am. Chem. Soc. 2009, 131, 8394. [DOI] [PubMed] [Google Scholar]; h Murarka S.; Deb I.; Zhang C.; Seidel D. J. Am. Chem. Soc. 2009, 131, 13226. [DOI] [PubMed] [Google Scholar]; i Dunkel P.; Turos G.; Benyei A.; Ludanyi K.; Matyus P. Tetrahedron 2010, 66, 2331. [Google Scholar]; j Zhou G.; Zhang J. Chem. Commun. 2010, 46, 6593. [DOI] [PubMed] [Google Scholar]; k Kang Y. K.; Kim S. M.; Kim D. Y. J. Am. Chem. Soc. 2010, 132, 11847. [DOI] [PubMed] [Google Scholar]; l Cao W. D.; Liu X. H.; Wang W. T.; Lin L. L.; Feng X. M. Org. Lett. 2011, 13, 600. [DOI] [PubMed] [Google Scholar]; m Haibach M. C.; Deb I.; De C. K.; Seidel D. J. Am. Chem. Soc. 2011, 133, 2100. [DOI] [PubMed] [Google Scholar]; n Mori K.; Ehara K.; Kurihara K.; Akiyama T. J. Am. Chem. Soc. 2011, 133, 6166. [DOI] [PubMed] [Google Scholar]; o Barluenga J.; Fananas-Mastral M.; Fernandez A.; Aznar F. Eur. J. Org. Chem. 2011, 1961. [Google Scholar]; p Zhou G. H.; Liu F.; Zhang J. L. Chem.—Eur. J. 2011, 17, 3101. [DOI] [PubMed] [Google Scholar]; q He Y. P.; Du Y. L.; Luo S. W.; Gong L. Z. Tetrahedron Lett. 2011, 52, 7064. [Google Scholar]; r Jurberg I. D.; Peng B.; Woestefeld E.; Wasserloos M.; Maulide N. Angew. Chem., Int. Ed. 2012, 51, 1950. [DOI] [PubMed] [Google Scholar]; s Sugiishi T.; Nakamura H. J. Am. Chem. Soc. 2012, 134, 2504. [DOI] [PubMed] [Google Scholar]; t Wang Y.; Chi Y.; Zhang W. X.; Xi Z. F. J. Am. Chem. Soc. 2012, 134, 2926. [DOI] [PubMed] [Google Scholar]; u Han Y. Y.; Han W. Y.; Hou X.; Zhang X. M.; Yuan W. C. Org. Lett. 2012, 14, 4054. [DOI] [PubMed] [Google Scholar]; v Chen L. J.; Zhang L.; Lv J.; Cheng J. P.; Luo S. Z. Chem.—Eur. J. 2012, 18, 8891. [DOI] [PubMed] [Google Scholar]; w He Y. P.; Wu H.; Chen D. F.; Yu J.; Gong L. Z. Chem.—Eur. J. 2013, 19, 5232. [DOI] [PubMed] [Google Scholar]; x Kang Y. K.; Kim D. Y. Chem. Commun. 2014, 50, 222. [DOI] [PubMed] [Google Scholar]; y Mori K.; Kurihara K.; Akiyama T. Chem. Commun. 2014, 50, 3729. [DOI] [PubMed] [Google Scholar]; z Mori K.; Kurihara K.; Yabe S.; Yamanaka M.; Akiyama T. J. Am. Chem. Soc. 2014, 136, 3744. [DOI] [PubMed] [Google Scholar]
- Selected reviews on azomethine ylide chemistry:; a Padwa A.; Pearson W. H.. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Wiley: Chichester, U.K., 2002; Vol. 59. [Google Scholar]; b Najera C.; Sansano J. M. Curr. Org. Chem. 2003, 7, 1105. [Google Scholar]; c Coldham I.; Hufton R. Chem. Rev. 2005, 105, 2765. [DOI] [PubMed] [Google Scholar]; d Pandey G.; Banerjee P.; Gadre S. R. Chem. Rev. 2006, 106, 4484. [DOI] [PubMed] [Google Scholar]; e Pinho e Melo T. M. V. D. Eur. J. Org. Chem. 2006, 2873. [Google Scholar]; f Bonin M.; Chauveau A.; Micouin L. Synlett 2006, 2349. [Google Scholar]; g Najera C.; Sansano J. M. Top. Heterocycl. Chem. 2008, 12, 117. [Google Scholar]; h Stanley L. M.; Sibi M. P. Chem. Rev. 2008, 108, 2887. [DOI] [PubMed] [Google Scholar]; i Nyerges M.; Toth J.; Groundwater P. W. Synlett 2008, 1269. [Google Scholar]; j Burrell A. J. M.; Coldham I. Curr. Org. Synth. 2010, 7, 312. [Google Scholar]; k Anac O.; Gungor F. S. Tetrahedron 2010, 66, 5931. [Google Scholar]; l Adrio J.; Carretero J. C. Chem. Commun. 2011, 47, 6784. [DOI] [PubMed] [Google Scholar]
- Mannich C.; Krösche W. Arch. Pharm. 1912, 250, 647. [Google Scholar]
- These compounds have been prepared by alternate means. For instance, see:Suginome M.; Uehlin L.; Yamamoto A.; Murakami M. Org. Lett. 2004, 6, 1167. [DOI] [PubMed] [Google Scholar]
- Cromwell N. H.; Burch J. S. J. Am. Chem. Soc. 1944, 66, 872. [Google Scholar]
- Compound 6, although known, had never been fully characterized. Characterization data for 6 are provided in the Supporting Information.
- For a related discussion, see:Shi S.-L.; Wei X.-F.; Shimizu Y.; Kanai M. J. Am. Chem. Soc. 2012, 134, 17019. [DOI] [PubMed] [Google Scholar]
- In a number of experiments conducted under various conditions, we have been unable to isomerize either 6 or 8 into the corresponding redox-Mannich products. Also, attempts to convert mixtures of either pyrrolidine or THIQ with 7 or 5 to redox-Mannich products were unsuccessful.
- Apparently, under the optimized conditions for THIQ, keto/enol tautomerization of the nucleophile (e.g., acetophenone), a known requirement of the Mannich process, is sufficiently slow to allow for iminium isomerization.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.