Abstract
STUDY QUESTION
Can morphologic measurements (width, length and surface area) of the uterine septum predict healing-dependent abnormal anatomic results [ARs; residual septum (RS) and intrauterine adhesions in other locations (IUA-OLs)] after complete hysteroscopic metroplasty (HM)?
SUMMARY ANSWER
Significant predictors of ARs are the septal width and, to a lesser extent, septal surface area.
WHAT IS KNOWN ALREADY
Anatomic results after hysteroscopic metroplasty have very large variation. A RS >1 cm and IUA-OLs can aggravate reproductive outcomes, resulting in the need for reoperation. New criteria for diagnosing a uterine septum according to the European Society of Human Reproduction and Embryology (ESHRE) and European Society for Gynaecological Endoscopy (ESGE) have been suggested (ESHRE-ESGE criteria). Autocross-linked hyaluronic acid gel (autocross-linked polysaccharide) has an antiadhesive effect.
STUDY DESIGN, SIZE, DURATION
A prospective, observational cohort study was performed with 96 women consecutively enrolled between 2007 and 2012.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women who had uterine septum and previous miscarriage or infertility presented for evaluation at a university hospital, private hospital or private medical center were included. Preoperative septal width, length and surface area were determined with three-dimensional sonohysterography. Women were treated by hysteroscopy in a standardized manner with three- or four-dimensional transrectal ultrasound guidance (complete resection). Patients received either no adhesion barrier (49 patients) or adhesion barrier with autocross-linked polysaccharide (47 patients). Anatomic results were assessed with three-dimensional sonohysterography and second-look hysteroscopy. Healing-dependent ARs were reported using both American Society of Reproductive Medicine (ASRM) criterion of RS length >1 cm (ASRM>1 cm criterion) and ESHRE-ESGE criteria. Univariate and multivariate logistic regression were used to identify predictors of RS, IUA-OLs and ARs.
MAIN RESULTS AND ROLE OF CHANCE
In patients who had no adhesion barrier, ARs were diagnosed in 11 of 49 patients (23%) using the ASRM > 1 cm criterion and in 20 of 49 patients (41%) using the ESHRE-ESGE criteria for RS [odds ratio (OR)ESHRE-ESGE:ASRM, 2.4, P = 0.05]. In the patients who had autocross-linked polysaccharide, ARsASRM > 1 cm were diagnosed in 2 of 47 patients (4%) and ARsESHRE-ESGE in 4 of 47 patients (9%). RSESHRE-ESGE was diagnosed significantly more often than RSASRM > 1 cm 19 of 96 (20%) versus 5 of 96 (5%) in all patients (ORESHRE-ESGE:ASRM > 1 cm = 4.5, P < 0.01). In patients who had no adhesion barrier, logistic regression with ASRM > 1 cm and ESHRE-ESGE criteria showed that the width and surface area were predictors of ARs. Models adjusted by patient group confirmed the significance of width as a predictor of ARsASRM > 1 cm [OR for width, 3.5 (P < 0.01); OR for group, 0.22 (P < 0.01)], width as a predictor of ARsESHRE-ESGE [OR for width, 2.2 (P < 0.01); OR for group, 0.26 (P < 0.01)] and surface area as a predictor of ARsASRM > 1 cm [OR for surface area, 1.5 (P < 0.01)]; OR for group, 0.32 (P < 0.01). In patients who had autocross-linked polysaccharide, these predictors were not significant. Receiver-operating characteristic curves showed cutoff values for ARsASRM > 1 cm (septal width, 3.42 cm; septal surface area, 4.68cm2) and ARsESHRE-ESGE (septal width, 3.42 cm; septal surface area, 3.51cm2).
LIMITATIONS AND REASONS FOR CAUTION
Patients were enrolled in the adhesion barrier group in a time-dependent, consecutive and non-randomized manner.
WIDER IMPLICATIONS OF THE FINDINGS
A wide septum and large surface area may be indications for adhesion barrier. The use of autocross-linked polysaccharide reduces the risk of ARs. The ESHRE-ESGE criteria may cause greater frequency of recognition of RS than the ASRM > 1 cm criterion, which could result in more frequent reoperations with use of the ESHRE-ESGE criteria, possibly without any significant effect on reproductive performance.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by Jagiellonian University (grant no. K/ZDS/003821). The authors have no competing interest to declare.
Keywords: septate uterus, intrauterine adhesions, classification system, autocross-linked hyaluronic acid gel, three-dimensional sonohysterography
Introduction
Hysteroscopic metroplasty is common treatment for women with uterine septa who have had recurrent spontaneous abortions (Homer et al., 2000; Valle and Ekpo, 2013). It is also performed in infertile women (Pabuçcu and Gomel, 2004; Mollo et al., 2009). However, there is controversy about whether to offer hysteroscopic metroplasty to women with uterine septa, or wait until miscarriage occurs or pregnancy fails, because no randomized controlled trial has identified the benefits and risks of this procedure (Bosteels et al., 2010; Chan et al., 2011; Kowalik et al., 2011).
Hysteroscopic metroplasty may have good obstetric outcomes and rare complications as reported in non-controlled reports (Nouri et al., 2010; Valle and Ekpo, 2013), but abnormal anatomic results (ARs) occur with wide variation (Porcu et al., 2000; Mollo et al., 2011; Yang et al., 2013; Ludwin et al., 2014a). Abnormal anatomic results may include a residual septum (RS) at the uterine fundus and isolated intrauterine adhesions in other locations (IUA-OLs) (Ludwin et al., 2014a). A RS may be caused by incomplete resection or adhesions. Due to the surgeons' subjective assessments of the completeness of resection and a lack of standardised post-operative evaluation of the uterine cavity, there is a high risk of bias. In reality, the incidence of healing-dependent RS is unknown.
Various methods have been used to prevent adhesions after hysteroscopic metroplasty, but there is no unequivocal recommendation (Vercellini et al., 1989; Homer et al., 2000; Taskin et al., 2000; Nawroth et al., 2002; Nappi et al., 2007). Some data are available about the effectiveness of autocross-linked polysaccharide, a cross-linked gel derived from hyaluronic acid (De Iaco et al., 2001), during hysteroscopic metroplasty (Guida et al., 2004; Mais et al., 2012). However, there is no information available about the frequency of healing-dependent anatomic outcomes or the effects of uterine parameters such as uterine septum morphology on healing-dependent anatomic results. The varied morphology of the septum may affect the formation of adhesions and may be an indication for the use of targeted adhesion prevention.
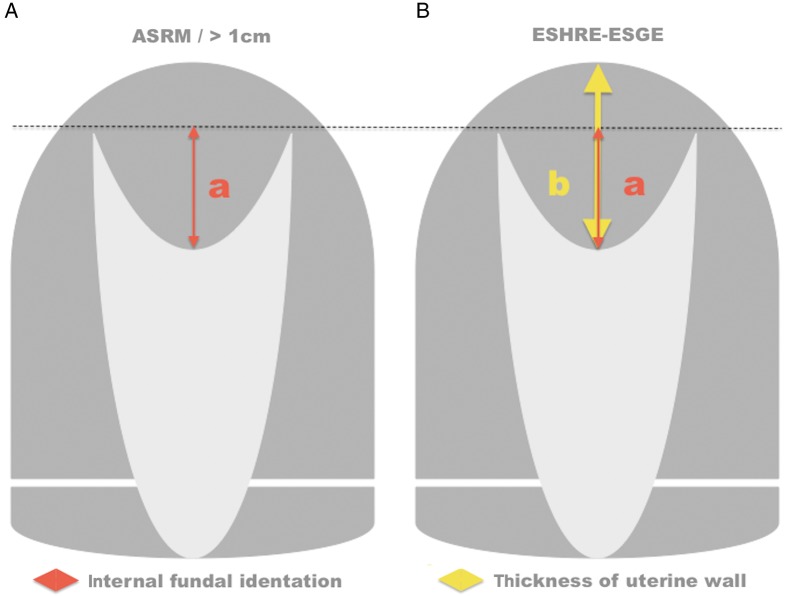
The purpose of this study was to determine the frequency of healing-dependent abnormal anatomic results after complete hysteroscopic septal resection and to assess the effect of uterine septal morphology (width, length and surface area) on these results. In addition, we evaluated the effect of using different criteria for the diagnosis of uterine RS including (i) the American Society for Reproductive Medicine (ASRM) (Buttram et al., 1988) criterion with an internal fundal indentation length >1 cm (Fedele et al., 1996; Ludwin et al., 2014a) and (ii) the European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy (ESHRE-ESGE) classification of female genital tract congenital anomalies with an internal indentation at the fundal midline >50% myometrial thickness (Grimbizis et al., 2013). The effect of adhesion barrier on healing-dependent ARs was also determined.
Materials and Methods
Patients
This prospective observational study was performed as part of a larger research project to assess the use of ultrasonographic and endoscopic methods in the diagnosis and treatment of congenital uterine anomalies (Ludwin et al., 2014a). Patients were recruited from three centers: the Department of Gynecology and Oncology, Jagiellonian University; Ludwin & Ludwin—Private Medical Center and Centermed—Private Hospital. Included patients (i) had a uterine septum, (ii) were of reproductive age, (iii) had a history of ≥1 miscarriage or infertility; (iv) intended to have children in the future and (v) provided informed consent for the study. Patients were excluded for (i) pregnancy; (ii) menopause; (iii) precancerous state or malignancy of the reproductive organs; (iv) benign lesions in the myometrium of the uterine fundus (myomas or adenomyosis) (Hirai et al., 1995; Anderson, 1999) or lesions distorting the uterine cavity (submucosal myomas or intrauterine adhesions) on ultrasonography; (v) withdrawal of patient consent; (vi) rare complex uterine congenital anomalies without an ASRM classification such as uterine septum with double cervix (Ludwin et al., 2013b); (vii) presence of external intercornual cleft; (viii) incomplete (internal fundal identation >10 mm) or non-standardized septum resection (internal fundal indentation <10 mm and uterine myometrial thickness >10 mm at the fundus), confirmed intraoperatively and (ix) excessive fundal incision (uterine myometrial thickness <6 mm at the fundus confirmed intraoperatively) (Ludwin et al., 2014a).
The patients were divided into two groups: (i) the no adhesion barrier group, who were women recruited before December 2009 and who were treated hysteroscopically with no adhesion barrier (Nawroth et al., 2002) and (ii) the autocross-linked polysaccharide group, who were women recruited after December 2009 and who received an intrauterine application of autocross-linked polysaccharide (Hyalobarrier Gel, Fidia Advanced Biopolymers, Abano Terme, Italy) (Guida et al., 2004). The study was approved by the Jagiellonian University Review Board and informed consent was obtained from all participants.
Evaluation
All patients of reproductive age who presented for diagnosis and/or treatment for miscarriage, infertility or uterine septum (suspected or diagnosed by hysteroscopy, hysterosalpingography, two- or three-dimensional transvaginal sonography, sonohysterography or magnetic resonance imaging) had three-dimensional transvaginal sonography in a standardized manner from certified examiners. The diagnosis of uterine septum was confirmed by three-dimensional sonohysterography (3D-SIS) (performed by I.L., K.P. or A.L.) in the early proliferation phase in all patients who had a septate, bicornuate or arcuate uterus noted on three-dimensional transvaginal sonography. Uterine septa were classified according to the descriptive classification of ASRM, including the distinction between complete (class VA) and partial (class VB) uterine septum (Buttram et al., 1988), and additional morphometric criteria for differentiating between septate and bicornuate (Homer et al., 2000; Salim et al., 2003a; Ludwin et al., 2011; 2013a, 2014a) or septate, arcuate and normal uteri (Bermejo et al., 2010; Ludwin et al., 2011, 2013a, 2014a; Gergolet et al., 2012). A uterine septum was diagnosed when the uterine cavity was divided into two parts at >1.5 cm from the interostial line (Bermejo et al., 2010; Ludwin et al., 2013a, 2014a), with a normal or nearly normal external outline and intercornual cleft depth <1 cm (Salim et al., 2003a,b; Ludwin et al., 2013a, 2014a).
All three-dimensional volume acquisitions were performed in a standardized manner after obtaining a sagittal view of the central part of the uterus (maximum sweep angle, 180°; approximate angle between uterine axis and ultrasound beam, 90°; ultrasound probe held steadily) (Ludwin et al., 2014a). Preoperative, intraoperative and post-operative ultrasonography was performed with an ultrasound system (Voluson E8 Expert or 730 Expert, GE Healthcare Ultrasound, Milwaukee, WI, USA) with volumetric intravaginal probes (GE RIC 5–9 MHz 3D/4D; GE Healthcare Ultrasound). For 3D-SIS, saline was used as the contrast medium in the uterine cavity. For three- or four-dimensional transrectal ultrasonography (3D/4D-TRUS) during hysteroscopic metroplasty, a mixture of mannitol and sorbitol (Purisol, Fresenius, Bad Homburg, Germany) was used as a contrast and distention medium. All measurements were taken after obtaining a coronal view with visible intramural parts of both fallopian tubes.
Septal width, length and surface area were determined with preoperative 3D-SIS (Fig. 1). The septal width was measured between the inner contours of the apices of the horns of the uterine cavity. The length of the septum was determined by measuring the distance between the interostial line and the parallel line running through the lowest point of the septum in the lower part of the uterus. The septal surface area was determined by outlining the entire surface of the myometrium forming the septum, including the lateral edges of the septum and septal base formed by the interostial line.
Figure 1.
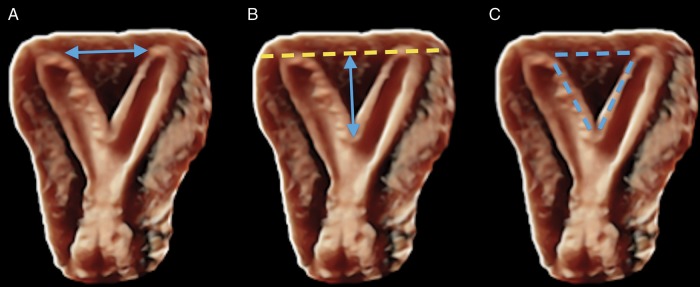
Preoperative three-dimensional sonohysterography to measure morphological parameters of the uterine septum. (A) Width. (B) Length. (C) Surface area.
Hysteroscopic metroplasty with three- or four-dimensional transrectal ultrasonography
Women were treated with hysteroscopic metroplasty in a standardized manner guided by information from 3D/4D-TRUS. In all patients, the bowel was prepared using a 150-ml sodium dihydrophosphate-sodium hydrophosphate enema (Laboratorium Galenowe, Olsztyn, Poland) 2 h before transrectal ultrasonography (Ludwin et al., 2013c). Antibacterial prophylaxis was with cefuroxime (1.5 g intravenous) (Biofuroksym, Polpharma, Poland). No patient received any hormonal treatment during the 3 months before or after hysteroscopy.
The procedures were performed during the early proliferation phase by the same surgeon (A.L.), who had 10–15 years’ experience in hysteroscopic metroplasty, using the same operative technique, hysteroscope (diameter, 4 mm; 30°), monopolar resectoscope, needle electrode (Collins, Karl Storz, Tuttlingen, Germany) and device parameters. The septum was cut from its lower to upper part until the optimal uterine cavity shape was obtained, tailored to the anatomy of the fundus. In patients who had a complete (class VA) uterine septum associated with a vaginal septum, the procedure began with resection of the vaginal septum and lower part of the cervical septum with an electrosurgical knife before hysteroscopic resection of the remaining part of the cervical and uterine septa. Resection of the uterine septum in the upper part of the uterus was performed with guidance from 3D/4D-TRUS until a uniform fundal myometrial thickness (6–10 mm) was obtained (Supplementary data, Fig. S1) (Ludwin et al., 2014a). The maximum cutoff corresponded to that previously used during ultrasound-guided hysteroscopic metroplasty (Querleu et al., 1990). The minimum cutoff was based on our experience during 10 years with two- and three-dimensional transrectal ultrasonography (no uterine rupture during pregnancy) (Ludwin et al., 2014a).
Completion of septal resection during and at the end of the procedure was confirmed by measuring the myometrium in the central fundus using 3D/4D-TRUS (I.L.). The shape of the uterine cavity was assessed in the coronal view with 3D/4D-TRUS. The intrauterine application of autocross-linked polysaccharide gel (10 mL) was performed immediately after this procedure using a 3-mm-diameter silicone tube cut with scissors to a length of 10–12 cm. Patients were hospitalized for 1 day after the procedure.
Post-operative evaluation
Post-operative evaluation included 3D-SIS and second-look hysteroscopy. All women were monitored for possible occurrence of early post-operative complications such as bleeding, infection, fever and allergic reactions. The 3D-SIS (I.L.) and office second-look hysteroscopy (A.L.) were performed at 6–8 weeks after hysteroscopic metroplasty to assess the anatomic results, consistent with general practice (Homer et al., 2000). Both 3D-SIS and hysteroscopy were used for post-operative diagnosis to evaluate the comparative accuracy of 3D-SIS and hysteroscopy, reported elsewhere (Ludwin et al., 2014a). Anatomic parameters assessed by post-operative 3D-SIS were (i) myometrial thickness in the central fundus and (ii) internal fundal indentation/fundal notch length/RS. The latter parameters were evaluated by measuring the distance between the interostial line and a parallel line running through the lowest point of the uterine cavity fundus. Hysteroscopic findings of IUA-OLs were reported in the present study because hysteroscopy had a higher (non-significant) diagnostic accuracy than 3D-SIS in detecting intrauterine adhesions (Ludwin et al., 2014a).
Measurements of internal fundal indentation and myometrial thickness of the uterine fundus enabled additional categorization of anatomic results (Table I, Fig. 2). This categorization of uterine cavity shape was justified because of common present use (ASRM classification; Buttram et al., 1988; Bermejo et al., 2010; Ludwin et al., 2011, 2013a, 2014a), reproductive outcome results (RS ≥1 cm; Fedele et al., 1996), expert consensus (ESHRE-ESGE system; Grimbizis et al., 2013) and the known effects of intrauterine adhesions on fertility.
Table I.
Classification of anatomic results after hysteroscopic metroplasty.
| Classification | Uterine cavity shape | Intrauterine adhesions in other locations |
|---|---|---|
| ASRMa,b | ||
| Normal | Normal (internal fundal indentation/RS <1 cm) | Absent |
| Abnormalc | Abnormal (internal fundal indentation/RS ≥1 cm) | Present |
| ESHRE-ESGEb | ||
| Normal | Normal [internal fundal indentation <50% myometrial (uterine wall) thickness] | Absent |
| Abnormalc | Residual septate uterus [internal fundal indentation >50% myometrial (uterine wall) thickness] | Present |
ASRM, American Society for Reproductive Medicine; ESHRE-ESGE, European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy.
aASRM classification system and criterion of RS by length >1 cm after hysteroscopic metroplasty (Fedele et al., 1996; Ludwin et al., 2014a).
bModified to include the presence/absence of intrauterine adhesions in other locations (Ludwin et al., 2014a).
cPresence the RS or adhesions in other locations or both.
Figure 2.
Diagnosis of RS. (A) American Society of Reproductive Medicine (ASRM) classification system with additional morphometric criterion of internal fundal indentation >1 cm (ASRM > 1 cm). (B) European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy (ESHRE-ESGE) classification system of female tract congenital anomalies and internal fundal indentation >50% myometrial (uterine wall) thickness.
Additional surgical intervention was performed during second-look hysteroscopy (A.L.), including removal of any observed adhesions and resection of residual septa with the length ≥10 mm (Fedele et al., 1996). The goals of this intervention were to achieve a uterine cavity shape as close to normal as possible and a myometrial thickness of 6–10 mm. Myometrial thickness was controlled indirectly by second-look hysteroscopy calibrated with a 5-French hysteroscopic probe (intraoperative myometrial thickness during additional resection = difference between fundal thickness assessed by 3D-SIS and intraoperative measurement of the resected RS).
The mean fundal myometrial thickness was measured during the initial hysteroscopy, immediately after resection, and after a 6–8-week healing period (Homer et al., 2000). The thickness was compared within and between groups and within the entire study population.
The frequency of RS, IUA-OLs, ARs and additional surgical interventions performed during second-look hysteroscopy were determined and compared between groups. The results were compared with the initial morphology of the uterine septum, including the ASRM classification of complete or partial septum, septal width in the upper part of the uterus, septal length and surface area. The frequency of RS was compared with and without the inclusion of IUA-OLs (Table I).
Validation of reproducibility among diagnostic methods
The validity of all ultrasonographic imaging methods (three-dimensional transvaginal sonography, 3D-SIS and 3D/4D-TRUS) was assessed by determining interrater/intrarater agreement (off-line blinded diagnosis) in 30 patients selected randomly from the study population. This showed almost perfect correlation for the differentiation of septate uterus and measurement of septal width, length and surface area (3D-SIS), measurement of myometrial thickness (3D-SIS and 3D/4D-TRUS) (unpublished data) and post-operative evaluation of uterine cavity shape and length of fundal notch/RS (3D-SIS) (Ludwin et al., 2014a).
Sample size
The sample size a priori was determined according to the main objective of the study (to determine the prevalence of healing-dependent abnormal anatomic results after hysteroscopic metroplasty) based on the assumption that the prevalence of ARs ≠ 50% (frequency of intrauterine adhesions or ARs in previous reports, a retrospective analysis in women treated by surgeons with average experience, and a small preliminary study) and the anticipated prevalence of healing-dependent ARs is 27% (based on a larger retrospective analysis of operator- and healing-dependent ARs by ASRM > 1 cm criteria in women treated by an expert surgeon; post-operative two- or three-dimensional sonohysterography). Assuming the alternative hypothesis to test for equality (0.27true ≠ 0.50reference), with α = 0.05 and 1 − β = 0.90, the required group size was 46 (Chow et al., 2012). For this sample size, the anticipated prevalence of ARs, with a confidence level of 95%, the margin of error calculated post hoc using Kish formula was 13% (Kish, 1965). With these assumptions, the prevalence of ARs may vary from 14 to 40%, significantly different from 50%. Thus, we found that the margin of error and sample size would be sufficient. A more precise estimation of prevalence of healing-dependent ARs with a common 5% margin of error and 95% confidence level, and based on the study results (prevalence of ARs according to ASRM > 1 cm criteria), would require a very large sample of 269 patients with septate uterus (Kish, 1965), which exceeded our recruitment opportunities. Additional patients were enrolled in a group of comparable size in which autocross-linked polysaccharide was used to achieve the other objectives of the study.
Statistical analysis
All analyses were performed with statistical software (STATISTICA, version 10.0, StatSoft, Inc., Tulsa, OK, USA; R, version 2.15.2, R Foundation, Vienna, Austria). Continuous measurements are presented as mean ± standard deviation for normally distributed data (age, weight, septal area, intraoperatively evaluated myometrial thickness of uterine fundus after metroplasty and myometrial thickness of uterine fundus after healing period), and as median values with lower and upper quartiles for non-normally distributed data (body mass index, septal length, RS length after healing period, procedure time and medium absorption). Minimum and maximum values also are shown. Categorical variables are presented as numbers of subjects and percentages. The Shapiro–Wilk test was used to evaluate the normality of distribution of continuous variables. The t-test was used to compare differences between normally distributed continuous variables. Differences between non-normally distributed variables were tested using the non-parametric Mann–Whitney test. Comparisons between measurements of fundal myometral thickness at two different times (intraoperative after resection and follow-up) were made with paired t-test. To compare differences between categorical variables, the Pearson χ² test (chi-square test) or Fisher exact test was used. Pearson product moment and Spearman rank correlation were used to assess the functional dependence between the order of inclusion of the patient in the study, procedure duration and medium absorption. The effects of RS, IUA-OLs and ARs were evaluated using univariate and multivariate logistic regression, with morphologic parameters serving as independent variables [categorical data: subclassification of septate uterus by ASRM [subclass, VA or VB); continuous variables: width (cm), length (cm), surface area [cm2)]. Models were created for ASRM > 1 cm and ESHRE-ESGE criteria for all 96 patients and separately for the two groups. Stepwise and forward methods were used for multivariate logistic regression. Group-adjusted models were created for all data models. The results are shown as odds ratios (normal-to-abnormal state) with 95% confidence intervals and P values determined by Wald test. Group-adjusted models were created. Receiver-operating characteristic curves were created to determine cutoff values for continuous parameters such as RS, IUA-OLs and ARs. Statistical significance was defined by two-sided P ≤ 0.05.
Results
Patients were enrolled between January 2007 and December 2011 and data collection was completed in February 2012. There were 161 patients who were potentially eligible for the study, and 108 patients included in the study. Finally, data of 96 women [no adhesion barrier group, N = 49 (university hospital—27 patients; private hospital or private medical center—22 patients); autocross-linked polysaccharide group, N = 47 [university hospital—20 patients; private hospital or private medical center—27 patients)] were analyzed (Supplementary data, Fig. S2).
The mean age and median body mass index were similar between patients who had autocross-linked polysaccharide or no adhesion barrier (Table II). There were 56 patients (58%) who had miscarriages, 31 patients (32%) who were infertile, and 9 patients (9%) who were infertile after miscarriage. The procedure duration, medium absorption, and preoperative and intraoperative (after resection) morphologic characteristics of uterine septa were similar between patients who had no adhesion barrier or autocross-linked polysaccharide (Tables II and III). There was no significant correlation between the order of patient inclusion in the study and procedure duration or medium absorption in either surgical center. There were no intraoperative or early post-operative complications observed.
Table II.
Clinical parameters of patients who had hysteroscopic metroplastya.
| Parameter | Total | No antiadhesion | Autocross-linked polysaccharide | P |
|---|---|---|---|---|
| Age (years) | 29 ± 5 (20–41) | 29 ± 5 (20–41) | 29 ± 5 (20–41) | 0.47 |
| Body mass index (kg/m²) | 22 [20–24] (17–38) | 23 [20–24] (18–38) | 21 [20–24] (17–34) | 0.82 |
| Procedure duration (min) | 17 [15–20] (9–41) | 17 [15–18] (9–40) | 17 [16–21] (10–41) | 0.1 |
| Medium absorption (ml) | 310 [230–400] (50–750) | 290 [230–380] (50–700) | 310 [240–400] (50–750) | 0.62 |
aN = 96 patients. Data reported as mean ± SD or median [interquartile range] (range, minimum–maximum).
Table III.
Characteristics of uterine septum before, fundal myometrial thickness during metroplasty, fundal myometrial thickness and fundal notch/RS after a 6–8-week healing perioda.
| Assessment | Variable | Total | No antiadhesion | Autocross-linked polysaccharide | P |
|---|---|---|---|---|---|
| No. patients | 96 | 49 | 47 | ||
| Preoperative | ASRM subclass | ||||
| VA | 19 (20%) | 9 (18%) | 10 (21%) | 0.72 | |
| VB | 77 (80%) | 40 (82%) | 37 (79%) | ||
| Width (cm) | 3.3 ± 0.8 (1.8–4.8) | 3.3 ± 0.8 (1.8–4.7) | 3.4 ± 0.8 (1.8–4.8) | 0.48 | |
| Height (cm) | 2.2 [1.9–3.2] (1.5–7.1) | 2.2 [1.9–3.2] (1.5–7.1) | 2.2 [1.9–2.5] (1.7–6.8) | 0.88 | |
| Surface area (cm2) | 4 ± 1 (1.4–7.6) | 4 ± 1 (1.4–7.6) | 4 ± 1 (1.6–7.4) | 0.57 | |
| Intraoperative after resection | Myometrial thickness (mm) | 8 ± 1 (6–10) | 8 ± 1 (6–10) | 8 ± 1 (6–10) | 0.99 |
| Follow-up | Myometrial thickness after healing (mm) | 12 ± 4 (7–24) | 13 ± 4 (7–24) | 11 ± 3 (7–18) | <0.01 |
| Length of fundal notch/RS (mm) | 4.5 [3.0–7.1] (1–15) | 4.9 [4.2–7.9] (1–15) | 3.2 [2.1–4.5] (1–8) | <0.01 |
aData reported as number (%), mean ± SD (range, minimum–maximum), or median [interquartile range] (range, minimum to maximum). Assessments: preoperative parameters assessed with three-dimensional sonohysterography; intraoperative myometrial thickness after septum resection assessed with three-dimensional transrectal ultrasonography; follow-up (6–8 weeks) myometrial thickness and RS length assessed with three-dimensional sonohysterography.
The fundal myometrial thickness measured after the healing period was significantly greater than that measured immediately after hysteroscopic metroplasty in both the groups (no adhesion barrier, P < 0.01; autocross-linked polysaccharide, P < 0.01) and the entire sample (P < 0.01) (Table III). However, at follow-up, the mean myometrial thickness and RS length were significantly lower in patients who had autocross-linked polysaccharide than patients who had no adhesion barrier (Table III). According to the ASRM classification with morphometric criteria, uterine cavity shape after the healing period was normal in 91 (95%) patients and arcuate (RS by ASRM > 1 cm criterion) in 5 patients (5%, all had no adhesion barrier). There were no healing-dependent uterus septate (internal fundal indentation > 15 mm) observed according to the ASRM classification with morphometric criteria (Ludwin et al., 2013a).
Independent of the criteria used for RS detection (ASRM > 1 cm or ESHRE-ESGE), residual septa and ARs were detected significantly more frequently in patients who had no adhesion barrier than patients who had autocross-linked polysaccharide (Table IV). The frequency of IUA-OLs did not differ significantly between the two patient groups (Table IV).
Table IV.
Abnormal anatomic results after complete hysteroscopic metroplastya.
| Anatomic result | Total | No antiadhesion | Autocross-linked polysaccharide | P |
|---|---|---|---|---|
| No. patients | 96 | 49 | 47 | |
| Intrauterine adhesions in other locations | 9 (9%) | 7 (14%) | 2 (4%) | 0.16 |
| ASRM and morphometric criteria (RS > 1 cm) | ||||
| Residual septum | 5 (5%) | 5 (10%) | – | 0.03 |
| Abnormal anatomic result | 13 (14%) | 11 (23%) | 2 (4%) | 0.02 |
| ESHRE-ESGE criteria | ||||
| Residual septum | 19 (20%) | 16 (33%) | 3 (6%) | <0.01 |
| Abnormal anatomic result | 24 (25%) | 20 (41%) | 4 (9%) | <0.01 |
ASRM, American Society of Reproductive Medicine; ESHRE-ESGE, European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy.
aData reported as number (%). Abnormal anatomic result defined as RS or/and intrauterine adhesions in other locations.
RS was diagnosed significantly more frequently with the ESHRE-ESGE classification than with the ASRM > 1 cm criteria in all patients (odds ratio, 4.5; 95% confidence interval, 1.6–12.6; P < 0.01) (Table IV). Intrauterine adhesions occurred as isolated changes, and the inclusion of frequency of occurrence of intrauterine adhesions in the analysis weakened the significance of the effect of the diagnostic criteria for RS (ASRM > 1 cm versus ESHRE-ESGE) on the frequency of ARs (odds ratio, 2.1; 95% confidence interval, 1.01–4.49; P = 0.05).
Univariable logistic regression with the dependent variable RS determined by either criteria (ASRM > 1 cm or ESHRE-ESGE criteria) demonstrated that septal width (cm) and surface area (cm2) were predictors of RS in patients who had no adhesion barrier and in the entire study population. For patients who had no adhesion barrier, RS was significantly predicted by width (both criteria) and surface area (ASRM > 1 cm criteria only) (Table V). Group-adjusted models (odds ratio for no adhesion barrier to autocross-linked polysaccharide group) for RS (ESHRE-ESGE criteria) confirmed the significance of septal width and weakened the significance of septal surface area (Table V). Models for the autocross-linked polysaccharide group and adjusted by group for RS (ASRM > 1 cm criterion) could not be performed because no patient in the autocross-linked polysaccharide group had an RS > 1 cm.
Table V.
Univariate analysis of predictors of RS and abnormal anatomic results in patients who had no adhesion barrier during hysteroscopic metroplastya and group-adjusted modelsb.
| Parameter | Criteria | Predictors | No antiadhesion |
||
|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P | |||
| RS | ESHRE-ESGE | Width | 3.2 | 1.7–6.5 | <0.01 |
| Surface area | 1.3 | 1.0–1.8 | 0.07 | ||
| ASRM > 1 cm | Width | 9.7 | 2.4–21.0 | 0.02 | |
| Surface area | 1.8 | 1.2–3.3 | 0.02 | ||
| Group-adjusted models for RSc,d | ESHRE-ESGE | Width | 2.0 | 1.3–3.1 | <0.01 |
| Group | 0.30 | 0.14–0.60 | <0.01 | ||
| Surface area | 1.2 | 0.9–1.5 | 0.18 | ||
| Group | 0.33 | 0.16–0.63 | <0.01 | ||
| Abnormal anatomic resultse | ESHRE-ESGE | Width | 3.4 | 1.8–6.9 | <0.01 |
| Surface area | 1.4 | 1.0–1.9 | 0.04 | ||
| ASRM > 1 cm | Width | 3.1 | 1.6–6.7 | <0.01 | |
| Surface area | 1.7 | 1.2–2.6 | <0.01 | ||
| Group-adjusted models for abnormal anatomic results | ESHRE-ESGE | Width | 2.2 | 1.5–3.5 | <0.01 |
| Group | 0.26 | 0.12–0.55 | <0.01 | ||
| Surface area | 1.2 | 1.0–1.5 | 0.10 | ||
| Group | 0.31 | 0.16–0.57 | <0.01 | ||
| ASRM > 1 cm | Width | 3.5 | 1.9–7.5 | <0.01 | |
| Group | 0.22 | 0.07–0.55 | <0.01 | ||
| Surface area | 1.5 | 1.2–2.1 | <0.01 | ||
| Group | 0.32 | 0.13–0.69 | <0.01 | ||
ASRM, American Society of Reproductive Medicine; ESHRE-ESGE, European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy.
aN = 49 patients; bN = 96 patients.
cRatio between no adhesion barrier to autocross-linked polysaccharide group.
dModels adjusted by group for RS with the ASRM > 1 cm criterion could not be performed because no patient in the autocross-linked polysaccharide group had an RS > 1 cm.
eRS or/and intrauterine adhesions in other locations.
Univariate logistic regression in patients who had no adhesion barrier with the dependent variable abnormal anatomic results (RS and/or IUA-OLs) showed that septal width and surface area were significant predictors of ARs (both ESHRE-ESGE and ASRM > 1 cm criteria) (Table V). Group-adjusted models for abnormal anatomic results (odds ratio for no adhesion barrier to autocross-linked polysaccharide group) confirmed the significance of septal width and weakened the significance of surface area for the ESHRE-ESGE criteria, and confirmed the significance of septal width and surface area for the ASRM > 1 cm criteria (Table V).
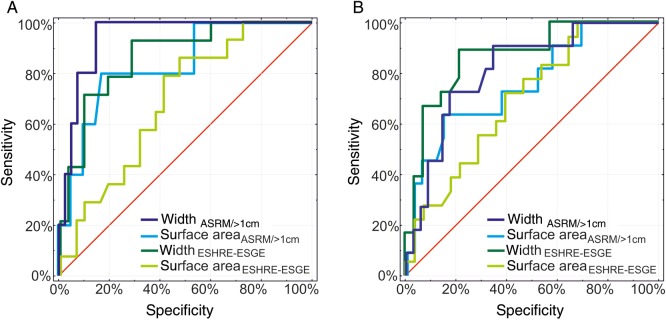
In patients who had no adhesion barrier, receiver-operating characteristic curve analysis revealed the cutoff values for RS with the greatest sensitivity for width (ASRM > 1 cm criteria) and greatest specificity for width and surface area (ASRM > 1 cm criteria) (Table VI, Fig. 3), as well as the cutoff values for abnormal anatomic results with the greatest sensitivity for width (ASRM > 1 cm criteria) and greatest specificity for surface area (ASRM > 1 cm criteria) (Table VI, Fig. 3).
Table VI.
Cutoff values from receiver-operating characteristic curves in patients who had no antiadhesion prevention adhesion barrier during hysteroscopic metroplastya.
| Result | Criteria | Parameter | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Residual septum | ESHRE/ESGE | Width (cm) | 3.4 | 81 | 73 |
| Surface area (cm²) | 3.2 | 81 | 55 | ||
| ASRM > 1 cm | Width (cm) | 3.84 | 100 | 86 | |
| Surface area (cm²) | 4.73 | 80 | 84 | ||
| Abnormal anatomic result | ESHRE/ESGE | Width (cm) | 3.42 | 80 | 79 |
| Surface area (cm²) | 3.51 | 70 | 62 | ||
| ASRM > 1 cm | Width (cm) | 3.42 | 91 | 68 | |
| Surface area (cm²) | 4.68 | 64 | 87 |
ASRM, American Society of Reproductive Medicine; ESHRE-ESGE, European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy.
aN = 49 patients.
Figure 3.
Receiver-operating characteristic curves of septal width and surface area to predict healing outcomes after complete hysteroscopic hysteroplasty. (A) RS. (B) Abnormal anatomic results (RS and intrauterine adhesions in other location). ASRM > 1 cm, American Society of Reproductive Medicine (ASRM) classification with additional morphometric criterion of internal fundal indentation >1 cm; ESHRE-ESHE, European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy classification.
Univariate logistic regression in all patients identified septal width as a predictor of intrauterine adhesion in other locations (odds ratio, 1.9; 95% confidence interval, 1.1 to 3.4; P < 0.01). The model adjusted by group confirmed the significance of this parameter (odds ratio for width, 2.2; 95% confidence interval, 1.2–4.2; P ≤ 0.02; odds ratio for group, 0.4; 95% confidence interval, 0.15–0.93; P ≤ 0.05). These results were obtained for the whole group (N = 96). Receiver-operating characteristic curves showed that the cutoff value was a septal width of 3.42 cm (sensitivity, 86%; specificity, 62%). In eight of nine adhesions, the septal width was > 3.42 cm. Separate models for the two patient groups had a low predictive value because of the small numbers of IUA-OLs, especially in patients who received autocross-linked polysaccharide.
No other morphologic factor, either alone or in group-adjusted analyses, had a significant effect on RS, ARs or IUA-OLs. No multivariate model including the morphologic factors studied had a predictive value for the occurrence of healing-dependent RS, IUA-OLs or general healing-dependent abnormal anatomic results after hysteroscopic metroplasty.
Additional surgical interventions were performed during repeat hysteroscopy in 13 patients (14%) who had abnormal anatomic results by ASRM > 1 cm criteria. These interventions were performed significantly less frequently in the autocross-linked polysaccharide group [2 of 47 patients (4%)] than in patients who had no polysaccharide [11 of 49 patients (23%); P ≤ 0.05)].
Discussion
This prospective study is the first to show the frequency of occurrence of healing-dependent ARs after complete hysteroscopic metroplasty using the ESHRE-ESGE classification as well as ASRM and morphometric criteria. In patients who had no adhesion barrier, ARs occurred in 23% of patients according to the ASRM > 1 cm criterion or in 41% of patients according to the ESHRE-ESGE criteria for diagnosis of RS (Table IV). The most important study findings were that septal width and, to a lesser extent, septal surface area were significant predictors of abnormal anatomic results (Table V, Fig. 4). The risk of ARs increased significantly above the cutoff values determined for these parameters, and these cutoff values can be used to target adhesion prophylaxis and plan future studies about the effectiveness of new antiadhesion methods. In the entire sample, the study showed that autocross-linked polysaccharide significantly decreased the frequency of ARs (Table IV).
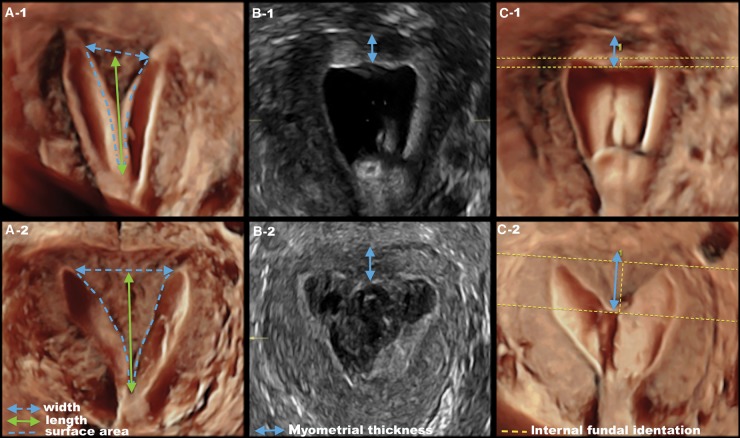
Figure 4.
Representative uterine septa from two patients who were treated with hysteroscopic metroplasty and no antiadhesion prophylaxis. Top row (33-year-old woman): (A-1) Preoperative three-dimensional sonohysterography showing the uterine width <3.5 cm and surface area <4.5 cm2. (B-1) Myometrial thickness achieved at the completion of surgery (three- or four-dimensional transrectal ultrasonography). (C-1) After healing, RS was absent. Bottom row (30-year-old woman): (A-2) Preoperative three-dimensional sonohysterography showing uterine width >3.5 cm and surface area >4.5 cm2; the length was similar to other patient in (A-1). (B-2) Myometrial thickness achieved at the completion of surgery (three- or four-dimensional transrectal ultrasonography). A similar myometrial thickness was achieved as in other patient in (B-1). (C-2) After healing, RS was present.
The measurements of myometrial thickness of the uterine fundus immediately after metroplasty and at follow-up also may be useful (Table III). These measurements confirm that adhesion processes may be a natural component of healing after hysteroscopic metroplasty. Furthermore, adhesion processes may be a natural attempt to restore primary anatomy that may cause a fundal notch, an RS or intrauterine adhesions in other locations. The observed changes in myometrial thickness of the uterine fundus confirm the findings of a recent report of de novo adhesion formation in most women after hysteroscopic metroplasty (Yang et al., 2013). However, the extent of these adhesions may not necessarily be important and they are not diagnosed in most patients.
In contrast with patients who had no adhesion barrier, septal width and surface area were not significant predictive risk factors for ARs after metroplasty in patients who received autocross-linked polysaccharide. The use of autocross-linked polysaccharide may not completely eliminate ARs, but may reduce the extent of adhesions, reduce the RS size and improve anatomic outcomes after hysteroscopic metroplasty.
In the present study, the ESHRE-ESGE classification criteria for the assessment of hysteroscopic metroplasty resulted in a higher frequency of diagnosis of RS (20%) than the ASRM > 1 cm criterion (5%) (Table IV). The ESHRE-ESGE criteria for diagnosis of uterine septum are based on expert consensus, but not validated by any prospective or retrospective studies, and have not been evaluated in practice (Ludwin et al., 2014b). Therefore, the ESHRE-ESGE criteria should be used with caution when considering reoperation for a uterine septum that is diagnosed after hysteroscopic metroplasty and when the absolute length of the RS is < 1 cm. These criteria may cause the diagnosis of all internal fundal indentations > 50% wall thickness as a uterine septum. In a patient who has an average (12.3 mm) myometrial thickness of the uterine fundus after hysteroscopic metroplasty, a fundal notch slightly >6 mm would be classified as an RS according to the ESHRE-ESGE criteria. However, there are no data available that show that an RS (Fedele et al., 1996) or internal indentation <1 cm affects reproductive outcomes or that these small findings require surgical correction (Tomazevic et al., 2007; Gubbini et al., 2009; Gergolet et al., 2012). The two classification systems originally refer to the natural preoperative status of the uterus, not the uterus after any surgical correction. It is paradoxical that the ESHRE-ESGE criteria applied before treatment (internal indentation 6 mm; uterine wall thickness 10 mm) may be an indication for surgery as uterus septate but the identical morphology after hysteroscopic metroplasty has been defined as a satisfactory outcome (Fedele et al., 1996).
The ESHRE-ESGE classification did not contain recommendations about how to measure the thickness of the uterine wall, which may vary at different regions of the uterus (Grimbizis et al., 2012, 2013; Ludwin et al., 2014b). Temporary guidelines have been suggested, with measurement of the mean thickness of the anterior and posterior wall (Grimbizis et al., 2014). However, this may not solve the basic problem with the ESHRE-ESGE criteria, because linking the internal indentation and thickness of the uterine wall may generate more frequent diagnosis of uterine septum or RS in women who have a thin wall, and less frequent diagnosis in women who have a thick wall, than a real degree of distortion of uterine cavity architecture (Salim et al., 2003b). However, women of childbearing age typically have wall thickness <20 mm (Traiman et al., 1996; Youm et al., 2011), and the risk of overdiagnosis of RS or septate uterus may be higher with the ESHRE-ESGE criteria than with the classification of ASRM and simple criterion to differentiate between normal and abnormal uterine cavity shape after metroplasty (Ludwin et al., 2014a) or between normal (internal identation <1 cm), arcuate and septate uteri (Bermejo et al., 2010; Ludwin et al., 2013a,b,c, 2014b).
Further studies with reproductive outcomes may verify whether the ESHRE-ESGE criteria are relevant for the diagnosis and treatment of septate uterus. The ideal classification should suggest appropriate therapy and avoid inadequate or unnecessary surgery (Acién and Acién, 2011).
A strength of the present study was the use of standardized and reproducible three-dimensional imaging including preoperative and post-operative 3D-SIS, intraoperative 3D/4D-TRUS and complementary imaging for the prospective assessment of adhesion formation upon the completion of hysteroscopic metroplasty. This assessment is important because hysteroscopy has low reproducibility in assessing the shape of the uterine cavity (Smit et al., 2013). The use of intraoperative 3D/4D-TRUS enabled unification of the range of septum resection by achieving similar fundal myometrial thicknesses in the tested patients. However, this may not eliminate the risk of incomplete or non-standardized resection caused by early termination of the procedure because of hypoosmolar negative (>1 L) medium balance or excessive bleeding. Nevertheless, intraoperative 3D/4D-TRUS provided an objective measure of resection completion and minimized subjective or arbitrary evaluation by the surgeon. A limitation of this method is that the diagnostic accuracy of 3D/4D-TRUS is unknown; this method is based on advanced volume imaging technology and negative contrast of the uterine cavity by the medium, and visualization may be impaired by the high mobility of the uterus, blood clots, gas bubbles or ultrasonic interference with a resectoscope (Ludwin et al., 2013c).
The suggested optimal range of 6–10 mm for intraoperative fundal myometrial thickness was based on our personal experience (Ludwin et al., 2014a). There are no data that suggest that thinner fundal myometrium may be associated with an increased risk of uterine rupture during pregnancy. Other surgeons have suggested that hysteroscopic metroplasty may be completed when the internal fundal indentation is <10 mm and the fundal myometrial thickness >10 mm.
Limitations of the present study included the lack of randomization of patients who had autocross-linked polysaccharide, which may limit the importance of observations about the effectiveness of autocross-linked polysaccharide in decreasing healing-dependent abnormal anatomic results. In addition, the patient groups were defined by earlier or later inclusion, and unidentified time-dependent factors may have introduced bias, even though consistent standardization of procedures was feasible. All septal resections were performed by the same surgeon who had reached a plateau on the learning curve of hysteroscopic metroplasty. Although sufficient statistical power (1.0) was achieved for comparisons between groups, a double-blinded randomized controlled trial is justified to better assess the efficacy of autocross-linked polysaccharide. Nevertheless, the present results with autocross-linked polysaccharide are useful initial outcomes that helped determine the sample size needed for the double-blinded randomized controlled trial that was started after completing this study.
The occurrence of abnormal anatomic results in patients who did not have adhesion barrier was similar to the worst outcomes (reoperations in 23% women) presented in previous reports (Litta et al., 2004). In a literature review, Nouri et al. (2010) reported that reoperations were conducted in 6% of 1324 women who underwent hysteroscopic metroplasty. Most previous studies have been retrospective and have lacked qualification criteria for repeat hysteroscopy, specific methods and validation of post-operative assessment. It is important to identify which patients may obtain the greatest benefit of antiadhesion treatments such as autocross-linked polysaccharide (Acunzo et al., 2003; Guida et al., 2004; Ducarme et al., 2006; Deans and Abbott, 2010; Di Spiezio Sardo et al., 2011). Autocross-linked polysaccharide may have good efficacy in the prevention of intrauterine adhesions after hysteroscopic metroplasty, polypectomy and myomectomy (Guida et al., 2004). Adhesion barriers may be considered in patients who have an increased risk of adhesion formation.
The most important clinical implication of the present study is the identification of septal width as the primary factor that may predict healing-dependent abnormal anatomic results after hysteroscopic metroplasty. This finding may help target adhesion barrier and optimize the cost of care by avoiding antiadhesion methods in patients who are at low risk of developing abnormal anatomic results and by minimizing reoperation in patients who are at high risk for ARs. In addition, the present results showed that caution may be required in using the ESHRE-ESGE classification for the post-operative diagnosis of problems related to uterine septa. Additional studies are needed to evaluate the effects of septal morphology, the suitability of the ESGE-ESGE criteria in evaluating uterine septa, the use of autocross-linked polysaccharide and the effects of various anatomic results on reproductive outcomes such as frequency of pregnancy, miscarriage and live birth.
In the present study, additional surgical interventions were performed during second-look hysteroscopy. The potential effects of these interventions on the final reproductive outcomes may have neutralized the effects of autocross-linked polysaccharide. This issue may be addressed in a further study that includes patients who have no corrective intervention during second-look hysteroscopy.
Using advanced three-dimensional imaging to determine preoperative parameters, the septal width was the most important morphologic parameter. The septal width is the only tested parameter that may also be determined easily with two-dimensional ultrasonography.
Conclusion
The uterine septal width and, to a lesser extent, surface area may predict the development of healing-dependent abnormal anatomic results after complete hysteroscopic metroplasty. These factors may help determine which patients may benefit from targeted antiadhesion prophylaxis. The use of autocross-linked polysaccharide reduces the risk of abnormal anatomic results.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
A.L.: substantial contributions to conception, design, acquisition of data, analysis and interpretation of data; drafting and revision of the article and final approval of the version to be published. I.L. and K.P.: substantial contributions to acquisition of data, article revision and final approval of the version to be published. K.P.: substantial contributions to acquisition of data, article revision and final approval of the version to be published. T.B. substantial contributions to analysis and interpretation of data, article revision and final approval of the version to be published. R.J.: substantial contributions to interpretation of data, article revision and final approval of the version to be published.
Funding
This work was supported in part by Jagiellonian University (grant no. K/ZDS/003821). Funding to pay the Open Access publication charges for this article was provided by Jagiellonian University.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Material
Acknowledgements
We thank Ms Justyna Stefaniak (MSc, mathematician, biostatistician) of Data Management and Statistical Analysis (Krakow, Poland) for data processing and statistical analysis. We also thank Professor Grimbizis and The CONUTA (CONgenital UTerine Anomalies) Working Group for their contribution to the development of knowledge about congenital abnormalities of the female genital tract, which was the inspiration for part of our analysis, reflection and further research.
References
- Acién P, Acién MI. The history of female genital tract malformation classifications and proposal of an updated system. Hum Reprod Update. 2011;17:693–705. doi: 10.1093/humupd/dmr021. [DOI] [PubMed] [Google Scholar]
- Acunzo G, Guida M, Pellicano M, Tommaselli GA, Di Spiezio Sardo A, Bifulco G, Cirillo D, Taylor A, Nappi C. Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective randomized, controlled study. Hum Reprod. 2003;18:1918–1921. doi: 10.1093/humrep/deg368. [DOI] [PubMed] [Google Scholar]
- Anderson J. The myometrium. In: Anderson J, editor. Gynecologic Imaging. Philadelphia, PA, USA: Churchill Livingstone; 1999. p. 243. [Google Scholar]
- Bermejo C, Martínez Ten P, Cantarero R, Diaz D, Pérez Pedregosa J, Barrón E, Labrador E, Ruiz López L. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35:593–601. doi: 10.1002/uog.7551. [DOI] [PubMed] [Google Scholar]
- Bosteels J, Weyers S, Puttemans P, Panayotidis C, Van Herendael B, Gomel V, Mol BW, Mathieu C, D'Hooghe T. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Hum Reprod Update. 2010;16:1–11. doi: 10.1093/humupd/dmp033. [DOI] [PubMed] [Google Scholar]
- Buttram VC, Jr, Gomel V, Siegler A, DeCherney A, Gibbons W, March C. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49:944–955. doi: 10.1016/s0015-0282(16)59942-7. [DOI] [PubMed] [Google Scholar]
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, Coomarasamy A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update. 2011;17:761–771. doi: 10.1093/humupd/dmr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S-Ch, Wang H, Shao J. Sample Size Calculations in Clinical Research. New York, USA: Chapman & Hall/CRC; 2012. [Google Scholar]
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17:555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- De Iaco PA, Muzzupapa G, Bigon E, Pressato D, Donà M, Pavesio A, Bovicelli L. Efficacy of a hyaluronan derivative gel in postsurgical adhesion prevention in the presence of inadequate hemostasis. Surgery. 2001;130:60–64. doi: 10.1067/msy.2001.115102. [DOI] [PubMed] [Google Scholar]
- Di Spiezio Sardo A, Spinelli M, Bramante S, Scognamiglio M, Greco E, Guida M, Cela V, Nappi C. Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J Minim Invasive Gynecol. 2011;18:462–469. doi: 10.1016/j.jmig.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Ducarme G, Davitian C, Zarrouk S, Uzan M, Poncelet C. Interest of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a case–control study [in French] J Gynecol Obstet Biol Reprod (Paris) 2006;35:691–695. doi: 10.1016/s0368-2315(06)76465-1. [DOI] [PubMed] [Google Scholar]
- Fedele L, Bianchi S, Marchini M, Mezzopane R, Di Nola G, Tozzi L. Residual uterine septum of less than 1 cm after hysteroscopic metroplasty does not impair reproductive outcome. Hum Reprod. 1996;11:727–729. doi: 10.1093/oxfordjournals.humrep.a019242. [DOI] [PubMed] [Google Scholar]
- Gergolet M, Campo R, Verdenik I, Kenda Suster N, Gordts S, Gianaroli L. No clinical relevance of the height of fundal indentation in subseptate or arcuate uterus: a prospective study. Reprod Biomed Online. 2012;24:576–582. doi: 10.1016/j.rbmo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Grimbizis GF, Campo R, Gordts S, Brucker S, Gergolet M, Tanos V, Li TC, De Angelis C, Di Spiezio Sardo A Scientific Committee of the Congenital Uterine Malformations (CONUTA) Common ESHRE/ESGE Working Group. Clinical approach for the classification of congenital uterine malformations. Gynecol Surg. 2012;9:119–129. doi: 10.1007/s10397-011-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbizis GF, Gordts S, Di Spiezio Sardo A, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Brölmann H, Gianaroli L, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod. 2013;28:2032–2044. doi: 10.1093/humrep/det098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbizis GF, Gordts S, Di Spiezio Sardo A, Brucker SY, De Angelis C, Gergolet M, Li TC, Tanos V, Brölmann HH, Gianaroli L, et al. Reply: Are the ESHRE/ESGE criteria of female genital anomalies for diagnosis of septate uterus appropriate? Hum Reprod. 2014;29:868–869. doi: 10.1093/humrep/deu002. [DOI] [PubMed] [Google Scholar]
- Gubbini G, Di Spiezio Sardo A, Nascetti D, Marra E, Spinelli M, Greco E, Casadio P, Nappi C. New outpatient subclassification system for American Fertility Society Classes V and VI uterine anomalies. J Minim Invasive Gynecol. 2009;16:554–561. doi: 10.1016/j.jmig.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Guida M, Acunzo G, Di Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, Cerrota G, Cirillo D, Nappi C. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Hum Reprod. 2004;19:1461–1464. doi: 10.1093/humrep/deh238. [DOI] [PubMed] [Google Scholar]
- Hirai M, Shibata K, Sagai H, Sekiya S, Goldberg BB. Transvaginal pulsed and color Doppler sonography for the evaluation of adenomyosis. J Ultrasound Med. 1995;14:529–532. doi: 10.7863/jum.1995.14.7.529. [DOI] [PubMed] [Google Scholar]
- Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertil Steril. 2000;73:1–14. doi: 10.1016/s0015-0282(99)00480-x. [DOI] [PubMed] [Google Scholar]
- Kish L. Survey Sampling. New York, USA: John Wiley & Sons; 1965. [Google Scholar]
- Kowalik CR, Goddijn M, Emanuel MH, Bongers MY, Spinder T, de Kruif JH, Mol BW, Heineman MJ. Metroplasty versus expectant management for women with recurrent miscarriage and a septate uterus. Cochrane Database Syst Rev. 2011;6:CD008576. doi: 10.1002/14651858.CD008576.pub3. [DOI] [PubMed] [Google Scholar]
- Litta P, Pozzan C, Merlin F, Sacco G, Saccardi C, Ambrosini G, Capobianco G, Dessole S. Hysteroscopic metroplasty under laparoscopic guidance in infertile women with septate uteri: follow-up of reproductive outcome. J Reprod Med. 2004;49:274–278. [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Banas T, Knafel A, Miedzyblocki M, Basta A. Diagnostic accuracy of sonohysterography, hysterosalpingography and diagnostic hysteroscopy in diagnosis of arcuate, septate and bicornuate uterus. J Obstet Gynaecol Res. 2011;37:178–186. doi: 10.1111/j.1447-0756.2010.01304.x. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Pityński K, Ludwin I, Banas T, Knafel A. Two- and three-dimensional ultrasonography and sonohysterography versus hysteroscopy with laparoscopy in the differential diagnosis of septate, bicornuate, and arcuate uteri. J Minim Invasive Gynecol. 2013a;20:90–99. doi: 10.1016/j.jmig.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Pityński K, Banas T, Jach R. Differentiating between a double cervix or cervical duplication and a complete septate uterus with longitudinal vaginal septum. Taiwan J Obstet Gynecol. 2013b;52:308–310. doi: 10.1016/j.tjog.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Pityński K, Basta P, Basta A, Banas T, Jach R, Wiecheć M, Grabowska R, Stangel-Wójcikiewicz K, et al. Transrectal ultrasound-guided hysteroscopic myomectomy of submucosal myomas with a varying degree of myometrial penetration. J Minim Invasive Gynecol. 2013c;20:672–685. doi: 10.1016/j.jmig.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Kudla M, Pityński K, Banas T, Jach R, Knafel A. Diagnostic accuracy of three-dimensional sonohysterography compared with office hysteroscopy and its interrater/intrarater agreement in uterine cavity assessment after hysteroscopic metroplasty. Fertil Steril. 2014;101:1392–1399. doi: 10.1016/j.fertnstert.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Pityński K, Jach R, Banas T. Are the ESHRE/ESGE criteria of female genital anomalies for diagnosis of septate uterus appropriate? Hum Reprod. 2014b;29:867–868. doi: 10.1093/humrep/deu001. [DOI] [PubMed] [Google Scholar]
- Mais V, Cirronis MG, Peiretti M, Ferrucci G, Cossu E, Melis GB. Efficacy of auto-crosslinked hyaluronan gel for adhesion prevention in laparoscopy and hysteroscopy: a systematic review and meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2012;160:1–5. doi: 10.1016/j.ejogrb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Mollo A, De Franciscis P, Colacurci N, Cobellis L, Perino A, Venezia R, Alviggi C, De Placido G. Hysteroscopic resection of the septum improves the pregnancy rate of women with unexplained infertility: a prospective controlled trial. Fertil Steril. 2009;91:2628–2631. doi: 10.1016/j.fertnstert.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Mollo A, Nazzaro G, Granata M, Clarizia R, Fiore E, Cadente C, Castaldo G, Conforti S, Locci M, De Placido G. Combined hysteroscopic findings and 3-dimensional reconstructed coronal view of the uterus to avoid laparoscopic assessment for inpatient hysteroscopic metroplasty: pilot study. J Minim Invasive Gynecol. 2011;18:112–117. doi: 10.1016/j.jmig.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Nappi C, Di Spiezio Sardo A, Greco E, Guida M, Bettocchi S, Bifulco G. Prevention of adhesions in gynaecological endoscopy. Hum Reprod Update. 2007;13:379–394. doi: 10.1093/humupd/dml061. [DOI] [PubMed] [Google Scholar]
- Nawroth F, Schmidt T, Freise C, Foth D, Römer T. Is it possible to recommend an “optimal” postoperative management after hysteroscopic metroplasty? A retrospective study with 52 infertile patients showing a septate uterus. Acta Obstet Gynecol Scand. 2002;81:55–57. doi: 10.1046/j.0001-6349.2001.10228.x. [DOI] [PubMed] [Google Scholar]
- Nouri K, Ott J, Huber JC, Fischer EM, Stögbauer L, Tempfer CB. Reproductive outcome after hysteroscopic septoplasty in patients with septate uterus—a retrospective cohort study and systematic review of the literature. Reprod Biol Endocrinol. 2010;8:52. doi: 10.1186/1477-7827-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabuçcu R, Gomel V. Reproductive outcome after hysteroscopic metroplasty in women with septate uterus and otherwise unexplained infertility. Fertil Steril. 2004;81:1675–1678. doi: 10.1016/j.fertnstert.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Porcu G, Cravello L, D'Ercole C, Cohen D, Roger V, de Montgolfier R, Blanc B. Hysteroscopic metroplasty for septate uterus and repetitive abortions: reproductive outcome. Eur J Obstet Gynecol Reprod Biol. 2000;88:81–84. doi: 10.1016/s0301-2115(99)00126-8. [DOI] [PubMed] [Google Scholar]
- Querleu D, Brasme TL, Parmentier D. Ultrasound-guided transcervical metroplasty. Fertil Steril. 1990;54:995–998. doi: 10.1016/s0015-0282(16)53993-4. [DOI] [PubMed] [Google Scholar]
- Salim R, Woelfer B, Backos M, Regan L, Jurkovic D. Reproducibility of three-dimensional ultrasound diagnosis of congenital uterine anomalies. Ultrasound Obstet Gynecol. 2003a;21:578–582. doi: 10.1002/uog.127. [DOI] [PubMed] [Google Scholar]
- Salim R, Regan L, Woelfer B, Backos M, Jurkovic D. A comparative study of the morphology of congenital uterine anomalies in woman with and without a history of recurrent first trimester miscarriage. Human Reprod. 2003b;18:162–166. doi: 10.1093/humrep/deg030. [DOI] [PubMed] [Google Scholar]
- Smit JG, Kasius JC, Eijkemans MJ, Veersema S, Fatemi HM, Santbrink van EJ, Campo R, Broekmans FJ. The international agreement study on the diagnosis of the septate uterus at office hysteroscopy in infertile patients. Fertil Steril. 2013;99:2108–2113.e2. doi: 10.1016/j.fertnstert.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Taskin O, Sadik S, Onoglu A, Gokdeniz R, Erturan E, Burak F, Wheeler JM. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7:351–354. doi: 10.1016/s1074-3804(05)60478-1. [DOI] [PubMed] [Google Scholar]
- Tomazevic T, Ban-Frangez H, Ribic-Pucelj M, Premru-Srsen T, Verdenik I. Small uterine septum is an important risk variable for preterm birth. Eur J Obstet Gynecol Reprod Biol. 2007;135:154–157. doi: 10.1016/j.ejogrb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Traiman P, Saldiva P, Haiashi A, Franco M. Criteria for the diagnosis of diffuse uterine myohypertrophy. Int J Gynaecol Obstet. 1996;54:31–36. doi: 10.1016/0020-7292(96)02676-8. [DOI] [PubMed] [Google Scholar]
- Valle RF, Ekpo GE. Hysteroscopic metroplasty for the septate uterus: review and meta-analysis. J Minim Invasive Gynecol. 2013;20:22–42. doi: 10.1016/j.jmig.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Arcaini L, Rognoni MT, Candiani GB. Value of intrauterine device insertion and estrogen administration after hysteroscopic metroplasty. J Reprod Med. 1989;34:447–450. [PubMed] [Google Scholar]
- Yang JH, Chen MJ, Chen CD, Chen SU, Ho HN, Yang YS. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil Steril. 2013;99:2092–2096.e3. doi: 10.1016/j.fertnstert.2013.01.137. [DOI] [PubMed] [Google Scholar]
- Youm HS, Choi YS, Han HD. In vitro fertilization and embryo transfer outcomes in relation to myometrial thickness. J Assist Reprod Genet. 2011;28:1135–1140. doi: 10.1007/s10815-011-9640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.