Abstract
In this report we review the health effects of three short-lived greenhouse pollutants—black carbon, ozone, and sulphates. We undertook new meta-analyses of existing time-series studies and an analysis of a cohort of 352 000 people in 66 US cities during 18 years of follow-up. This cohort study provides estimates of mortality effects from long-term exposure to elemental carbon, an indicator of black carbon mass, and evidence that ozone exerts an independent risk of mortality. Associations among these pollutants make drawing conclusions about their individual health effects difficult at present, but sulphate seems to have the most robust effects in multiple-pollutant models. Generally, the toxicology of the pure compounds and their epidemiology diverge because atmospheric black carbon, ozone, and sulphate are associated and could interact with related toxic species. Although sulphate is a cooling agent, black carbon and ozone could together exert nearly half as much global warming as carbon dioxide. The complexity of these health and climate effects needs to be recognised in mitigation policies.
Introduction
Short-lived greenhouse pollutants emitted largely from fuel combustion account directly or indirectly for a large proportion of present global warming. They also account for most of the direct damage to human health from energy use worldwide. These pollutants include two important health-damaging agents—sulphates and organic-carbon aerosols—which generally have global-cooling characteristics. Another aerosol, black carbon, is also health damaging, but is a warming agent. Other short-lived greenhouse pollutants include warming agents in the form of health-damaging gases such as ozone, a secondary pollutant formed after complex photochemical reactions, and other gases that contribute to ozone formation such as carbon monoxide, non-methane volatile organic compounds, methane, and nitrogen oxides. Most of these precursors to ozone also exert direct effects on human health.
Conversely, carbon dioxide, the most important greenhouse gas, and nitrous oxide and halocarbons, the other long-lived greenhouse gases, have little direct effect on health. Nitrous oxide and halocarbons arise mainly from sources outside the energy supply system.
All short-lived greenhouse pollutants, whether warming or cooling, have effects on health when people are exposed to them or, in the case of methane, their atmospheric byproduct, ozone. Patterns of emissions and exposures vary greatly, and because the pollutants are short-lived, their health effects depend on the location of sources in relation to local and regional factors such as weather patterns, geography, and population distribution. Localised concentrations of ozone and black carbon have been identified as agents increasing the urban heat island effect by trapping heat and interacting with urban carbon dioxide concentrations.1 In turn, these short-lived greenhouse pollutants might increase health burdens from heat waves in urban cores.1
Several of the short-lived greenhouse pollutants have substantial effects on the human-managed and natural biosphere through acid precipitation (sulphate and nitrate),2 eutrophication (nitrate), direct damage to organisms (ozone), and in the case of black carbon deposition, accelerated melting of ice and snow. Increased forest growth through eutrophication could lead to interactions with the global carbon cycle through, for example, enhanced growth and carbon dioxide uptake, suggesting a cooling effect on climate,3 which might in turn be partly offset by ozone’s negative effect on carbon uptake in ecosystems.4
Many important sources emit more than one short-lived greenhouse pollutant, and in some cases, control of one and not another is difficult—eg, black carbon and organic carbon from combustion of biomass, coal, and diesel fuel. Additionally, ozone and sulphate are not emitted directly, but are secondary products from transformations of precursor emissions in the atmosphere. The net effect of control measures on climate warming can thus be difficult to estimate because of simultaneous changes in both warming and cooling agents. This challenge is amplified by the scientific and policy complexities related to the widely different temporal patterns that characterise the burden of such pollutants compared with carbon dioxide control, which is usually the major consideration in climate negotiations and discussions.
Aggressive policies directed towards carbon dioxide reduction, although necessary for the long term, are by themselves insufficient to reduce the rate of warming in the next few decades because of the long atmospheric lifetime of this gas.5 Thus, governments will need to reduce warming from short-lived greenhouse gas pollutants when considering climate change mitigation policies.6,7 Choice of policies with positive health and ecosystem effects provides the opportunity for substantial co-benefits (eg, reductions in ozone concentrations will diminish warming while also providing substantial health benefits to human populations and ecosystems) and climate protection. Alternatively, poor choices could result in major net additional risks to human health and ecosystems.
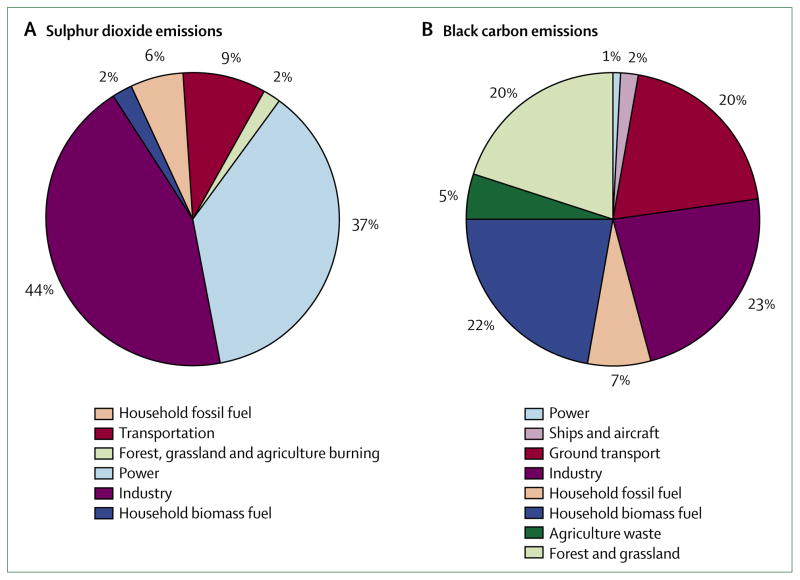
The short-lived greenhouse pollutants climate primer (panel) summarises the issues related to these pollutants. We examine the present state of health evidence for three important short-lived greenhouse pollutants: black carbon, sulphate, and ozone. The figure shows the relative importance of different sectors that are thought to cause most anthropogenic emissions for black carbon and the precursor of sulphate, sulphur dioxide. The relative emissions differ greatly by sector, which has important policy implications for control measures. We focus on the health effects of ambient pollution and not on exposures from indoor sources, such as household fuels, which also substantially affect health worldwide.12
Figure. Relative contributions of human sources to sulphur dioxide (A) and black carbon (B) emissions.
In each case, the distribution of climate effects differs from the distribution of the primary pollutant. For sulphur dioxide, the extent of transformation to the climate-active species, sulphate, will vary by location. For black carbon, the different sources produce cooling organic-carbon aerosols in varying amounts. The climate implication is due to the net radiative forcing of the two linked emissions. Only half the total forest and grassland emissions are counted here as an estimate of the proportion that is due to human activities. 2005 sulphur dioxide estimates are interpolated between the 1995 and 2030 estimates calculated by Unger and colleagues.10 Since there are no official inventories and because methods vary across investigators, these estimates should be regarded as approximate. Estimates of black carbon emissions are from the black carbon emissions inventory.11
We discuss the present state of knowledge of health effects on the basis of toxicological evidence in controlled settings and from observational epidemiological studies. This review includes new meta-analyses of time-series studies and new evidence for relative mortality effects of long-term exposures to sulphates, elemental carbon, and ozone from a national US cohort study. We conclude by discussing cross-cutting issues such as the benefits of removing remaining uncertainties and the need for analyses that incorporate both climate and health implications of control policies.
Review of health effects
Sulphates
Respirable ambient particles have been associated with increased mortality and a wide range of morbidity effects.13 Most evidence relates to undifferentiated particulate matter with aerodynamic diameter 10 μm or less (PM10) and 2·5 μm or less (PM2·5), and these metrics of particle size are the basis of most health-based standards and impact assessments for particles. Although many scientists believe that particle toxicity is also affected by particle number and chemical composition and not just particle size, these differences are difficult to quantify.14
Sulphur emissions from human activities are dominated by fossil-fuel combustion (figure). Although emitted as sulphur dioxide, much is converted to sulphate, depending on local conditions.15,16 Human emissions are falling in most parts of the world because of air pollution regulations.10 The potential role of sulphate in driving the hazard of air pollution has been addressed in several long-term and short-term animal exposure experiments, especially in relation to effects on the pulmonary system. In 1992, the UK Advisory Group on the Medical Aspects of Air Pollution Episodes concluded that even fairly high concentrations of inhaled sulphur dioxide, sulphate, and aerosols of sulphuric acid are well tolerated by many animal species.17 The results of subsequent studies collectively lend support to this original conclusion.18 The concentrations used in these studies are much higher than the typical concentrations measured in the environment, even during pollution episodes. Thus, at the concentrations recorded in most countries, sulphate and related sulphur compounds are unlikely to have substantial pulmonary toxicity.
Panel: Climate primer for energy-related short-lived greenhouse pollutants.
In this panel we present a short summary of the climate issues surrounding each short-lived greenhouse pollutant, and carbon dioxide for comparison. Although only the health effects of sulphates, black carbon, and ozone are discussed in the main text, all the other short-lived greenhouse pollutants also have direct or indirect health effects, or both. The figure in the webappendix p 1, which is taken directly from the most recent authoritative international scientific assessment,8 shows the estimated pattern across these agents of global warming in 2005 due to all human emissions since 1750. The greenhouse pollutants are compared by radiative forcing (RF), which is a metric used for the quantitative comparisons of the strength of different human and natural agents in causing climate change.
To distinguish between direct and indirect RF is important. Direct RF results when the emitted substance is a greenhouse pollutant itself, such as carbon dioxide. Indirect RF results when the emitted pollutant is not a greenhouse pollutant but takes part in chemical reactions within the atmosphere to form a greenhouse pollutant or to change the global distribution of a greenhouse pollutant. Sulphur dioxide is an example because it is transformed in the atmosphere to form aerosol sulphates that act to produce a negative RF. Nitrogen oxide emissions act to increase the oxidising capacity of the troposphere, reducing methane (negative RF), but adding to tropospheric ozone (positive RF), whereas methane, carbon monoxide, and non-methane volatile organic carbons contribute to tropospheric ozone. As a result, although not emitted directly, ozone in total is ranked as the third most important human-influenced greenhouse gas in the atmosphere (after carbon dioxide and methane).
Aerosol particles affect RF directly through the reflection and absorption of solar and infrared radiation. Some aerosols, such as black carbon, cause a positive RF (climate warming) whereas others, such as sulphates, cause a negative RF (climate cooling). The direct RF summed over all aerosol types is negative. Aerosols also cause a negative RF indirectly through the changes that they cause in cloud properties, which are not shown in webappendix p 1. The observed global temperature records for the 20th century cannot be explained with global climate models without a substantial cooling term after World War 2 from the burning of sulphur-containing fossil fuels that offset partially the global warming from the greenhouse gases. As nitrogen and sulphur oxides are reduced globally owing to health and acid-precipitation concerns, their cooling effect will reduce, thus unmasking the climate warming due to other greenhouse pollutants that is now counteracted.
Additionally, because control of black carbon emissions is difficult without simultaneously doing so for the associated organic carbon particle emissions from the same combustion sources, the worldwide climate benefits of carbon-particle control measures, including improved combustion efficiency, depend on the ratio of black carbon to organic carbon of each source. Due to recent observations and modelling,9 however, the estimated warming of black carbon might increase above that in the most recent Intergovernmental Panel on Climate Change (IPCC), which is shown in the figure. This increase would have policy implications because a much broader range of combustion sources would then have net climate benefits from combined control of black and organic carbon.
Since methane and carbon dioxide are well mixed globally, emissions in all places and seasons can be treated as essentially equal, which led to the deployment of so-called global warming potentials by the IPCC, which are used to weight the relative importance of emissions of different greenhouse gases in treaties, inventories, and international carbon-offset programmes. The complexity, short life, and local dependence of the short-lived greenhouse pollutants, however, make it difficult if not impossible to establish official global warming potentials for use in policy making. Thus, at present, short-lived greenhouse pollutants other than methane are not included in many international climate policy deliberations, although they have been the subject of much research and media attention and are featured prominently in scientific assessments.
Long-lived greenhouse pollutants (hundreds of years)
Carbon dioxide poses a low direct health hazard and is the weakest greenhouse pollutant by mass; however, because of the magnitude of emissions it is the most important overall. It also has a much longer lifetime in the atmosphere than do any of the other energy-related pollutants—most is gone in 100 years or so but a proportion of emissions is thought to remain in the atmosphere for thousands of years.
Medium-lived greenhouse pollutants (tens of years and thus globally mixed)
Methane is the second most important greenhouse pollutant. It is produced from a range of energy-related sources, including leakage from oil and gas facilities and coal mines, and from incomplete combustion of biomass and fossil fuels. Its main sources, however, are agriculture and poor waste management. Although not directly damaging to health, methane is a precursor to the global rise in tropospheric ozone concentrations, which is a concern in parts of Asia and is generally separate from urban sources. Although having a shorter overall lifetime than carbon dioxide, because of its large direct and indirect effects on warming, a tonne of methane will have a much bigger warming effect than would a tonne of carbon dioxide for the first few decades after emission.
Short-lived greenhouse pollutants (days to weeks and thus effects depend on local conditions)
Carbon monoxide is mainly a product of incomplete combustion, and although not having a direct climate effect, acts to sweep up hydroxyl radicals in the atmosphere, thus effectively increasing the lifetime of methane and adding to tropospheric ozone. The effects of carbon monoxide on methane and tropospheric ozone are both potentially climate warming.
Non-methane volatile organic compounds come from several human-generated sources, including incomplete combustion and evaporation from fuels. Emissions of these pollutants contribute to ozone formation and act to reduce the oxidising capacity of the atmosphere, which extends the lifetime of methane. Both of these effects increase global warming. These compounds can also have direct human health effects.
Nitrogen oxides derive from fuel combustion and have a complex relation to and indirect effect on both climate warming and cooling by affecting ozone, methane, and particle concentrations. Emissions from nitrogen oxides act to decrease the oxidising capacity of the troposphere increasing the methane lifetime, but also are a major precursor to tropospheric ozone. Nitrate particles, as with those of sulphate, are lighter in colour and thus generally cooling. There also seems to be a small increase in carbon capture in natural ecosystems due to eutrophication from deposited nitrate.
Sulphur dioxide, which derives mainly from combustion of fuels, partly converts to sulphate aerosols in the atmosphere. Although these aerosols are potentially damaging to health, they are generally thought to exert a net cooling effect on the climate. As with organic carbon, sulphates can sometimes be coated with black carbon to create brown carbon, which has warming potential.
Black carbon, which is fine particulate matter of dark colour containing a large fraction of elemental carbon, is derived exclusively from incomplete combustion. It is strongly warming in the atmosphere, and increases heat absorption if deposited on ice and snow—eg, on Himalayan glaciers or in the Arctic.
Organic carbon aerosol, a less dark carboneous particulate matter, is produced mainly from incomplete combustion, but also from secondary processes involving biogenic volatile organic compounds. Although not well characterised and sometimes physically combined with black carbon, it is thought generally to produce a net cooling effect globally, although with much local variation. Organic carbon is a major form of health-damaging small particles worldwide.
Very short-lived greenhouse pollutants (hours to days)
Tropospheric ozone is a health-damaging secondary pollutant formed through complex photochemical reactions involving nitrogen oxides and volatile organic compounds, including methane in the presence of sunlight. Stratospheric ozone generally has different sources and, although also warming, protects earth’s surface from health-damaging and ecosystem-damaging UV radiation.
Little toxicological evidence suggests that sulphate itself is toxic to the cardiovascular system. A few experimental studies have investigated the effect of sulphate-containing compounds or mixtures on the cardiovascular system. Such studies have included concentrated air particles,19 metal sulphates,20 soluble particulate matter extracts,21 and residual oil fly ash.22 Although several cardiovascular variables have been measured across these studies, none has associated the sulphate component itself with cardiovascular effects.
The 1992 Advisory Group on the Medical Aspects of Air Pollution Episodes report, however, identified that “exposure of animals to sulphur dioxide in combination with particulates may be more damaging than the effects of the gas or sulphuric acid alone”.17 Such studies also used high particulate concentrations in animal experiments and focused on pulmonary toxicity, making results difficult to interpret in terms of human health effects from ambient sulphate in the troposphere.
In a new systematic review and meta-analysis of ten (eight from the USA and Canada and two from Europe) single-city time-series studies of sulphate and daily all-cause mortality, a pooled random-effects estimate of a 0·21% (95% CI 0·11–0·30) increase in mortality per 1 μg/m3 increase in sulphate was obtained (table 1 and webappendix pp 26–27). Positive associations were also detected in the fewer studies of cause-specific mortality. The sulphate estimates tended to be independent of other particle metrics and pollutant gases such as ozone. Findings from a meta-analysis of panel studies showed positive associations between sulphate exposure and daily measures of lung function, symptoms, and asthma drug use.16
Table 1.
Estimates of mortality effects of ambient particulate matter (sulphate and black smoke) and ozone from cohort and time-series studies
| Outcome | Percentage change in mortality per 1 μg/m3 change in pollutant (95% CI) | |
|---|---|---|
|
Particle sulphate
| ||
| American Cancer Society cohort23 | All-cause | 0·88 (0·67 to 1·10) |
| American Cancer Society cohort23 | Cardiopulmonary | 1·01 (0·75 to 1·38) |
| Time-series studies | ||
| Single-city estimates (n=10) | All-cause | 0·21 (0·11 to 0·30) |
| Multi-city studies | ||
| 10 Canadian cities | All-cause | 0·40 (0·10 to 0·70) |
| 6 Californian counties | All-cause | 0·12 (−0·76 to 1·00) |
| 6 US communities | All-cause | 0·22 (0·13 to 0·31) |
| Time-series studies | ||
| Single-city estimates (n=5) | Cardiovascular | 0·09 (−0·04 to 0·21) |
| Multi-city studies | ||
| 6 Californian counties | Cardiovascular | 0·36 (−0·91 to 1·65) |
| Time-series studies | ||
| Single-city estimates (n=4) | Respiratory | 0·37 (−0·15 to 0·90) |
| Multi-city studies | Respiratory | 0·70 (−1·65 to 3·11) |
| 6 Californian counties | ||
|
| ||
|
Black smoke
| ||
| NLCS-AIR Study24 | All-cause | 0·49 (0·00 to 1·05) |
| NLCS-AIR Study24 | Cardiovascular | 0·39 (−0·51 to 1·23) |
| NLCS-AIR Study24 | Respiratory | 2·01 (−0·10 to 4·14) |
| PAARC25 | All-cause | 0·68 (0·30 to 0·96) |
| PAARC25 | Cardiopulmonary | 0·49 (0·00 to 1·14) |
| Time-series studies | ||
| Single-city estimates (n=25) | All-cause | 0·05 (0·03 to 0·07) |
| Multi-city studies | ||
| APHEA 1 (12 European cities) | All-cause | 0·03 (0·02 to 0·03) |
| APHEA 2 (29 European cities) | All-cause | 0·06 (0·03 to 0·08) |
| 13 Spanish cities | All-cause | 0·08 (0·04 to 0·12) |
| 9 Scottish sites | All-cause | 0·17 (0·07 to 0·26) |
| Time-series studies | ||
| Single-city estimates (n=20) | Cardiovascular | 0·04 (0·01 to 0·06) |
| Multi-city studies | ||
| APHEA 2 (15 cities) | Cardiovascular | 0·06 (0·03 to 0·09) |
| 13 Spanish cities | Cardiovascular | 0·03 (−0·02 to 0·08) |
| 9 Scottish sites | Cardiovascular | 0·04 (−0·10 to 0·18) |
| Time-series studies | ||
| Single-city estimates (n=20) | Respiratory | 0·04 (−0·02 to 0·11) |
| Multi-city studies | ||
| APHEA 2 (15 cities) | Respiratory | 0·08 (0·01 to 0·16) |
| 13 Spanish cities | Respiratory | 0·11 (0·04 to 0·18) |
| 9 Scottish sites | Respiratory | 0·52 (0·29 to 0·76) |
|
| ||
|
Ozone
| ||
| American Cancer Society cohort26 | All-cause | 0·02 (−0·08 to 0·14) |
| American Cancer Society cohort26 | Cardiovascular | 0·22 (0·06 to 0·46) |
| American Cancer Society cohort26 | Respiratory | 0·57 (0·20 to 0·94) |
|
| ||
|
Ozone (8 h)
| ||
| Time-series studies | ||
| Single-city estimates (n=22) | All-cause | 0·03 (0·02 to 0·04) |
| Multi-city study | ||
| APHEA 2 (23 cities) | All-cause | 0·003 (−0·018 to 0·024) |
| Time-series studies | ||
| Single-city estimates (n=19) | Cardiovascular | 0·04 (0·03 to 0·05) |
| Time-series studies | ||
| Single-city estimates (n=19) | Respiratory | 0·04 (0·01 to 0·07) |
Time-series coefficients for daily mortality are based on a systematic review and meta-analysis (webappendix pp 9–25).
The only long-term exposure studies of sulphate and mortality are from the USA. The most extensive is based on the American Cancer Society (ACS) Cancer Prevention Study II (CPS II).23 Investigators of this study, which included more than 500 000 participants, reported increased mortality from all natural causes, cardiopulmonary and cardiovascular disease, and lung cancer associated with long-term exposure.23 The Harvard Six-Cities study reported similar results.27 These findings have been corroborated and extended in our case study.
Black carbon
The term black carbon is used rather loosely with varied meanings in different disciplines.28 Whereas the colour (albedo) is the key factor for climate scientists, health scientists have shown growing interest in the chemical composition of this greenhouse pollutant. Both groups are concerned with size distributions and atmospheric lifetimes. Elemental carbon is interchangeable with black carbon insofar as it represents the largest proportion of heat-absorbing components in undifferentiated particulate matter.29 Unlike the major greenhouse pollutants, no official inventories of black carbon emissions have been produced by the UN Framework Convention on Climate Change (UNFCCC), so estimates vary. With data drawn from a widely-cited inventory,11 the figure shows that most black carbon emissions come from incomplete combustion of biomass or fossil fuel. As regulations for air pollution tighten, global black carbon emissions from fossil-fuel combustion will probably continue to fall. The largest anthropogenic category is household combustion of biomass and coal in developing countries, followed by incomplete combustion of coal in industry and diesel transport.
Studies in volunteers suggest that short-term exposure to diluted diesel exhaust impairs vascular function and induces ischaemic and thrombotic mechanisms.30–32 The role of elemental carbon is unclear, however, because whole-diesel emissions contain much organic carbon and other aerosol components in both the particle and gaseous phases. Studies in animals33,34 and in vitro34,35 with black carbon particles show similar effects, but these particles have very large surface areas compared with atmospheric black carbon. Animal studies have also shown that black carbon can accelerate atherosclerotic plaque formation,35 suggesting a long-term effect on cardiovascular health.
In-vivo36 and in-vitro37,38 modelling studies focusing on pulmonary responses have shown that ultrafine or nano-sized (less than 100 nm) particles are more likely to be toxic per unit mass, in terms of inducing inflammation and oxidative stress, than are larger particles of the same composition,39 presumably because they penetrate more deeply into the respiratory system. However, the relation between increasing toxic effects and decreasing particle size is not limited to carbon, but also applies for other low-solubility, low-toxicity materials such as titanium dioxide and polystyrene beads.40 Thus, it is not possible to isolate the size fraction mode from the chemical composition of carbon particles as the source of toxicity.41
Epidemiological evidence linking measured black carbon and elemental carbon to health outcomes is scarce, partly because routine government monitoring is not done in a sufficient number of locations and no common measurement method is agreed upon. Most evidence relates to the black smoke index—a standard method that relies on the light-absorbing characteristics of particles less than 4 μm in diameter. The webappendix pp 3–8 provides details of this method. Black carbon and black smoke are highly correlated, suggesting that black smoke is a good marker for black carbon.42,43 However, epidemiological evidence relating to particulate matter containing black carbon measured as PM10 or PM2·5 cannot realistically be used as a quantitative surrogate for black carbon, because the ratio of black carbon to total particulate matter varies greatly according to the mixture of sources (ie, industrial, diesel exhaust, wood smoke, refuse burning), which in turn varies by season, site, and time. For example, Li and colleagues44 investigated carbonaceous aerosol emissions from combustion of household fuels (wheat and woody fuels) in biomass stoves and noted that percentages of black carbon in PM2·5 were generally greater for emissions from woody fuel than for emissions from crop waste (3·6% to 71·2% vs 1·7% to 33·5%).44
Our systematic review and meta-analysis of short-term exposure time-series studies of black smoke and daily mortality detected significant, positive associations with all-cause, cardiovascular, and respiratory mortality (table 1 and webappendix pp 9–25). Although the results of the time-series meta-analysis suggest larger effects of sulphate than of black smoke, this distinction is not so clear in the few studies that have measured both.16 There is strong evidence that these health effects are not confined to mortality, but also include effects on morbidity outcomes such as hospital admissions for cardiopulmonary disorders.13
Two European cohort studies have shown positive associations between black smoke and mortality (table 1),24,25 and these results are supported by a small area analysis in the UK.45 Studies of occupational exposure to black carbon provide little evidence of increased risk of lung cancer,46 although abnormal chest radiographs have been reported.47 Occupational exposure to diesel exhaust is associated with increased risk of mortality from lung cancer and chronic obstructive pulmonary disease (COPD),48,49 but the role of elemental carbon specifically is unclear since diesel exhaust is itself, as mentioned, a complex mixture of particles and gases that contains many other species including organic carbon.
Ozone
Ozone is a secondary pollutant that is formed through complex photochemical reactions involving nitrogen oxides and volatile organic compounds in the presence of ultraviolet sunlight. Background tropospheric concentrations of ozone have doubled worldwide since pre-industrial times. Remote areas now display concentrations in the range of 30 parts per billion compared with earlier preindustrial estimates of 10–15 parts per billion. Although plants and other natural sources such as forest fires contribute precursors, the major reason for the doubling is anthropogenic release of methane and the emission of nitrogen oxides from fossil-fuel burning (webappendix p 1).8,50
Exposure of the airways to ozone can cause effects through two primary mechanisms: direct oxidative stress or by damaging the pulmonary system and draining energy from normal cell functions towards defence mechanisms.51 Such mechanisms can induce inflammation within cells that can contribute to formation of or exacerbate existing pulmonary disease.52,53 Human chamber-exposure studies and animal studies suggest that ozone could elicit biological responses at or near environmental concentrations.52–54
Time-series studies of daily mortality have consistently noted associations with ozone in Europe, the USA, and Canada (table 1).55–57 Ozone has also been associated with morbidity, including asthma exacerbation58 and hospital admissions for respiratory causes.59 Little evidence exists for associations between long-term exposure to ozone and mortality. A cohort study undertaken in the midwest and eastern USA reported an inverse association between ozone and mortality.27 Reanalysis of this study replicated these findings, but also suggested a positive association with warm-season ozone exposure.60 A study of about 6000 non-smoking Seventh-Day Adventists living in southern California recorded increased risks for men after long-term ozone exposure,61 but this finding was based on a small number of deaths.
An extension of the ACS CPS II with improved exposure data identified a significant association between long-term ozone exposure and cardiovascular, cardiopulmonary, and respiratory mortality (table 1). Effects were most pronounced for respiratory mortality and were insensitive to adjustment for several confounding variables and the co-pollutant PM2·5.26 Other findings of chronic effect lend support to the idea that ozone might target respiratory systems, including evidence of lung function deficits in children,62 increased asthma incidence,63 and impaired pulmonary function.64,65
Case study: effects of sulphate, elemental carbon, and ozone on mortality in the USA
Methods and data
We present a new analysis examining the relative strength of association between major mortality outcomes and short-lived greenhouse pollutants in a national US study. We used data from the ACS CPS II cohort.66 The analytical cohort for this research included 352 242 participants in 66 metropolitan statistical areas of the USA, with follow-up from 1982 to 2000 (webappendix pp 26–27).23 We included ozone measurements from the second and third quarters (warm season), PM2·5, sulphate, and elemental carbon using government monitors in each of the metropolitan areas. More detail about the exposure estimates for PM2·5, sulphate, and ozone is available elsewhere.23,67 For elemental carbon, we downloaded data from tabulations prepared by the Health Effects Institute for 2003–05, with maximum coverage across metropolitan statistical areas that had ACS participants with other available pollution data. Although these years are after the end of follow-up, the overall spatial patterns in particulate matter are fairly stable over time.68 Exposures were assigned to individuals on the basis of their metropolitan statistical area of residence at enrolment. We estimated mortality effects with models for independent pollutants and various combinations of co-pollutants. We tested two-way linear interactions between all pollutants.
We used multilevel random-effects Cox proportional hazards models to assess the risk of mortality in relation to pollution exposures, stratifying for age (single-year groupings), sex, and race in the baseline hazard.23 Some 20 variables with 44 terms were included to control for individual characteristics that might confound the association between air pollution and mortality. The spatial unit of analysis was the metropolitan statistical area for random-effects estimation (webappendix pp 26–27 provides more details).
Results
Tables 2 and 3 show the results of single-pollutant and multiple-pollutant models for the four pollutant estimates for all-cause and cardiopulmonary mortality. Relative risks (RRs) presented in the first row for each cause of death show the effects in single-pollutant models, whereas subsequent rows under each cause of death indicate pollutants simultaneously included in the survival model. We have included PM2·5 for comparison with the other pollutants and with previously published studies.23 PM2·5 is not included in any of the co-pollutant models because the other particle metrics are constituents of it. Additionally, multiple-pollutant results for PM2·5 and ozone have been investigated elsewhere.26 To provide a basis for comparison, all RRs are assessed over a 1 μg/m3 change in each pollutant measured as the percentage excess RR, specifically, as (RR–1)×100. Because the distributions of pollutants vary greatly, table 3 shows the percentage increase in the RR assessed against the IQR of the pollutant distribution used to generate the risk estimates. This analysis provides a basis for comparison of relative effects of the pollutants that more accurately indicates the real-world difference over their distributions in the USA than does the 1 μg/m3 change.
Table 2.
Percentage changes of relative risk based on μg/m3 range of pollutant concentration by selected causes of death for single-pollutant and multiple-pollutant models
| PM2·5 (1·0 μg/m3) | Ozone (1·0 μg/m3) | Sulphate (1·0 μg/m3) | Elemental carbon (1·0 μg/m3) | |
|---|---|---|---|---|
|
All-cause mortality (deaths=93 358)
| ||||
| Single-pollutant | 0·58 (0·22 to 0·95) | 0·04 (−0·01 to 0·09) | 1·11 (0·78 to 1·44) | 5·51 (0·74 to 10·51) |
| Multiple-pollutant | ·· | 0·01 (−0·06 to 0·07) | ·· | 5·16 (−0·51 to 11·17) |
| Multiple-pollutant | ·· | 0·02 (−0·01 to 0·06) | 1·09 (0·76 to 1·43) | ·· |
| Multiple-pollutant | ·· | ·· | 1·06 (0·73 to 1·40) | 2·70 (−1·01 to 6·57) |
| Multiple-pollutant | ·· | 0·01 (−0·04 to 0·06) | 1·07 (0·73 to 1·40) | 2·11 (−2·44 to 6·89) |
|
| ||||
|
Cardiopulmonary mortality (deaths=46 168)
| ||||
| Single-pollutant | 1·27 (0·76 to 1·79) | 0·12 (0·03 to 0·21) | 1·55 (1·03 to 2·08) | 10·60 (2·92 to 18·86) |
| Multiple-pollutant | ·· | 0·08 (−0·02 to 0·18) | ·· | 6·55 (−2·05 to 15·91) |
| Multiple-pollutant | ·· | 0·10 (0·04 to 0·16) | 1·54 (1·05 to 2·03) | ·· |
| Multiple-pollutant | ·· | ·· | 1·46 (0·94 to 1·97) | 7·05 (1·11 to 13·35) |
| Multiple-pollutant | ·· | 0·09 (0·01 to 0·17) | 1·51 (1·01 to 2·01) | 2·09 (−4·53 to 9·18) |
Data from the American Cancer Society Cancer Prevention II cohort (n=352 242), with follow-up from 1982 to 2000. Spatial survival model included random effects at the 66 metropolitan statistical areas that had all pollutants recorded for the national cohort. Survival model is stratified by age (1 year), sex, and race. Pollution effects adjusted for 44 covariates measured at the individual level and seven covariates measured at the ecological level for the zip code area of residence and for the zip code area deviated from the metropolitan area average. Relative risks presented in the first row for each cause of death are from single-pollutant models, whereas those in subsequent rows indicate pollutants simultaneously included in survival models. See webappendix pp 26–27 for details. PM2·5=particulate matter with aerodynamic diameter 2·5 μm or less.
Table 3.
Percentage changes of relative risk based on IQR of pollutant concentration by selected causes of death for single-pollutant and multiple-pollutant models
| PM2·5 (4·30 μg/m3) | Ozone (22·38 μg/m3) | Sulphate (3·75 μg/m3) | Elemental carbon (0·31 μg/m3) | |
|---|---|---|---|---|
|
All-cause mortality (deaths=93 358)
| ||||
| Single-pollutant | 2·56 (0·96 to 4·18) | 0·93 (−0·35 to 2·23) | 4·23 (2·96 to 5·51) | 1·67 (0·22 to 3·14) |
| Multiple-pollutant | ·· | 0·16 (−1·34 to 1·69) | ·· | 1·57 (−0·15 to 3·33) |
| Multiple-pollutant | ·· | 0·57 (−0·38 to 1·54) | 4·18 (2·90 to 5·46) | ·· |
| Multiple-pollutant | ·· | ·· | 4·05 (2·77 to 5·36) | 0·83 (−0·31 to 1·99) |
| Multiple-pollutant | ·· | 0·25 (−0·92 to 1·45) | 4·07 (2·78 to 5·38) | 0·65 (−0·76 to 2·08) |
|
| ||||
|
Cardiopulmonary mortality (deaths=46 168)
| ||||
| Single-pollutant | 5·60 (3·31 to 7·95) | 2·83 (0·84 to 4·86) | 5·96 (3·91 to 8·04) | 3·17 (0·89 to 5·49) |
| Multiple-pollutant | ·· | 1·86 (−0·46 to 4·24) | ·· | 1·98 (−0·64 to 4·67) |
| Multiple-pollutant | ·· | 2·42 (0·99 to 3·87) | 5·90 (4·01 to 7·82) | · |
| Multiple-pollutant | ·· | ·· | 5·59 (3·60 to 7·62) | 2·13 (0·34 to 3·95) |
| Multiple-pollutant | ·· | 2·10 (0·34 to 3·88) | 5·79 (3·87 to 7·74) | 0·64 (−1·42 to 2·75) |
Data from the American Cancer Society Cancer Prevention II cohort (n=352 242), with follow-up from 1982 to 2000. Spatial survival model included random effects at the 66 metropolitan statistical areas that had all pollutants recorded for the national cohort. Survival model is stratified by age (1 year), sex, and race. Pollution effects adjusted for 44 covariates measured at the individual level and seven covariates measured at the ecological level for the zip code area of residence and for the zip code area deviated from the metropolitan area average. Relative risks presented in the first row for each cause of death are from single-pollutant models, whereas those in subsequent rows indicate pollutants simultaneously included in survival models. See webappendix pp 26–27 for details. PM2·5=particulate matter with aerodynamic diameter 2·5 μm or less.
Sulphate, PM2·5, and elemental carbon have positive, significant associations with all-cause mortality. On the basis of the 1 μg/m3 contrast, the percentage increase in all-cause mortality for PM2·5 was 0·58 (95% CI 0·22–0·95). Sulphate effects were about twice those of PM2·5, and effects of elemental carbon about ten times greater, although this estimate has poor precision (table 2). We noted a small increased risk for ozone with all-cause mortality (table 2). For all causes of death, when assessed against the IQR in the pollutants, effect sizes were largest for sulphate, followed by PM2·5, elemental carbon, and ozone.
For cardiopulmonary mortality, effect sizes of PM2·5 and sulphate were similar over the 1 μg/m3 exposure contrast, with sulphate having slightly larger effects than those of PM2·5 (table 2). Elemental carbon effects were roughly seven or eight times greater than were those of sulphate and PM2·5, respectively (table 2). We also noted significantly increased risks of cardiopulmonary death from ozone exposure (table 2). Assessed against the IQR distributions, both PM2·5 and sulphate had significantly increased risks of 5–6%, whereas the increased risks were about 3% for ozone and elemental carbon (table 3). For the all-cause multiple-pollutant models, only sulphate remained significantly increased. In cardiopulmonary models, ozone and sulphates were both raised and significant in two-pollutant models, whereas ozone was confounded in a model containing elemental carbon alone (table 2). Sulphate and elemental carbon remained significantly raised in two-pollutant models. In the three-pollutant model, only ozone and sulphate were significantly increased (table 2).
Discussion of new evidence
Sulphate has the most robust association with all-cause and cardiopulmonary mortality. Ozone was significantly associated with cardiopulmonary mortality only, although this association was confounded by inclusion of elemental carbon. Elemental carbon has the largest effects on all-cause and cardiopulmonary mortality per μg/m3, followed by sulphate, PM2·5, and ozone. These rankings change when the actual US distributions employed to generate the risk estimates are used, with sulphate having the largest effects, followed by PM2·5, elemental carbon, and ozone. In all instances, the confidence intervals overlap, and we were unable to establish conclusively whether any of the pollutant effects differed significantly from each other. Moreover, definitive conclusions about the relative importance of each pollutant were difficult to make because of high amounts of correlation between pollutant estimates. Sulphate seems to have the most robust effects on mortality when account is taken of confounding in the multiple-pollutant models.
We fitted a model estimating a multiplicative interaction between ozone and sulphate (data not shown). The results suggested that cardiopulmonary mortality associated with ozone was greatest at low concentrations of sulphate, and vice versa. This finding might indicate differences in the spatial patterns of both pollutants across the USA: sulphate concentrations are high in the midwest and northeast USA, whereas ozone concentrations are high in southwest regions, particularly in California. The single-pollutant models suggest that both pollutants are associated with increased mortality. Thus, if valid, we would infer that, if one pollutant is less prominent, the effects of the other become more pronounced. Further investigation of multiple-pollutant interactions seems to be warranted.
Conclusions
The findings from toxicology and epidemiology with respect to sulphates differ substantially. In most toxicology studies, which use a pure form of sulphate, the effects are negligible, whereas epidemiological studies that use measured particle sulphates, representing a mixture of sulphates and other species from combustion sources such as metals, find significant associations. Some characteristic of this mixture, associated or interacting with sulphates, probably explains the differences between these two sets of findings.16 Therefore, the epidemiological associations should be regarded as quantifying the health effects of particle species and other co-pollutants associated with sulphate. Furthermore, since control measures might not affect all components equally, we cannot assume that control of sulphur emissions will result in a directly corresponding fall in health effects. The assessment of policies to reduce emissions of sulphur provides, however, good evidence that these interventions do have major health benefits.69,70 Additionally, sulphur is initially emitted from fuel combustion in the form of sulphur dioxide—a gas that does not actively affect climate itself, but does probably exert an independent effect on health.13
Unlike sulphate, pure black carbon does seem to exert effects in toxicological studies. Nevertheless, as with sulphate, it should be regarded as an indicator of a mixture of pollutants from combustion, especially when interpreting associations between proxies, such as black smoke, and health effects.
Plausible biological mechanisms link ozone exposure to direct oxidative stress and secondary damage through associated inflammatory processes. Evidence from human and animal toxicology studies lends support to these mechanisms. Epidemiological evidence suggests a wide range of health effects. The ACS case study indicates ozone effects on the cardiopulmonary system, although these effects are not easily separated from those of elemental carbon. As with black carbon and sulphates, however, the toxicology of pure ozone probably does not replicate the actual effects in ambient settings in which ozone is closely associated with other oxidative pollutants.
Evidence associating ozone with morbidity strengthens the case for causality and intervention justification. Such evidence might also identify effects on special subgroups (eg, children with asthma). However, in most cost–benefit analyses of air pollution that are used to justify public health interventions, mortality dominates estimates of benefit. Until recently, the mortality effects of long-term ozone exposure were not clear,13 but new studies67 and the analysis in this report provide evidence that the mortality effects from long-term exposures are real, and probably independent from those of sulphate. In view of the large risks noted in these long-term studies, the co-benefits of reductions in ozone might be greater than those previously estimated. Further replication in other cohorts and settings is needed to ensure estimates of benefits are accurate and reliable for policy decisions.71
Elemental carbon seems to exert the strongest effects per unit mass in the presence of other major co-pollutants for chronic exposures in the ACS case study, but sulphate seems to have larger effects per unit mass in the time-series studies (tables 2 and 3). Although the results of the time-series meta-analysis suggest larger effects of sulphate than of black smoke per μg/m3, this distinction is less clear in the few studies that have measured both (table 1). Additionally, most studies of black smoke were done in Europe and most sulphate studies in North America, so comparisons are difficult. Furthermore, comparison of the two metrics on a mass basis is variable because the black smoke measurement is based on an optical, not gravimetric, technique.
Differential amounts of measurement error might also be present because sulphate, a secondary pollutant formed in the atmosphere, tends to vary regionally, but not within cities compared with concentrations of elemental carbon or black smoke. For this reason, estimates of elemental or black carbon are likely to have more measurement error than are the assigned sulphate exposures. Additionally, because it is highly reflective, sulphate can cause negative artifacts for reflectance-based measurements of black carbon that might vary by day, season, and location, complicating health-effect comparisons of sulphate versus black smoke or elemental and black carbon.72 When measurement error is present in a regression analysis, variables measured with high precision will tend to overpower effects from other variables measured with less precision.73 This notion might partly explain why sulphate seems to be more robust to confounding by other co-pollutants than was elemental carbon in the ACS study and also in other chronic studies that have used black smoke. Table 4 qualitatively summarises the evidence presented in this paper about the health, climate, and other characteristics of the three short-lived greenhouse pollutants; however, we cannot confidently quantify the differences in effects between them.14,16
Table 4.
Summary of health, climate, emissions, and ecosystem issues related to sulphate, black carbon, and ozone
| Main human sources | Measurement | Toxicology | Epidemiology (mortality) | Climate | Ecosystems | Control | |
|---|---|---|---|---|---|---|---|
| Sulphate particles | Power plants, industry, and transport from sulphur in fuels; concentrations falling worldwide | Little ambiguity, although emissions are mainly sulphur dioxide, complicating calculation of extent and location of transformation | Pure sulphate not shown to be toxic at concentrations encountered in the environment | Could have larger relative effects than undifferentiated fine particles that seem to be independent of other pollutants; sulphur dioxide, the emitted precursor, probably has additional effects | Generally cooling with some difference by location and complexity when in mixtures | Acid precipitation is of little uncertainty, but wide difference in effect by location | Control of sulphur dioxide has some, but not large interaction with other types of control |
| Black carbon particles | Household solid fuels, industrial coal, forest/grassland burning, and diesel from incomplete combustion; fossil-fuel proportion falling slowly | Basic measurement methods and metrics in some confusion across disciplines | Pure EC not very toxic in human and animal studies at environmental levels | Measured as EC, might have larger effects than undifferentiated fine particles, but results are not stable when other pollutants are included in models | Major uncertainties, but high warming potential that is complicated by location, short life, and mixtures with other aerosols | Melting and warming effects if it falls on ice or snow, particularly in Arctic and Himalayas; not yet well understood | Controls also reduce organic carbon emissions, which are generally cooling; net climate effect thus varies depending on source OC/BC ratio |
| Ozone (tropospheric) | Precursors: methane and NMVOCs with combustion and non-combustion sources and NOx mainly from combustion; concentrations rising worldwide | Ozone itself has little uncertainty, but precursor emission measurements are uncertain; might be formed far from sources; needs sunlight to form | Oxidative stress and inflammation pathways established for toxicity of pure ozone at or near environmental concentrations | Might have mortality effects that are independent of major types of small particles; evidence is more extensive for short-term exposure, but results from one large cohort study26 suggest much larger effects from long-term exposure | Warming potential well established, but total effect shared across precursor emissions in complex ways | Adversely affects agriculture and ecosystems; might reduce carbon storage | Complex atmospheric chemistry determines importance of VOCs vs NOx control locally |
As noted in the text, epidemiological methods are not able to identify the mortality effects of the pure material in the environment, but rather the mixture of pollutants for which each material is an indicator. See panel for more details on climate interactions. EC=elemental carbon. OC=organic carbon. BC=black carbon. NMVOC=non-methane volatile organic compounds. NOx=nitrogen oxides. VOC=volatile organic compounds.
The distinction between short-lived greenhouse pollutants with regard to emissions, ambient concentrations, and effects is important. What is emitted is not exactly what is detected in the environment in all cases, because of both atmospheric transformations and closely associated co-pollutants. For example, several geographic, meteorological, and seasonal factors, such as the interaction with clouds, can transform emissions and change climate effects. Health effects can vary because of the differences in intake fraction or exposure efficiency—ie, people might breathe more pollution from sources nearer to them than from those at a greater distance, even if those at greater distance contribute importantly to environmental concentrations.74
Black carbon could have effects both chemically and as a generic function of being a particle. The toxicological evidence suggests that the ultrafine fraction is more toxic than other fractions, but the chemical composition might account for the health effects recorded in epidemiological studies. In the troposphere, ultrafine concentrations often quickly fall after the primary emission source,75 and black carbon is often detected in sizes greater than the ultrafine fraction. For example, the peak black carbon concentration of anthropogenic haze over the Indian Ocean (far away from sources) occurred at 0·3–1·0 μm diameter, which is larger than is the size of black carbon particles detected near sources—eg, in fresh engine exhaust (0·1–0·2 μm).76,77 Even within a city, ambient black carbon particles are generally smaller than are those found far away from sources—eg, 0·2–0·4 μm in ambient air in Los Angeles, CA, USA.78 So far, neither toxicology nor epidemiology provide a clear answer as to the relative toxic effects of black carbon by size, number, or chemical composition, including any differences for combustion particles associated with black carbon, such as those termed organic carbon.14
One of the recurring suggestions to slow climate change is the deliberate injection of sulphates into the atmosphere because of their climate-cooling properties and low toxic effects.79,80 Any such so-called geoengineering needs to be analysed carefully and implemented cautiously. The present epidemiological evidence for mortality effects of sulphate is not conclusive because of the strong association with other pollutants from the same combustion sources. Moreover, studies are needed to understand how artificially injected sulphate from non-combustion processes transforms in the atmosphere to affect human beings and ecosystems. Another widely discussed option for mitigation of climate change is to use biofuels instead of fossil fuels. Research suggests that a switch from fossil fuels to ethanol could increase emission of volatile organic compounds (aldehydes), which are known precursors of tropospheric ozone. Thus, the health effects could potentially rival those of the fossil fuels that ethanol is intended to replace.81
Black carbon is always emitted along with particles of organic carbon, which are cooling but also health-damaging. Thus the effects of an emissions reduction of black carbon on climate will depend partly on the ratio of black to organic carbon emissions—reducing emissions from a source with a low ratio of organic to black carbon such as diesel exhaust would have greater benefit for climate than would reducing emissions with a high ratio such as biomass smoke. For health, however, both types of particles have effects.
Climate mitigation planning for short-lived greenhouse pollutants is increasingly difficult because of the absence of official climate-weighting factors for these pollutants (panel). As the world seeks to reduce global warming risks in ways that are cost effective and compatible with other goals, including protection from outdoor air pollutants, some version of weighting factors will be needed, although creation of these factors might need acceptance of rough average values for effects that vary in time and space. Alternatively, in addition to those for long-lived green-house gases, completely separate agreements for short-lived greenhouse pollutants may be needed.
Integrated models incorporating global warming and air pollution health effects are needed to enable the exploration of the wide range of options available for control of short-lived greenhouse pollutants. Such models would benefit from additional research to quantify the relative toxic effects of specific climate-relevant components of air pollution mixtures, including ozone and the elemental and organic components of fine particulate matter. Finally, to establish policy, multidisciplinary approaches are needed to model and assess the health and climate co-benefits of aerosol control, both primary and secondary, which could in some cases lead to undesirable effects in one or the other sector.
Supplementary Material
Key messages.
Short-lived greenhouse pollutants need to be controlled in addition to regulating carbon dioxide emissions because they collectively create a substantial proportion of all human-contributed global warming and directly damage health. Importantly, control of some short-lived greenhouse pollutants may lead to quick reductions in global warming.
Short-lived greenhouse pollutants include gases such as the directly health-damaging carbon monoxide and non-methane volatile organic compounds, and others responsible for ozone creation in the lower atmosphere such as methane. Aerosols of short-lived greenhouse pollutants include sulphate, organic carbon, and black carbon particles, which have differing climate implications: the first two cooling, but the third strongly warming.
The toxicology of sulphate and black carbon in pure form does not adequately indicate their health effects in ambient conditions where they are closely associated with other pollutants. The epidemiological effects of atmospheric sulphate and black carbon therefore should be interpreted as representing mixtures.
Meta-analyses of time-series studies of short-term exposure suggest larger mortality effects per unit mass of sulphate than of black smoke, an optical measure correlated with black carbon. Although measurements of black smoke correlate well with estimates of black carbon in some studies, black smoke measurements do not provide a reliable quantitative indicator of black carbon concentrations because of large variations by site, season, and year.
Our analysis of a 66-city, 18-year nationwide US cohort provides estimates of the mortality effects of long-term exposure to elemental carbon, the best available measure of black carbon. This analysis shows stronger effects for elemental carbon than for undifferentiated fine particles (PM2·5), but the model estimates are unstable with respect to inclusion of other pollutants.
Differential mortality effects between various components of PM2·5 are difficult to assess. Our analysis, however, does not lend support to the view that sulphate has smaller mortality effects than does undifferentiated PM2·5 and provides new evidence that long-term exposures to sulphates and ozone exert adverse effects on mortality that are independent of other constituents.
Acknowledgments
External funding for this work was obtained solely from the government agencies and non-profit foundations listed below. The project that led to this Series was funded by the Wellcome Trust (coordinating funder); UK Department of Health, National Institute for Health Research; the Royal College of Physicians; the Academy of Medical Sciences; the Economic and Social Research Council; the US National Institute of Environmental Health Sciences; and WHO. The Royal College of Physicians was supported by an unrestricted educational grant from Pfizer. Additional support was obtained from the California Air Resources Board, the US National Institute of Environmental Health Sciences Center grant (number ES00260), Health Effects Institute’s National Particle Component Toxicity Initiative, and the Clean Air Task Force. The funders had no role in the design, analysis, or interpretation of the study. The views expressed are those of the authors and do not necessarily reflect the position of the funding bodies or the Health Effects Institute or its sponsors. We thank Milena Simic-Lawson, St George’s, University of London, for her work on the time-series meta-analysis; Bernard Beckerman, University of California, Berkeley, for his help with the ACS spatial analysis; Yuanli Shi, University of Ottawa, who did the statistical modelling for the case studies; Zev Ross, of ZevRoss Spatial Analysis, for assistance with preparation of the elemental carbon estimates; and Tami Bond, University of Illinois, for data from her emissions inventory database. Daniel Krewski holds the NSERC Chair in Risk Science.
Footnotes
Contributors
KRS conceived of the paper and wrote the abstract, introduction, climate primer, parts of the conclusion, and some of the tables. He also edited contributions from other authors and contributed interpretive expertise on the epidemiological results. MJ led the American Cancer Society (ACS) analysis, wrote all sections of the ACS analysis, assisted with the meta-analysis summary, wrote the ozone toxicology section, wrote portions of the conclusion, edited contributions from other authors, and coordinated the response to the review comments. HRA and RWA led the meta-analysis, drafted text related to the meta-analysis, supplied expert medical-epidemiological advice about interpretation of toxicology and epidemiology studies, and edited the paper. RTB designed the random effects program used for the ACS statistical analysis, supervised the statistical modelling, assisted with preparation of the tables, interpreted results, undertook quality assurance tests on the ACS results, and edited the paper. VS wrote the toxicology sections on sulphates and black carbon and supplied edits to the paper. RD wrote sections of the climate primer, supplied expert commentary and review of climate and pollution measurement sections, and edited the paper. AC helped to outline the paper, wrote parts of the conclusion, supplied detailed edits on each draft, and assisted with all revisions. SBS wrote parts of the sections on black carbon, contributed to the interpretation of the ACS results, wrote parts of the conclusions, edited contributions from other authors, helped to coordinate the response to the review comments, and wrote the primer on black smoke, black carbon, and elemental carbon metrics in the webappendix. DK edited the document and assisted with the ACS analysis and the statistical modelling. CAP edited the document and assisted with the ACS analysis and the interpretation of the ACS results. MJT collected all the ACS data, provided quality assurance, edited the document, and supplied expert medical-epidemiological advice. GT assisted with compilation of the sulphate and elemental carbon data, wrote parts of the conclusion, and supplied expertise on interpretation of the epidemiological results.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Jacobson MZ. On the causal link between carbon dioxide and air pollution mortality. Geophys Res Lett. 2008;35:1–5. [Google Scholar]
- 2.Schindler D. Effects of acid rain on freshwater ecosystems science. Science. 1988;239:149–57. doi: 10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- 3.Reay DS, Dentener F, Smith P, Grace J, Feely RA. Global nitrogen deposition and carbon sinks. Nat Geosci. 2008;1:430–37. [Google Scholar]
- 4.Sitch S, Cox PM, Collins WJ, Huntingford C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature. 2007;448:791–94. doi: 10.1038/nature06059. [DOI] [PubMed] [Google Scholar]
- 5.Molina M, Zaelke D, Madhava Sarma K, Andersen SO, Ramanathan V, Kaniaru D. Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions. Proc Nat Acad Sci USA. 2009 doi: 10.1073/pnas.0902568106. published online Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KR. Methane controls before risky geoengineering, please. New Sci. 2009:2714. [Google Scholar]
- 7.Tollefson J. Atmospheric science: climate’s smoky spectre. Nature. 2009;460:29–32. doi: 10.1038/460029a. [DOI] [PubMed] [Google Scholar]
- 8.Forster P, Ramaswamy, Artaxo V, et al. Changes in atmospheric constituents and in radiative forcing. Cambridge and New York: Cambridge University Press; 2007. [Google Scholar]
- 9.Ramanathan V, Carmichael G. Global and regional climate changes due to black carbon. Nat Geosci. 2008;1:221–27. [Google Scholar]
- 10.Unger N, Shindell DT, Koch DM, Streets DG. Air pollution radiative forcing from specific emissions sectors at 2030. J Geophys Res. 2008;113:1–12. [Google Scholar]
- 11.Bond TC. [accessed Feb 14, 2009];Black carbon emissions inventory, version 7.1.1. 2009 Feb 14; http://www.hiwater.org/
- 12.Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor smoke from household solid fuels. In: Ezzati M, Rodgers AD, Lopez AD, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease due to selected major risk factors. Geneva: World Health Organization; 2004. pp. 1435–93. [Google Scholar]
- 13.WHO. Air quality guidelines: global update 2005, particulate matter, ozone, nitrogen dioxide and sulphur dioxide. Copenhagen: World Health Organization Regional Office for Europe; 2006. [Google Scholar]
- 14.WHO. Health relevance of particulate matter from various sources: report on a WHO workshop, Bonn, Germany, March 2007. Copenhagen: World Health Organization Regional Office for Europe; 2007. [Google Scholar]
- 15.Khoder MI. Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere. 2002;49:675–84. doi: 10.1016/s0045-6535(02)00391-0. [DOI] [PubMed] [Google Scholar]
- 16.COMEAP. Long-term exposure to air pollution: effect on mortality. London: Committee on the Medical Effects of Air Pollutants (COMEAP), Department of Health; 2009. [Google Scholar]
- 17.MAAPE. Sulphur dioxide, acid aerosols and particulates. London, UK: Advisory Group on the Medical Aspects of Air Pollution Episodes; 1992. [Google Scholar]
- 18.Schlesinger RB, Cassee F. Atmospheric secondary inorganic particulate matter: the toxicological perspective as a basis for health effects risk assessment. Inhal Toxicol. 2003;15:197–235. doi: 10.1080/08958370304503. [DOI] [PubMed] [Google Scholar]
- 19.Godleski JJ, Verrier RL, Koutrakis P, Catalano PJ. Mechanisms of morbidity and mortality from exposure to ambient air particles: report number 91. Boston: Health Effects Institute; 2000. [PubMed] [Google Scholar]
- 20.Muggenburg BA, Benson JM, Barr EB, Kubatko J, Tilley LP. Short-term inhalation of particulate transition metals has little effect on the electrocardiograms of dogs having preexisting cardiac abnormalities. Inhal Toxicol. 2003;15:357–71. doi: 10.1080/08958370304456. [DOI] [PubMed] [Google Scholar]
- 21.Bagate K, Meiring JJ, Cassee FR, Borm PJ. The effect of particulate matter on resistance and conductance vessels in the rat. Inhal Toxicol. 2004;16:431–36. doi: 10.1080/08958370490439588. [DOI] [PubMed] [Google Scholar]
- 22.Campen MJ, Nolan JP, Schladweiler MC, Kodavanti UP, Costa DL, Watkinson WP. Cardiac and thermoregulatory effects of instilled particulate matter-associated transition metals in healthy and cardiopulmonary-compromised rats. J Toxicol Environ Health A. 2002;65:1615–31. doi: 10.1080/00984100290071694. [DOI] [PubMed] [Google Scholar]
- 23.Krewski D, Jerrett M, Burnett RT, et al. Extended analysis of the American Cancer Society study of particulate air pollution and mortality. Boston: Health Effects Institute; 2009. [PubMed] [Google Scholar]
- 24.Beelen R, Hoek G, van den Brandt PA, et al. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study) Environ Health Perspect. 2008;116:196–202. doi: 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filleul L, Rondeau V, Vandentorren S, et al. Twenty five year mortality and air pollution: results from the French PAARC survey. Occ Environ Med. 2005;62:453–60. doi: 10.1136/oem.2004.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerrett M, Burnett RT, Pope CA, 3rd, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–95. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dockery DW, Pope AC, Xu X, et al. An association between air pollution and mortality in six US cities. N Engl J Med. 1993;329:1753–59. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 28.Highwood EJ, Kinnersley RP. When smoke gets in our eyes: the multiple impacts of atmospheric black carbon on climate, air quality and health. Environ Int. 2006;32:560–66. doi: 10.1016/j.envint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Bailey DLR, Clayton P. The measurement of suspended particle and total carbon concentrations in the atmosphere using standard smoke shade methods. Atmos Environ. 1982;16:2683–90. [Google Scholar]
- 30.Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 31.Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–36. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 32.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–36. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 33.Gilmour PS, Ziesenis A, Morrison ER, et al. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol. 2004;195:35–44. doi: 10.1016/j.taap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–40. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- 35.Niwa Y, Hiura Y, Murayama T, Yokode M, Iwai N. Nano-sized carbon black exposure exacerbates atherosclerosis in LDL-receptor knockout mice. Circ J. 2007;71:1157–61. doi: 10.1253/circj.71.1157. [DOI] [PubMed] [Google Scholar]
- 36.Li XY, Brown D, Smith S, MacNee W, Donaldson K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal Toxicol. 1999;11:709–31. doi: 10.1080/089583799196826. [DOI] [PubMed] [Google Scholar]
- 37.Brown DM, Donaldson K, Borm PJ, et al. Calcium and reactive oxygen species-mediated activation of transcription factors and TNFa cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004;286:L344–53. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- 38.Stone V, Shaw J, Brown DM, MacNee W, Faux SP, Donaldson K. The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol In Vitro. 1998;12:649–59. doi: 10.1016/s0887-2333(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson K, Tran L, Jimenez LA, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberdoerster G, Stone V, Donaldson K. Toxicology of nanoparticles: a historical perspective. Nanotoxicology. 2007;1:2–25. [Google Scholar]
- 41.Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M. The health relevance of ambient particulate matter characteristics: coherence of toxicological and epidemiological inferences. Inhal Toxicol. 2006;18:95–125. doi: 10.1080/08958370500306016. [DOI] [PubMed] [Google Scholar]
- 42.Quincey P. A relationship between black smoke index and black carbon concentration. Atmos Environ. 2007;41:7964–68. [Google Scholar]
- 43.Schaap M, van der Gon H. On the variability of black smoke and carbonaceous aerosols in the Netherlands. Atmos Environ. 2007;41:5908–20. [Google Scholar]
- 44.Li X, Wang S, Duan L, Hao J, Nie Y. Carbonaceous aerosol emissions from household biofuel combustion in China. Environ Sci Technol. 2009;43:6076–81. doi: 10.1021/es803330j. [DOI] [PubMed] [Google Scholar]
- 45.Elliott P, Shaddick G, Wakefield JC, de Hoogh C, Briggs DJ. Long-term associations of outdoor air pollution with mortality in Great Britain. Thorax. 2007;62:1088–94. doi: 10.1136/thx.2006.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straif K, Keil U, Taeger D, et al. Exposure to nitrosamines, carbon black, asbestos, and talc and mortality from stomach, lung, and laryngeal cancer in a cohort of rubber workers. Am J Epidemiol. 2000;152:297–306. doi: 10.1093/aje/152.4.297. [DOI] [PubMed] [Google Scholar]
- 47.van Tongeren MJ, Gardiner K, Rossiter CE, Beach J, Harber P, Harrington MJ. Longitudinal analyses of chest radiographs from the European Carbon Black Respiratory Morbidity Study. Eur Respir J. 2002;20:417–25. doi: 10.1183/09031936.02.00224502. [DOI] [PubMed] [Google Scholar]
- 48.Hart JE, Laden F, Schenker MB, Garshick E. Chronic obstructive pulmonary disease mortality in diesel-exposed railroad workers. Environ Health Perspect. 2006;114:1013–17. doi: 10.1289/ehp.8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipsett M, Campleman S. Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am J Public Health. 1999;89:1009–17. doi: 10.2105/ajph.89.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finlayson-Pitts BJ, Pitts JN., Jr Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science. 1997;276:1045–52. doi: 10.1126/science.276.5315.1045. [DOI] [PubMed] [Google Scholar]
- 51.Royal Society. Ground-level ozone in the 21st century: future trends, impacts and policy implications. London: The Royal Society; 2008. [Google Scholar]
- 52.Chen TM, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: ozone health effects. Am J Med Sci. 2007;333:244–48. doi: 10.1097/MAJ.0b013e31803b8e8c. [DOI] [PubMed] [Google Scholar]
- 53.Mudway IS, Kelly FJ. An investigation of inhaled ozone dose and the magnitude of airway inflammation in healthy adults. Am J Respir Crit Care Med. 2004;169:1089–95. doi: 10.1164/rccm.200309-1325PP. [DOI] [PubMed] [Google Scholar]
- 54.Gong H, Jr, Wong R, Sarma RJ, et al. Cardiovascular effects of ozone exposure in human volunteers. Am J Respir Crit Care Med. 1998;158:538–46. doi: 10.1164/ajrccm.158.2.9709034. [DOI] [PubMed] [Google Scholar]
- 55.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–45. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology. 2005;16:446–57. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- 57.Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology. 2005;16:458–68. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- 58.Delfino RJ, Quintana PJ, Floro J, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112:932–41. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q, Chen Y, Shi Y, Burnett RT, McGrail KM, Krewski D. Association between ozone and respiratory admissions among children and the elderly in Vancouver, Canada. Inhal Toxicol. 2003;15:1297–308. doi: 10.1080/08958370390241768. [DOI] [PubMed] [Google Scholar]
- 60.Krewski D, Burnett R, Goldberg MS, et al. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of particulate air pollution and mortality, part II: sensitivity analysis: a special report of the Institute’s Particle Epidemiology Reanalysis Project. Boston: Health Effects Institute; 2000. [Google Scholar]
- 61.Abbey DE, Nishino N, McDonnell WF, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Resp Crit Care. 1999;159:373–82. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 62.Thurston GD, Lippmann M, Scott MB, Fine JM. Summertime haze air pollution and children with asthma. Am J Respir Crit Care Med. 1997;155:654–60. doi: 10.1164/ajrccm.155.2.9032209. [DOI] [PubMed] [Google Scholar]
- 63.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–91. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 64.Spektor DM, Lippmann M, Lioy PJ, et al. Effects of ambient ozone on respiratory function in active, normal children. Am Rev Respir Dis. 1988;137:313–20. doi: 10.1164/ajrccm/137.2.313. [DOI] [PubMed] [Google Scholar]
- 65.Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Kunzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology. 2005;16:751–59. doi: 10.1097/01.ede.0000183166.68809.b0. [DOI] [PubMed] [Google Scholar]
- 66.Thun M, Calle E, Namboodiri M, et al. Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst. 1992;84:491–500. doi: 10.1093/jnci/84.19.1491. [DOI] [PubMed] [Google Scholar]
- 67.Jerrett M, Finkelstein MM, Brook JR, et al. A cohort study of traffic-related air pollution and mortality in Toronto, Ontario, Canada. Environ Health Perspect. 2009;117:772–77. doi: 10.1289/ehp.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jerrett M, Burnett RT, Ma R, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–36. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 69.Clancy L, Goodman P, Sinclair H, Dockery DW. Effect of air-pollution control on death rates in Dublin, Ireland: an intervention study. Lancet. 2002;360:1210–14. doi: 10.1016/S0140-6736(02)11281-5. [DOI] [PubMed] [Google Scholar]
- 70.Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. Lancet. 2002;360:1646–52. doi: 10.1016/s0140-6736(02)11612-6. [DOI] [PubMed] [Google Scholar]
- 71.NAS. Estimating mortality risk reduction and economic benefits from controlling ozone air pollution. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 72.Chow JC. Measurement methods to determine compliance with ambient air-quality standards for suspended particles. J Air Waste Manage Assoc. 1995;45:320–82. doi: 10.1080/10473289.1995.10467369. [DOI] [PubMed] [Google Scholar]
- 73.Zidek JV, Wong H, Le ND, Burnett R. Causality, measurement error and multicollinearity in epidemiology. Environmetrics. 1996;7:441–51. [Google Scholar]
- 74.Bennett DH, McKone TE, Evans JS, et al. Defining intake fraction. Environ Sci Technol. 2002;36:207A–11A. [PubMed] [Google Scholar]
- 75.Moffet R, Prather K. In-situ measurments of the mixing state and optical properties of soot with implications for radiative forcing estimates. Proc Natl Acad Sci USA. 2009;106:11872–77. doi: 10.1073/pnas.0900040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kleeman MJ, Schauer JJ, Cass GR. Size and composition distribution of fine particulate matter emitted from motor vehicles. Environ Sci Technol. 2000;34:1132–42. [Google Scholar]
- 77.Ramanathan V, Crutzen PJ, Lelieveld J, et al. Indian Ocean Experiment: an integrated analysis of the climate forcing and effects of the great Indo-Asian haze. J Geophys Res. 2001;106:28371–98. [Google Scholar]
- 78.Kleeman MJ, Cass GR, Eldering A. Modeling the airborne particle complex as a source-oriented external mixture. J Geophys Res. 1997;102:21355–72. [Google Scholar]
- 79.Rasch PJ, Tilmes S, Turco RP, et al. An overview of geoengineering of climate using stratospheric sulphate aerosols. Phil Trans R Soc A. 2008;366:4007–37. doi: 10.1098/rsta.2008.0131. [DOI] [PubMed] [Google Scholar]
- 80.Tuck AF, Donaldson DJ, Hitchman MH, et al. On geoengineering with sulphate aerosols in the tropical upper troposphere and lower stratosphere. Climatic Change. 2008;90:315–31. [Google Scholar]
- 81.Jacobson MZ. Effects of ethanol (E85) versus gasoline vehicles on cancer and mortality in the United States. Environ Sci Technol. 2007;41:4150–57. doi: 10.1021/es062085v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.