Abstract
Over the past decade, the function of the cytoskeleton has been extensively studied in developing and in mature neurons. Actin, a major cytoskeletal protein, is indispensable for the structural integrity and plasticity of neurons and their synapses. Disruption of actin dynamics has significant consequence for neurons, neuronal circuits, and the functions they govern. In particular, cell adhesion molecules (CAMs), members of the Rho family of GTPases, and actin binding proteins (ABPs) are important modulators of actin dynamics and neuronal as well as behavioral plasticity. In this review, we discuss recent advances in Drosophila that highlight the importance of actin regulatory proteins in mediating fly behaviors such as circadian rhythm, courtship behavior, learning and memory, and the development of drug addiction.
Keywords: Actin, behavior, Drosophila, genetics
Introduction
The central nervous system (CNS) comprises between 200,000 neurons in Drosophila melanogaster and 100 billion neurons in humans (Leyssen and Hassan, 2007). These neurons are interconnected into functional circuits that underlie the formation of our thoughts, memories and behaviors with basic neuronal functions conserved across species. The ability of neurons to communicate within these circuits is largely mediated through specialized cell junctions called synapses (Lamprecht and LeDoux, 2004). Synapses mediate electrochemical communication within neural networks and pass information directly from pre-synaptic axon terminals to post-synaptic dendritic regions. The precise formation and maintenance of synapses is critical for accurate neural network activity and normal brain function (Hotulainen and Hoogenraad, 2010). Strengthening or weakening of synapses helps to regulate the storage of information in the brain. Alterations in synapse efficacy are accompanied by structural changes in both pre- and post-synaptic terminals, such as the growth or shrinkage/disappearance of pre-existing synapses and/or the appearance of new synapses (Cingolani and Goda, 2008; Dillon and Goda, 2005; Hotulainen and Hoogenraad, 2010). Because actin is the major cytoskeletal protein found in the pre- and post-synaptic terminals, genes that affect actin dynamics can drive cytoarchitectural changes in neuronal circuits and can affect the behaviors they govern. In this review, we summarize how the genetic model organism Drosophila melanogaster (vinegar fly) has contributed to recent advances in the understanding of how the actin cytoskeleton affects behaviors such as courtship, circadian rhythm, learning and memory, and the development of drug addiction.
Drosophila melanogaster as a Model Organism
The vinegar fly is widely used in genetic studies for many reasons. Flies are small, inexpensive to maintain, and easy to grow in the laboratory. Their generation time is short, requiring only about 2 weeks to go from a freshly laid egg to a reproducing adult. A single female lays about 800 eggs in a lifetime, at a rate of one egg per 30 minute at optimum (Rubin and Lewis, 2000). With these characteristics, flies have long represented an excellent model organism to conduct large-scale mutagenesis screens to isolate genes regulating a particular biological process of interest. Engineering transgenic flies to rescue mutations, or conduct structure/function experiments has been available for some time (Rubin and Spradling, 1982). The Gal4/UAS system was introduced as another milestone for reverse genetics (Brand and Perrimon, 1993). It utilizes the yeast transcriptional activator Gal4, expressed under the control of a defined promoter, to activate an effector trangsene, under the control of the Gal4-target UAS. This allows for expression in spatially restricted patterns, to ask which brain regiosn, or neurotransmitter systems are involved in a given process. It also permits expression in a conditional manner to ask, for example, about the developmental versus adult requirement of a given gene. A further expansion, useful for reverse genetic analysis, has been the systematic generation of transgenic stocks carrying a UAS-regulated transgene expressing RNAi (double-stranded interfering RNA; Dietzl et al., 2007) for almost every gene in Drosophila. These stocks facilitate rapidly testing any gene’s involvement in a given process. In addition, since RNAi does not generally lead to complete loss-of-function mutants, homologous recombination has also been used to engineer knock-out mutants, including for whole gene families (eg. Chan et al., 2011). Finally, the Drosophila genome has been fully sequenced, annotated, and shows extensive gene conservation with humans, though with less genetic redundancy (Adams et al., 2000). The fact that an estimated 70–80% of human disease genes have obvious, conserved orthologs in Drosophila, has confirmed the vinegar fly as an excellent genetic model organism, including for probing, and deepening our mechanistic understanding of human diseases (Pandey and Nichols, 2011).
Drosophila synapses and actin
Apart from the genetic conservation between Drosophila and humans, similarities in their brains are also evident. Even though the Drosophila CNS is anatomically distinct and clearly of lesser complexity than the mammalian CNS, evidence for some deep evolutionary homology regarding the ancestry and function of whole brain regions continues to emerge (eg. Strausfeld and Hirth, 2013). On a cellular level, the brains are built from similar components (Leyssen and Hassan, 2007; Pandey and Nichols, 2011). For instance, neurons and glia form the main building blocks of the CNS and have conserved chemical neurotransmitters (such as dopamine, serotonin, acetylcholine, and glutamate) for synaptic neural communication (Pandey and Nichols, 2011). Additionally, both the Drosophila and mammalian CNS have conserved cytoskeletal elements that help to maintain cell shape and size. These cytoskeletal elements (actin, intermediate filaments, and microtubules) are indispensable in developmental functions including neuron division, axon guidance, and synapse formation while also functioning in vesicular trafficking, endo/exocytosis, and neurotransmitter release (Lamprecht and LeDoux, 2004). Taking advantage of the powerful genetic tools offered by Drosophila, studies have unraveled the importance of the actin cytoskeleton in organizing neural circuitries and synapses, and the behaviors they govern.
The actin cytoskeleton is one of the major components of the cellular scaffold that is essential for maintaining cell shape and size (Hotulainen and Hoogenraad, 2010). Actin dynamics support a myriad of processes ranging from cell migration, division and morphogenesis to intracellular protein trafficking (Cingolani and Goda, 2008). In developing neurons, the actin cytoskeleton has a key role in axon guidance, neurite extension/branching, and synapse formation. Actin exists in two forms: globular (G) and filamentous (F)-actin. G-actin is the monomeric subunit which polymerizes to form an asymmetric two-stranded helical filament called F-actin (Dillon and Goda, 2005). The assembly and disassembly of F-actin can be spontaneous, due to the weak non-covalent interactions of G-actin. However, at steady state and at a given cellular G-actin concentration, the differences in polymerization rates give rise to two ends: a net loss of actin monomers at the pointed (or minus) end and a net gain of F-actin at the barbed (or plus) end. This phenomenon, known as actin treadmilling, leads to rapid turnover of G-actin while maintaining the length of F-actin at steady state (Dillon and Goda, 2005).
A variety of actin-binding proteins (ABPs) influence actin dynamics and the organization of the actin cytoskeleton. Capping proteins like tropomodulin and CapZ bind to filament ends and can modify filament turnover to affect their length (Lamprecht and LeDoux, 2004; Cingolani et al., 2008). Cross-linking proteins such as α-actinin, filamin, Arp2/3, and spectrin can arrange F-actin into distinct arrays of networks (Dillon and Goda, 2005). Other ABPs such as profilin promote F-actin polymerization while ADF/Cofilin depolymerizes F-actin. Cellular signaling pathways employ these ABPs to modify the synaptic architecture in response to changes in synaptic activity (Cingolani et al., 2008).
In mature neurons, actin is highly enriched in both pre- and post-synaptic terminals. The importance and organization of actin at these terminals is evolutionarily conserved in Drosophila and mammals, and actin is vital for maintaining and regulating synaptic vesicle pools at pre-synaptic terminals (Dillon and Goda, 2005). These vesicles are organized into at least two functionally distinct pools: the readily releasable pool (RRP) and the reserve pool (RP). The readily releasable pool consists of vesicles that are docked and primed for neurotransmitter release at the active zone of the pre-synaptic terminal. In larval Drosophila neuromuscular junction (NMJ) boutons, F-actin is required for endocytosis and recruiting synaptic vesicles into the RRP. For instance, fly strains with a loss of function mutation or expressing the dominant negative form of N-ethylmaleimide sensitive factor (NSF), a protein essential for disassembly and recycling of soluble NSF attachment protein receptor (SNARE) complex driving synaptic vesicle fusion, causes a reduction in vesicle mobility and F-actin levels at their NMJ boutons (Delgado et al., 2000; Nunes et al., 2006).
The RP are pools of synaptic vesicles released during intense stimulation. These pools are located at the center of the pre-synapse, where they are interlinked to each other by short F-actin filaments and synapsin (a pre-synaptic scaffolding protein) into clusters. Studies suggest that this meshwork of F-actin, synapsin and vesicles creates a barrier separating the RRP from the RP (Cingolani and Goda, 2008). This is evident from analyses of Drosophila larval NMJ boutons pretreated with Cytochalasin D, which inhibits polymerization of F-actin, leading to the elimination of the RP and reduced synaptic transmission evoked by high frequency stimulation (Kuromi and Kidokoro, 1998; Siechen et al., 2009). In mice, knock-out of synapsin I, II, or both genes leads to alterations in synaptic structure and functional plasticity (Rosahl et al., 1993). Surprisingly, flies lacking synapsin show neither structural, nor physiological defects at the NMJ (Godenschwege et al., 2004). They do, however, display impairments in a number of simple and complex behaviors, including faster habituation to an olfactory jump response, enhanced rapid ethanol tolerance, and significant defects in learning and memory paradigms including conditioned courtship suppression, heat-box learning, and olfactory learning (Godenschwege et al., 2004). These data suggest that although the loss of synapsin in flies appears to cause more subtle deficits than in mice, it is involved in synaptic plasticity, with proper function requiring synapsin’s phosphorylation in both animals (Michels et al., 2011).
In post-synaptic terminals, actin is highly enriched in dendritic spines and at the post-synaptic density (PSD) (Cingolani and Goda, 2008; Lamprecht and LeDoux, 2004). Dendritic spines are small protrusions formed on the main dendrite shaft and receive inputs from excitatory pre-synaptic terminals such as glutamate and acetylcholine. As in mammals, Drosophila dendritic spines take on various shapes ranging from thin or stubby to mushroom or cuplike (Leiss et al., 2009), and are thought to correlate with the strength and activity of the synapse (Bourne and Harris, 2008). Dendritic spines are highly dynamic and their formation, maturation, and plasticity depend heavily on actin cytoskeletal remodeling (Korobova and Svitkina, 2010). This includes the requirement for actin polymerization in dendritic spines upon electrophysiological theta-burst stimulation, which causes long-term potentiation (LTP) of synaptic strength, in rat hippocampal slices (Kramar et al., 2006). Drosophila is less amenable to neuron-to-neuron electrophysiological recordings, but a broad number of experiments, including many behavioral in vivo studies, have underlined the importance of actin regulatory proteins in behavioral plasticity, and in neural structure and function.
Cell adhesion molecules
At synapses, pre- and post-synaptic cells contact each other and the surrounding extracellular matrix via cell adhesion molecules (CAMs). Many different classes of CAMs, including cadherins, protocadherins, neuroligins, neurexins, integrins, and immunoglobulin adhesion proteins are localized to synapses (Dityatev et al., 2008). CAMs regulate synaptic strength by recruiting scaffolding proteins, neurotransmitter receptors, and synaptic vesicles in response to coupling with like (homophilic) or other (heterophilic) cell adhesion receptors across the synaptic cleft (Brunton et al., 2004; Thalhammer and Cingolani, 2013). Neuroligins, synaptic cell adhesion molecules (SynCAMs) and integrins, are enriched at the center of the synapse (Mortillo et al., 2012), while others, like members of the cadherin family, are preferentially localized at the outer rims of pre-synaptic active zones and PSDs (Uchida et al., 1996).
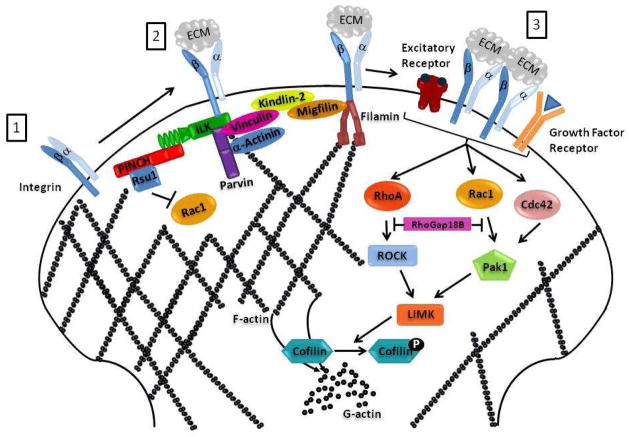
Integrins are a class of transmembrane extracellular matrix (ECM) receptors that function as αβ heterodimers and activate bidirectional-signaling cascades across the cell membrane (Grashoff et al., 2004). Integrins transduce information to the actin cytoskeleton via their direct and indirect interactions with ABPs. For instance, activation of the integrin receptor leads to the formation of cell adhesion complexes, consisting of many cytoplasmic proteins including talin, vinculin, paxillin, integrin-linked-Kinase (ILK), parvins and PINCH (particularly-interesting-cysteine- and histidine-rich protein), binding to the cytoplasmic tail of the β-integrin receptor subunit (Figure 1) (Legate et al., 2006). These complexes interact and activate ABPs like α-actinin (Honda et al., 1998; Legate et al., 2006; Pavalko and Burridge, 1991) and filamin (Loo et al., 1998; Sharma et al., 1995), which are proteins that can bind to the cytoplasmic tail of β-integrin receptor subunit and function to cross-link actin filaments to actin bundles and networks. Through these complexes, integrin-linked ABPs attach to signaling molecules and function as stable platforms for connecting the actin cytoskeleton to the ECM and for maintaining cell-ECM contacts (Figure 1). The link from integrin activation to F-actin filaments is highlighted by the finding that in rat hippocampal slices, LTP induction, and the concomitant increase in dendritic F-actin can be inhibited by anti-β1 integrin antibody incubation in hippocampal slices (Kramar et al., 2006).
Figure 1. Model of the Integrin receptor and Rho GTPases modulation of actin in a mature dendritic spine.
Upon activation of the integrin receptor by an ECM ligand, the integrin receptor (1) undergoes a conformational change leading to the formation of a cell adhesion complex at the cytoplasmic domain of the β-integrin subunit (2). Various proteins interact, and activate ABPs such as α-actinin and filamin to cross-link and connect actin filament bundles to the integrin receptor. Activation of the integrin receptor leads to the clustering a integrin receptors that can activate various growth factor receptors and affect various signaling pathways (3). Changes in the cellular actin cytoskeleton after integrin engagement are mediated through the Rho family of GTPases, Rac1, Cdc42 and Rho. Rac1 and Cdc42 phosophate Pak1 leading to LIMK-mediated phosphoryilation, and inactivation of cofilin, which prevents depolymerization of F-actin to G-actin.
In flies, integrins are highly expressed in a subpopulation of synaptic boutons at the CNS neuropil such as the mushroom bodies and a subset of synaptic boutons at the NMJs (Grotewiel et al., 1998; Rohrbough et al., 2000). It thus seems likely that loss of integrin signaling to the actin cytoskeleton would prevent regulation of dendritic spine growth and sprouting. Indeed, this is the case, since loss of the α-integrin gene volado (vol) leads to a significant increase in synapse size and number, overgrowth of synaptic terminals, and increased dendritic branching in flies (Rohrbough et al., 2000). Additionally, vol mutant flies display abnormally elevated evoked transmission amplitudes and altered Ca2+ dependence of transmission at the NMJ, suggesting that integrin is required for normal short-term synaptic facilitation processes (Rohrbough et al., 2000). Similar to these fly studies, mammalian hippocampal culture studies support integrin’s role in dendritic spine growth and plasticity. Using peptide inhibitors of integrin-ECM ligand interaction, the phenotypes observed include aberrant stability of LTP, and actin-mediated structural remodeling, which were rescued by blocking N-Methyl-D-Aspartate receptor (NMDAR) function (Bahr et al., 1997; Shi and Ethell, 2006). Since NMDAR are required for the induction of LTP and structural plasticity, these data indicate a crucial role for integrin-mediated cell-ECM adhesion in spine formation, as well as a role in neurotransmission-dependent morphological and physiological plasticity. Disruption of integrin signaling can therefore have profound effects on synapse plasticity and neural circuits that underlie certain behaviors.
Flies can learn to avoid specific odors previously associated with electric shock (Pavlovian aversive olfactory conditioning; Quinn et al., 1974). They can learn to reduce their courtship advances after experiencing rejection from females (conditioned courtship suppression; Siegel and Hall, 1979). In flies, single session training in courtship suppression, or aversive olfactory conditioning, results in short-term (STM) and mid-term memory (MTM) retention (DeZazzo and Tully, 1995). Protein synthesis dependent long-term memory (LTM), on the other hand, is elicited only with repetitive spaced training and lasts for at least a week (Tully et al., 1994). For example, a one-hour pairing of a male fly with a mated female leads to 2–3 hours of conditioned courtship suppression, whereas three 1-hour pairings or one 5-hour pairing lead to conditioned courtship suppression that lasts 9 days (McBride et al., 1999). The capacity to learn has made Drosophila a good model for isolating and studying genes necessary for memory retention, including genes encoding CAMs. The vol gene, for example, is required for proper formation of STM (Grotewiel et al., 1998). vol mutant flies were assayed for aversive olfactory classical conditioning, where flies receive an electric shock (unconditioned stimulus, US) in the presence of one odor (conditioned stimulus, CS+), and subsequently were presented with a second odor (CS−) without shock. After training, flies were allowed to choose between CS+ and CS− odors in a T- maze. Compared to wild type, vol mutant flies showed memory deficits 3 minutes after training, suggesting that the formation, stability, or retrieval of STM is dependent on integrin function (Grotewiel et al., 1998). Another neural CAMs implicated in the formation of STM in Drosophila is Fasciclin II (the fly ortholog of NCAM2). Strains carrying mutations in fasciclin II (fasII) also show an STM defect (Cheng et al., 2001). fasII and vol are both expressed preferentially in the mushroom bodies (MB), fly structures crucial for olfactory learning and memory (Waddell and Quinn, 2001; Sokolowski, 2001; Heisenberg, 2003). Taken together, these studies support a model where integrin’s activation and signaling through ABPs enable the formation, and/or stability of activity- and experience-dependent changes in synapse strength and spine structure essential for behavioral plasticity.
One of the strongest ways to change animal behavior is via exposure to drugs of abuse, which highjack circuits normally engaged by natural rewards such as food and sex. When used repeatedly, drugs elicit molecular and structural changes at the synapse that promote continued drug craving, and this can supplant almost all other of the animal’s behavioral goals (Hyman, 2005). These experience-, and drug-dependent reorganizations of neural circuitry require molecular mechanisms including CAM signaling. CAMs are also implicated in acute drug-induced behaviors such as sensitivity to ethanol-induced sedation. For example, the fasII gene is required for normal ethanol sensitivity in Drosophila (Cheng et al., 2001). Fly strains carrying mutations in fasII, when exposed to vaporized ethanol, take a shorter time than wild type flies to lose postural control, and then fall on their backs unable to right themselves (loss of righting or LOR), indicative of their ethanol-sensitivity. Similarly, flies carrying mutations in either the α-integrin receptor gene scab (scb) or β-integrin receptor gene myospheroid (mys) also cause increased ethanol sensitivity (Bhandari et al., 2009). A characteristic behavioral plasticity seen after acute ethanol exposure is the development of tolerance. Tolerance is defined as a decrease in the effect of a drug after repeated exposure, leading to a need for increased dosage to attain the same effect (Rodan and Rothenfluh, 2010). Tolerance is important in the development of drug dependence and addiction, and actin-dependent alterations in synapse structure are believed to play a major role. For instance, integrin’s modulation of actin-mediated structural plasticity also plays a role in ethanol tolerance. scb and mys mutant flies, which are initially sensitive to ethanol, show increased tolerance to ethanol-induced loss of postural control 4 hours after the first ethanol exposure, when compared to wild type (Bhandari et al., 2009).
Activation of integrin can lead to the activation of various growth factor receptors such as epidermal growth factor (EGF), insulin receptor (InR), and vascular endothelial growth factor (VEGF), which also are implicated in learning and memory processes and the development of drug abuse (Brunton et al., 2004; Corl et al., 2009; McClure et al., 2011; see Figure 1). Although integrin receptors have many functions in various signaling pathways, dramatic changes in the cellular actin cytoskeleton after integrin engagement has been attributed to its signaling through the Rho family of GTPases. For instance, the icarus (ics) gene, which encodes the fly ortholog of mammalian Ras suppressor 1 (Rsu1; Kadrmas et al., 2004), regulates ethanol-induced sedation downstream of the integrin receptor, and flies lacking ics are resistant to ethanol-induced sedation (Ojelade et al., 2013). Loss of Rsu1 in Drosophila cell culture leads to an increase in F-actin polymerization, suggesting that Rsu1 affects actin dynamics. Indeed, Rsu1 directly binds to the small actin-regulatory GTPase Rac1, and acts upstream of Rac1 to inhibit ethanol resistance (Ojelade et al., 2013). Loss of Rsu1 also affects the way flies drink ethanol. When given a choice between ethanol-containing and non-ethanol foods in a 2-bottle choice assay called CAFÉ, similar to 2-bottle choice assays use in rodent studies (Ja et al., 2007), wild-type flies show progressively increasing ethanol-preference from day 1 to day 4, showing little to no preference on day 1 and a high and stable preference on day 3 and 4 (Devineni and Heberlein, 2009). ics flies, on the other hand, show high preference for ethanol starting the first day and maintained through day 4 (Ojelade et al., 2013). These studies show that CAMs, such as integrin, molecularly regulate actin dynamics, and that they modulate both acute responses to drugs of abuse, as well as drug-induced behavioral plasticity such as tolerance and ethanol consumption preference.
Rho Family GTPases
As mentioned above, behavioral plasticity coincides with synaptic changes, including structural rearrangements. Postsynaptic dendritic spines commonly mature from filapodia (Figure 2), finger-like projections made up of bundled actin filaments, which establish the initial contact with axons (Korobova and Svitkina, 2010). Dendritic patches, where filapodia will form, contain a mixed network of linear and branched actin filaments, while the head of mature spines contains an actin meshwork similar to the one observed in lamellipodia, structures found in many dynamic cells (Halpain, 2000; Tada and Sheng, 2006; Sekino et al., 2007; Hotulainen and Hoogenraad, 2010; Korobova and Svitkina, 2010). The major regulator of actin-dependent protrusions, morphogenesis, and structure is the Rho family of small GTPases, comprising Rho, Rac, and Cdc42. These GTPases act as molecular switches by cycling between an inactive GDP (guanosine diphosphate) form and an active GTP (guanosine triphosphate) form. The proportions of GTP-, or GDP-binding is determined by three classes of regulatory proteins: guanine nucleotide exchange factors (GEFs) enhance the exchange of bound GDP for GTP; the GTPase activating proteins (GAPs) serve as negative regulators by increasing the rate of hydrolysis of bound GTP; and guanine nucleotide dissociation inhibitors (GDIs) inhibit both GTP exchange and the hydrolysis of GTP (Saneyoshi and Hayashi, 2012). Rho family GTPases play critical roles in the activity dependent formation and structural modification of dendritic spines in flies. For instance, loss of all three Rac genes, Rac1, Rac2, and Mtl, in Drosophila MB neurons results in a significant reduction in dendritic branching and length (Ng et al., 2002). Analysis of Cdc42 clones in vertical system (VS) neurons demonstrated a requirement for Cdc42 in regulating dendritic morphology, branching, and guidance (Scott et al., 2003). These phenotypes are similar to analyses of Cdc42 and Rac1 in cultured hippocampal neurons, where dominant-negative expression of Cdc42 and Rac1 leads to a decrease in spine density (Impey et al., 2010; Irie and Yamaguchi, 2002; Tashiro et al., 2000), and expression of constitutive active Cdc42 and Rac1 cause an increase in spine density (Impey et al., 2010; Tashiro et al., 2000). In contrast to Cdc42 and Rac1, the constitutive active form of RhoA decreases dendritic spine density and increases spine length, while a dominant negative form of RhoA increases spine density (Impey et al., 2010).
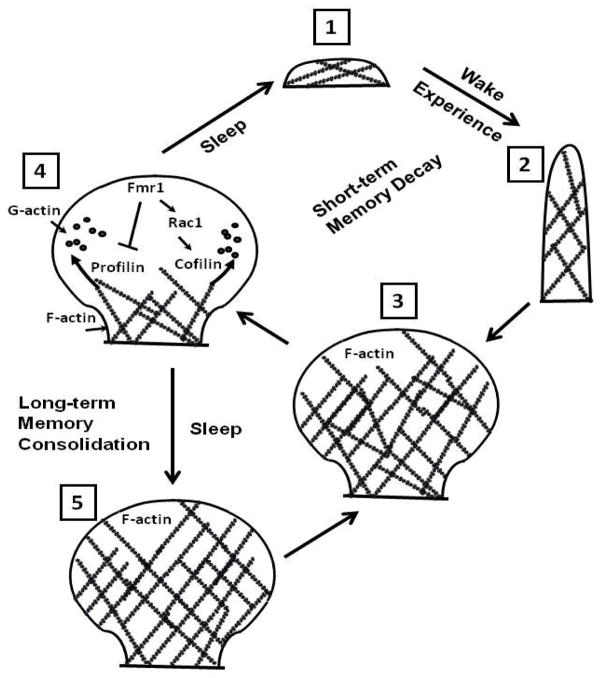
Figure 2. Fmr1 and Rac1 function in dendritic spine plasticity.
Experiences acquired from the environment lead to de novo formation of a mature dendritic spine (3) from a dendritic filopodia (2) or patch (1), via actin-dependent protrusions and morphogenesis regulated by synaptic transmission. Fmr1 functions to reduce synaptic strength via actin-mediated decrease in spine size and number, for example during sleep-mediated synaptic homeostasis. Fmr1 also interacts with Rac1 GTPase and is involved in short-term memory decay (1) and long-term memory consolidation (5) through their activation of cofilin and through their inhibition of Profilin (4).
Within a single spine, the activities of RhoA and Cdc42 were analyzed in cultured slices of rat hippocampus during induction of LTP (Murakoshi et al., 2011). As the dendritic spine expands, the activity of both RhoA and Cdc42 were elevated for at least 30 minutes, depending on NMDAR and the Ca2+/calmodulin-dependent kinase (CaMKII), which are both essential for LTP. Activation of Cdc42 localized specifically to the stimulated spines, while RhoA diffused out from those stimulated spines (Murakoshi et al., 2011). Rac1 is also required for the formation and maintenance of LTP, since both mutant mice lacking the Rac1 gene, as well as inhibition of Rac1 using pharmacological inhibitors affect spine structure and impair synaptic plasticity in the hippocampus, concomitant with hippocampus-dependent spatial learning defects (Haditsch et al., 2009; Rex et al., 2009). A particularly striking, and direct example of the importance of proper actin regulation in synaptic plasticity and behavioral learning was published recently by Huang and colleagues (2013). mTORC (target of rapamycin complex) is activated by numerous growth factor receptors. mTORC1 contains the protein raptor, and is involved in cell growth and protein translation. Less well understood is mTORC2, which contains Rictor (rapamycin insensitive companion of mTOR). Mice with forebrain-specific Rictor knock out do not show long-lasting L-LTP, and learn poorly in contextual fear conditioning (where mice normally learn to associate an environmental box with foot shocks, and therefore acquire box-induced freezing behavior). Similarly, flies lacking a functional rictor gene show normal STM, but no spaced training-induced LTM. Rictor knock out mice show decreased Rac1 activation, and a reduced F- to G-actin ratio, as well as fewer dendritic spines. Amazingly, these defects (fear memory, L-LTP, and F-/G-actin ratio) could be rescued by application of jasplakinolide to brain slices, or direct injection into the brain. This marine sponge toxin promotes actin polymerization, and in normal mice can also turn sub-threshold electro-physiological stimulation into L-LTP, as well as behavioral under-training into strong memories (Huang et al., 2013), illustating the direct impact of actin polymerization on neural plasticity. Together, these studies suggest that (NMDA, integrin, and/or growth factor) receptor-mediated signaling pathways act via Rho family GTPases to regulate F-actin reorganization and spine morphology involved in synaptic, and behavioral plasticity, as well as learning and memory.
Acquired memory that is not reinforced by repetitive learning is vulnerable to being erased or forgotten (Shuai and Zhong, 2010). A recent report showed that Rac1 contributes to both passive memory decay and forgetting in Drosophila (Shuai et al., 2010). Over-expression of a dominant negative form of Drosophila Rac1, Rac1DN in neurons led to normal memory acquisition in the first 30 minutes after training, but significantly slowed memory decay at later time points from 2 hours to 24 hours (Shuai et al., 2010). This delay in memory decay is independent of protein synthesis and therefore does not resemble LTM. The Rac1DN expressing flies also did not forget previously trained odor even when perturbed 1.5 hours later, by training with a new aversive odor (interference-learning paradigm). Conversely, over-expression of constitutively active form, Rac1CA, accelerated memory decay. In wild-type flies, Rac1 activation also correlated with memory decay, suggesting that memory can be bi-directionally regulated through the manipulation of Rac1 (Shuai et al., 2010). Interestingly, in conjunction with previous studies discussed, these experiments also suggest that Rac1 has a critical role in both the acquisition, as well as in the active erasing/forgetting of memories. It also highlights the importance of not only controlled synapse strengthening, but also weakening and elimination in the normal context of daily experiences.
Rho GTPases and their effectors also play a role in ethanol-induced behaviors in Drosophila. For instance, neuronal loss of Rac1 activity leads to sensitivity to ethanol-induced behaviors while expression of activated Rac1 GTPase leads to resistance (Peru et al., 2012; Rothenfluh et al., 2006). Flies carrying mutations in RhoGAP18B, a protein that deactivates Rho-family GTPases such as Rac1 and Rho (Figure 1), display resistance to ethanol-induced sedation (Rothenfluh et al., 2006). Flies with decreased Rac1 function are sensitive to ethanol-induced sedation (Rothenfluh et al., 2006). Flies lacking Arf6, a member of the Arf family of GTPases that functions in membrane trafficking, and actin organization, are also sensitive to ethanol-induced sedation (Peru y Colón de Portugal et al., 2012). Rac1 functionally connects to Arf6 via the BAR domain protein Arfaptin, which can directly bind to Rac1 as well as Arf6. Flies lacking Arfaptin are also ethanol-sensitive (Peru y Colón de Portugal et al., 2012), and they show synaptic undergrowth at the Drosophila NMJ (Chang et al., 2013), again linking behavior, synapse structure and actin dynamics.
These mutants, with their altered synaptic structures, may well predispose the animals to react differently to ethanol exposures. But are there effects of ethanol on the actin cytoskeleton? It has indeed been known for a while that exposure in cell culture leads to profound changes in cell shape. For instance, chronic exposure of primary astrocytes to ethanol (30 mM for 7 days) alters the actin cytoskeleton, with a marked increase in F-actin near the plasma membrane (Tomas et al., 2003). The ethanol-induced changes in actin are likely due to an ethanol-induced decrease in Rho family GTPase activity, especially RhoA, since treatment with lysophosphatic acid (LPA), an activator of RhoA (Tomas et al., 2003), or transfection with activated RhoA (Guasch et al., 2003) blocks the ethanol-induced effects. Conversely, astrocyte cultures treated acutely with ethanol (100mM for 10 minutes) have reduced stress fibers, which are rich in F-actin (Allansson et al., 2001; Guasch et al., 2003), suggesting a rapid change in RhoA activity. One potential mechanism for reduced RhoA activity is via upregulation of p190 RhoGAP, converting active RhoA-GTP to inactive RhoA-GDP. Chronic alcohol exposure increases p190 RhoGAP activity and redistributes it to the plasma membrane (Selva and Egea, 2011), but the precise mechanism(s) remains unclear. Nevertheless, these data suggest that acute ethanol has a negative effect on F-actin stability, and that the observed long-term increases in plasma membrane actin filaments may be a compensatory reaction to prolonged ethanol exposure (Rothenfluh and Cowan, 2013).
Insights into the acute effects of ethanol on neuronal function have come from a number of studies. Popp and Dertien (2008) reported that a brief, 30 second, pre-exposure of cultured cerebellar granule cells to ethanol potentiated subsequent NMDAR inhibition by ethanol, even when the pretreatment was applied intracellularly. Phalloidin, an F-actin stabilizer, prevented this potentiation, while latrunculin A (latA), an actin depolymerizer, mimicked the effect (Popp and Dertien, 2008). These findings suggest that acute ethanol leads to F-actin instability, and causes a decrease in NMDAR current, which was indeed found in cerebellar granule cell slices (Offenhauser et al., 2006). Knocking out EGF receptor pathway substrate 8 (EPS8) in mice, an actin capping protein, suppressed both ethanol-induced NMDAR current rundown and F-actin instability. Behaviorally, EPS8 knockout mice were resistant to ethanol-induced loss of righting and showed increased alcohol consumption in a 2-bottle choice assay (Offenhauser et al., 2006). EPS8 localizes to postsynaptic densities in cerebellar granule neurons, and can activate the small GTPase Rac1 (Offenhauser et al., 2006). Similar to mammals, loss of the fly ortholog of EPS8, called arouser, also affects ethanol-induced LOR, and it also affects synapse number (Eddison et al., 2011), once more relating actin to neuronal structure and function.
Aside from alcohol, members of the Rho family of GTPases are also linked to other drugs of abuse, such as nicotine and cocaine, in both flies and mammals. Loss of RhoGAP18B makes flies resistant to both nicotine and cocaine-induced LOR, for example (Rothenfluh et al., 2006). Recently, Dietz et al. (2012) showed that the small GTPase Rac1 affects cocaine reward in the nucleus accumbens (NAc). They found that acute intraperitoneal injections of cocaine in mice led to transient reduction in active Rac1, and expression of dominant-negative Rac1 enhanced both cocaine-induced place preference as well as dendritic spine numbers (Dietz et al., 2012). These studies suggest that the same molecules that are involved in learning and memory also particiate in drug-induced plasticity, even though in the case of Rac1 the seem to have opposite effects, with dominant-negative Rac1 enhancing cocaine-induced plasticity, while normal Rac1 activity is required for L-LTP and fear conditioning (Huang et al., 2013). This highlights both the importance of this actin-regulating small GTPase, as well as the requirement for its fine-tuned regulation for proper neuronal and behavioral plasticity.
Effect of ABPs and other Actin Regulatory Genes on Drosophila Behavior
One of the downstream effectors of Rac1 is the actin-severing protein cofilin. It is inactivated by phosphorylation, which can be triggered by Rho family GTPases. GTP-bound Rac1 and Cdc42 activate p21-activated kinase (PAK), which in turn phosphorylates and activates Lin11/Isl-1/Mec3 kinase (LIMK), which in turn inactivates cofilin. Rho1 can activate LIMK via activation of Rho-associated kinase ROCK (Schubert and Dotti, 2007). Within spines, cofilin is thought to be critically involved in the structural changes triggered by experiences leading to stable modifications in synaptic responses (Figure 1 and 2). Cocaine-conditioned place preference is suppressed by photo-activated Rac1, which is mediated by cofilin inactivation. Photo-activation of Rac1 causes phosphorylation of cofilin, and expression of dominant-negative (pseudo-phosphorylated) cofilin recapitulates the behavioral suppression seen with Rac1 (Dietz et al., 2012). LIMK and cofilin also affect ethanol-induced sedation in flies (S.A.O. and A.R., unpublished results). Furthermore, cofilin also functions downstream of Rac1 to regulate memory decay and forgetting, since neuronal expression of the constitutively active form of cofilin enhanced 3 hour memory performance similar to Rac1 inhibition (Shuai et al., 2010).
Behavioral and functional plasticity is also affected by actin capping proteins, as illustrated by the β-adducin knockout mouse, which has defects in hippocampal LTP and LTD, as well as deficits in several learning assays (Rabenstein et al., 2005). Hts, the fly ortholog of this actin capping protein found at pre-synaptic terminals has not been shown to affect learning and memory, but loss of hts results in a dramatic increase in the number of synaptic retractions, as well as a generalized overgrowth of large-diameter glutamatergic type Ib boutons at the larval NMJ (Pielage et al., 2011; Stevens and Littleton, 2011). As mentioned earlier, the actin capping protein EPS8 is involved in ethanol responses in both flies and mice, and a number of other ABPs affect both drug-induced behaviors, and learning and memory. For example, filamin, an actin cross-linking protein previously discussed as binding to the β-subunit of integrin (Figure 1), is necessary for learning and memory, and drug-induced behaviors since loss of filamin (cheerio mutants) causes sensitivity to ethanol-induced sedation, and deficits in LTM formation (Berger et al., 2008; Bolduc et al., 2010). Formin3, an ABP that nucleates the formation of unbranched actin filaments also regulates ethanol sensitivity, tolerance, and LTM formation in flies (Berger et al., 2008).
All these studies suggest that common neurobiological mechanisms contribute to the development of synaptic, and dendritic spine plasticity, and these mechanisms are required for both drug addiction and for learning and memory. Indeed, many fly mutants isolated by their behavioral defects in associative learning and memory also show defects in ethanol-induced behaviors such as tolerance, or acute ethanol sensitivity (Berger et al., 2008). This is not surprising, however, since the current view is that drugs of abuse highjack natural reward centers in the CNS. This artificially reinforces the drug-associated experiences, and thereby causes long-lasting changes in the brain that underlie the behavioral abnormalities associated with drug addiction (Hyman, 2005). Common experiences of environmental stimuli normally induce memory formation, and stable changes in the brain as well. Drug addiction can thus be viewed as a disease of pathological learning (Nestler, 2002), utilizing existing plasticity mechanisms, including actin-mediated structural alterations.
Fmr1 and Drosophila Behavior
The most common inherited learning disability in people is fragile X syndrome, which is caused by CGG triplet expansion in the FXR1 gene (Verkerk et al., 1991). Fxr1 knock out mice show an unusual abundance of dendritic spines, especially long immature ones, suggesting that the missing FMRP protein regulates spine maturation and pruning (Comery et al., 1997). These mice also display learning deficits, reiterating the connection between behavioral and dendritic spine plasticity. The FMRP protein regulates mRNA transport and is a repressor of protein translation. FMRP-containing granules are enriched in F-actin-rich compartments, such as filapodia and spines where they contain ribosomes, FMRP-regulated target RNAs such as messages for the synaptic plasticity proteins PSD-95 and CaMKII (Antar et al., 2005; see above), but also other proteins such as CYFIP1 and 2 (cytosplasmic FMRP interacting protein). Drosophila CYFIP, a subunit of the WAVE/SCAR complex required for Arp2/3-dependent actin nucleation, interacts biochemically and genetically with Fmr1 and Rac1 (Galy et al., 2011). CYFIP protein is expressed specifically in the nervous system, and mutations affect dendritic spines much like mutations in Drosophila Fmr1 and Rac1 (Schenck et al., 2003). Murine fibroblasts lacking FMRP show changes in Rac1-induced actin remodeling and an accumulation of uphospholylated, active cofilin (Castets et al., 2005). The F-actin regulatory protein profilin is also upregulated in Fmr1 mutant flies, and loss of profilin phenocopies FMRP over-expression (Reeve et al., 2005). In mice, Profilin2a knock outs suggest that the protein is required for stabilizing dendritic spines (Michaelsen et al., 2010), where the protein normally accumulates upon NMDAR activation (Ackermann & Matus, 2003) and fear conditioning (Lamprecht et al., 2006). Overall, the emerging model is that synaptic maturation, strengthening and growth downregulate FMRP activity, which in turn allows translation of plasticity and structure-relevant proteins, including a number of actin regulatory proteins.
Given the role of FMRP in spine plasticity, it is not surprising that the Drosophila Fmr1 gene has been implicated in a number of behaviors, including in behavioral plasticity. Fmr1 mutant males spend significantly less time trying to court females (Dockendorff et al., 2002). However, just like wild-type males, when they are unsuccessfully courting a mated female, they learn to decrease their (rejected) courtship advances. When wild-type males are then put together with a (receptive) female, they remember their prior experience and show continued courtship depression. Fmr1 mutants, on the other hand, do not remember, and immediately go back to naïve courtship levels (McBride et al., 2005). This suggests that flies lacking FMRP can learn, but cannot stably encode, or recall memories.
Fmr1 mutant flies also have a defect in their circadian clock, and most mutant flies are arrhythmic, with concomitant morphological aberrations such as axonal overextension and excessive branching (Morales et al., 2002; Dockendorff et al., 2002). One of the physiological outputs regulated by circadian rhythms is sleep. Like mammals, flies display criteria of sleep including long bouts of immobility and increased arousal threshold at particular times during the circadian day. Fly sleep is also under homeostatic regulation, since sleep deprivation is followed the next day by a compensatory sleep rebound (Hendricks et al., 2000). Although the function of sleep remains unknown, one current hypothesis is that sleep is required for synaptic homeostasis. A consequence of staying awake is a progressive increase in synaptic strength, which results from learning and adapting to environmental stimuli (Huber et al., 2004). Such potentiation of synapses by information encoded in the brain cannot be sustained indefinitely and therefore, sleep may serve an essential function in promoting a homeostatic reduction in synaptic strength to baseline levels (Huber et al., 2004), which could explain why sleep is under homeostatic regulation (Hendricks et al., 2000). As mentioned previously, these increases in synaptic strength are associated with actin-mediated changes in synaptic structure, including synapse size and number. Experiments carried out in Drosophila support the hypothesis of sleep being required for synaptic homeostasis. The overall levels of synaptic proteins in the fly brain increases after wake and decrease after sleep, suggesting a reduction in synaptic strength (Gilestro et al., 2009). Also, studies in three distinct neuronal circuits in Drosophila show that synapse size and number increase after hours of wakefulness, and decrease only if flies are allowed to sleep (Bushey et al., 2011). Studies with Fmr1 show that actin-mediated structural plasticity at the synapse plays a role in the homeostatic reduction of synaptic strength that occurs during sleep (Bushey et al., 2009). For instance, dFMRP expression levels increase in the adult fly brain during wake compared to sleep, and is independent of circadian time (Bushey et al., 2009). Also, over-expression of dFMRP in either the MBs or the entire fly brain is associated with a ~30% decrease in sleep duration (Bushey et al., 2009), and while sleep deprivation increases spine number and branch length in wild-type flies, this is suppressed in dFMRP overexpressing flies (Bushey et al., 2011), suggesting that dFMRP functions as a synapse pruner to regulate sleep-dependent homeostatic reduction in synaptic strength (Figure 2).
Conclusion
Neural circuits in the brain are the substrates for sensory processing and integration, which ultimately lead to animal behavior. These behaviors, and the changes that result from experience, are dependent on which neurons communicate with each other, and how these (mostly) synaptic communications change with experience. The plasticity of synapses, including the changes in the postsynaptic dendrites and their spines is highly contingent on the structure of those compartments. This neural morphology, and its dynamic change, depends on proper growth and retraction of actin filaments. It is thus not surprising that a large number of actin regulatory proteins also affect numerous behaviors. In this review, we have highlighted a selection of these proteins, and the behaviors they modulate, with an emphasis on the model organism Drosophila melanogaster. Other than stressing the link between actin dynamics, and structural, and behavioral plasticity, we hope to have reiterated the usefulness of this genetically tractable model system. Both as a tool to find novel genes involved in given behaviors of choice, as well as a way to test the in vivo relevance of molecularly characterized proteins and signaling cascades.
Biographies
Shamsideen Ojelade received his B.S. in Biology from the University of Houston. He is presently, a graduate student in the neuroscience program and works in the laboratory of Dr. Rothenfluh, Department of Psychiatry, University of Texas-Southwestern Medical Center in Dallas, TX 75235. He has received funding from NIH through a NIDA institutional grant (T32 DA7290) and currently is completing his thesis dissertation under a F31-NIAAA fellowship (F31 AA021340).
Summer F. Acevedo graduated with a BA in Biochemistry from the University of Northern Colorado, Greeley, CO and a PhD in Genetics from Texas A&M University, College Station, TX. She then completed a NIDA post-doctoral fellowship in Behavioral Neuroscience at Oregon Health & Science University in Portland, OR, before moving on as faculty in the department of Pharmacology, Physiology & Toxicology, Program in Psychology at Ponce School of Medicine & Health Sciences in Puerto Rico. She is currently on the faculty in the Department of Psychiatry at UT Southwestern Medical Center, Dallas, TX.
Adrian Rothenfluh received his Diploma in molecular biology from the Biocenter, University of Basel, Switzerland, and his Ph.D. in genetics from Rockefeller University in New York. Following postdoctoral training at UCSF, he became an assistant professor in the Department of Psychiatry at UT Southwestern Medical Center in Dallas, TX in 2007. He is funded by the NIH (R01AA019526), the Brain & Behavior Research Foundation, and the Endowed Scholars Program at UTSW.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–2000. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The Genome Sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J Neurochem. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P, Kendler KS, Bettinger JC, Davies AG, Grotewiel M. An assay for evoked locomotor behavior in Drosophila reveals a role for integrins in ethanol sensitivity and rapid ethanol tolerance. Alcohol Clin Exp Res. 2009;33:1794–1805. doi: 10.1111/j.1530-0277.2009.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P, Chelly J. From Fragile X Mental Retardation Protein to Rac1 GTPase: New Insights from Fly CYFIP. Neuron. 2003;38:843–845. doi: 10.1016/s0896-6273(03)00362-3. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Rosenfelt C, Cox H, Tully T. Fragile x mental retardation 1 and filamin a interact genetically in Drosophila long-term memory. Front Neural Circuits. 2010;3:22. doi: 10.3389/neuro.04.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted Gene-Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bushey D, Cirelli C. From genetics to structure to function: exploring sleep in Drosophila. Int Rev Neurobiol. 2011;99:213–244. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29:1948–1961. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. Sleep and Synaptic Homeostasis: Structural Evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets M, Schaeffer C, Bechara E, Schenck A, Khandjian EW, Luche S, Moine H, Rabilloud T, Mandel JL, Bardoni B. FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics in murine fibroblasts. Hum Mol Genet. 2005;14:835–844. doi: 10.1093/hmg/ddi077. [DOI] [PubMed] [Google Scholar]
- Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, Perkins TJ, Brankatschk M, Rothenfluh A, Buszczak M, Hiesinger PR. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Current Biology. 2011;21:1704–1715. doi: 10.1016/j.cub.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kreko T, Davison H, Cusmano T, Wu Y, Rothenfluh A, Eaton BA. Normal dynactin complex function during synapse growth in Drosophila requires membrane binding by Arfaptin. Mol Biol Cell. 2013;24:1749–1764. doi: 10.1091/mbc.E12-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J, Tully T. Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Bukalo O, Schachner M. Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 2008;4:197–209. doi: 10.1017/S1740925X09990111. [DOI] [PubMed] [Google Scholar]
- Dockendorff TC, Su HS, McBride SMJ, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila Lacking dfmr1 Activity Show Defects in Circadian Output and Fail to Maintain Courtship Interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- Eddison M, Guarnieri DJ, Cheng L, Liu CH, Moffat KG, Davis G, Heberlein U. arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Galy A, Schenck A, Sahin HB, Qurashi A, Sahel JA, Diebold C, Giangrande A. CYFIP dependent actin remodeling controls specific aspects of Drosophila eye morphogenesis. Dev Biol. 2011;359:37–46. doi: 10.1016/j.ydbio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BRE, Martin JR, et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin’s mysterious partner. Curr Opin Cell Biol. 2004;16:565–571. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Guasch RM, Tomas M, Minambres R, Valles S, Renau-Piqueras J, Guerri C. RhoA and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. J Neurosci Res. 2003;72:487–502. doi: 10.1002/jnr.10594. [DOI] [PubMed] [Google Scholar]
- Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM, McConnell SK, Palmer TD. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol Cell Neurosci. 2009;41:409–419. doi: 10.1016/j.mcn.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 2000;23:141–146. doi: 10.1016/s0166-2236(00)01576-9. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BSS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila Is a Sleep-like State. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and invasion. Journal of Cell Biology. 1998;143:277–277. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43:146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR, Beckerle MC. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol. 2004;167:1019–1024. doi: 10.1083/jcb.200408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006;9:481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fussler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Leiss F, Koper E, Hein I, Fouquet W, Lindner J, Sigrist S, Tavosanis G. Characterization of dendritic spines in the Drosophila central nervous system. Dev Neurobiol. 2009;69:221–234. doi: 10.1002/dneu.20699. [DOI] [PubMed] [Google Scholar]
- Leyssen M, Hassan BA. A fruitfly’s guide to keeping the brain wired. EMBO Rep. 2007;8:46–50. doi: 10.1038/sj.embor.7400869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DT, Kanner SB, Aruffo A. Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction. J Biol Chem. 1998;273:23304–23312. doi: 10.1074/jbc.273.36.23304. [DOI] [PubMed] [Google Scholar]
- McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- McClure KD, French RL, Heberlein U. A Drosophila model for fetal alcohol syndrome disorders: role for the insulin pathway. Dis Model Mech. 2011;4:335–346. doi: 10.1242/dmm.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels B, Chen YC, Saumweber T, Mishra D, Tanimoto H, Schmid B, Engmann O, Gerber B. Cellular site and molecular mode of synapsin action in associative learning. Learn Mem. 2011;18:332–344. doi: 10.1101/lm.2101411. [DOI] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Mortillo S, Elste A, Ge Y, Patil SB, Hsiao K, Huntley GW, Davis RL, Benson DL. Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic beta1-integrin. J Comp Neurol. 2012;520:2041–2052. doi: 10.1002/cne.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Nunes P, Haines N, Kuppuswamy V, Fleet DJ, Stewart BA. Synaptic vesicle mobility and presynaptic F-actin are disrupted in a N-ethylmaleimide-sensitive factor allele of Drosophila. Mol Biol Cell. 2006;17:4709–4719. doi: 10.1091/mbc.E06-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D’Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Ojelade SA, Rodan AR, Kadrmas JL, Ruggeri B, Tianye J, Cattrell A, Desrivieres S, Banaschewski T, Barker GJ, Buchel C, et al. Ras Suppressor 1 Acts Downstream of Integrin to Regulate Rac1 Activity and Ethanol Consumption in Drosophila and Humans (Submitted) [Google Scholar]
- Ou H, Lei T. A novel strategy for conditional gene knockout based on ΦC31 integrase and Gal4/UAS system in Drosophila. IUBMB Life. 2013;65:144–148. doi: 10.1002/iub.1119. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peru y Colón de Portugal RL, Acevedo SF, Rodan AR, Chang LY, Eaton BA, Rothenfluh A. Adult neuronal Arf6 controls ethanol-induced behavior with Arfaptin downstream of Rac1 and RhoGAP18B. J Neurosci. 2012;32:17706–17713. doi: 10.1523/JNEUROSCI.1944-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 2011;69:1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp RL, Dertien JS. Actin depolymerization contributes to ethanol inhibition of NMDA receptors in primary cultured cerebellar granule cells. Alcohol. 2008;42:525–539. doi: 10.1016/j.alcohol.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned Behavior in Drosophila melanogaster. Proc Natl Acad Sci U A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci. 2005;25:2138–2145. doi: 10.1523/JNEUROSCI.3530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve SP, Bassetto L, Genova GK, Kleyner Y, Leyssen M, Jackson FR, Hassan BA. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr Biol. 2005;15:1156–1163. doi: 10.1016/j.cub.2005.05.050. [DOI] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan AR, Rothenfluh A. The genetics of behavioral alcohol responses in Drosophila. Int Rev Neurobiol. 2010;91:25–51. doi: 10.1016/S0074-7742(10)91002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin’? Curr Opin Neurobiol. 2013;23:507–512. doi: 10.1016/j.conb.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Lewis EB. A Brief History of Drosophila’s Contributions to Genome Research. Science. 2000;287:2216–2218. doi: 10.1126/science.287.5461.2216. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Hayashi Y. The Ca2+ and Rho GTPase signaling pathways underlying activity-dependent actin remodeling at dendritic spines. Cytoskeleton. 2012;69:545–554. doi: 10.1002/cm.21037. [DOI] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 Controls Neuronal Connectivity in Drosophila and Links the Rac1 GTPase Pathway to the Fragile X Protein. Neuron. 2003;38:887–898. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–212. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- Scott EK, Reuter JE, Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J Neurosci. 2003;23:3118–3123. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Selva J, Egea G. Ethanol increases p190RhoGAP activity, leading to actin cytoskeleton rearrangements. J Neurochem. 2011;119:1306–1316. doi: 10.1111/j.1471-4159.2011.07522.x. [DOI] [PubMed] [Google Scholar]
- Sharma CP, Ezzell RM, Arnaout MA. Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J Immunol. 1995;154:3461–3470. [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–589. doi: 10.1016/j.cell.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Shuai Y, Zhong Y. Forgetting and small G protein Rac. Protein Cell. 2010;1:503–506. doi: 10.1007/s13238-010-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siechen S, Yang S, Chiba A, Saif T. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc Natl Acad Sci USA. 2009;106:12611–12616. doi: 10.1073/pnas.0901867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. Conditioned-Responses in Courtship Behavior of Normal and Mutant Drosophila. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Littleton JT. Synaptic growth: dancing with adducin. Curr Biol. 2011;21:R402–405. doi: 10.1016/j.cub.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hirth F. Deep Homology of Arthropod Central Complex and Vertebrate Basal Ganglia. Science. 2013;340:157–161. doi: 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Thalhammer A, Cingolani LA. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.015. (in press) [DOI] [PubMed] [Google Scholar]
- Tomas M, Lazaro-Dieguez F, Duran JM, Marin P, Renau-Piqueras J, Egea G. Protective effects of lysophosphatidic acid (LPA) on chronic ethanol-induced injuries to the cytoskeleton and on glucose uptake in rat astrocytes. J Neurochem. 2003;87:220–229. doi: 10.1046/j.1471-4159.2003.01993.x. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]