Abstract
About one half of malignant peripheral nerve sheath tumors (MPNST) have Neurofibromin 1 (NF1) mutations. NF1 is a tumor suppressor gene essential for negative regulation of RAS signaling. Survival for MPNST patients is poor and we sought to identify an effective combination therapy. Starting with the mTOR inhibitors rapamycin and everolimus, we screened for synergy in 542 FDA approved compounds using MPNST cells with a native NF1 loss in both alleles. We further analyzed the cell cycle and signal transduction. In vivo growth effects of the drug combination with local radiation therapy were assessed in MPNST xenografts. The synergistic combination of mTOR inhibitors with bortezomib yielded a reduction in MPNST cell proliferation. The combination of mTOR inhibitors and bortezomib also enhanced the anti-proliferative effect of radiation in vitro. In vivo, the combination of mTOR inhibitor (everolimus) and bortezomib with radiation therapy decreased tumor growth and proliferation, and augmented apoptosis. The combination of approved mTOR and proteasome inhibitors with radiation showed a significant reduction of tumor growth in an animal model and should be investigated and optimized further for MPNST therapy.
Keywords: MPNST, mTOR signaling, proteasome inhibitor, drug combination
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) are an aggressive soft-tissue sarcoma and about 50% of MPNSTs arise in Neurofibromatosis Type 1 patients (NF1). NF1 patients have 8–13% lifetime risk of developing a MPNST. Despite aggressive treatment strategy, MPNST has a high rate of recurrence and distant metastases. The 5-year survival rate ranges from 39 to 60 % [1–3]. These tumors have poor clinical prognosis, despite efforts to find better targeted therapy [4].
Neurofibromin 1 (NF1) is a tumor suppressor gene essential for negative regulation of RAS protein activity and is the most frequently mutated gene in MPNSTs [5, 6]. Loss of NF1 up-regulates PI3K/Akt/Mammalian target of rapamycin (mTOR) signaling and sensitizes cells to mTOR inhibitors [7]. Overexpression of mTOR downstream signaling components are observed in MPNST human samples and are associated with poor prognosis in MPNST patients [8, 9]. Preclinical data showed an anti-tumoral effect of allosteric inhibitors of mTOR in MPSNT mouse models. However, blocking mTOR signaling promoted a cytostatic rather than cytotoxic effect [10].
Combinatorial targeted therapy has been previously tested in preclinical MPNST models. Johansson et al. observed that the mTOR inhibitor everolimus combined with doxorubicin did not reduce MPNST tumor growth in vivo. Everolimus plus erlotinib, an EGFR inhibitor, slightly reduced growth when compared with single treatment in a xenograft MPNST model [11]. Recently, the combination of the mTOR inhibitor rapamycin and HSP90 inhibitors resulted in tumor regression in MPNST transgenic mice, through a mechanism that involved endoplasmatic reticulum stress and subsequent cell death [12]. Preclinical data showed an antiproliferative effect with HSP90 inhibition in other tumor types and several HSP90 inhibitors are in clinical trials [13, 14].
In this study, we sought to identify potential combination therapies and test their efficacy in an MPNST preclinical model. We focused on FDA approved drugs to accelerate any possible translational to clinical trials. We first screened 542 inhibitors seeking synergy with rapamycin or everolimus to reduce MPNST cell proliferation. We then tested the drug combination with radiation in vitro and in a preclinical xenograft MPNST model.
MATERIALS AND METHODS
Cell culture, transfection and ionizing radiation
Human NF1-associated MPNST cell lines NF90.8 and ST88-14 were provided by Dr Michael Tainsky (Wayne University, Detroit, MI), T265-2C was obtained from Dr Steven Porcelli (Albert Einstein College of Medicine, Bronx, NY), and sNF96.2 was purchased from ATCC (Manassas, VA) [15, 16]. MPNST cells were maintained in RPMI 1640 medium (Sigma) containing 10% of fetal bovine serum (BenchMarck, Gemini Bio-Products, West Sacramento, CA) and 1% penicillin- streptomycin (Invitrogen Life Technologies, Carlsbad, CA) at 37°C in an humidified 5% CO2 atmosphere. MPNST cells were transiently transfected with 10 nM of siRNA duplex mTOR#13 (CCAAAGUCAAUGUGCAGGAUCUUCCCA) or mTOR#19 (GCCAAGACACAGUAGCGAAUGUCAGGG) (Integrated DNA Technology, Coralville, IA) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Non-targeting GFP siRNA (IDT DNA Technologies) was used as control. Cells were irradiated in a Caesium137 (2.24 Gy/min) radiation source Gammacell 1000 (MDS Nordion, Ottawa, ON, Canada). All compound were purchased from LC Laboratories (LC Laboratories, Woburn, MA), with exception of dichloroacetic acid (Tocris, Ellisville, MO) and dissolved in dimethyl sufoxide (DMSO) (Sigma).
Cell proliferation and drug synergism
MPNST cell proliferation was performed using the fluorescence cell-based alamarBlue assay (Invitrogen). Fluorescence was read in Victor-3 automated plate reader (Perkim-Elmer, Turku, Finland), with a 540-nm excitation/590-nm emission filter. Half inhibitory concentration (IC50) was calculated using GraphPad Prism 5.0 (Prism, La Jolla, CA) as previously described [17]. Effects of drug combinations were calculated using CompuSyn Software (ComboSyn, Paramus, NJ) [18].
Drug screening
Cell-based drug screening was assessed by using the NINDS discovery library (National Institute of Neurological Disorders and Stroke) and the Approved Oncology Drug Set III library (National Cancer Institute) (Supplementary table 1) – in combination with rapamycin or everolimus, as previously described [19]. NF90.8 cells were seeded (103 cells/well) in 96-well black flat-bottom plates, allowed to attach for 24 hours, individually treated with libraries compounds (4 M) and rapamycin or everolimus at 25% of IC50 (12.5 and 5.2 M, respectively). Cell proliferation was analyzed 72 hours after drug exposure using alamarBlue assay (Invitrogen). As a secondary screening dose response curves (with at least nine different concentrations) were generated for each drug and cell proliferation was analyzed by alamarBlue assay. IC50 was calculated using GraphPad Prism 5.0 (Prism, La Jolla, CA, USA) as described above.
Proteasome activity
MPNST cells were seeded at 104 cells/well in 96-well plates in triplicate and treated with bortezomib. Chymotrypsin-like activity of the 20S proteasome was determined according to the manufactory’s instructions (Proteasome-Glo, Promega). Luminescence was read in Victor-3 automated plate reader (Perkim-Elmer).
Cell cycle analysis
Cell cycle analysis was performed as previously described [20]. MPNST cells (5 × 105) were subjected to radiation, seeded in triplicate, and treated with everolimus and bortezomib. After 48 hours DNA was stained with propidium iodide (20 ìg/mL) (Sigma) and measured in flow cytometer BD Calibur (BD Bioscience, San Jose, CA). Data were analyzed in BD CellQuest Pro software (BD Bioscience); pulse width and pulse area were used to exclude doublets.
Western blot
Total protein lysates were extracted using RIPA buffer (Sigma) containing protease and phosphatase inhibitor cocktails (Thermo Scientific, Rockford, IL) and protein concentration was measured using the BCA protein kit assay (Thermo Scientific). Protein were separated by 4–12% SDS-PAGE and blotted in a PVDF membrane (Bio-Rad, Hercules, CA) as previously described [21]. The following primary antibodies were used: mTOR (1:1000), cleaved PARP (Asp214) (1:1000), PARP (1:1000), S6 (1:1000), pS6 (S240/244) (1:1000) (Cell Signaling, Danvers, MA), GAPDH (1:1000) (FL-335), BCL-xL (1:1000) (S-18) (Santa Cruz Biotechnology, Santa Cruz, CA), BAX (1:1000) (06-499) (Upstate, Lake Placid, NY), and Actin (1:1000) (Sigma). The antigen-antibody complexes were visualized using a horseradish peroxidase–conjugated secondary antibody (Invitrogen) and an enhanced chemiluminescence system (Thermo).
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed on formalin-fixed paraffin- embedded (FFPE) sections from blocks of xenograft MPNSTs. Immuno detection of cleaved caspase-3 (1:400), and phospho-70S6K (thr389) (p70S6K) (1:100) (Cell Signaling) expression was performed by an indirect 3-stage immunoenzymatic method as previously described [17].
Xenograft MPNST and radiation therapy in vivo
Animal studies were approved by Johns Hopkins Animal Care and Use Committee. Female athymic nude mice (5–6 weeks) purchased from National Cancer Institute (Bethesda, MD) were kept in a pathogen-controlled environment with access to food and water ad libitum. For in vivo experiments, tumor fragments (approximately 2 mm2) were implanted s.c. into the right flank of the mice. Animals were randomized in (a) Control (n = 4); (b) Everolimus (1.5 mg/kg, orally 5 days a week) + Bortezomib (1.3 mg/m2, intraperitoneally 2 days a week) (n=4); (c) Radiation Therapy (RT) (n=5); (d) RT + Everolimus (1.5 mg/kg) (n=5); (e) RT + Bortezomib (1.3 mg/m2) (n=5); (f) RT + Everolimus (1.5 mg/kg) + Bortezomib (1.3 mg/m2) (n=5). Ten days after tumor implantation local radiotherapy was performed by using the Small Animal Radiation Research Platform (SARRP), which mimics the isocentric external-beam used to deliver image-guided radiotherapy in patients [22]. In brief, a 2.3 mm CT-Spot (Beekley Medical, Bristol, CT) was used to locate the tumor in the microcomputer tomography (CT) system. Next, the tumor was locally subjected to 10 Gy of radiation in a single dose (3-mm×3-mm beam) 4 mm below the mouse skin. The first dose of everolimus and bortezomib were administrated five hours after RT. Dry powder compounds were dissolved in 1% DMSO and 0.9% sterile saline and stored in aliquots at −20°C. Tumor volume was determined by caliper measurements once or twice a week according to the following formula: length×width2×0.5.
Statistical analysis
The statistical analysis was performed by using GraphPad Prism 5.0 (Prism). The data were expressed as mean ± SD. Two-way ANOVA followed by Tukey tests was used for multiple comparisons. P < 0.05 was considered as statistically significant.
RESULTS
Screening of 542 approved compounds with mTOR inhibitors identifies anti-proliferative combinations
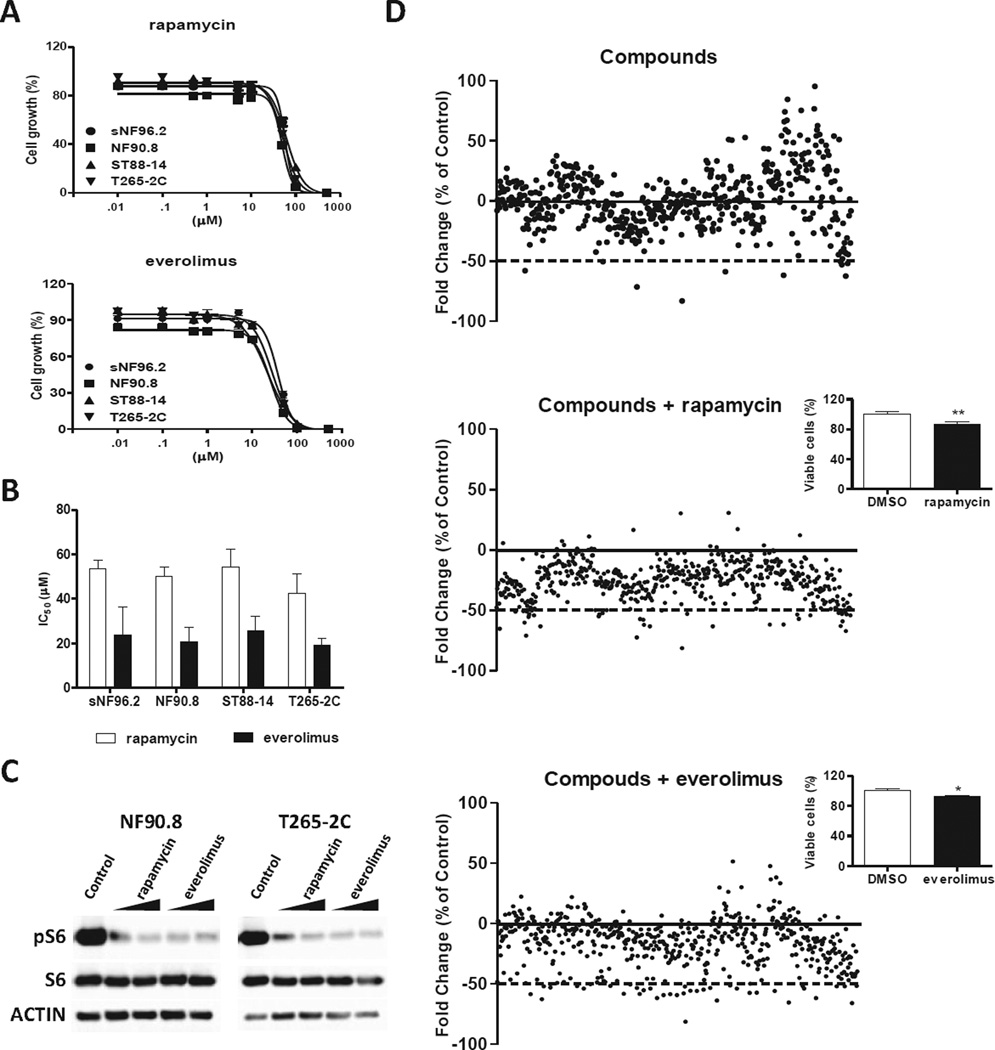
We first determined the effect of mTOR signaling inhibitors as a single agent. Dose-dependent growth inhibition was observed in MPNST cells treated with either rapamycin or everolimus (Fig. 1a). As a single agent mTOR inhibitors showed a very broad range of IC50 values in tumor cell lines without an apparent relation to tumor histotype [23–26]. Figure 1b shows the IC50 values in MPNST cells harboring inactivating NF1 mutations. Rapamycin and everolimus mTOR inhibitors strongly decreased pS6 protein expression in NF 90.8 and T265-2C cells (Fig. 1c). We then tested the combination of mTOR inhibitors with 542 compounds in NF90.8 cells, seeking potential candidates for drug combination (Fig. 1d). We selected 10 compounds for further study, based on their ability to decrease cell proliferation at 4 µM in combination with mTOR inhibitors at 25% of IC50 in additional cells, sNF96.2 and T265-2C (Supplementary Table 1). When optimizing across a range of doses, the calculated IC50 values for toremifen, riluzole and bortezomib were reduced in combination with mTOR inhibitors in human MPNST cells (Fig. 2a and Supplementary Table 2).
Figure 1. Cell-based drug screening assay identifies compounds with anti-proliferative effect in combination with mTOR inhibitors.
(A) MPNST cells were treated with nine different concentrations of rapamycin or everolimus (10 nM to 500 µM) and proliferation was analyzed by alamarBlue assay 72 hours after drug exposure. (B) IC50 values at 72 hours of four MPNST cell lines treated with rapamycin and everolimus. Data are represented as average ± standard deviation of four independent experiments with each containing three replicates. (C) NF90.8 and T265-2C cells were treated with rapamycin at 25% and 12.5% of IC50 values (12.5 µM and 6.2 µM for NF90.8 and 10 µM and 5 µM for T265-2C, respectively) or everolimus (5 µM and 2.5 µM for both MPNST cells); after 48 hours of drug exposure total protein lysates cells were separated by SDS-PAGE and analyzed by Western blot for pS6, total S6, and ACTIN. (D) Cell-based screening of drug libraries was performed in independent experiments, testing the libraries alone (upper plot) or the libraries in combination with rapamycin (middle plot) or everolimus (lower plot) at 25% of IC50 (12.5 µM and 5 µM, respectively) in NF90.8 cells. Each dot represents the cell proliferation change of individual compounds. Data represented as fold change vs. control group (DMSO). Insets show the effect of mTOR inhibitors as a single agent in cell proliferation.
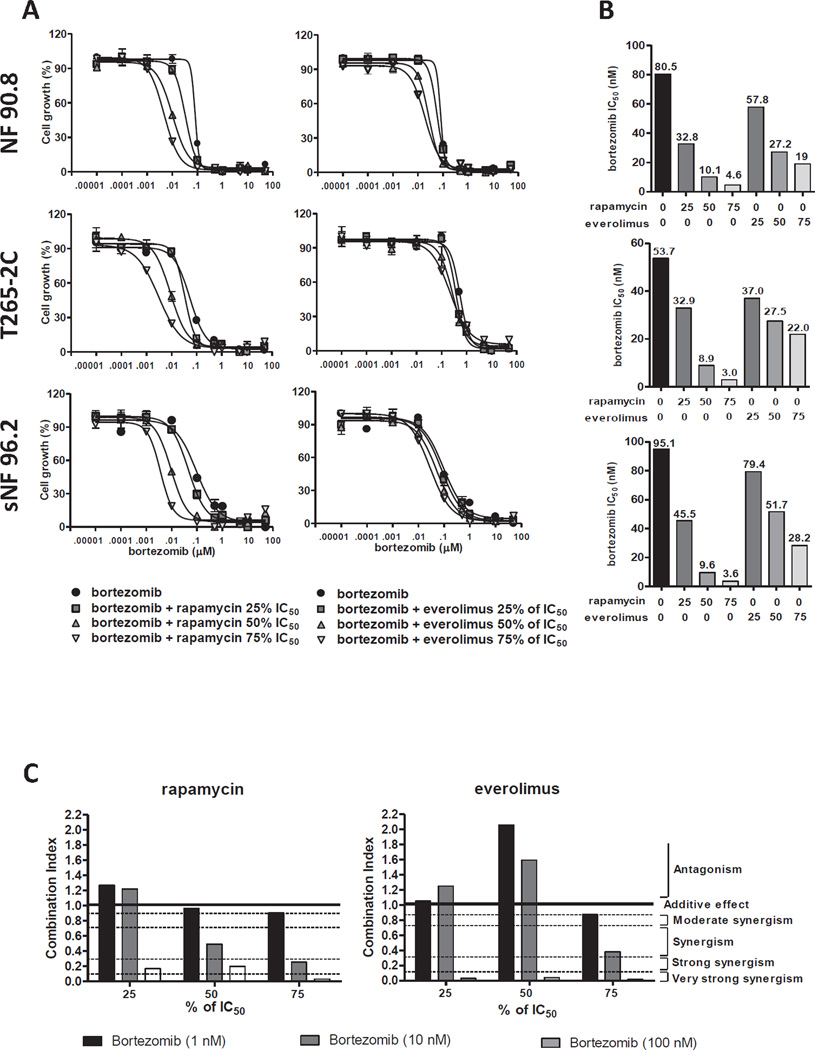
Figure 2. mTOR inhibitors sensitive the anti-proliferative effect induced by bortezomin in human MPNST cells.
(A) MPNST cells were treated with different concentrations of bortezomib (0.001 nM to 50 µM) plus rapamycin or everolimus at the indicated concentration and cell growth was analyzed by alamarBlue assay 72 hours after drugs exposure. (B) MPNST cells were treated with ten different concentrations of bortezomib in combination with rapamycin (25, 50, and 75% of IC5012.5, 25, and 37.5 µM, respectively) or everolimus (25, 50, 75% of IC505, 10, and 15 µM, respectively) and bortezomib IC50 value were calculated 72 hours after drug combination exposure, similar bortezomib IC50 results were obtained 96 hours after drug combination exposure. (C) Synergistic activity of mTOR inhibitors (25, 50 or 75% of IC50) and bortezomib (1, 10 and 100 nM) in NF90.8 cells was calculated using the method of Chou and Talalay. Combination index (CI) provides a quantitative definition for additive effect (CI: 0.9 to 1.1), synergism (CI: < 0.9), and antagonism (CI: > 1.1) in drug combinations studies.
Combinations of mTOR inhibitors and bortezomib decreased human MPNST cells proliferation
We identified bortezomib as a potential compound against MPNST cell proliferation in combination with mTOR inhibitors. The dose response curve of bortezomib in combination with different concentrations of mTOR inhibitors showed a leftward shift in the cell viability curve, indicating an interaction between these drugs in MPNST cell growth inhibition (Fig. 2a). Rapamycin and everolimus, at 75% of IC50, reduced the bortezomib IC50 value in 94 to 96% and 59 to 76% in MPNST cells, respectively (Fig. 2b). Bortezomib, at a concentration of 25% of the IC50reduced the IC50 for mTOR inhibitors in MPNST cells (Supplementary table 3). The synergistic combination was verified by using the Chou-Talalay method for drug combination. Although bortezomib at low concentration (1nM) showed moderate synergism or antagonism, higher concentration of bortezomib with a range of concentration of mTOR inhibitors yielded very strong synergism in MPNST cells (Fig. 2b and Supplementary table 4). Bortezomib as a single agent reduced chymotrypsin-like activity of the 20S proteasome MPNST cells (Supplementary figure 1).
Synergistic combination of mTOR inhibitors and bortezomib radiosensitizes MPNST cells
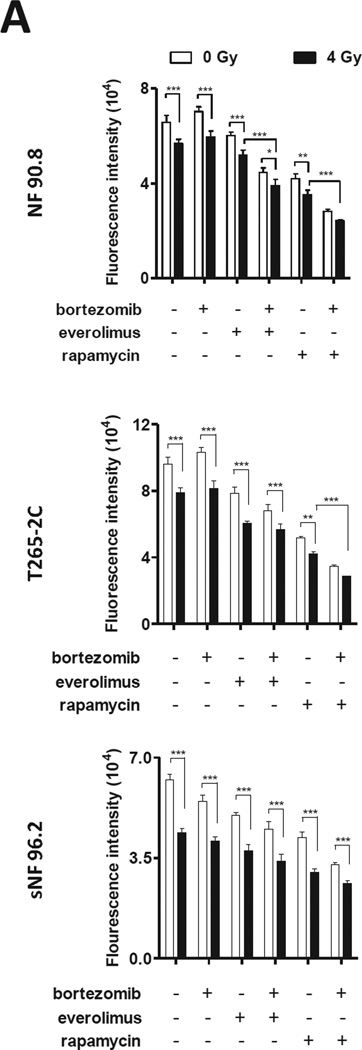
Since mTOR inhibition radio-sensitizes soft tumor sarcoma and the currently treatment of MPNST involves radiation therapy [4, 27], we reasoned that mTOR inhibition and bortezomib treatment might enhance the effects of radiation on MPNST cells. A reduction in cell viability after radiation was observed in a dose-dependent manner in all MPNST cells tested (Supplementary figure 2). When testing the combination of radiation (12 Gy) with inhibition of mTOR by drugs or siRNA mediated gene silencing; we observed a reduction in MPNST cell proliferation (Supplementary figure 4 and 5). Therefore, we examined the combination of mTOR inhibitors with bortezomib plus radiation in MPNST cells. We tested a lower radiation dose (4Gy) to determine if we could observe synergism between drugs and radiation in a low dose. The triple treatment was the most effective combination to reduce MPNST cell proliferation (Fig. 3).
Figure 3. The drug combination with radiation further decreases MPNST cell proliferation.
(A) MPNST cells were subjected to radiation (4 Gy) Gammacell 1000 (2.24 Gy/min; radiation source: Caesium137), treated with bortezomib (5 nM) plus rapamycin at 50% of IC50 (25 M for NF90.8; 21 M for T265-2C cells and 27 M for sNF96.2) or everolimus 50% of IC50 (10 M for NF90.8; 9.6 M for T265-2C cells and 11.8 M for sNF96.2) and after 96 hours cell growth was assessed by alamarBlue assay. (*) P < 0.05; (**) P < 0.01; (***) P < 0.0001.
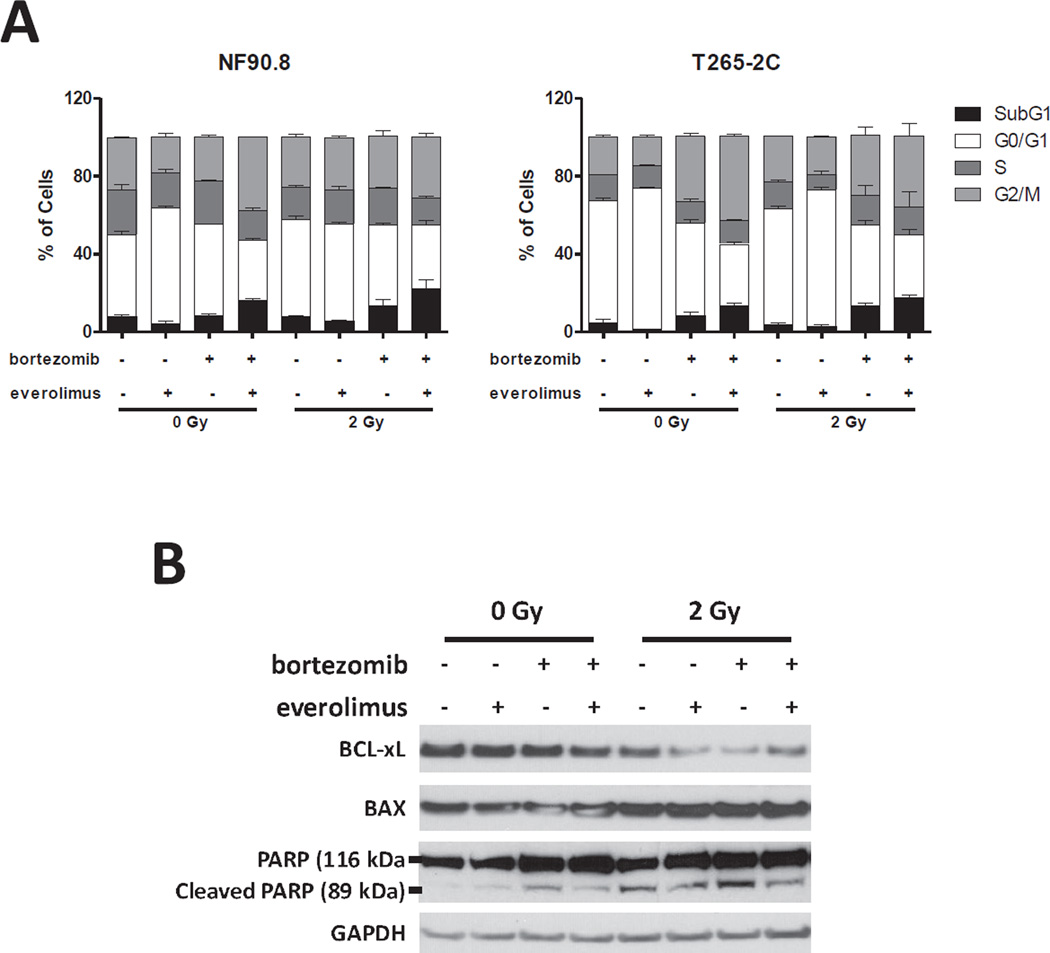
To assess whether this growth inhibition induced by drug combination and radiation was associated with cell cycle modulation, we analyzed the cell cycle distribution by flow cytometry. As expected, everolimus as a single agent induces G0/G1 cell cycle arrest. The combination of everolimus and bortezomib plus radiation shows a G2/M arrest and increased SubG1 fraction in MPNST cells (Fig. 4a). To provide further evidence that the therapy combination modulates apoptosis, we performed a protein expression analysis of apoptosis regulators and cleaved-PARP. BCL-xL expression decreased and BAX protein expression slightly increased in cells treated with the combination of everolimus, bortezomib and radiation in NF90.8 cells. In addition, Western blot analysis showed an increased in cleaved-PARP after radiation treatment in NF90.8 cells (Fig. 4b).
Figure 4. The drug combination plus radiation regulates cell cycle and apoptosis.
(A) NF90.8 and T265-2C cell were submitted to radiation (2 Gy) Gammacell 1000 (2.24 Gy/min; radiation source: Caesium137), treated with bortezomib (5 nM) plus everolimus at 50% of IC50 (10 µM for NF90.8 and 9.6 µM for T265-2C cells) and after 48 hours DNA were stained with propidium iodide (PI) and analyzed by flow cytometry. Data are expressed as average ± standard deviation (n=3) of two independent experiments performed in triplicate. (B) NF90.8 cell line was submitted to radiation (2 Gy), treated with bortezomib (5 nM) plus everolimus (50% of IC50) (10 µM for NF90.8) and after 48 hours total protein lysates cells were separated by SDS-PAGE and analyzed by Western blot for BAX, BCL-xL, cleaved-PARP (89 kDa), PARP (116 kDa) and GAPDH.
The combination of mTOR inhibition, bortezomib, and radiation decreases MPNST growth in vivo
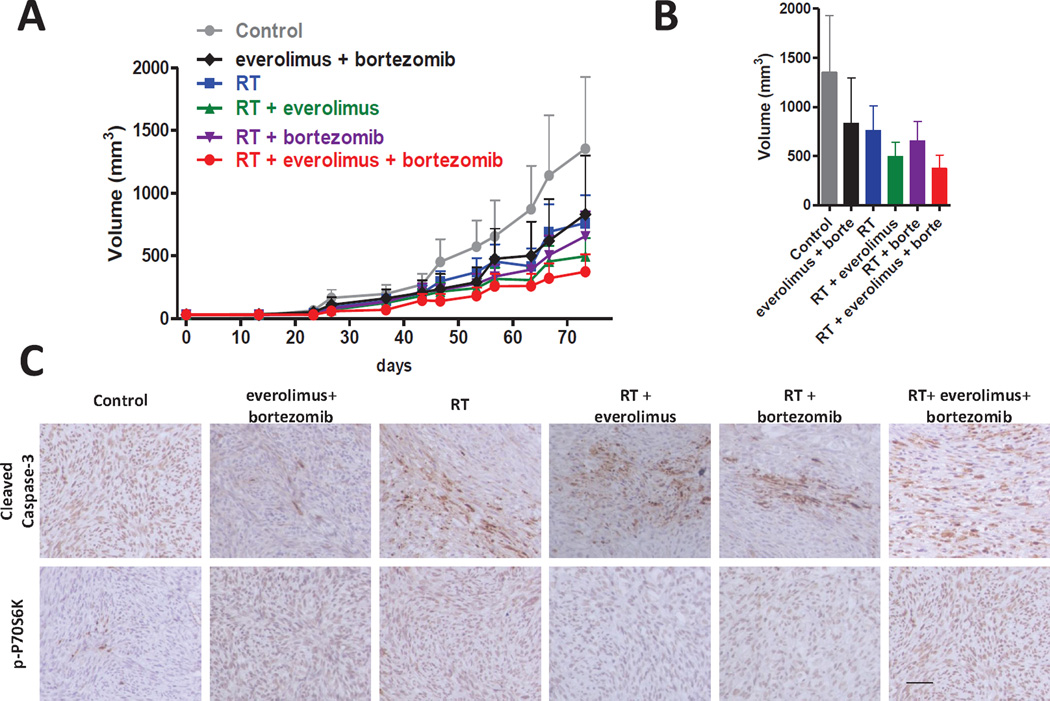
Encouraged by our in vitro data, we tested if the drug combination with radiation could reduce growth of flank MPNST xenografts. A preliminary toxicity test was performed to test if drug combination induces a systemic toxicity. Animals treated with everolimus (1.5 mg/kg) in combination with different concentrations of bortezomib (0.65; 0.95; and 1.3 mg/m2) did not show weight loss (Supplementary figure 5), suggesting that the combination of everolimus and bortezomib did not induced obvious systemic toxicity. Therefore, we implanted NF90.8 cells subcutaneously nude mice and treated with radiotherapy and/or drugs. In order to mimic the human doses, the drugs administered in mice were similar to the FDA approved human doses according with body surface mass for bortezomib and body mass for everolimus [28]. In this experiment the combination of everolimus and bortezomib decreased the tumor volume in 38% when compared with control group, a similar effect was observed in radiation therapy (RT) mice with a reduction in tumor volume in 43% when compared with control group. RT combined with everolimus or bortezomib decreased the tumor volume in 63% and 51%, respectively, when compared with control group. The mice treated with RT + everolimus + bortezomib had the lower mean tumor volume, decreasing 72% when compared with control group (Fig. 5a and 5b). We confirmed the mTOR signaling inhibition in xenograft MPNST model by the immunostaining of phospho-P70S6K (p-P70S6K) expression. The groups treated with the everolimus slightly reduced p-P70S6K expression. In addition, cleaved caspase-3 was increased in all groups subjected to radiation, suggesting that radiation therapy predominantly induces apoptosis in xenograft MPNST (Fig. 5c).
Figure 5. The drug combination plus radiation decreases MPNST xenograft tumor growth in vivo.
(A) NF90.8 tumor was implanted subcutaneously in nude mice and tumor volume was measured over time. RT (10 Gy) was performed ten days after xenograft implantation using the Small Animal Radiation Resource Platform. Tumor volume curves were plotted against days of treatment. (B) Histogram represents the average final tumor volume. (C) Representative tissue sections of immunohistochemical analysis of phosphor-p70S6K, and cleaved caspase-3 in NF90.8 xenografts. RT: radiation therapy. Original magnification: x200; Scale bar: 50 µm.
DISCUSSION
The pathological molecular effects that occur after NF1 loss, such as increased mTOR signaling, provide possible opportunities for treating NF1-associated tumors such as MPNSTs [10, 29]. However, a combination approach should be considered during the early stages of therapeutic strategy design to help combat the resistance that quickly develops to single agent targeted therapy. This preclinical investigation of combination therapy for MPNST has as its foundation mTOR inhibition. Our drug screen with mTOR inhibitors identified synergistic compounds that decrease cell proliferation. We further characterized a triple treatment in vitro and in vivo by adding radiation to mTOR inhibitors and the synergistic bortezomib. This combination decreased, but unfortunately did not halt, growth of MPNST flank xenografts.
Aberrant activation of mTOR signaling is observed in several types of cancer and currently several drugs targeting mTOR signaling are in clinical tests [30]. mTOR signaling plays a central role in MPNST and it is a reasonable target for this tumor type. However, cancer cells acquire drug resistance against single agent therapy [31]. Therefore, drug combination is often a necessary strategy to overcome drug resistance [30, 32]. In addition, drugs with strong synergy might theoretically improve efficacy and decrease normal cell toxicity by reducing required drug concentrations [33].
NF1 plays an important role in MPNST tumorigenesis, and drugs that show preferential sensitivity for this mutation have potential clinical utility [34, 35]. Cancer cell lines harboring NF1 mutation are more sensitive to bortezomib, suggesting that the proteasome is a useful target for MPNST associated with the NF1 genetic alteration [35]. The combination of an mTOR inhibitor and bortezomib shows synergistic activity in non-solid tumors cells (mantle lymphoma and B-acute lymphocytic leukemia) and in hepatocellular carcinoma cells [24, 26, 36]. In addition, the combination of temsirolimus, an analogue of rapamycin, with bortezomib in relapsed and refractory multiple myeloma patients showed clinical benefit and manageable adverse effects [37]. Although mTOR inhibitors with bortezomib showed synergy in different tumor types, additional investigation is needed.
The addition of radiation in our combination therapy helped reduce cell growth. Down-regulation of BCL-xL and a slight increased in BAX expression was observed in vitro, suggesting that MPNST apoptosis cell was triggered by the combination therapy. Interestingly, BCL-xL is overexpressed in human MPNST samples and NF1 deficiency upregulates BCL-xL in vitro. Furthermore, BCL-xL pharmacological inhibition decreased MPNST cell growth in combination with doxorubicin [38]. Therefore, modulating levels of BCL-xL directly, or by targeting upstream regulators, could be an effective inducer of apoptosis. The induction of apoptosis was confirmed by the increase of cleaved caspase-3 in vivo, suggesting that inhibition of MPNST cell growth is due at least in part to apoptosis. Furthermore, adjuvant local radiotherapy improves control of local disease, while small molecule combinations can reach metastatic disease [3, 39].
In summary, our preclinical study yielded some important insights. First, cell-based screening assay for identification of approved compounds that cooperates to decrease cell proliferation is a potentially valuable tool that could accelerate the translational of a therapy to the clinic. Second, using this approach we identified possible drug combination candidates for MPNST treatment. Finally, pharmacologic and side effects for these drugs are known in human, supporting faster translation to the clinic. These preclinical data support a combination therapy, that might include some or all of the modalities tested here, for possible clinical trials in MPNST patients.
Supplementary Material
Acknowledgment
The NF90.8 and ST88-14 cells were donated by Dr Michael Tainsky (Wayne University, Detroit, MI) and T265-2C was donated by Dr Steven Porcelli (Albert Einstein College of Medicine, Bronx, NY). The authors thank for Dr William Tadeu Lara Festuccia for their valuable suggestions to improve the quality of paper.
Funding
Funding was provided by the U.S. Department of Defense grant W81XWH-10-1-0387 (GJR), the Virginia and D.K. Ludwig Fund for Cancer Research, the Irving J Sherman Research Professorship (GJR) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), graduate student’s scholarship grant (BEX-5939/11-9) to ASY.
Footnotes
Disclosures/Conflict of Interest Statement
The authors declare no conflict of interest that would prejudice the impartiality of this scientific work.
REFERENCES
- 1.Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, Wasif N. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–885. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 2.Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, Pollock RE, Lev D. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 3.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–360. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 4.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, Towers R, Gleeson M, Steiger C, Kirby A. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 6.DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 7.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE, Slopis JM, Meric- Bernstam F, Peng Z, Bornmann WG, Mills GB, Lazar AJ, Pollock RE, Lev D. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 9.Endo M, Yamamoto H, Setsu N, Kohashi K, Takahashi Y, Ishii T, Iida K, Matsumoto Y, Hakozaki M, Aoki M, Iwasaki H, Dobashi Y, Nishiyama K, Iwamoto Y, Oda Y. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1067. [DOI] [PubMed] [Google Scholar]
- 10.Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, Rioth MJ, McClatchey A, Ryeom S, Cichowski K. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 11.Johansson G, Mahller YY, Collins MH, Kim MO, Nobukuni T, Perentesis J, Cripe TP, Lane HA, Kozma SC, Thomas G, Ratner N. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–1245. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, Maertens O, Jeong SM, Bronson RT, Lebleu V, Kalluri R, Normant E, Haigis MC, Manning BD, Wong KK, Macleod KF, Cichowski K. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im JS, Tapinos N, Chae GT, Illarionov PA, Besra GS, DeVries GH, Modlin RL, Sieling PA, Rambukkana A, Porcelli SA. Expression of CD1d molecules by human schwann cells and potential interactions with immunoregulatory invariant NK T cells. J Immunol. 2006;177:5226–5235. doi: 10.4049/jimmunol.177.8.5226. [DOI] [PubMed] [Google Scholar]
- 16.Sun D, Haddad R, Kraniak JM, Horne SD, Tainsky MA. RAS/MEK- independent gene expression reveals BMP2-related malignant phenotypes in the Nf1-deficient MPNST. Mol Cancer Res. 2013;11:616–627. doi: 10.1158/1541-7786.MCR-12-0593. [DOI] [PubMed] [Google Scholar]
- 17.Baia GS, Caballero OL, Orr BA, Lal A, Ho JS, Cowdrey C, Tihan T, Mawrin C, Riggins GJ. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012;10:904–913. doi: 10.1158/1541-7786.MCR-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 19.Trembath DG, Lal A, Kroll DJ, Oberlies NH, Riggins GJ. A novel small molecule that selectively inhibits glioblastoma cells expressing EGFRvIII. Mol Cancer. 2007;6:30. doi: 10.1186/1476-4598-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-beta by repressing SMAD4 in thyroid cancer. Oncogene. 2011;31:1910–1922. doi: 10.1038/onc.2011.381. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, Baia GS, Kimura ET. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6:197–205. doi: 10.1593/tlo.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, Gray O, Verhaegen F, McNutt T, Ford E, DeWeese TL. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoi H, Dilling MB, Liu LN, Danks MK, Shikata T, Sekulic A, Abraham RT, Lawrence JC, Jr, Houghton PJ. Studies on the mechanism of resistance to rapamycin in human cancer cells. Mol Pharmacol. 1998;54:815–824. doi: 10.1124/mol.54.5.815. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Gao D, Guo K, Kang X, Jiang K, Sun C, Li Y, Sun L, Shu H, Jin G, Sun H, Wu W, Liu Y. Novel synergistic antitumor effects of rapamycin with bortezomib on hepatocellular carcinoma cells and orthotopic tumor model. BMC Cancer. 2012;12:166. doi: 10.1186/1471-2407-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly T, McSheehy PM. Biomarker Development for the Clinical Activity of the mTOR Inhibitor Everolimus (RAD001): Processes, Limitations, and Further Proposals. Transl Oncol. 2010;3:65–79. doi: 10.1593/tlo.09277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders P, Cisterne A, Weiss J, Bradstock KF, Bendall LJ. The mammalian target of rapamycin inhibitor RAD001 (everolimus) synergizes with chemotherapeutic agents, ionizing radiation and proteasome inhibitors in pre- B acute lymphocytic leukemia. Haematologica. 2011;96:69–77. doi: 10.3324/haematol.2010.026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JD, Spalding AC, Somnay YR, Markwart S, Ray ME, Hamstra DA. Inhibition of mTOR radiosensitizes soft tissue sarcoma and tumor vasculature. Clin Cancer Res. 2009;15:589–596. doi: 10.1158/1078-0432.CCR-08-1019. [DOI] [PubMed] [Google Scholar]
- 28.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S, Gianino SM, Gao F, Christians U, Gutmann DH. Interpreting mammalian target of rapamycin and cell growth inhibition in a genetically engineered mouse model of Nf1-deficient astrocytes. Mol Cancer Ther. 2011;10:279–291. doi: 10.1158/1535-7163.MCT-10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 31.Broxterman HJ, Gotink KJ, Verheul HM. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Updat. 2009;12:114–126. doi: 10.1016/j.drup.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Sano D, Matsumoto F, Valdecanas DR, Zhao M, Molkentine DP, Takahashi Y, Hanna EY, Papadimitrakopoulou V, Heymach J, Milas L, Myers JN. Vandetanib restores head and neck squamous cell carcinoma cells' sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. 2011;17:1815–1827. doi: 10.1158/1078-0432.CCR-10-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olaussen KA, Commo F, Tailler M, Lacroix L, Vitale I, Raza SQ, Richon C, Dessen P, Lazar V, Soria JC, Kroemer G. Synergistic proapoptotic effects of the two tyrosine kinase inhibitors pazopanib and lapatinib on multiple carcinoma cell lines. Oncogene. 2009;28:4249–4260. doi: 10.1038/onc.2009.277. [DOI] [PubMed] [Google Scholar]
- 34.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, Jr, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, Liu Q, Iorio F, Surdez D, Chen L, Milano RJ, Bignell GR, Tam AT, Davies H, Stevenson JA, Barthorpe S, Lutz SR, Kogera F, Lawrence K, McLaren-Douglas A, Mitropoulos X, Mironenko T, Thi H, Richardson L, Zhou W, Jewitt F, Zhang T, O'Brien P, Boisvert JL, Price S, Hur W, Yang W, Deng X, Butler A, Choi HG, Chang JW, Baselga J, Stamenkovic I, Engelman JA, Sharma SV, Delattre O, Saez-Rodriguez J, Gray NS, Settleman J, Futreal PA, Haber DA, Stratton MR, Ramaswamy S, McDermott U, Benes CH. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haritunians T, Mori A, O'Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 37.Ghobrial IM, Weller E, Vij R, Munshi NC, Banwait R, Bagshaw M, Schlossman R, Leduc R, Chuma S, Kunsman J, Laubach J, Jakubowiak AJ, Maiso P, Roccaro A, Armand P, Dollard A, Warren D, Harris B, Poon T, Sam A, Rodig S, Anderson KC, Richardson PG. Weekly bortezomib in combination with temsirolimus in relapsed or relapsed and refractory multiple myeloma: a multicentre, phase 1/2, open-label, dose-escalation study. Lancet Oncol. 2011;12:263–272. doi: 10.1016/S1470-2045(11)70028-6. [DOI] [PubMed] [Google Scholar]
- 38.Park HJ, Lee SJ, Sohn YB, Jin HS, Han JH, Kim YB, Yim H, Jeong SY. NF1 deficiency causes Bcl-xL upregulation in Schwann cells derived from neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Int J Oncol. 2013;42:657–666. doi: 10.3892/ijo.2012.1751. [DOI] [PubMed] [Google Scholar]
- 39.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co- opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.